Abstract
Background
The influence of airborne particulate matter (PM) on skin has primarily been studied in patients with skin diseases such as atopic dermatitis. Recently, the effect of PM on healthy human skin has gained attention.
Objective
To evaluate the relationship between PM concentration and objective skin changes in healthy subjects.
Methods
This prospective study enrolled 25 healthy volunteers without any skin disease. Data regarding daily meteorological parameters and air pollution were collected during a high-PM period and a low-PM period for 14 days. Environmental and lifestyle factors that might influence skin conditions of subjects were also collected during the study period. Biophysical parameters of the skin such as transepidermal water loss (TEWL), hydration, erythema index, and melanin index were measured. Pores, wrinkles, sebum, and skin tone were evaluated using a facial analysis system.
Results
Mean TEWL value during the high-PM period was significantly higher than that during the low-PM period (10.16 g/m2/h vs. 5.99 g/m2/h; p=0.0005). Mean erythema index was significantly higher in the high-PM period than that in the low-PM period (4.3 vs. 3.42; p=0.038). For facial analysis system indices, uniformity of skin tone was higher in the low-PM period than that in the high-PM period (p<0.0001). In addition, with increasing PM10 and PM2.5, TEWL also showed increase when other environmental components were constant (regression coefficient [RC]=0.1529, p<0.0001 for PM10; RC=0.2055, p=0.0153 for PM2.5).
Conclusion
Increased PM concentrations may contribute to disturbed barrier function, increased facial erythema, and uneven skin tone even in healthy human skin.
Keywords: Comparative study, Particulate matter, Skin
INTRODUCTION
Among various environmental factors, particulate matter (PM) has gained attention in recent years. Airborne PM can be categorized by median aerodynamic particle diameters1. PM10 is a particle smaller than 10 µm in diameter. It comprises dust, industrial emissions, and traffic emissions2. PM2.5, also called fine PM, is a particle with a diameter less than 2.5 µm. It is composed of organic carbon compounds, nitrates, and sulfates2,3. Many clinical, epidemiological and toxicological studies have demonstrated that PM exposure is associated with a high risk for cancer, respiratory diseases, pulmonary diseases and cardiovascular diseases4,5,6,7,8. Although effects of PM on skin have been recently reported, the number of such studies is relatively small compared to studies on effects of PM on other organs9,10,11.
Recent studies have shown that high concentrations of PM10 and PM2.5 are associated with the development and exacerbation of various skin diseases such as atopic dermatitis, allergic skin diseases, and skin aging 3,7,10,12,13. Our previous study has evaluated daily skin changes using a smartphone application and revealed that the cumulative effect of PM2.5 is significantly associated with increased wrinkle in volunteers without any skin disease14. A self-portrait smartphone application has an advantage in that it is easy to obtain daily skin condition. However, it has limitation in that it cannot clearly reveal the degree of pigmentation, erythema or hydration of the face since it is evaluated by resolution of the photograph. In practice, patients often complain of skin discomfort, such as troubles and blemishes, during days with high PM concentrations. Therefore, a comprehensive evaluation of facial skin characteristics using various non-invasive instruments and skin analysis systems would be useful to determine influences of PM on healthy human skin.
The aim of this study was to quantitatively measure the facial skin characteristics according to PM concentration. To determine the specific influence of PM on skin, data regarding other daily uses, lifestyle, and environmental factors that might influence the overall skin condition were also collected and adjusted in the analysis. Therefore, facial skin characteristics of the same person were evaluated during high- and low-PM periods to differentiate the exact effect of PM on skin.
MATERIALS AND METHODS
Determination of high-PM and low-PM periods
As daily PM concentration varies, it is difficult to determine high and low PM on a daily basis. Previous studies have indicated that the average concentration of PM in Seoul is higher in spring than in other seasons due to transport of aerosols from the Asian continent15,16. In contrast, in summer, frequent precipitation and low transport from the Asian continent will lead to a lower concentration of PM than in other seasons16. Cumulative effects of PM should also be considered in the analysis. Therefore, the high- and low-PM periods should be at least one month apart to wash out the cumulative effect. For these reasons, days with a high average PM concentration during spring and those with a low PM concentration during early summer were selected as study periods.
Meteorological measurements and air pollution
This study was performed in a Gangnam-gu, Seoul metropolitan region of Korea, located in the eastern part of Asia. Air pollution and meteorological information in Gangnam-gu were obtained from Korea Meteorological Administration (KMA) and AIRKOREA (www.airkorea.or.kr). KMA provides information regarding hourly outdoor temperature, relative humidity, wind velocity and ultraviolet (UV) index based on data measured at 76 automatic weather stations throughout Seoul area. Average daily values were used for each study period. The AIRKOREA website is operated by the Korean Ministry of Environment. It provides information regarding air quality level including PM10, PM2.5, O3, NO2, CO, and SO3. Average daily concentration of each pollutant was calculated from the data acquired at the station located in Gangnam-gu. These methods of collecting meteorological information and air pollution data have been previously reported14.
Study population and study design
This study was conducted in accordance with the Declaration of Helsinki for ethical conduct of research involving human subjects. The study protocol was approved by the Institutional Review Board of Samsung Medical Center (2017-10-0085). Volunteers working and residing in Gangnam-gu of Seoul who were outdoors more than one hour a day during their commute were enrolled in this study. Subjects with significant skin disease (i.e., atopic dermatitis, allergic skin diseases or psoriasis), those who had complaints of sensitive skin, those who were taking medications that could interfere with study results, and those who had undergone any skin treatment within 3 months prior to participating in this study were excluded. All volunteers provided written informed consent before this study. During the study period, personal conditions were required as consistent as possible for better analysis. During high- and low-PM periods, volunteers were asked to record their daily life events that could affect skin condition (such as sleeping less than 6 hours, working at night for more than 6 hours, consuming alcohol, smoking, and having high glycemic index diet) every day for two weeks. Participants who performed outdoor activities for more than 8 hours were encouraged to detail their activities. They were required not to take a vacation that involved sunlight exposure. They were also asked not to change their lifestyles. They were instructed to apply regularly a sunscreen during study periods. This study was conducted for two periods in each individual. The high-PM period was occurred during spring of either 2017 or 2018, and the low-PM period was during early summer of either 2017 or 2018. Objective skin measurements were performed on day 1 and day 14 for each period. Since no specific treatment was applied during these periods, and there were no changes in external factors other than meteorological factors, analysis was performed using average values of measured data on day 1 and day 14.
Assessment of facial skin characteristics
At each visit, volunteers were asked to wash their face and arms followed by drying for 15~20 minutes before skin assessments. They were allowed to relax. They were then assessed in the same room, with temperature maintained between 20℃ and 22℃ and a relative humidity between 30% and 40%17,18.
Transepidermal water loss (TEWL), melanin index and erythema index were measured at the front of both cheeks, which was the point with distance of 3 cm vertically from the mid-pupillary line. TEWL (g/m2/h) was measured using a Tewameter® TM210 (Courage and Khazaka, Koln, Germany). The instrument was placed vertically on the skin, and the pressure was applied for 30~45 seconds. Using a Mexameterr® MX16 (Courage and Khazaka), melanin and erythema indices were also measured. A slight pressure was applied vertically to the skin. Three repetitive measurements were performed at each site and their average values were used in further analyses. The skin on the upper inner arm was also measured. Its value was used as a reference value to adjust for seasonal influence. Values of erythema and melanin indices used for analyses were calculated as delta value between those measured on the face and the inner part of upper arm.
A comprehensive facial-analysis system (Janusr®; PIE Co., Ltd, Seoul, Korea) was used in this study. It had a 10-megapixel, ultra-high-resolution camera for analyzing skin pore, wrinkle, sebum, and skin tone under normal, polarized, and UV light. It has Janusr® light-control technology that uses a brightness reference to capture only within a certain brightness range to increase reproducibility of the data. This system uses the light source that is most suitable for each skin measurement parameter to increase reliability of the data. The area of the measurement site was analysed using a 100-point scale to determine the area occupied by pores or wrinkles. In particular, when using an UV light, it is possible to evaluate the degree of sebum as porphyrin produced by Propionibacterium acne in sebum emits orange light. Higher scores correspond to higher numbers of pores, larger degrees of wrinkles, and larger amounts of sebum. Average values of red, green, and blue values of cheeks, nose, eyes, and forehead were calculated and converted to a 100-point skin tone grading, with a higher value indicating a brighter and more uniform skin.
Statistical analysis
Mean value and standard deviation (SD) were calculated for each outcome measured (24-hour averages for meteorological measurements and air pollution, and averages of day 1 and day 14 for facial skin characteristics). Wilcoxon signed-rank test was used to compare demographic characteristics of the study population. Differences of meteorological variable between the two periods were tested using an autoregressive error model with first-order auto-correlation. The first-order autocorrelation was examined with Durbin-Watson statistics. Facial skin characteristics were also plotted for two periods and compared between the two periods using paired t-test or Wilcoxon signed-rank test. Effects of Meteorological measurements and air pollution on facial skin characteristics were analysed using generalized estimating equations due to repeated measurements for both univariable and multivariable analyses. Multicollinearity among meteorological variables was checked with variance influence factor (VIF) before the multivariable analysis. Because temperature, NO2, and CO had multicollinearity (VIF>4) with other meteorological variables, they were excluded from the multivariate analysis. SAS software, version 9.4 (SAS Institute, Cary, NC, USA) was used for all analyses. Statistical significance was considered when defined as p-value was less than 0.05. All statistical analyses were conducted by two biostatistics specialists (SW Kim and MJ Kim).
RESULTS
Study population
Baseline demographic characteristics of volunteers are presented in Table 1. Enrolled volunteers were 25 non-smoking females with a mean age of 36.7 years (range, 23~56 years). No participant spent any time outside the Gangnamgu area during the study period. Of 700 total person-records, 350 were collected during the high-PM period and 350 were collected during the low-PM period. The total number of person-visits for measurement of facial skin characteristics was 100 (50 in the high-PM period and 50 in the low-PM period). There were no statistically significant differences in sleeping time, night-working time, or alcohol intake between the two periods. However, the frequency of high glycemic index diet was different during the two periods (1.32 times per 2 weeks in the high-PM period vs. 2.44 times per 2 weeks in the low-PM period).
Table 1. Demographic characteristics of study population.
| Variable | High-PM period | Low-PM period | p-value |
|---|---|---|---|
| Total no. of volunteers | 25 | ||
| Age (yr), mean (range) | 36.7 (23∼56) | ||
| Outdoor activity ≥8 h* | 0.72 | 0.40 | 0.3125 |
| Sleeping time ≤6 h* | 2.24 | 2.52 | 0.6589 |
| Night working ≥6 h* | 0.16 | 0.44 | 0.5000 |
| Drinking* | 1.08 | 1.48 | 0.2451 |
| Smoking* | 0 | 0 | |
| High glycemic index diet* | 1.32 | 2.44 | 0.0042 |
Wilcoxon signed-rank test was used. PM: particulate matter. *Average times per 2 weeks. Each individual recorded data daily for 14 days during high-PM period and low-PM period.
Changes in meteorological measurements and air pollution
Meteorological data during high-PM and low-PM periods are summarized in Table 2. In the high-PM period, average temperature, humidity, and wind velocity were 14.26℃±5.17℃, 58.49%±16.11%, and 1.90±0.56 m/s, respectively. In the low-PM period, average temperature, humidity, and wind velocity were 22.33℃±4.77℃, 63.91%±12.12%, and 2.05±0.67 m/s, respectively. Daily temperatures were significantly higher in the low-PM period (p=0.004). The mean±SD UV index were 4.75±1.94 in the high-PM period, and 4.37±1.39 in the low-PM period. Mean values of PM10 and PM2.5 in high-PM period were 57.25±29.68 µg/m3 and 31.86±20.24 µg/m3, respectively. These values were 26.5±13.76 µg/m3 and 14.54±8.56 µg/m3 in the low-PM period (p<0.0001 for PM10, p=0.0001 for PM2.5). Mean concentration of O3 was 17.98±5.39 ppb in the high-PM period and 18.21±7.87 ppb in the low-PM period. Mean concentration of NO2 was 44.26±14.96 ppb in the high-PM period, and 25.89±8.63 ppb in the low-PM period (p<0.0001). Mean concentration of CO was 0.6±0.13 ppm in the high-PM period, and 0.39±0.10 ppm in the low-PM period (p<0.0001). The mean concentration of SO3 was 4.56±1.53 ppb in the high-PM period, and 4.71±0.58 ppb in the low-PM period.
Table 2. Levels of meteorological measurements and air pollutants during high-PM period and low-PM period.
| Variable | High-PM period | Low-PM period | p-value | |
|---|---|---|---|---|
| Meteorological measurements & air pollutants | ||||
| Temperature (oC) | 14.26±5.17 | 22.33±4.77 | 0.0040* | |
| (2.1∼24.0) | (11.4∼31.4) | |||
| Relative humidity (%) | 58.49±16.11 | 63.91±12.12 | 0.0989 | |
| (22.9∼97.0) | (42.5∼95.6) | |||
| Wind velocity (m/s) | 1.90±0.56 | 2.05±0.67 | 0.2046 | |
| (1.1∼4.1) | (1.2∼4.0) | |||
| PM10 (μg/m3) | 57.25±29.68 | 26.5±13.76 | <0.0001* | |
| (10∼127) | (6∼70) | |||
| PM2.5 (μg/m3) | 31.86±20.24 | 14.54±8.56 | 0.0001* | |
| (5∼102) | (4∼40) | |||
| O3 (ppb) | 17.98±5.39 | 18.21±7.87 | 0.7701 | |
| (6∼37) | (7∼46) | |||
| NO2 (ppb) | 44.26±14.96 | 25.89±8.63 | <0.0001* | |
| (16∼94) | (12∼48) | |||
| CO (ppm) | 0.6±0.13 | 0.39±0.10 | <0.0001* | |
| (0.3∼0.9) | (0.2∼0.6) | |||
| SO3 (ppb) | 4.56±1.53 | 4.71±0.58 | 0.5939 | |
| (2∼8) | (1∼6) | |||
| UV index (AU) | 4.75±1.94 | 4.37±1.39 | 0.3286 | |
| (0.5∼10.0) | (1.9∼7.4) | |||
Values are presented as mean±standard deviation (range). All values are 24-hour averages. PM: particulate matter, UV: ultraviolet. *Significance of differences between high-PM and low-PM periods by autoregressive error model with first-order autocorrelation (p<0.05).
Changes in facial skin characteristics
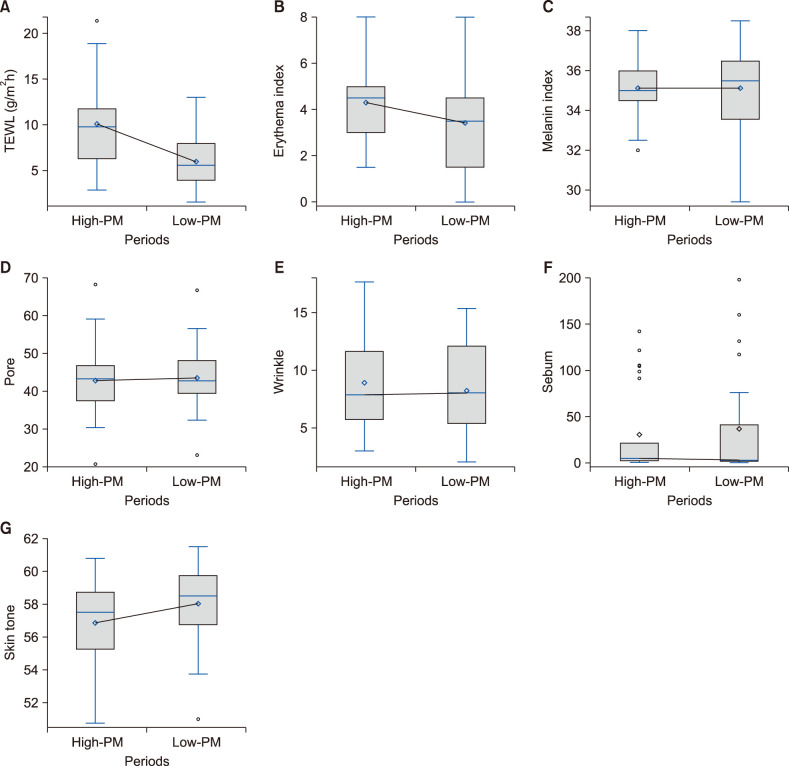
Changes in facial skin characteristics between high- and low-PM periods were analysed. Results are shown in Fig. 1. The mean TEWL value during the high-PM period was significantly higher than that during the low-PM period (10.16±4.77 g/m2/h vs. 5.99±2.87 g/m2/h; p=0.0005). The erythema index was also significantly higher in the high-PM period than in the low-PM period (4.3±1.53 vs. 3.42±2.08; p=0.0383). The melanin index was 35.12±1.53 for the high-PM period and 35.12±2.18 for the low-PM period. The mean Janusr® index was 42.8±9.31 for pores, 8.93±4.08 for wrinkles, 30.38±47.06 for sebum, and 56.87±2.59 for skin tone during the high-PM period. It was 43.59±9 for pores, 8.24±3.71 for wrinkles, 36.56±56.32 for sebum, and 58.04±2.57 for skin tone during the low-PM period. Skin tone was significantly higher in the low-PM period than that in the high-PM period (p<0.0001).
Fig. 1. Changes in facial skin characteristics according to high-particulate matter (PM) period and low-PM period. (A) Transepidermal water loss (TEWL), (B) erythema index, (C) melanin index, (D) pore, (E) wrinkle, (F) sebum, and (G) skin tone. Facial skin characteristics were plotted for two periods and compared between the two periods using paired t-test or Wilcoxon signed-rank test. Box indicates lower and upper quartiles. Central line indicates median value; Black points at the ends of the whisker indicate upper and lower extreme values. Values are presented as mean±standard deviation.
Effects of meteorological factors and air pollutants on facial skin characteristics
As PM10 increased, TEWL also significantly increased after adjusting for relative humidity, wind velocity, O3, SO3, and UV index (slope [standard error, SE]=0.1529 (0.0375); p<0.0001). As PM2.5 increased, TEWL also significantly increased adjusting for relative humidity, wind velocity, O3, SO3, and UV index (slope [SE]=0.2055 [0.0848]; p<0.0001). As PM10 increased, the erythema index also increased after adjusting for relative humidity, wind velocity, O3, SO3, and UV index (slope [SE]=0.042 [0.0132]; p=0.7522). As PM2.5 increased, erythema index also increased after adjusting for relative humidity, wind velocity, O3, SO3, and UV index (slope [SE]=0.017 [0.0196]; p=0.3867). As PM10 increased, skin tone also increased after adjusting for relative humidity, wind velocity, O3, SO3, and UV index (slope [SE]=0.0175 [0.0201]; p=0.3836). As PM2.5 increased, skin tone also increased after adjusting for relative humidity, wind velocity, O3, SO3, and UV index (slope [SE]=0.006 [0.0338]; p=0.8593).
DISCUSSION
The present study revealed that mean PM concentrations in spring were higher than those in summer, consistent with other studies10,15,16,19. In East Asia, PM10 levels peak in March through April20. In Korea, high PM concentrations are uncommon in summer due to frequent precipitation and less transport from the Asian continent16. Thus, effects of PM were observed by having high-PM (spring) and low-PM (early summer) periods in the present study. When dividing the study period, seasonal variation definitely influences the skin. Therefore, temperature, relative humidity, wind velocity and UV index were also taken into consideration for the evaluation of actual effects of PM on skin. Between the two study periods, temperature was statistically significantly different. However, relative humidity, wind velocity, and UV index failed to show significant differences. Dusts and sandstorms can exacerbate outdoor air pollution in Seoul from spring to early summer14. Thus, besides PM, other air pollutants such as O3, NO2, CO, and SO3, should also be evaluated. Both NO2 and CO levels in the high-PM period were significantly higher than those in the low-PM period. However, their levels were too low to show any significant association with facial skin characteristics.
TEWL can be used to assess skin barrier function and reflects the barrier activity of components of the stratum corneum and tight junctions. In this study, TEWL was significantly higher in the high-PM period than that in the low-PM period. The multivariate analysis after adjusting other factors showed a considerable increase of TEWL with increasing PM10 or PM2.5. How PM interacts with skin and eventually causes health problems remains controversial21,22. Araviiskaia et al.23 have suggested that harmful effects of air pollutants on the skin are influenced by direct penetration into the skin surface or by indirect distribution with the systemic distribution through respiratory system. They reviewed the literature to report biochemical effects of air pollutants including PM by increasing reactive oxygen species via the aryl hydrocarbon receptor23,24. Several epidemiological studies have shown that these pollutants including PM will eventually affect the exacerbation of symptoms of inflammatory diseases (e.g. eczema including atopic dermatitis) and skin aging9,10,14,25. Our results suggest that PM might impair the epidermal barrier function, resulting in the development of skin irritation and exacerbation of skin diseases. However, the slope of PM in our study was too small. Thus the effect of PM on healthy skin might be less than that on underlying skin disease. Further investigation is needed to elucidate this possibility.
This study has strengths in that dermatological impact of atmospheric pollution, with a focus on PM, was assessed for each individual during a precise period of time. Such an approach provides a dynamic monitoring of environmental effects while classical clinical studies generally compare data obtained in a static situation. Therefore, even if the number of volunteers is limited, information provided by this study might enrich the knowledge on pollution effects on skin.
Nevertheless, this study also has several limitations. First, the study duration was restricted to two weeks in spring and two weeks in early summer, making it difficult to recruit a sufficient cohort within that time frame. Although a large number of volunteers were not enrolled in this study, all 25 subjects adhered to the study protocol while daily PM concentrations were monitored. Second, hormonal levels could also be different based on seasonal variation. Particularly, not only glucocorticoids level, the periodic variation in estrogen levels over a volunteer's menstrual cycle could influence results. Third, we were unable to measure the amount of PM exposure for each subject individually. However, previous studies have shown that indoor PM environment in Seoul is highly dependent on outdoor conditions. Therefore, daily average PM concentration in the Gangnam-gu area can be expected to be applicable to each participant in the study26,27. Fourth, there was a statistically significant difference in high glycemic index diet between the high- and low-PM periods. The reason why the participants' dietary habits were statistically significant by period could not be found. It is possible that the number of volunteers was small, so it could have been statistically significant. However, the participants tended to have low glycemic index diet during the high-PM period compared to low-PM period, so a high glycemic index diet would not have been a factor affecting the outcome of facial skin characteristics.
To the best of our knowledge, this is the first study to assess correlations between PM concentration and objective facial skin characteristics measured using various non-invasive instruments by comparing intra-subject data between low- and high-PM periods. In the future, large numbers of clinical cohort studies on healthy skin and physiological studies on human skin are necessary to confirm these findings.
ACKNOWLEDGMENT
We thank SW Kim and MJ Kim of Samsung Biostatistics Institutes for assistance with statistical analysis.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This study was supported by a grant (2017R1D1A1B03032881) of the National Research Foundation of Korea. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
This study was previously preprinted in Research Square, contrary to the authors' intention during the process of submission to another journal earlier (https://www.researchsquare.com/article/rs-41713/v1)
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Schwarze PE, Ovrevik J, Låg M, Refsnes M, Nafstad P, Hetland RB, et al. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Hum Exp Toxicol. 2006;25:559–579. doi: 10.1177/096032706072520. [DOI] [PubMed] [Google Scholar]
- 2.Noh J, Sohn J, Cho J, Cho SK, Choi YJ, Kim C, et al. Short-term effects of ambient air pollution on emergency department visits for asthma: an assessment of effect modification by prior allergic disease history. J Prev Med Public Health. 2016;49:329–341. doi: 10.3961/jpmph.16.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngoc LTN, Park D, Lee Y, Lee YC. Systematic review and meta-analysis of human skin diseases due to particulate matter. Int J Environ Res Public Health. 2017;14:1458. doi: 10.3390/ijerph14121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 7.Peng F, Xue CH, Hwang SK, Li WH, Chen Z, Zhang JZ. Exposure to fine particulate matter associated with senile lentigo in Chinese women: a cross-sectional study. J Eur Acad Dermatol Venereol. 2017;31:355–360. doi: 10.1111/jdv.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyzga RE, Rohr AC. Long-term particulate matter exposure: attributing health effects to individual PM components. J Air Waste Manag Assoc. 2015;65:523–543. doi: 10.1080/10962247.2015.1020396. [DOI] [PubMed] [Google Scholar]
- 9.Vierkötter A, Schikowski T, Ranft U, Sugiri D, Matsui M, Krämer U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol. 2010;130:2719–2726. doi: 10.1038/jid.2010.204. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132:495–498. doi: 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Noma H, Kurai J, Sano H, Iwata K, Hantan D, et al. Association of short-term exposure to ambient fine particulate matter with skin symptoms in schoolchildren: a panel study in a rural area of Western Japan. Int J Environ Res Public Health. 2017;14:299. doi: 10.3390/ijerph14030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh I, Lee J, Ahn K, Kim J, Kim YM, Sim CS, et al. Association between particulate matter concentration and symptoms of atopic dermatitis in children living in an industrial urban area of South Korea. Environ Res. 2018;160:462–468. doi: 10.1016/j.envres.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Otani S, Onishi K, Mu H, Yokoyama Y, Hosoda T, Okamoto M, et al. The relationship between skin symptoms and allergic reactions to Asian dust. Int J Environ Res Public Health. 2012;9:4606–4614. doi: 10.3390/ijerph9124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JH, Oh SJ, Lee JH. Effects of particulate matter on healthy human skin: a panel study using a smartphone application measuring daily skin condition. J Eur Acad Dermatol Venereol. 2019;33:1363–1368. doi: 10.1111/jdv.15517. [DOI] [PubMed] [Google Scholar]
- 15.Jin Q, Fang X, Wen B, Shan A. Spatio-temporal variations of PM2.5 emission in China from 2005 to 2014. Chemosphere. 2017;183:429–436. doi: 10.1016/j.chemosphere.2017.05.133. [DOI] [PubMed] [Google Scholar]
- 16.Choi SH, Ghim YS, Chang YS, Jung K. Behavior of particulate matter during high concentration episodes in Seoul. Environ Sci Pollut Res Int. 2014;21:5972–5982. doi: 10.1007/s11356-014-2555-y. [DOI] [PubMed] [Google Scholar]
- 17.Piérard GE, Piérard-Franchimont C, Marks R, Paye M, Rogiers V. EEMCO guidance for the in vivo assessment of skin greasiness. The EEMCO Group. Skin Pharmacol Appl Skin Physiol. 2000;13:372–389. doi: 10.1159/000029945. [DOI] [PubMed] [Google Scholar]
- 18.Rogiers V EEMCO Group. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Skin Physiol. 2001;14:117–128. doi: 10.1159/000056341. [DOI] [PubMed] [Google Scholar]
- 19.Kim YM, Kim J, Jung K, Eo S, Ahn K. The effects of particulate matter on atopic dermatitis symptoms are influenced by weather type: application of spatial synoptic classification (SSC) Int J Hyg Environ Health. 2018;221:823–829. doi: 10.1016/j.ijheh.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Hong YJ, Szulejko JE, Kang CH, Chambers S, Feng X, et al. Airborne iron across major urban centers in South Korea between 1991 and 2012. Sci Total Environ. 2016;550:309–320. doi: 10.1016/j.scitotenv.2015.11.109. [DOI] [PubMed] [Google Scholar]
- 21.Larese Filon F, Mauro M, Adami G, Bovenzi M, Crosera M. Nanoparticles skin absorption: new aspects for a safety profile evaluation. Regul Toxicol Pharmacol. 2015;72:310–322. doi: 10.1016/j.yrtph.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Kim KE, Cho D, Park HJ. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Araviiskaia E, Berardesca E, Bieber T, Gontijo G, Sanchez Viera M, Marrot L, et al. The impact of airborne pollution on skin. J Eur Acad Dermatol Venereol. 2019;33:1496–1505. doi: 10.1111/jdv.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afaq F, Zaid MA, Pelle E, Khan N, Syed DN, Matsui MS, et al. Aryl hydrocarbon receptor is an ozone sensor in human skin. J Invest Dermatol. 2009;129:2396–2403. doi: 10.1038/jid.2009.85. [DOI] [PubMed] [Google Scholar]
- 25.Li A, Fan L, Xie L, Ren Y, Li L. Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south-western China: a time series analysis. J Eur Acad Dermatol Venereol. 2018;32:486–494. doi: 10.1111/jdv.14730. [DOI] [PubMed] [Google Scholar]
- 26.Fuller CH, Brugge D, Williams PL, Mittleman MA, Lane K, Durant JL, et al. Indoor and outdoor measurements of particle number concentration in near-highway homes. J Expo Sci Environ Epidemiol. 2013;23:506–512. doi: 10.1038/jes.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Ryu SH, Lee G, Bae GN. Indoor-to-outdoor particle concentration ratio model for human exposure analysis. Atmos Environ. 2016;127:100–106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.