Dear Editor:
Female pattern hair loss (FPHL) is the most common form of alopecia in women. With increase in its incidence, attention to the treatment of FPHL has also increased. Several medications have been used for the treatment of FPHL, including topical minoxidil, oral antiandrogen, and finasteride1,2. Finasteride, a 5α-reductase inhibitor, is widely used for the treatment of androgenetic alopecia. However, the efficacy of oral finasteride in female patients with FPHL is still controversial and recommended finasteride dosage varies widely (1~5 mg/day). In this study, we aimed to evaluate the efficacy of oral finasteride treatment for female patients with FPHL by performing a meta-analysis.
We conducted comprehensive literature search of MEDLINE, Embase Cochrane library databases on articles published before May 4, 2019 using the following keywords: “finasteride”, “5α-reductase inhibitor”, “female pattern hair loss”, “female pattern baldness”, and “female androgenetic alopecia”. Search strategy used for each database is described in Supplementary Materials. Two reviewers independently evaluated the titles and abstracts of the retrieved studies, and a final decision was made through a consensus. Nine relevant studies were identified and the related PRISMA flow diagram is presented in the Supplementaty Fig. 1. The data extracted from each study included the study year, study setting, study population, age, drug dose and duration, changes in hair density, patients' self-assessment of treatment, investigators' assessment of treatment, global photographic assessments (GPA) of hair growth, and adverse effects.
Hair density and improvement rates were assessed in the meta-analysis to evaluate the efficacy of finasteride. Hair density was defined as the number of hairs per square centimeter. The differences in hair density before and after finasteride treatment were analyzed. Response was defined as “GPA ≥1 (slightly increased)” or more than mild improvement according to the investigators' assessment. Subgroup analyses were performed according to dose of finasteride (<2.5 mg or ≥2.5 mg). A fixed effects model was used to analyze the changes in the hair density and treatment response. The heterogeneity of the included studies was calculated using the I2 static for inconsistency. All effects were computed using the R software, version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
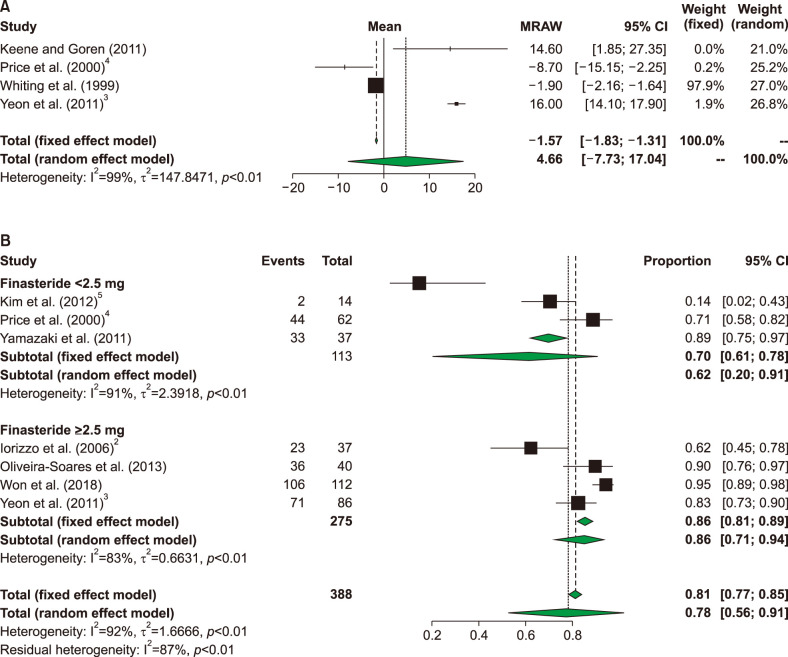
Nine articles, involving a total of 490 patients, were included in the meta-analysis. The characteristics of the studies were summarized in Table 1. Four studies that included 250 patients reported hair density and seven studies that included 388 patients reported either GPA or investigators' assessment of treatment. The results of metaanalysis showed no significant increase in hair density after the treatments (−1.90 hair count/cm2; 95% confidence internal=−0.216 to −1.64 hair count/cm2) (Fig. 1A). The heterogeneity of the studies was high (I2=99%). Because of limited number of studies and prominent high heterogeneity, subgroup analysis did not perform for hair density. The results of meta-analyzed response rate were summarized in Fig. 1B. The overall response rate with finasteride treatment was 81% (70% in <2.5 mg group and 86% in ≥2.5 mg group). The heterogeneity of the studies was high (I2=92% [overall], 91% [<2.5 mg subgroup], and 83% [≥2.5 mg subgroup]). The response rate was higher in the high dose (≥2.5 mg) group than in the low dose (<2.5 mg) group. Five of the nine studies reported adverse events. Yeon et al.3 reported that four patients presented with headache, menstrual irregularity, dizziness, and increased body hair. Price et al.4 reported one patient with folliculitis as a drug-related adverse event.
Table 1. Characteristics of the nine studies included in the meta-analysis.
| Study (year) | Country | Study type | No. of patient | Age (yr) | Pre/postmenopausal | Finasteride dose (mg/d) | Duration (mo) | Methods of evaluating efficacies | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|
| Iorizzo et al. (2006)2 | Italy | Prospective | 37 | 19∼50 | Premenopausal | 2.5 | 12 | GPA (−3∼3) | None |
| Patients' self-assessment | |||||||||
| Keene and Goren (2011) | United States | Randomized controlled | 8 | - | Postmenopausal | 1 | 6 | Hair density | None |
| Kim et al. (2012)5 | Republic of Korea | Prospective | 14 | 46.3 | Pre/postmenopausal | 1.25 | 7 | Hair thickness | - |
| GPA (−3∼3) | |||||||||
| Patients' self-assessment | |||||||||
| Oliveria-Soares et al. (2013) | Portugal | Prospective | 40 | Postmenopausal | 5 | 18 | GPA (−3∼3) | - | |
| Investigators' assessment | |||||||||
| Price et al. (2000)4 | United States | Randomized controlled | 62 | 41∼60 | Postmenopausal | 1 | 12 | Hair density, GPA (−3∼3) | 1 patient |
| Investigators' assessment | |||||||||
| Patients' self-assessment | |||||||||
| Whiting et al. (1999) | United States | Randomized controlled | 94 | 41∼60 | Postmenopausal | 1 | 12 | Hair density | - |
| Won et al. (2018) | Republic of Korea | Retrospective | 112 | 30∼73 | Pre/postmenopausal | 2.5 | - | Investigators' assessment (0∼2) | None |
| Yeon et al. (2011)3 | Republic of Korea | Retrospective | 85 | 21∼69 | Pre/postmenopausal | 5 | 12 | Hair density | 4 patients |
| Hair thickness | |||||||||
| GPA (−3∼3) | |||||||||
| Yamazaki et al. (2011) | Japan | Prospective | 37 | 29∼75 | Pre/postmenopausal | 1 | 6 | GPA (−3∼3) | - |
GPA: global photographic assessments.
Fig. 1. Forest plot of meta-analysis for clinical outcome measures. (A) Changes in hair density before and after finasteride administration. (B) Response rates of finasteride with subgroup analysis. CI: confidence interval.
FPHL differs from male pattern hair loss in its epidemiology, clinical presentation, and management. Various oral medications, including finasteride, spironolactone, and cyproterone acetate, are used for this condition3. Several reports on the treatment of FPHL using oral finasteride have showed varying degrees of efficacies5. In the nine studies included in this meta-analysis, finasteride showed no effect on hair density of FPHL patients; rather hair density was decreased with finasteride treatment. However, with high heterogeneity and limited number of contained studies, reliability of result is poor. The response rate evaluated by the investigators was around 81%. The response rate increased in proportion to finasteride dose, however limitation of single arm study, it is hard to comparing two groups' efficacy. Our findings indicated that finasteride exhibited an overall therapeutic effect in FPHL patients; however, it was not effective for promoting hair density. However, in clinical practice, evaluation of treatment response was more meaningful with rating by GPA or patients' self-assessment of treatment, not by change of hair density or thickness. In this point, high dose finasteride (≥2.5 mg) showed better response rate than low dose (<2.5 mg), and high dose finasteride would be better choice for FPHL treatment. There are limitations to this meta-analysis. First, most of the included studies exhibited high heterogeneity. Second, all three of the randomized controlled studies were conducted with a finasteride dose of 1 mg. Moreover, higher number of randomized control studies with better design and administration of higher dose of finasteride in FPHL patients was need. In recent, dutasteride also used in FPHL, and several research reported efficacy of dutasteride in FPHL6. However, there was lack of meta-analysis of efficacy of dutasteride, as well as finasteride. Further researches with better design and comparing efficacy of finasteride and dutasteride will need.
This study was exempted for a review by the institutional review board of Kyung Hee University Hospital at Gangdong in accordance with the exemption criteria (IRB no. KHNMC 2021-03-018).
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: None.
DATA SHARING STATEMENT
Research data are not shared.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-33-304-s001.pdf.
PRISMA flow diagram of literature search and study selection for the systematic review of studies on finasteride administration for female pattern hair loss.
References
- 1.Olsen EA. Female pattern hair loss. J Am Acad Dermatol. 2001;45(3 Suppl):S70–S80. doi: 10.1067/mjd.2001.117426. [DOI] [PubMed] [Google Scholar]
- 2.Iorizzo M, Vincenzi C, Voudouris S, Piraccini BM, Tosti A. Finasteride treatment of female pattern hair loss. Arch Dermatol. 2006;142:298–302. doi: 10.1001/archderm.142.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Yeon JH, Jung JY, Choi JW, Kim BJ, Youn SW, Park KC, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211–214. doi: 10.1111/j.1468-3083.2010.03758.x. [DOI] [PubMed] [Google Scholar]
- 4.Price VH, Roberts JL, Hordinsky M, Olsen EA, Savin R, Bergfeld W, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 Pt 1):768–776. doi: 10.1067/mjd.2000.107953. [DOI] [PubMed] [Google Scholar]
- 5.Kim WJ, Song M, Ko HC, Kim BS, Kim MB. Efficacy of finasteride 1.25 mg on female pattern hair loss; pilot study. Ann Dermatol. 2012;24:370–372. doi: 10.5021/ad.2012.24.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boersma IH, Oranje AP, Grimalt R, Iorizzo M, Piraccini BM, Verdonschot EH. The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2014;80:521–525. doi: 10.4103/0378-6323.144162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA flow diagram of literature search and study selection for the systematic review of studies on finasteride administration for female pattern hair loss.
Data Availability Statement
Research data are not shared.