Abstract
Background
The effect of solid organ transplantation (SOT) on the severity and mortality of coronavirus disease 2019 (COVID-19) remained controversial. There is still no consensus on whether solid organ transplantation (SOT) recipients with COVID-19 are at greater risk of developing severe or fatal COVID-19. Therefore, we conducted a systematic review and meta-analysis to investigate the association between SOT, severe COVID-19 illness, and mortality.
Methods
A systemically comprehensive search in Pubmed, Embase, the Cochrane Library, Web of Science, and China National Knowledge Infrastructure was performed for relevant studies and articles. Consequently, we pooled the odds ratio (OR) from individual studies and performed heterogeneity, quality assessment and subgroup/sensitivity analysis.
Results
A total number of 15 articles with 265,839 participants were included in this study. Among the total number of participants, 1485 were SOT recipients. The meta-analysis results showed that transplant patients with COVID-19 were remarkably associated with a higher risk of intensive care unit admission than non-transplant patients (OR = 1.57, 95%CI: 1.07 to 2.31, P = 0.02). On the other hand, there were no statistically significant differences between SOT recipients and non-SOT recipients in mechanical ventilation need (OR = 1.55, 95%CI: 0.98 to 2.44, P = 0.06). In addition, we found that SOT recipients with COVID-19 had 1.40-fold increased odds of mortality than non-SOT recipients (OR = 1.40, 95%CI: 1.10 to 1.79, P = 0.007). Moreover, pooled analysis of adjusted results revealed that SOT recipients had a greater risk of mortality compared with non-SOT patients (HR = 1.54, 95%CI: 1.03 to 2.32, P = 0.037).
Limitations
The main limitations in our study are attributed to the relatively small sample size, short follow-up period, and the fact that most of the studies included were retrospective in design.
Conclusions
The results of this study indicate that SOT recipients with COVID-19 had a more significant risk of COVID-19 severity and mortality than the general population.
Keywords: COVID-19, Solid organ transplantation, Severe, Mortality, Meta-analysis
Abbreviations: SOT, solid organ transplantation; COVID-19, coronavirus disease-2019; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; ARDS, acute respiratory distress syndrome; MODS, multiple organ dysfunction syndrome; MERS, Middle East Respiratory Syndrome; SARS, Severe Acute Respiratory Syndrome; CKD, chronic kidney disease; CAD, chronic artery disease; OR, odds ratio; NOS, Newcastle-Ottawa Scale; CI, confidence intervals; CNKI, China National Knowledge Infrastructure
1. Introduction
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), has now claimed over 1.6 million lives worldwide and has led to an unprecedented global health crisis. The clinical manifestations of COVID-19 may range from asymptomatic or mild symptoms to severe pneumonia, including acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS) [1]. Several risk factors for severe or fatal COVID-19 were identified, including old age, male gender, obesity, hypertension, cardiovascular disease, chronic kidney disease, and chronic lung disease [[2], [3], [4]]. Besides, recent studies indicated that the magnitude of specific immunity is associated with the severity of COVID-19 [5].
Most SOT recipients have one or several associated risk factors of severe or death COVID-10, such as hypertension, cardiovascular disease, and chronic kidney disease [6]. Moreover, SOT recipients require several immunosuppressive drugs, such as calcineurin inhibitors, antimetabolites, and steroids, which may moderate their immune system and significantly increase their susceptibility to viral infections and bacterial and fungal superinfection [7]. Hence, some studies reported that SOT recipients had a higher COVID-19 related mortality rate than non-transplant patients [8,9]. In contrast, some other studies found that both mortality and severity rates of COVID-19 among SOT recipients were similar to the general population [10,11]. Severe COVID-19 manifestations were mainly associated with a disproportionate hyperinflammatory reaction due to cytokine release syndrome. Thus, the serum concentrations of proinflammatory cytokines were found to be of higher levels in severe cases compared to mild ones [12,13]. Subsequently, under such circumstances, commencing the immunosuppressive agents may modulate and reduce the inflammatory response by blunting excessive cytokine release, thus promoting the prevention of severe complications in SOT patients [14,15].
It remains highly controversial whether SOT recipients were more prone to develop severe or fatal COVID-19 or whether immunosuppression could protect them from “cytokine storm” and prevent potential severe complications. To the best of our knowledge, no existing meta-analysis compared the severity and mortality of COVID-19 in SOT recipients and the general population. Therefore, we aimed to perform a systematic review and meta-analysis to investigate the association between severe or fatal COVID-19 and SOT.
2. Material and methods
2.1. Search strategy, selection criteria, and outcome
This meta-analysis was carried out in accord with the PRISMA guidelines [16]. We systematically searched relevant studies in PubMed, Embase, the Cochrane Library, Web of Science, and China National Knowledge Infrastructure (CNKI), using the keywords “solid organ transplant recipients” or “transplantation” AND “novel coronavirus” or “coronavirus disease 2019” or “COVID-19” or “SARS-CoV-2” from their inception up to 26th March 2021 without language restrictions. We included studies that fulfilled the following entry criteria: (1) patients were diagnosed with COVID-19 infection; (2) provided clinical outcomes of transplant recipients versus non-transplant controls. Meanwhile, study exclusion criteria included: (1) studies without clinical outcomes (severe versus non-severe patients, death versus survival); (2) letters, case reports, review articles, abstracts, comments. Data extraction included study characteristics (e.g., name of the first author, country, sample size, type of SOT, and duration of follow-up) and baseline patient characteristics (e.g., age, gender, and comorbidities, adjusted variables). Outcome data extraction included the severity of the disease (intensive care unit admission and mechanical ventilation need), mortality, and the results of multivariable regression analyses (including the level of statistical adjustment). Disagreements were resolved by consensus or by a third investigator. The severity of the disease was mainly determined by developing specific symptoms (e.g. intubation and mechanical ventilation need or intensive care unit admission). Two investigators (YW and GA) evaluated the risk of bias/quality of studies using the Newcastle-Ottawa Scale (NOS). A total score of ≥7 was used to indicate a high-quality study, while a study with a total score of <7 was considered a low-quality study. This study is registered with PROSPERO, number CRD42020207387.
2.2. Statistical analysis
The literature search, study selection and data extraction were performed independently by two investigators (YW and QX). Disagreements were resolved by discussion. Review Manager 5.3 (Cochrane Collaboration) and Stata 15.0 (StataCorp) were applied to calculate odds ratios (OR) and their 95% confidence intervals. We used the adjusted hazard ratios (HR) with 95% confidence intervals (CI) for the overall effect estimate, if possible, to reduce the effects of potential confounders. We assessed between-study heterogeneity among combined study results with Cochran's Q test and the I2 statistic, with an I2 < 25%, 25% to 50%, and greater than 50% indicated low, moderate, and high heterogeneity, respectively. When I2 < 50%, we used a fixed effect model; otherwise, a random-effect model was used. We also preformed sub-analysis based on countries and sensitivity analysis by excluding one study at a time to explore the cause of heterogeneity and evaluate the stability of the results of this meta-analysis. Publication bias was assessed by Begg's adjusted rank correlation test and Egger's regression asymmetry test (P < 0.10 was considered indicative of statistically significant heterogeneity). P < 0.05 was considered statistically significant.
3. Results
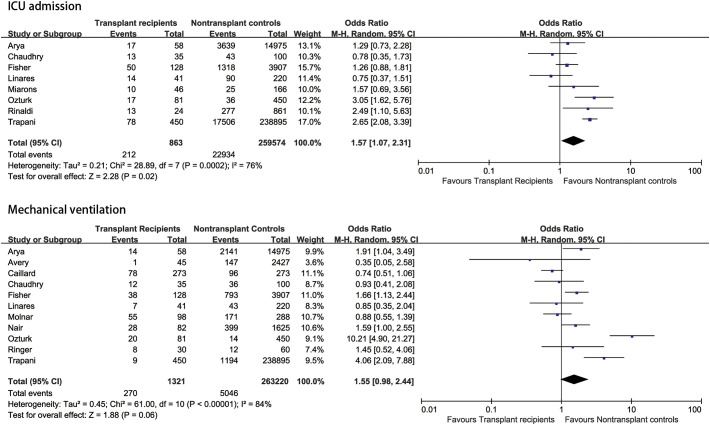
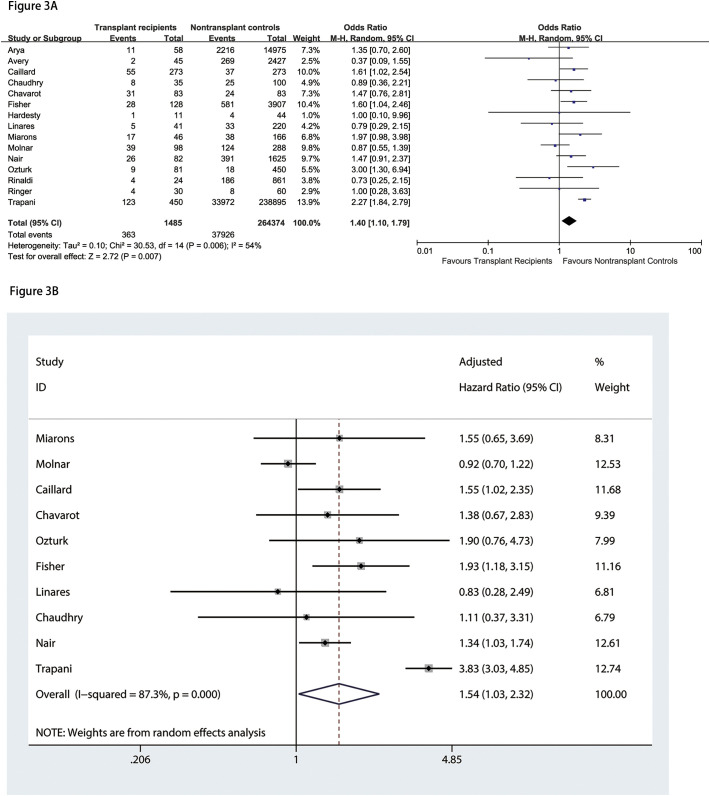
The database search resulted in 584 studies, with two studies identified through manual searching of reference lists from extracted studies. After removing duplicates, we conducted a review of the titles and abstracts of the 491 articles. As a result, 491 studies were excluded based on the title and abstract. After further evaluation of the remaining 58 full-text eligible papers, we excluded 43 papers due to insufficient data for calculation. Eventually, a total number of 15 papers, including 265,839 participants, were involved in this meta-analysis [[8], [9], [10], [11],[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]] (Fig. 1 ). All studies were published in 2020. Eight studies were from America, while other studies from European and Asian countries. One study [21] from Italy, had the largest sample size with 239,325 populations, using the Italian integrated COVID-19 surveillance system. The final number of SOT recipients was 1485. Four studies [8,17,20,25] included kidney transplant recipients with COVID-19, and other studies involved several types of SOT recipients. Additionally, only one single study was prospective in design, while all other studies were retrospective. All studies were published in English language. The characteristics of the study are demonstrated in Table 1 . The overall quality of available literature was moderate with NOS scores ranging from 7 to 9. Characteristics of SOT and non-SOT recipients with COVID-19 are summarized in Table 2 . Included patients were predominately male. SOT recipients were more likely to present with higher proportions of co-morbidities, including hypertension and diabetes mellitus. The quality of the included articles is evaluated and shown in Table 3 . Eight studies [9,10,13,18,20,21,26,27] provided data on patients who required admission to intensive care unit (ICU). The meta-analysis showed that transplant patients with COVID-19 were associated with a higher risk of ICU admission than non-transplant patients (OR = 1.57, 95%CI: 1.07 to 2.31, P = 0.02; I2 = 76%) (Fig. 2A). According to the subgroup analysis based on countries, analysis of studies from America did not reveal significant differences in ICU admission between transplant patients and non-transplant patients; however, the heterogeneity decreased to none (OR = 1.19, 95%CI: 0.89 to 1.58, P = 0.24; I2 = 0%). In addition, eleven studies [8,10,11,[19], [20], [21], [22], [23], [24],26,27] provided results of patients who needed mechanical ventilation. We found no significant differences in mechanical ventilation need between SOT recipients and non-SOT recipients (OR = 1.55, 95%CI: 0.98 to 2.44, P = 0.06; I2 = 84%) (Fig. 2B). Subgroup analysis of studies from America did not indicate significant differences in mechanical ventilation need between transplant patients and non-transplant patients, however the heterogeneity decreased significantly (OR = 1.26, 95%CI: 1.00 to 1.59, P = 0.05; I2 = 33%). Moreover, we found that SOT recipients with COVID-19 had 1.40-fold increased odds of mortality than non-SOT recipients (OR = 1.40, 95%CI: 1.10 to 1.79, P = 0.007; I2 = 54%) (Fig. 3A). Pooled analysis of adjusted results [[8], [9], [10], [11],17,20,21,23,24,26] also revealed that SOT recipients had a higher risk of mortality compared with non-SOT patients (HR = 1.54, 95%CI: 1.03 to 2.32, P = 0.037) with substantial heterogeneity (I2 = 87.3%) (Fig. 3B). Excluding the one study [21] (Trapani et al.) with the largest sample size did not alter the overall estimate; however, the heterogeneity decreased significantly (HR = 1.27, 95%CI: 1.09 to 1.47, P = 0.002; I2 = 24.8%). In addition, pooled analysis of three studies [8,17,26] that matched SOT recipients with the general population based on age, sex and comorbidities also indicated that SOT recipients were associated with increased risk of mortality compared with non-SOT patients (HR = 1.42, 95%CI: 1.01 to 2.00, P = 0.046, I2 = 0%). Sensitivity analyses by excluding each study at a time did not significantly alter the overall results. Egger's or Begg's tests did not reveal significant publication bias in the analysis of mortality (Egger P = 0.502 and Begg P = 0.858).
Fig. 1.
Flow diagram of literature search and study selection.
Table 1.
Characteristics of included studies.
| Studya | Country | Sample size |
Study design | Transplant recipients | Follow-up duration (days) | Definition of severity used | Definition of COVID-19 infection used | Adjusted variables | |
|---|---|---|---|---|---|---|---|---|---|
| Transplant recipients | Nontransplant controls | ||||||||
| Arya [27] | America | 58 | 14,975 | Retrospective | 38 kidney, 8 liver, 5 heart, 3 pancreases, 4 multiorgan transplants | 184 | ICU admission, mechanical ventilation | SARS-CoV-2 RT-PCR (+) | NR |
| Avery [19] | America | 45 | 2427 | Retrospective | NR | 173 | Invasive mechanical ventilation | SARS-CoV-2 RT-PCR (+) | NR |
| Caillard [8] | France | 273 | 273 | Retrospective | Kidney | 64 (55–71) | Mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Age, BMI, cardiovascular and respiratory diseases, cancer, and diabetes |
| Chaudhry [10] | America | 35 | 100 | Retrospective | 38 kidneys and 9 non-kidney organs | 35 (20–36) | ICU admission, mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Age, coexisting conditions, transplant status, and Henry Ford Hospital COVID-19 severity score |
| Chavarot [17] | France | 83 | 83 | Retrospective | Kidney | 13 (7–30) | defined as death and/or need for ICU | SARS-CoV-2 RT-PCR (+) | Age, sex, body mass index, diabetes mellitus, preexisting cardiopathy, chronic lung disease and basal renal function. |
| Fisher [23] | America | 128 | 3907 | Retrospective | 106 kidneys, 9 livers, 6 hearts, 4 combined kidneys/pancreas, 3 combined kidney/livers | Patients were followed until the first of hospital discharge, death or September 1, 2020 | ICU admission, mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Age, sex, race, ethnicity, BMI, hypertension, diabetes mellitus, congestive heart failure, and obesity (defined as BMI ≥ 30 kg/m2). |
| Hardesty [25] | America | 11 | 44 | Retrospective | Kidney | 80 | NR | SARS-CoV-2 RT-PCR (+) | Age and sex |
| Linares [26] | Spain | 41 | 220 | Retrospective | 32 kidney, 4 liver, 3 heart, 2 combined kidney/liver | 80 | ICU admission, mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Age, sex, hypertension, lung disease, use of anti-cytokine therapies, baseline clinical status and clinical status during hospitalization |
| Miarons [9] | Spain | 46 | 166 | Retrospective | 30 kidneys, 3 livers, 13 lungs | 28 | ICU admission | SARS-CoV-2 RT-PCR (+) | Sex, age and age-adjusted Charlson's Index |
| Molnar [11] | America | 98 | 288 | Retrospective | 67 kidneys, 13 livers, 13 hearts, 4 lungs, and 1 pancreas | 28 | Invasive mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Age, gender, race, ethnicity, BMI, comorbidities and medication use prior to hospital admission |
| Nair [24] | America | 82 | 1625 | Retrospective | 69 kidneys, 3 livers, 6 hearts, 1 lung, 2 combined kidneys/pancreas, 1 combined kidney/liver | Patients were followed from the date of initial hospitalization until the first outcome of either death, discharge from the hospital, transfer to another center, or end of the study | Invasive mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Age, gender, diabetes, hypertension, and cardiovascular disease (defined as any of coronary artery disease, peripheral arterial disease, or heart failure) |
| Ozturk [20] | Turkey | 81 | 450 | Retrospective | Kidney | 28 | ICU admission, mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Age, gender, presence of diabetes, hypertension, cardiovascular disease, COPD and eGFR |
| Rinaldi [18] | Italy | 24 | 861 | Prospective | 22 kidneys, 2 livers | 30 | ICU admission | SARS-CoV-2 RT-PCR (+) | NR |
| Ringer [22] | America | 30 | 60 | Retrospective | 26 kidneys, 3 livers, 1 heart | 28 | Invasive mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Age, BMI and comorbidities (hypertension and diabetes mellitus with hemoglobin A1c >8.0%) |
| Trapani [21] | Italy | 450 | 238,875 | Retrospective | 285 kidneys, 89 livers, 54 hearts, 15 lungs, 8 pancreases | 365 | ICU admission, mechanical ventilation | SARS-CoV-2 RT-PCR (+) | Gender and age |
Age data presented as median (IQR) or mean (SD); ICU: intensive care units; BMI: body mass index; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; SARS-CoV-2: 2019 severe acute respiratory syndrome coronavirus 2; RT-PCR: Real-time reverse transcription polymerase chain reaction; NR, not reported.
Table 2.
Clinical characteristics of solid organ transplant patients and non-transplant patients with COVID-19.
| Author | Agea |
Male (%) |
CKD (%) |
CAD (%) |
Chronic lung disease (%) |
Chronic liver disease (%) |
Obesity (%) |
Malignancy (%) |
Hypertension (%) |
Diabetes (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | NT | T | NT | T | NT | T | NT | T | NT | T | NT | T | NT | T | NT | T | NT | T | NT | |
| Arya [27] | 57.4 | 52.3 | 36 (62) | 6739 (45) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Avery [19] | 59 (48, 65) | 59 (43, 72) | 24 (53.3) | 1259 (51.9) | 36 (83.7) | 455 (19.4) | NR | NR | 8 (18.6) | 531 (22.6) | 15 (34.9) | 200 (8.5) | 15 (34.9) | 745 (31.7) | 10 (23.3) | 257 (10.9) | 31 (68.9) | 1076 (44.3) | 27 (60) | 820 (33.8) |
| Caillard [8] | 62.0 (53.0–69.0) | 63.0 (48.0–74.0) | 181 (66.3) | 173 (63.4) | NR | NR | NR | NR | 38 (19.9) | 45 (16.5) | NR | NR | 177 (64.8) | 181 (66.3) | 34 (12.5) | 26 (9.5) | 232 (91.3) | 136 (49.8) | 101 (37) | 98 (35.9) |
| Chaudhry [10] | 62 (48–71) | 60 (51–72) | 23 (65.7) | 50 (50.0) | 31 (88.6) | 57 (57) | 5 (14.3) | 12 (12) | 6 (17.1) | 13 (13) | NR | NR | NR | NR | 4 (11.4) | 13 (13) | 33 (94.3) | 72 (72) | 23 (65.7) | 23 (23) |
| Chavarot [17] | 67.2 (58.5–74.1) | 65.1 (56.1–79.3) | 52 (82.6) | 55 (82.7) | 61 (73.5) | 55(66.4) | 15 (18.1) | 16 (19.2) | 10 (12) | 10 (12) | NR | NR | NR | NR | NR | NR | 68 (81.9) | 62 (74.7) | 41 (49.4) | 44 (53) |
| Fisher [23] | 60 (50, 68) | 60 (51, 69) | 79 (61.7) | 2411 (61.7) | 74 (57.8) | 295 (7.6) | 3 (2.3) | 80 (2.1) | 3 (2.3) | 160 (4.1) | 2 (1.6) | 22 (0.6) | 11 (8.6) | 336 (8.6) | 0 (0) | 33 (0.8) | 76 (59.4) | 2320 (59.4) | 72 (56.2) | 2198 (56) |
| Hardesty [25] | 55 (34–68) | 55 (33–68) | 4 (36.3) | 17 (38.6) | 9 (81.8) | 4 (9.1) | 4 (36.3) | 5 (11.4) | 0 | 13 (29.5) | NR | NR | 7 (63.6) | 19 (43.2) | NR | NR | 10 (90.9) | 26 (59.1) | 7 (63.6) | 19 (43.2) |
| Linares [26] | 58 (33–86) | 63 (51–72) | 27 (66) | 144 (66) | 14 (34) | 11 (5) | 10 (24) | 29 (13) | 8 (20) | 38 (17) | NR | NR | NR | NR | NR | NR | 33 (81) | 98 (45) | 34 (16) | 13 (32) |
| Miarons [9] | 62.7 ± 12.6 | 66.0 ± 12.7 | 33 (71.7) | 122 (73.5) | 36 (78.3) | 30 (18.1) | NR | NR | 16 (35.8) | 34 (20.5) | 4 (8.7) | 3 (1.8) | 10 (21.7) | 37 (22.6) | 10 (21.7) | 40 (24.1) | 36 (78.3) | 94 (56.6) | 20 (44.4) | 58 (35.2) |
| Molnar [11] | 58 (52–69) | 61 (51–70) | 72 (73) | 205 (71) | 55 (56) | 143 (50) | 26 (27) | 78 (27) | 19 (19) | 52 (18) | 12 (12) | 23 (8) | NR | NR | 7 (7) | 18 (6) | 82 (84) | 235 (82) | 64 (65) | 189 (66) |
| Nair [24] | 61.8 ± 11.7 | 62.7 ± 11.5 | 56 (68.3) | 1117 (68.7) | NR | NR | 20 (24.4) | 335 (20.6) | 2 (2.4) | 117 (7.2) | NR | NR | 22 (26.8) | 684 (42.1) | NR | NR | 69 (84.1) | 1365 (84) | 51 (62.2) | 1005 (61.8) |
| Ozturk [20] | 48 (38–56) | 51 (38–63) | 48 (59.3) | 246 (54.7) | NR | NR | 13 (17.1) | 40 (9.3) | 5 (6.5) | 44 (10.1) | 0 | 4 (0.9) | NR | NR | 2 (2.6) | 20 (4.6) | 57 (72.2) | 132 (30) | 20 (25.3) | 68 (15.5) |
| Rinaldi [18] | 62 (48–67) | 70 (57–80) | 15 (62.5) | 577 (67) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Ringer [22] | 60 (29–78) | 60.5 (23–83) | 16 (53) | 36 (60) | NR | NR | 18 (60) | 21 (35) | 10 (33) | 21 (35) | NR | NR | NR | NR | NR | NR | 28 (93) | 56 (93) | 12 (40) | 24 (40) |
| Trapani [21] | 61 (53–67) | 61 (47–80) | 340 (75.6) | 109,261 (45.7) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
T, transplant; NT, non-transplant; CKD, chronic kidney disease; CAD, chronic artery disease; NR, not reported.
Data presented as either median (lower quartile, upper quartile), or mean (standard deviation).
Table 3.
Study quality assessment using the Newcastle-Ottawa Scale.
| First author, year of publication (reference) | Selection |
Comparability | Outcome |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of nonexposed cohort | Ascertainment of exposure | Outcome of interest absent at start of study | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow-up | Total score | ||
| Arya [27] | * | * | * | * | * * | * | … | … | 7 |
| Avery [19] | * | * | * | * | * * | * | … | … | 7 |
| Caillard [8] | * | * | * | * | * * | * | … | … | 7 |
| Chaudhry [10] | * | * | * | * | * * | * | … | … | 7 |
| Chavarot [17] | * | * | * | * | * * | * | … | * | 8 |
| Fisher [23] | * | * | * | * | * * | * | … | … | 7 |
| Hardesty [25] | * | * | * | * | * * | * | … | * | 8 |
| Linares [26] | * | * | * | * | * * | * | … | * | 8 |
| Miarons [9] | * | * | * | * | * * | * | … | … | 7 |
| Molnar [11] | * | * | * | * | * * | * | … | … | 7 |
| Nair [24] | * | * | * | * | * * | * | … | … | 7 |
| Ozturk [20] | * | * | * | * | * * | * | … | * | 8 |
| Rinaldi [18] | * | * | * | * | * * | * | … | * | 8 |
| Ringer [22] | * | * | * | * | * * | * | … | … | 7 |
| Trapani [21] | * | * | * | * | * * | * | * | * | 9 |
Fig. 2.
Association between solid organ transplantation and severe COVID-19 (2A: ICU admission; 2B: Mechanical ventilation).
Fig. 3.
Association between solid organ transplantation and mortality (3A: analysis of unadjusted results; 3B: analysis of adjusted results).
4. Discussion
Solid organ transplant recipients face a substantial threat of COVID-19 infection due to the chronic use of immunosuppression agents and increased comorbidities risks. Previous studies have suggested no significant differences between immunocompromised and ordinary patients in the outcomes of Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) infections [[28], [29], [30], [31]]. However, our study showed a higher risk of developing severe COVID-19 infection and mortality in SOT patients compared with non-SOT patients with COIVD-19.
Cytokine release syndrome is considered a significant cause of severe COVID-19 infection, including acute respiratory distress syndrome (ARDS) and organs dysfunction [32,33]. The chronically suppressed immune system in transplant recipients may blunt the effect of Inflammatory cascades and cytokines/chemokines release. Studies have been reported that immunosuppression in SOT patients can effectively reduce the hyperinflammatory in the clinical course of COVID-19 and potentially serve as a protecting factor to prevent inflammation and cytokine release syndrome [34,35]. In addition, some of the immunosuppressive drugs especially cyclosporine and mycophenolic mofetil have also been demonstrated to have anti-viral activity [[36], [37], [38]]. A recent study has suggested that cyclosporin A (CsA) may reduce the risk of death in severe COVID-19 patients [39]. Romero et al. also found that CsA may be an adjuvant to steroid treatment for COVID-19 patients [40]. However, immunosuppression in transplant recipients during COVID-19 may change. Innate and adaptive immunity may be altered in solid organ transplant recipients taking combinations of immunosuppressive drugs for an extended period, putting them at risk of infection. Furthermore, the use of immunosuppression had made these patients more susceptible to viral respiratory infections. Some studies have also demonstrated that SOT recipients with COVID-19 are more likely to develop bacterial and fungal coinfections [8,18]. Oltean et al. performed a systematic review of the case series of kidney transplant recipients with COVID-19. Their study indicates that transplanted patients with COVID-19 shared several clinical characteristics with the general population, such as a higher proportion of men gender and advanced age as risk factors. However, their main finding was the very high mortality rates in hospitalized kidney transplant recipients with COVID-19, which significantly increased with age [41]. Moreover, SOT recipients have a higher prevalence of comorbidities, such as hypertension, diabetes, chronic kidney disease, and others, which will increase the severity and mortality of COVID-19 [42]. In a large cohort study, the mortality among hospitalized SOT recipients with COVID-19 was 20.5%. The author pointed out that age and underlying comorbidities rather than immunosuppression intensity-related measures were significant mortality drivers among SOT recipients [43]. Besides, Coll et al. found that the mortality rate among transplant recipients with COVID-19 was 27%. Such a high mortality rate may be predominantly influenced by the demographic profile, comorbidity burden, and type of transplant organ than by the direct impact of transplantation or associated immunosuppression [44]. In our study, a large proportion of included COVID-19 positive SOT recipients are kidney transplant patients with renal anemia, renal hypertension, renal bone disease, and other kidney related complications. Given that these factors could increase the risk of severe and death in COVID-19 [45], some of the included studies [8,17,26] matched SOT recipients with the general population based on age, sex and comorbidities to reduce potential confounders' effects. In addition, most included studies adjusted confounding risk factors, and our pooled analysis of adjusted results indicated that SOT recipients had higher risk of mortality compared with non-SOT patients. However, it is still insufficient to consider that the different outcome in SOT recipients is attributed to the use of immunosuppressive drugs rather than potential risk factors. It is noted that included studies adjusted similar but not the same variables and adjustments for those classical risk factors may not be sufficient enough to reduce all the potential confounders that could influence the outcomes. Further large well-designed studies are needed to explore the underlying mechanisms of pharmacological effects of immunosuppressive drugs on the treatment of SOT recipients with COVID-19.
Although we performed a comprehensive review of the recent literature, some limitations to this study should be noted. The studies' sample size was relatively small, and most of the studies were retrospective in design, which also limited the number of included studies assignable to specific subgroups. The included observational studies were subject to potential confounders that may weaken the effect estimate. The proportion of SOT recipients was relatively small (0.5%), and the lack of sufficient data on different types of SOT hindered analyzing the possibility of different clinical outcomes. Additionally, one study [9] involved patients who had been already admitted to the intensive care unit, which may have potentially impacted the results of both disease severity and mortality. All included studies had a relatively short follow-up; therefore, we could not further analyze the short-term versus long-term follow-up clinical outcomes. Apart from that, this meta-analysis is not an individual patient data meta-analysis. Although the final total number of SOT recipients was 1485, there may be an overlap in the SOT recipients in which one patient may have been involved in more than one individual study. Finally, despite the high heterogeneity in the meta-analysis, we further conducted sub-analysis based on countries and sensitivity analysis to explain the cause of heterogeneity.
To the best of our knowledge, this study is the first systematic literature review to explore the association between severe or fatal COVID-19 and SOT recipient. In conclusion, the results of this study indicated that SOT recipients with COVID-19 had a higher risk of developing severe COVID-19 and mortality compared with the general population. Further research is needed to shed more light on the risk-benefit balance of using specific immunosuppression and immunomodulatory agents in this particular setting.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Authorship contributions
G.A. participated in the research design, data collection, data analysis, writing and submission of manuscript.
Y.W. participated in the research design, data collection, data analysis, writing and submission of manuscript.
B.N. participated in data collection and writing of the paper.
M.B. participated in data collection and data analysis.
M.G. participated in data collection.
X.Q. participated in data collection.
D.X. participated in the research design, helped with coordination of study groups, writing and submission of manuscript.
Funding/Acknowledgments
None.
Declaration of Competing Interest
None.
References
- 1.Xie P., Ma W., Tang H., Liu D. Severe COVID-19: a review of recent progress with a look toward the future. Front Public Health. 2020;8:189. doi: 10.3389/fpubh.2020.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soeroto A.Y., Soetedjo N.N., Purwiga A., et al. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: a systematic review and meta-analysis [published online ahead of print, 2020 Sep 28] Diabetes Metab Syndr. 2020;14:1897–1904. doi: 10.1016/j.dsx.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H., Hou K., Xu R., et al. Clinical characteristics and risk factors of cardiac involvement in COVID-19. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danziger-Isakov L., Blumberg E.A., Manuel O., Sester M. Impact of COVID-19 in solid organ transplant recipients [published online ahead of print, 2020 Dec 14] Am J Transplant. 2020 doi: 10.1111/ajt.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burra P., Becchetti C., Germani G. NAFLD and liver transplantation: disease burden, current management and future challenges. JHEP Rep. 2020;2:100192. doi: 10.1016/j.jhepr.2020.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam S., Rotstein C., AST infectious disease community of practice Candida infections in solid organ transplantation: guidelines from the American Society of transplantation infectious diseases community of practice. Clin Transpl. 2019;33:e13623. doi: 10.1111/ctr.13623. [DOI] [PubMed] [Google Scholar]
- 8.Caillard S., Chavarot N., Francois H., et al. Is Covid-19 infection more severe in kidney transplant recipients? [published online ahead of print, 2020 Dec 1] Am J Transplant. 2020 doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miarons M., Larrosa-García M., García-García S., et al. COVID-19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation. 2021;105:138–150. doi: 10.1097/TP.0000000000003460. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry Z.S., Williams J.D., Vahia A., et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a cohort study. Am J Transplant. 2020;20:3051–3060. doi: 10.1111/ajt.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar M.Z., Bhalla A., Azhar A., et al. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am J Transplant. 2020;20:3061–3071. doi: 10.1111/ajt.16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin S.H., Zhao Y.S., Zhou D.X., Zhou F.C., Xu F. Coronavirus disease 2019 (COVID-19): cytokine storms, hyper-inflammatory phenotypes, and acute respiratory distress syndrome. Genes Dis. 2020;7:520–527. doi: 10.1016/j.gendis.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaza G., Leventhal J., Signorini L., Gambaro G., Cravedi P. Effects of antirejection drugs on innate immune cells after kidney transplantation. Front Immunol. 2019;10:2978. doi: 10.3389/fimmu.2019.02978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts M.B., Fishman J.A. Immunosuppressive agents and infectious risk in transplantation: managing the "net state of immunosuppression" [published online ahead of print, 2020 Aug 17] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1189. ciaa1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Chavarot N., Gueguen J., Bonnet G., et al. COVID-19 severity in kidney transplant recipients is similar to non-transplant patients with similar comorbidities [published online ahead of print, 2020 Nov 30] Am J Transplant. 2020 doi: 10.1111/ajt.16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinaldi M., Bartoletti M., Bussini L., et al. COVID-19 in solid organ transplant recipients: no difference in survival compared to general population [published online ahead of print, 2020 Jul 20] Transpl Infect Dis. 2020 doi: 10.1111/tid.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avery R.K., Chiang T.P., Marr K.A., et al. Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: a retrospective cohort [published online ahead of print, 2020 Dec 7] Am J Transplant. 2020 doi: 10.1111/ajt.16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozturk S., Turgutalp K., Arici M., et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35:2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapani S., Masiero L., Puoti F., et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide population-based study [published online ahead of print, 2020 Dec 5] Am J Transplant. 2020 doi: 10.1111/ajt.16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ringer M., Azmy V., Kaman K., et al. A retrospective matched cohort single-center study evaluating outcomes of COVID-19 and the impact of immunomodulation on COVID-19-related cytokine release syndrome in solid organ transplant recipients [published online ahead of print, 2020 Dec 30] Transpl Infect Dis. 2020 doi: 10.1111/tid.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher A.M., Schlauch D., Mulloy M., et al. Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States [published online ahead of print, 2021 Jan 6] Clin Transpl. 2021 doi: 10.1111/ctr.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair V., Jandovitz N., Hirsch J.S., et al. An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients [published online ahead of print, 2020 Dec 16] Am J Transplant. 2020 doi: 10.1111/ajt.16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardesty A., Pandita A., Vieira K., et al. Coronavirus disease 2019 in kidney transplant recipients: single-center experience and case-control study. Transplant Proc. 2021 Jan;13 doi: 10.1016/j.transproceed.2021.01.002. S0041–1345(21)00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linares L., Cofan F., Diekmann F., et al. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS One. 2021 Mar 3;16 doi: 10.1371/journal.pone.0247251. [eCollection 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arya A., Li M., Aburjania N., et al. COVID-19 in solid organ transplantation: disease severity and clinical update. Transplant Proc. 2021 Feb 25 doi: 10.1016/j.transproceed.2021.02.014. S0041-1345(21)00136-6. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raja M.A., Mendoza M.A., Villavicencio A., et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature [published online ahead of print, 2020 Nov 14] Transplant Rev (Orlando) 2020;35:100588. doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan J.W., Ng C.K., Chan Y.H., et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J.E., Jung S., Kim A., Park J.E. MERS transmission and risk factors: a systematic review. BMC Public Health. 2018;18:574. doi: 10.1186/s12889-018-5484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vishnevetsky A., Levy M. Rethinking high-risk groups in COVID-19. Mult Scler Relat Disord. 2020;42:102139. doi: 10.1016/j.msard.2020.102139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16:308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibabaw T. Inflammatory cytokine: IL-17A signaling pathway in patients present with COVID-19 and current treatment strategy. J Inflamm Res. 2020;13:673–680. doi: 10.2147/JIR.S278335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romanelli A., Mascolo S. Immunosuppression drug-related and clinical manifestation of coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020;20:1947–1948. doi: 10.1111/ajt.15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee D., Popoola J., Shah S., Ster I.C., Quan V., Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho J., Yi H., Jang E.Y., et al. Mycophenolic mofetil, an alternative antiviral and immunomodulator for the highly pathogenic avian influenza H5N1 virus infection. Biochem Biophys Res Commun. 2017;494:298–304. doi: 10.1016/j.bbrc.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Uematsu J., Sakai-Sugino K., Kihira-Nakanishi S., et al. Inhibitions of human parainfluenza virus type 2 replication by ribavirin and mycophenolate mofetil are restored by guanosine and S-(4-nitrobenzyl)-6-thioinosine. Drug Discov Ther. 2019;13:314–321. doi: 10.5582/ddt.2019.01084. [DOI] [PubMed] [Google Scholar]
- 38.Xiao J., Song X., Deng J., et al. Inhibition of cyclophilin A suppresses H2O2-enhanced replication of HCMV through the p38 MAPK signaling pathway. FEBS Open Bio. 2016;6:961–971. doi: 10.1002/2211-5463.12105. Published 2016 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guisado-Vasco P., Valderas-Ortega S., Carralón-González M.M., et al. Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: a retrospective observational study (COQUIMA cohort) EClinicalMedicine. 2020;28:100591. doi: 10.1016/j.eclinm.2020.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gálvez-Romero J.L., Palmeros-Rojas O., Real-Ramírez F.A., et al. Cyclosporine A plus low-dose steroid treatment in COVID-19 improves clinical outcomes in patients with moderate to severe disease: a pilot study [published online ahead of print, 2020 Dec 3] J Intern Med. 2020 doi: 10.1111/joim.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oltean M., Søfteland J.M., Bagge J., et al. Covid-19 in kidney transplant recipients: a systematic review of the case series available three months into the pandemic. Infect Dis (Lond) 2020;52:830–837. doi: 10.1080/23744235.2020.1792977. [DOI] [PubMed] [Google Scholar]
- 42.Aziz H., Lashkari N., Yoon Y.C., et al. Effects of coronavirus disease 2019 on solid organ transplantation. Transplant Proc. 2020;52:2642–2653. doi: 10.1016/j.transproceed.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kates O.S., Haydel B.M., Florman S.S., et al. COVID-19 in solid organ transplant: a multi-center cohort study [published online ahead of print, 2020 Aug 7] Clin Infect Dis. 2020:ciaa1097. [Google Scholar]
- 44.Coll E., Fernández-Ruiz M., Sánchez-Álvarez J.E., et al. COVID-19 in transplant recipients: the Spanish experience [published online ahead of print, 2020 Oct 23] Am J Transplant. 2020 doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carminatti M., Tedesco-Silva H., Silva Fernandes N.M., Sanders-Pinheiro H. Chronic kidney disease progression in kidney transplant recipients: a focus on traditional risk factors. Nephrology (Carlton) 2019;24:141–147. doi: 10.1111/nep.13483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.