Abstract
Background
The novel severe acute respiratory syndrome coronavirus-2 virus, which has led to the global coronavirus disease-2019 (COVID-19) pandemic is known to adversely affect the cardiovascular system through multiple mechanisms. In this international, multicenter study conducted by the World Alliance Societies of Echocardiography, we aim to determine the clinical and echocardiographic phenotype of acute cardiac disease in COVID-19 patients, to explore phenotypic differences in different geographic regions across the world, and to identify parameters associated with in-hospital mortality.
Methods
We studied 870 patients with acute COVID-19 infection from 13 medical centers in four world regions (Asia, Europe, United States, Latin America) who had undergone transthoracic echocardiograms. Clinical and laboratory data were collected, including patient outcomes. Anonymized echocardiograms were analyzed with automated, machine learning–derived algorithms to calculate left ventricular (LV) volumes, ejection fraction, and LV longitudinal strain (LS). Right-sided echocardiographic parameters that were measured included right ventricular (RV) LS, RV free-wall strain (FWS), and RV basal diameter. Multivariate regression analysis was performed to identify clinical and echocardiographic parameters associated with in-hospital mortality.
Results
Significant regional differences were noted in terms of patient comorbidities, severity of illness, clinical biomarkers, and LV and RV echocardiographic metrics. Overall in-hospital mortality was 21.6%. Parameters associated with mortality in a multivariate analysis were age (odds ratio [OR] = 1.12 [1.05, 1.22], P = .003), previous lung disease (OR = 7.32 [1.56, 42.2], P = .015), LVLS (OR = 1.18 [1.05, 1.36], P = .012), lactic dehydrogenase (OR = 6.17 [1.74, 28.7], P = .009), and RVFWS (OR = 1.14 [1.04, 1.26], P = .007).
Conclusions
Left ventricular dysfunction is noted in approximately 20% and RV dysfunction in approximately 30% of patients with acute COVID-19 illness and portend a poor prognosis. Age at presentation, previous lung disease, lactic dehydrogenase, LVLS, and RVFWS were independently associated with in-hospital mortality. Regional differences in cardiac phenotype highlight the significant differences in patient acuity as well as echocardiographic utilization in different parts of the world.
Keywords: Echocardiography, WASE, International, COVID-19, Mortality, Strain
Abbreviations: 2CH, Two-chamber; 4CH, Four-chamber; ACC, American College of Cardiology; AI, Artificial intelligence; ASE, American Society of Echocardiography; AUC, Area under the curve; BNP, Brain natriuretic peptide; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; EF, Ejection fraction; EACVI, European Association of Cardiovascular Imaging; FWS, Free-wall strain; ICU, Intensive care unit; LDH, Lactic dehydrogenase; LV, Left ventricular; LS, Longitudinal strain; LVEDV, Left ventricular end-diastolic volume; LVEF, Left ventricular ejection fraction; LVESV, Left ventricular end-systolic volume; LVLS, Left ventricular longitudinal strain; MICE, Multiple imputations by chained equations; OR, Odds ratio; Q1, Quartile 1; Q3, Quartile 3; ROC, Receiver-operating characteristic; RV, Right ventricular, ventricle; RVBD, Right ventricular basal diameter; RVFWS, Right ventricular free-wall strain; RVLS, Right ventricular longitudinal strain; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; TTE, Transthoracic echocardiogram; ULN, Upper limit of normal; WASE, World Alliance Societies of Echocardiography
Attention ASE Members:
Login at www.ASELearningHub.org to earn continuing medical education credit through an online activity related to this article. Certificates are available for immediate access upon successful completion of the activity and postwork. This activity is free for ASE Members, and $25 for nonmembers.
The year 2020 has been marked by the global coronavirus disease-2019 (COVID-19) pandemic, with over 108 million cases and 2.4 million deaths in 223 countries to date.1 The novel virus known as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has spread quickly across the globe and has inflicted historic levels of morbidity and mortality. While the respiratory system is the most directly affected, growing evidence suggests that COVID-related cardiovascular disease plays a significant role in disease severity and patient outcomes.2, 3, 4
Of note, the number of cases, deaths, and mortality rates vary considerably across various countries and regions. The reasons for such diversity have not yet been fully elucidated but could be socioeconomic, genomic,5 or multifactorial in nature.
The International World Alliance Societies of Echocardiography (WASE) COVID-19 study was designed to identify echocardiographic parameters that would be prognostic of clinical outcomes in patients with COVID-19 infection and to determine whether COVID-19 heart disease presents differently in various geographic regions around the world. In this initial report of the WASE COVID-19 study we aim to describe the clinical characteristics and echocardiographic phenotype of acute cardiac disease in patients with COVID-19 infection and to explore their in-hospital prognostic value.
Methods
Data Collection
Adult patients (≥18 years old) with a diagnosis of SARS-CoV-2 infection (including a positive antigen or polymerase chain reaction test) during the first wave of the pandemic (January-September 2020) were considered for the study if a transthoracic echocardiogram (TTE) was performed during the initial COVID-19-related hospitalization. The data regarding hospital care and echocardiograms were collected retrospectively. Patients were enrolled at 13 medical centers in four world regions (Asia, Europe, United States, Latin America). The echocardiograms were ordered and acquired based on local clinical practices. Acceptable TTEs included both comprehensive and limited studies as long as at least the apical four-chamber (4CH) view was acquired. The study was approved by the local ethics or institutional review board committee.
Basic clinical information and DICOM cardiac ultrasound images were collected from the medical records, PACS systems, or echo machines, deidentified, and transferred via a web-based system (Ultromics, Oxford, UK) for central analysis by a core group lead by the principal investigators.
Clinical information including demographic data, medical history, vital signs, and biomarkers were collected by local investigators and stored in a secure web-based system (Castor EDC, London, UK). Blood pressure and heart rate were obtained at the time of the echocardiographic exam. Biomarkers were collected by each site whenever deemed clinically appropriate, and all biomarkers collected within 72 hours of echocardiographic acquisition were included in the analysis. These biomarkers included troponin, lactic dehydrogenase (LDH), brain natriuretic peptide (BNP), D-dimer, and C-reactive protein (CRP). To account for different biomarker assays used at each center, each biomarker (troponin, BNP, LDH, D-dimer, and CRP) was classified as either normal, borderline abnormal (<2 × upper limit of normal [ULN]), or abnormal (>2 × ULN), based on normal reference values for the particular center at which the patient was enrolled.
Image Analysis
Image transfer was facilitated by a two-step anonymization process to a cloud-based image analysis software. Left ventricular (LV) analyses were performed through commercially available artificial intelligence (AI) algorithms created by machine learning (Ultromics), which generated an initial LV ejection fraction (LVEF), LV end-systolic and end-diastolic volumes (LVESV, LVEDV), and LV longitudinal strain (LVLS); see additional details in the Supplemental Material. As a means of quality control and validation, all LV measurements were repeated manually twice by an independent British Society of Echocardiography accredited echocardiographer (human reads 1 and 2). The two human reads were performed independently by the same echocardiographer (to derive intraobserver variability) who was randomly selected from a pool of seven independent operators, blinded to other echo reads and clinical data. All LV volumes and EFs were obtained by performing endocardial tracings and using the biplane method of disks (modified Simpson's rule).6 Only cases with acceptable-quality LV views were included, which was defined as lack of apical foreshortening with adequate visualization of all segments in the apical 4CH view. Longitudinal strain was calculated as the average of all available segments from the 4CH and two-chamber (2CH) views, as a long-axis view was not obtained in the vast majority of cases. Cutoffs for mild, moderately, and severely reduced LVEF as well as normal and abnormal LVLS were determined by the 2015 American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) guidelines for cardiac chamber quantification.6 Normal LVLS was defined as <–19%, mildly abnormal LVLS was defined as >–19% and <–15%, and severely abnormal LVLS was defined as >–15%.6 , 7
Right ventricular (RV) analysis included RV global LS (RVLS), RV free-wall strain (RVFWS), and RV basal diameter (RVBD) and was performed by the Core Laboratory with a semiautomated right ventricle– (RV-) specific package from TOMTEC Image Arena (build no. 494368, TOMTEC, Unterschleissheim, Germany). Only cases with acceptable-quality RV views were included. Acceptable imaging quality was defined as presence of an RV-focused view with adequate visualization of the RV free wall. Abnormal RVFWS was defined as >–20%.8
Statistical Analysis
The primary outcome was defined as in-hospital death. Continuous variables were expressed as mean (±SD) or median (interquartile range) according to data distribution and compared using the Student's t test or Wilcoxon rank-sum test, as appropriate. Categorical data, presented as number and percentages, were compared using χ2 test. Binomial generalized linear models were used to evaluate the univariate and multivariate association between covariates (age, sex, ethnicity, region, baseline comorbidities, etc.), echocardiographic parameters, and the outcome. Forward stepwise binomial regression models (in which the choice of variables was carried out by an automatic procedure) were performed to evaluate the association between confounders, multiple variables, and outcome and included the following univariate significant covariates: previous heart disease, previous lung disease, age, geographical region, LVEF, LVLS, RVFWS, LDH (log), BNP (log), CRP (log), shock, and admission to an intensive care unit (ICU). In this stepwise regression model, the choice of variables was carried out autonomously, by which the addition of each stepwise selected variable gave the most statistically significant improvement of the fit. This multivariate modeling method selects the best-fitting model. All significant univariate variables were offered as input to the model initially, which then statistically applies an independent stepwise hierarchy to selecting the best-fitting covariate from this input list. Results from regression models are reported as odds ratios (ORs) and 95% CIs.
As echocardiograms in the context of the COVID-19 pandemic were performed with operator safety in mind,9 not all traditional views were uniformly acquired. In order to determine accurate values and to build a homogeneous database, missing data for calculation of biplane LVEF and LVLS were determined using a multiple imputation model, following guidelines from the European Medicines Agency on confirmatory clinical trials.10 Specifically, a multiple imputation by chained equations (MICE) method was used to derive the 2CH values for cases with a 4CH value but missing 2CH (n = 89), in order to calculate biplane measures. The mean of the three LV reads (automated AI, human read 1, and human read 2) was taken as the final value. An AI analysis was used to derive interobserver (human vs AI) variability. Reproducibility of LV measurements was tested on the entire cohort and in 40 random cases for the RV. Inter- and intraobserver variability were assessed using intraclass correlation coefficients, coefficients of variation (calculated as the absolute difference between pairs of repeated measurements in the percentage of their mean value), and mean of differences (see Supplemental Table 1).
Results
Over a 9-month period (January to September 2020), 870 patients were enrolled at 13 centers in nine countries. Baseline patient demographic and clinical characteristics are listed in Table 1 . The median age was 60 years old, and 56.1% of patients were male. One hundred twenty-five patients (14.3%) were enrolled in the United States, 238 (27.4%) in Latin America, 160 (18.4%) in Europe, and 347 (39.9%) in Asia. The distribution of enrolled cases from each of the four global regions according to calendar month in 2020 is shown in Figure 1 , which depicts the rapid expansion of the pandemic during its first wave, starting in China, followed by Europe, the United States, and Latin America in that chronological order.
Table 1.
Baseline characteristics, all patients (N = 870)
| Characteristic | Value |
|---|---|
| Age, years, median (Q1-Q3) | 60 (50-70) |
| Gender, n (%) | |
| Female | 381 (43.8) |
| Male | 488 (56.1) |
| Race, n (%) | |
| White non-Hispanic | 197 (22.6) |
| White Hispanic | 152 (17.5) |
| Black | 136 (15.6) |
| Asian | 271 (31.1) |
| Mixed | 72 (8.3) |
| Other | 34 (3.9) |
| Unknown | 8 (0.9) |
| Days to echo,∗ median (Q1-Q3) | 3 (1-9) |
| Geographic region (n, %) | |
| United States | 125 (14.4) |
| Europe | 160 (18.4) |
| Asia | 347 (39.9) |
| Latin America | 238 (27.5) |
| Blood pressure, mean ± SD | |
| Systolic, mm Hg | 123.3 ± 19.3 |
| Diastolic, mm Hg | 74.6 ± 12.1 |
| Heart rate, bpm, mean ± SD | 85.4 ± 15.4 |
| Previous medical conditions, n (%) | |
| Cardiac (all) | 513 (58.9) |
| Heart failure | 64 (7.3) |
| Coronary artery disease | 120 (13.8) |
| Stroke | 32 (3.6) |
| Diabetes | 175 (20.1) |
| Hypertension | 374 (42) |
| Lung | 126 (14.5) |
| Kidney | 75 (8.6) |
| Biomarkers, n (%) | |
| Troponin | |
| Normal | 18 (6.0) |
| Borderline | 68 (22.6) |
| Abnormal | 215 (71.4) |
| CRP | |
| Normal | 106 (13.4) |
| Borderline | 51 (6.4) |
| Abnormal | 635 (80.2) |
| BNP/N-terminal pro b-type BNP | |
| Normal | 153 (42.6) |
| Borderline | 46 (12.8) |
| Abnormal | 160 (44.6) |
| LDH, U/L | |
| Normal | 117 (22.3) |
| Borderline | 255 (48.7) |
| Abnormal | 152 (29.0) |
| D-dimer | |
| Normal | 85 (13.8) |
| Borderline | 98 (16.0) |
| Abnormal | 431 (70.2) |
| Condition at time of echo, n (%) | |
| Ventilation | 236 (27.1) |
| Hemodynamic support | 155 (17.8) |
| ICU | 402 (46.2) |
| Admitted, non-ICU | 468 (53.8) |
Time to echo is measured from time of admission for COVID. Normal refers to values below the ULN specific for the test utilized at site of enrollment. Borderline refers to values within 1-2 × ULN. Abnormal refers to values > 2 × ULN.
Figure 1.
Temporal trends in study enrollment in each of the four world regions. In the early months of the pandemic (January-February), the majority of COVID-19 admissions occurred in Asia, followed shortly thereafter by Europe and the United States (March-May), and finally Latin America (May-September).
By protocol design, all patients were hospitalized. At the time of echocardiogram acquisition, 46.2% were admitted to an ICU, 27.1% required mechanical ventilation, and 17.9% required hemodynamic support (inotropic drugs, vasopressors, intra-aortic balloon pump; Table 1).
Troponin was tested in 301 patients and was elevated in 94% (borderline, 22.6%; abnormal, 71.4%). C-reactive protein was recorded in 792 patients and was elevated in 87% (borderline, 6.4%; abnormal, 80.2%), while BNP was recorded in 359 patients and was elevated in 57% (borderline, 12.8%; abnormal, 44.6%). Lactic dehydrogenase was recorded in 524 patients and was elevated in 78% (borderline, 48.7%; abnormal, 29%), and D-dimer was recorded in 614 patients and was elevated in 86% (borderline, 16% abnormal, 70.2%).
Echocardiographic Practices
Echocardiograms were obtained a median of 3 days after admission (interquartile range, 1-9). Due to safety concerns related to transmission of the COVID-19 virus, enrolling centers followed different echocardiographic acquisition protocols and therefore completeness of the echocardiographic studies was variable: 10 of the 13 centers performed limited exams as their primary COVID in-patient practice and three out of the 13 centers performed comprehensive exams. The median number of video loops acquired per exam was 32 (quartile 1 [Q1] to quartile 3 [Q3], 19-42). The 4CH view was evaluable in 722 exams (83% of enrolled patients), while both the 2CH and 4CH views were evaluable in 633 of them (75% of enrolled patients). The remaining 89 cases had 4CH without 2CH views, for which the 2CH value was estimated by multiple imputation, following guidelines from the European Medicines Agency, as described in the methods section. Only cases in which a 4CH view was available for quantification were used for LV analysis. Baseline characteristics and outcomes of these 722 patients were similar to the entire cohort and those excluded from LV analysis (Supplemental Table 2). Of the available cases, the percentage of echocardiograms with good image quality by region was 88.2% for Asia, 86.6% for Europe, 86.7% for the United States, and 69.3% for Latin America. Echocardiograms included a dedicated RV-focused view of sufficient quality for strain analysis in 589 patients (68% of enrolled patients). The mean monthly LVEF and RVFWS measurements remained relatively constant throughout the calendar year (Supplemental Figure 1).
Echocardiographic Findings
The mean LVEF was 60.2% (±12.3), the mean LVEDV was 107.9 (±45.1), and the mean LVESV was 44.8 mL (±33.7; Table 2 ). Eighty-three percent of the patients had LVEF > 50%, 11% had mild LV dysfunction (LVEF, 40%-50%), 5% had moderate dysfunction (LVEF, 30%-40%), and 3% had severe LV dysfunction (LVEF < 30%). The mean LVLS was –18.7% (±5.3), and mean RVFWS was –22.8% (±6.1). Severely abnormal LVLS (>–15%) and abnormal RVFWS (>–20%) were present in 22% and 29% of patients, respectively. The mean RVBD was 40 mm (±25), and 33% had a dilated RV (RVBD > 42 mm). The overall distribution of patients according to ventricular function (LVLS and RVFWS) and region can be seen in Supplemental Figure 2. Among patients in whom both LVLS and RVFWS could be evaluated (n = 466), 19.7% had biventricular dysfunction, 51.1% had normal function of both ventricles, 21.8% had LV dysfunction with normal RV function, and 6.2% had RV dysfunction with normal LV function. Compared to patients not admitted to the ICU at the time of their echocardiogram, those admitted to the ICU had worse mean LVEF (61.2% ± 12.0% vs 59.1% ± 12.8%, P = .029), LVLS (–19.3% ± 5.25% vs –18.0% ± 5.58%, P = <.001), and RVFWS (–24.0% ± 5.80% vs –21.2% ± 6.19%, P = <.001).
Table 2.
Echocardiographic characteristics
| Characteristic | All patients |
ICU patients |
Non-ICU patients |
|---|---|---|---|
| N = 722 | n = 326 | n = 396 | |
| LVEF, % | 60.2 (±12.3) | 59.1 (±12.9) | 61.1 (±11.7) |
| LVEDV, mL | 107.9 (45.1) | 110 (±45.9) | 106 (±44.5) |
| LVESV, mL | 44.8 (±33.7 | 47.4 (±33.4) | 42.8 (±33.8) |
| LVLS, % | –18.7 (±5.3) | –17.9 (±5.6) | –19.4 (±5.0) |
| N = 509 | n = 234 | n = 275 | |
|---|---|---|---|
| RVFWS, % | –22.8 (±6.1) | –21.3 (±6.2) | –24.0 (±5.8) |
| RVBD, cm | 4 (±2.5) | 4.2 (±2.3) | 4 (±2.7) |
| Pericardial effusion, n (%) | 145 (19.4) | 78 (23.2) | 67 (16.3) |
Reproducibility of Echocardiographic Measurements
Inter and intra-observer variability analyses, as defined by intraclass correlation coefficients, coefficient of variance, and mean of differences, are presented in Supplemental Tables 1 and 3. Reproducibility was excellent for intraobserver LV variables and good for interobserver LVEF and LVLS. Both intra- and interobserver reproducibility of RVFWS and RVBD were excellent, while they were very good for RVLS.
Regional Differences
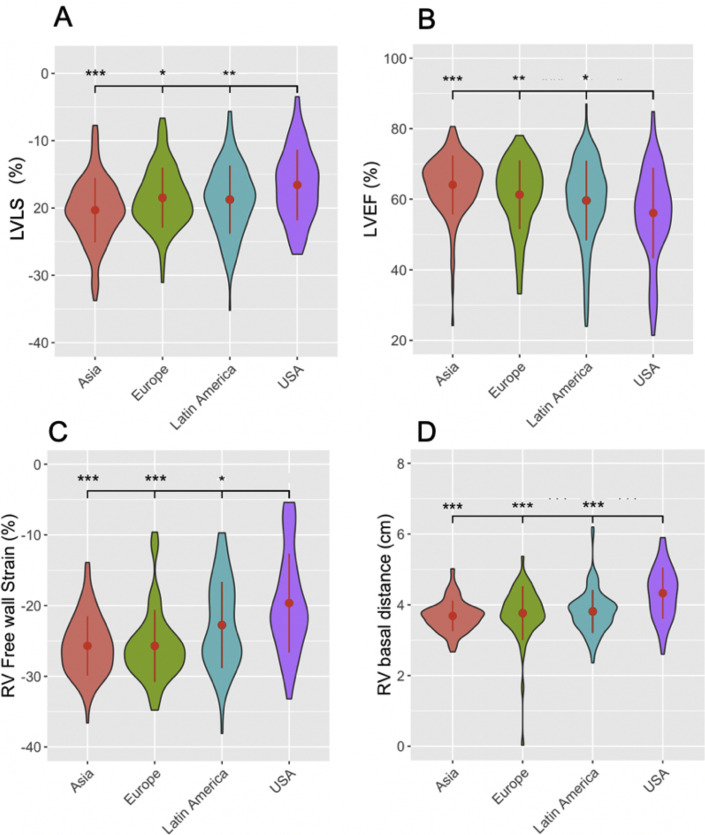
Comparison of baseline clinical, treatment, and echocardiographic characteristics suggests significant regional differences in patients with acute COVID-19 infection throughout the first wave of the pandemic. Compared to those enrolled in the United States, Latin America, and Europe, patients enrolled in Asia had fewer comorbid conditions (heart and lung disease; Figure 2 ) and a better biomarker profile (LDH, BNP, and D-dimer; Figure 3 ) and required less hemodynamic/LV support and mechanical ventilation (Supplemental Figure 3). Importantly, regional differences were also observed in terms of LVEF, LVLS, RVFWS, and RVBD, with best values observed in Asia, followed in worsening order by Europe, Latin America, and the United States. The LV and RV measurements by region are shown in Figure 4 .
Figure 2.
Regional differences in heart and lung disease. (A) Regional differences in rates of heart disease among COVID-19 patients who underwent echocardiograms, with the highest rates observed in the United States (73.8%), followed by Latin America (68.1%), Europe (55.2%), and Asia (46.9%). (B) Regional differences in rates of lung disease among COVID-19 patients who underwent echocardiograms, with the highest rates observed in the United States (36.9%), followed by Latin America (11.8%), Europe (17.7%), and Asia (3.9%). Data are shown as a pie chart depicting percentage of patients with heart and lung disease, respectively, in each world region.
Figure 3.
Regional differences in biomarkers. Violin plots depicting regional differences in LDH (A), BNP (B), and D-dimer (C) biomarkers. In all cases, the highest values were observed in the United States, followed by Europe, Latin America, and then Asia, with ranges depicted in the plots above. Central dots and lines represent mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 compared with United States.
Figure 4.
Regional differences in LVEF, LVLS, RVFWS, and RVBD. Violin plots depicting regional differences in LVEF (A), LVLS (B), RVFWS (C), and RVBDs (D). Better functional average values were observed in Asia, followed by Europe, Latin America, and the United States. Central dots and lines represent means ± SD. Widths of the plots are directly proportionate to the number of cases. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 compared with United States.
In-Hospital Mortality
Overall in-hospital mortality was 21.6% (188 patients). In-hospital mortality in each region is shown in Figure 5 . As assessed by univariate analysis (Table 3 ), clinical factors significantly associated with in-hospital mortality included age; prior cardiovascular disease; prior lung disease; serum levels of BNP, LDH, D-dimer, and CRP; admission to the ICU; need for mechanical ventilation; and need for hemodynamic support. The echocardiographic parameters associated with mortality in univariate analysis were LVEF, LVLS (when severely reduced, as defined by LVLS > –15%), RVFWS, and RVBD.
Figure 5.
Regional differences in in-hospital mortality. Pie chart demonstrating in-hospital mortality rates in different geographic regions (11% in Asia, 19% in Europe, 27% in Latin America, and 26% in the United States).
Table 3.
Multiple regression analysis for associations with death
| Univariate analysis | ||
|---|---|---|
| Risk factor | OR (95% CI) | P value |
| Clinical parameters | ||
| Prior heart disease | 1.819 (1.276, 2.625) | .001 |
| Prior lung disease | 1.787 (1.174, 2.690) | .006 |
| Prior kidney disease | 0.884 (0.480, 1.545) | .679 |
| Hypoxemia | 1.324 (0.505, 3.120) | .540 |
| Body mass index | 0.980 (0.953, 1.005) | .128 |
| Age | 1.041 (1.028, 1.054) | <.001 |
| Gender | 1.057 (0.763, 1.470) | .739 |
| Ethnicity (vs white non-Hispanic) | ||
| White Hispanic | 1.248 (0.733, 2.145) | .417 |
| Black | 1.039 (0.593, 1.825) | .894 |
| Asian | 0.591 (0.353, 0.997) | .046 |
| Mixed | 1.889 (1.010, 3.537) | .046 |
| Other | 0.769 (0.286, 1.863) | .579 |
| On ventilation | 9.638 (5.798, 16.264) | <.001 |
| In ICU | 5.721 (3.454, 9.784) | <.001 |
| On hemodynamic support | 7.641 (4.416, 13.374) | <.001 |
| LV echocardiography | ||
| EF | ||
| Continuous | 0.974 (0.956, 0.992) | .005 |
| Mildly reduced | 1.049 (0.586, 1.804) | .868 |
| Moderately reduced | 1.267 (0.567, 2.637) | .540 |
| Severely reduced | 3.764 (1.544, 9.278) | .003 |
| LS | ||
| Continuous | 1.030 (0.997, 1.064) | .076 |
| Mild abnormal | 1.340 (0.714, 2.510) | .359 |
| Severe abnormal | 3.045 (1.684, 5.586) | <.001 |
| RV echocardiography | ||
| FWS | 0.936 (0.904, 0.968) | <.001 |
| Global LS | 0.924 (0.888, 0.960) | <.001 |
| Basal diameter | 1.500 (1.154, 1.954) | .0025 |
| Biomarkers, vs normal | ||
| BNP, abnormal | 5.530 (3.065, 10.552) | <.001 |
| BNP, borderline | 1.756 (0.535, 4.990) | .314 |
| LDH, abnormal | 11.200 (4.857, 32.530) | <.001 |
| LDH, borderline | 4.441 (1.759, 13.587) | .003 |
| D-dimer, abnormal | 28.306 (6.184, 501.854) | <.001 |
| D-dimer, borderline | 20.720 (3.454, 397.374) | .005 |
| CRP, abnormal | 4.748 (2.309, 11.480) | <.001 |
| CRP, borderline | 0.918 (0.0472, 5.743) | .939 |
| Troponin, abnormal | 1.385 (0.809, 2.415) | .242 |
| Troponin, borderline | 1.885 (0.615, 5.601) | .254 |
| Region, vs Asia | ||
| Europe | 1.936 (0.913, 4.185) | .086 |
| Latin America | 3.077 (1.615, 6.164) | <.001 |
| United States | 2.940 (1.420, 6.270) | <.001 |
| Multivariate Analysis | ||
| Model 1 (LV) | ||
| Age | 1.118 [1.051, 1.219] | .003 |
| LVLS | 1.179 [1.045, 1.358] | .012 |
| LDH (log) | 6.17 [1.744, 28.734] | .009 |
| Previous lung disease | 7.322 [1.561, 42.152] | .015 |
| Model 2 (RV) | ||
| LDH (log) | 5.691 [1.898, 20.844] | .003 |
| Age | 1.080 [1.034, 1.141] | .002 |
| RVFWS | 1.136 [1.037, 1.256] | .007 |
Univariate and multivariate relationships assessed by binomial generalized linear models. Forward-stepwise binomial regression models were performed to evaluate the association between confounders. Model 1 included LVLS instead of RVFWS as the co-correlate. Model 2 included RVFWS instead of LVLS as the co-correlate. Left ventricular support refers to the use of inotropes or vasopressors. Ejection fraction mildly reduced, 40%-50%; EF moderately reduced, 30%-40%; EF severely reduced, <30%; LS mildly abnormal, –19% to –15%; LS severely abnormal, > –15%. Normal refers to values within ULN. Borderline refers to values within 1-2 × ULN. Abnormal refers to values > 2 × ULN.
In the multivariate analysis, the independent clinical parameters were age, LDH, and previous lung disease. Furthermore, LVLS and RVFWS demonstrated independent associations with in-hospital mortality (Table 3). Since LVLS and RVFWS appeared to be co-correlates, these variables were modeled separately.
Receiver-operating characteristic (ROC) curves were used to generate the optimal cutoff values for continuous variables (age, LDH, LVLS, and RVFWS) for associations with mortality (Supplemental Figure 4). The optimal cutoff values were 64 years for age (area under the curve [AUC] = 0.71), 389 U/L for LDH (AUC = 0.74), –16.7% for LVLS (AUC = 0.59), and –20.2% for RVFWS (AUC = 0.65). Using a composite ROC model with all these optimal cutoffs, we identified the best-performing combination for association with mortality, which had 80.0% sensitivity, 66.5% specificity, and an AUC = 0.80 (Supplemental Figure 5).
Discussion
In this report from the global WASE COVID-19 study, we have shown that LV dysfunction is observed in approximately 20% and RV dysfunction in approximately 30% of patients with acute SARS-CoV-2 infection and that age at presentation, previous lung disease, LDH, LVLS, and RVFWS are independently associated with in-hospital mortality. This is the first large, international study including 13 medical centers spanning nine countries and four world regions and designed to characterize cardiac phenotype in patients with acute COVID-19 infection. Significant diversity in cardiac phenotype and echocardiographic utilization was observed among different world regions, with the worst metrics of LV and RV function (LVEF, LVLS, RVLS, and RVFWS) noted in the United States, followed by Latin America and Europe, while Asia consistently showed the most normal biventricular metrics.
Overall, these observations can be explained by regional differences in baseline comorbidities and patient acuity or may, in fact, reflect a population bias stemming from changes in echocardiographic practices as a result of growing concern for viral spread and more ubiquitous implementation of safe practices. A few months into the global COVID-19 pandemic, providers were urged by ASE/American College of Cardiology (ACC) recommendations to defer all elective examinations and to perform problem-focused, limited echocardiograms whenever possible, and only for urgent or emergent indications.9 The regional differences observed in the WASE COVID-19 study may partially reflect the different echocardiographic practices in various parts of the world, with American providers performing TTEs primarily on the most critically ill patients (as recommended by the ASE/ACC) compared with a broader patient population in other regions of the world. Another possible explanation is that given the earlier onset of the pandemic, patients recruited from Asia may have demonstrated “burned-out” COVID-19 disease on echocardiography that was not as severe compared with other regions, which may eventually show similarly improved cardiac phenotypes. Through future studies and continued follow-up of our patient cohort, we will aim to determine whether cardiac involvement has changed over time since the initial months of the pandemic.
Early studies out of Wuhan, China, demonstrated a link between myocardial injury (as determined by troponin elevation) and increased risk of mechanical ventilation and mortality.11 , 12 Similarly, a subsequent study from New York City demonstrated that a significant proportion of patients admitted with COVID-19 infection had cardiac involvement (36%) and that troponin elevation was associated with higher risk of death.4 In our population, troponin was not associated with death, while other biomarkers were significant in the univariate analysis (LDH, BNP, D-dimer, and CRP), although LDH was the only independent parameter associated with mortality.
The lack of association with mortality for troponin in our study may have several explanations. There may be a component of selection bias, as it was tested only in 35% of the patients and was elevated in nearly all of them. However, the elevated troponin contrasts with the overall good LV and RV function of the population. In patients with sepsis, such as those with severe COVID-19 infection, elevations in troponin may not necessarily be the result of direct necrosis of cardiomyocytes but may be the result of increased cell permeability leading to the release of troponin degradation products through the cell membrane in nonnecrotic cardiomyocytes.13 , 14 While several studies have concluded that elevated troponin is predictive of mortality in sepsis, other studies did not find it to be an independent predictor of mortality.13 , 15, 16, 17 Given the severity of COVID-19 infection of our patient population, it is possible that while elevated troponin served as a marker of severe illness, it was in many cases not reflective of direct myocardial damage or injury, as suggested by a weak correlation between troponin and LVLS, and was therefore by itself not associated with mortality.
More recently, reports from the American Heart Association COVID-19 Cardiovascular Disease Registry described that cardiovascular comorbidities including coronary artery disease, hypertension, and diabetes dramatically increased the risk of in-hospital mortality in patients with COVID-19 infection and that the risk of death was particularly high for older, nonwhite males.3 , 18 Although these results are consistent with our findings, the WASE COVID-19 study is unique in that we included multiple cardiac markers during the acute phase of the infection to describe clinical and echocardiographic parameters associated with short-term outcomes. In addition, the cardiac phenotypic data were obtained by an independent, centralized analysis using advanced echocardiographic techniques including automated, AI-driven technologies. A recent international survey conducted by the EACVI in April 2020 on 1,216 patients with presumed or confirmed COVID-19 infection showed a slightly higher proportion of left- and right-sided involvement (39% and 33%, respectively) than that observed in our study.19 This survey, however, was conducted during a short 2-week time period during the height of the pandemic, while the WASE COVID-19 study was conducted over a longer period spanning a larger portion of the pandemic and included only patients with laboratory confirmed SARS-CoV-2 infection; echocardiograms were analyzed centrally by a core group. Of note, the percentage of left-sided involvement seen in the COVID-19 patients in our study was similar to that observed in a recent study by Churchill et al.,20 although slightly less than that observed by Sud et al. 21
Multiple mechanisms have been proposed for myocardial injury following COVID-19 infection, including direct injury caused by binding of the SARS-CoV-2 spike protein to the ACE-2 receptor in the myocardium leading to a myocarditis-like syndrome, as well as indirect injury caused by an inflammatory storm induced by the body's immune response.22 , 23 Given the known link between COVID-19 and cardiovascular disease, identification of the underlying cardiac abnormalities in patients with COVID-19 infection and myocardial injury using available imaging modalities is critically important. In a small series of patients with cardiac magnetic resonance imaging, a significant proportion of cardiac involvement in patients recovered from COVID-19 infection was noted, as evidenced by myocardial edema in 54% of patients and late gadolinium enhancement in 31% of patients.22 In a recent study of 305 patients from New York City and Milan who had undergone TTE examinations, Giustino et al. 24 showed that patients with myocardial injury had an increased prevalence of major echocardiographic abnormalities and that following multivariable adjustment, myocardial injury with TTE abnormalities was associated with a higher risk of death compared with myocardial injury without TTE abnormalities. In the WASE COVID-19 study, we have performed central, blinded analyses of echocardiograms that included LVEF and strain of both ventricles and have shown that both left-sided and right-sided markers of function (LVLS and RVFWS) are independently associated with mortality, while an elevated troponin level was not. Using a composite ROC model, we showed that the sensitivity of the model for association with mortality is improved when adding strain-based echocardiographic parameters to clinical parameters. Importantly, LVEF in our study failed to show independent association with mortality in a model that included strain variables (which differed from recent findings published by Faridi et al. 25), while LVLS and RVFWS did, further highlighting the importance of advanced echocardiographic assessment of COVID-19 patients to obtain a more complete risk assessment. Importantly, beyond its association with mortality, echocardiography is an important diagnostic tool in COVID-19 that can help point the provider toward a particular diagnosis, for example, ischemic heart disease in the setting of regional wall motion abnormalities or sepsis in the setting of hyperdynamic LV function.
Because the lungs are the main target organ of SARS-CoV-2 and given the large prevalence of acute respiratory distress syndrome in critically ill patients with COVID-19 infection, the RV is thought to be particularly susceptible to dysfunction following COVID-19 infection.26 Previous studies have demonstrated RV failure as a sequelae of acute lung injury and acute respiratory distress syndrome,27 as the RV is easily affected by changes in pulmonary vascular resistance.28 Investigators from Israel showed that in 100 patients diagnosed with COVID-19 infection who had undergone TTE, RV dilatation and dysfunction were the most common echocardiographic findings and that worsening RV function was the most common finding in patients with clinical deterioration.29 A group of investigators from Wuhan, China, found that RVLS was the most predictive of mortality in patients with COVID-19 infection compared with other conventional RV parameters.26 In view of the above, and because the lungs are the main target organ of the SARS-CoV-2 virus, our finding that RVFWS is independently associated with mortality in COVID-19 patients worldwide makes sense from a pathophysiologic standpoint and confirms the findings from Israel and China.
Importantly, our study's findings reflect the cardiac manifestations of COVID-19 infection during the early months (first wave) of the global pandemic, starting in January 2020. Since that time, we have seen rapid improvement in early diagnosis, therapeutics, and management strategies, resulting in improved mortality rates across health systems, even when adjusted for demographic and clinical factors.30 In the WASE COVID-19 study, we did see an improvement in overall in-hospital mortality from July to September (Supplemental Figure 6), which could be attributed to seasonal factors as well as to improved therapeutics as noted above.
Finally, the WASE COVID-19 study was unique in its use of AI and machine learning to calculate the main left-sided parameters (LVEF and LVLS) in this study. We also conducted a robust reproducibility analysis with several rounds of manual measurements to ensure that the AI-based results were both reliable and reproducible. To our knowledge, this is the largest multicenter, echocardiographic-based study on patients with COVID-19 infection that has employed AI and machine learning for determination of both LV and RV function.
Limitations
The limitations of our study include a relatively small number of patients compared with the overall scale of the global COVID-19 pandemic. In spite of this limitation, our patient cohort included patients from nine different countries representing most continents, including some of the most affected regions worldwide. Additionally, overall image quality of echocardiograms was less than optimal compared with nonpandemic standards, reflecting widespread safety concerns during the early months of the pandemic as well as the lack of a standardized, universal acquisition protocol. As a result, some of the studies did not have dedicated RV-focused views for measurement of RVLS or RVFWS. Despite this limitation, RV metrics were obtained in 68% of the enrolled patients. For the same safety reasons, most studies were limited echocardiograms that were missing the apical long-axis view; therefore LS was calculated only from the apical 4CH and 2CH views, as opposed to the standard three apical views. Similarly, most of the echocardiograms did not include sufficient information to assess the left atrium, diastolic function, and pulmonary pressures. Thus, the association of these parameters with outcome cannot be assessed in this data set. The authors have observed that the image quality of echocardiograms performed on patients with COVID-19 infection has improved considerably since the beginning of the pandemic, likely reflecting the increased comfort level of sonographers and physicians. To that end, the ASE recently published a guidelines statement focused on the safety and protection of patients and echocardiography service providers during the novel COVID-19 pandemic.9 It is appreciated that our patient cohort is limited to those with clinical indications for echocardiography and that these patients are more likely to have cardiac involvement compared with the general population of patients with COVID-19 infection. We also acknowledge that while LVLS and RVFWS were independently associated with mortality, their individual sensitivity is limited, therefore affecting the ROC analysis (AUC = 0.59 and 0.65, respectively, as seen in Supplemental Figure 4), and thus should be used in conjunction with other clinical prognostic factors such as age and LDH. Finally, it is possible that the clinical indications for echocardiograms resulted in selection bias, with our study population reflecting mostly patients in the severe spectrum of the disease. Nevertheless, this study provides valuable insights into the global utilization of echocardiography during the COVID-19 pandemic and demonstrates that in cases where echocardiography is clinically indicated, it provides crucial prognostic information.
Conclusion
This study confirms the profound effect of the SARS-CoV-2 virus on the cardiovascular system and highlights the significant regional differences in both cardiac phenotype and echocardiographic utilization. Left ventricular LS, RVFWS, age, LDH, and previous lung disease were independently associated with in-hospital mortality, while LVEF was not.
Acknowledgments
We thank Katie Ions, Nancy Spagou, Alex Hudson, Jake Kenworthy, Lokken Wong, Angela Mumith, Will Hawkes, and Rizwan Sarwar from Ultromics; Marcus Schreckenberg, Niklas Hitschrich, and Sabrina Zoernack from TOMTEC; Andrea Van Hoever from the American Society of Echocardiography; and Patricia Marques, Gaynor Jones, Seamus Walker, Gurpreet Dhadday, Aiko Nepomuceno, Jherick Tay, Claire Mitchell, Sarah Hastings, and Emma-Jayne Robins.
Footnotes
Conflicts of Interests: G.M.W. and T.D. are employees of Ultromics. M.J.M. is on the advisory board and speaker's bureau for Bracco and Philips. R.M.L. is on the advisory board and speaker's bureau for Philips and the advisory board for Caption Health. F.M.A. received institutional (MedStar Health) research grants from TOMTEC, Ultromics, GE, and Caption Health and nonpaid scientific advisory committee for Ultromics. All other authors have no conflicts to disclose related to this work.
This work was supported by the American Society of Echocardiography Foundation, University of Chicago, and MedStar Health with in-kind support from Ultromics and TOMTEC.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.echo.2021.05.010.
Contributor Information
WASE-COVID Investigators:
Vince Ryan V. Munoz, Rafael Porto De Marchi, Sergio M. Alday-Ramirez, Consuelo Orihuela, Anita Sadeghpour, Jonathan Breeze, Amy Hoare, Carlos Ixcanparij Rosales, Ariel Cohen, Martina Milani, Ilaria Trolese, Oriana Belli, Benedetta De Chiara, Michele Bellino, and Giuseppe Iuliano
Additional WASE COVID Investigators
Vince Ryan V. Munoz, MD, Philippine Heart Center, Quezon City, Philippines; Rafael Porto De Marchi, MD, Radiology Institute of the University of Sao Paulo Medical School, São Paulo, Brazil; Sergio M. Alday-Ramirez, PhD, and Consuelo Orihuela, MD, Instituto Nacional de Ciencias Medicas y Nutricion, CDMX, Mexico; Anita Sadeghpour, MD, FASE, Rajaie Cardiovascular Medical and Center, Echocardiography Research Center, IUMS, Tehran, Iran; Jonathan Breeze, MD, and Amy Hoare, King's College Hospital, London, UK; Carlos Ixcanparij Rosales, MD, Centro Nacional 20 de Noviembre, ISSSTE, CDMX, Mexico; Ariel Cohen, MD, Hôpitaux de l'est parisien St Antoine-Tenon, Universite Pierre et Marie Curie, Paris, France; Martina Milani, MD, Ilaria Trolese, RDCS, Oriana Belli, MD, and Benedetta De Chiara, MD, Ospedale Niguarda, Milan, Italy; Michele Bellino, MD, and Giuseppe Iuliano, MD, University of Salerno, Salerno, Italy.
Supplementary data
References
- 1.World Health Organization . 2020. Coronavirus disease (COVID-19) pandemic.https://covid19.who.int/ Available at: [Google Scholar]
- 2.Uriel N., Sayer G., Clerkin K.J. Myocardial injury in COVID-19 patients: the beginning or the end? J Am Coll Cardiol. 2020;76:547–549. doi: 10.1016/j.jacc.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navar A.M. Impact of cardiovascular disease on outcomes among hospitalized COVID-19 patients: results from >14,000 patients across the United States. Virtual AHA Scientific Sessions. 2020 https://www.ahajournals.org/doi/10.1161/CIR.0000000000000940 Available at: [Google Scholar]
- 4.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyoshima Y., Nemoto K., Matsumoto S., Nakamura Y., Kiyotani K. SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J Hum Genet. 2020;65:1075–1082. doi: 10.1038/s10038-020-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang H., Wright L., Negishi T., Negishi K., Liu J., Marwick T.H. Research to practice: assessment of left ventricular global longitudinal strain for surveillance of cancer chemotherapeutic-related cardiac dysfunction. JACC Cardiovasc Imaging. 2018;11:1196–1201. doi: 10.1016/j.jcmg.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.H., Park J.H. Strain analysis of the right ventricle using two-dimensional echocardiography. J Cardiovasc Imaging. 2018;26:111–124. doi: 10.4250/jcvi.2018.26.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel Coronavirus outbreak: endorsed by the American College of Cardiology. J Am Soc Echocardiogr. 2020;33:648–653. doi: 10.1016/j.echo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Medicines Agency . 2011. Guideline on missing data in confirmatory clinical trials.guideline-missing-data-confirmatory-clinical-trials_en.pdf [Google Scholar]
- 11.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosjo H., Varpula M., Hagve T.A., Karlsson S., Ruokonen E., Pettila V. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med. 2011;37:77–85. doi: 10.1007/s00134-010-2051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu A.H. Increased troponin in patients with sepsis and septic shock: myocardial necrosis or reversible myocardial depression? Intensive Care Med. 2001;27:959–961. doi: 10.1007/s001340100970. [DOI] [PubMed] [Google Scholar]
- 15.Tiruvoipati R., Sultana N., Lewis D. Cardiac troponin I does not independently predict mortality in critically ill patients with severe sepsis. Emerg Med Australas. 2012;24:151–158. doi: 10.1111/j.1742-6723.2011.01530.x. [DOI] [PubMed] [Google Scholar]
- 16.Lim W., Cook D.J., Griffith L.E., Crowther M.A., Devereaux P.J. Elevated cardiac troponin levels in critically ill patients: prevalence, incidence, and outcomes. Am J Crit Care. 2006;15:280–288. quiz 289. [PubMed] [Google Scholar]
- 17.Yucel T., Memis D., Karamanlioglu B., Sut N., Yuksel M. The prognostic value of atrial and brain natriuretic peptides, troponin I and C-reactive protein in patients with sepsis. Exp Clin Cardiol. 2008;13:183–188. [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez F., Solomon N., de Lemos J.A., Das S.R., Morrow D.A., Bradley S.M. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.052278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dweck M.R., Bularga A., Hahn R.T., Bing R., Lee K.K., Chapman A.R. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churchill T.W., Bertrand P.B., Bernard S., Namasivayam M., Churchill J., Crousillat D. Echocardiographic features of COVID-19 illness and association with cardiac biomarkers. J Am Soc Echocardiogr. 2020;33:1053–1054. doi: 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sud K., Vogel B., Bohra C., Garg V., Talebi S., Lerakis S. Echocardiographic findings in patients with COVID-19 with significant myocardial injury. J Am Soc Echocardiogr. 2020;33:1054–1055. doi: 10.1016/j.echo.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giustino G., Croft L.B., Stefanini G.G., Bragato R., Silbiger J.J., Vicenzi M. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76:2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faridi K.F., Hennessey K.C., Shah N., Soufer A., Wang Y., Sugeng L. Left ventricular systolic function and inpatient mortality in patients hospitalized with coronavirus disease 2019 (COVID-19) J Am Soc Echocardiogr. 2020;33:1414–1415. doi: 10.1016/j.echo.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Li H., Zhu S., Xie Y., Wang B., He L. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osman D., Monnet X., Castelain V., Anguel N., Warszawski J., Teboul J.L. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009;35:69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 28.Repesse X., Charron C., Vieillard-Baron A. Right ventricular failure in acute lung injury and acute respiratory distress syndrome. Minerva Anestesiol. 2012;78:941–948. [PubMed] [Google Scholar]
- 29.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I. Spectrum of cardiac manifestations in covid-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz L.I., Jones S.A., Cerfolio R.J., Francois F., Greco J., Rudy B. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16:90–92. doi: 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.