Abstract
Background and aim
Cytokine release syndrome is a dangerous complication of the coronavirus disease 2019 (COVID-19). This study aimed to evaluate the efficacy and safety of tofacitinib in the management of this complication.
Methods
The retrospective study included COVID-19 patients with C-reactive protein (CRP) levels of 60–150 mg/L.
Results
Thirty-two patients who received tofacitinib (TOF group) and 30 patients who did not receive any anti-cytokine drugs (control [CON] group) were enrolled. Mortality and the incidence of admission to the intensive care unit were lower in the TOF group than in the CON group (16.6% vs. 40.0%, p = 0.009; and 15.6% vs. 50.0%, p = 0.004). There was a significant decrease in the volume of the affected part of the lungs (p = 0.022) and a significant increase in oxygen saturation (p = 0.012) in the TOF group than in the CON group 7–10 days after the beginning tofacitinib administration. CRP level was lower in the TOF group than in the CON group (7 [3–22] vs. 20 [5–52] mg/L; p = 0.048) 7–10 days after the start of the administration of tofacitinib. During this period, the number of patients requiring mechanical ventilation or those in the prone position increased in the CON group compared to those in the TOF group (26.7% vs. 0.0%, p = 0.002; 33.3% vs. 6.7%, p = 0.020). There was no significant difference in the development of secondary infections, liver or kidney injury, and cytopenia between the two groups.
Conclusion
Tofacitinib was effective and safe for managing the cytokine release syndrome in COVID-19. Randomized controlled double-blind trials with tofacitinib with and without the simultaneous use of glucocorticoids are required to confirm our findings.
Keywords: COVID-19, Cytokine release syndrome, Janus kinase inhibitor, Tofacitinib, Interleukin 6
Nomenclature
- COVID-19
coronavirus disease 2019
- CRP
C-reactive protein
- CT
computed tomography
- ICU
Intensive care unit
1. Introduction
The coronavirus disease 2019 (COVID-19) has become a huge challenge for medicine worldwide. It can have systemic complications, and cytokine release syndrome is one of the most dangerous complications [[1], [2], [3]]. Several anti-cytokine drugs have been proposed to manage this complication [4]. Interleukin-6 antagonist tocilizumab being the most studied [5]. However, the results of randomized trials have been inconsistent and its place in the treatment of COVID-19 is yet to be established [5].
Several experts suggested that attention be paid to the small molecules that are blockers of intracellular transmitters of inflammatory signals. Janus kinase inhibitors are the most promising of these molecules. Janus kinase is connected to cytokine receptors and their signal is transmitted into the cell [6,7]. The blockade of this kinase leads to a blockage in signaling pathways dependent on its cytokines. Therefore, Janus kinase inhibitors occupy an intermediate place between broad immune suppressors (glucocorticoids) and single cytokine inhibitors (tocilizumab).
Recently, the results of a large randomized placebo-controlled trial showed that the addition of the Janus kinase inhibitor baricitinib to remdesivir improved the course of COVID-19 but had no significant effect on mortality [8]. Another Janus kinase inhibitor, ruxolitinib, also did not show a significant effect on mortality in a randomized placebo-controlled trial in COVID-19 [9].
However, no controlled studies have been published describing the efficacy and safety of Janus kinase inhibitor tofacitinib for the management of COVID-19, which was the aim of our study.
2. Materials and methods
This was a retrospective study. All patients signed informed consent for the use of off-label drugs. The study was approved by the local ethical committee.
2.1. Patients
The study included patients admitted to the Clinic of internal medicine, gastroenterology, and hepatology of Sechenov University with suspected COVID-19 following the recommendations of the World health organization [10] from April to July 2020 and presenting with cytokine release syndrome. Unfortunately, there are no generally accepted criteria for the diagnosis of cytokine release syndrome. According to the Russian clinical guidelines [11], the indication for prescribing anti-cytokine drugs for COVID-19 is the presence of a C-reactive protein (CRP) level of above 60 mg/L. However, our doctors considered patients with severe cytokine release syndrome (CRP>150 mg/L) as candidates for the use of other anti-cytokine drugs. Thus, the criteria for inclusion in the study were as follows: age over 18 years, laboratory-confirmed COVID-19 (a positive result of polymerase chain reaction) or suspected COVID-19 (based on the complex of clinical, imagine and epidemiological data) [10,11], the absence of pregnancy, the signing of informed consent to the administration of drugs off-label, CRP level above 60 mg/L. Patients who were on other anti-cytokine drugs or with CRP levels of above 150 mg/L were excluded.
2.2. Intervention
Tofacitinib was used orally on the first day at a dose of 10 mg two times a day, then for 4 days at 5 mg two times a day.
2.3. Controls
The control group (CON group) consisted of patients who did not receive anti-cytokine therapy.
Tofacitinib was intermittently available at the Clinic's pharmacy. Thus, the patient's allocation into the tofacitinib group (TOF group) or the CON group depended primarily on luck. Both groups were recruited at almost the same time.
Patients in both groups also underwent antiviral, antibacterial, anticoagulant, and dexamethasone treatment depending on the indications and contraindications (Table S1).
2.4. Outcomes
Survival or death of the patient was considered as the primary outcome.
The duration of hospitalization, total duration of the disease, the incidence of admission to the intensive care unit (ICU) and mechanical ventilation, the change in the values of key biomarkers, chest computed tomography (CT) and respiratory function 7–10 days after starting tofacitinib administration were considered as secondary outcomes. The main side effects (cytopenia, secondary infections, thrombosis, increased transaminases, and cholestasis) were also evaluated.
The volume of the affected lungs was determined by chest CT and consisted of the sum of ground glass and consolidation volumes. Oxygen saturation was measured using a pulse oximeter.
The value of the main biomarkers of the disease was evaluated at two points: first, at 1–3 days before the administration of tofacitinib and second, 7–10 days after its administration.
The average day of hospitalization when tofacitinib was administered was determined; this day of hospitalization +/− 1 day was used as point 1 in the CON group. The value of biomarkers 7–10 days after this point was considered as point 2.
2.5. Statistical analysis
Results are presented as median [interquartile range]. Group comparison for continuous data and categorical data was performed using the Mann–Whitney test and Chi-square test, respectively. The Wilcoxon test was used to assess the changes in the continuous biomarkers. Survival was assessed using the Kaplan-Meier estimator and Cox's F-test. A p-value ≤0.050 was taken as the criterion for significance. Statistical calculations were performed using STATISTICA 10 (TIBCO Software, Palo Alto, CA).
3. Results
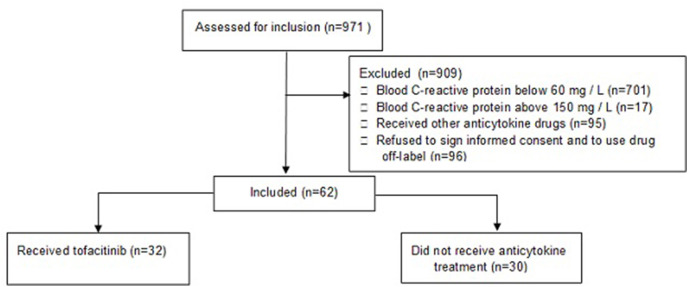
The study included 32 patients who received tofacitinib (TOF group) and 30 people who did not receive any anti-cytokine treatment (CON group) (Fig. 1 ). There was no significant difference between the patient groups in terms of age, sex distribution, body mass index, body temperature at admission, total duration of disease, length of hospital stay, symptoms of COVID-19, the incidence of comorbidities, and the frequency of taking other drugs used to treat COVID-19 (Table 1 , Table S1). Patients who received tofacitinib were less likely to require admission at the ICU than patients who did not receive anti-cytokine drugs. The difference between the groups in the frequency of need for mechanical ventilation almost reached the limits of significance (Table 1).
Fig. 1.
CONSORT 2010 flow diagram.
Table 1.
Main characteristics of patients by groups.
| Group TOF (n = 32) | Group CON (n = 30) | p | |
|---|---|---|---|
| Age, years | 64 [56–73] | 68 [57–78] | 0.117 |
| Male/Female | 19/13 | 16/14 | 0.632 |
| Body temperature at admission, 0C | 37.4 [37.2–37.9] | 37.7 [37.2–38.1] | 0.442 |
| Body mass index, kg/m2 | 29.2 [27.1–34.9] | 31.0 [27.4–33.4] | 0.970 |
| Length of hospital stay, days | 18 [15–23] | 17 [11–22] | 0.200 |
| Total duration of disease, days | 27 [21–35] | 23 [19–30] | 0.121 |
| Death | 5 (15.6%) | 12 (40.0%) | 0.009 |
| Admission to intensive care unit | 5 (15.6%) | 15 (50.0%) | 0.004 |
| The need for mechanical ventilation | 5 (15.6%) | 11 (36.7%) | 0.059 |
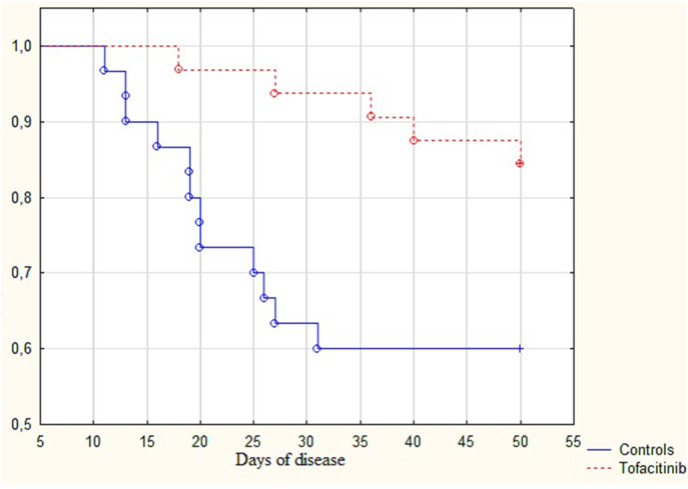
Patients who received tofacitinib had better survival rates than those who did not receive anti-cytokine treatment (84.4% vs. 60.0%; p = 0.009) (Fig. 2 ). Patients in the TOF group died later than patients in the CON group (36 [27–40] days vs. 20 [15–26] days; p = 0.035).
Fig. 2.
Survival curves for patients with COVID-19 and cytokine release syndrome that did not receive anticytokine treatment (Control group) and received tofacitinib (Tofacitinib group).
There was no significant difference between patient groups in the value of biomarkers at point 1 (Table 2 ). Seven to 10 days after the start of tofitcinib administration, a significant decrease in the activity of lactate dehydrogenase and creatinine level, and the volume of the affected part of the lungs, and a significant increase in oxygen saturation were observed in the TOF group. A significant decrease in body temperature and CRP level, and an increase in white blood cell and neutrophil count were observed in the two groups. However, the decrease in CRP level was significantly greater in the TOF group than in the CON group. Alanine aminotransferase activity increased significantly only in the CON group (Table 2).
Table 2.
Change in the values of the main biomarkers 7–10 days after the beginning of the tofacitinib use. Point 1 was 1–3 days before the beginning of the tofacitinib use or equivalent days in the group CON. Point 2 was 7–10 days after the beginning of tofacitinib use or equivalent days in group CON.
| Group |
Group TOF (n = 32) |
Group CON (n = 30) |
p |
p |
||||
|---|---|---|---|---|---|---|---|---|
| Point | 1 | 2 | pa | 1 | 2 | pa | 1 | 2 |
| Lung lesion volume, % | 44 [34–63] | 38 [28–50] | 0.022 | 44 [35–63] | 38 [37–63] | 0.760 | 0.762 | 0.457 |
| C-reactive protein, mg/L | 95 [73–140] | 7 [3–22] | <0.001 | 110 [94–131] | 20 [5–52] | 0.001 | 0.458 | 0.048 |
| Oxygen saturation, % | 93 [91–95] | 96 [91–98] | 0.012 | 92 [87–95] | 92 [82–97] | 0.561 | 0.200 | 0.073 |
| Body temperature, 0C | 36.9 [36.7–38.0] | 36.6 [36.4–36.7] | <0.001 | 37.5 [36.7–38.0] | 36.6 [36.4–36.7] | <0.001 | 0.402 | 0.857 |
| White blood cells, 109/L | 6.9 [4.9–10.7] | 9.1 [7.3–13.1] | 0.001 | 7.0 [5.7–8.9] | 10.7 [7.0–14.1] | 0.001 | 0.927 | 0.773 |
| Neutrophils, 109/L | 5.2 [3.7–6.6] | 6.4 [4.5–10.8] | 0.009 | 5.3 [4.2–7.8] | 7.5 [4.2–12.0] | 0.005 | 0.442 | 0.767 |
| Lymphocytes, 109/L | 1.2 [0.7–1.7] | 1.7 [0.9–2.1] | 0.161 | 0.9 [0.7–1.2] | 1.3 [0.5–2.2] | 0.010 | 0.098 | 0.477 |
| Platelets, 109/L | 194 [165–283] | 275 [219–364] | 0.007 | 224 [189–254] | 233 [140–440] | 0.154 | 0.577 | 0.434 |
| Creatinine, μmol/L | 102 [92–115] | 91 [74–104] | <0.001 | 93 [83–110] | 84 [73–97] | 0.090 | 0.156 | 0.519 |
| ALT, U/L | 36 [19–66] | 46 [27–87] | 0.147 | 44 [24–59] | 54 [35–81] | 0.048 | 0.606 | 0.947 |
| LDH, U/L | 518 [396–824] | 415 [372–570] | 0.019 | 663 [474–978] | 511 [354–957] | 0.440 | 0.205 | 0.336 |
| Supplemental oxygen | 22 (68.8%) | 15 (46.7%) | 0.076 | 20 (66.7%) | 9 (30.0%) | 0.005 | 0.861 | 0.173 |
| Mechanical ventilation | 0 (0.0%) | 0 (0.0%) | – | 0 (0.0%) | 8 (26.7%) | 0.002 | – | 0.002 |
| No respiratory support | 10 (31.2%) | 17 (53.1%) | 0.076 | 10 (33.3%) | 13 (43.3%) | 0.426 | 0.861 | 0.441 |
| Prone position | 7 (21.9%) | 6 (18.8%) | 0.756 | 2 (6.7%) | 10 (33.3%) | 0.010 | 0.089 | 0.190 |
ALT - Alanine aminotransferase; AST - Asparaginaminotransferase; LDH - Lactate dehydrogenase; ICU - intensive care unit.
*- Interleukin 6 was not tested in all included patients.
- Difference between points 1 and 2.
There was a significant decrease in interleukin-6 level within 7–10 days after the start of tofacitinib (36.8 [25.0–64.9] vs. 10.4 [3.1–23.2] pg/ml; p = 0.023). Unfortunately, this biomarker was not studied in the control group.
The proportion of patients who no longer needed supplemental oxygen increased 7–10 days after the start of tofacitinib administration, and this increase almost reached the limit of significance. The proportion of patients who required mechanical ventilation significantly increased in the CON group compared to those in the tofacitinib group. The proportion of patients who were in the prone position increased significantly in the control group and did not change significantly in the tofacitinib group (Table 2).
There were no cytopenias and extrapulmonary infections in both groups. There was no significant difference between both groups in the frequency of detection of signs of pulmonary embolism, liver, and kidney injury (Table 3 ).
Table 3.
Complications of COVID-19 and its treatment in patients who received tofacitinib (group TOF) and did not receive anticytokine drugs (group CON).
| Complication | Group TOF (n = 32) | Group CON (n = 30) | p |
|---|---|---|---|
| Pulmonary embolism | 4 | 6 | 0.422 |
| Acute kidney injury | 5 | 2 | 0.265 |
| ALT > ULN | 19 | 21 | 0.382 |
| ALT > 3 ULN | 8 | 9 | 0.660 |
| ALT > 10 ULN | 2 | 5 | 0.195 |
| Cholestasisa | 2 | 0 | 0.164 |
ALT- Alanine aminotransferase; ULN - Upper limit of normal.
- Increased activity of gamma-glutamate transferase and alkaline phosphatase above ULN.
4. Discussion
Cytokine release syndrome is one of the most dangerous complications of COVID-19 [2,3]. Despite the encouraging results from the use of tocilizumab in low-quality studies, recent randomized trials have produced conflicting results [4,5]. Thus, the place of tocilizumab, the most studied anti-cytokine drug, in the treatment of COVID-19 remains to be established [5].
In this regard, it is relevant to study the effect of other groups of anti-cytokine drugs, including Janus kinase inhibitors [6]. Unlike monoclonal antibodies, which include tocilizumab, these drugs are specially designed small molecules against Janus kinase, an enzyme that transmits a proinflammatory signal from the membrane cytokine receptor into the cell [12].
Tofacitinib was the first drug from this group to be used clinically. It was used for the treatment of rheumatoid arthritis and inflammatory bowel diseases. Among the various types of Janus kinases, tofacitinib mostly blocks JAK3, whereas, the signal from the interleukin-6 receptor is transmitted by JAK1 [13]. Therefore, the molecules with a greater affinity for this Janus kinase (baricitinib and ruxolitinib) were used for the treatment of cytokine release syndrome in COVID-19 before tofacitinib. However, a recent randomized placebo-controlled trial found no significant effect of baricitinib and ruxolitinib on patient mortality [8,9].
These failures made us consider the use of tofacitinib, which has a slightly different spectrum of blocked cytokine pathways. To our knowledge, no study has been published on its efficacy and safety in the management of COVID-19; thus, our study is the first to do so.
Unfortunately, there is no generally accepted criterion for the development of cytokine release syndrome. The ideal criterion might be the level of proinflammatory cytokines, in particular interleukin-6, but these tests are expensive and are not yet available in most clinics. We used CRP as a marker for the development of this syndrome because it is the main biomarker of inflammation and can be easily determined in all clinics in the world.
In our study, tofacitinib reduced mortality, the rate of admission to the ICU, the volume of affected lungs, and the level of systemic inflammation. In addition, it also prevented the deterioration of respiratory function. It should be noted that in our study most patients received glucocorticoids, which could have contributed significantly to the improvement of most biomarkers. However, there was no significant difference in the frequency of administration of these drugs between the two groups. Therefore, this difference in mortality cannot be explained by the different frequency of usage of these drugs. However, it is possible that the beneficial effect of tofacitinib was associated with its synergistic action with glucocorticoids in this study.
Baricitinib and ruxolitinib block the effect of a very large number of cytokines (interferon-gamma, interleukins 2, 3, 4, 5, 6, 7, 9, 11, 12, 13, 15, 19, 20, 21, 22, 23, 26, 27, 28, 31, 35, and others) including interleukin-10 that is a main anti-inflammatory factor. Tofacitinib blocks the pathways of a fewer number of cytokines (interleukins 2, 4, 7, 9, 15, and 21), and does not block pathways of interleukin-10 [14]. This difference possibly explains the difference in the results of these drugs in the treatment of COVID-19.
The use of tofacitinib was not accompanied by the development of significant side effects.
A limitation of our study is its retrospective and non-randomized nature. Although the study groups did not differ in the main baseline parameters, selection biases cannot be excluded.
5. Conclusions
In conclusion, tofacitinib was effective and safe for the management of cytokine release syndrome in COVID-19 in our pilot study. Randomized controlled double-blind trials of tofacitinib with and without the simultaneous use of glucocorticoids are required to confirm our findings.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pupt.2021.102039.
Author contributions
Vladimir Ivashkin - research idea, data analysis, article writing and editing; Roman Maslennikov - data collection and analysis, article writing; Ekaterina Vasilieva, Maxim Chipurik, Polina Semikova, Victoria Semenets, Tatyana Russkova, Anna Levshina, Diana Grigoriadis, Shamil Magomedov, Irina Efremova, Natiya Dzhakhaya - data collection, article editing.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J. Microbiol. Immunol. Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 3.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burrage D.R., Koushesh S., Sofat N. Immunomodulatory drugs in the management of SARS-CoV-2. Front. Immunol. 2020;11:1844. doi: 10.3389/fimmu.2020.01844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosn L., Chaimani A., Evrenoglou T., Davidson M., Graña C., et al. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst. Rev. 2021 Mar 18;3 doi: 10.1002/14651858.CD013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai Y.C., Tsai T.F. Oral disease-modifying antirheumatic drugs and immunosuppressants with antiviral potential, including SARS-CoV-2 infection: a review. Therapeutic Advances in Musculoskeletal. 2020;12 doi: 10.1177/1759720X20947296. 1759720X2094729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satarker S., Tom A.A., Shaji R.A., Alosious A., Luvis M., Nampoothiri M. Jak-STAT pathway inhibition and their implications in COVID-19 therapy. PGM (Postgrad. Med.) 2020;16:1–19. doi: 10.1080/00325481.2020.1855921. Published online December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N. Engl. J. Med. 2020;11 doi: 10.1056/NEJMoa2031994. Published online December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y., Wei J., Zou L., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146(1):137–146. doi: 10.1016/j.jaci.2020.05.019. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19 WHO/2019-nCoV/clinical/2020.5 Available from:
- 11.Interim guidelines for the prevention, diagnosis and treatment of new coronaviral infection (COVID-19) https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/051/777/original/030902020_COVID-19_v8.pdf Available from:
- 12.Kontzias A., Kotlyar A., Laurence A., Changelian P., O'Shea J.J. Jakinibs: a new class of kinase inhibitors in cancer and autoimmune disease. Curr. Opin. Pharmacol. 2012;12(4):464–470. doi: 10.1016/j.coph.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadina M., Hilton D., Johnston J.A., et al. Signaling by Type I and II cytokine receptors: ten years after. Curr. Opin. Immunol. 2001;13(3):363–373. doi: 10.1016/s0952-7915(00)00228-4. [DOI] [PubMed] [Google Scholar]
- 14.Hammarén H.M., Virtanen A.T., Raivola J., Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine. 2019;118:48–63. doi: 10.1016/j.cyto.2018.03.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.