Abstract
Subacute thyroiditis (SAT) is a self-limiting thyroid dysfunction of viral origin. Relatively little is known about its occurrence in SARS CoV-2 infected COVID-19 patients. Herein, we report a case of SAT in a 58-year-old patient that was apparently triggered by infection with SARS CoV-2. Clinical, laboratory and imaging features of the patient are presented. The patient was vitally stable with a slightly tender and warm thyroid gland, which was painful on swallowing. His free thyroxine (FT4) was elevated, thyroid stimulating hormone (TSH) was below normal and free triiodothyronine (FT3) was in the physiological range. Previous thyroid exam conducted as a part of routine annual physical checkup was normal. The patient was put on prednisolone and recovered completely within three weeks.
Key indexing terms: COVID-19, SARS CoV-2, Subacute thyroiditis
Introduction
On 31st of December 2019, a novel coronavirus, later named Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV)-2, was detected in the Wuhan city of China in patients who suffered from an atypical pneumonia.1 The disease caused by the virus was named the Coronavirus Disease of 2019 (COVID-19). The virus is highly contagious and has spread rapidly all over the World infecting millions of people. Like a typical coronavirus, SARS-CoV-2 is a single stranded positive sense RNA virus and belongs to beta genus of the Coronaviridae family. The coronaviruses of this genus have caused two epidemics in the last two decades in humans: SARS in 2002 and MERS (Middle East Respiratory Syndrome) in 2012.2 It has been found that SARS-CoV-2 enters target host cells via angiotensin converting enzyme (ACE)−2 receptors. As ACE2 is expressed on a wide variety of cell types and tissues in the body,3 it is not surprising that COVID-19 patients present with a wide range of clinical symptoms such as fever, chills, cough, dyspnea, myalgia, headache and diarrhea, etc.4 Apart from being the metabolic regulator of the body, thyroid gland dysfunction affects innate immunity and may contribute to the pathogenesis of COVID-19.5 Subacute thyroiditis (SAT), also known as De-Quervain's thyroiditis, is a self-limiting acute inflammatory disease of the thyroid that is accompanied by symptoms such as neck pain, fever and other indicators of thyroid dysfunction. It is often triggered in viral infections such as influenza virus, human immunodeficiency virus (HIV), hepatitis E virus (HEV) and hepatitis C virus (HCV), etc.5 , 6 However, relatively little is known about potential effects of the SARS-CoV-2 infection on the thyroid gland.7 , 8 Here we report the case of a COVID-19 patient that developed SAT.
Case presentation
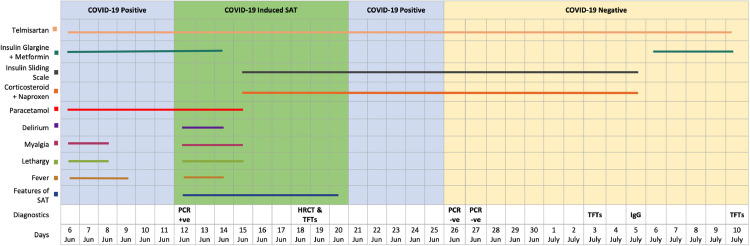
A 58-year-old diabetic and hypertensive male presented with mild fever. The chronic diseases were controlled with tablet metformin (500 mg TDS) plus injection glargine (insulin) (30 units OD) and tablet telmisartan (80 mg OD). On 12th June 2020, he underwent reverse transcription polymerase chain reaction (RT-PCR) testing of his nasopharyngeal swab for the SARS-CoV-2 RNA and was found positive (Fig. 1 ). The patient was suspected for the viral infection as he had lived with his son who had tested positive for the virus on June 5, 2020 and was home quarantined. Furthermore, on June 6, he also developed low grade fever (37.2–37.7 °C), mild myalgia and fatigue. There were no other symptoms, and his oxygen saturation levels were above 95 percent. The patient was receiving azithromycin (500 mg OD) and paracetamol (1 g TDS); his fever was touching base line and his overall health status was improving. Six days following his symptoms, i.e. on June 12, 2020, the patient experienced high grade fever (39.4–40 °C) along with extreme fatigue and delirium. A chest X-ray was performed which indicated infection and the patient was put on IV cefepime (1 g BD) and oral moxifloxacin (400 mg OD) for three days. Meanwhile, the patient experienced staring gaze, weakness with 5 kg of unintentional weight loss over 12 days, along with tender thyroid and odynophagia, but no symptomatic tachycardia. Physical examination revealed febrile, tachypneic patient with pulse rate of 98 beats/minute. Simultaneous local examination of the thyroid showed warmth and tenderness with odynophagia. Upon clinical suspicion of SAT, IV Dexamethasone (4 mg BD) and naproxen were initiated as a treatment for SAT. To minimize the side effects on glycemic control, insulin on sliding scale was started with close blood pressure monitoring with the same dose of telmisartan. Meanwhile, thyroid function tests (free T3, free T4 and thyroid stimulating hormone [TSH]) were advised whose results were suggestive of hyperthyroidism (Fig. 1). Notably, the patient's first routine thyroid function test, conducted earlier on May 8, 2020, as a part of routine annual medical exam was normal.
FIGURE 1.
Evolution of clinical symptoms, treatment and diagnostic parameters over the course of time. Abbreviations: TFT, Thyroid function tests; PCR, PCR test for SARS-CoV-2 RNA; IgG, Anti SARS-CoV-2 IgG.
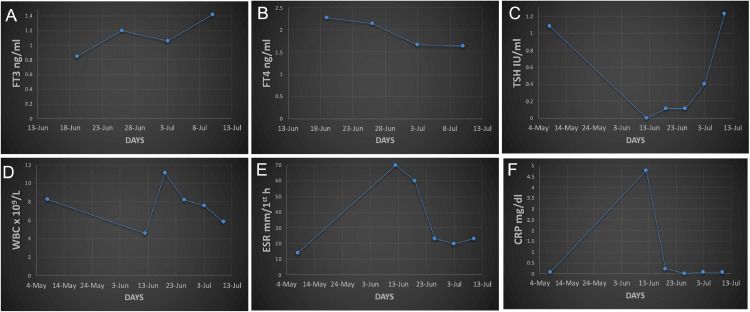
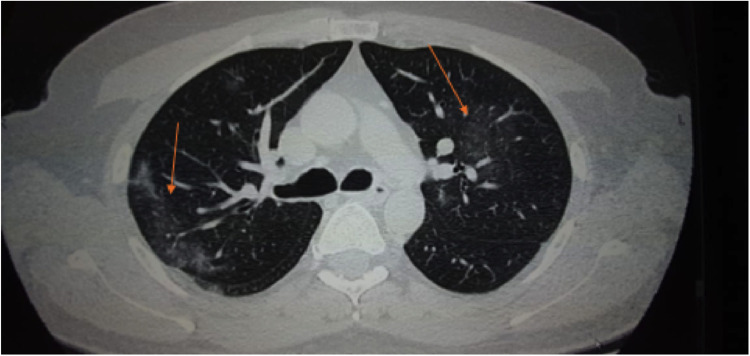
IV dexamethasone was discontinued after 3 days and the patient was put on oral prednisolone (15 mg BD) for 1 week followed by stepwise tapering off. Meanwhile, the patient's serial blood pressure and glycemic control were regularly monitored and remained in physiological limits with medication. Moreover, among hematological and biochemical parameters, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin level, and free T4 levels were elevated. One week after the initial thyroid exam, a fresh thyroid evaluation was performed, which also indicated hyperthyroidism (Fig. 2 ). However, the thyroid function tests conducted for the next two consecutive weeks were found to be in normal ranges and hyperthyroidism was resolved. Moreover, the tenderness and warmth of the gland also disappeared after 4 days, and prednisone was tapered off (i.e. 10 mg BD for 5 days, 10 mg OD for 3 days and finally 5 mg OD for 3 days). Two consecutive RT-PCR tests for the SARS-CoV-2 conducted on June 26 and June 27 were found to be negative, and the test for COVID-19 specific antibody (IgG) performed on July 5 was positive (Fig. 1). A HRCT was carried out on June 19 to visualize any COVID-19-related lung pathology and though the patient was completely asymptomatic, without any respiratory issue, his HRCT revealed ground glass appearance in both lungs even on day 14 (Fig. 3 ). The last evaluation was conducted on July 10, 2020, upon which the patient was asymptomatic. The thyroid functional tests and inflammatory markers were also normal.
FIGURE 2.
Clinical lab parameters over the course of time.Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone or thyrotropin; WBC, white blood cells; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein. Reference ranges: FT3 ng/ml (0.80–2.00), FT4 ng/ml (0.93–1.70), TSH lU/ml (0.27–4.27), WBC x109 cells/L (4.00–11.00), ESR mm/1st h (0.00–10.00), CRP mg/dl (< 0.05).
FIGURE 3.
HRCT of the patient fourteen days after initiation of clinical symptoms. The arrows show ground glass appearance.
Discussion
Herein we report the case of a SARS-CoV-2-infected COVID-19 patient who developed SAT in the course of the infection. The condition was not pre-existing in the patient. Many viruses such as influenza virus, Epstein-Barr virus (EBV), hepatitis E virus (HEV), human immunodeficiency virus (HIV), human cytomegalovirus (HCMV), etc., can lead to SAT.9 In 2002′s outbreak of SARS-CoV-1 infection, autopsies performed on infected patients showed thyroid injury, but the virus infection was never associated with SAT.10 In the case of SARS-CoV-2 pandemic, only a few cases of SAT in COVID-19 patients have been reported. The first such case was reported in Italy by Brancatella et al. in an 18 years old SARS-CoV-2-infected female. The patient recovered from symptoms within one week of taking prednisone. Her thyroid functions and inflammatory markers took 40 days to resolve completely.10 Ruggeri et al. reported another case of de novo SAT occurring in a SARS-CoV-2 infected woman. She developed the condition six weeks following the upper respiratory tract infection.11 The condition resolved progressively within 4 weeks upon corticosteroid therapy. More recently, Mattar et al. described a case of SAT occurring in a hospitalized COVID-19 patient.12 The authors reported rapid resolution upon treatment with corticosteroids. The patient showed upper neck pain along with respiratory tract symptoms. Another de novo case of SAT in a hospitalized COVID-19 patient was reported by Ippolito et al.13 Interestingly, the patient experienced no neck pain (despite enlarged and hypoechoic thyroid) and was treated with pain killers, hydroxychloroquine plus lopinavir/ritonavir and low-flow oxygen therapy. SAT resolved upon treatment with high dose steroids. Interestingly, the patient's RT-PCR tested positive even after all the symptoms disappeared. Despite rarity of SAT in SARS-CoV-2 infected COVID-19 patients, a retrospective study has shown that a majority of COVID-19 patients had decreased levels of TSH and FT3 compared to those in controls.7 Interestingly, the degree of the decrease in these levels correlated with severity of the disease. These studies suggest that thyroid function becomes compromised in a majority of the SARS-CoV-2-infected patients. However, SAT could also occur in some of these patients. It has been well documented that certain individuals are genetically prone to the development of SAT. For example, HLA-B35 is the most common haplotype found in SAT patients.14 Thus, it would be interesting to see whether the COVID-19 patients who develop SAT inherit this haplotype.
SAT leads to severe pain, fever, myalgia and fatigue along with difficulty in swallowing, and with symptoms of hyperthyroidism (tachycardia, palpitation and sweating). ESR and CRP both are elevated in this condition while anti-TPO and anti-TG antibodies are lacking. FT3 and FT4 both remain in high levels while TSH levels are low.15 SAT mainly affects middle aged persons with female predominance. Three clinical phases are usually observed during SAT: the first phase is presented with hyperthyroidism followed by second phase of hypothyroidism, which lasts for approximately 6 months and thereafter, the third phase (euthyroid) appears.16 However, in long term follow up of this patient, neither symptomatically nor laboratory features became suggestive of hypothyroidism (Fig. 1). This finding was in line with the study stating that only 30% of SAT patients will undergo a hypothyroid phase.17 Moreover, diagnosis of SAT is made on clinical history, cytology and thyroid scanning reports. The reports reveal low iodine uptake while ultrasonography shows hypo-echogenicity of the gland. Treatment plan for SAT depends on clinical condition of the patient. In mild cases, no treatment is required while in severe cases glucocorticoid therapy is suggested. Forty mg prednisone is given once daily for a week which is reduced gradually over next 6 weeks. Thyroxin is suggested for patients who develop permanent hypothyroidism after a SAT episode.18
In the case reported here by us, the patient's thyroid function test was normal a month earlier before testing positive for the SARS-CoV-2. The patient had developed symptoms of neck pain and hyperthyroidism after one week of being positive for the viral infection with normal procalcitonin levels. He took prednisone and became asymptomatic within one week of the treatment, which was then gradually tapered off. His-inflammatory markers and thyroid functions took almost 22 days to resolve completely. Hence, in COVID-19 cases with fever and pain in the anterior neck (enlarged, tender and warm thyroid) testing with TSH and at least a free T4 measurement should be done to rule out SAT.
One of the limitations in the case was the inability to test for thyroid ultrasound or radio-iodine uptake scan (RAIU) of the patient. The tests were abandoned as it may have exposed the medical staff and other patients to the virus. Moreover, Xie et al. have reported that thyroid ultrasound may not always provide conclusive evidence for diagnosis of SAT and its differentiation from Graves’ disease.19 Another study, suggests RAIU is variable and can range from depressed to increased uptake depending on the extent of follicular destruction.20
Conclusions
SAT could occur in COVID-19 patients. Therefore, clinicians should also focus on testing thyroid function in these patients with clinical features of hyperthyroidism, fever, anterior neck pain, and/or previous history of thyroid dysfunction.
Financial support
Study was supported by Shaikh Zayed Medical Complex, Lahore, Pakistan.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We thank the patient, whose written informed consent was obtained and agreed to collaborate in this study. We also thank Muhammad Ashraf for his intellectual help. We would also like to thank Dr. Shahroze Arshad, Dr. Abeer Bin Awais and Dr. Hassan Riaz for proofreading this article.
Authors contribution statement
S.A: Data curation, Writing – original draft, Writing – review & editing; M.A.I: Data curation, Writing – original draft, Writing – review & editing; S.A: Writing – original draft, Writing – review & editing; H.H: Data curation, Writing – original draft, Writing – review & editing; S.K: Writing – original draft, Writing – review & editing; M.K.A: Writing – original draft, Writing – review & editing; M.G: Writing – original draft, Writing – review & editing; K.K.C: Writing – original draft, Supervision, Writing – review & editing; A.A: Writing – original draft, Supervision, Writing – review & editing; M.I: Writing – original draft, Supervision, Writing – review & editing
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docea A.O., Tsatsakis A., Albulescu D., et al. A new threat from an old enemy: re‑emergence of coronavirus (review) Int J Mol Med. 2020;45(6):1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Wang Y., Luo W., et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci. 2020;17(11):1522–1531. doi: 10.7150/ijms.46695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariyanto T.I., Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1429–1430. doi: 10.1016/j.dsx.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desailloud R., Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M., Zhou W., Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid. 2021;31(1):8–11. doi: 10.1089/thy.2020.0363. [DOI] [PubMed] [Google Scholar]
- 8.Dworakowska D., Grossman A.B. Thyroid disease in the time of COVID-19. Endocrine. 2020;68(3):471–474. doi: 10.1007/s12020-020-02364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimos G., Pappas G., Akritidis N. Subacute thyroiditis in the course of novel H1N1 influenza infection. Endocrine. 2010;37(3):440–441. doi: 10.1007/s12020-010-9327-3. [DOI] [PubMed] [Google Scholar]
- 10.Brancatella A., Ricci D., Viola N., et al. Subacute thyroiditis After Sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105(7) doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri R.M., Campennì A., Siracusa M., et al. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones. 2021;20(1):219–221. doi: 10.1007/s42000-020-00230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattar S.A.M., Koh S.J.Q., Rama Chandran S., et al. Subacute thyroiditis associated with COVID-19. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ippolito S., Dentali F., Tanda M.L. SARS-CoV-2: a potential trigger for subacute thyroiditis? J Endocrinol Invest. 2020;43(8):1171–1172. doi: 10.1007/s40618-020-01312-7. Insights from a case report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahar S.A., Shahid M., Sarfaraz A., et al. Presentations and outcome of thyroiditis from a tertiary care hospital of Karachi. J Coll Physicians Surg Pak. 2015;25(10):717–720. doi: 10.2015/JCPSP.717720. [DOI] [PubMed] [Google Scholar]
- 15.Dalugama C. Asymptomatic thyroiditis presenting as pyrexia of unknown origin: a case report. J Med Case Rep. 2018;12(1):51. doi: 10.1186/s13256-018-1590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saklamaz A. Is there a drug effect on the development of permanent hypothyroidism in subacute thyroiditis? Acta Endocrinol. 2017;13(1):119–123. doi: 10.4183/aeb.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuels M.H. Subacute, silent, and postpartum thyroiditis. Med Clin North Am. 2012;96(2):223–233. doi: 10.1016/j.mcna.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney L.B., Stewart C., Gaitonde D.Y. Thyroiditis: an integrated approach. Am Fam Physician. 2014;90(6):389–396. [PubMed] [Google Scholar]
- 19.Xie P., Xiao Y., Liu F. Real-time ultrasound elastography in the diagnosis and differential diagnosis of subacute thyroiditis. J Clin Ultrasound. 2011;39(8):435–440. doi: 10.1002/jcu.20850. [DOI] [PubMed] [Google Scholar]
- 20.Slatosky J., Shipton B., Wahba H. Thyroiditis: differential diagnosis and management. Am Fam Physician. 2000;61(4):1054. [PubMed] [Google Scholar]