To the Editor:
We read with interest the recent reports1 , 2 describing the development of minimal change disease (MCD) following the first injection of the BNT162b2 vaccine (Pfizer-BioNTech) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).3 Here, we report a case of nephrotic syndrome relapse in a patient with known MCD following the first injection of the Pfizer-BioNTech coronavirus 19 (COVID-19) vaccine.
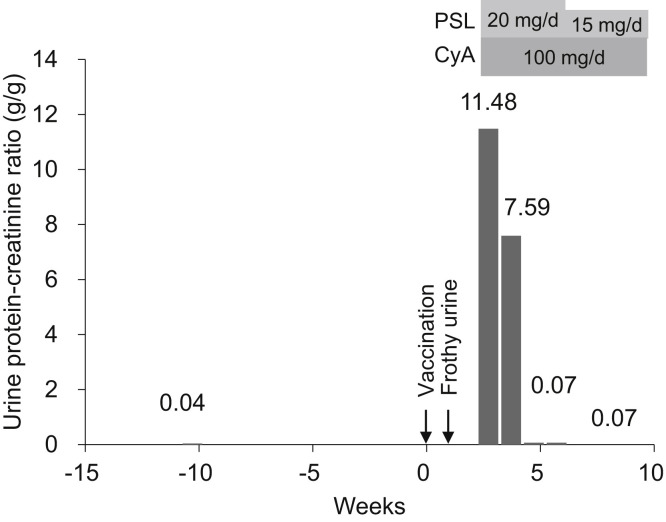
A man in his mid-sixties was followed up at our division for MCD, which was diagnosed by kidney biopsy when he developed nephrotic syndrome at the age of 40. He had been treated with prednisolone and cyclosporine; after long-term remission, the medications were tapered and discontinued 4 and 2 years prior, respectively. Recently, 8 days after receiving the first injection of the Pfizer-BioNTech vaccine, he noticed frothy urine. On the 19th day after vaccination, he visited our clinic, where laboratory tests revealed stable kidney function (serum creatinine 0.99 mg/dL), hypoalbuminemia (2.8 g/dL), and massive proteinuria (urinary protein-creatinine ratio, 11.48 g/g) with high selectivity (selectivity index 0.096), making a relapse of MCD the most likely diagnosis. As measured by the Elecsys immunoassay (Roche Diagnostics), the level of serum antibodies to the SARS-CoV-2 spike protein was markedly elevated (196 U/mL), indicating an immune response to vaccination. Prednisolone (20 mg/d) and cyclosporine (100 mg/d) were restarted, and his proteinuria resolved within 2 weeks (Fig 1).
Figure 1.
Clinical course of the patient. Abbreviations: CyA, cyclosporine; PSL, prednisolone.
The Pfizer-BioNTech vaccine is reported to induce robust T-cell activation and cytokine release along with strong antibody responses,4 which might have contributed to MCD relapse in our patient. However, whether SARS-CoV-2 vaccines could trigger a relapse of MCD or other forms of nephrotic syndrome is currently unclear. Additional case reports and studies are required to address this important question.
Article Information
Support
None.
Financial Disclosure
Dr Komaba has received honoraria, consulting fees, and/or grant support from Bayer Yakuhin, Chugai Pharmaceutical, Japan Tobacco, Kyowa Kirin, Novartis, and Ono Pharmaceutical. Dr Wada has received honoraria, consulting fees, and/or grant support from AstraZeneca K.K., Daiichi Sankyo, Kyowa Kirin, Mitsubishi Tanabe Pharma, and Otsuka Pharmaceutical. Dr Fukagawa has received honoraria, consulting fees, and/or grant support from Astellas Pharma, Bayer Yakuhin, Kissei Pharmaceutical, Kyowa Kirin, Ono Pharmaceutical, and Torii Pharmaceutical.
Peer Review
Received May 11, 2021. Direct editorial input from a Deputy Editor. Accepted in revised form May 15, 2021.
References
- 1.Lebedev L, Sapojnikov M, Wechsler A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(1):142-145. [DOI] [PMC free article] [PubMed]
- 2.Maas RJ, Gianotten S, van der Meijden WAG. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 Vaccine. Am J Kidney Dis. 2021;78(2):312. [DOI] [PMC free article] [PubMed]
- 3.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin U., Muik A., Derhovanessian E. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]