Abstract
Background and aims
There are increasing case reports of rhino-orbital mucormycosis in people with coronavirus disease 2019 (COVID-19), especially from India. Diabetes mellitus (DM) is an independent risk factor for both severe COVID-19 and mucormycosis. We aim to conduct a systematic review of literature to find out the patient's characteristics having mucormycosis and COVID-19.
Methods
We searched the electronic database of PubMed and Google Scholar from inception until May 13, 2021 using keywords. We retrieved all the granular details of case reports/series of patients with mucormycosis, and COVID-19 reported world-wide. Subsequently we analyzed the patient characteristics, associated comorbidities, location of mucormycosis, use of steroids and its outcome in people with COVID-19.
Results
Overall, 101 cases of mucormycosis in people with COVID-19 have been reported, of which 82 cases were from India and 19 from the rest of the world. Mucormycosis was predominantly seen in males (78.9%), both in people who were active (59.4%) or recovered (40.6%) from COVID-19. Pre-existing diabetes mellitus (DM) was present in 80% of cases, while concomitant diabetic ketoacidosis (DKA) was present in 14.9%. Corticosteroid intake for the treatment of COVID-19 was recorded in 76.3% of cases. Mucormycosis involving nose and sinuses (88.9%) was most common followed by rhino-orbital (56.7%). Mortality was noted in 30.7% of the cases.
Conclusion
An unholy trinity of diabetes, rampant use of corticosteroid in a background of COVID-19 appears to increase mucormycosis. All efforts should be made to maintain optimal glucose and only judicious use of corticosteroids in patients with COVID-19.
Keywords: Mucormycosis, Diabetes mellitus, Corticosteroids, COVID-19, Systematic review
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been associated with a wide range of opportunistic bacterial and fungal infections [1]. Both Aspergill us and Candida have been reported as the main fungal pathogens for co-infection in people with COVID-19 [2]. Recently, several cases of mucormycosis in people with COVID-19 have been increasingly reported world-wide, in particular from India. The primary reason that appears to be facilitating Mucorales spores to germinate in people with COVID-19 is an ideal environment of low oxygen (hypoxia), high glucose (diabetes, new-onset hyperglycemia, steroid-induced hyperglycemia), acidic medium (metabolic acidosis, diabetic ketoacidosis [DKA]), high iron levels (increased ferritins) and decreased phagocytic activity of white blood cells (WBC) due to immunosuppression (SARS-CoV-2 mediated, steroid-mediated or background comorbidities) coupled with several other shared risk factors including prolonged hospitalization with or without mechanical ventilators.
Phycomycosis or zygomycosis was first described in 1885 by Paltauf [3] and later coined as Mucormycosis in 1957 by Baker [4] an American pathologist for an aggressive infection caused by Rhizopus. Mucormycosis is an uncommon but a fatal fungal infection that usually affects patients with altered immunity. Mucormycosis is an angioinvasive disease caused by mold fungi of the genus Rhizopus, Mucor, Rhizomucor, Cunninghamella and Absidia of Order- Mucorales, Class- Zygomycetes [5]. The Rhizopus Oryzae is most common type and responsible for nearly 60% of mucormycosis cases in humans and also accounts for 90% of the Rhino-orbital-cerebral (ROCM) form [6]. Mode of contamination occurs through the inhalation of fungal spores.
Globally, the prevalence of mucormycosis varied from 0.005 to 1.7 per million population, while its prevalence is nearly 80 times higher (0.14 per 1000) in India compared to developed countries, in a recent estimate of year 2019–2020 [[7], [8], [9]]. In other words, India has highest cases of the mucormycosis in the world. Notwithstanding, India is already having second largest population with diabetes mellitus (DM) and was the diabetes capital of the world, until recently [10]. Importantly, DM has been the most common risk factor linked with mucormycosis in India, although hematological malignancies and organ transplant takes the lead in Europe and the USA [9]. Nevertheless, DM remains the leading risk factor associated with mucormycosis globally, with an overall mortality of 46% [11]. Indeed, presence of DM was an independent risk factor (Odds ratio [OR] 2.69; 95% Confidence Interval 1.77–3.54; P < 0.001) in a large 2018 meta-analysis of 851 cases of rarely occurring mucormycosis, and the most common species isolated was Rhizopus (48%) [11]. While long term use of corticosteroids have often been associated with several opportunistic fungal infection including aspergillosis and mucormycosis, even a short course of corticosteroids has recently been reported to link with mucormycosis especially in people with DM. A cumulative prednisone dose of greater than 600 mg or a total methyl prednisone dose of 2–7 g given during the month before, predisposes immunocompromised people to mucormycosis [12]. There are few case reports of mucormycosis resulting from even a short course (5–14 days) of steroid therapy, especially in people with DM [13]. Surprisingly, 46% of the patients had received corticosteroids within the month before the diagnosis of mucormycosis in the European Confederation of Medical Mycology study [14].
These findings need a relook in the context of COVID-19 pandemic where corticosteroids are often being used. There has been a steep rise in case reports/series of mucormycosis in people with COVID-19 especially from India. Similarly, many cases are being reported from other parts of globe. Several anecdotal cases are also being reported in grey literature such as the print and electronic media. These finding are unprecedented and carry an immense public health importance, primarily because fatality rate with mucormycosis is pretty high. Especially the intracranial involvement of mucormycosis increases the fatality rate to as high as 90% [15]. Moreover, rapidity of dissemination of mucormycosis is an extraordinary phenomenon and even a delay of 12 h in the diagnosis could be fatal, the reason 50% of cases of mucormycosis have been historically diagnosed only in the post-mortem autopsy series [16]. This prompted us to conduct a systematic review of published case reports/series of mucormycosis in people with COVID-19, to know its temporal associations in relation to comorbidities, association with drugs being used in COVID-19 and overall characteristics of patients with its outcome. We additionally postulated a mechanistic explanation as to why mucormycosis could be increasingly linked to COVID-19 and is being reported increasingly from India.
2. Methods
A systematic literature search was conducted in the electronic database of PubMed and Google Scholar from inception until May 13, 2021 using keyword “COVID-19”, “SARS CoV-2”, AND “Mucormycosis”, “Zygomycosis”, “Phycomycosis, “Mucorales”, “Mucor”, “Rhizopus”, “Rhizomucor”, “Cunninghamella”, and “Absidia”. Details of all the cases that reported mucormycosis (both confirmed and suspected) in people with COVID-19 so far, were retrieved. Characteristics of each patient was collected on excel sheet and analyzed on various endpoints and outcomes. Two authors independently checked the veracity of data.
3. Results
Overall, 28 articles were found to report the original case(s) from the database of PubMed (24/28) and Google Scholar (4/28) [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]]. A total of 101 cases of mucormycosis (including confirmed [95/101] and suspected [6/101]) in people with confirmed (RT-PCR diagnosis) COVID-19 were retrieved (Table 1 ). Largely, 82 cases (81.2%) of mucormycosis in patients with COVID-19 were reported from India, followed by 9 cases (8.9%) from USA and 3 cases (3.1%) from Iran. Only 19 (18.8%) cases as of now were reported from other parts of the world. One study by Satish et al. [25] that reported 11 case-series of mucormycosis in people with COVID-19 from India lacked granular detail of every patient and therefore excluded from some of the analysis. Pooled data from this study showed mucormycosis was predominantly seen in males (78.9%), both in people who were active (59.4%) or recovered (40.6%) from COVID-19. Recovered COVID-19 was defined as those who were either discharged from hospital or in-hospital but 2-weeks had passed post-detection, although there was evident overlap across the cases. Hyperglycemia at presentation (due to pre-existing DM or new-onset hyperglycemia or new-onset diabetes or diabetic ketoacidosis [DKA]) was the single most important risk factor observed in majority of cases (83.3%) of mucormycosis in people with COVID-19, followed by cancer (3.0%). Pre-existing DM accounted for 80% of cases, while concomitant DKA was present in nearly 15% of people with mucormycosis and COVID-19. History of corticosteroid intake for the treatment of COVID-19 was present in 76.3% of cases, followed by remdesivir (20.6%) and tocilizumab (4.1%). Commonest organ involved with mucormycosis was nose and sinus (88.9%), followed by rhino-orbital (56.7%) and ROCM type (22.2%). Overall mortality was noted in 30.7% of the cases. Table 2 summarizes the findings from 101 cases of mucormycosis in people with COVID-19.
Table 1.
Mucormycosis in COVID-19 – Summary of 101 cases reported world-wide till May’ 2021.
| First author |
Place (of report) |
N |
Age, range, M/F |
Comorbidities |
Confirmed/Suspected COVID-19 (Active/Recovered) | Treatment received for COVID-19 |
Confirmed/Suspected Mucor | Location of mucormycosis |
Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | Cancer | Steroid | Tocilizumab | Remdesivir | Nasal/Sinus | Orbit | CNS | Bone | Lung | GIT | |||||||
| Case report/series from India | |||||||||||||||||
| Mehta et al.17 | Mumbai | 1 | 60, M | Y | N | Confirm, A | Y | Y | N | Confirm | Y | Y | N | N | N | N | Death |
| Garg et al.18 | Chandigarh | 1 | 55, M | Y | N | Confirm, A | Y | N | Y | Confirm | N | N | N | N | Y | N | Improving |
| Maini et al.19 | Mumbai | 1 | 38, M | N | N | Confirm, R | Y | N | Y | Confirm | Y | Y | N | N | N | N | Improved |
| Saldanha et al.20 | Mangalore | 1 | 32, F | Y | N | Confirm, A | NR | NR | NR | Confirm | Y | Y | N | N | N | N | Improved |
| Revannavar et al.21 | Mangalore | 1 | Middle age, F | Y, NDD | N | Confirm, A | N | N | N | Confirm | Y | Y | Y | N | N | N | Improving |
| Sen et al.22 | Mumbai | 6 | 46.2–73.9, M: 6 | Y: All | N | Confirm, A: 1 R: 5 |
Y: 5 N: 1 |
N | N | Confirm: 5, Suspect: 1 |
Y: All | Y: All | Y: 5 N: 1 |
N | N | N | Improving |
| Sarkar et al.23 | Puducherry | 10 | 27-67, M: 8 F: 2 |
Y: All, DKA: 9 |
N | Confirm, A: 10 | Y: 10 | N | Y: 5 N: 5 |
Confirm: 6, Suspect: 4 | Y: All | Y: All | Y: 1 | N | N | N | Death: 4, Improved: 2, Unchanged: 4 |
| Mishra et al.24 | Bangalore | 10 | 37-78, M: 9 F: 1 |
Y: 8 N: 2 |
N | Confirm, A: 10 |
Y: 6 N: 4 |
Y: 1 N: 9 |
Y: 6 N: 4 |
Confirm: All | Y: All | Y: 2 | N | Y: 1 | N | N | Death: 4 Improved: 5 LFU: 1 |
| Satish et al.25 | Bangalore | 11 | 30-74, M: NR F: NR |
Y: Majority | Y: 1 (Leukemia) | Confirm, A: 11 |
N | N | N | Confirm: All | Y: Majority | Y: Majority | Y: NR | N | N | N | Death: 2 LAMA: 5 Improving: 4 |
| Moorthy et al.26 | Bangalore | 17 | 39-73, M: 15 F: 2 |
Y: 15 N: 2 |
N | Confirm, A: 4 R: 13 |
Y: 15 N: 2 |
N | N | Confirm: All | Y: All | Y: 11 N: 6 |
Y: 8 N: 9 |
Y: 14 N: 3 |
N | N | Death: 7 Alive: 9 LFU: 1 |
| Sharma et al.27 |
Jaipur |
23 |
NR M: 15 F: 8 |
Y: 21 N: 2 |
N |
Confirm, A: 4 R: 19 |
Y: All |
N |
N |
Confirm: All |
Y: All |
Y: 10 |
Y: 2 |
N |
N |
N |
Death: 0 LFU: 2 Alive: 21 |
| Case report/series from other parts of world | |||||||||||||||||
| Hanley et al.28 | UK | 1 | 22, M | N | N | Confirm, A | NR | NR | NR | Confirm: Autopsy | N | N | N | N | Y | N | Autopsy report |
| Dallalzadeh et al.29 | USA | 2 | 36, M 48, M |
Y:2 DKA: 2 |
N | Confirm, A: 2 |
Y:2 | N | Y:2 | Confirm: 1 Suspected: 1 |
Y | Y | Y | N | N | N | Death: 1 Unchanged: 1 |
| Werthman-E et al.30 | USA | 1 | 33, F | N, DKA | N | Confirm, A | N | N | N | Confirm | Y | Y | N | N | N | N | Improving |
| Placik et al.31 | USA | 1 | 49, M | N | N | Confirm, A | Y | Y | Y | Confirm | N | N | N | N | Y | N | Death |
| Mekkonen et al.32 | USA | 1 | 60, M, | T1DM | N | Confirm, A | Y | N | Y | Confirm | Y | Y | N | N | N | N | Death |
| Alekseyev et al.33 | USA | 1 | 41, M | T1DM, DKA | N | Confirm, A | Y | N | N | Confirm | Y | N | Y | N | N | N | Recovered |
| Johnson et al.34 | USA | 1 | 79, M | Y | N | Confirm, A | Y | N | Y | Confirm, AF + |
N | N | N | N | Y | N | Improving |
| Kanwar et al.35 | USA | 1 | 56, M | N | N | Confirm, A | Y | Y | N | Confirm | N | N | N | N | Y | N | Death |
| Khatri et al.36 | USA | 1 | 68, M | Y | N, (HT) | Confirm, R | Y | N | N | Confirm | N | N | N | N, Skin |
N | N | Death |
| Monte Junior et al.37 | Brazil | 1 | 86, M | N | N | Confirm, A | N | N | N | Confirm | N | N | N | N | N | Y | Death |
| Pasero et al.38 | Italy | 1 | 66, M | N | N | Confirm, A | N | N | N | Confirm | Y | N | N | N | Y | N | Death |
| Bellanger et al.39 | France | 1 | 55, M | N | Y, (Lymphoma) | Confirm, A | N | N | N | Confirm, AF + |
N | N | N | N | Y | N | Death |
| Karimi-G et al.40 | Iran | 1 | 61, M | N, NOD | N | Confirm, R | Y | N | Y | Confirm | Y | Y | N | N | N | N | Improving |
| Veisi et al.41 | Iran | 2 | 40, F:1; 54, M:1 |
N: 1 Y:1 |
N | Confirm, A: 2 |
Y: 2 | N: 2 | Y: 2 | Confirm, All | Y: 2 | Y: 2 | Y: 1 N: 1 |
N | N | N | Death: 1 Recovered: 1 |
| Sargin et al.42 | Turkey | 1 | 56, F | Y, DKA | N | Confirm, R | Y | N | N | Confirm | Y | Y | Y | N | N | N | Death |
| Waizel-H et al.43 | Mexico | 1 | 24, F | N, DKA | N | Confirm, A | N | N | N | Confirm | Y | Y | N | N | N | N | Death |
| Zurl et al.44 | Austria | 1 | 53, M | N | Y, (Leukemia) | Confirm, A | N | N | N | Confirm, Autopsy | N | N | N | N | Y | N | Death |
DM: Diabetes mellitus, CNS: Central nervous system, GIT: Gastro-intestinal tract, M: Male, F: Female, T1DM: Type 1 diabetes mellitus, DKA: Diabetic ketoacidosis, NOD: New-onset diabetes, NDD: Newly detected diabetes, A: Active COVID-19, R: Recovered COVID-19, Y: Yes, N: No, HT: Heart transplant, AF: Aspergillosis fungi, LFU: Lost to follow-up, LAMA: Left against medical advice.
Table 2.
Characteristics of 101 patients of mucormycosis with COVID-19.
| Confirmed mucormycosis, N = 101 | n, (%) | Remarks and limitations | |
|---|---|---|---|
| Country reported (Published) | India | 82 (81.2) | Highest cases reported from India. ≈ denotes nearest rounded of value. |
| USA | 9 (8.9) | ||
| Iran | 3 (≈3.0) | ||
| UK | 1 (≈1.0) | ||
| France | 1 (≈1.0) | ||
| Italy | 1 (≈1.0) | ||
| Brazil | 1 (≈1.0) | ||
| Turkey | 1 (≈1.0) | ||
| Mexico | 1 (≈1.0) | ||
| Austria |
1 (≈1.0) |
||
| Age (Years) |
Range 22-86 |
– |
|
| Sex | Male | 71/90 (78.9) | More commonly observed in males. |
| Female |
19/90 (21.1) |
||
| COVID-19 status | Active | 60/101 (59.4) | Exact definition of active and recovered cases of COVID-19 was different and not unanimous. |
| Recovered |
41/101 (40.6) |
||
| Risk factors | Hyperglycemia at presentation | 75/90 (83.3) | No unanimous definition of hyperglycemia. |
| Malignancy | 3/101 (3.0) | 2 Leukemia, 1 Lymphoma | |
| Post-transplant |
1/101 (1.0) |
1 Heart transplant |
|
| Hyperglycemia at presentation | Pre-existing DM | 72/90 (80.0) | Unless reported as insulin-dependent or type 1 diabetes, all cases were assumed as type 2 diabetes. Lack of baseline HbA1c data and duration of diabetes for majority of DM patients. |
| Types of DM# | – | ||
| Type 2 diabetes | 70/72 (97.2) | ||
| Type 1 diabetes | 2/72 (2.8) | ||
| New-onset DM/hyperglycemia | 2/90 (2.2) | ||
| Presented with DKA |
15/101 (14.9) |
||
| Treatment history of COVID-19 | Steroid | 74/97 (76.3) | Few cases were received all 3 drugs for COVID-19. |
| Tocilizumab | 4/97 (4.1) | ||
| Remdesivir |
20/97 (20.6) |
||
| Mucormycosis | Confirmed | 95/101 (94.1) | Confirmed denotes microbiological or histopathological diagnosis. |
| Suspected |
6/101 (5.9) |
||
| Location of mucormycosis | Nasal/Sinus | 80/90 (88.9) | There appears to have an overlap between Nasal/Sinus only and Rhino-orbital variety. |
| Rhino-orbital | 51/90 (56.7) | ||
| Rhino-orbito-cerebral | 20/90 (22.2) | ||
| Bone involvement | 15/101 (14.9) | ||
| Pulmonary | 8/101 (7.9) | ||
| Gastrointestinal | 1/101 (1.0) | ||
| Cutaneous |
1/101 (1.0) |
||
| Outcomes | Alive (Improved/Improving) | 56/101 (55.4) | Outcomes is difficult to assess considering that several cases were still under in-hospital treatment and their final outcome are not yet known. |
| Unchanged | 5/101 (5.0) | ||
| Death | 31/101 (30.7) | ||
| Status unknown (LFU, LAMA) | 9/101 (8.9) | ||
DM: Diabetes mellitus, DKA: Diabetic ketoacidosis, LFU: Lost to follow-up, LAMA: Left against medical advice.
4. Discussion
Although mucormycosis is an extremely rare in healthy individuals but several immunocompromised conditions predispose it. This includes uncontrolled DM with or without DKA, hematological and other malignancies, organ transplantation, prolonged neutropenia, immunosuppressive and corticosteroid therapy, iron overload or hemochromatosis, deferoxamine or desferrioxamine therapy, voriconazole prohylaxis for transplant recipients, severe burns, acquired immunodeficiency syndrome (AIDS), intravenous drug abusers, malnutrition and open wound following trauma [45]. Mucormycosis can involve nose, sinuses, orbit, central nervous system (CNS), lung (pulmonary), gastrointestinal tract (GIT), skin, jaw bones, joints, heart, kidney, and mediastinum (invasive type), but ROCM is the commonest variety seen in clinical practice world-wide [45]. It should be noted that term ROCM refers to the entire spectrum ranging from limited sino-nasal disease (sino-nasal tissue invasion), limited rhino-orbital disease (progression to orbits) to rhino-orbital-cerebral disease (CNS involvement) [46]. The area of involvement may differ due to underlying condition. For example, ROCM is frequently observed in association with uncontrolled diabetes and DKA, whereas pulmonary involvement is often observed in patients having neutropenia, bone marrow and organ transplant, and hematological malignancies, while GIT gets involved more in malnourished individuals. Giant cell invasion, thrombosis and eosinophilic necrosis of the underlying tissue is the pathological hallmark of mucormycosis. Microbiological identification of the hyphae based on diameter, presence or absence of septa, branching angle (right or acute branching), and pigmentation, differentiates it from other fungal infections. The 1950 Smith and Krichner [47] criteria for the clinical diagnosis of mucormycosis are still considered to be gold standard and include:
-
(i)
Black, necrotic turbinate's easily mistaken for dried, crusted blood,
-
(ii)
Blood-tinged nasal discharge and facial pain, both on the same side,
-
(iii)
Soft peri-orbital or peri-nasal swelling with discoloration and induration,
-
(iv)
Ptosis of the eyelid, proptosis of the eyeball and complete ophthalmoplegia and, (v) Multiple cranial nerve palsies unrelated to documented lesions.
A 2019 nationwide multi-center study of 388 confirmed or suspected cases of mucormycosis in India prior to COVID-19, Prakash et al. found that 18% had DKA and 57% of patients had uncontrolled DM [48]. Similarly, in a data of 465 cases of mucormycosis without COVID-19 in India, Patel et al. [49] has shown that rhino-orbital presentation was the most common (67.7%), followed by pulmonary (13.3%) and cutaneous type (10.5%). The predisposing factors associated with mucormycosis in Indians include DM (73.5%), malignancy (9.0%) and organ transplantation (7.7%) [49]. Presence of DM significantly increases the odds of contracting ROCM by 7.5-fold (Odds ratio 7.55, P = 0.001) as shown in a prospective Indian study, prior to COVID-19 pandemic [50]. In a recent systematic review conducted until April 9, 2021 by John et al. [51] that reported the findings of 41 confirmed mucormycosis cases in people with COVID-19, DM was reported in 93% of cases, while 88% were receiving corticosteroids. These findings are consistent with our findings of even larger case series of 101 mucormycosis cases (95 confirmed and 6 suspected) in Covid-19, where 80% cases had DM, and more than two-third (76.3%) received a course of corticosteroids. Collectively, these findings suggest a familiar connection of mucormycosis, diabetes and steroid, in people with COVID-19.
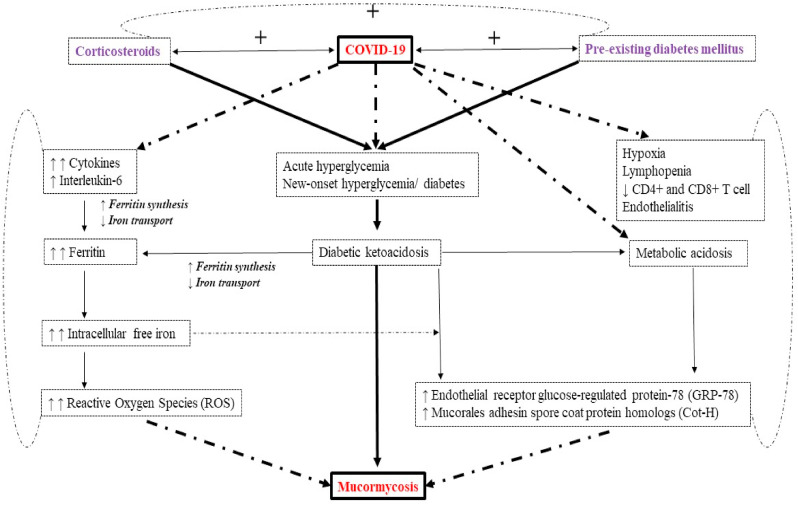
Since there are no studies that compared patients of mucormycosis in non-diabetic COVID-19 who did not receive steroids versus COVID-19 patients who received steroids and developed mucormycosis, it is difficult to establish a causal effect relationship between COVID-19 and mucormycosis in relation to corticosteroids. Nonetheless, there appears to be a number of triggers that may precipitate mucormycosis in people with COVID-19 in relation to corticosteroids:
-
(i)
Presence of DM with or without DKA increases the risk of contracting mucormycosis and DM is often associated with an increased severity of COVID-19,
-
(ii)
Uncontrolled hyperglycemia and precipitation of DKA is often observed due to corticosteroid intake. Low pH due to acidosis is a fertile media for mucor spores to germinate. Moreover, steroid use reduces the phagocytic activity of WBC (both first line and second line defense mechanism), causes impairment of bronchoalveolar macrophages migration, ingestion, and phagolysosome fusion, making a diabetic patient exceptionally vulnerable to mucormycosis.
-
(iii)
COVID-19 often causes endothelialitis, endothelial damage, thrombosis, lymphopenia, and reduction in CD4+ and CD8+ T-cell level and thus predisposes to secondary or opportunistic fungal infection,
-
(iv)
Free available iron is an ideal resource for mucormycosis. Hyperglycemia causes glycosylation of transferrin and ferritin, and reduces iron binding allowing increased free iron. Moreover, increase in cytokines in patients with COVID-19 especially interleukin-6, increases free iron by increasing ferritin levels due to increased synthesis and decreased iron transport. Furthermore, concomitant acidosis increases free iron by the same mechanism and additionally by reducing the ability of transferrin to chelate iron,
-
(v)
High glucose, low pH, free iron, and ketones in presence of decreased phagocytic activity of WBC, enhances the growth of mucor. In addition, it enhances the expression of glucose-regulator protein 78 (GRP-78) of endothelium cells and fungal ligand spore coating homolog (CotH) protein, enabling angio-invasion, hematogenous dissemination and tissue necrosis [52].
Fig. 1 depicts the postulated mechanism of increased propensity of having mucormycosis infection in COVID-19 patients.
Fig. 1.
Postulated interaction of diabetes, corticosteroid and COVID-19 with mucormycosis.
There are certain limitations to conduct this kind of systematic review based on case reports/series subject to publication biases and considerable heterogeneity in reporting cases. It is highly likely that reported cases of mucormycosis may be an underrepresentation of the real burden owing to difficulty in making a microbiological or histopathological diagnosis especially in a raging pandemic setting. While some case reports had every minute detail, other did not report important parameter, for example – duration of DM, lack of baseline HbA1c data in majority of cases. Secondly, the lack of a denominator value may not allow the true estimation of mucormycosis incidence in people with COVID-19 compounded by the lack of control. Thirdly, defining active and recovered COVID-19 and its relation to the onset of mucormycosis could be difficult considering the lower sensitivity of confirmatory RT-PCR. Finally, evaluating the outcomes in people with mucormycosis and COVID-19 could be difficult at the moment because these case reports have been published while many of these patients are still under treatment. Other minor limitations have been highlighted in Table 2.
5. Conclusions
Increase in mucormycosis in Indian context appears to be an unholy intersection of trinity of diabetes (high prevalence genetically), rampant use of corticosteroid (increases blood glucose and opportunistic fungal infection) and COVID-19 (cytokine storm, lymphopenia, endothelial damage). All efforts should be made to maintain optimal hyperglycemia and only judicious evidence-based use of corticosteroids in patients with COVID-19 is recommended in order to reduce the burden of fatal mucormycosis.
Funding
No funding.
Author's contribution
AKS conceptualized, searched the literature and wrote first draft; RS made the tables, analyzed the data and revised the first draft, SRJ and AM edited the final draft. All authors agreed mutually to submit for publication.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship and take responsibility for the integrity of the work. They confirm that this paper will not be published elsewhere in the same form, in English or in any other language, including electronically.
Declaration of competing interest
We hereby declare that we have no conflict of interest, related to this article titled “Mucormycosis in COVID-19: A Systematic Review of Cases Reported Worldwide and in India”.
References
- 1.Kubin C.J., McConville T.H., Dietz D., et al. Open Forum Infectious Diseases; 2021. Characterization of bacterial and fungal infections in hospitalized patients with COVID-19 and factors associated with healthcare-associated infections. ofab201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song G., Liang G., Liu W. Fungal Co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020 Aug;185(4):599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paltauf A. Mycosis mucorina. Virchows Arch Pathol Anat Physiol Klin Med. 1885;102:543–564. [Google Scholar]
- 4.Baker R.D. Mucormycosis-a new disease? J Am Med Assoc. 1957;163:805–808. doi: 10.1001/jama.1957.02970450007003. [DOI] [PubMed] [Google Scholar]
- 5.Eucker J., Sezer O., Graf B., Possinger K. Mucormycoses. Mycoses. 2001;44(7):253–260. [PubMed] [Google Scholar]
- 6.Sugar A.M. In: Mandell, Douglas, and Bennett's principles and practice of infectious diseases. fifth ed. Mandell G.L., Bennett J.E., Dolin R., editors. Churchill Livingstone; New York, USA: 2000. [Google Scholar]
- 7.Skiada A., Pavleas I., Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an Update. J Fungi. 2020;6(4):265. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chander J., Kaur M., Singla N., et al. Mucormycosis: battle with the deadly enemy over a five-year period in India. J. Fungi. 2018;4(2):46. doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5:26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Diabetes Federation Idf diabetes atlas. 2019. https://diabetesatlas.org/en/resources/ Available online:
- 11.Jeong W., Keighley C., Wolfe R., et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Lionakis M.S., Kontoyiannis D.P. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 13.Hoang K., Abdo T., Reinersman J.M., Lu R., Higuita N.I.A. A case of invasive pulmonary mucormycosis resulting from short courses of corticosteroids in a well-controlled diabetic patient. Med Mycol Case Rep. 2020;29(1):22–24. doi: 10.1016/j.mmcr.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skiada A., Pagano L., Groll A., et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European confederation of medical Mycology (ECMM) working group on zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch P.G., Whittaker J., Prasad S. Invasive and non-invasive fungal rhinosinusitis—a review and update of the evidence. Medicina. 2019;55:1–14. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maartens G., Wood M.J. The clinical presentation and diagnosis of invasive fungal infections. J Antimicrob Chemother. 1991;28(13–22):17–44. doi: 10.1093/jac/28.suppl_a.13. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020 Sep 30;12(9) doi: 10.7759/cureus.10726. e10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg D., Muthu V., Sehgal I.S., Ramachandran R., et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021 May;186(2):289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maini A., Tomar G., Khanna D., Kini Y., Mehta H., Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep. 2021 May 4;82:105957. doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saldanha M., Reddy R., Vincent M.J. Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg. 2021 Apr 22:1–4. doi: 10.1007/s12070-021-02574-0. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revannavar S.M., PS S., Samaga L. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021 Apr 27;14(4) doi: 10.1136/bcr-2021-241663. e241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sen M., Lahane S., Lahane T.P., et al. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69:244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar S., Gokhale T., Choudhury S.S., Deb A.K. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021 Apr;69(4):1002–1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra N., Mutya V.S.S., Thomas A., et al. A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021 May;7(5):867–870. [Google Scholar]
- 25.Satish D., Joy D., Ross A., Balasubramanya Mucormycosis coinfection associated with global COVID-19: a case series from India. Int J Otorhinolaryngol Head Neck Surg. 2021 May;7(5):815–820. [Google Scholar]
- 26.Moorthy A., Gaikwad R., Krishna S., et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021 Mar 6:1–8. doi: 10.1007/s12663-021-01532-1. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021 Apr 8:1–6. doi: 10.1017/S0022215121000992. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanley B., Naresh K.N., Roufosse C., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020 Oct;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dallalzadeh L.O., Ozzello D.J., Liu C.Y., Kikkawa D.O., Korn B.S. Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit. 2021 Mar 23:1–4. doi: 10.1080/01676830.2021.1903044. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264. doi: 10.1016/j.ajem.2020.09.032. e5–264.e8, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Rep. 2020 Nov;15(11):2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mekonnen Z.K., Ashraf D.C., Jankowski T., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37 doi: 10.1097/IOP.0000000000001889. e40–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alekesyev K., Didenko L., Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases. 2021;12(3):85–89. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson A.K., Ghazarian Z., Cendrowski K.D., Persichino J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021 Jun;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanwar A., Jordan A., Olewiler S., Wehberg K., Cortes M., Jackson B.R. A fatal case of Rhizopus azygosporus pneumonia following COVID-19. J Fungi (Basel) 2021 Feb 28;7(3):174. doi: 10.3390/jof7030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khatri A., Chang K.M., Berlinrut I., Wallach Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient - case report and review of literature. J Mycol Med. 2021 Apr 2;31(2):101125. doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monte Junior E.S.D., Santos M.E.L.D., Ribeiro I.B., et al. Rare and fatal gastrointestinal mucormycosis (zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020 Nov;53(6):746–749. doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasero D., Sanna S., Liperi C., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020:1–6. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellanger A.P., Navellou J.C., Lepiller Q., et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect Dis News. 2021 Jan 27 doi: 10.1016/j.idnow.2021.01.010. S2666-9919(21)00030-0. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karimi-Galougahi M., Arastou S., Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19) Int Forum Allergy Rhinol. 2021 Mar 13 doi: 10.1002/alr.22785. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veisi A., Bagheri A., Eshaghi M., Rikhtehgar M.H., Rezaei Kanavi M., Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur J Ophthalmol. 2021 Apr 10 doi: 10.1177/11206721211009450. 11206721211009450. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sargin F., Akbulut M., Karaduman S., Sungurtekin H. Severe rhinocerebral mucormycosis case developed after COVID 19. J Bacteriol Parasitol. 2021;12:386. [Google Scholar]
- 43.Waizel-Haiat S., Guerrero-Paz J.A., Sanchez-Hurtado L., et al. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021;13 doi: 10.7759/cureus.13163. e13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zurl C., Hoenigl M., Schulz E., et al. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. J Fungi (Basel). 2021 Jan 27;7(2):88. doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugar A.M. Mucormycosis. Clin Infect Dis. 1992;14:S126–S129. doi: 10.1093/clinids/14.supplement_1.s126. [DOI] [PubMed] [Google Scholar]
- 46.Peterson K.L., Wang M., Canalis F.R., Abemayor E. Rhinocerebral mucormycosis: evolution of the disease and treatment options. Laryngoscope. 1997;107:855–862. doi: 10.1097/00005537-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Smith H.W., Kirchner J.A. Cerebral mucor-mycosis: a report of 3 cases. Arch Otolaryngol. 1950;68:715–726. doi: 10.1001/archotol.1958.00730020739010. [DOI] [PubMed] [Google Scholar]
- 48.Prakash H., Ghosh A.K., Rudramurthy S.M., et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57:395–402. doi: 10.1093/mmy/myy060. [DOI] [PubMed] [Google Scholar]
- 49.Patel A., Kaur H., Xess I., et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(7) doi: 10.1016/j.cmi.2019.11.021. 944.e9-944.e15. [DOI] [PubMed] [Google Scholar]
- 50.Bala K., Chander J., Handa U., et al. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol. 2015 Apr;53(3):248–257. doi: 10.1093/mmy/myu086. [DOI] [PubMed] [Google Scholar]
- 51.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel) 2021 Apr 15;7(4):298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldin C., Ibrahim A.S. Molecular mechanisms of mucormycosis -The bitter and the sweet. PLoS Pathog. 2017;13(8) doi: 10.1371/journal.ppat.1006408. e1006408. [DOI] [PMC free article] [PubMed] [Google Scholar]