Abstract
Objective
The present study assessed if individuals ≥ 60 years of age with periodontitis are more likely to develop stroke or ischemic heart diseases, or at a higher risk of death for 17 years.
Material and methods
At baseline individuals ≥ 60 received a dental examination including a panoramic radiograph. Periodontitis was defined as having ≥ 30% sites with ≥ 5-mm distance from the cementoenamel junction to the marginal bone level. Medical records were annually reviewed from 2001 to 2018. Findings from the medical records identifying an ICD-10 code of stroke and ischemic heart diseases or death were registered.
Results
Associations between periodontitis and incidence of ischemic heart disease were found in this 17-year follow-up study in all individuals 60–93 years (HR: 1.5, CI: 1.1–2.1, p = 0.017), in women (HR: 2.1, CI: 1.3–3.4, p = 0.002), and in individuals 78–96 years (HR: 1.7, CI: 1.0–2.6, p = 0.033). Periodontitis was associated with mortality in all individuals (HR: 1.4, CI: 1.2–1.8, p = 0.002), specifically in men (HR: 1.5, CI: 1.1–1.9, p = 0.006) or in ages 60–72 years (HR: 2.2, CI: 1.5–3.2, p = 0.000). Periodontitis was more prevalent among men (OR: 1.8, CI: 1.3–2.4, p = 0.000).
Conclusions
Individuals with periodontitis have an increased risk for future events of ischemic heart diseases and death.
Clinical relevance
Improving periodontal health in older individuals may reduce overall mortality and ischemic heart diseases. Both dental and medical professionals should be aware of the associations and ultimately cooperate.
Keywords: Periodontitis, Ischemic heart disease, Mortality, Epidemiology
Introduction
Periodontitis is a chronic disease with an infectious etiology, causing an inflammatory response resulting in the breakdown of soft and hard tissues around teeth [1]. Severe periodontitis has been identified as the sixth most prevalent disease in the world [2]. In the USA, the prevalence of periodontitis is reported to be 47% [3]. In older individuals, it is even more prevalent [4].
Cardiovascular diseases (CVDs) include all diseases associated with the heart and blood vessels, such as stroke, coronary heart disease, and heart failure [5]. CVDs are the most common causes of death in the USA [6]. Atherosclerosis is considered the leading cause of CVDs [7, 8]. Periodontal infections may cause bacteremia triggering host systemic inflammatory responses and chronic inflammation and related to the pathogenesis of atherosclerosis [9]. Data suggest that periodontitis is associated with subclinical atherosclerosis [7, 8, 10].
Periodontitis has been associated with an increased risk for CVDs [8, 11–13]. Having periodontitis has been reported to increase the risk for stroke [14–16]. Periodontal disease has been pointed out as a risk factor for stroke, especially in men and in younger subjects [17]. In a review and meta-analysis of the literature, periodontitis was reported to be associated with the occurrence of stroke [18]. Periodontitis has also been associated with myocardial heart infarction [19, 20]. Individuals diagnosed with the first event of acute myocardial infarction (AMI) were matched with subjects with no evidence of AMI. A clear association was reported between periodontitis and AMI [19]. In another control-matched study by Rydén et al. 2016[20], an increased risk for a first myocardial infarction was reported among individuals with periodontitis. In a review by Dietrich et al. [21], six case-control and cohort epidemiological studies described an increased risk for a first coronary event in individuals with diagnosed periodontitis. In general, epidemiologic data are linking periodontitis to CVDs. Periodontitis is presently believed as a CVD risk factor. Several studies assessing associations between periodontitis and CVDs are however cross-sectional cohort or case-control studies [7, 8, 16]. Few studies with a prospective longitudinal study design have been reported.
Accordingly, the present study aimed to assess if individuals ≥ 60 years of age with periodontitis are more likely to develop stroke or ischemic heart diseases or are at a higher risk of death over 17 years.
Material and methods
Study individuals
Inclusion criteria selected the study individuals from the Swedish National Study of Aging and Care (SNAC). SNAC is a population-based, prospective longitudinal study in which SNAC-Blekinge is one participating research center. At the baseline in 2001–2003, an equal number of study individuals in age cohorts of 60, 66, 72, and 78 were randomly selected from the Swedish population database for Karlskrona City (The Swedish Tax Agency electronic database) and were invited by regular mail. All individuals in the community at age 81, 84, 87, 90, 93, and 96 years were also invited to participate at baseline, representing the older population in Karlskrona, Sweden. In total, 1402 individuals agreed to participate. All participants signed an informed consent. The principles of the Helsinki declarations were followed. The Ethics Committee Lund, Sweden approved the study (LU 604-00, LU 744-00). Baseline inclusion criteria were as follows: (i) age between 60 and 96 years and living in the community of Karlskrona, Sweden, (ii) dentate, with one or more teeth. Exclusion criterion was (i) non-readable panoramic radiographs.
Medical and dental research teams at a research center in Karlskrona, Sweden, examined the study participants. The overall response rate was 62%, representing approximately 10% of the entire population ≥ 60 years of age in the community. The proportion per group was 53% in individuals 60–78 years (the randomly selected), and the response rate in 81–96 years was 47%.
Radiographic measurement
An analogue panoramic radiograph using a standard exposure of 75 kV/10 mA was obtained using an Orthopantomograph (OP 100, Instrumentarium, Tuusula, Finland; film Kodak T-Mat G/RA, intensifying screen Kodak Lanex Regular, film processor Durr XR 24). The radiographic measurements were made from the panoramic radiographs exposed at baseline between 2001 and 2003. Among the initial 1402 radiographs, 858 readable radiographs meeting the inclusion criteria of presenting with at least one tooth were included in the present study.
An independent, experienced examiner (REP) performed the radiographic measurements. The examiner was masked to medical conditions, gender, age, and survival status of the study individuals. Bone loss was measured, based on the number of interproximal sites, as percent loss of bone from the enamel cement junction (CEJ) to the highest marginal bone level on the mesial and distal surfaces of each tooth. Alveolar bone loss ≥ 5-mm distance from CEJ to marginal bone level on ≥ 30% of sites was used as the definition of periodontitis. Intra-class coefficient (ICC) analysis between randomly selected cases for double assessments regarding the reproducibility of the distance between CEJ to the apex was 0.93 (95% CI: 0.91–0.96, p < 0.01) between the first and second reading.
Medical examination
Cerebrovascular diseases (stroke) and ischemic heart diseases were registered from an electronic medical database at the research center of the general hospital in Karlskrona and following the International Statistical Classification of Diseases and Related Health Problems 10th revision ICD-10 codes (ICD-10): ICD I60-69 for stroke and ICD I20-25 for ischemic heart diseases. A physician (JB) annually reviewed the medical database, including all medical records between 2001 and 2018, assessing medical records of the participating individuals in the 17 years following the baseline examination. Any findings from the medical records identifying an ICD-10 code were identified as a positive finding. Death or the first event of a stroke or ischemic heart disease was recorded as an event. Information on diabetes type 2, hypertension, or smoking was identified from a self-reported questionnaire on medical history at baseline, with a focus on a history of acute myocardial infarction (AMI) or a history of stroke. Smoking included both current and former smoking versus non-smoking included only those individuals with no current nor former history of smoking.
Statistics
The Statistical Package for the Social Sciences (SPSS) Predictive Analytics Software (PASW) 25.0 statistical software package (SPSS Inc., Armonk, NY, USA) for personal computer (PC) was used in the analyses. The data were analyzed using descriptive and inferential statistics. Dichotomous data were analyzed using the Pearson χ2 test, and by Mantel–Haenszel common odds ratio. Survival statistics with Cox regression analysis, method enter, was used to study adjusted associations. A multivariable adjustment was made for age, body mass index (BMI) ≥ 30, diagnosis of diabetes mellitus type 2, gender, hypertension, history of acute myocardial infarction (AMI), history of stroke, periodontitis, and smoking. Proportional hazards assumption was evaluated graphically with “log-log” plots. Time was defined as months from inclusion (dental examination) to either stroke, respectively ischemic heart diseases or death outcome or censoring due to emigration, death, or end of follow-up. Statistical significance was declared at p < 0.05.
Results
Demographic data
Data were derived from 858 individuals (women 53.5%). During the 17-year follow-up period, 492/858 (57.3%) died, and 51/858 (5.9%) moved away. The ages at baseline varied between 60 and 93 years with median age 72.0 years. On average, the individuals had 18.6 remaining teeth (SD: ± 7.5). Approximately half of the individuals 428/838 (51.1%) reported that they did not or had never smoked. At the baseline examination, periodontitis was declared in 212/858 (24.7%) (Table 1). Men had a higher prevalence of periodontitis than women, 121/212 (57.1%) (OR: 1.8, CI: 1.3–2.4, p = 0.000). Data were derived from 858 individuals (women 53.5%) with 471/858 (54.9%) in ages 60-72 years, young old (YO), and 378/858 (45.1%) in ages 78-96 years, old-old (OO) 78–96 years age group, including 471/858 (54.9%) respectively 387/858 (45.1%) individuals.
Table 1.
Baseline 2001–2003, characteristics of the study individuals
| ≥ 1 remaining tooth, dental examination, readable panoramic radiograph n = 858 | |
|---|---|
| Variables | |
| Median age (years) | 72.0 (SD: ± 9.3) |
| Gender (women) | 53.5% |
| Mean remaining teeth | 18.6 (SD: ± 7.5). |
| Periodontitis | 212/858 (24.7%) |
| No current nor former smoker | 428/838 (51.1%) |
| BMI ≥ 30 | 192/853 (22.4%) |
| Hypertension | 260/847 (30.3%) |
| History of stroke | 35/853 (4.1%) |
| History of AMI | 66/850 (7.7%) |
| Diabetes type 2 | 66/856 (7.7%) |
AMI acute myocardial infarction, BMI body mass index, Periodontitis = alveolar bone loss ≥ 5-mm distance from the CEJ to marginal bone level on ≥ 30% of sites. Self-reported at baseline: no current nor former smoker, hypertension, history of stroke, history of AMI, and diabetes type 2
Periodontitis and incidence of ischemic heart diseases or stroke during the follow-up period
The cumulative incidence of ischemic heart diseases between 2001 and 2018 was 203/858 (23.7%), with men 102/203 (50.2%) and women 101/203 (49.8%) (OR: 1.2, CI: 0.9–1.7, p = 0.221). The incidence of ischemic heart diseases was 57.2 incidences per year and 6668 per 100,000 and year. The cumulative incidence of stroke was 118/858 (13.8%), with 60/118 (50.8%) men respectively 58/118 (49.2%) women (OR:1.2, CI: 0.8-1.8, p=0.308). Stroke incidence was 24.86 per year, which corresponds to 2898 strokes per 100,000 persons and year.
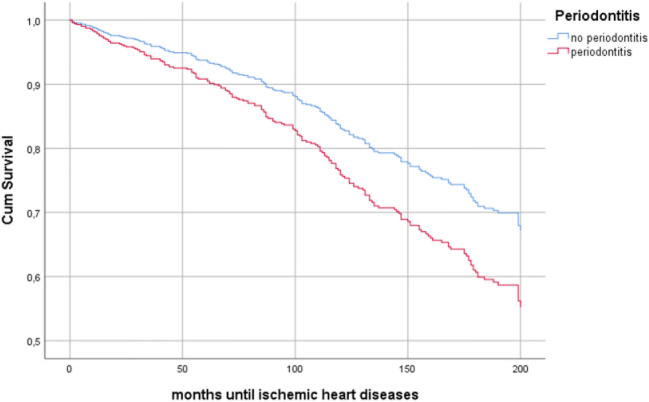
Cox regression analysis based on baseline data with periodontitis as an independent variable and incidence of the first event of a stroke or ischemic heart diseases as the dependent variable and with adjustment for the variables age group, BMI ≥ 30, diabetes type 2, gender, hypertension, history of acute myocardial infarction, history of stroke, and smoking, was used. Periodontitis increased the risk for ischemic heart diseases in all individuals (HR: 1.5, CI: 1.1–2.1, p = 0.017) (Fig. 1), in women (HR: 2.1, CI: 1.3–3.4, p = 0.002), and in the OO group (HR: 1.7, CI: 1.0–2.6, p = 0.033) (Table 2). No significant association was identified between periodontitis and stroke, in neither of all individuals, women, men, YO, and OO (Table 3).
Fig. 1.
Cox regression curves: 17-year cumulative ischemic heart disease survival of the total population, comparing individuals with and without periodontitis
Table 2.
Associations between ischemic heart diseases and different independent variables including periodontitis by Cox regression analysis
| Independent variables at baseline | All individuals (n = 858) HR, CI, p value |
Women (n = 459) HR, CI, p value |
Men (n = 399) HR, CI, p value |
YO (n = 471) HR, CI, p value |
OO (n = 387) HR, CI, p value |
|---|---|---|---|---|---|
| Age category | HR: 1.9, CI: 1.4–2.5, p = 0.000** | HR: 2.5, CI: 1.6–3.8, p = 0.000** | HR: 1.4, CI: 0.9–2.2, p = 0.098 | ||
| BMI ≥ 30 | HR: 1.2, CI: 0.9–1.7, p = 0.246 | HR: 1.2, CI: 0.8–2.0, p = 0.417 | HR: 1.3, CI: 0.8–2.2, p = 0.304 | HR: 1.3, CI: 0.8–2.0, p = 0.284 | HR: 1.2, CI: 0.7–2.1, p = 0.479 |
| Diabetes type 2 | HR: 1.5, CI: 1.0–2.4, p = 0.079 | HR: 1.5, CI: 0.8–3.0, p = 0.243 | HR: 1.4, CI: 0.7–2.6, p = 0.310 | HR: 1.6, CI: 0.8–2.9, p = 0.153 | HR: 1.4, CI: 0.7–3.0, p = 0.311 |
| Gender | HR: 1.2, CI: 0.9–1.6, p = 0.349 | HR: 1.4, CI: 1.0–2.2, p = 0.079 | HR: 0.9, CI: 0.6–1.5, p = 0.768 | ||
| Hypertension | HR: 1.5, CI: 1.1–2.0, p = 0.009** | HR: 1.8, CI: 1.2–2.8, p = 0.006** | HR: 1.2, CI: 0.7–1.8, p = 0.498 | HR: 1.5, CI: 1.0–2.3, p = 0.049* | HR: 1.5, CI: 1.0–2.3, p = 0.079 |
| History of AMI | HR: 2.3, CI: 1.5–3.5, p = 0.000** | HR: 2.5, CI: 1.1–5.8, p = 0.033* | HR: 2.4, CI: 1.4–4.1, p = 0.001** | HR: 2.5, CI: 1.2–5.0, p = 0.010* | HR: 2.2, CI: 1.2–4.0, p = 0.006** |
| Periodontitis | HR: 1.5, CI: 1.1–2.1, p = 0.017* | HR: 2.1, CI: 1.3–3.4, p = 0.002** | HR: 1.1, CI: 0.7–1.7, p = 0.749 | HR: 1.3, CI: 0.8–2.2, p = 0.229 | HR: 1.7, CI: 1.0–2.6, p = 0.033* |
| Smoking | HR: 1.1, CI: 0.8–1.5, p = 0.634 | HR: 1.0, CI: 0.7–1.6, p = 0.900 | HR: 1.2, CI: 0.8–1.8, p = 0.475 | HR: 1.3, CI: 0.9–2.0, p = 0.181 | HR: 0.9, CI: 0.5–1.4, p = 0.579 |
AMI acute myocardial infarction, BMI body mass index, Periodontitis = alveolar bone loss ≥ 5-mm distance from the CEJ to marginal bone level on ≥ 30% of sites, YO young-old 60–72 years, OO old-old 78–93 years, *p < 0.0.5, **p < 0.01
Table 3.
Associations between stroke and different independent variables including periodontitis by Cox regression analysis
| Independent variables at baseline | All individuals (n = 858) HR, CI, p value |
Women (n = 459) HR, CI, p value |
Men (n = 399) HR, CI, p value |
YO (n = 471) HR, CI, p value |
OO (n = 387) HR, CI, p value |
|---|---|---|---|---|---|
| Age cut | HR: 3.4, CI: 2.3–5.1, p = 0.000** | HR: 3.6, CI: 2.0–6.4, p = 0.000** | HR: 3.2, CI: 1.8–5.7, p = 0.000** | ||
| BMI ≥ 30 | HR: 0.7, CI: 0.4–1.1, p = 0.157 | HR: 0.7, CI: 0.3–1.4, p = 0.282 | HR: 0.8, CI: 0.4–1.6, p = 0.479 | HR: 1.0, CI: 0.5–2.0, p = 0.953 | HR: 0.5, CI: 0.3–1.1, p = 0.098 |
| Diabetes type 2 | HR: 0.8, CI: 0.4–1.7, p = 0.613 | HR: 0.6, CI: 0.2–2.2, p = 0.474 | HR: 1.0, CI: 0.4–2.7, p = 0.924 | HR: 0.4, CI: 0.1–1.8, p = 0.245 | HR: 1.2, CI: 0.5–2.8, p = 0.704 |
| Gender | HR: 1.3, CI: 0.9–1.9, p = 0.188 | HR: 1.2, CI: 0.6–2.2, p = 0.587 | HR: 1.3, CI: 0.8–2.2, p = 0.294 | ||
| Hypertension | HR: 1.8, CI: 1.2–2.6, p = 0.006** | HR: 2.1, CI: 1.2–3.6, p = 0.013* | HR: 1.6, CI: 0.9–2.8, p = 0.118 | HR: 1.7, CI: 0.9–3.1, p = 0.128 | HR: 1.8, CI: 1.1–3.1, p = 0.019* |
| History of stroke | HR: 3.7, CI: 2.0–6.9, p = 0.000** | HR: 2.7, CI: 1.0–7.5, p = 0.052 | HR: 4.6, CI: 2.1–10.2, p = 0.000** | HR: 6.2, CI: 2.7–14.4, p = 0.000** | HR: 2.3, CI: 0.9–6.1, p = 0.088 |
| Periodontitis | HR: 1.2, CI: 0.8–1.8, p = 0.442 | HR: 1.3, CI: 0.7–2.4, p = 0.485 | HR: 1.2, CI: 0.6–2.1, p = 0.627 | HR: 1.1, CI: 0.5–2.3, p = 0.865 | HR: 1.2, CI: 0.7–2.0, p = 0.535 |
| Smoking | HR: 1.0, CI: 0.7–1.5, p = 0.915 | HR: 0.8, CI: 0.4–1.4, p = 0.381 | HR: 1.2, CI: 0.7–2.1, p = 0.544 | HR: 1.2, CI: 0.7–2.3, p = 0.496 | HR: 0.9, CI: 0.6–1.6, p = 0.834 |
BMI body mass index, Periodontitis = alveolar bone loss ≥ 5-mm distance from the CEJ to marginal bone level on ≥ 30% of sites, YO young-old 60–72 years, OO old-old 78–93 years, *p < 0.05, **p < 0.01
Associations to mortality during the follow-up period
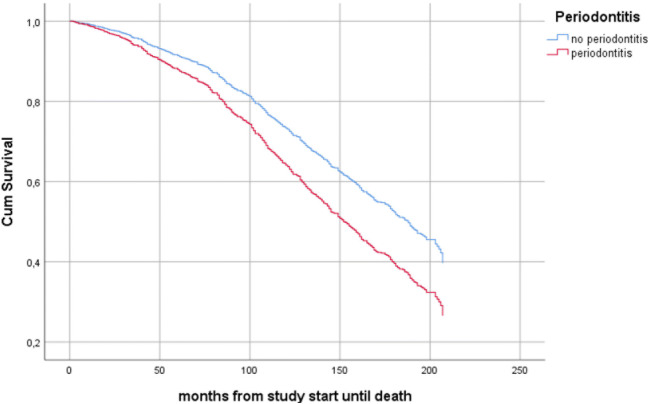
Data on the cause of death was not available, but during the 17-year follow-up period, 492/858 (57.3%) had died. Among those who had died, 160/492 (32.5%) had periodontitis whereas among those who were alive, 52/366 (14.2%) had periodontitis (OR: 2.9, CI: 2.1–41.1, p = 0.000). In individuals with periodontitis, 160/212 (75.5%) (62 women and 98 men) had died and 52/212 (24.5%) (29 women and 23 men) were still alive at the end of the study in 2018 (OR: 1.7, CI: 1.1–2.7, p = 0.028). Cox regression analysis with periodontitis as the independent variable and mortality as the dependent variable and with adjustment for the variables age group, BMI ≥ 30, diabetes type 2, gender, hypertension, history of acute myocardial infarction (AMI), history of stroke, and smoking, was used. Periodontitis increased the risk for all-cause mortality in all individuals (HR: 1.4, CI:1.2–1.8, p = 0.002) (Fig. 2), in men (HR: 1.5, CI: 1.1–1.9, p = 0.006), and in the YO group (HR: 2.2, CI: 1.5–3.2, p = 0.000). No associations in women or the OO individuals between periodontitis and mortality were identified (HR: 1.4, CI: 1.0–1.9, p = 0.055), respectively (HR: 1.2, CI: 1.0–1.6, p = 0.102) (Table 4).
Fig. 2.
Cox regression curves: 17-year cumulative death survival of the total study population, comparing individuals with and without periodontitis
Table 4.
Associations between mortality and different independent variables including periodontitis by Cox regression analysis
| Independent variables at baseline | All individuals (n = 858) HR, CI, p value |
Women (n = 459) HR, CI, p value |
Men (n = 399) HR, CI, p value |
YO (n = 471) HR, CI, p value |
OO (n = 387) HR, CI, p value |
|---|---|---|---|---|---|
| Age cut | HR: 5.7, CI: 4.6–7.0, p = 0.000** | HR: 5.9, CI: 4.3–7.9, p = 0.000** | HR: 5.6, CI: 4.2–7.5, p = 0.000** | ||
| BMI ≥ 30 | HR: 1.0, CI: 0.8–1.3, p = 0.863 | HR: 1.0, CI: 0.8–1.5, p = 0.675 | HR: 1.0, CI: 0.7–1.3, p = 0.808 | HR: 1.4, CI: 0.9–2.0, p = 0.117 | HR: 0.9, CI: 0.7–1.2, p = 0.422 |
| Diabetes type 2 | HR: 1.1, CI: 0.8–1.5, p = 0.591 | HR: 1.2, CI: 0.7–2.0, p = 0.554 | HR: 1.1, CI: 0.7–1.7, p = 0.835 | HR: 1.0, CI: 0.6–1.9, p = 0.887 | HR: 1.1, CI: 0.7–1.6, p = 0.671 |
| Gender | HR: 1.3, CI:1.1–1.6, p = 0.009** | HR: 1.3, CI: 0.9–1.9, p = 0.112 | HR: 1.3, CI: 1.0–1.7, p = 0.025* | ||
| Hypertension | HR: 1.2, CI: 1.0–1.5, p = 0.033* | HR: 1.4, CI: 1.1–1.9, p = 0.012* | HR: 1.1, CI: 0.9–1.5, p = 0.371 | HR: 1.2, CI: 0.8–1.7, p = 0.444 | HR: 1.3, CI: 1.0–1.6, p = 0.057 |
| History of AMI | HR: 1.1, CI: 0.8–1.5, p = 0.540 | HR: 1.4, CI: 0.7–2.7, p = 0.287 | HR: 1.0, CI: 0.7–1.4, p = 0.996 | HR: 1.3, CI: 0.7–2.6, p = 0.397 | HR: 1.0, CI: 0.7–1.5, p = 0.813 |
| History of stroke | HR: 1.9, CI: 1.3–2.9, p = 0.002** | HR: 1.5, CI: 0.8–2.8, p = 0.225 | HR: 2.3, CI: 1.3–3.9, p = 0.004** | HR: 1.8, CI: 0.9–3.5, p = 0.088 | HR: 1.9, CI: 1.1–3.2, p = 0.013* |
| Periodontitis | HR: 1.4, CI: 1.2–1.8, p = 0.002** | HR: 1.4, CI: 1.0–1.9, p = 0.055 | HR: 1.5, CI: 1.1–1.9, p = 0.006** | HR: 2.2, CI: 1.5–3.2, p = 0.000** | HR: 1.2, CI: 1.0–1.6, p = 0.102 |
| Smoking | HR: 1.0, CI: 0.8–1.2, p = 0.977 | HR: 0.9, CI: 0.6–1.2, p = 0.333 | HR: 1.1, CI: 0.9–1.5, p = 0.321 | HR: 1.1, CI: 0.8–1.5, p = 0.624 | HR: 1.0, CI: 0.8–1.2, p = 0.810 |
AMI acute myocardial infarction, BMI body mass index, Periodontitis = alveolar bone loss ≥ 5-mm distance from the CEJ to marginal bone level on ≥ 30% of sites, YO young-old 60–72 years, OO old-old 78–93 years, *p < 0.05, **p < 0.01
A significant association was found between periodontitis and the number of lost teeth (OR: 1.1, CI: 1.1–1.1, p = 0.000) using binary logistic regression analysis. In a second Cox regression analysis, the number of lost teeth at baseline was used as a proxy for periodontitis with the number of lost teeth as an independent variable and incidence of the first event of a stroke or ischemic heart diseases as the dependent variable and with adjustment for the following variables: age group, BMI ≥ 30, diabetes type 2, gender, hypertension, history of acute myocardial infarction, history of stroke, and smoking. The number of lost teeth increased the risk for ischemic heart diseases in all individuals (HR: 1.0, CI: 1.0–1.1, p = 0.006), in women (HR: 1.0, CI: 1.0–1.1, p = 0.019), and in the YO group (HR: 1.0, CI: 1.0–1.1, p = 0.006) (Table 5). No significant association was identified between the number of lost teeth and stroke, in neither of all individuals, women, men, YO, and OO (Table 6). Using the number of lost teeth as an independent variable and incidence of mortality as the dependent variable adjusting for the same variables as above, the number of lost teeth increased the risk for mortality in all individuals (HR: 1.0, CI: 1.0–1.0, p = 0.000) and in women (HR: 1.0, CI: 1.0–1.0, p = 0.000) (Table 7).
Table 5.
Associations between ischemic heart diseases and different independent variables including the number of lost teeth by Cox regression analysis
| Independent variables at baseline | All individuals (n = 813) HR, CI, p value |
Women (n = 430) HR, CI, p value |
Men (n = 383) HR, CI, p value |
YO (n = 452) HR, CI, p value |
OO (n = 361) HR, CI, p value |
|---|---|---|---|---|---|
| Age cut | HR: 1.7, CI: 1.2–2.3, p = 0.002** | HR: 2.2, CI: 1.4–3.4, p = 0.001** | HR: 1.3, CI: 0.8–2.0, p = 0.291 | ||
| BMI ≥ 30 | HR: 1.2, CI: 0.8–1.6, p = 0.356 | HR: 1.1, CI: 0.7–1.8, p = 0.686 | HR: 1.3, CI: 0.8–2.1, p = 0.320 | HR: 1.2, CI: 0.8–1.9, p = 0.370 | HR: 1.1, CI: 0.7–1.9, p = 0.666 |
| Diabetes type 2 | HR: 1.3, CI: 0.8–2.1, p = 0.257 | HR: 1.4, CI: 0.7–2.8, p = 0.379 | HR: 1.3, CI: 0.7–2.5, p = 0.427 | HR: 1.3, CI: 0.7–2.5, p = 0.380 | HR: 1.2, CI: 0.6–2.5, p = 0.571 |
| Gender | HR: 1.2, CI: 0.9–1.7, p = 0.201 | HR: 1.5, CI: 1.0–2.3, p = 0.036* | HR: 1.0, CI: 0.6–1.6, p = 0.995 | ||
| Hypertension | HR: 1.5, CI: 1.1–2.0, p = 0.012* | HR: 1.8, CI: 1.2–2.8, p = 0.008** | HR: 1.2, CI: 0.7–1.8, p = 0.514 | HR: 1.5, CI: 1.0–2.3, p = 0.072 | HR: 1.5, CI: 0.9–2.3, p = 0.090 |
| History of AMI | HR: 2.2, CI: 1.4–3.5, p = 0.000** | HR: 2.4, CI: 1.0–5.5, p = 0.040* | HR: 2.3, CI: 1.4–3.9, p = 0.002** | HR: 2.2, CI: 1.1–4.4, p = 0.03* | HR: 2.2, CI: 1.3–3.9, p = 0.007** |
| Smoking | HR: 1.1, CI: 0.8–1.5, p = 0.567 | HR: 1.1, CI: 0.7–1.7, p = 0.700 | HR: 1.2, CI: 0.8–1.8, p = 0.494 | HR: 1.3, CI: 0.9–2.0, p = 0.202 | HR: 0.9, CI: 0.6–1.5, p = 0.690 |
| Tooth loss | HR: 1.0, CI: 1.0–1.1, p = 0.006** | HR: 1.0, CI: 1.0–1.1, p = 0.019** | HR: 1.0, CI: 1.0–1.1, p = 0.161 | HR: 1.0, CI: 1.0–1.1, p = 0.006** | HR: 1.0, CI: 1.0–1.0, p = 0.285 |
AMI acute myocardial infarction, BMI body mass index, Tooth loss = number of lost teeth, YO young-old 60–72 years, OO old-old 78–93 years, *p < 0.05, **p < 0.01
Table 6.
Associations between stroke and different independent variables including the number of lost teeth by Cox regression analysis
| Independent variables at baseline | All individuals (n = 817) HR, CI, p value |
Women (n = 431) HR, CI, p value |
Men (n = 385) HR, CI, p value |
YO (n = 447) HR, CI, p value |
OO (n = 366) HR, CI, p value |
|---|---|---|---|---|---|
| Age cut | HR: 3.3, CI: 2.1–5.1, p = 0.000** | HR: 3.2, CI: 1.7–6.0, p = 0.000** | HR: 3.4, CI: 1.9–6.2, p = 0.000** | ||
| BMI ≥ 30 | HR: 0.7, CI: 0.4–1.1, p = 0.140 | HR: 0.7, CI: 0.3–1.3, p = 0.256 | HR: 0.8, CI: 0.4–1.5, p = 0.454 | HR: 1.0, CI: 0.5–2.0, p = 0.961 | HR: 0.5, CI: 0.3–1.1, p = 0.088 |
| Diabetes type 2 | HR: 0.8, CI: 0.4–1.7, p = 0.542 | HR: 0.6, CI: 0.2–2.1, p = 0.422 | HR: 1.0, CI: 0.4–2.6, p = 0.958 | HR: 0.4, CI: 0.1–1.8, p = 0.232 | HR: 1.1, CI: 0.5–2.7, p = 0.754 |
| Gender | HR: 1.3, CI: 0.9–2.0, p = 0.151 | HR: 1.2, CI: 0.7–2.2, p = 0.554 | HR: 1.3, CI: 0.8–2.3, p = 0.272 | ||
| Hypertension | HR: 1.7, CI: 1.2–2.6, p = 0.007** | HR: 2.0, CI: 1.1–3.5, p = 0.020* | HR: 1.6, CI: 0.9–2.7, p = 0.129 | HR: 1.6, CI: 0.8–3.1, p = 0.147 | HR: 1.8, CI: 1.1–3.1, p = 0.021* |
| History of stroke | HR: 3.6, CI: 1.9–6.6, p = 0.000** | HR: 2.5, CI: 0.9–6.9, p = 0.070 | HR: 4.6, CI: 2.1–10.1, p = 0.000** | HR: 6.1, CI: 2.6–14.1, p = 0.000** | HR: 2.2, CI: 0.9–5.8, p = 0.103 |
| Smoking | HR: 1.0, CI: 0.7–1.5, p = 0.909 | HR: 0.8, CI: 0.4–1.4, p = 0.353 | HR: 1.2, CI: 0.7–2.2, p = 0.483 | HR: 1.2, CI: 0.7–2.2, p = 0.535 | HR: 1.0, CI: 0.6–1.6, p = 0.881 |
| Tooth loss | HR: 1.0, CI: 1.0–1.0, p = 0.593 | HR: 1.0, CI: 1.0–1.1, p = 0.249 | HR: 1.0, CI: 1.0–1.0, p = 0.717 | HR: 1.0, CI: 1.0–1.1, p = 0.552 | HR: 1.0, CI: 1.0–1.0, p = 0.921 |
BMI body mass index, Tooth loss = number of lost teeth, YO young-old 60–72 years, OO old-old 78–93 years, *p < 0.05, **p < 0.01
Table 7.
Associations between mortality and different independent variables including the number of lost teeth by Cox regression analysis
| Independent variables at baseline | All individuals (n = 810) HR, CI, p value |
Women (n = 427) HR, CI, p value |
Men (n = 383) HR, CI, p value |
YO (n = 448) HR, CI, p value |
OO (n = 362) HR, CI, p value |
|---|---|---|---|---|---|
| Age cut | HR: 5.0, CI: 4.0–6.2, p = 0.000** | HR: 4.8, CI: 3.5–6.6, p = 0.000** | HR: 5.2, CI: 3.8–7.1, p = 0.000** | ||
| BMI ≥ 30 | HR: 1.0, CI: 0.8–1.2, p = 0.913 | HR: 1.0, CI: 0.7–1.4, p = 0.839 | HR: 0.9, CI: 0.7–1.3, p = 0.658 | HR: 1.3, CI: 0.9–1.9, p = 0.219 | HR: 0.9, CI: 0.6–1.2, p = 0.347 |
| Diabetes type 2 | HR: 1.0, CI: 0.7–1.3, p = 0.806 | HR: 1.0, CI: 0.6–1.8, p = 0.815 | HR: 0.9, CI: 0.6–1.4, p = 0.641 | HR: 0.8, CI: 0.4–1.5, p = 0.528 | HR: 1.0, CI: 0.7–1.5, p = 0.943 |
| Gender | HR: 1.4, CI: 1.1–1.7, p = 0.002** | HR: 1.5, CI: 1.0–2.1, p = 0.022 | HR: 1.4, CI: 1.1–1.7, p = 0.014 | ||
| Hypertension | HR: 1.2, CI: 1.0–1.5, p = 0.040* | HR: 1.4, CI: 1.0–1.9, p = 0.025* | HR: 1.1, CI: 0.8–1.5, p = 0.393 | HR: 1.1, CI: 0.8–1.6, p = 0.597 | HR: 1.3, CI: 1.0–1.6, p = 0.060 |
| History of AMI | HR: 1.1, CI: 0.8–1.6, p = 0.403 | HR: 1.5, CI: 0.8–2.9, p = 0.212 | HR: 1.0, CI: 0.7–1.5, p = 0.813 | HR: 1.2, CI: 0.6–2.3, p = 0.580 | HR: 1.1, CI: 0.7–1.5, p = 0.710 |
| History of stroke | HR: 1.7, CI: 1.1–2.5, p = 0.012* | HR: 1.3, CI: 0.7–2.4, p = 0.426 | HR: 2.0, CI: 1.2–3.5, p = 0.013* | HR: 1.7, CI: 0.8–3.3, p = 0.149 | HR: 1.8, CI: 1.1–2.9, p = 0.032 |
| Smoking | HR: 1.0, CI: 0.8–1.2, p = 0.956 | HR: 0.9, CI: 0.6–1.2, p = 0.337 | HR: 1.2, CI: 0.9–1.5, p = 0.311 | HR: 1.1, CI: 0.8–1.6, p = 0.555 | HR: 1.0, CI: 0.8–1.2, p = 0.828 |
| Tooth loss | HR: 1.0, CI: 1.0–1.0, p = 0.000** | HR: 1.0, CI: 1.0–1.1, p = 0.000** | HR: 1.0, CI: 1.0–1.0, p = 0.050 | HR: 1.1, CI: 1.0–1.1, p = 0.000** | HR: 1.0, CI: 1.0–1.0, p = 0.090 |
AMI acute myocardial infarction, BMI body mass index, Tooth loss = number of lost teeth, YO young-old 60–72 years, OO old-old 78–93 years, *p < 0.05, **p < 0.01
Discussion
The present study identified that over the 17-year follow-up period, periodontitis increased the risk of future incidences of ischemic heart diseases in all individuals, in women, and in the OO age group. In woman with periodontitis, the HR for the incidence of ischemic heart disease was 2.1. Another study has confirmed that after the menopause, women have a higher incidence of AMI compared to age-matched men [22] which is in agreement with the results of the present study in older people. There are few other longitudinal studies concerning periodontitis and incidences of CVDs. In a Danish national register–based cohort study with a follow-up period of 15 years, patients with periodontitis were reported to have an increased risk of CVDs [23]. In the study by Hansen et al. (2013), ICD codes were used to define periodontitis. This classification of periodontitis is different from the one used in the present study. Additionally, the age of the study population was > 18 years, and gender differences were not reported making comparisons between results observed in the present study impossible. A Korean nationwide cohort follow-up study of 7.6 years showed a dose-dependent association with tooth loss and incident myocardial infarction, heart failure, and ischemic stroke, especially in individuals with periodontitis [24]. The circumstances between the study above and our study are not the same. The classification of periodontitis was in the study by Lee et al. [24] not defined, and the study individuals included were from 20 years, and the mean age not mentioned. Also, the included individuals had a history of a CVD event, meaning that they were at an increased risk for a subsequent CVD [25].
Another main finding in this study was that individuals with periodontitis were at a higher risk to die during the 17-year follow-up compared to individuals without a diagnosis of periodontitis. Recently published data have shown that periodontitis increased the risk for all-cause mortality within 15 years [23]. In a 3-year follow-up study, individuals with severe periodontitis developed the combined endpoint (myocardial infarction, stroke/transient ischemic attack, cardiovascular death, and death caused by stroke) more often compared to individuals without periodontitis (18.9% versus 14.2%) [26]. As we were unaware of the exact reasons for death in the present study, such a combined endpoint was not possible to include. In the present study, it was demonstrated that in men and the YO (60–72 years), there was an association between periodontitis and mortality. Young individuals (30–40 years) with periodontitis and missing molars have been reported to have an increased risk for early death over 16 years [27]. Missing teeth, due to periodontal disease, could be a proxy for a previous inflammation and partly explain the identified association with an increased risk for mortality among young-old in the present study that is in line with the results from the study by Lee et al. [24]. In another recent study, ≥ nine missing teeth were also associated with mortality [28]. In the present study, an association between periodontitis and the number of lost teeth at baseline was found. When lost teeth at baseline were used as a proxy for periodontitis, an association between all-cause mortality in all individuals was found. Such an association is in line with the results obtained using periodontitis as the independent variable in the Cox regression analysis. However, lost teeth at baseline were also associated with mortality among women and to an increased risk for ischemic heart diseases in all individuals, in women, and in the YO group. Accordingly, using lost teeth as a proxy for periodontitis does not seem to be an adequate approximation for periodontal disease.
Deaths by CVDs have decreased during the latest 60 years, as a result of preventive care and advances in medicine [29]. Many older individuals are using preventive medications that may delay or even prevent a CVD event from occurring [30].
Recent data reported that the numbers of missing teeth were related to heart failure and myocardial infarction. In contrast, the number of teeth missing was not significantly related to stroke in a longitudinal study with a median follow-up time of 15.8 years [31]. In another study, the same tendency was reported; missing teeth (≥ five missing teeth) were statistically associated with an event of coronary heart disease and acute myocardial infarction. In contrast, tooth loss was not associated with stroke in a 13-year prospective longitudinal study [28].
In the literature, different classifications and parameters for periodontitis have been used. Missing teeth have also been proposed as a proxy for current or past periodontitis as it is considered to reflect an accumulation of oral inflammation [32]. Correctly, if untreated, periodontitis may result in tooth loss and is one of the primary reasons for tooth loss in adulthood [33]. In a recent study by Lee et al. [24], edentulous individuals demonstrated the highest cardiovascular risk. It is, however, difficult to be sure of the reason for missing teeth unless it is reported in the dental records. In the present study, it was not possible to within certainty decide the reason for tooth loss. Alveolar bone loss, as used in the present study as a proxy for periodontal disease, is an indicator of a patient who has had periodontal inflammation during a period.
The prevalence of periodontitis defined by loss of alveolar bone was relatively low (24.7%). The reported prevalence of periodontitis in individuals 65 years and older has been reported to be 70% in the USA and with increasing prevalence with increasing age [3]. Differences in prevalence may be related to the different classifications of periodontitis used. Eke et al. [3], when defining “total periodontitis,” included mild, moderate, and severe stages of periodontitis and used clinical attachment–level loss as an indicator for periodontitis. The definition of periodontitis used in the present study is based on bone loss of ≥ 5 mm from the CEJ to the alveolar bone level on ≥ 30% of sites. The use of the 5-mm level was chosen to account for possible measurement errors. Individuals defined as mild periodontitis cases in the study by Eke et al. [3] were not defined as periodontitis patients in the present study, which to some degree may explain the differences in prevalence figures reported in the two studies. Individuals affected with bone loss in older ages may reflect a long history of periodontitis and accordingly a long time of an inflammatory response.
The new accepted classification of periodontitis includes clinical variables (CAL and probing pocket depth) as well as bone loss in radiographs [34]. The bone loss reflects the accumulated progressive effect of periodontitis over a long time [35, 36]. Clinical parameters partly reflecting the inflammatory activity could be more transient, giving the information of the periodontal status at that specific moment. Studies have reported that the mean proportion of bone loss increased with age, but the proportion of teeth with periodontal pockets remained unmodified. In another study, the alveolar bone loss progressed with age but was limited after the age of 50 [37]. In older individuals, gingival recession is the main reason for attachment loss [38]. Crestal bone height and CAL have shown a good correlation [39]. It has been proven that attachment loss precedes radiographic evidence of crestal alveolar bone loss during periods of periodontal disease activity [40] whereas, over time, these differences seem to level off [39]. In our study, the limit of alveolar bone loss ≥ 5-mm distance from CEJ to marginal bone level reflects a definitive bone loss and such a bone loss is present on ≥ 30% of sites corresponding to a general disease distribution.
One limitation with the present study was that the most fragile and medically compromised individuals were not able to participate. The fact that the most fragile individuals did not participate may have affected the results, possibly lowering the associations between periodontitis and CVDs. The individuals in the present study were 60 years or older. While the follow-up period was long, obviously many died during the study. The causes of death were not known, but the main reasons for death are still CVDs [41]. The consequence of not knowing the reasons for death makes it impossible to include mortality by CVDs in a combined endpoint, among others, which should have to strengthen the associations between periodontitis and CVDs. The risk factors in the Cox regression analysis were adjusted only from the baseline data, which is another limitation in the study.
The long-time follow-up and that CVD events were easy to follow and control is a strength with the present study. It would be interesting to study if well-designed preventive dental programs can influence the incidence of CVDs in long-term studies. Intervention studies are needed to verify a valid link between periodontitis and CVDs.
In conclusion, this study demonstrated that in older adults, periodontitis was a statistical risk indicator for ischemic heart diseases. Over the time studied, periodontitis was significantly associated with mortality. This is the first long-time follow-up report on periodontitis and the incidence of cardiovascular diseases and death.
Acknowledgments
We acknowledge the contribution by Associate Professor Rigmor Persson (REP), University of Washington, who analyzed the radiographs. We also want to acknowledge Ms. Ingrid Jonasson, Blekinge Institute of Technology, Karlskrona, Sweden, who collected the clinical dental data and Ms. Johanna Renvert, University of Kristianstad, for data management. We are specifically grateful to the study participants. The study was accomplished within the context of the Swedish National Graduate School for Competitive Science on Aging and Health (SWEAH). The Ministry of Health and Social Affairs, Sweden; the participating county councils, municipalities, and university departments; and the Swedish National Study on Aging and Care (SNAC) (www. snac.org) supported the study. The study was also supported by the Research Foundation at Kristianstad University, Sweden.
Funding
Open Access funding provided by Kristianstad University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study, involving human participants, were following the ethical standards of the regional research ethics committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
All individuals included in the study signed an informed consent.
Disclaimer
The sponsors had no role in any aspect of the study design or reporting of findings.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Viveca Wallin Bengtsson, Email: viveca.wallin_bengtsson@hkr.se.
Gösta Rutger Persson, Email: rutger.persson@hkr.se.
Johan Sanmartin Berglund, Email: johan.sanmartin.berglund@bth.se.
Stefan Renvert, Email: stefan.renvert@hkr.se.
References
- 1.Bartold PM, van Dyke TE. Periodontitis: a host-mediated disrupture of microbial homeostasis unlearning learned concepts. Periodontology. 2013;2000(62):203–217. doi: 10.1111/j.1600-0757.2012.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. CDC Periodontal Disease Surveillance workgroup: James Beck, Gordon Douglass Roy Page. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 4.Eke PI, Wei L, Borgnakke WS, Thornton-Evans G, Zhang X, Lu H, McGuire LC, Genco RJ. Periodontitis prevalence in adults, ≥ 65 years of age, in the USA. Periodontology. 2016;2000(72):76–95. doi: 10.1111/prd.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters AM, Trinh L, Chau T, Bourchier M, Moon L. Latest statistics on cardiovascular disease in Australia. Clin Exp Pharmacol Physiol. 2013;40(347–356):10.111/1440–11681.12079. doi: 10.1111/1440-1681.12079. [DOI] [PubMed] [Google Scholar]
- 6.Roth GA, Johnson CO, Abate KH, et al. Global burden of cardiovascular diseases collaboration, the burden of cardiovascular diseases among US states, 1990-2016. JAMA Cardiol. 2018;3:375–389. doi: 10.1001/jamacardio.2018.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR Jr, Papapanou PN, Sacco RL. Oral Infections and Vascular Disease Epidemiology Study (INVEST). Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Stroke. 2003;34:2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockhart PB, Bolger AF, Papapanou PN, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Council on Clinical Cardiology. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 2012;125:2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama N, Suzuki JI, Kobayashi N, Hanatani T, Ashigaki N, Yoshida A, Shiheido Y, Sato H, Kumagai H, Ikeda Y, Akazawa H, Komuro I, Izumi Y, Isobe M. Periodontitis deteriorates peripheral arterial disease in Japanese population via enhanced systemic inflammation. Heart Vessel. 2017;32:1314–1319. doi: 10.1007/s00380-017-1003-6. [DOI] [PubMed] [Google Scholar]
- 10.Söder PO, Söder B, Nowak J, Jogestrand T. Early carotid atherosclerosis in subjects with periodontal diseases. Stroke. 2005;36:1195–2000. doi: 10.1161/01.STR.0000165916.90593.cb. [DOI] [PubMed] [Google Scholar]
- 11.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Annuals of Periodontology. 2003;8:38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Kinane D, Bouchard P, Group E of European Workshop on Periodontology J Clin Periodontol. 2008;35:333–337. doi: 10.1111/j.1600-051X.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 13.Cullinan MP, Seymour GJ. Periodontal diseases and health: consensus report of the Sixth European Workshop on Periodontology. Periodontology 2000. 2013;62:271–286. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- 14.Reyes L, Herrera D, Kozarov E, Roldán S, Progulske-Fox AJ. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. Clinical Periodontology. 2013;40:S30–S50. doi: 10.1111/jcpe.12079. [DOI] [PubMed] [Google Scholar]
- 15.Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. Jorunal of Clinical Periodontology. 2013;40:S51–S69. doi: 10.1111/jcpe.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonetti MS, Van Dyke TE. Working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84(4 Suppl):24–29. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 17.Lafon A, Pereira B, Dufour T, et al. Periodontal disease and stroke: a meta-analysis of cohort studies. Eur J Neurol. 2014;21(1155–61):e66–e67. doi: 10.1111/ene. [DOI] [PubMed] [Google Scholar]
- 18.Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, Lutz R, Bültmann S, Preusch M, Dörfer CE. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D. [DOI] [PubMed] [Google Scholar]
- 19.Persson GR, Ohlsson O, Pettersson T. Chronic periodontitis, a significant relationship with acute myocardial infarction. Eur Heart J. 2003;24(23):2108–2115. doi: 10.1016/j.ehj.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Rydén L, Buhlin K, Ekstrand E, et al. Periodontitis increases the risk of a first myocardial infarction: a report from the PAROKRANK study. Circulation. 2016;133:576–583. doi: 10.1161/CIRCULATIONAHA.115.020324. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich T, Sharma P, Walter C. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol. 2013;14:S70–S84. doi: 10.1111/jcpe.12062. [DOI] [PubMed] [Google Scholar]
- 22.Stramba-Badiale M, Fox KM, Priori SG, Collins P, Daly C, Graham I, Jonsson B, Schenck-Gustafsson K, Tendera M. Cardiovascular diseases in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J. 2006;27:994–1005. doi: 10.1093/eurheartj/ehi819. [DOI] [PubMed] [Google Scholar]
- 23.Hansen GM, Egeberg A, Holmstrup P, Hansen PR. Relation of periodontitis to risk of cardiovascular and all-cause mortality (from a Danish Nationwide Cohort Study) Am J Cardiol. 2016;118:489–493. doi: 10.1016/j.amjcard.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, Choi EK, Park JB, Han KD, Oh S. Tooth loss predicts myocardial infarction, heart failure, stroke, and death. J Dent Res. 2019;98:164–170. doi: 10.1177/0022034518814829. [DOI] [PubMed] [Google Scholar]
- 25.Park HW, Yoon CH, Kang SH, Choi DJ, Kim HS, Cho MC, Kim YJ, Chae SC, Yoon JH, Gwon HC, Ahn YK, Jeong MH, KAMIR/KorMI Registry Early- and late-term clinical outcome and their predictors in patients with ST-segment elevation myocardial infarction and non-ST segment elevation myocardial infarction. Int J Cardiol. 2013;169:254–261. doi: 10.1016/j.ijcard.2013.08.132. [DOI] [PubMed] [Google Scholar]
- 26.Reichert S, Schulz S, Benten AC, Lutze A, Seifert T, Schlitt M, Werdan K, Hofmann B, Wienke A, Schaller HG, Schlitt A. Periodontal conditions and incidence of new cardiovascular events among patients with coronary vascular disease. J Clin Periodontol. 2016;43:918–925. doi: 10.1111/jcpe.12611. [DOI] [PubMed] [Google Scholar]
- 27.Söder B, Jin LJ, Klinge B, Söder PO. Periodontitis and premature death: a 16-year longitudinal study in a Swedish urban population. J Periodontal Res. 2007;42:361–366. doi: 10.1111/j.1600-0765.2006.00957.x. [DOI] [PubMed] [Google Scholar]
- 28.Liljestrand JM, Havulinna AS, Paju S, Männistö S, Salomaa V, Pussinen PJ. Missing teeth predict incident cardiovascular events, diabetes, and death. J Dent Res. 2015;94:1055–1062. doi: 10.1177/0022034515586352. [DOI] [PubMed] [Google Scholar]
- 29.GBD 2017 Risk Factor Collaborators (2018) Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1923–1994. 10.1016/S0140-6736(18)32225-6 Erratum in: Lancet 2019; 393:132. Lancet 2019;393:e44 [DOI] [PMC free article] [PubMed]
- 30.van der Vorst EPC, Peters LJF, Müller M, Gencer S, Yan Y, Weber C, Döring Y. G-protein coupled receptor targeting on myeloid cells in atherosclerosis. Front Pharmacology. 2019;10:531. doi: 10.3389/fphar.2019.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmlund A, Lampa E, Lind L. Oral health and cardiovascular disease risk in a cohort of periodontitis patients. Atherosclerosis. 2017;262:101–106. doi: 10.1016/j.atherosclerosis.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Holmlund A, Lind L. Number of teeth is related to atherosclerotic plaque in the carotid arteries in an elderly population. J Periodontol. 2012;83:287–291. doi: 10.1902/jop.2011.110100. [DOI] [PubMed] [Google Scholar]
- 33.Natto ZS, Aladmawy M, Alasqah M, Papas A. Factors contributing to tooth loss among the elderly: a cross-sectional study. Singap Dent J. 2014;35:17–22. doi: 10.1016/j.sdj.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Papapanou PN, Sanz M, Buduneli NS, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, Greenwell H, Herrera D, Kao RT, Kebschull M, Kinane DF, Kirkwood KL, Kocher T, Kornman KS, Kumar PS, Loos BG, Machtei E, Meng H, Mombelli A, Needleman I, Offenbacher S, Seymour GJ, Teles R, Tonetti MS. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):173–182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 35.Needleman I, Garcia R, Gkranias N. Mean annual attachment, bone level, and tooth loss: a systematic review. J Periodontol. 2018;89(Suppl 1):120–139. doi: 10.1002/JPER.17-0062. [DOI] [PubMed] [Google Scholar]
- 36.Reddy MS, Geurs NC, Jeffcoat RL, Proskin H, Jeffcoat MK. Periodontal disease progression. J Periodontol. 2000;71:1583–1590. doi: 10.1902/jop.2000.71.10.1583. [DOI] [PubMed] [Google Scholar]
- 37.Persson RE, Hollender LG, Persson GR. Assessment of alveolar bone levels from intraoral radiographs in subjects between ages 15 and 94 years seeking dental care. J Clin Periodontol. 1998;25:647–654. doi: 10.1111/j.1600-051X.1998.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 38.Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, Dye BA. Age-dependent distribution of periodontitis in two countries: findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J Periodontol. 2018;89(Suppl. 1):140–158. doi: 10.1002/JPER.17-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machtei EE, Hausmann E, Grossi SG, Dunford R, Genco RJ. The relationship between radiographic and clinical changes in the periodontium. J Periodontal Res. 1997;32(8):661–666. doi: 10.1111/j.1600-0765.1997.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 40.Goodson JM, Haffajee AD, Socransky SS. The relationship between attachment level loss and alveolar bone loss. Journal of Clinal Periodontology. 1984;11:348–359. doi: 10.1111/j.1600-051X.1984.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 41.GBD Causes of Death Collaborators (2018) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2017;10:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]