Abstract
The adverse neurocognitive sequelae following clinical radiation therapy (RT) for CNS malignancies are often long-lasting without any clinical recourse. Despite recent progress, the cellular mechanisms mediating RT-induced cognitive deficits (RICD) are poorly understood. The complement system is an immediate sensor of a disturbed inflammatory environment and a potent mediator of gliosis with a range of non-immune functions in the CNS, including synaptic pruning which is detrimental if dysregulated. We hypothesize that complement-mediated changes in glial cell function significantly contribute to RICD. The underlying alterations in CNS complement cascade proteins (C1q, C3), TLR4 and, co-labeling with glia (IBA1, GFAP) were examined using gene expression, immunofluorescence and in silico modeling approaches in the adult mouse brain following 9 Gy cranial RT. 3D volumetric quantification showed elevated molecular signatures of gliosis at short- and long-term post-RT times. We found significant elevations in complement C1q, C3 and TLR4 post-RT accompanied by increased co-labeling of astrocytes and microglia. To address the mechanism of RT-induced complement cascade activation, neuroinflammation, and cognitive dysfunction, we used a genetic approach – conditional, microglia-selective C1q (Flox) knockdown mice – to determine whether a glia-specific, upstream complement cascade contributes to RICD. C1q-Flox mice exposed to cranial RT showed no cognitive deficits compared to irradiated WT mice. Further, irradiated C1q-Flox mice were protected from RT-induced microglial activation and synaptic loss, elevation of anaphylatoxin C5a receptor, astrocytic-C3, and microglial-TLR4 expression in the brain. Our findings demonstrate for the first time a microglia-specific mechanism of RICD involving an upstream complement cascade component, C1q.
Keywords: Cranial radiation therapy, normal tissue toxicity, cognitive dysfunction, complement cascade, C1q, C3, astrocytes, microglia, gliosis, hippocampus, neuroinflammation, SV2, synaptophysin
INTRODUCTION
Clinical radiation therapy (RT) remains a frontline treatment for primary and metastatic brain cancers, along with surgery and chemotherapy. Unfortunately, longer-term survival comes at a cost, as there are now numerous clinical (1,2) and preclinical studies (3) that have established the adverse side effects of such treatment on cognition without any clinical recourse, imposing significant socioeconomic burdens and impacting long-term quality of life (4). Therefore, cellular mechanisms and strategies for preventing RT-induced cognitive deficits (RICD) following cancer treatments such as cranial RT are clearly needed. Pre-clinical studies conducted by us and others have found that the irradiated CNS exhibits robust and dynamic cycles of secondary reactive processes that follow complex temporal dynamics (5,6). Many of these changes can be linked to increased ROS (6), reduced hippocampal neurogenesis, damaged neuronal morphology, and elevated neuro-inflammation involving a range of cytokines along with persistent microglial activation (7–10). While these factors are likely to disrupt CNS functionality, our current knowledge regarding the cellular mechanisms of RT-induced brain injury and neurocognitive sequelae are limited. Since cranial RT causes microglial activation, we hypothesized that detrimental change in the glial function leads to long-term cognitive dysfunction. Astrocytes form complex astroglial networks and modulate synaptic plasticity and network activity through the tripartite synapse (11). Disruption of astrocytic function following irradiation or brain injury may cause an imbalance in the global homeostasis of the brain leading to functional deficits (12). Our data and reports in the literature have shown that radiation-induced elevation in inflammatory cytokines and reduced synaptic adenosine accompanied by astrogliosis are biochemical indicators of RICD (13–15). In addition, persistent activation of microglia is implicated in chronic neurodegeneration and spine loss in the irradiated brain (7,16,17). Thus, perturbations in glial function significantly contribute to RICD.
The complement system is comprised of over 40 proteins. Activation of the complement system generates powerful effector molecules of the immune system that protect tissues from infection, and also play important roles during development by synaptic pruning (18). Yet, an imbalance in complement cascades can also provoke a pro-inflammatory response in many degenerative conditions including rheumatoid arthritis, traumatic brain injury (TBI), and Alzheimer’s disease (AD) (18,19). C1q synthesis is elevated as an early response to a variety of neurodegenerative conditions in both humans and rodents (19). Predominant microglial synthesis of C1q affords a reparative role of microglia to maintain normal brain function. Conversely, activation of C1 in complex with serine proteases (C1r, C1s) initiates an enzymatic cascade that generates C3a and C5a, both of which can mediate inflammation that is associated with synapse and neuronal loss, and cognitive dysfunction (20–22). As such, neuronal injury or radiation-induced activation of the complement cascade can potentially trigger collateral pro-inflammatory polarization resulting in damaging microglial activation (15). Past studies from our laboratory have established the damaging role of radiation-induced chronic, elevated microglial activation in the irradiated adult brain (14,16,17). Astrocytes also express C1q under pathological conditions including multiple sclerosis (23), temporal lobe epilepsy (24) and AD (25). Based on these observations, we hypothesize that cranial RT-induced aberrant activation of the CNS complement cascade contributes to brain injury. Using gene expression, dual-immunofluorescence and in silico 3D reconstruction and volumetric quantification approaches, this report addresses detrimental neuro-inflammatory consequences of complement activation in the irradiated brain. In addition, our data show that conditional, microglia-selective deletion of C1q in the mouse brain is neuroprotective against the detrimental neurocognitive impact of cranial irradiation, establishing a mechanistic link between RT-induced complement cascade activation and cognitive dysfunction.
MATERIALS AND METHODS
Detailed methods are provided in the Supplemental Information section.
Animals, cranial Irradiation and tissue analysis:
All animal use procedures and safety protocols were approved by UCI Institutional Animal Care and Use Committee and were consistent with NIH guidelines. Adult (5 to 6-month-old) male WT mice (C57Bl/6J) were randomly divided into two groups: 0 or 9 Gy head-only irradiation using a 137Cs-γ irradiator at the dose rate of 1.2 Gy/min with shielding to the eyes, body and the cerebellum. A recent study showed that the adult female brain is protected from RICD and neuronal damage (26) thus, as a proof of principle, we have used male mice to determine the detrimental consequences of cranial RT on complement cascade. Mice were divided into the following experimental groups (8–10 mice/group): 2 hours, 24 hours, 48 hours, 1-, 2-, 3- and 4-weeks post-irradiation. An additional, 6-month post-RT group was also included for the gene expression analysis. Concurrent sham-irradiated controls were included at each time point. At the select post-RT times, brains were collected after intra-cardiac perfusion, fixed using 4% paraformaldehyde and cryo-sectioned (30 μm, coronal). For gene expression analysis, the micro-dissected hippocampus was flash-frozen and stored at −80 C° until analyzed. For RT-PCR, total mRNA was extracted from micro-dissected hippocampi (3–6 mice/group). Primers and experimental conditions for gliosis markers were optimized and followed as described (27), (Suppl. Table T1, Suppl. Information). Gene expression data are presented as 2^-(ΔΔCt) values.
C1q-flox mice and behavior testing:
Microglia-selective deletion of the C1qa gene was achieved by crossing C1qaFl/Fl and microglia-specific deleter strain (Cx3cr1CreERT2/WganJ) to generate C1qaFL/FL:Cx3cr1CreERT2/WganJ (C1q-Flox) mice as described (28). We have shown previously that C1q-Flox display CNS and microglia-specific depletion of C1q, without the need of tamoxifen treatment, by the age of 8-weeks. The ablation of the C1qa gene did not impact peripheral or circulating C1q levels thereby enabling the mechanistic determination of CNS C1q-mediated events in the irradiated brain. Adult (4-months old, male) littermate control (WT) and C1q-Flox mice were randomly divided into 0 or 9 Gy head-only irradiation groups (12–16 mice/group). Our past studies have shown that the group size of 8–10 mice/group for behavior and 4–6 mice/group for the molecular or tissue analyses achieved sufficient power (α=0.05) to reach the statistical significance (15,16,29). 1-month post-RT, animals were administered a light-dark box (LDB) test to assess anxiety and a spatial memory testing using the novel place recognition (NPR) task as described (30,31). The NPR task depends on animal’s inherent preference to explore a novel location of an object and relies on intact hippocampal function (32,33). Time spent interacting with the familiar versus novel object location was recorded and the data calculated as the discrimination index (DI): ([Novel location exploration time/Total exploration time] – [Familiar location exploration time/Total exploration time]) × 100. For the LDB, the time spent in light and dark chamber and number of transitions between compartments was assessed. A separate cohort of mice was also administered the fear extinction memory test as described (see Supplemental Information). All tests were scored by observers blind to the experimental groups to avoid bias. After conclusion of behavior testing, mice were euthanized via intra-cardiac perfusion and brains were fixed using 4% PFA for immunohistochemistry (IHC).
Dual-immunofluorescence staining, confocal microscopy, 3D algorithm-based volumetric quantification and cytokine analysis:
PFA-fixed brains from each treatment group underwent dual-immunofluorescence staining (3–4 sections/brain, 6–8 brains/group) including GFAP, IBA1, C1q, C3, C5aR1, TLR4, CD68, synaptophysin and SV2a. We used unbiased, 3D blinded deconvolution and volumetric quantification of glial surfaces, complement and receptor puncta using AutoQuant X3 and Imaris modules. Details of primary and secondary antibodies, immunostaining and in silico modeling are provided in the Supplemental Information section. We did not observe significant differences between control groups (0 Gy) from each post-irradiation time point (2h to 4wks); thus, pooled control data from 48h and 4wks were used for all the comparisons. Data were expressed as mean percent immunoreactivity relative to the unirradiated control group. For the cytokine analysis, freshly dissected hemi-brains (3–6 brains/group) were homogenized in presence of protease inhibitor, centrifuged and supernatants were assayed for cytokines using a magnetic bead-based customized kit (Thermo Fisher Scientific).
Data analysis:
Statistical analyses were performed using one-way ANOVA to confirm overall significance (GraphPad Prism, v8.0, RRID:SCR_002798). All data are expressed as the mean ± SEM. For comparisons between the controls (0 Gy) and irradiated groups, non-parametric, two-tailed unpaired t tests were performed with Holm-Sidak’s correction. For the analysis of C1q deletion and cranial irradiation, two-way ANOVA along with Bonferroni’s multiple comparisons test were performed. The Kruskal-Wallis test was used for the analysis of cytokine and synaptic proteins data. Wilcoxon matched-pairs signed rank test was used to compare exploration of familiar versus novel places by the same animals. All analyses considered a value of P ≤ 0.05 to be statistically significant.
RESULTS
Cranial irradiation elevates astrogliosis
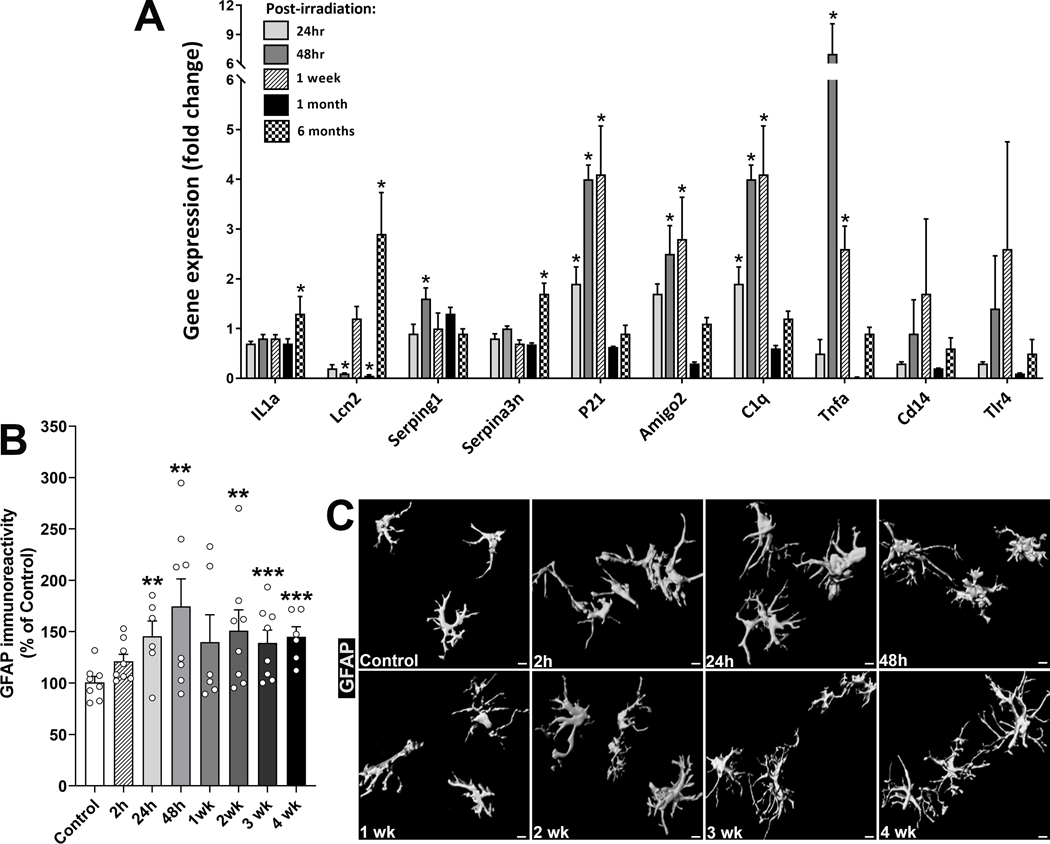
To determine the impact of cranial RT on the hippocampal neuroinflammation status as a function of time, gene expression (RT-PCR, Fig. 1A) for gliosis and neuroinflammation-related genes and astrocytic morphology were characterized at early and delayed post-irradiation times (Fig. 1B–C). A subset of these genes and gene products are shown to be linked with inflammation-mediated microglial and astrocytic crosstalk leading to synaptic damage (e.g. IL1a, Lcn2, Serping1, Serpina3n, Amigo2, C1q and Tnfa) (27). RT significantly increased expression (2–6 fold) of gliosis-related genes Serping1, P21, Amigo2, Tnfa and C1q at early post-RT times (24h to 1wk, Fig. 1A). IL1a, Lcn2 and Serpina3n were increased (1.5–3 fold) at the delayed 6-month post-RT time, indicating a long-term effect of cranial RT that coincides with cognitive impairments (16,17). Lcn2 expression was also reduced at 48h and 1 month post-RT. To determine the dynamics of astrocytic morphology as a function of time post-RT, the volume and area of individual astrocytes were quantified using 3D algorithm-based blinded deconvolution of high-resolution confocal z-stacks and unbiased volumetric quantification of reconstructed GFAP+ astrocytic surface (Fig. 1B–C). We found 1.3–1.7 fold increases in astrocytic immunoreactivity in the hippocampus as early as 48h that remained elevated at 4-weeks post-RT (Fig. 1B). The morphology of astrocytes showed longer and thicker processes in the irradiated brain compared to controls that is characteristic of astrocytic hypertrophy (Fig. 1C) (34). Together, analysis of gene expression and astrocytic morphology indicate RT-induced persistent gliosis that is coincident with cognitive deficits (16,17) indicating elevated neuroinflammation.
Figure 1. Cranial irradiation elevates expression of complement and inflammation-related genes and astrogliosis.
(A) Micro-dissected hippocampi from the irradiated (IRR) and control mice were analyzed for mRNA levels at early (24h, 48h and 1-week) and delayed (1- and 6-months) post-irradiation times. Elevated expression for inflammation (IL1a, C1q, Tnfa, Tlr4), gliosis (Lcn2, Serping1, Serpina3n, Amigo2) and apoptosis (P21)-related genes was found at short-term post-irradiation. (B) Volumetric analysis of GFAP+ hippocampal astrocytes (dentate hilus, dentate gyrus and CA1) showed elevated immunoreactivity at early (2, 24, 48 h) and delayed (1, 2, 3 and 4 weeks) post-IRR as compared to controls, indicating ongoing astrogliosis. (C) 3D algorithm-based z-stack deconvolution, surface reconstruction and volumetric quantification of GFAP+ astrocytes showed thicker and longer stelae indicating IRR-induced astrocytic hypertrophy. Mean ± SEM (3 to 4 observations per group, A; 6 to 8 observations per group, B). P-values are derived from one-way ANOVA, unpaired, two-tailed t-test with Holm-Sidak’s correction. *, P≤0.05, **, P<0.03, ***, P<0.01 vs. control. Scale: 5μm.
Radiation exposure increases glial levels of complement C1q
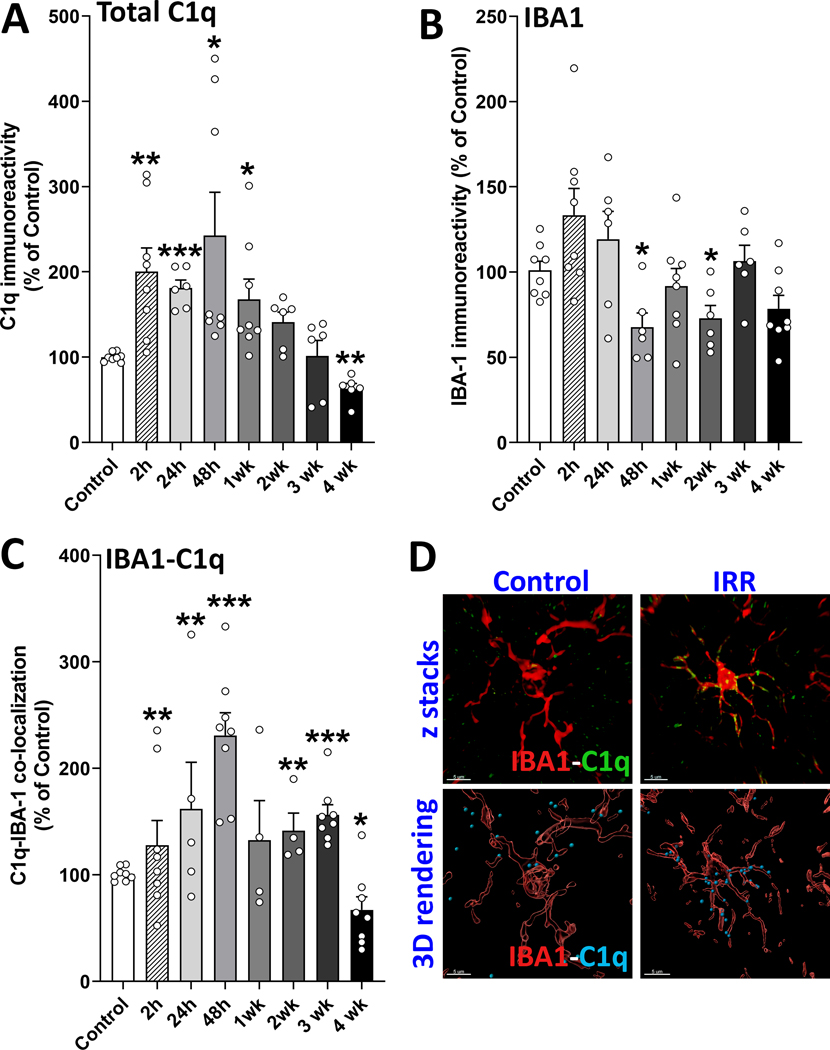
In the murine brain, resident microglia are the major source of C1q (28). Moreover, C1q also co-localized with astrocytes in multiple sclerosis (23), temporal lobe epilepsy (24) and AD (25,35). Interestingly, the status of C1q has not been characterized in context of normal tissue responses following cranial RT. Therefore, we characterized the time-dependent microglial (Fig. 2A-D) and astrocytic (Fig. 3A-B) immunoreactivity of C1q at early and delayed post-irradiation times in the hippocampus. RT elicited a 2-fold increase in the total immunoreactivity of C1q at 2 and 24h post-exposure (P<0.01 and 0.001, respectively, Fig. 2A) that reached a peak level at 48h (2–3 fold, P < 0.02). However, by 2–3 weeks post-RT the gross C1q immunoreactivity dropped to control levels and to less than control levels by 4 weeks post-irradiation (63%, P<0.01). The volume of IBA1+ microglial immunoreactivity did not change except decreases at 48h and 2wks post-exposure (66% and 73% of controls, respectively; P<0.02; Fig. 2B). Importantly, volumetric quantification of C1q+ puncta co-localized within 0.5 μm of IBA1+ microglial surfaces indicated a 1.3-fold increase at 2h post-irradiation (P<0.01, Fig. 2C). The co-labeling with IBA1+ microglia reached a peak level at 48h post-RT (2.3-fold, P<0.001) and then declined at 1 through 3-weeks (1.3–1.6 folds) and dropped below the control level by 4-weeks post-exposure (67%, P<0.02) (Fig. 2C–D). Concurrently, quantification of C1q+ puncta co-localized with GFAP+ astrocytic surfaces (Fig. 3A) followed a similar pattern as that observed for microglia. Increased co-labeling of C1q with GFAP+ astrocytes was observed at 2h post-exposure that peaked between 24 to 48h (P<0.002) and then declined in weeks 1, 2, and 3 post-RT (P<0.002) before returning to control levels at 4-weeks post-exposure. As shown above (Fig. 1C), astrocytic hypertrophy was also evident in the C1q+ GFAP+ ramified processes of astrocytes in the irradiated brain (Fig. 3B).
Figure 2. Elevated microglial expression of complement component C1q in the irradiated brain.
(A) 3D algorithm-based volumetric quantification of the total overall immunoreactivity of C1q+ puncta in the irradiated hippocampus (dentate hilus, dentate gyrus and CA1) showed increased C1q short-term post-exposure (2–24 h) that reached the peak at 48 h, and subsequently declined to control levels by 3 weeks post-irradiation. (B) Radiation exposure reduced IBA1+ microglial immunoreactivity at 48 h and 2-week post-exposure. (C) Volumetric analysis of IBA1+ microglia showed a significantly elevated expression of complement component C1q from 2–48 h post-irradiation that gradually declined from1 to 4-weeks post-irradiation. (D) The confocal z stacks (upper panels) for microglia (IBA1, red) and C1q (green) and; 3D surface and spot reconstruction (lower panels) for the microglial (IBA1, red) co-labeling with C1q (sky blue) are shown for the control and irradiated groups at the 48 h time point. Data are presented as mean ± SEM (N = 4 to 8 observations per group). P values are derived from one-way ANOVA and unpaired, two-tailed t test with Holm-Sidak’s correction. *, P < 0.02; **, P < 0.01 and ***, P < 0.001 compared to control. Scale bar, 5 μm (D).
Figure 3. Increased glial C1q and C3 co-labeling in the irradiated brain.
(A) Quantification of C1q+ puncta on the surface of GFAP+ astrocytes showed radiation-induced elevation in the hippocampus (dentate hilus, dentate gyrus and CA1) at 2 and 24 hours post-exposure that peaked at 48 h and, declined with time to the control levels by 4-weeks post-irradiation. (B) The confocal z stacks (upper panels) for astrocytes (GFAP, red) and C1q (green) and; 3D surface and spot reconstruction (lower panels) for the astrocytic C1q (GFAP, red; C1q white) are shown for the control and irradiated groups at the 48 h time point. (C) Volumetric quantification of the overall total C3 immunoreactivity (C3d) in the irradiated hippocampus showed consistently increased C3 expression levels at all post-exposure times. (D) Volumetric analysis of GFAP+ astrocytes showed significantly elevated levels of complement component C3 at all post-irradiation times. (D) The confocal z stacks (upper panels) and 3D surface and spot reconstruction for astrocytic C3 (GFAP, magenta; C3 green) are shown for the control and irradiated groups at the 48 h time point. Data are presented as mean ± SEM (N = 5 to 8 observations per group). P values are derived from one-way ANOVA and unpaired, two-tailed t test with Holm-Sidak’s correction. *, P < 0.002; and **P < 0.001 compared to control. Scale bar, 5 μm (B and E).
Elevated astrocytic expression of complement C3 in the irradiated brain
In the brain, complement C3 is primarily expressed by astrocytes (36–38) and a recent report suggested that complement receptor 3 (CR3) knockout mice were protected against radiation-induced neuronal damage (26). Thus, we characterized the astrocytic expression of complement C3 via C3d chain immunoreactivity in the hippocampus following cranial RT as a function of time (Fig. 3C–E). We found a significant elevation in total C3 protein levels as early as 2h that remained elevated for over 4-weeks post-irradiation (2.5-fold, P<0.002; Fig. 3C). Quantification of astrocyte-specific C3 similarly showed a 2.5–3.5-fold increase in expression from 2h to 4-weeks after radiation exposure (P<0.002; Fig. 3D). Typical C3+ immunofluorescent puncta were co-localized with the GFAP+ astrocytic processes in both control and irradiated cohorts (Fig. 3E).
Increased glial expression of TLR4 in the irradiated brain
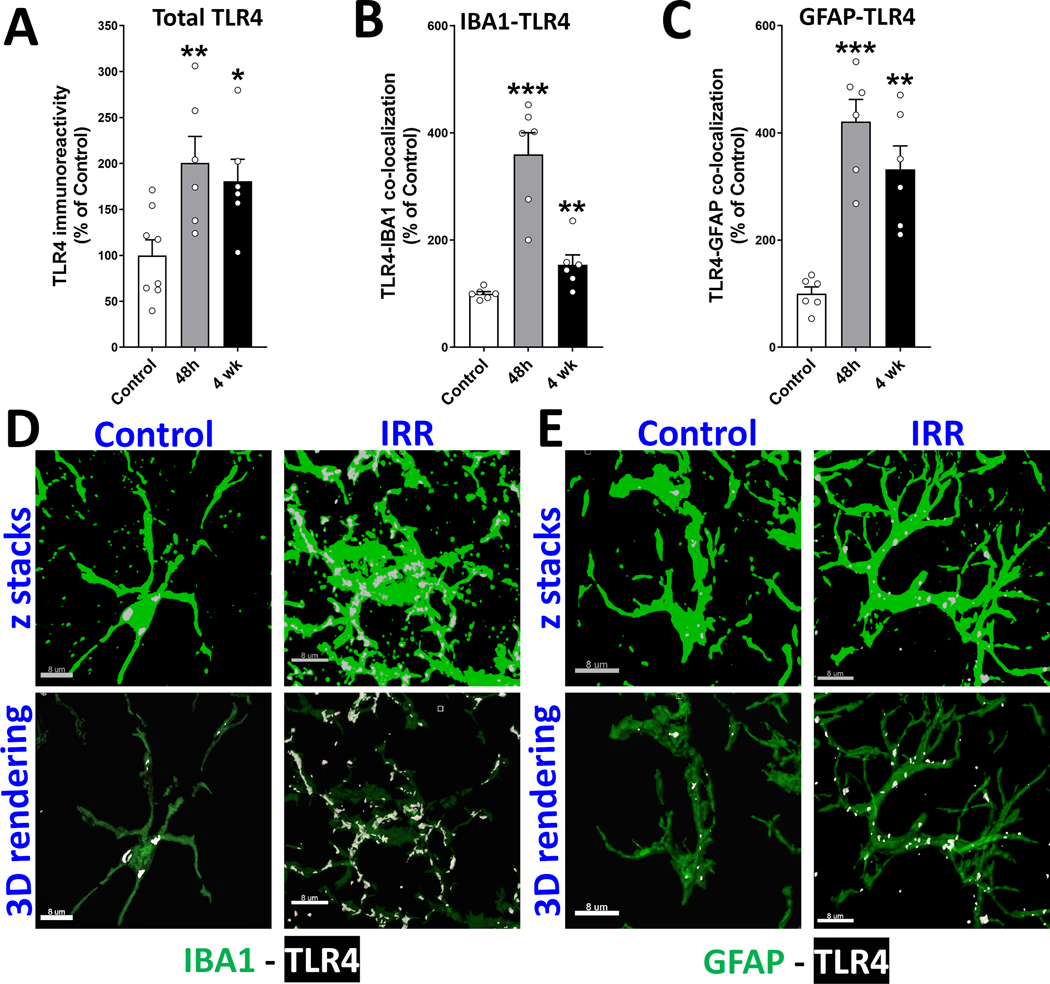
Toll-like receptor 4 (TLR4) plays important roles in innate and acquired immune responses, particularly triggering microglial activation in the brain (39). The foregoing data showed peak increase in complement proteins (C1q, C3; Figs. 2–3) and astrogliosis (Fig. 1B) at 48h post-exposure. We showed a correlation between microglial activation and RICD one month later (16). Based on these observations, we assessed TLR4 at key post-irradiation time points 48h and 1-month (Fig. 4). We found a 2-fold elevation in total TLR4 immunoreactivity in the hippocampus at 48h and 1-month post-irradiation (P<0.01; Fig. 4A). Further, quantification of glial-specific TLR4 indicated a 3.5–4-fold increase in microglial and astrocytic TLR4 expression (IBA1+ and GFAP+, respectively; P<0.001, Fig. 4B, C) at 48h post-exposure. TLR4 expression remained high in astrocytes (3-fold, P<0.001) and microglia at 1-month post-irradiation (3-fold and 1.5-fold respectively; P<0.001). Typical TLR4+ immunofluorescent puncta were co-localized with the IBA1+ microglial and GFAP+ astrocytic processes in both control and irradiated cohorts (Fig. 4D, E). Such persistent TLR4 elevation may contribute significantly to glial activation in the irradiated brain.
Figure 4. Glial expression of toll-like receptor 4 (TLR4) is increased in the irradiated brain.
(A) Volumetric quantification of the overall TLR4 immunoreactivity in the irradiated hippocampus (dentate hilus, dentate gyrus and CA1) showed increased TLR4 at early- (48 h) and delayed (4-weeks) post-exposure times. (B-C) Volumetric analysis of IBA1+ microglia and GFAP+ astrocytes showed a significantly elevated glia-specific TLR4 expression at 48 h and 4-weeks post-irradiation. (D-E) The confocal z stacks (upper panels) and 3D surface and spot reconstruction for microglial (IBA1, green, E) and astrocytic (GFAP, green, D) co-labeling with TLR4 (white) are shown for the control and irradiated groups at the 48 h time point. Data are presented as mean ± SEM (N = 5 to 8 observations per group). P values are derived from one-way ANOVA and unpaired, two-tailed t test with Holm-Sidak’s correction. *, P < 0.04; **P < 0.001 and ***P < 0.0001 compared to control. Scale bar, 8 μm.
In summary, these data describing the time-dependent dynamics of astrogliosis (Fig. 1), complement component C1q and C3 (Fig. 2–3) and TLR4 (Fig. 4) expression in the irradiated brain clearly indicate elevated neuroinflammation. Radiation-induced neuroinflammation has been shown to be detrimental to neuron and synaptic structure and cognitive function (17). Our new data on astrogliosis and complement activation thus provide functional relevance to our past data showing that cranial RT (9 Gy)-induced significant impairments in cognitive function and microglial activation (CD68)(16). Altogether, these data strongly suggest that RT-induced activation of the CNS classical complement cascade triggers neuroinflammation leading to functional impairments in the CNS.
Microglia-selective deletion of C1q prevents RT-induced cognitive impairments
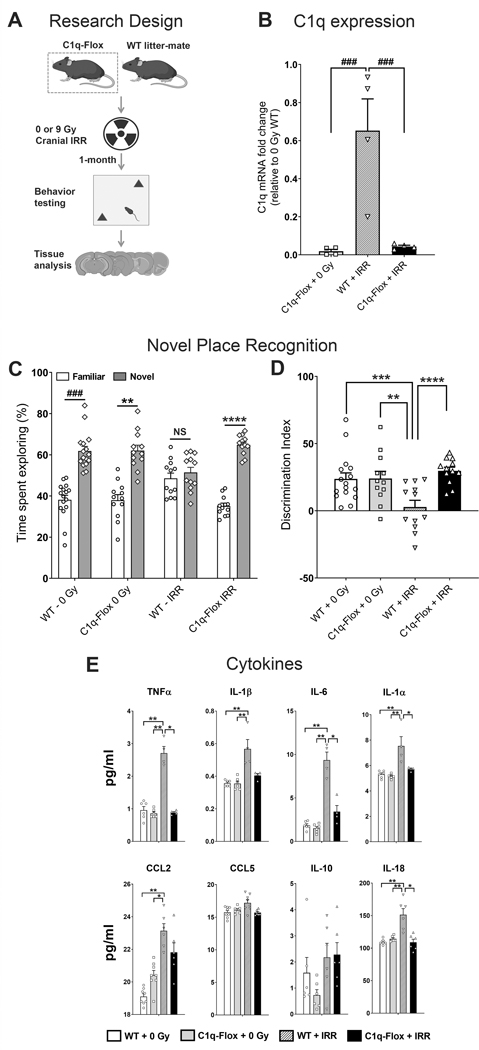
To determine whether RT-induced CNS complement cascade activation leads to cognitive decrements, microglia-specific C1q knockdown mice (C1q-Flox) were exposed to cranial irradiation (Fig. 5A). C1q-Flox mice show a complete depletion of CNS C1q in the absence of tamoxifen by the age of 8-weeks without alteration in circulating C1q (28). Analysis of C1q expression (qPCR) in the hippocampus showed nearly complete ablation (<2–4%) in the 0 or 9 Gy irradiated C1q-Flox groups (Fig. 5B). 1-month post-irradiation, C1q-flox and littermate control (WT) mice were habituated and tested on the hippocampal-dependent NPR task (Fig. 5C–D). Comparison of the exploration of familiar and novel location exploration times revealed significant differences between the WT (0 Gy, P=0.0001), C1q-Flox (0 Gy, P=0.02) and irradiated C1q-Flox (P=0.001) mice, but not for the WT mice exposed to cranial irradiation (WT–IRR), indicating reduced exploration of the novel spatial location (Fig. 5C). We also found significant overall group differences between the cohorts for the discrimination index (Fig. 5D; F(3, 48)=6.31, P=0.001). Irradiated WT mice (WT–IRR) showed a significantly reduced preference for the novel location compared to the 0 Gy WT (P=0.001), 0 Gy C1q-Flox (P=0.02) and irradiated C1q-Flox (P=0.001) mice. Importantly, irradiated C1q-Flox mice did not show reductions in the discrimination index and the novel place exploration was comparable to the unirradiated WT and C1q-Flox mice. In addition, a significant interaction for the genetic knockdown of C1q was found (F(1, 48)=8.42, P=0.01). For the LDB test, we did not find significant overall differences between groups for the time spent exploring the open compartment or number of transition between the dark and light compartments (Suppl. Fig. S1, A-B). Moreover, for the fear extinction test, WT–IRR mice showed elevated freezing compared to WT–0 Gy mice (P=0.03, Suppl. Fig. S2). Interestingly, both 0 and 9 Gy irradiated C1q-Flox mice showed elevated freezing on the extinction test (P=0.03 and 0.001 respectively, Suppl. Fig. S2). In summary, genetic depletion of microglial C1q is neuroprotective against cranial radiation-induced, hippocampal-dependent, cognitive impairments.
Figure 5. Microglia-selective deletion of complement C1q protect against radiation-induced cognitive impairments and elevated pro-inflammatory cytokines.
(A) Four month old C1q-Flox (C1qaFL/FL:Cx3cr1CreERT2) and littermate control (WT) mice were exposed to 0 or 9 Gy cranial irradiation and one month post-irradiation mice were administered hippocampal-dependent novel place recognition task. (B) Quantification of C1q expression (qPCR) in vivo showed a significant reductions (96–98%, P’s = 0.0001) in the C1q-Flox 0 and 9 Gy irradiated brains. (C) Irradiated (IRR) WT mice spent significantly less time exploring novel spatial location. Time spent exploring the novel placements of objects during the test phase of the novel place recognition task show that 0 Gy WT, 0 Gy C1q-Flox and irradiated C1q-Flox mice spent significantly more time exploring novel versus familiar location whereas irradiated WT mice spent comparable time exploring both locations. (D) The tendency to explore novel spatial location was derived from the Discrimination Index, calculated as ([Novel location exploration time/Total exploration time] – [Familiar location exploration time/Total exploration time]) × 100. Cranial irradiation significantly impaired cognitive function in the WT mice as shown by the reduced preference towards the novel place compared to the 0 Gy WT, 0 Gy C1q-Flox and irradiated C1q-Flox mice (P = 0.01, P= 0.02 and P = 0.001 respectively). Importantly, irradiated C1q-Flox mice did not show a decline on the spatial exploration. (E) Multiplex quantification of cytokines show radiation-induced elevations in the pro-inflammatory factors TNFα, IL-1β, IL-6, IL-1α, CCL2 and IL-18 in the WT-IRR brains whereas irradiated C1q-Flox mice did not show increases in the cytokine levels. Data are presented as mean ± SEM (N = 4 observations per group, B; N = 16 observations per group, C-D; N = 3 to 5 observations per group, E). P values were derived from Kruskal-Wallis test (B and E), Wilcoxon matched-pairs signed rank test (C) and, two-way ANOVA and Bonferroni’s post hoc test (D). NS, not significant; *, P = 0.03; **, P=0.02, ***, P =0.01; ****, P = 0.001; ### P = 0.0001.
Microglial C1q knockdown reduced neuroinflammation
To determine if microglia-specific knockdown of C1q prevents radiation-induced neuroinflammation we evaluated cytokine levels (Fig. 5E), CD68 (microglial activation marker, Fig. 6A), C5aR1 (a downstream complement or anaphylatoxin receptor, Fig. 6B), astrocytic C3 (GFAP-C3, Fig. 6C) and microglial TLR4 (IBA1-TLR4, Fig. 6D). Exposure to cranial RT significantly increased levels of pro-inflammatory cytokines in the WT–IRR brains (e.g. TNFα, IL-1β, IL-6, IL-1α, CCL2, and IL-18; Fig. 5E) as compared to irradiated C1q-Flox mice. Further, we found significant overall group effects for the quantification of CD68 (F(3, 20)=33.57, P<0.0001). CD68 expression in the WT–IRR group was significantly greater than that of the 0 Gy WT, 0 Gy C1q-Flox and irradiated C1q-Flox mice (P<0.0001, Fig. 6A). A significant overall group effect was found for C5aR1 immunoreactivity (F(3, 28)=13.52, P<0.0001). C5aR1 expression was significantly elevated in the WT–IRR hippocampus compared to WT–0 Gy (P=0.002), C1q-Flox 0 Gy (P=0.0001) and C1q-Flox IRR (P=0.002) groups (Fig. 6B). Radiation exposure did not increase CD68 or C5aR1 in C1q-Flox mice. Cranial irradiation significantly elevated astrocytic co-labeling of C3 in the WT–IRR brains (P=0.001) whereas C3 expression was comparable in the WT–0 Gy, C1q-Flox 0 Gy and C1q-Flox IRR mice. Similarly, quantification of microglial TLR4 immunoreactivity indicated radiation-induced elevation in the WT–IRR group (P=0.04 and 0.002 compared to WT–0 Gy and C1q-Flox 0 Gy respectively). Conversely, irradiated C1q-Flox mice showed a significant decrease in microglial TLR4 expression compared to the WT–IRR group (P=0.04). Overall, these data show that microglia-specific deletion of C1q prevented cranial RT-induced neuroinflammation and gliosis that was coincided with intact cognitive function.
Figure 6. Microglial C1q knockdown prevents radiation-induced neuroinflammation.
Immunofluorescence staining, confocal microscopy and 3D algorithm-based, volumetric quantification for the CD68 (A) and C5aR1 (B) immunoreactivity (CD68 and C5aR1, red, blue, DAPI nuclear counter stain; a1-a4 and b1-b4 respectively) show that cranial irradiation (IRR) significantly elevated CD68 (P = 0.001, A) and C5aR1 (P = 0.002, B) in the WT hippocampus (DH, dentate hilus; DG, dentate gyrus) compared to unirradiated controls. Exposure of C1q-Flox mice to the cranial IRR did not elevate CD68 or C5aR1 immunoreactivity indicating prevention of inflammation (A and B). Similarly, volumetric analysis for C3-GFAP (C, c1-c4) and IBA1-TLR4 co-localization (D, d1-d4) showed radiation-induced elevation in the co-labeling of GFAP+ astrocytic surfaces with C3 (GFAP surface rendering, magenta and C3 spot rendering, yellow, c1-c4) and, IBA1+ microglial surfaces with TLR4 (IBA1, green; red, TLR4 and blue, DAPI, d1-d4) in the hippocampal DH. Irradiated C1q-Flox brains did not show elevated C3-GFAP or IBA-TLR4 indicating reduced neuroinflammation. Data are presented as mean ± SEM (N = 6 to 8 observations per group). P values were derived from ANOVA and Bonferroni’s post hoc test. Scale bars, 40 μm (a1-a4, b1-b4) and 5 μm (c1-c4, d1-d4).
C1q knockdown protects against radiation-induced synaptic loss
C1q has been shown to play an important role in synapse elimination in pathological conditions (18). To determine the downstream impact of deleting microglial C1q expression in the irradiated brain, we quantified immunoreactivity of synaptic markers synaptophysin and SV2a (Fig. 7A–B). Exposure to cranial RT significantly reduced synaptophysin and SV2a immunoreactivity in the CA1 stratum radiatum (Fig. 7, a1–a4 and b1–b4) in WT–IRR mice compared to the unirradiated WT controls (P=0.01 and 0.03 respectively). In contrast, irradiated C1q-Flox mice did not show decreased syanptophysin or SV2a immunoreactivity indicating a neuroprotective role of CNS C1q ablation against radiation-induced synaptic loss.
Figure 7. C1q depletion in microglia prevents radiation-induced synaptic loss.
(A-B) Immunofluorescence staining, confocal microscopy and 3D algorithm-based quantification for the synaptic proteins synaptophysin (red, DAPI nuclear counter stain; A, a1-a4) and SV2a (red, DAPI+ nucleus; B, b1-b4) show that cranial irradiation (IRR) significantly decreased synaptic proteins in the WT CA1 sub-region (pyr, pyramidal cell layer; sr, stratum radiatum) compared to unirradiated controls, 0 Gy C1q-Flox and irradiated C1q-Flox groups (A and B). Exposure of C1q-Flox mice to the cranial IRR did not reduce synaptic protein immunoreactivity indicating neuroprotection against irradiation (A and B). Data are presented as mean ± SEM (N = 5 to 8 observations per group). P values were derived from ANOVA and Bonferroni’s post hoc test. Scale bars, 40 μm.
DISCUSSION
Over the past two decades, numerous studies have shown cranial RT-induced early and delayed neuropathologies that culminate in long-term cognitive impairments (3,7) linked with elevated neuroinflammation (7,16,17,40–42). The current study sought to address the underlying radiation-induced pro-inflammatory signaling mechanisms leading to the brain injury. In the CNS, complement factors C1q and C3 serve not only as mediators of innate immunity, but also coordinate microglia-mediated synapse pruning during circuit development (18,43). In the diseased or injured state, activation of the complement system is linked with the loss of spines and increased neuroinflammation (6–9). To the date, two studies have shown the adverse effects of hippocampal C3 or CR3 signaling in radiation-induced brain injury models (26,44). We characterized radioresponse of complement C1q and C3 that may, in presence of injured cells, induce tagging of damaged or “weak” synapses for pruning or elimination by microglia and trigger long-term neuroinflammation (via C3a or C5a). Importantly, microglia-selective deletion of the upstream complement C1q allowed us to study the CNS-specific contribution of classical complement cascade activation in the RICD, neuroinflammation and synaptic loss.
Radiation exposure induces astrogliosis.
Pre-clinical experimental models and clinical cases have provided sporadic evidence that astrocytes play a role in neuropathological conditions (27,45). However, unlike microglial activation, astrogliosis is still an understudied area of research in CNS radiobiology. Our past data and the literature have shown that radiation-induced elevation in inflammatory cytokines, and reduced synaptic adenosine accompanied by astrogliosis, are functional indicators of radiation-induced brain injury and cognitive deficits (5,14). Moreover, Barres and colleagues showed that microglia-mediated astrocytic activation via C1q, IL-1a and TNFα upregulated the production of C3 and neurotoxins that were destructive to synapses (27). The molecular signatures of astrogliosis were substantiated by acute and delayed alterations morphologic characterization of individual astrocytes (Fig. 1B), as illustrated by elongation and thickening of the astrocyte intermediate filaments (GFAP). Acute reactive astrogliosis observed within hours after irradiation is thought to be neuroprotective by repairing BBB integrity, detoxifying ROS, reducing peripheral immune cell infiltration and slowing down neurodegeneration (34). However, persistent astrogliosis at delayed times has been shown to be neurotoxic where elevated levels of pro-inflammatory cytokines, complement components and activated microglia limit regenerative neuronal, synaptic and glial responses.
We characterized in vivo gliosis, inflammation and apoptosis/cell cycle arrest (P21)-related gene expression in the hippocampus from 24h to 6-months post-irradiation (Fig. 1). We found increased expression of Serping1, Amigo2, P21, TNFα and C1qa genes at early (24h to 1 week) times, suggesting an early trigger for radiation-induced inflammation. Serping1 (encoding C1-INH) regulates complement cascade activation by inhibiting activated C1r and C1s (46). A recent report showed that knockdown of circulating C1-INH caused upregulation of CNS C1-INH that led to neurovascular impairment and loss of BBB integrity, elevated astrogliosis and hippocampal-dependent cognitive deficits (47). The latter two degenerative characteristics have also been observed in the irradiated brain (14,16). Amigo2 regulates the PDK1-Akt signaling pathway that regulates cell survival, adhesion, neurite development, axon tract formation and angiogenesis (48). In the irradiated hippocampus, elevated expression of Serging1 and Amigo2 (24h post-RT) was synchronized with increased P21 and C1q genes, suggesting an adaptive CNS response that may facilitate the removal of apoptotic cells, cellular debris and facilitate repair of the irradiated tissue bed at 24h post-exposure time, but at 48h elicits persistent and damaging inflammation (TNFα). Elevated classical complement pathway proteins (C1, C4 and C3) were implicated in neurodegeneration, synapse elimination and cognitive impairments (43). Our present and past studies on late CNS radio-responses (16,17) found similar increases in pro-inflammatory gene expression and gliosis along with behavioral deficits at 1- and 6-months post-irradiation respectively, linking CNS activation of the complement cascade with cognitive impairment. In fact, we found long-term hippocampal elevation in cytokine IL1α and lipocalin 2 (Lcn2). Secretion of Lcn2 by reactive astrocytes was shown to damage neurons and elicit neurodegeneration (49). McBride and colleagues characterized a sharp elevation in pro-inflammatory cytokines including TNFα and IL-1α 4h after exposure to high acute dose cranial-RT (25 Gy) with recurrent cyclic upregulation between 48h and 1-month post-RT (5,50). We also found elevated pro-inflammatory cytokines (TNFα, IL-1β, IL-6, IL-1α, CCL2, and IL-18, Fig. 5E) 1-month post-RT, coinciding with RICD. Those data provided premise to characterize time-dependent changes in gliosis and complement cascade proteins at early post-irradiation times. While the resultant short- and long-term gene expression profiles (Fig. 1A) remain to be validated at the protein level, they do suggest a radiation-induced pro-inflammatory astrocytic response that may lead to neurotoxic events in vivo.
Taken together, our new data and the literature suggest two key points in the context of the CNS radio-response: i) early upregulation of inflammation, apoptosis, complement-related gene expression and gliosis are suggestive of an adaptive, reparative radio-response, but that ii) persistent inflammation and reactive astrogliosis are detrimental responses to RT that may lead to functional deficits in cognition.
Radiation elevates glial co-labeling of complement cascade proteins.
We found a sharp elevation in C1q immunoreactivity in the early phases (hours) of the radiation response that receded by 1-month (Fig. 2), consistent with mRNA expression (Fig. 1). Our past genetic study using the microglia-selective C1q-Flox mouse, with intact peripheral C1q levels, showed that resident microglia are the prominent source of C1q in the CNS (28). Indeed, dual staining for IBA+ and microglial C1q showed similar patterns of C1q upregulation that peaked at 48h and declined by 1-month post-irradiation. These data indicate that radiation-induced complement activation may synergize with elevated cytokine signaling at a short-term (2 to 48h) post-irradiation interval. Radiation-induced brain injury may also induce activation of other complement proteins, including C3a, iC3b and C5a, leading to the pro-inflammatory polarization of microglia and astrocytic hypertrophy. We found elevated C3 in the irradiated brain (Fig. 3) that coincides with elevated expression of the danger recognition receptor TLR4 and pro-inflammatory cytokine signaling (Fig. 4 and microglial activation in the short-term and, subsequently long-term cognitive deficits (1-month) (Fig. 5C, D). These data suggest RT-induced complement cascade activation lead to long-term inflammatory injury.
Interestingly, we found elevated C1q also associated with astrocyte intermediate filaments (GFAP, Fig. 3). Recent studies using LPS-induced CNS insult have suggested that microglia-mediated astrogliosis via C1q, IL-1a or TNFα increased the production of C3 and neurotoxins that were destructive to synapses (27). Similarly, we and others have shown persistent microglial activation that may share some commonalities with the LPS-induced inflammatory cascade (7,16,41). C3 gene expression was found to be elevated following 20 Gy RT in the juvenile brain (51), but genetic C3 knockout prevented radiation-induced learning disabilities and gliosis (44). Further, Hinkle and colleagues showed neuroprotective effects of the CR3 knockdown against radiation-induced damage to neuron structure, spine loss and microglial activation (26) further suggesting a neurotoxic role of iC3b in RICD. A caveat of this study is that the global knockdown of CD11b gene to produce mutant CR3 that does not allow mechanistic characterization of peripheral- versus CNS-specific effects of complement cascade-mediated signaling in vivo. Nonetheless, this study revealed that radiation-induced detrimental complement signaling leads to synaptic damage. In total, radiation-induced glial elevation in complement cascade proteins are linked with cognitive impairments and neuroinflammation.
Microglial C1q knockdown protects against cranial RT-induced cognitive deficits.
RT-induced complement cascade activation, commencing with C1q (Fig. 2), triggers detrimental microglial activation in the irradiated hippocampus that may lead to cognitive decline as shown in other neurodegeneration models. Thus, to validate the pro-inflammatory role of complement system in the irradiated brain, we utilized conditional C1q-Flox mice. We have shown selective knockdown of microglial C1q by 8-week of age, in absence of tamoxifen treatment (28) and, established microglia as a major source of C1q in the brain. Our study also showed selective depletion of C1q in the brain without altering C1q in the blood (28) thus allowing the delineation of CNS-specific C1q-mediated events in the irradiated brain. Indeed, cranially irradiated C1q-Flox mice did not exhibit cognitive impairments on the hippocampal-dependent NPR task, as indicated by comparable behavior with the 0 Gy control and C1q-Flox mice (Fig. 5C–D). In contrast, littermate controls exposed to cranial RT showed significant decrements on a hippocampal-dependent novel spatial recognition task. Using a fear extinction test, we found increased freezing behavior by the WT-IRR mice indicating RT-induced disruption in the dissociative learning process (Suppl Fig. S2) whereas WT-0 Gy mice were able to show reduced freezing. Interestingly, both 0 Gy and 9 Gy irradiated C1q-Flox mice showed elevated freezing on the extinction test. To address this finding, we quantified cytokines in the WT and C1q-Flox brains exposed to cranial RT. Activated microglia and elevated pro-inflammatory cytokines play a disruptive role in fear memory consolidation (52–56). Our multiplex data (Fig. 5E, Suppl. Fig. S2) showed significant elevations in TNFα, IL-1β, IL-6, IL-1α, CCL2, and IL-18 in the WT-IRR brains that suggest pro-inflammatory cytokine-mediated disruption in dissociative learning. However, we did not observe significant differences in the cytokine levels in the 0 or 9 Gy irradiated C1q-Flox brains. Additional cytokine analyses in the brains of WT and C1q-Flox mice with or without experiencing foot shock and after fear extinction training also did not show any significant differences (Suppl. Fig. S3). These behavior and cytokine data may indicate that cytokines are not playing any role in the elevated freezing observed in the C1q-Flox mice and that possible impact of the C1q gene inactivation on the dissociative learning and memory consolidation processes in the brain. In fact, a recent study, using CD55-mediated inhibition of classical CNS complement pathway, suggested the dependency of dissociative learning or memory consolidation processes on the microglial C1q (57).
Importantly, analysis of irradiated C1q-Flox mouse brains (with undetectable C1q, Fig. 5B) did not show microglial activation (CD68, Fig. 6A), C5aR1 (Fig. 6B), astrocytic-C3 or microglial-TLR4 (Fig. 6C–D) elevation compared to irradiated littermate control mice providing evidence that deletion of microglial C1q protected the brain from the downstream detrimental effects of cranial RT. Activation of the complement cascade, starting form C1q, produces effector anaphylatoxins, C3a and C5a, which are chemotactic for microglia (37,58). Of these, C5a is more potent in triggering neuroinflammation and C5aR1 signaling also synergizes with the danger recognition TLRs (19,59). C1 complex, along with C3b and iC3b, also play important roles in microglia-mediated synaptic pruning (43). Radiation-induced aberrant activation of complement cascade may also facilitate overzealous synaptic pruning leading to functional impairments. Importantly, cranial RT-induced loss of synaptic proteins synaptophysin and SV2a (Fig. 7) was not found in the irradiated C1q-Flox mice, indicating neuroprotective effect of ablating C1q. These data also show that microglia-selective targeting of C1q prevented radiation-induced downstream damaging events.
Conclusion.
This study provides novel insights into induction of complement cascade components by radiation exposure that impact brain function and lead to cognitive impairments. Our studies linking activation of the complement cascade shortly after radiation exposure to the long-term neuroinflammation and cognitive dysfunction have a unique potential for impacting the quality of life for cancer survivors. Particularly, our genetic approach, microglia-selective deletion of C1q, addresses the contribution of an upstream CNS complement cascade mechanism playing a detrimental role in RICD. Importantly, our strategy to target the CNS complement cascade also needs to be confirmed in presence of tumor, as activation of the complement cascade has been proposed to promote glioma stem-like cell proliferation, decreased apoptosis, immunosuppression and angiogenesis within tumor vasculature, thereby promoting invasion (60–64). Moreover, the impact of fractionated RT should be studied to evaluate the nature of complement and microglia activation in the normal tissue bed. Nonetheless, our study establishes the link between radiation-induced gliosis, neuroinflammation and cognitive dysfunction (16) with complement activity. These data highlight the intricacies of the aberrant glial responses following radiation exposure in the brain and provide a unique opportunity to develop pathway branch point-specific therapeutics that may not need to directly affect neuronal function in the irradiated brain.
Supplementary Material
SIGNIFICANCE.
Clinically-relevant radiotherapy induces aberrant complement activation leading to brain injury. Microglia-selective genetic deletion of CNS complement C1q ameliorates radiation-induced cognitive impairments, synaptic loss, and neuroinflammation, highlighting the potential for C1q as a novel therapeutic target.
ACKNOWLDEGMENTS
This work was support by American Cancer Society (ACS) Research Scholar grant (RSG-17-146-01-CCE), UCI School of Medicine Faculty Pilot Research award (19900), University of California Cancer Research Coordinating Committee award (CRR-19-585293) and HESI-Thrive award (Health and environmental Sciences Institute) to M.M.A. and, National Institutes of Health (NIH) award R01 AG 60148 to A.J.T. We also thank the support of the UCI Chao Family Comprehensive Cancer Center Biostatistics shared resource supported by the National Cancer Institute (NIH, award P30CA062203). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of Potential Conflicts of Interest
Authors declares no conflicts of interest.
REFERENCES
- 1.Anderson VA, Godber T, Smibert E, Weiskop S, Ekert H. (2000). Cognitive and academic outcome following cranial irradiation and chemotherapy in children: a longitudinal study. Br J Cancer 2000;82:255–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol 2006;24:1305–9 [DOI] [PubMed] [Google Scholar]
- 3.Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: A review. Frontiers in oncology 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. The Lancet Oncology 2011;12:703–8 [DOI] [PubMed] [Google Scholar]
- 5.McBride WH. Cytokine cascades in late normal tissue radiation responses. Int J Radiat Oncol Biol Phys 1995;33:233–4 [DOI] [PubMed] [Google Scholar]
- 6.Acharya MM, Lan ML, Kan VH, Patel NH, Giedzinski E, Tseng BP, et al. Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radical Biology & Medicine 2010;49:1846–55 [DOI] [PubMed] [Google Scholar]
- 7.Parihar VK, Acharya MM, Roa DE, Bosch O, Christie LA, Limoli CL. Defining functional changes in the brain caused by trageted stereotaxic radiosurgery. Translational Cancer Research 2014;3:124–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol 2009;19:122–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizumatsu S, Monje M, Morhardt D, Rola R, Palmer T, Fike J. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res 2003;63:4021–7 [PubMed] [Google Scholar]
- 10.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in hippocampal neurogenesis. Radiat Res 2004;Submitted manuscript [DOI] [PubMed] [Google Scholar]
- 11.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 2014;81:728–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 2009;57:343–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys 2006;66:860–6 [DOI] [PubMed] [Google Scholar]
- 14.Acharya MM, Baulch JE, Lusardi T, Allen BD, Chmielewski NN, Baddour AAD, et al. Adenosine Kinase Inhibition Protects against Cranial Radiation-Induced Cognitive Dysfunction. Frontiers in molecular neuroscience 2016;9:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montay-Gruel P, Markarian M, Allen BD, Baddour JD, Giedzinski E, Jorge PG, et al. Ultra-High-Dose-Rate FLASH Irradiation Limits Reactive Gliosis in the Brain. Radiat Res 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, et al. Elimination of microglia improves cognitive function following cranial irradiation. Scientific reports 2016;6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A 2019;116:10943–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007;131:1164–78 [DOI] [PubMed] [Google Scholar]
- 19.Tenner AJ. Complement-Mediated Events in Alzheimer’s Disease: Mechanisms and Potential Therapeutic Targets. Journal of immunology 2020;204:306–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016;352:712–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, et al. Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. J Neurosci 2015;35:13029–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 2016;534:538–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingram G, Loveless S, Howell OW, Hakobyan S, Dancey B, Harris CL, et al. Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta Neuropathol Commun 2014;2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aronica E, Boer K, van Vliet EA, Redeker S, Baayen JC, Spliet WG, et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiology of disease 2007;26:497–511 [DOI] [PubMed] [Google Scholar]
- 25.Iram T, Trudler D, Kain D, Kanner S, Galron R, Vassar R, et al. Astrocytes from old Alzheimer’s disease mice are impaired in Abeta uptake and in neuroprotection. Neurobiology of disease 2016;96:84–94 [DOI] [PubMed] [Google Scholar]
- 26.Hinkle JJ, Olschowka JA, Love TM, Williams JP, O’Banion MK. Cranial irradiation mediated spine loss is sex-specific and complement receptor-3 dependent in male mice. Scientific reports 2019;9:18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017;541:481–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca MI, Chu SH, Hernandez MX, Fang MJ, Modarresi L, Selvan P, et al. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. Journal of neuroinflammation 2017;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leavitt RJ, Acharya MM, Baulch JE, Limoli CL. Extracellular vesicle-derived miR-124 resolves radiation-induced brain injury. Cancer Res 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen BD, Syage AR, Maroso M, Baddour AAD, Luong V, Minasyan H, et al. Mitigation of helium irradiation-induced brain injury by microglia depletion. Journal of neuroinflammation 2020;17:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acharya MM, Baulch JE, Klein PM, Baddour AAD, Apodaca LA, Kramar EA, et al. New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose-Rate, Neutron Radiation. eNeuro 2019;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 2007;27:2948–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci 2011;31:10721–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 2014;94:1077–98 [DOI] [PubMed] [Google Scholar]
- 35.Orre M, Kamphuis W, Osborn LM, Jansen AHP, Kooijman L, Bossers K, et al. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiology of aging 2014;35:2746–60 [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Fonseca MI, Pisalyaput K, Tenner AJ. Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer’s disease. J Neurochem 2008;106:2080–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu T, Dejanovic B, Gandham VD, Gogineni A, Edmonds R, Schauer S, et al. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell reports 2019;28:2111–23 e6 [DOI] [PubMed] [Google Scholar]
- 38.Guttenplan KA, Weigel MK, Adler DI, Couthouis J, Liddelow SA, Gitler AD, et al. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nature communications 2020;11:3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. Journal of neuroinflammation 2015;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng X, Jopson TD, Paladini MS, Liu S, West BL, Gupta N, et al. Colony-stimulating factor 1 receptor blockade prevents fractionated whole-brain irradiation-induced memory deficits. Journal of neuroinflammation 2016;13:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belarbi K, Jopson T, Arellano C, Fike JR, Rosi S. CCR2 deficiency prevents neuronal dysfunction and cognitive impairments induced by cranial irradiation. Cancer Res 2013;73:1201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moravan MJ, Olschowka JA, Williams JP, O’Banion MK. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res 2011;176:459–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenner AJ, Stevens B, Woodruff TM. New tricks for an ancient system: Physiological and pathological roles of complement in the CNS. Mol Immunol 2018;102:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalm M, Andreasson U, Bjork-Eriksson T, Zetterberg H, Pekny M, Blennow K, et al. C3 deficiency ameliorates the negative effects of irradiation of the young brain on hippocampal development and learning. Oncotarget 2016;7:19382–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mciver SR, Faideau M, Haydon PG. Neural-Immune Interactions in Brain Function and Alcohol Related Disorders. Cui C, editor. New York: Springer Science+Business Media; 2012. [Google Scholar]
- 46.Heit C, Jackson BC, McAndrews M, Wright MW, Thompson DC, Silverman GA, et al. Update of the human and mouse SERPIN gene superfamily. Hum Genomics 2013;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farfara D, Feierman E, Richards A, Revenko AS, MacLeod RA, Norris EH, et al. Knockdown of circulating C1 inhibitor induces neurovascular impairment, glial cell activation, neuroinflammation, and behavioral deficits. Glia 2019;67:1359–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park H, Lee S, Shrestha P, Kim J, Park JA, Ko Y, et al. AMIGO2, a novel membrane anchor of PDK1, controls cell survival and angiogenesis via Akt activation. The Journal of cell biology 2015;211:619–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci U S A 2013;110:4069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. International journal of radiation biology 1997;72:45–53 [DOI] [PubMed] [Google Scholar]
- 51.Kalm M, Fukuda A, Fukuda H, Ohrfelt A, Lannering B, Bjork-Eriksson T, et al. Transient inflammation in neurogenic regions after irradiation of the developing brain. Radiat Res 2009;171:66–76 [DOI] [PubMed] [Google Scholar]
- 52.Yu Z, Fukushima H, Ono C, Sakai M, Kasahara Y, Kikuchi Y, et al. Microglial production of TNF-alpha is a key element of sustained fear memory. Brain Behav Immun 2017;59:313–21 [DOI] [PubMed] [Google Scholar]
- 53.Schubert I, Ahlbrand R, Winter A, Vollmer L, Lewkowich I, Sah R. Enhanced fear and altered neuronal activation in forebrain limbic regions of CX3CR1-deficient mice. Brain Behav Immun 2018;68:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, et al. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun 2010;24:243–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen BD, Apodaca LA, Syage AR, Markarian M, Baddour AAD, Minasyan H, et al. Attenuation of neuroinflammation reverses Adriamycin-induced cognitive impairments. Acta Neuropathol Commun 2019;7:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, et al. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiology of disease 2006;23:127–39 [DOI] [PubMed] [Google Scholar]
- 57.Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020;367:688–94 [DOI] [PubMed] [Google Scholar]
- 58.Hernandez MX, Jiang S, Cole TA, Chu SH, Fonseca MI, Fang MJ, et al. Prevention of C5aR1 signaling delays microglial inflammatory polarization, favors clearance pathways and suppresses cognitive loss. Mol Neurodegener 2017;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 2007;110:228–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Parsa AT. Cancer and the complement cascade. Molecular cancer research : MCR 2010;8:1453–65 [DOI] [PubMed] [Google Scholar]
- 61.Sayegh ET, Bloch O, Parsa AT. Complement anaphylatoxins as immune regulators in cancer. Cancer Med 2014;3:747–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74 [DOI] [PubMed] [Google Scholar]
- 63.Bouwens van der Vlis TAM, Kros JM, Mustafa DAM, van Wijck RTA, Ackermans L, van Hagen PM, et al. The complement system in glioblastoma multiforme. Acta Neuropathol Commun 2018;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, et al. Modulation of the antitumor immune response by complement. Nature immunology 2008;9:1225–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.