Abstract
Chimeric antigen receptor (CAR) engineering of T cells has revolutionized the field of cellular therapy for the treatment of cancer. Despite this success, autologous CAR-T cells have recognized limitations that have led to the investigation of other immune effector cells as candidates for CAR modification. Recently, natural killer (NK) cells have emerged as safe and effective platforms for CAR engineering. In this article, we review the advantages, challenges and preclinical and clinical research advances in CAR-NK cell engineering for cancer immunotherapy. We also briefly consider the feasibility and potential benefits of applying other immune effector cells as vehicles for CAR expression.
INTRODUCTION
The field of cellular therapy for cancer is growing at an unprecedented pace, especially since the advent of chimeric antigen receptor (CAR) engineered T cells. This strategy has proven effective against B cell malignancies and is showing promising activity in clinical trials for other hematologic cancers, with the potential for inducing beneficial responses in solid tumors.(1) Some of the obstacles faced in the clinical application of CAR-T cell therapy are cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS), both of which increase the length of patient hospitalization and the cost of therapy.(2) Other limitations of CAR-T cell therapy are the logistic hurdles of generating an autologous product(3) and the fact that further genetic modification is necessary to produce a safe allogeneic T cell product, given the risk of graft versus host disease (GVHD) mediated by the T-cell receptor.(4) The suboptimal results of CAR-T cell therapy for solid tumors have been attributed mainly to the unique challenges presented by the immunosuppressive microenvironment and the physical barriers imposed by the tumor stroma.(5) Given these shortcomings of conventional CAR transduced αβ-T cells, there has been a surge of interest in other candidate immune cell subsets in both the innate and adaptive compartments, as well as improved strategies of CAR engineering. Natural killer (NK) cells have gained much recent attention as a promising alternative platform for CAR engineering owing to their unique biological attributes, their specialized cytotoxicity against tumors, their safety profile and their potential use as an off-the-shelf cellular therapy.(3)
In this article, we review the development and performance of CAR-modified NK cells in cancer immunotherapy, discussing their advantages, including both mechanisms of anti-tumor activity and safety features, as well as the roadblocks and challenges that must be overcome before CAR-NK cells can become a central player in the struggle against cancer. We also discuss the progress being made in exploring other immune effector cells as platforms for CAR-based therapies.
CAR NK CELLS IN CANCER THERAPY
NK cells, which represent 5–10% of peripheral blood lymphocytes, are an essential component of the innate immune system and play a critical role in our first-line defense against pathogens and cancer cells.(6) As their name implies, NK cells are specialized killers with a natural ability to eliminate abnormal cells that have been compromised by viral infections or malignant transformation. Unlike T cells, NK cells lack expression of the T cell receptor (TCR) and CD3 and can be identified by surface expression of CD56 and CD16.(7) Depending on their level of CD56 and CD16 expression, NK cells can be further subdivided into two major subsets: CD16+CD56dim, which represents a more mature and cytotoxic subset mostly present in the peripheral blood, and CD16−CD56bright, a less mature and more immunoregulatory subset found in tissues.(8)
Intrinsic killing capacity
NK cells are highly specialized cytotoxic cells that rely on the integration of multiple signals from activating receptors, inhibitory receptors, cytokine receptors and chemokine receptors to recognize and eliminate their targets.(9) Once committed to a cytotoxic state, NK cells (unlike T cells) do not require any prior antigen priming before they attack their targets, and therefore can quickly and effectively kill their target cells through a variety of mechanisms.(8) NK cells can release cytotoxic granules containing perforin and granzyme, leading to target cell lysis.(10) In addition, by releasing members of the tumor necrosis factor (TNF) family of molecules, NK cells upregulate death ligands on their surface, including FAS ligand and TRAIL, which can bind to death receptors on tumor cells, thus activating the caspase pathway to induce apoptosis of target cells.(11) Furthermore, upon engagement, NK cells produce interferon gamma (IFN-γ), which activates the adaptive immune response through its pleiotropic effects on other immune effector cells, such as macrophages and dendritic cells.(9) Finally, NK cells have the ability to kill cancer cells through antibody-dependent cellular cytotoxicity (ADCC) mediated by CD16, which binds to the Fc portion of IgG1 antibodies opsonized on the surface of tumor cells.(12)
Engineered killing capacity
Engineering immune cells to express a CAR redirects their specificity and focuses their killing capacity on a particular antigen. The basic structure of a CAR molecule includes an antigen recognition domain comprised of a single-chain variable fragment (scFv), an extracellular hinge domain, a transmembrane domain and an intracellular signaling domain. Incremental improvements in CAR design can be appreciated from differences in the multiple generations of these constructs. While first generation CARs consist of the basic structure with one signaling region,(13) second-generation CARs contain an additional costimulatory domain such as CD28 or 4–1BB,(14,15) and third generation CARs possess multiple costimulatory domains.(16,17) Several costimulatory domains have been investigated, including members of the immunoglobulin superfamily (CD28 and ICOS), members of the TNF receptor superfamily (4–1BB, CD27, OX40, and CD40), and others such as CD40L and TLRs.(18) Early pre-clinical studies exploring CAR-NK cells used CAR constructs optimized for T cell signaling and function. While some signaling/costimulatory domains used in CAR design such as CD3ζ and 4–1BB are shared between NK cells and T cells, the role of other costimulatory molecules such as CD28 in NK cells is less well understood.(19) This led many investigators to study costimulatory domains with greater specificity for NK cell signaling, such as DAP10, DAP12 or 2B4 (20,21) (Figure 1). DAP10 and DAP12 are adaptor molecules that contain immunoreceptor tyrosine-based activation motifs (ITAMs) and transmit activating signals to NK cells; DAP10 is critical for transmitting signals for the activating receptor NKG2D, while DAP12 mediates signaling through NKG2C, NKp44 and activating killer immunoglobulin receptors (KIRs). 2B4, another activating receptor, belongs to the signaling lymphocytic activation molecule (SLAM) family of proteins, and upon binding to its natural ligand CD48, recruits adaptor molecules such as SLAM-associated protein (SAP) through its immunoreceptor tyrosine-based switch motif (ITSM) to mediate signal transduction.(22) NK cells transduced with an anti-CD19 CAR containing either a DAP10 or CD3ζ signaling domain were shown to successfully trigger NK cell cytotoxicity,(23) but the best response was achieved when both domains were included in the CAR construct.(24) Likewise, CAR-NK cells targeting prostate stem cell antigen (PSCA) displayed enhanced cytotoxicity when DAP12 was incorporated into the CAR construct, compared to results when CD3ζ alone was used alone.(25) Other studies reported superior in vitro and in vivo activity of NK cells expressing 2B4 containing CAR constructs targeting mesothelin(26) or CD5(27) in the relevant tumor models.
Figure 1.
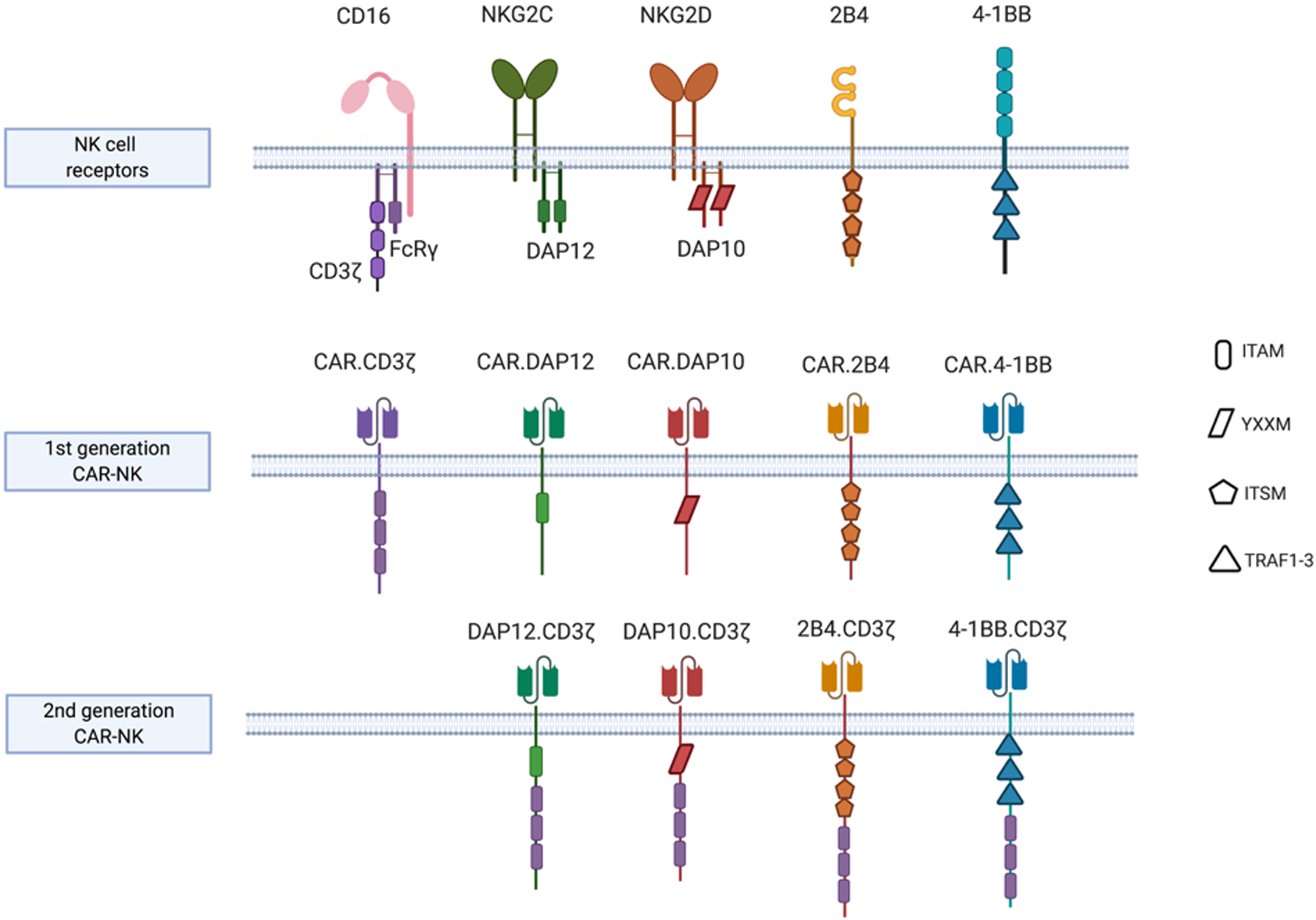
NK cell specific signaling domains incorporated within CAR intracellular domains
Image created in BioRender.com
Abbreviations: DAP12, DNAX Activating Protein of 12KDa; DAP10, DNAX Activating Protein of 12KDa; ITAM, immunoreceptor tyrosine-based activation motif; ITSM, immunoreceptor tyrosine-based switch motif; YXXM, Y stands for tyrosine, X for any amino acid residue, and M for methionine; TRAF1–3, tumor necrosis factor receptor-associated factors 1, 2 and 3.
More recently, fourth-generation CARs, also known as ‘armored CARs’ or TRUCKs (T-cells redirected for Universal Cytokine Killing), have been investigated.(28) These constructs incorporate a transgenic “payload” designed to improve the proliferation, persistence and anti-tumor activity of CAR engineered NK cells. Results published to date support the superior activity of these latest revisions to CAR design, with the promise of broadening the role of CAR-engineered NK cells within the available repertoire of cancer immunotherapies.(29,30)
SAFETY FEATURES OF CAR-NK CELLS
Intrinsic features
NK cell killing is specific for transformed or otherwise abnormal cells in the body. To recognize and spare healthy cells, NK cells rely primarily on inhibitory receptors (such as killer cell immunoglobulin-like receptors [KIRs] and NKG2A) that bind to major histocompatibility complex (MHC) class I or class-I like molecules that are constitutively present on normal cells and act as a safety switch to inhibit NK cell cytotoxicity.(9,31) As opposed to T cells, NK cells do not induce graft-versus-host disease (GVHD), opening the way for their broad application in the allogeneic settings and for the generation of off-the-shelf cellular therapy products.(3) More recently, NK cells were also demonstrated to have an important edge over T cells as platforms for CAR engineering, as they lacked any evidence of serious toxicities, including CRS or ICANS.(29)
Engineered features
Suicide genes have been investigated as safety switches mainly in long-lived genetically modified effectors, such as CAR-T cells. NK cells, by contrast, are typically short-lived and safe, raising questions as to the need for a suicide switch in adoptive NK cell therapy.(32) Use of inducible caspase 9 (iCas9), activated by a chemical inducer of dimerization (CID),(33) has proven effective at eliminating CAR-NK cells both in vitro and in vivo in preclinical studies.(30,34) In the first-in-human clinical trial of CAR-NK cells for the treatment of relapsed/refractory B-cell lymphoid malignancies, our group tested cord blood derived NK cells transduced with a retroviral vector encoding a single chain variable fragment (scFv) against CD19, IL-15 to enhance proliferation and in vivo persistence and iCas9 as a cell suicide switch. Given further advances in construct design, non-viral methods of cell engineering and gene editing technologies, it is likely that future generations of CAR-T and CAR-NK cells will induce fewer toxicities, eliminating the need for suicide genes or other types of safety switches; nonetheless, it is possible that fourth-generation CARs could cause unanticipated toxicity due to excessive cytokine production, supporting incorporation of a suicide gene in certain cases, depending on the construct and the cytokine transgene.
CHALLENGES FACING CAR-NK CELL THERAPY
Limited persistence
One of the major limitations of adoptive NK cell therapy is the lack of in vivo persistence of the infused cells in the absence of cytokine support. While this feature might be desirable from a safety standpoint, it may well limit the efficacy of the NK cell immunotherapy. The administration of exogenous cytokines has been shown to enhance the proliferation and persistence of adoptively infused NK cells; however, it can also induce undesirable toxicities(35) as well as the expansion of other immune subsets that might be immunosuppressive, such as regulatory T cells (Tregs).(36) Hence, several groups have reported encouraging results by engineering NK cells with transgenes encoding for cytokines that are either expressed on the membrane or secreted constitutively. In one study, NK-92 cell lines or primary NK cells transduced with retroviral vectors expressing IL-2 or IL-15 showed enhanced proliferation and persistence in tumor-bearing mice.(37) Our group has shown that incorporating the IL-15 transgene into the CAR construct enhances NK cell proliferation and in vivo persistence with improved anti-tumor activity, without increasing systemic levels of IL-15 or toxicity in patients with high-risk lymphoid malignancies.(29,30) Other armored CAR-NK cells engineered with cytokine transgenes are under development, but published reports are not yet available. Another strategy to improve the persistence of NK cells is to endow them with a memory-like phenotype, for example by briefly preactivating them with a cytokine cocktail (IL-12, IL-15 and IL-18) to induce differentiation into cytokine-induced memory-like NK cells.(38,39) Recently these memory-like NK cells were engineered to express a CAR directed against CD19 and showed enhanced responses against NK resistant B-cell lymphoma in vitro and in vivo.(40)
Trafficking to tumor sites
Rapid homing to tumor beds is critical for the efficacy of adoptive cellular therapy and is regulated by complex interactions between chemokines secreted by NK cells with those secreted by tumor cells.(41) Controversy over the effectiveness of NK cell homing to tumor sites has stimulated efforts to improve this property.(42) For example, transfer of the chemokine receptor CCR7 from K562 feeder cells to NK cells via trogocytosis resulted in enhanced homing of NK cells to lymph nodes.(43) Similarly, upregulation of CXCR3 on NK cells following ex vivo expansion with irradiated EBV-LCL feeder cells and IL-2 resulted in enhanced trafficking and improved anti-tumor activity in a xenograft mouse model of CXCL10-transfected melanoma.(44) Since then, several groups have explored different engineering strategies to improve NK cell homing. For example, to improve migration of NK cells towards lymph nodes that express the chemokine CCL19, NK cells were electroporated with mRNA coding for the chemokine receptor CCR7.(45) Similarly, NK cells transduced with a CXCR2-encoding viral vector had improved migration to renal cell carcinoma tumors expressing cognate ligands such as CXCL1, CXCL2, CXCL5, CXCL6 and CXCL8.(46) Another strategy applied an NK cell–recruiting protein-conjugated antibody (NRP-body) that incorporates a cleavable CXCL16 molecule to increase trafficking and infiltration of NK cells into pancreatic tumors.(47) This chemokine is cleaved by furin, an endoprotease expressed on the surface of pancreatic cancer cells, leading to induction of NK cell infiltration through RhoA activation via the ERK signaling cascade. This approach was shown to enhance NK cell mediated tumor control in a mouse model of pancreatic cancer.(47) CAR-NK cells are also being engineered to enhance their trafficking to sites of tumor. Müller et al, for example, showed that anti-EGFRvIII CAR-NK cells engineered to express CXCR4 conferred specific chemotaxis to CXCL12/SDF-1α secreting glioblastoma cells, leading to improved tumor regression and survival in a mouse model of glioblastoma.(48) Finally, NKG2D CAR-NK cells engineered to express CXCR1 significantly increased anti-tumor responses in mice bearing established peritoneal ovarian cancer xenografts.(49) Thus, a number of innovative strategies to increase the trafficking of NK cells to the tumor sites have been tested in mouse models; the efficacy of these approaches to improve the success of NK cell immunotherapy against solid tumors in patients will need to be validated in clinical trials.
The immunosuppressive tumor microenvironment
The tumor microenvironment poses major obstacles to successful CAR-NK cell therapy, including immunosuppressive soluble substances, immunosuppressive cells and an unfavorable milieu for adequate immune cell function. With regard to soluble factors, the tumor microenvironment is rich in immunosuppressive cytokines and metabolites, such as transforming growth factor β (TGF-β), adenosine, indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2), all of which can adversely affect NK cell function.(50) Certain types of cells present in this malignant milieu, including regulatory T cells, regulatory B cells, myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), platelets and fibroblasts also induce immunosuppression,(51) as do a number of unfavorable metabolic factors, including hypoxia, acidity, and nutrient deprivation.(52) Thus, efforts are under way to engineer CAR-NK cells so that they can circumvent some of these immunosuppressive factors. One promising approach has been to engineer NK cells to render them resistant to the action of TGF-β. Our group used CRISPR-Cas9 technology to delete the TGF-β receptor 2 gene (TGFβR2) in primary human NK cells, which rendered them resistant to this immunosuppressive growth factor without loss of their effector activity against acute myeloid leukemia (AML).(53) Similarly, NK cells modified to express a dominant-negative TGF-β receptor, a high-affinity non-signal transducing receptor derived from TGFβR2, antagonized the suppressive effects of TGF-β on NK cells and restored their cytotoxicity.(54) Another strategy, using an anti-miR against miR-27a-5p, a micro-RNA that is upregulated by TGF-β in NK cells, (55) increased NK cell effector function both in vitro and in vivo.(55) Finally, adenosine, an important immunosuppressive metabolite generated from ATP by the ectonucleotidases CD73 and CD39 in response to hypoxia and stress,(56) has been targeted by blocking the high affinity A2A adenosine receptor on NK cells, resulting in more potent antitumor activity in mouse models of breast cancer, melanoma and fibrosarcoma.(57,58)
Checkpoint molecule engagement is another key mechanism by which the tumor microenvironment induces NK cell dysfunction.(59) Gene editing technologies are therefore being used to delete checkpoint molecules within NK cells as a means to enhance their function. For instance, TIGIT knockout was shown to protect against NK cell exhaustion and improve outcome in tumor-bearing mice.(60) Others have targeted NKG2A and reported enhanced cytotoxicity of NKG2Anull NK cells against HLA-E expressing tumors.(61,62) Work by our group and others suggests that an effective approach to enhancing NK cell anti-tumor activity would be to combine CAR engineering with checkpoint deletion (by targeting CIS, a negative regulator of cytokine signaling).(63–66) Other inhibitory molecules under investigation in NK cells include the classical T-cell checkpoints PD-1 and CTLA-4.(67,68) The application of checkpoint blockade to enhance NK cell effector function is reviewed in depth by Vivier at al in this issue of the journal.(69)
The studies discussed above all indicate that the biological limitations of NK cells and the challenges imposed by the tumor microenvironment can be circumvented by innovative engineering techniques and gene editing technologies. Strategies to enhance NK cell persistence, trafficking to tumor sites, and effector function in a hostile and malignant milieu are likely to transform adoptive therapy with NK cells from a safe treatment with only modest efficacy to a serious contender for a frontline role in cancer immunotherapy.
EVOLUTION OF CAR-NK CELL THERAPY
A variety of different cell sources have been used to generate CAR-expressing NK cells. These include peripheral blood, cord blood, stem cells including hematopoietic or induced pluripotent stem cells and NK cell lines, each with their unique advantages and disadvantages (Table 1).
Table 1.
Sources of CAR-NK cells under investigation in the clinic.
| Cell source | Advantages | Disadvantages | Potential for OTS |
|---|---|---|---|
| Peripheral blood |
|
|
|
| Cord blood |
|
|
|
| iPSC |
|
|
|
| NK cell line (NK-92) |
|
|
|
Abbreviations: CB, cord blood; iPSC, induced pluripotent stem cells; OTS, off-the-shelf; ADCC, antibody dependednt cellular cytotoxicity.
Strategies to combat hematologic malignancies
As with CAR-modified T lymphocytes, CAR-NK cells were first tested against hematologic malignancies, with preclinical data confirming their activity against leukemia, lymphoma and myeloma. To treat B-cell malignancies, we engineered NK cells from cord blood using a retroviral vector that expressed a fourth generation CAR vector (iC9.CAR19.CD28-ζ-IL-15)(70) targeting the CD19 antigen and incorporating the IL-15 transgene to enhance proliferation and the inducible caspase 9 transgene (iC9) to provide a safety switch.(30) The resultant CAR-NK cells showed significantly enhanced persistence and antitumor efficacy in a xenograft mouse model of Raji lymphoma, compared to non-transduced NK cells.(30) Other groups engineered NK-92 cell lines with an anti-CD19 CAR construct that incorporated a CD3ζ signaling endodomain either alone or with a costimulatory domain (CD28 or CD137), and found that they displayed effective anti-tumor activity against B-cell malignancies in vitro and in vivo in a Raji lymphoma mouse model.(71,72)
In addition, CAR-NK cells could offer an advantage over CAR-T cells in the treatment of T-lymphoid cancers because they circumvent the issue of fratricide arising from shared antigens between normal and malignant T cells. Indeed, NK-92 or NK-92MI (engineered to express IL-2)(73) cells transduced with a lentiviral vector encoding a second or third generation CAR targeting CD5 and a CD3ζ signaling domain containing a CD28, 4–1BB or 2B4 costimulatory domain produced effective in vitro cytotoxicity against patient-derived T-cell malignancies and improved survival in a xenograft mouse model of Jurkat lymphoma.(74,75) Similarly, NK-92MI cells transduced with anti-CD7 CARs, designed using nanobody technology, had potent in vitro and in vivo activity against T-cell leukemia.(76)
Unfortunately, despite the remarkable activity against lymphoid malignancies, CAR-engineered T-cells have been much less effective in AML.(77) These disappointing results can be attributed in part to shared expression of antigen (e.g., CD123) on AML blasts and normal HSCs, and heterogeneous expression of target antigen (e.g., CD33) on blasts.(78) Fortuitously, AML blasts are susceptible to NK cell mediated killing since they express ligands recognized by activating receptors on NK cells.(79) Hence, CAR-NK cells may possess features that could overcome some of the obstacles related to antigen escape and heterogeneous gene expression that have thwarted efforts to improve the outcome of AML immunotherapy. To meet this challenge, our group and others have demonstrated the anti-leukemic activity of third (CD28.BBζ) or fourth generation (CD28ζ-IL-15) CAR-NK cells targeting CD123 both in vitro and in vivo. (80,81) Others targeted CD4, a T cell marker that can also be expressed by a subset of AML blasts, using third generation CAR-NK cells (CD28.BBζ), leading to potent anti-leukemic activity both in vitro and in a xenograft mouse model of CD4+ AML.(82) It is important to note that AML stem cells have also evolved mechansims to evade NK cell recognition, such as downregulation of NKG2D ligands, which may in turn limit the efficacy of CAR-NK cell therapy.(83)
Multiple myeloma may also be especially sensitive to CAR-NK cell therapy, as myeloma cells express multiple ligands for NK receptors.(84) In fact, NK-92 cells transduced with a lentiviral vector encoding a first generation anti-CD138 CAR or a second generation anti-CS1 CAR.CD28.ζ exerted effective in vitro and in vivo activity against myeloma.(85,86)
In summary, the intrinsically favorable biologic features of NK cells and the added specificity afforded by CAR expression, leading to potent in vitro and in vivo activity against various hematologic cancer models provided the impetus to initiate phase 1 and 2 clinical trials of this therapy and to undertake exploratory studies of CAR NK cells against solid tumors.
Strategies to combat solid tumors
In contrast to liquid tumors, solid tumors and their supporting stroma possess the means to evade all but the most innovative immunotherapies. These tactics include the secretion of inhibitory cytokines (e.g., TGF-β) by tumors cells or by cells in the immediate microenvironment (or both), extreme cellular heterogeneity with an abundance of resistant stem-like progenitor cells, and the downregulation of cognate ligands that can stimulate a response from immune effectors such as NK cells. Thus, Kruschinski et al. engineered HER-2-specific CAR-NK cells (HER-2-CD3ζ-CD28) that mediated effective in vitro and in vivo activity in a mouse model of ovarian cancer.(87) To overcome the deficient trafficking of NK cells to the tumor site,(87) Han et al administered EGFR-redirected CAR-NK cells intracranially and confirmed their safety and efficacy in a mouse model of glioblastoma.(88) In efforts to enhance the potency of NK cells and strengthen their CAR-specific signaling, Li et al. devised an anti-mesothelin CAR containing the CD3ζ signaling domain and the 2B4 co-stimulatory domain, together with the transmembrane domain of the activating NK cell receptor NKG2D. When transduced with this construct, iPSC-derived NK cells mediated strong antitumor effects in an ovarian cancer xenograft model.(89) These successes have not been matched by other investigators using different preclinical solid tumor models. For instance, while NK cells expressing a second- or third-generation GD2-directed CAR (GD2-t2B4ζ, GD2-BBζ or GD2-t2B4.BBζ) could kill several GD2+ cancer cell lines in vitro, they failed to control tumors in a xenograft mouse model.(90) This suboptimal activity was subsequently linked to the upregulation of HLA-G (the ligand for the inhibitory KIR2DL4) in studies with Ewing sarcoma cells, leading to NK cell inhibition and immune escape.(90,91)
One of the major obstacles to reliable preclinical evaluation of CAR therapy for solid cancers is the lack of clinically relevant animal models that recapitulate the complexity of the interactions within the tumor microenvironment. Most studies have relied on transplanted xenografts derived from human tumor cell lines in immune-compromised NOD scid gamma null (NSG) mice that lack a competent immune system. While the extant NSG models are useful for rapid evaluation of CAR effector function and persistence, they fail to establish a clinically relevant tumor microenvironment that would yield accurate estimate of CAR-NK cell function and persistence and allow the exploration of the cross-talk among the different immune cells and the tumor.
Clinical evaluation of CAR NK cells (Table 2)
Table 2.
Clinical trials of CAR engineered immune effector cells
| CAR vehicle NCT identifier | Clinical trial phase | Cancer type | Antigen target | Cell source | Construct/Method | Dose | Status | Location |
|---|---|---|---|---|---|---|---|---|
| NK | ||||||||
| NCT03692767 | Early phase 1 | Refractory B-cell lymphoma | CD22 | unknown | unknown | 50–600 × 103/kg | Not yet recruiting | unknown |
| NCT03690310 | Early phase 1 | Refractory B-cell lymphoma | CD19 | unknown | unknown | 50–600 × 103/kg | Not yet recruiting | unknown |
| NCT03824964 | Early phase 1 | Refractory B-cell lymphoma | CD19/CD22 | unknown | unknown | 50–600 ×103/kg | Not yet recruiting | unknown |
| NCT02892695 | Phase 1/2 | ALL, CLL, follicular lymphoma, mantle cell lymphoma, B-PLL, DLBCL | CD19 | NK-92 cell line | CAR.CD19-CD28–41BB- CD3ζ | unknown | Unknown | China |
| NCT03056339 | Phase 1/2 | ALL, CLL, NHL | CD19 | Cord blood | CAR.CD19-CD28- CD3ζ .iCasp9-IL15 | 3 dose levels: 105/kg 106/kg 107/kg |
Phase I portion completed. Phase 2 recruiting | MD Anderson Cancer Center, Houston, TX USA |
| NCT01974479 | Phase 1 | B-ALL | CD19 | Haploidentical donor | CAR.19–41BB-CD3ζ | 0.5 – 5 × 107/kg, and up to 1 × 108/Kg | Suspended for interim review | Singapore |
| NCT00995137 | Phase 1 | B-ALL | CD19 | Haploidentical donor | CAR.19–41BB-CD3ζ | unknown | Completed | St Jude Children’s Research Hospital, Memphis, TN USA |
| NCT04245722 | Phase 1 | B-cell lymphoma, CLL | CD19 +/− CD20 antibody (Rituximab or obinutuzumab) | iPSC derived NK cells | CAR.19-NKG2D-2B4-CD3ζ-IL15RF-hnCD16 | Dose escalation, exact doses unknown | Recruiting | University of Minnesota Masonic Cancer Center, Minnesota USA |
| NCT02742727 | Phase 1/2 | AML, preT-ALL, T-PLL, T-cell LGL, PTCL, angioimmunoblastic T-cell lymphoma, extranodal NK/T-cell lymphoma (nasal type), enteropathy-type intestinal T-cell lymphoma, hepatosplenic T-cell lymphoma | CD7 | NK-92 cell line | CAR.CD7-CD28–41BB-CD3ζ | unknown | Unknown | China |
| NCT03940833 | Phase 1/2 | Multiple myeloma | BCMA | NK-92 cell line | unknown | unknown | Recruiting | China |
| NCT02944162 | Phase 1/2 | AML | CD33 | NK-92 cell line | CAR.CD33-CD28–41BB-CD3ζ | unknown | Unknown | China |
| NCT02839954 | Phase 1/2 | Hepatocellular carcinoma, non-small cell lung cancer, pancreatic carcinoma, triple-negative invasive, breast carcinoma, glioblastoma, colorectal carcinoma, gastric carcinoma | MUC1 | unknown | unknown | unknown | Unknown | China |
| NCT03692663 | Early phase 1 | Castration-resistant prostate cancer | PSMA | unknown | unknown | 0.5–3 × 106/kg | Not yet recruiting | unknown |
| NCT03692637 | Early phase 1 | Epithelial ovarian cancer | Mesothelin | unknown | unknown | 0.5–3 × 106/kg | Not yet recruiting | unknown |
| NCT03415100 | Phase 1 | Solid tumors | NKG2D ligands | Autologous or allogeneic NK | mRNA electroporation | unknown | Recruiting | China |
| NCT03940820 | Phase 1/2 | Solid tumors | ROBO1 | unknown | unknown | Recruiting | China | |
| NCT03941457 | Phase 1/2 | Pancreatic cancer | ROBO1 | unknown | unknown | unknown | Recruiting | China |
| NCT03383978 | Phase 1 | Glioblastoma | HER2 | NK-92 | CAR.HER2. CD28.CD3ζ | 1×107–1×108 intracranial infusion | Recruiting | Germany |
| iNKT cells | ||||||||
| NCT03774654 | Phase 1 | Relapsed/refractory B-cell malignancies | CD19 | Allogeneic NKT cells | CAR.19-CD28-CD3ζ-IL15 | 4 dose levels: 1×107/m2 3×107/m2 1×108/m2 3×108/m2 |
Not yet recruiting | Baylor-Methodist-Texas Children’s, USA |
| NCT03294954 | Phase 1 | Relapsed/refractory neuroblastoma | GD2 | Autologous NKT cells | CAR.GD2-CD28-CD3ζ-IL15 | 4 dose levels: 3×106/m2 1×107/m2 3×107/m2 1×108/m2 |
Recruiting | Baylor-Methodist-Texas Children’s, USA |
| γδ T cells | ||||||||
| NCT02656147 | Phase 1 | B-cell leukemia and lymphoma | CD19 | Allogeneic γδ T cells | unknown | unknown | Not yet recruiting | China |
| NCT04107142 | Phase 1 | Solid tumors | NKG2D ligands | Haploidenticalor allogeneic γδ T cells | unknown | 3 × 108– 3 × 109 cells | Not yet recruiting | Malaysia |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin lymphoma; T-LGL, T-cell large granular leukemia; PLL, prolymphocytic leukemia; PTCL, prolymphocytic T cell leukemia; BCMA, B-cell maturation antigen; DLBCL, diffuse large B cell lymphoma; PSMA, prostate specific membrane antigen; hnCD16, high-affinity, non-cleavable CD16; IL15RF, IL-15 receptor alpha
Despite growing experimental evidence to support the use of anti-CD19 CAR NK cells in patients with B-cell malignancies, this concept was not tested in the clinic until recently. Using HLA-mismatched NK cells from cord blood, our group led a first-in-human phase 1 and 2 clinical trial of CAR-NK cell therapy in patients with relapsed/refractory B-cell hematologic malignancies (ClinicalTrials.gov number, NCT03056339). The NK cells were transduced with a retroviral vector encoding (i) a CAR against the CD19 antigen, (ii) IL-15 to enhance NK cell persistence and function, and (iii) inducible caspase 9 (iCas9) as suicide gene, safety switch.(29) Of the 11 heavily pre-treated patients with NHL or CLL, 8 responded for an overall response rate of 73%, with 7 achieving a complete remission (64%). These responses were rapid and seen at all dose levels (1 × 105 to 1 × 107 per kg). Response durations could not be reliably assessed because patients received other post-remission therapies. Importantly, serious toxicities, including CRS, ICANS or GVHD, did not develop in any of the patients, obviating the need to activate the safety switch in the small group of patients treated to date on this trial.(29) In summary, the interim results of this trial show that CAR-NK cells can induce complete responses in patients with high risk CD19+ cancers with relatively few adverse side-effects. Larger multicenter studies are planned to validate the safety and efficacy of this approach in lymphoid malignancies and to ascertain the long-term durability of the response in the absence of maintenance or consolidation therapy. Our group is also working to use this platform to target antigens beyond CD19.
Data on the use of CAR-NK cell therapy to target other hematologic and solid malignancies are limited. In a phase I study of NK-92 cells transduced with a third-generation CAR targeting CD33 with CD28 and 4–1BB co-stimulatory domains, Tang et al treated 3 patients with relapsed or refractory AML(92). The investigators report the safety of this approach but no durable responses. As pointed out by the authors, one of the disadvantages of using NK-92 cells for cellular therapy is the need for irradiating this cancer-derived cell line, which may have affected the persistence and efficacy of the infused product.(92) Among the nearly 20 clinical trials now evaluating CAR-NK cells as therapy for a diverse set of cancer (Table 2), 8 are targeting CD19 or CD22 (B-cell malignancies), 1 BCMA (multiple myeloma; NCT03940833), 1 CD33 (AML; NCT02944162), 1 CD7 (T-cell leukemia and lymphoma; NCT02742727), 1 HER-2 (glioblastoma; NCT03383978), 1 targeting prostate specific membrane antigen (PSMA) (prostate cancer; NCT03692663), 1 mesothelin (ovarian cancer; NCT03692637), 1 MUC1 (multiple solid tumors; NCT02839954); 1 NKG2D (multiple solid tumors; NCT03415100) and 2 ROBO1 (1 for multiple solid tumors; NCT03940820 and 1 for pancreatic cancer; NCT03941457). With few exceptions, these trials are testing strategies that target single antigens only. It will be interesting to learn if the risk of immune escape with CAR-NK cells will be lower than with CAR-T cells owing to the innate ability of NK cells to recognize tumors through their germline-encoded receptors.
ALTERNATIVE CELL TYPES FOR CAR EXPRESSION
Just as NK cells have emerged as promising candidates for CAR expression, other cell populations possess specific advantages that may augment the current repertoire of CAR-based therapies (Figure 2). Indeed, a number of early-phase clinical trials are anticipated or underway to investigate these new immune effectors (Table 2).
Figure 2.

Track record of alternative CAR based therapies
Image created in BioRender.com
iNKT cells
Invariant NKT cells (iNKT) represent a small subset of specialized T cells that possess unique features of both innate and adaptive immune cells. They can mount a rapid response to antigenic exposure much like innate immune cells, but can also display precise antigen recognition in the manner of adaptive cells.(93) Similar to conventional T cells, iNKT cells undergo thymic development and selection and harbor a T cell receptor (TCR) to recognize antigens; however, unlike conventional T cells, their TCR recognizes lipid antigens presented by CD1d, a monomorphic MHC class 1 like molecule.(93) In addition to TCR stimulation, iNKT cells can be activated through a variety of cytokine receptors (e.g. IL-12, IL-18, IL-23 and IL-25) similar to innate immune cells such as NK cells and innate lymphoid cells (ILCs).(94) Hence, in the tumor microenvironment, upon sensing a breach in tissue integrity via recognition of endogenous lipids, iNKT cells become activated and can mediate effective antitumor immunity via direct tumor lysis or indirectly through secretion of an array of cytokines that can modulate other immune cells in the malignant milieu, thus aiding in the host immune response.(95) Another advantage of iNKT cells over conventional T cells is the fact that they are not associated with GVHD,(96) making them an attractive option as allogeneic off-the-shelf cell therapy product.
iNKT cells may also be useful platforms for CAR engineering.(97,98) Heczey et al demonstrated that when transduced with an anti-GD2 CAR, especially one with a third generation CD28–41BBζ containing endodomain, iNKT cells displayed potent anti-tumor activity in a metastatic neuroblastoma mouse model, without evidence of GVHD.(98) Given a hallmark of iNKT cells is their production of both Th1 (inflammatory) and Th2 (anti-inflammatory) cytokines, the authors showed that a CD3ζ only or a CD28-CD3ζ endodomain skewed the CAR-iNKT cell towards Th2 production, whereas 41BB-CD3ζ or CD28–41BB-CD3ζ polarized the iNKT cells towards a Th1 cytokine profile.(98) The same group also demonstrated that co-expression of IL-15 with an anti-GD2 CAR (with CD28 or 4–1BB co-stimulatory domain) enhances iNKT cell persistence and anti-tumor activity in a neuroblastoma xenograft mouse model.(99) These results led to an ongoing phase 1 clinical trial to evaluate iNKT cells transduced with a vector expressing a GD2-specific CAR and IL-15 in children with neuroblastoma (NCT03294954). Early results are encouraging with one of two patients treated to date reported to have achieved tumor regression without toxicity.(100) The same approach is now being applied in a phase 1 clinical trial of CAR19-CD28- CD3ζ-IL-15 transduced iNKT cells for lymphoid malignancies (NCT03774654), expected to enroll patients soon.
γδ T cells
γδ T cells link innate and adaptive immune responses. They represent 1–5% of all circulating T cells, but are the predominant lymphocyte at epithelial surfaces.(101) Contrary to αβ TCR activation, which requires MHC-bound peptides, γδ TCRs are triggered in an MHC-independent fashion.(102) For instance, γ9δ2 T cells that are highly prevalent in the adult peripheral blood are activated by aminobisphosphonates, such as the cholesterol precursor isopentylpyrophosphate.(101) Zoledronic acid (ZOL) interferes with cholesterol synthesis, resulting in accumulation of isopentylpyrophosphate, and therefore can be incorporated into expansion protocols to generate large numbers of these γδ T cells from PBMCs.(103) Given that this approach may lead to the preferential amplification of γ9δ2+ T cells, several groups have developed alternative protocols to allow for expansion of multiple γδ T cell subtypes concomitantly. Some of the reported strategies include expansion with the help of feeder cells such as K562 cells engineered to express costimulatory ligands and membrane-bound IL-15, the use of an anti-CD2 monoclonal antibody, or the T cell mitogen concanavalin A (ConA).(104) Collectively, these protocols enable the production of large numbers of γδ T cells needed for an effective adoptive T-cell therapy.
The unique features described above put γδ T cells in the spotlight as potential platforms for CAR engineering. In the first published report, investigators expanded γ9δ2 T cells from peripheral blood using ZOL, transduced them with a retroviral vector encoding an anti-GD2 or anti-CD19 first-generation CAR, and showed an enhanced anti-tumor response against Ewing sarcoma cells and B cell (CD19+) tumor cell lines in vitro.(105) Another group showed that CAR-transduced Vδ2 T-cells can retain the ability to cross-present the processed peptides from uptaken tumor antigens to responder αβ T cells.(106) Another strategy, designed by Deniger et al., relied on PBMCs electroporated with a sleeping beauty transposon/transposase system to express a second-generation anti-CD19 CAR with a CD28-CD3ζ endodomain; CAR+ γδ T cells were sort-purified and propagated using K562-based feeder cells.(107) The authors showed that these anti-CD19 CAR-transduced γδ T cells can improve tumor control in a CD19+ leukemia (NALM6) xenograft mouse model.(107)
Two phase I clinical studies of γδ T cells are anticipated. One will evaluate the safety and efficacy of allogeneic anti-CD19 CAR γδ T cells for the treatment of B-cell hematologic malignancies (NCT02656147), while the other will test escalating doses of haploidentical or allogeneic CAR-transduced γδ T cells targeting the natural killer group 2D ligand (NKG2DL) in patients with relapsed or refractory solid tumors (NCT04107142).
Macrophages
Macrophages (Greek for ‘big eaters’) are specialized innate immune cells responsible for detecting and engulfing cellular debris, pathogens and cancer cells.(108) Unlike T cells and other immune effectors, macrophages have the advantage of being able to readily penetrate solid tumors(109) in response to tumor-derived chemokines such as colony-stimulating factor-1 (CSF-1), CCL2, CCL3, CCL4, CCL5, CCL8 and vascular endothelial growth factor (VEGF).(110) Recent preclinical data demonstrate that macrophages can be engineered with a CAR (CAR-Ms) incorporating a CD3ζ intracellular domain to direct their phagocytic activity specifically against tumors.(111,112) The authors of this study used an adenovirus vector (Ad5f35) to transduce macrophages with a CAR and showed that this strategy polarized the macrophages into the M1 immunostimulatory phenotype without reversion to the M2 immunosuppressive phenotype. In a xenograft mouse model of HER2+ ovarian cancer (SKOV3), mice treated with CAR-Ms targeting HER2 had better tumor control and improved survival compared to untreated mice or mice treated with unmodified control macrophages.(111,112) Another group showed that engineering macrophages with a CAR-P (P, phagocytosis) by using intracellular domains from engulfment receptors (Megf10 and FcRɣ) could induce specific engulfment of tumors expressing the antigen to which the scFv is targeted.(113) These investigators found that adding a tandem PI3K recruitment domain to the CAR-P intracellular signaling enhanced tumor engulfment further. This directed phagocytosis led to better tumor control in vitro than that induced by unmodified macrophages.(113) Preclinical animal data are needed to further test the in vivo antitumor activity of these CAR-P macrophages. Still others, using lentiviral or adenoviral vectors, engineered monocyte-derived human macrophages with a CAR construct (MOTO-CAR) that contains a Toll/IL-1 receptor (TIR) signaling domain, demonstrating enhanced phagocytosis (compared to the control) against TK1 expressing non-small cell carcinoma cell lines.(114) These encouraging preclinical data suggest that CAR-engineered macrophages have the potential to become useful immunotherapeutic agents, especially in the setting of typically resistant solid tumors.
Dendritic cells
Dendritic cells (DCs) are professional antigen presenting cells that efficiently process antigens and present them on their surface to activate the adaptive immune system.(115) They also possess the unique ability to interact with multiple immune cell subsets and regulate their function, including CD4 and CD8 T cells in lymph nodes, resulting in downstream B cell activation and antibody secretion, as well as activation of NK cells and phagocytes.(116) Because of these unique features, DCs have been used in multiple clinical trials as therapeutic vaccines in the setting of various solid tumors (e.g. prostate cancer, melanoma, glioblastoma multiforme).(117) This clinical experience using DCs as cancer vaccines established their safety as immunotherapeutic agents with some evidence of efficacy. More recently, to demonstrate the feasibility of CAR engineering of DCs, a group of investigators differentiated DCs in vitro using a combination of Flt3-ligand, GM-CSF and IL-4 and then transduced the cells with a lentiviral construct containing a 4–1BB signaling domain.(118) This work demonstrated that DCs transduced with a 4–1BB CAR directed against CD33 have higher frequencies of CD141+/Cleg9a+ cells, which play an important role in induction of intra-tumoral T-cell cytotoxicity, compared to control DCs. The authors of this study demonstrated that combining CAR-DC with CAR-T cells can enhance CAR-T cell function and cytotoxicity, leading to improved tumor control in a xenograft mouse model of AML, compared to results with CAR-T cells alone. These findings, if validated in future studies, could support the addition of CAR-DCs to the armamentarium of next-generation adoptive cellular therapies.
THE NEXT FRONTIER: OFF-THE-SHELF CELLULAR THERAPY FOR CANCER PATIENTS
Since allogeneic NK cells do not cause GVHD, they offer the opportunity for off-the-shelf therapy with potential advantages, such as large-scale manufacturing and the production of multiple doses from a single donor, thus, making the therapy cheaper and immediately available for use. However, in contrast to T cells and many other human cells, NK cells are particularly susceptible to cryo-injury, with impairment in their effector function post-thaw.(119) Thus, before the promise of “on demand” off-the-shelf CAR-NK cell therapy can be fulfilled, the development of optimized cryopreservation protocols for efficient freezing and banking is critical. Factors that could influence cryopreservation outcomes include the metabolic state of the cells, cell culture density, cytokine-driven vs. non-stimulated cultures and cell age, to name a few. While to date, progress in the cryopreservation of NK cells has been modest, recent advancements in the basic science of cryobiology and its application, the development of novel cryoprotectants and optimization of GMP protocols for rate-controlled cooling and thawing will likely ensure efficient manufacturing and banking of these cellular therapeutics for off-the-shelf therapy.
CONCLUSIONS AND FUTURE PROSPECTS
The cellular therapy field has undergone a shift in its guiding paradigm since the advent of CAR engineering. As the first example of CAR-engineered immune effector cells to yield promising results in the clinic, CAR T cells have set the pace for future developments in CAR-based immunotherapy. Despite this success, CAR-T cells possess distinct shortcomings that have catalyzed studies of other immune effector cells, both in the innate and adaptive compartments, as alternative platforms for CAR engineering. Although definitive clinical trials of these new strategies are still under way, the results to date carry the promise of an improved safety profile without loss of efficacy and the feasibility of an off-the-shelf CAR-engineered therapeutic product. While a number of immune cell subsets have properties and activities that clearly support their candidacy as novel platforms for CAR expression, NK cells appear especially attractive given the strength of their intrinsic antitumor lytic activity and their relative lack of toxic side-effects. In the near future, we are likely to see a plethora of strategies combining CAR engineering with other approaches, such as checkpoint blockade or gene editing technologies, to further enhance the function of immune effector cells, especially in solid tumor microenvironments that are fraught with obstacles to successful immunotherapy.
SIGNIFICANCE.
CAR engineering can redirect the specificity of immune effector cells, converting them to a much more potent weapon to combat cancer cells. Expanding this strategy to immune effectors beyond conventional T lymphocytes could overcome some of the limitations of CAR-T cells, paving the way for safer and more effective off-the-shelf cellular therapy products.
FUNDING
Supported by the generous philanthropic support of MD Anderson Cancer Center Moonshot program, by grants from CPRIT (RP160693), the Leukemia Lymphoma Society (6555-18), by a Stand Up To Cancer Dream Team Research Grant (Grant Number: SU2C-AACR-DT-29-19), by grants (1 R01 CA211044-01, 5 P01CA148600-03, and P50CA100632-16) from the National Institutes of Health (NIH), and by a grant (CA016672) to the MD Anderson Cancer Center from the NIH. The SU2C research grant is administered by the American Association for Cancer Research, the scientific partner of SU2C.
Footnotes
CONFLICT OF INTEREST
K.R., M.D. and The University of Texas MD Anderson Cancer Center (MDACC) have an institutional financial conflict of interest with Takeda Pharmaceutical for the licensing of the technology related to the research mentioned here. MD Anderson has implemented an Institutional Conflict of Interest Management and Monitoring Plan to manage and monitor the conflict of interest with respect to MDACC’s conduct of any other ongoing or future research related to this relationship.
References
- 1.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018;359(6382):1361–5 doi 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 2.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev 2019;34:45–55 doi 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daher M, Rezvani K. Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr Opin Immunol 2018;51:146–53 doi 10.1016/j.coi.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin Cancer Res 2017;23(9):2255–66 doi 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez M, Moon EK. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol 2019;10:128 doi 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol 2018;9:1869 doi 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001;22(11):633–40 doi 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 8.Malmberg KJ, Carlsten M, Bjorklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol 2017;31:20–9 doi 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008;9(5):495–502 doi 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol 2019;105(6):1319–29 doi 10.1002/JLB.MR0718-269R. [DOI] [PubMed] [Google Scholar]
- 11.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol 2016;17(9):1025–36 doi 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front Immunol 2015;6:368 doi 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, et al. Antitransgene Rejection Responses Contribute to Attenuated Persistence of Adoptively Transferred CD20/CD19-Specific Chimeric Antigen Receptor Redirected T Cells in Humans. Biology of Blood and Marrow Transplantation 2010;16(9):1245–56 doi 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai C, Mihara K, Andreansky M, Nicholson I, Pui C, Geiger T, et al. Chimeric receptors with 4–1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18(4):676. [DOI] [PubMed] [Google Scholar]
- 15.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nature biotechnology 2002;20(1):70. [DOI] [PubMed] [Google Scholar]
- 16.Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. International journal of cancer 2011;129(12):2935–44. [DOI] [PubMed] [Google Scholar]
- 17.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences of the United States of America 2009;106(9):3360–5 doi 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology 2019;8(5):e1049-e doi 10.1002/cti2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang S, Vujanovic NL, Wollenberg B, Whiteside TL. Absence of B7.1-CD28/CTLA-4-mediated co-stimulation in human NK cells. Eur J Immunol 1998;28(3):780–6 doi . [DOI] [PubMed] [Google Scholar]
- 20.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nature immunology 2003;4(6):557–64 doi 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 21.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 1998;391(6668):703–7 doi 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima H, Colonna M. 2B4: an NK cell activating receptor with unique specificity and signal transduction mechanism. Hum Immunol 2000;61(1):39–43 doi 10.1016/s0198-8859(99)00170-6. [DOI] [PubMed] [Google Scholar]
- 23.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005;106(1):376–83 doi 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res 2013;73(6):1777–86 doi 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 25.Topfer K, Cartellieri M, Michen S, Wiedemuth R, Muller N, Lindemann D, et al. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. Journal of immunology (Baltimore, Md : 1950) 2015;194(7):3201–12 doi 10.4049/jimmunol.1400330. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018;23(2):181–92 e5 doi 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Liu Q, Zhong M, Wang Z, Chen Z, Zhang Y, et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol 2019;12(1):49 doi 10.1186/s13045-019-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther 2015;15(8):1145–54 doi 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 29.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med 2020;382(6):545–53 doi 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018;32(2):520–31 doi 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahaweni NM, Ehlers FAI, Bos GMJ, Wieten L. Tuning Natural Killer Cell Anti-multiple Myeloma Reactivity by Targeting Inhibitory Signaling via KIR and NKG2A. Front Immunol 2018;9:2848 doi 10.3389/fimmu.2018.02848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, et al. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol 2015;6:21 doi 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di SA, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011;365(18):1673–83 doi 10.1056/NEJMoa1106152 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oelsner S, Waldmann A, Billmeier A, Roder J, Lindner A, Ullrich E, et al. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive B-ALL and inhibit in vivo leukemia growth. Int J Cancer 2019;145(7):1935–45 doi 10.1002/ijc.32269. [DOI] [PubMed] [Google Scholar]
- 35.Antony GK, Dudek AZ. Interleukin 2 in cancer therapy. Current medicinal chemistry 2010;17(29):3297–302. [DOI] [PubMed] [Google Scholar]
- 36.Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cellular & molecular immunology 2013;10(3):222–9 doi 10.1038/cmi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viral Matosevic S. and Nonviral Engineering of Natural Killer Cells as Emerging Adoptive Cancer Immunotherapies. J Immunol Res 2018;2018:4054815 doi 10.1155/2018/4054815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016;8(357):357ra123 doi 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 2012;209(13):2351–65 doi 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gang M, Marin ND, Wong P, Neal CC, Marsala L, Foster M, et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 2020. doi 10.1182/blood.2020006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castriconi R, Carrega P, Dondero A, Bellora F, Casu B, Regis S, et al. Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front Immunol 2018;9:2324 doi 10.3389/fimmu.2018.02324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res 2011;17(4):678–89 doi 10.1158/1078-0432.CCR-10-2173. [DOI] [PubMed] [Google Scholar]
- 43.Somanchi SS, Somanchi A, Cooper LJ, Lee DA. Engineering lymph node homing of ex vivo-expanded human natural killer cells via trogocytosis of the chemokine receptor CCR7. Blood 2012;119(22):5164–72 doi 10.1182/blood-2011-11-389924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother 2015;64(2):225–35 doi 10.1007/s00262-014-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlsten M, Levy E, Karambelkar A, Li L, Reger R, Berg M, et al. Efficient mRNA-Based Genetic Engineering of Human NK Cells with High-Affinity CD16 and CCR7 Augments Rituximab-Induced ADCC against Lymphoma and Targets NK Cell Migration toward the Lymph Node-Associated Chemokine CCL19. Front Immunol 2016;7:105 doi 10.3389/fimmu.2016.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kremer V, Ligtenberg MA, Zendehdel R, Seitz C, Duivenvoorden A, Wennerberg E, et al. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer 2017;5(1):73 doi 10.1186/s40425-017-0275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J, Kang TH, Yoo W, Choi H, Jo S, Kong K, et al. An Antibody Designed to Improve Adoptive NK-Cell Therapy Inhibits Pancreatic Cancer Progression in a Murine Model. Cancer Immunol Res 2019;7(2):219–29 doi 10.1158/2326-6066.CIR-18-0317. [DOI] [PubMed] [Google Scholar]
- 48.Muller N, Michen S, Tietze S, Topfer K, Schulte A, Lamszus K, et al. Engineering NK Cells Modified With an EGFRvIII-specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1alpha-secreting Glioblastoma. J Immunother 2015;38(5):197–210 doi 10.1097/CJI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng YY, Tay JCK, Wang S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol Ther Oncolytics 2020;16:75–85 doi 10.1016/j.omto.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2016;16(1):7–19 doi 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 51.Murray S, Lundqvist A. Targeting the tumor microenvironment to improve natural killer cell-based immunotherapies: On being in the right place at the right time, with resilience. Hum Vaccin Immunother 2016;12(3):607–11 doi 10.1080/21645515.2015.1096458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis 2018;7(1):10 doi 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daher M, Basar R, Shaim H, Gokdemir E, Uprety N, Kontoyiannis A, et al. The TGF-β/SMAD Signaling Pathway As a Mediator of NK Cell Dysfunction and Immune Evasion in Myelodysplastic Syndrome. Blood 2017;130(Suppl 1):53-. [Google Scholar]
- 54.Yvon ES, Burga R, Powell A, Cruz CR, Fernandes R, Barese C, et al. Cord blood natural killer cells expressing a dominant negative TGF-beta receptor: Implications for adoptive immunotherapy for glioblastoma. Cytotherapy 2017;19(3):408–18 doi 10.1016/j.jcyt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Kim TD, Lee SU, Yun S, Sun HN, Lee SH, Kim JW, et al. Human microRNA-27a* targets Prf1 and GzmB expression to regulate NK-cell cytotoxicity. Blood 2011;118(20):5476–86 doi 10.1182/blood-2011-04-347526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review). Int J Oncol 2008;32(3):527–35. [PubMed] [Google Scholar]
- 57.Young A, Ngiow SF, Gao Y, Patch A-M, Barkauskas DS, Messaoudene M, et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer research 2018;78(4):1003–16 doi 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 58.Mittal D, Young A, Stannard K, Yong M, Teng MWL, Allard B, et al. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer research 2014;74(14):3652–8 doi 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 59.Sun H, Sun C. The Rise of NK Cell Checkpoints as Promising Therapeutic Targets in Cancer Immunotherapy. Front Immunol 2019;10:2354 doi 10.3389/fimmu.2019.02354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018;19(7):723–32 doi 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 61.Figueiredo C, Seltsam A, Blasczyk R. Permanent silencing of NKG2A expression for cell-based therapeutics. J Mol Med (Berl) 2009;87(2):199–210 doi 10.1007/s00109-008-0417-0. [DOI] [PubMed] [Google Scholar]
- 62.Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. The Journal of clinical investigation 2019;129(5):2094–106 doi 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daher M, Basar R, Gokdemir E, Baran N, Uprety N, Nunez Cortes AK, et al. CIS checkpoint deletion enhances the fitness of cord blood derived natural killer cells transduced with a chimeric antigen receptor. Blood in Press [Google Scholar]
- 64.Delconte RB, Kolesnik TB, Dagley LF, Rautela J, Shi W, Putz EM, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol 2016;17(7):816–24 doi 10.1038/ni.3470. [DOI] [PubMed] [Google Scholar]
- 65.Rautela J, Surgenor E, Huntington ND. Efficient genome editing of human natural killer cells by CRISPR RNP. bioRxiv 2018:406934 doi 10.1101/406934. [DOI] [PubMed] [Google Scholar]
- 66.Zhu H, Blum RH, Bernareggi D, Ask EH, Wu Z, Hoel HJ, et al. Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-tumor Activity. Cell Stem Cell 2020;27(2):224–37 e6 doi 10.1016/j.stem.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest 2018;128(10):4654–68 doi 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stojanovic A, Fiegler N, Brunner-Weinzierl M, Cerwenka A. CTLA-4 is expressed by activated mouse NK cells and inhibits NK Cell IFN-gamma production in response to mature dendritic cells. J Immunol 2014;192(9):4184–91 doi 10.4049/jimmunol.1302091. [DOI] [PubMed] [Google Scholar]
- 69.Vivier E Cancer Discov 2020(This issue).
- 70.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010;24(6):1160–70 doi 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oelsner S, Friede ME, Zhang C, Wagner J, Badura S, Bader P, et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy 2017;19(2):235–49 doi 10.1016/j.jcyt.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med 2016;20(7):1287–94 doi 10.1111/jcmm.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tam YK, Maki G, Miyagawa B, Hennemann B, Tonn T, Klingemann HG. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum Gene Ther 1999;10(8):1359–73 doi 10.1089/10430349950018030. [DOI] [PubMed] [Google Scholar]
- 74.Chen KH, Wada M, Pinz KG, Liu H, Lin KW, Jares A, et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia 2017;31(10):2151–60 doi 10.1038/leu.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y, Liu Q, Zhong M, Wang Z, Chen Z, Zhang Y, et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. Journal of hematology & oncology 2019;12(1):49- doi 10.1186/s13045-019-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.You F, Wang Y, Jiang L, Zhu X, Chen D, Yuan L, et al. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am J Cancer Res 2019;9(1):64–78. [PMC free article] [PubMed] [Google Scholar]
- 77.Cummins KD, Gill S. Chimeric antigen receptor T-cell therapy for acute myeloid leukemia: how close to reality? Haematologica 2019;104(7):1302–8 doi 10.3324/haematol.2018.208751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Testa U, Pelosi E, Castelli G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers (Basel) 2019;11(9):1358 doi 10.3390/cancers11091358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baragano Raneros A, Lopez-Larrea C, Suarez-Alvarez B. Acute myeloid leukemia and NK cells: two warriors confront each other. Oncoimmunology 2019;8(2):e1539617 doi 10.1080/2162402X.2018.1539617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klöß S, Oberschmidt O, Morgan M, Dahlke J, Arseniev L, Huppert V, et al. Optimization of Human NK Cell Manufacturing: Fully Automated Separation, Improved Ex Vivo Expansion Using IL-21 with Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells. Human gene therapy 2017;28(10):897–913 doi 10.1089/hum.2017.157. [DOI] [PubMed] [Google Scholar]
- 81.Kerbauy LNAS, Liu E, Banejee PP, Wu Y, Shaim H, Wei Inng Lim FL, Basar R, Li L, Muftuoglu M, Daher M et al. Cord blood NK cells engineered to express a humanized CD123-targeted chimeric antigen receptor (CAR) and IL-15 as off-the-shelf therapy for acute myeloid Leukemia. Blood 2017;130(Supplement 1):4453. [Google Scholar]
- 82.Salman H, Pinz KG, Wada M, Shuai X, Yan LE, Petrov JC, et al. Preclinical Targeting of Human Acute Myeloid Leukemia Using CD4-specific Chimeric Antigen Receptor (CAR) T Cells and NK Cells. J Cancer 2019;10(18):4408–19 doi 10.7150/jca.28952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paczulla AM, Rothfelder K, Raffel S, Konantz M, Steinbacher J, Wang H, et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 2019;572(7768):254–9 doi 10.1038/s41586-019-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell KS, Cohen AD, Pazina T. Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma. Front Immunol 2018;9:2551- doi 10.3389/fimmu.2018.02551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol 2014;8(2):297–310 doi 10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014;28(4):917–27 doi 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B, et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proceedings of the National Academy of Sciences of the United States of America 2008;105(45):17481–6 doi 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Sci Rep 2015;5:11483- doi 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018;23(2):181–92.e5 doi 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kailayangiri S, Altvater B, Spurny C, Jamitzky S, Schelhaas S, Jacobs AH, et al. Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. Oncoimmunology 2016;6(1):e1250050–e doi 10.1080/2162402X.2016.1250050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spurny C, Kailayangiri S, Altvater B, Jamitzky S, Hartmann W, Wardelmann E, et al. T cell infiltration into Ewing sarcomas is associated with local expression of immune-inhibitory HLA-G. Oncotarget 2017;9(5):6536–49 doi 10.18632/oncotarget.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res 2018;8(6):1083–9. [PMC free article] [PubMed] [Google Scholar]
- 93.Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol 2018;18(9):559–74 doi 10.1038/s41577-018-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krovi SH, Gapin L. Invariant Natural Killer T Cell Subsets-More Than Just Developmental Intermediates. Front Immunol 2018;9:1393 doi 10.3389/fimmu.2018.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mallevaey T, Selvanantham T. Strategy of lipid recognition by invariant natural killer T cells: ‘one for all and all for one’. Immunology 2012;136(3):273–82 doi 10.1111/j.1365-2567.2012.03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mavers M, Maas-Bauer K, Negrin RS. Invariant Natural Killer T Cells As Suppressors of Graft-versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol 2017;8:900 doi 10.3389/fimmu.2017.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rotolo A, Caputo VS, Holubova M, Baxan N, Dubois O, Chaudhry MS, et al. Enhanced Anti-lymphoma Activity of CAR19-iNKT Cells Underpinned by Dual CD19 and CD1d Targeting. Cancer Cell 2018;34(4):596–610 e11 doi 10.1016/j.ccell.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 2014;124(18):2824–33 doi 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu XHW, Heczey A et al. NKT cells co-expressing a GD2-specific chimeric antigen receptor and IL-15 show enhanced in vivo persistence and antitumor activity against neuroblastoma. Clin Cancer Res 2019;19(0421). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heczey ACA, Tian G et al. Harnessing Natural and Engineered Properties of iNKT Cells for Adoptive Cancer Immunotherapy. Cancer immunol Research 2019;10(1158). [Google Scholar]
- 101.Nielsen MM, Witherden DA, Havran WL. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol 2017;17(12):733–45 doi 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adams EJ, Gu S, Luoma AM. Human gamma delta T cells: Evolution and ligand recognition. Cell Immunol 2015;296(1):31–40 doi 10.1016/j.cellimm.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nussbaumer O, Gruenbacher G, Gander H, Komuczki J, Rahm A, Thurnher M. Essential requirements of zoledronate-induced cytokine and gammadelta T cell proliferative responses. J Immunol 2013;191(3):1346–55 doi 10.4049/jimmunol.1300603. [DOI] [PubMed] [Google Scholar]
- 104.Fisher J, Anderson J. Engineering Approaches in Human Gamma Delta T Cells for Cancer Immunotherapy. Front Immunol 2018;9:1409 doi 10.3389/fimmu.2018.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rischer M, Pscherer S, Duwe S, Vormoor J, Jurgens H, Rossig C. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol 2004;126(4):583–92 doi 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 106.Capsomidis A, Benthall G, Van Acker HH, Fisher J, Kramer AM, Abeln Z, et al. Chimeric Antigen Receptor-Engineered Human Gamma Delta T Cells: Enhanced Cytotoxicity with Retention of Cross Presentation. Mol Ther 2018;26(2):354–65 doi 10.1016/j.ymthe.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deniger DC, Switzer K, Mi T, Maiti S, Hurton L, Singh H, et al. Bispecific T-cells expressing polyclonal repertoire of endogenous gammadelta T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther 2013;21(3):638–47 doi 10.1038/mt.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol 2015;33:643–75 doi 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 109.Long KB, Beatty GL. Harnessing the antitumor potential of macrophages for cancer immunotherapy. Oncoimmunology 2013;2(12):e26860 doi 10.4161/onci.26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66(2):605–12 doi 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 111.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol 2020;38(8):947–53 doi 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klichinsky MRM, Shestova O et al. Chimeric antigen receptor macrophages (CARMA) for adoptive cellular immunotherapy of solid tumors. Clin Cancer Res 2017;77(13):4575. [Google Scholar]
- 113.Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, et al. Chimeric antigen receptors that trigger phagocytosis. Elife 2018;7 doi 10.7554/eLife.36688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Velasquez EJLJE, Brindley TD et al. Macrophage Toll-like receptor-chimeric antigen receptors (MOTO-CARs) as a novel adoptive cell therapy for the treatment of solid malignancies. Clin Cancer Res 2018;78(13):2563. [Google Scholar]
- 115.Thery C, Amigorena S. The cell biology of antigen presentation in dendritic cells. Curr Opin Immunol 2001;13(1):45–51 doi 10.1016/s0952-7915(00)00180-1. [DOI] [PubMed] [Google Scholar]
- 116.Noubade R, Majri-Morrison S, Tarbell KV. Beyond cDC1: Emerging Roles of DC Crosstalk in Cancer Immunity. Front Immunol 2019;10:1014 doi 10.3389/fimmu.2019.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santos PM, Butterfield LH. Dendritic Cell-Based Cancer Vaccines. J Immunol 2018;200(2):443–9 doi 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suh HCPK, Javier APL et al. Effect of dendritic cells (DC) transduced with chimeric antigen receptor (CAR) on CAR T-cell cytotoxicity. Journal or Clinical Oncology 2017;35(7):144. [Google Scholar]
- 119.Klingemann H Challenges of cancer therapy with natural killer cells. Cytotherapy 2015;17(3):245–9 doi 10.1016/j.jcyt.2014.09.007. [DOI] [PubMed] [Google Scholar]


