Abstract
Patients with psychotic spectrum disorders (PSD) exhibit similar patterns of atrophy and microstructural changes that may be associated with common symptomatology (e.g., symptom burden and/or cognitive impairment). Gray matter concentration values (proxy for atrophy), fractional anisotropy (FA), mean diffusivity (MD), intracellular neurite density (Vic) and isotropic diffusion volume (Viso) measures were therefore compared in 150 PSD (schizophrenia, schizoaffective disorder, and bipolar disorder Type I) and 63 healthy controls (HC). Additional analyses evaluated whether regions showing atrophy and/or microstructure abnormalities were better explained by DSM diagnoses, symptom burden or cognitive dysfunction. PSD exhibited increased atrophy within bilateral medial temporal lobes and subcortical structures. Gray matter along the left lateral sulcus showed evidence of increased atrophy and MD. Increased MD was also observed in homotopic fronto-temporal regions, suggesting it may serve as a precursor to atrophic changes. Global cognitive dysfunction, rather than DSM diagnoses or psychotic symptom burden, was the best predictor of increased gray matter MD. Regions of decreased FA (i.e., left frontal gray and white matter) and Vic (i.e., frontal and temporal regions and along central sulcus) were also observed for PSD, but were neither spatially concurrent with atrophic regions nor associated with clinical symptoms. Evidence of expanding microstructural spaces in gray matter demonstrated the greatest spatial overlap with current and potentially future regions of atrophy, and was associated with cognitive deficits. These results suggest that this particular structural abnormality could potentially underlie global cognitive impairment that spans traditional diagnostic categories.
Keywords: Research Domain Criteria (RDoC), psychotic spectrum disorders, neurite orientation dispersion and density imaging, gray matter concentration, diffusion tensor imaging, biomarkers
1. Introduction
Traditional classification systems such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) were designed to increase diagnostic reliability rather than to study underlying biological mechanisms of disease states (Pearlson, 2015). For research purposes, there are potentially more optimal classifications of patients with psychotic spectrum disorders (PSD) based on a continuum of psychosis and dimensional biomarkers (e.g., cognition, clinical symptoms, imaging findings, or genetics; Clark et al., 2017; Hanlon et al., 2019). This perspective is not new (Crow, 1986; Coryell et al., 1984), yet has been re-conceptualized by the Research Domain Criteria (RDoC; Cuthbert, 2015; Pearlson, 2015) and Hierarchical Taxonomy of Psychopathology (HiTOP; Kotov et al., 2017) frameworks. This has renewed interest in the identification of biomarkers that can reliably stratify PSD along a continuum of psychosis and explain common symptomatology (e.g., symptom burden and cognitive impairment).
Atrophy and microstructural abnormalities are shared across psychotic spectrum diagnoses, have been related to psychosis and cognition, and evolve over the course of the disease (Bakhshi and Chance, 2015; Birur et al., 2017; Maggioni et al., 2017; Skudlarski et al., 2013; Vita et al., 2012; Walterfang et al., 2011). Thus, one of these abnormalities, or a combination of them, could potentially classify PSD along a continuum of symptom burden or cognitive impairment. It has been well established that PSD exhibit atrophy, including larger ventricles, reduced gray matter volume (potentially driven by cortical thinning; Rimol et al., 2012), and abnormal white matter connections, with the greatest atrophy occurring in frontotemporal lobes (Bakhshi and Chance, 2015; Birur et al., 2017; Maggioni et al., 2017; Altamura et al., 2018; Vita et al., 2012; Ivleva et al., 2013; Gupta et al., 2015; van Erp et al., 2018).
Decreased cortical volume in PSD is thought to be due to reduced neurites (axons and dendrites), neuronal number/size, neuropil (space between cells; i.e., reduced neuropil hypothesis; Selemon and Goldman-Rakic, 1999; Nazeri et al., 2020), and/or abnormal myelination, potentially supporting an underlying progressive neurodevelopmental abnormality rather than neurodegeneration per se due to the absence of gliosis or gross inflammatory processes (Bakhshi and Chance, 2015; Harrison et al., 2018; Iritani, 2013). However, few studies have examined what microstructural changes exist in conjunction with, or separately from, atrophy, and how both relate to clinical symptoms in PSD.
Diffusion tensor imaging (DTI) is arguably the most widely utilized diffusion magnetic resonance imaging (dMRI) technique for investigating these types of microstructure pathologies in vivo in PSD. The most common pattern of DTI findings across PSD (patients with schizophrenia [SP] and bipolar disorder Types I [BP-I] and II [BP-II]) is reduced fractional anisotropy (FA) and increased mean diffusivity (MD) in white matter (Birur et al., 2017; Skudlarski et al., 2013; Chang et al., 2018; Cui et al., 2011; O’Donoghue et al., 2017; Sussmann et al., 2009; Squarcina et al., 2017; Kelly et al., 2018) and increased MD in gray matter relative to healthy controls (HC; Anderson et al., 2013; Lee et al., 2009; Moriya et al., 2010). Reduced FA is thought to signify less organization/integrity of white matter bundles/myelin whereas increased MD represents increased extracellular spaces (e.g., neuroinflammation, atrophy, vasogenic, edema, changes in myelin, and/or abnormal connections/tracts; Pasternak et al., 2016). However, DTI assumes a Gaussian distribution of diffusion displacement (Jensen et al., 2005), measuring diffusion with a single b-value and modeling diffusion as a mono-exponential decay with tensors. Measuring independent and complimentary non-Gaussian diffusion information with more complex geometric models of diffusion such as neurite orientation dispersion and density imaging (NODDI; Zhang et al., 2012) should provide additional information on underlying biological mechanisms such as free water concentrations, neuroinflammation and changes in myelin content (Mayer et al., 2017; Fukutomi et al., 2018; Nazeri et al., 2020).
To date, there have only been a small number of dMRI studies that examine microstructural abnormalities in PSD (O’Donoghue et al., 2017; Pasternak et al., 2018; Nazeri et al., 2017), with most focusing on white rather than gray matter pathology (Pasternak et al., 2018). Only a single study (Nazeri et al., 2017) has examined both gray matter microstructure abnormalities and atrophy in PSD, reporting that patients with SP/schizoaffective disorder (SA) had reduced temporal gray matter neurite density, frontotemporal atrophy (reduced cortical thickness), as well as frontotemporal neurocognitive deficits (spatial working memory) compared to HC, whereas BP-I were intermediate between groups (no statistical difference). These biomarkers resulted in adequate prediction of diagnosis both individually and in combination (Nazeri et al., 2017). Thus, the predominant focus on white matter abnormalities (Pasternak et al., 2018) may have minimized potential relationships between biomarkers and expression of clinical symptoms. A critical next step is to examine atrophy and microstructural measures from not just gray matter (Nazeri et al., 2017), but also to incorporate white matter, examining their relationship to one another, as well as to clinical symptoms, across the psychosis continuum. Identifying these relationships may lead to an alternative dimensional approach of classifying PSD based on the RDoC framework (Cuthbert, 2015).
The current study therefore examined relationships between whole-brain (i.e., both white and gray matter) DTI (FA and MD), NODDI (intracellular neurite density volume fraction [Vic] and free isotropic diffusion volume fraction [Viso]) and voxel-based gray matter concentration (GMC) values in PSD. GMC was examined in lieu of volume as it is calculated regionally, similarly to the microstructural measures. We hypothesized that PSD would exhibit decreased FA and increased MD relative to HC (Pasternak et al., 2018; Birur et al., 2017; O’Donoghue et al., 2017), with the latter being more closely associated with a putative measure of gray matter atrophy (GMC). We further hypothesized that PSD would show reduced GMC and neurite density relative to HC in spatially overlapping frontotemporal regions (Nazeri et al., 2017). Finally, clinical symptoms (symptom burden and cognition) were expected to account for significantly more unique variance in terms of GMC and microstructural abnormalities relative to DSM diagnostic status (Hanlon et al., 2019).
2. Material and methods
2.1. Participants
One-hundred seventy-one PSD and 68 HC, between 18–54 years old, met inclusion criteria. PSD and HC were recruited to match on age and sex. Recruitment and exclusion criteria have previously been published (see also Supplemental Materials) for clinical data (Hanlon et al., 2019). Eighteen PSD and 5 HC did not complete the DTI scan, 2 PSD had scan data with unrecoverable issues and one PSD was removed due to excessive motion (>3 times the interquartile range on mean of the three translational motion parameters and the three rotational motion parameters, framewise displacement [FD]; Power et al., 2012). Therefore, 150 PSD (91 males; mean age=32.01±9.42 years) and 63 HC (41 males; mean age=33.30±8.27 years) were included in final analyses. PSD were diagnosed with SP (N=97), SA (N=12), or BP-I (N=41) by board-certified psychiatrists (J.B., S.S., or D.L.) based on the Structured Clinical Interview for DSM-IV-TR (SCID-II; First et al., 2012) and clinical records. The University of New Mexico Health Sciences Center’s Human Research Review Committee approved this study. Participants provided written informed consent according to institutional guidelines and were compensated for participating.
2.2. Assessments
Clinical symptoms assessments included the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impressions Scale (CGI), Montgomery and Asberg Depression Rating Scale (MADRS), Schizo-Bipolar Scale (SBS), Young Mania Rating Scale (YMRS), and a psychiatric medical history form. An olanzapine equivalence measure was calculated for PSD to determine medication load (Gardner et al., 2010). See Supplemental Materials for details and extrapyramidal symptoms assessments.
All participants completed the Wechsler Test of Adult Reading (WTAR) to estimate pre-morbid intelligence, a medical history form, and the Fagerstrom Test for Nicotine Dependence (FTND). Real-world functioning was assessed with the UCSD Performance-Based Skills Assessment Brief Version (UPSA-B) and the Quality of Life Questionnaire in Schizophrenia 18 (S-QoL 18). PSD family members (N=116) were contacted to complete the Specific Levels of Functioning Informant Scale (SLOF-I) for independent assessment of functioning (see Supplemental Materials). Finally, all participants completed the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB; Kern et al., 2004) to determine cognitive functioning.
2.3. Image Acquisition
Participants refrained from smoking for at least one hour prior to the MRI scan, with CO levels obtained with a Vitolograph BreathCO Monitor for all smokers. High resolution 5-echo multi-echo Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) T1-weighted images were collected on a Siemens 3 Tesla TrioTim scanner with a 32-channel head coil (details in Supplemental Materials). For each participant, structural images were reviewed for any abnormal findings by a board-certified neuroradiologist who was blinded to participant group.
High angular resolution dMRI scans were acquired using a twice-refocused spin-echo multi-shell sequence with 87 diffusion gradients (29 each at b=800 s/mm2, b=1600 s/mm2, b=2400 s/mm2) and the b=0 experiment repeated 4 times (details in Supplemental Materials). The data were collected across 2 runs, with reversed phase encoding direction for each run (A→P; P→A). Gradient directions were uniformly distributed over an electrostatic sphere using the CAMINO software package (Jones et al., 1999; Jansons and Alexander, 2003).
2.4. Image Processing
MRI and dMRI data were processed using AFNI (v17.1.01), FSL (v5.0.8), SPM (v12) and Matlab (vR2014a). Susceptibility artifacts (using reverse phase encoded images), eddy currents and head motion were first corrected by FSL TOPUP and EDDY. Resulting dMRI (from all three shells) were used for non-linear DTI calculations with AFNI to decrease noise-related errors of diffusion tensors and scalar measures (Cox and Glen, 2006). Each participant’s first unblurred b0 image was registered to their T1-weighted image using an affine transformation, with T1 images subsequently normalized to Talairach space (AFNI TT_N27) using a non-linear transformation (AFNI 3dQwarp). Both matrices were concatenated to bring DTI scalars into stereotaxic space.
Cellular microstructure was investigated via use of NODDI (Zhang et al., 2012) using fixed rates for isotropic (3.0×10−3 mm2/s) and intracellular (1.7×10–3 mm2/s) diffusion. Given the strong statistical relationship that exists between orientation dispersion index (ODI) and FA (Mayer et al., 2017; see secondary analyses), the two NODDI primary metrics of interest were the measureable intracellular (Vic) and isotropic (Viso) diffusion. All DTI and NODDI metrics were spatially normalized using the same concatenated matrices and blurred using a 6-mm FWHM Gaussian kernel. Voxel-based morphometry analysis of gray matter was performed on each participant’s T1 data using SPM12. Unmodulated (Ashburner and Friston, 2000) GMC images were blurred with a 6-mm FWHM Gaussian kernel (see Supplemental Materials).
2.5. GMC and dMRI Analyses
A voxel-wise regression model (AFNI’s 3dttest+) determined regions where GMC values varied as a function of Group (HC vs. PSD, dummy coded as 0 and 1) with mean FD as a single vector covariate. Next, a series of regression models were conducted using dMRI metrics as dependent variables (primary: FA, MD, Vic, Viso) with Group (HC vs. PSD, dummy coded as 0 and 1), mean FD (single vector covariate), and GMC values (voxel-wise covariate) as predictor variables. Unlike previous studies, the inclusion of GMC in dMRI models statistically controls for the effects of atrophy on microstructure values and isolates unique variance associated with the Group factor. Supplemental regressions including the GMC×Group (voxel-wise covariate) interaction term (hereafter referred to as interaction model) determined potential influence on dMRI main effects. Voxel-wise analyses were corrected for family-wise error (p<0.05) based on 10,000 Monte-Carlo simulations using parametric (p<0.001) and minimum cluster size (principal analyses=570 μl; GMC analysis=391 μl; interaction analyses=564 μl) using non-Gaussian spherical autocorrelation in AFNI (Cox et al., 2017).
Planned one-way ANCOVAs were implemented within the PSD sample to investigate whether the independent variables of DSM diagnosis (SP, BP-I, and SA), psychotic symptom load (PANSS positive and negative scale scores) or global cognition (MCCB total score) accounted for a significant proportion of the variance among the major congruous (i.e., more than one cluster, excluding Viso) directional differences in GMC (HC>PSD), MD (HC<PSD), FA (HC>PSD) or Vic (HC>PSD) presented in Table 2. The dependent variable consisted of a weighted mean of all regions showing similar patterns of pathology, resulting in four separate weighted models for each imaging metric (i.e., GMC/MD/FA/Vic), with age, sex, olanzapine equivalence, illness duration (years) and mean FD included as additional nuisance covariates.
Table 2:
Brain regions with Brodmann areas (denoted in parenthesis) showing significant Group (healthy controls [C] vs. patients diagnosed with a psychotic spectrum disorder [P]) differences for micro- and macro-structural measures.
| Pattern 1: | GMC (BA) | MD (BA) | Viso (BA) | FA (BA) | Vic (BA) |
|---|---|---|---|---|---|
| Right IFG/Sylvian cistern | C>P (47) | ||||
| Left entorhinal | C>P (34) | ||||
| Right entorhinal | C>P (34) | ||||
| Left ventral entorhinal | C>P (20/28/36/38) | ||||
| Right ventral entorhinal | C>P (28/38) | ||||
| Left V2 | P>C (18) | ||||
| Thalamus | C>P (NA) | ||||
| Pattern 2: | |||||
| Left insula/VLPFC | C>P (13/44/45) | P>C (13/44/45) | C>P (13/44) | ||
| Left STG | C>P (22/42) | P>C (22) | |||
| Pattern 3 (GM): | |||||
| Left DLPFC | C>P (10/46) | ||||
| Right VLPFC | P>C (47) | C>P (44) | |||
| Right insula | P>C (13) | ||||
| Bilateral MPFC | P>C (9/10/11) | C>P (10) | |||
| Left IFG/Sylvian cistern | P>C (38) | ||||
| Right SFG/MFG | C>P (6/8) | ||||
| Left ACG | C>P (24/32) | ||||
| Midline middle/posterior CG | C>P (24) | ||||
| Left superior precentral gyri/CS | C>P (4/6) | ||||
| Left pre/postcentral gyri/CS | C>P (3/4/6) | ||||
| Right pre/postcentral gyri/CS/DLPFC | C>P (3/4/6/9) | ||||
| Right STG/MTG/ITG/LS | P>C (21/22) | C>P (21/22/39/42) | |||
| Pattern 3 (WM): | |||||
| Right cerebral and superior cerebellar peduncle | P>C (NA) | ||||
| Left SLF | C>P (NA) | C>P (NA) | |||
| Left CC | C>P (NA) | ||||
| Left anterior corona radiata | C>P (NA) | ||||
Notes: ACG = anterior cingulate gyrus; BA = Brodmann area; C = healthy controls; CC = corpus callosum; CG = cingulate gyrus; CS = central sulcus; DLPFC = dorsolateral prefrontal cortex; FA = fractional anisotropy; GMC = gray matter concentration; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; LS = lateral sulcus; MD = mean diffusivity; MFG = medial frontal gyrus; MPFC = medial prefrontal cortex; MTG = middle temporal gyrus; NA = Not Applicable; P = patients diagnosed with a psychotic spectrum disorder; SFG = superior frontal gyrus; SLF = superior longitudinal fasciculus; STG = superior temporal gyrus; Vic = intracellular neurite density volume fraction; Viso = free isotropic volume fraction; VLPFC = ventral lateral prefrontal cortex; V2 = secondary visual cortex.
3. Results
3.1. Demographic, Clinical, and Functioning Data
Results for demographic, clinical, and functioning data are summarized in Table 1. Age and sex distribution was similar both between PSD and HC and within disorder sub-groups. PSD subgroups did not statistically differ in years of education, addiction to nicotine, quality of life, age of illness onset, illness duration, or manic, depressive or extrapyramidal symptoms. However, diagnostic sub-groups differed on pre-morbid intelligence (SP<BP-I), cognitive functioning (SP<BP-I), and real-world functioning (UPSA-B; SP<BP-I; SLOF-I; SP≈SA<BP-I). Among PSD, the PANSS positive (SP≈SA>BP-I), negative (SP>BP-I), and total (SP>BP-I) scores also significantly differed. Finally, SP were more clinically severe and took a higher antipsychotic medication dosage relative to BP-I.
Table 1:
Summary of demographics, functioning, and clinical data across all groups.
| HC (N = 63) | PSD (N = 150) | SP (N = 97) | SA (N = 12) | BP-I (N = 41) | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | p value | Mean (SD) | Mean (SD) | Mean (SD) | p value | |
| Demographics | |||||||
| Sex (Females/Males) | 22/41 | 59/91 | p = 0.545 | 37/60 | 4/8 | 18/23 | p = 0.742 |
| Age (years) | 33.30 (8.27) | 32.01 (9.42) | p = 0.344 | 31.79 (8.95) | 30.83 (9.52) | 32.85 (10.58) | p = 0.755 |
| Education (years) | 14.97 (2.19) | 13.27 (2.34) | p < 0.001 | 13.03 (2.52) | 13.25 (2.05) | 13.83 (1.87) | p = 0.187 |
| FTND | 0.49 (1.40) | 1.52 (2.41) | p < 0.001 | 1.76 (2.52) | 1.25 (2.09) | 1.02 (2.17) | p = 0.239 |
| Cognition | |||||||
| WTAR | 56.11 (6.79) | 51.21 (10.55) | p < 0.001 | 49.10 (11.14) | 54.83 (8.08) | 55.14 (8.29) | p = 0.004 |
| MCCB overall | 48.10 (9.67) | 35.37 (11.78) | p < 0.001 | 32.84 (11.07) | 38.08 (10.78) | 40.59 (12.06) | p = 0.001 |
| Functioning | |||||||
| UPSA-B total | 77.39 (11.89) | 69.10 (15.36) | p < 0.001 | 66.25 (15.58) | 72.89 (14.49) | 74.72 (13.50) | p = 0.008 |
| S-QoL 18 | 66.75 (9.59) | 55.42 (15.22) | p < 0.001 | 55.14 (15.18) | 55.73 (14.40) | 56.01 (15.87) | p = 0.952 |
| SLOF-I total | 89.62 (18.02) N=114 | 87.59 (18.71) N=73 | 75.29 (21.70) N=7 | 96.94 (12.45) N=34 | p = 0.003 | ||
| Clinical Measures | |||||||
| Age of onset (years) | 20.26 (5.74) | 21.03 (5.72) | 19.75 (5.51) | 18.59 (5.59) | p = 0.068 | ||
| Illness duration (years) | 11.75 (9.26) | 10.76 (8.65) | 11.08 (8.17) | 14.27 (10.59) | p = 0.122 | ||
| PANSS positive | 13.54 (5.21) | 14.32 (5.18) | 15.42 (5.73) | 11.15 (4.39) | p = 0.002 | ||
| PANSS negative | 13.48 (5.01) | 14.60 (5.24) | 11.50 (4.10) | 11.41 (3.80) | p = 0.001 | ||
| PANSS total | 52.87 (14.65) | 55.95 (15.30) | 52.83 (13.76) | 45.59 (10.40) | p = 0.001 | ||
| CGI | 3.44 (0.90) | 3.62 (0.90) | 3.42 (0.52) | 3.02 (0.88) | p = 0.002 | ||
| MADRS | 7.89 (7.02) | 8.63 (7.06) | 8.92 (9.06) | 5.83 (5.93) | p = 0.087 | ||
| YMRS | 3.75 (5.70) | 3.11 (4.95) | 5.33 (4.68) | 4.78 (7.29) | p = 0.176 | ||
| SBS | 5.85 (2.73) | 7.43 (1.46) | 5.58 (1.00) | 2.20 (1.63) | p < 0.001 | ||
| Olanzapine equivalent | 11.87 (10.82) | 13.90 (10.27) | 14.43 (17.99) | 6.30 (7.10) | p < 0.001 | ||
| Extrapyramidal Symptoms | N=149 | N=96 | N=12 | N=41 | |||
| AIMS | 0.51 (1.10) | 0.45 (0.93) | 1.00 (1.60) | 0.51 (1.29) | p = 0.263 | ||
| BAS | 0.42 (1.08) | 0.37 (0.89) | 1.08 (1.83) | 0.34 (1.18) | p = 0.085 | ||
| SAS | 0.93 (1.82) | 0.95 (1.87) | 1.25 (1.71) | 0.80 (1.76) | p = 0.753 | ||
Notes: HC = Healthy controls; PSD = Psychotic Spectrum Disorders; SP = Patients with schizophrenia; SA = Patients with schizoaffective disorder; BP-I = Patients with bipolar disorder, Type I; FTND = Fagerstrom Test for Nicotine Dependence; MCCB = Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery; WTAR = Wechsler Test of Adult Reading; UPSA-B = UCSD Performance Based Skills Assessment Brief Version; S-QoL 18 = Quality of Life Questionnaire in Schizophrenia 18; SLOF-I = Specific Levels of Functioning Informant Scale; PANSS = Positive and Negative Syndrome Scale; CGI = Clinical Global Impressions Scale; MADRS = Montgomery and Asberg Depression Rating Scale; YMRS = Young Mania Rating Scale; SBS = Schizo-Bipolar Scale; AIMS = Abnormal Involuntary Movement Scale (average total for the first 7 variables); BAS = Barnes Akathisia Scale; SAS = Simpson Angus Scale.
3.2. DTI, NODDI, and GMC Data
Importantly, mean FD did not differ between HC and PSD (p=0.456). Replication attempts (Mayer et al., 2017) for HC only (Figure S1 and Table S1) successfully validated a strong relationship between FA and ODI in both gray and white matter, providing additional empirical support to justify our choice for primary dMRI metrics. Vic was unrelated to FA and only moderately related to MD in both gray and white matter (Adjusted R2 range = 0.25–0.63; Ferguson, 2009). Only moderate relationships existed between Viso and MD in white matter, yet they showed a strong relationship in gray matter.
Three predominant patterns of group differences in GMC and dMRI were qualitatively observed (Table 2). The first pattern consisted of decreased GMC for PSD (i.e., increased atrophy) without any evidence of microstructural changes in the thalamus and bilateral entorhinal cortex, extending into inferior frontal gyri within the Sylvian cistern in the right hemisphere (Figure 1). In addition, PSD exhibited increased GMC relative to HC in the left secondary visual cortex.
Figure 1. Gray matter concentration (GMC):
Panel A depicts main effect of Group for greater GMC (dark blue: p<0.001; cyan: p<0.0001) values within the right (R) and left (L) entorhinal cortex (EC) and ventral entorhinal cortex (vEC) for healthy controls (HC) relative to patients with psychotic spectrum disorders (PSD). Coronal (Y) slice coordinates are given according to the Talairach atlas. The one region exhibiting increased GMC (red: p<0.001; yellow: p<0.0001) in PSD relative to HC is not shown. Panel B displays box-and-scatter plots of GMC for each region (PSD: red; HC: blue).
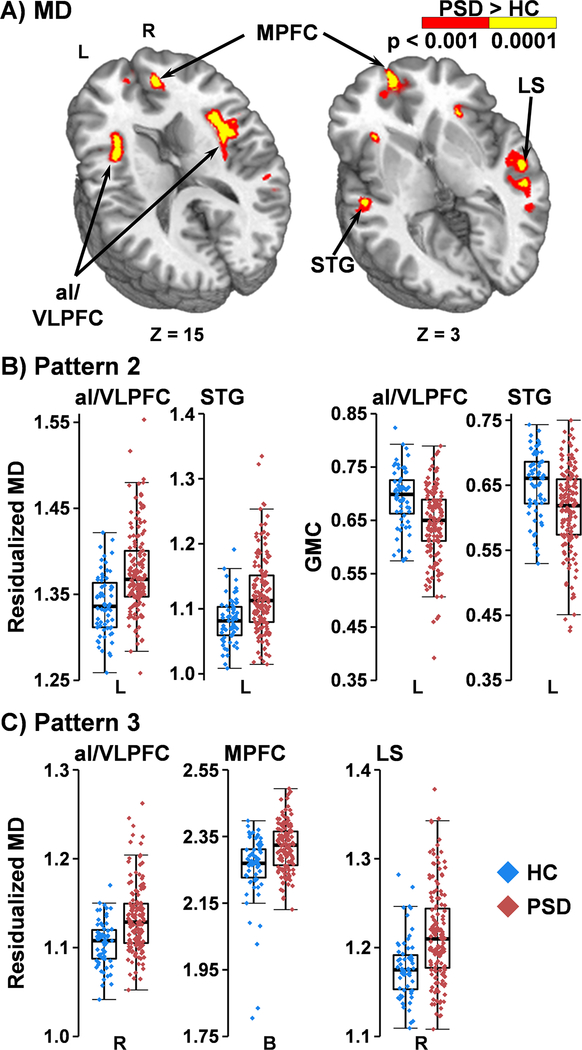
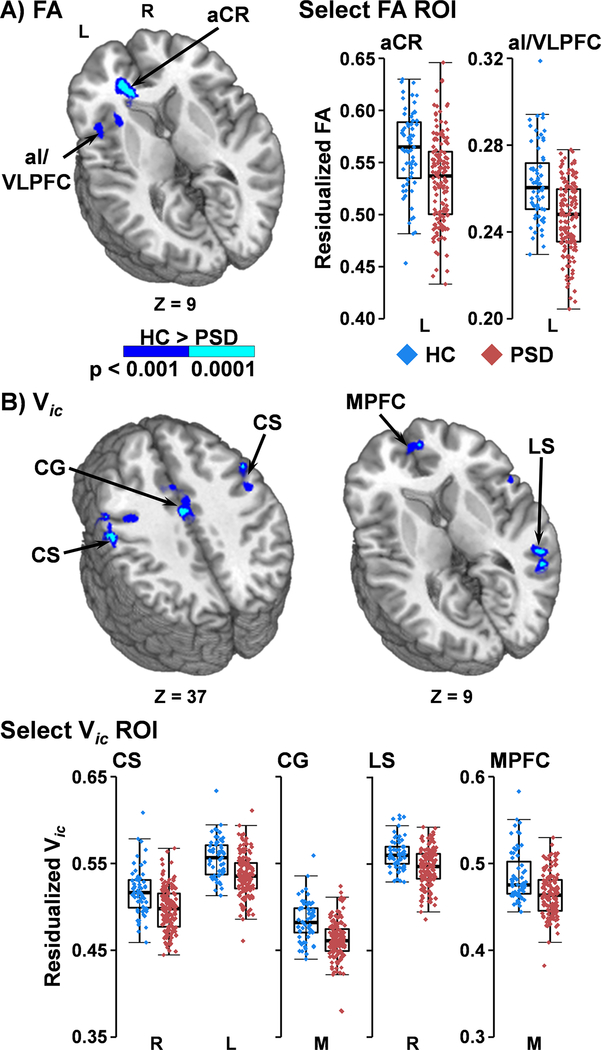
The second pattern (Figure 2A&B) indicated a combination of both atrophy (indicated by GMC main effects) and microstructural group differences in the left hemisphere. The left insula/VLPFC and STG were associated with decreased GMC and increased MD for PSD relative to HC. The left insula/VLPFC also exhibited a decrease in FA for PSD (Figure 3A).
Figure 2. Mean Diffusivity (MD):
Panel A depicts main effect of Group for greater MD (red: p<0.001; yellow: p<0.0001) in patients with psychotic spectrum disorders (PSD) relative to healthy controls (HC) within left (L) and right (R) hemispheres. Axial (Z) slice coordinates are provided according to the Talairach atlas. Areas include the anterior insula/ventrolateral prefrontal cortex (aI/VLPFC), superior temporal gyrus (STG), medial prefrontal cortex (MPFC), and temporal cortex along the lateral sulcus (LS). Panel B and C respectively displays box-and-scatter plots (PSD: red; HC: blue) for select regions that exhibited MD changes with (Panel B) or without (Panel C) overlapping changes in gray matter concentration (GMC).
Figure 3. Fractional Anisotropy (FA) and Intracellular Neurite Density (Vic):
Main effect of Group for greater FA (Panel A) and Vic volume (Panel B; dark blue: p<0.001; cyan: p<0.0001) for healthy controls (HC) relative to patients with psychotic spectrum disorders (PSD). Depicted regions include medial prefrontal cortex (MPFC), cingulate gyrus (CG), central sulcus (CS), anterior insular/ventrolateral prefrontal cortex (aI/VLPFC), anterior coronal radiata (aCR), and temporal cortex along the lateral sulcus (LS) within the left (L) or right (R) hemisphere. Axial (Z) slice coordinates are given according to the Talairach atlas. Box-and-scatter plots are displayed for select regions of interest for PSD (red) versus HC (blue).
The final pattern was characterized by changes in microstructure markers without evidence of atrophy (null GMC main effects). The majority of these findings involved either decreased Vic for PSD (Figure 3B; right superior/medial frontal gyrus, cingulate gyrus, and gray matter along the bilateral central sulci, extending into right DLPFC) or increased MD (Figure 2A&C; right insula; left inferior frontal gyrus/Sylvian cistern). Decreased Vic in conjunction with increased MD was observed in the right ventral lateral PFC, right STG/MTG/ITG along the lateral sulcus and bilateral medial PFC (Figure 3B). Decreased Vic in conjunction with decreased Viso was observed within the left superior longitudinal fasciculus. FA was decreased in the left corpus callosum, anterior corona radiata, DLPFC and ACG for PSD relative to HC (Figure 3A). Finally, Viso was increased in the right cerebral and cerebellar peduncle for PSD.
Supplemental analyses examined inclusion of the GMC×Group interaction term as a voxel-wise covariate. The Group main effects from principal and the supplementary analyses were similar for observed clusters (i.e., Figure S2 and Supplementary Results). Several ventricular, midline and sulcal regions demonstrated significant GMC×Group interactions for MD and Viso. However, additional testing indicated that these findings were primarily driven by 3 PSD with extreme atrophy (Figure S3), which was unexpected given the large sample size. Importantly, main effects of Group were similar with or without these three PSD in the analyses.
3.3. Relationship to Clinical Variables
Planned one-way ANCOVAs investigated whether DSM diagnosis, psychotic symptom load or global cognition were associated with the four major congruous directional differences observed in GMC, MD, FA or Vic. Overall models surviving Bonferroni-correction (0.05/4 = p<0.013) included GMC (F12,137=4.33, p≤0.001, partial-η2=0.28), MD (F12,137=2.54, p=0.005, partial-η2=0.18) and FA (F12,137=5.60, p≤0.001, partial-η2=0.33). Age accounted for the majority of significant variance in all models (all p’s≤0.017), while sex, olanzapine equivalence and illness duration were not significant. The only significant clinical effect (Figure S4) after controlling for nuisance variables involved a negative association between cognition and MD (β=−1.26×10−3; t=−2.53; p=0.013; partial-η2=0.04).
4. Discussion
Few studies have concurrently examined atrophy and microstructural abnormalities in gray and white matter in PSD, and how they in turn relate to traditional DSM diagnoses, symptom burden and cognition dysfunction (Nazeri et al., 2017). As expected, PSD exhibited decreased FA and Vic across gray and white matter, as well as increased MD in gray matter (Anderson et al., 2013; O’Donoghue et al., 2017; Pasternak et al., 2018; Nazeri et al., 2017), with the latter being more closely associated with GMC and cognitive impairment. PSD also exhibited evidence of increased atrophy within the bilateral medial temporal lobes and in subcortical structures surrounding the lateral ventricles (Table 2; Pattern 1).
Medial temporal lobe atrophy has been observed in several previous studies (Mathew et al., 2014; Bakhshi and Chance, 2015; Birur et al., 2017), including The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study (Mathew et al., 2014) and a recent mega-analysis on patterns of consistent GMC loss in SP (Gupta et al., 2015). This medial temporal lobe area did not show concurrent microstructural changes (Pattern 1) that were unrelated to atrophy. Neuropathological correlates of this atrophy include decreased size, number, and density of neurons, and are believed to be present at illness onset and non-progressive (static damage), supporting an early neurodevelopmental abnormality (Iritani, 2013; Takahashi and Suzuki, 2018). The medial temporal complex also plays a role in memory processes via its connections with frontal cortex (Morris et al., 1999; Vann et al., 2009), a connection that has previously been shown to be abnormal in PSD (O’Donoghue et al., 2017).
From a mechanistic perspective, a reduced neuropil in PSD should allow for an increase in extracellular free diffusion (MD/Viso), which typically would precede progressive atrophy (Bakhshi and Chance, 2015; Iritani, 2013; Narr et al., 2009). Consistent with this theory and similar to previous studies (Lee et al., 2009; Narr et al., 2009; Anderson et al., 2013), gray matter regions in the left STG and insula/VLPFC showed concurrent evidence of both increased atrophy and increased MD (Pattern 2). These regions have been reported to have the largest GMC deficits in SP by both meta- (Glahn et al., 2008) and mega- (Gupta et al., 2015) analysis. Moreover, evidence of increased MD in the absence of atrophy was present in homotopic fronto-temporal gray matter regions principally located along the right lateral sulcus, bilateral medial frontal cortex, right insula and VLPFC, and left Sylvian cistern. Increased MD may signal impending atrophy in certain regions not showing lower GMC (Pattern 3), which may become more apparent in larger (i.e., increased power; Gupta et al., 2015) or more elderly samples of PSD.
To the latter point, our purposeful recruitment of young to middle-aged PSD (maximum age of 54) may have diminished the strength of these emerging relationships (Bakhshi and Chance, 2015; Iritani, 2013). The predicted increased MD/Viso and deceased GMC were also evident when the interaction between diagnostic status and GMC values was modeled (Figure S3). However, the relationship within medial temporal and subcortical areas was primarily driven by a few patients with evidence of profound atrophy on structural scans (see Figure S3) rather than being representative of the entire PSD sample. Thus, caution must be employed when modeling the interaction between atrophy and dMRI findings, even with the relatively robust sample sizes used in the current study. Importantly, there was no evidence of changes in MD within white matter regions.
Other microstructural abnormalities included decreased Vic and FA for PSD. The distribution of neurites over the cortex has previously been found to positively correlate with estimated myelin content and negatively correlate with cortical thickness (Fukutomi et al., 2018). Thus, it was unexpected that gray matter regions showing reduced Vic in PSD did not co-occur with regions showing reduced GMC. A previous study in PSD (Nazeri et al., 2017) also found neurite density and cortical thickness to be independent of each other, with neurite density deficits observed in the temporal pole and parahippocampal region. In contrast, areas showing reduced Vic in PSD in the current study (i.e., frontal, lateral temporal regions, and along the central sulcus) were similar to those showing high neurite density and myelin content in HC (Fukutomi et al., 2018). Thus, current and previous results (Nazeri et al., 2017) suggest that decreased neurite density is mostly unrelated to atrophy and could potentially be a predictor of changes in myelin content. In contrast to other dMRI metrics that were primarily limited to gray matter, decreased FA was observed in both left frontal gray and white matter, but was not as widespread as some past studies have found. However, there is much heterogeneity in extent and location of FA abnormalities in PSD (Kelly et al., 2018; O’Donoghue et al., 2017; Pasternak et al., 2018). Current findings of reduced FA in the anterior corona radiata and corpus callosum are consistent with a large-scale meta-analysis of DTI data from SP that exhibited the greatest effect sizes (Cohen’s d=−0.40; Kelly et al., 2018).
Traditional DSM diagnosis, global cognitive scores and positive/negative symptom load were simultaneously entered into statistical models to predict observed atrophy and microstructural abnormalities. In contrast to previous PSD studies (Pearlson, 2015; Birur et al., 2017; Nazeri et al., 2017) demonstrating a linear trend (HC>BP-I>SA>SP), traditional DSM diagnoses were not associated with unique variance in terms of atrophy or microstructural changes. Similarly, a relationship between symptom burden and atrophy was not observed in current or previous studies in SP (Gupta et al., 2015), whereas B-SNIP reported an association between hippocampal volume and psychosis severity (Mathew et al., 2014). Current results of no significant relationships between DSM diagnosis and symptom load with atrophy and microstructural changes were not due to insufficient power according to effect sizes, but potentially a result of different statistical models (e.g., PSD vs. HC in the current study compared to linear model contrasts in previous studies). Interestingly, global cognitive functioning was associated with gray matter MD, further supporting the idea of MD being a potential marker of tissue integrity (Lee et al., 2009; Narr et al., 2009). There is limited previous research examining gray matter MD in relation to cognition in PSD. Spoletini et al. (2011) examined this relationship which was limited to subcortical structures in SP, and found increased MD in gray matter within thalamus, hippocampus and accumbens that correlated with working memory performance. Age, but not sex, illness duration, nor olanzapine equivalence, was a predictor of structural integrity, reinforcing this known relationship in normal aging (Pfefferbaum et al., 1994; Guerreri et al., 2019) and the need to account for age-related changes in these models while recognizing potential differences between groups. Collectively, current and previous (Hanlon et al., 2019) findings are generally consistent with the RDoC framework (Cuthbert, 2015), and suggest that symptom burden may better explain other state dependent measures (real-world functioning; Hanlon et al., 2019), while cognitive abilities may also be associated with long-term structural changes in gray matter.
Newer geometric models make several key assumptions about diffusion properties as well as the putative biological mechanisms that are measured (Jelescu and Budde, 2017), with some shown to create parameter bias (Lampinen et al., 2017). Importantly, our independent replication attempts (Mayer et al., 2017) within HC successfully validated that a strong statistical relationship (Ferguson, 2009) exists between ODI and FA, suggesting that both metrics measure similar underlying microstructure properties. In contrast, Vic was unrelated to FA and only moderately related to MD. Thus, although Vic was unrelated to clinical symptoms in the current study, it still provided unique information about the microstructural changes in PSD and other disease states (Mayer et al., 2017; Pasternak et al., 2018). Although moderate (white matter) or strong (gray matter) relationships existed between Viso and MD among HC, MD was both more sensitive to group differences and exhibited a strong relationship with cognitive deficits in PSD.
There are several limitations to the current study. First, differences in orders of magnitude exist between imaging tissue volume (e.g., 8 μl) versus the cellular milieu for a single axon (typically 8 × 10−9 μl), complicating the interpretation of dMRI measures of microstructure damage to actual cellular physiology (i.e., ground truth). Second, the current design cannot disambiguate several potential confounding factors (i.e., disease progression, continuity of treatments, and past substance abuse) or the true progression between atrophy and microstructural changes. In addition, while the inclusion of GMC as a covariate in the model was intended to statistically control for the effects of atrophy on diffusion measures, there is no way to completely account for this relationship or the effects atrophy may have on brain registration, which may potentially differ between groups. Third, SA patients were not well represented in the current sample in spite of our unbiased sampling methodology (i.e., all comers study). Importantly, the nosological status of SA has been controversial for some time (Madre et al., 2016; Maier, 2006), with poor diagnostic reliability, stability, and validity, resulting in excessive misdiagnosis for an intended uncommon disorder (Malaspina et al., 2013). Fourth, the series of regression models examining group comparisons of dMRI metrics did not correct for multiple comparisons, indicating the need for future replication. Finally, there are multiple statistical methodologies for analyzing RDoC datasets (Pearlson, 2015; Hanlon et al., 2019). The methodology adopted herein provides a direct method for probing unique variance in the relationship between structural markers, symptom burden, cognitive impairment and diagnostic status.
5. Conclusions
Current findings suggest spatially distinct patterns of gray matter atrophy relative to findings of decreased Vic and FA in a young/middle-aged sample of PSD. Both the spatial pattern of Vic findings and its relationship with more traditional DTI metrics in HC were unique, suggesting that Vic may provide complimentary information about changes in microstructural organization. Measurements corresponding to expanding microstructural spaces (MD) demonstrated the greatest spatial overlap with atrophy in the left STG and insula/VLPFC, with several homotopic regions also showing evidence of increased MD. The overarching purpose of the RDoC framework is to inform the field of more optimal classifications for common symptoms in mental illness based on dimensions of objective behavioral and neurobiological measures (Clark et al., 2017). To this end, increased gray matter MD was more closely linked with cognitive deficits relative to either symptom burden or traditional DSM diagnosis, suggesting that this particular structural abnormality potentially underlies global cognitive impairment that spans traditional diagnostic categories.
Supplementary Material
Acknowledgements and Funding Source:
This work was supported by the National Institutes of Health (grant number 1R01MH101512-03 to A.R.M.), who’s involvement was only financial.
Footnotes
Declarations of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability:
Data that support the findings of this study are openly available under the Research Domain Criteria (RDoC) Initiative at https://nda.nih.gov/edit_collection.html?id=2102.
References
- Altamura AC, Maggioni E, Dhanoa T, Ciappolino V, Paoli RA, Cremaschi L, Prunas C, Orsenigo G, Caletti E, Cinnante CM, Triulzi FM, Dell’Osso B, Yatham L, Brambilla P, 2018. The impact of psychosis on brain anatomy in bipolar disorder: A structural MRI study. J. Affect. Disord 233, 100–109. [DOI] [PubMed] [Google Scholar]
- Anderson D, Ardekani BA, Burdick KE, Robinson DG, John M, Malhotra AK, Szeszko PR, 2013. Overlapping and distinct gray and white matter abnormalities in schizophrenia and bipolar I disorder. Bipolar. Disord 15, 680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2000. Voxel-based morphometry--the methods. Neuroimage. 11, 805–821. [DOI] [PubMed] [Google Scholar]
- Bakhshi K, Chance SA, 2015. The neuropathology of schizophrenia: A selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neurosci 303, 82–102. [DOI] [PubMed] [Google Scholar]
- Birur B, Kraguljac NV, Shelton RC, Lahti AC, 2017. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder-a systematic review of the magnetic resonance neuroimaging literature. NPJ. Schizophr 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Womer FY, Edmiston EK, Bai C, Zhou Q, Jiang X, Wei S, Wei Y, Ye Y, Huang H, He Y, Xu K, Tang Y, Wang F, 2018. Neurobiological Commonalities and Distinctions Among Three Major Psychiatric Diagnostic Categories: A Structural MRI Study. Schizophr. Bull. 44, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Cuthbert B, Lewis-Fernandez R, Narrow WE, Reed GM, 2017. Three Approaches to Understanding and Classifying Mental Disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol. Sci Public Interest 18, 72–145. [DOI] [PubMed] [Google Scholar]
- Coryell W, Lavori P, Endicott J, Keller M, VanEerdewegh M, 1984. Outcome in schizoaffective, psychotic, and nonpsychotic depression. Course during a six- to 24-month follow-up. Arch. Gen. Psychiatry. 41, 787–791. [DOI] [PubMed] [Google Scholar]
- Cox R, Glen D, 2006. Efficient, robust, nonlinear, and guaranteed positive definite diffusion tensor estimation. Seattle, WA, Proceedings of the International Society for Magnetic Resonance and Medicine, 14th Scientific Meeting. [Google Scholar]
- Cox R, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI Clustering in AFNI: False Positive Rates Redux. Brain Connectivity. 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ, 1986. The continuum of psychosis and its implication for the structure of the gene. Br. J. Psychiatry. 149, 419–429. [DOI] [PubMed] [Google Scholar]
- Cui L, Chen Z, Deng W, Huang X, Li M, Ma X, Huang C, Jiang L, Wang Y, Wang Q, Collier DA, Gong Q, Li T, 2011. Assessment of white matter abnormalities in paranoid schizophrenia and bipolar mania patients. Psychiatry Res 194, 347–353. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, 2015. Research Domain Criteria: toward future psychiatric nosologies. Dialogues. Clin. Neurosci 17, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CJ, 2009. An effect size primer: A guide for clinicians and researchers. Professional Psychology: Research and Practice. 40, 532. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2012. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version, Administration Booklet American Psychiatric Pub, Arlington, VA. [Google Scholar]
- Fukutomi H, Glasser MF, Zhang H, Autio JA, Coalson TS, Okada T, Togashi K, Van Essen DC, Hayashi T, 2018. Neurite imaging reveals microstructural variations in human cerebral cortical gray matter. Neuroimage. 182, 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ, 2010. International consensus study of antipsychotic dosing. Am. J. Psychiatry. 167, 686–693. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT, 2008. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol. Psychiatry. 64, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreri M, Palombo M, Caporale A, Fasano F, Macaluso E, Bozzali M, Capuani S, 2019. Age-related microstructural and physiological changes in normal brain measured by MRI gamma-metrics derived from anomalous diffusion signal representation. Neuroimage. 188, 654–667. [DOI] [PubMed] [Google Scholar]
- Gupta CN, Calhoun VD, Rachakonda S, Chen J, Patel V, Liu J, Segall J, Franke B, Zwiers MP, Arias-Vasquez A, Buitelaar J, Fisher SE, Fernandez G, van Erp TG, Potkin S, Ford J, Mathalon D, McEwen S, Lee HJ, Mueller BA, Greve DN, Andreassen O, Agartz I, Gollub RL, Sponheim SR, Ehrlich S, Wang L, Pearlson G, Glahn DC, Sprooten E, Mayer AR, Stephen J, Jung RE, Canive J, Bustillo J, Turner JA, 2015. Patterns of Gray Matter Abnormalities in Schizophrenia Based on an International Mega-analysis. Schizophr. Bull. 41, 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon FM, Yeo RA, Shaff NA, Wertz CJ, Dodd AB, Bustillo JR, Stromberg SF, Lin DS, Abrams S, Liu J, Mayer AR, 2019. A symptom-based continuum of psychosis explains cognitive and real-world functional deficits better than traditional diagnoses. Schizophr. Res 208, 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Colbourne L, Harrison CH, 2018. The neuropathology of bipolar disorder: systematic review and meta-analysis. Mol. Psychiatry. 25, 1787–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani S, 2013. What happens in the brain of schizophrenia patients?: an investigation from the viewpoint of neuropathology. Nagoya J. Med. Sci 75, 11–28. [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, Moates AF, Lu H, Francis AN, Tandon N, Schretlen DJ, Sweeney JA, Clementz BA, Tamminga CA, 2013. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am. J. Psychiatry. 170, 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansons KM, Alexander DC, 2003. Persistent angular structure: new insights from diffusion magnetic resonance imaging data. Inverse Problems. 19, 1031. [DOI] [PubMed] [Google Scholar]
- Jelescu IO, Budde MD, 2017. Design and validation of diffusion MRI models of white matter. Front Phys 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K, 2005. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson. Med 53, 1432–1440. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A, 1999. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson. Med 42, 515–525. [PubMed] [Google Scholar]
- Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, Andreassen OA, Arango C, Banaj N, Bouix S, Bousman CA, Brouwer RM, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, Chen J, Chen JX, Chen X, Chiapponi C, Cho KK, Ciullo V, Corvin AS, Crespo-Facorro B, Cropley V, de RP, Diaz-Caneja CM, Dickie EW, Ehrlich S, Fan FM, Faskowitz J, Fatouros-Bergman H, Flyckt L, Ford JM, Fouche JP, Fukunaga M, Gill M, Glahn DC, Gollub R, Goudzwaard ED, Guo H, Gur RE, Gur RC, Gurholt TP, Hashimoto R, Hatton SN, Henskens FA, Hibar DP, Hickie IB, Hong LE, Horacek J, Howells FM, Hulshoff Pol HE, Hyde CL, Isaev D, Jablensky A, Jansen PR, Janssen J, Jonsson EG, Jung LA, Kahn RS, Kikinis Z, Liu K, Klauser P, Knochel C, Kubicki M, Lagopoulos J, Langen C, Lawrie S, Lenroot RK, Lim KO, Lopez-Jaramillo C, Lyall A, Magnotta V, Mandl RCW, Mathalon DH, McCarley RW, McCarthy-Jones S, McDonald C, McEwen S, McIntosh A, Melicher T, Mesholam-Gately RI, Michie PT, Mowry B, Mueller BA, Newell DT, O’Donnell P, Oertel-Knochel V, Oestreich L, Paciga SA, Pantelis C, Pasternak O, Pearlson G, Pellicano GR, Pereira A, Pineda ZJ, Piras F, Potkin SG, Preda A, Rasser PE, Roalf DR, Roiz R, Roos A, Rotenberg D, Satterthwaite TD, Savadjiev P, Schall U, Scott RJ, Seal ML, Seidman LJ, Shannon WC, Whelan CD, Shenton ME, Kwon JS, Spalletta G, Spaniel F, Sprooten E, Stablein M, Stein DJ, Sundram S, Tan Y, Tan S, Tang S, Temmingh HS, Westlye LT, Tonnesen S, Tordesillas-Gutierrez D, Doan NT, Vaidya J, van Haren NEM, Vargas CD, Vecchio D, Velakoulis D, Voineskos A, Voyvodic JQ, Wang Z, Wan P, Wei D, Weickert TW, Whalley H, White T, Whitford TJ, Wojcik JD, Xiang H, Xie Z, Yamamori H, Yang F, Yao N, Zhang G, Zhao J, van Erp TGM, Turner J, Thompson PM, Donohoe G, 2018. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry. 23, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Green MF, Nuechterlein KH, Deng BH, 2004. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr. Res 72, 11–19. [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, Brown TA, Carpenter WT, Caspi A, Clark LA, Eaton NR, Forbes MK, Forbush KT, Goldberg D, Hasin D, Hyman SE, Ivanova MY, Lynam DR, Markon K, Miller JD, Moffitt TE, Morey LC, Mullins-Sweatt SN, Ormel J, Patrick CJ, Regier DA, Rescorla L, Ruggero CJ, Samuel DB, Sellbom M, Simms LJ, Skodol AE, Slade T, South SC, Tackett JL, Waldman ID, Waszczuk MA, Widiger TA, Wright AGC, Zimmerman M, 2017. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J. Abnorm. Psychol 126, 454–477. [DOI] [PubMed] [Google Scholar]
- Lampinen B, Szczepankiewicz F, Martensson J, van WD, Sundgren PC, Nilsson M, 2017. Neurite density imaging versus imaging of microscopic anisotropy in diffusion MRI: A model comparison using spherical tensor encoding. Neuroimage. 147, 517–531. [DOI] [PubMed] [Google Scholar]
- Lee K, Yoshida T, Kubicki M, Bouix S, Westin CF, Kindlmann G, Niznikiewicz M, Cohen A, McCarley RW, Shenton ME, 2009. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a Diffusion Tensor Imaging study. Schizophr. Res 108, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madre M, Canales-Rodriguez EJ, Ortiz-Gil J, Murru A, Torrent C, Bramon E, Perez V, Orth M, Brambilla P, Vieta E, Amann BL, 2016. Neuropsychological and neuroimaging underpinnings of schizoaffective disorder: a systematic review. Acta Psychiatr. Scand 134, 16–30. [DOI] [PubMed] [Google Scholar]
- Maggioni E, Crespo-Facorro B, Nenadic I, Benedetti F, Gaser C, Sauer H, Roiz-Santianez R, Poletti S, Marinelli V, Bellani M, Perlini C, Ruggeri M, Altamura AC, Diwadkar VA, Brambilla P, 2017. Common and distinct structural features of schizophrenia and bipolar disorder: The European Network on Psychosis, Affective disorders and Cognitive Trajectory (ENPACT) study. PLoS. ONE. 12, e0188000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, 2006. Do schizoaffective disorders exist at all? Acta Psychiatr. Scand 113, 369–371. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Owen MJ, Heckers S, Tandon R, Bustillo J, Schultz S, Barch DM, Gaebel W, Gur RE, Tsuang M, van OJ, Carpenter W, 2013. Schizoaffective Disorder in the DSM-5. Schizophr. Res 150, 21–25. [DOI] [PubMed] [Google Scholar]
- Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ, Clementz B, Pearlson GD, Sweeney JA, Tamminga CA, Keshavan MS, 2014. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 71, 769–777. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ling JM, Dodd AB, Meier TB, Hanlon FM, Klimaj SD, 2017. A prospective microstructure imaging study in mixed-martial artists using geometric measures and diffusion tensor imaging: Methods and findings. Brain Imaging Behav 11, 698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya J, Kakeda S, Abe O, Goto N, Yoshimura R, Hori H, Ohnari N, Sato T, Aoki S, Ohtomo K, Nakamura J, Korogi Y, 2010. Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first-episode schizophrenia. Schizophr. Res 116, 196–203. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN, 1999. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta). Eur. J. Neurosci 11, 2506–2518. [DOI] [PubMed] [Google Scholar]
- Narr KL, Hageman N, Woods RP, Hamilton LS, Clark K, Phillips O, Shattuck DW, Asarnow RF, Toga AW, Nuechterlein KH, 2009. Mean diffusivity: a biomarker for CSF-related disease and genetic liability effects in schizophrenia. Psychiatry Res 171, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazeri A, Mulsant BH, Rajji TK, Levesque ML, Pipitone J, Stefanik L, Shahab S, Roostaei T, Wheeler AL, Chavez S, Voineskos AN, 2017. Gray Matter Neuritic Microstructure Deficits in Schizophrenia and Bipolar Disorder. Biol. Psychiatry. 82, 726–736. [DOI] [PubMed] [Google Scholar]
- Nazeri A, Schifani C, Anderson JAE, Ameis SH, Voineskos AN, 2020. In Vivo Imaging of Gray Matter Microstructure in Major Psychiatric Disorders: Opportunities for Clinical Translation. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging. 5, 855–864. [DOI] [PubMed] [Google Scholar]
- O’Donoghue S, Holleran L, Cannon DM, McDonald C, 2017. Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: A selective review of structural network analyses using diffusion MRI. J. Affect. Disord. 209, 217–228. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Kelly S, Sydnor VJ, Shenton ME, 2018. Advances in microstructural diffusion neuroimaging for psychiatric disorders. Neuroimage. 182, 259–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Kubicki M, Shenton ME, 2016. In vivo imaging of neuroinflammation in schizophrenia. Schizophr. Res 173, 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, 2015. Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annu. Rev. Clin. Psychol 11, 251–281. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO, 1994. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol 51, 874–887. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Nesvag R, Hagler DJ Jr., Bergmann O, Fennema-Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A, Melle I, Andreassen OA, Agartz I, Dale AM, 2012. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol. Psychiatry. 71, 552–560. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS, 1999. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 45, 17–25. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, Tamminga CA, Clementz BA, O’Neil K, Pearlson GD, 2013. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am. J. Psychiatry. 170, 886–898. [DOI] [PubMed] [Google Scholar]
- Spoletini I, Cherubini A, Banfi G, Rubino IA, Peran P, Caltagirone C, Spalletta G, 2011. Hippocampi, thalami, and accumbens microstructural damage in schizophrenia: a volumetry, diffusivity, and neuropsychological study. Schizophr. Bull. 37, 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarcina L, Bellani M, Rossetti MG, Perlini C, Delvecchio G, Dusi N, Barillari M, Ruggeri M, Altamura CA, Bertoldo A, Brambilla P, 2017. Similar white matter changes in schizophrenia and bipolar disorder: A tract-based spatial statistics study. PLoS. ONE. 12, e0178089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Munoz MS, Job D, Hall J, Bastin ME, Johnstone EC, Lawrie SM, McIntosh AM, 2009. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar. Disord 11, 11–18. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, 2018. Brain morphologic changes in early stages of psychosis: Implications for clinical application and early intervention. Psychiatry Clin. Neurosci 72, 556–571. [DOI] [PubMed] [Google Scholar]
- van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, Pearlson GD, Yao N, Fukunaga M, Hashimoto R, Okada N, Yamamori H, Bustillo JR, Clark VP, Agartz I, Mueller BA, Cahn W, de Zwarte SMC, Hulshoff Pol HE, Kahn RS, Ophoff RA, van Haren NEM, Andreassen OA, Dale AM, Doan NT, Gurholt TP, Hartberg CB, Haukvik UK, Jorgensen KN, Lagerberg TV, Melle I, Westlye LT, Gruber O, Kraemer B, Richter A, Zilles D, Calhoun VD, Crespo-Facorro B, Roiz-Santianez R, Tordesillas-Gutierrez D, Loughland C, Carr VJ, Catts S, Cropley VL, Fullerton JM, Green MJ, Henskens FA, Jablensky A, Lenroot RK, Mowry BJ, Michie PT, Pantelis C, Quide Y, Schall U, Scott RJ, Cairns MJ, Seal M, Tooney PA, Rasser PE, Cooper G, Shannon WC, Weickert TW, Morris DW, Hong E, Kochunov P, Beard LM, Gur RE, Gur RC, Satterthwaite TD, Wolf DH, Belger A, Brown GG, Ford JM, Macciardi F, Mathalon DH, O’Leary DS, Potkin SG, Preda A, Voyvodic J, Lim KO, McEwen S, Yang F, Tan Y, Tan S, Wang Z, Fan F, Chen J, Xiang H, Tang S, Guo H, Wan P, Wei D, Bockholt HJ, Ehrlich S, Wolthusen RPF, King MD, Shoemaker JM, Sponheim SR, de HL, Koenders L, Machielsen MW, van AT, Veltman DJ, Assogna F, Banaj N, de RP, Iorio M, Piras F, Spalletta G, McKenna PJ, Pomarol-Clotet E, Salvador R, Corvin A, Donohoe G, Kelly S, Whelan CD, Dickie EW, Rotenberg D, Voineskos AN, Ciufolini S, Radua J, Dazzan P, Murray R, Reis MT, Simmons A, Borgwardt S, Egloff L, Harrisberger F, Riecher-Rossler A, Smieskova R, Alpert KI, Wang L, Jonsson EG, Koops S, Sommer IEC, Bertolino A, Bonvino A, Di GA, Neilson E, Mayer AR, Stephen JM, Kwon JS, Yun JY, Cannon DM, McDonald C, Lebedeva I, Tomyshev AS, Akhadov T, Kaleda V, Fatouros-Bergman H, Flyckt L, Busatto GF, Rosa PGP, Serpa MH, Zanetti MV, Hoschl C, Skoch A, Spaniel F, Tomecek D, Hagenaars SP, McIntosh AM, Whalley HC, Lawrie SM, Knochel C, Oertel-Knochel V, Stablein M, Howells FM, Stein DJ, Temmingh HS, Uhlmann A, Lopez-Jaramillo C, Dima D, McMahon A, Faskowitz JI, Gutman BA, Jahanshad N, Thompson PM, Turner JA, 2018. Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol. Psychiatry. 84, 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA, 2009. What does the retrosplenial cortex do? Nat. Rev. Neurosci 10, 792–802. [DOI] [PubMed] [Google Scholar]
- Vita A, De PL, Deste G, Sacchetti E, 2012. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl. Psychiatry. 2, e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M, Velakoulis D, Whitford TJ, Pantelis C, 2011. Understanding aberrant white matter development in schizophrenia: an avenue for therapy? Expert. Rev. Neurother 11, 971–987. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC, 2012. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 61, 1000–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are openly available under the Research Domain Criteria (RDoC) Initiative at https://nda.nih.gov/edit_collection.html?id=2102.