Abstract
The average age at menarche declined in European and U.S. populations during the 19th and 20th centuries. The timing of pubertal events may have broad implications for chronic disease risks in aging women. Here we tested for associations of recalled menarcheal age with risks of 19 cancers in 536,450 women (median age, 60 years [range, 31-9 years]) in nine prospective US and European cohorts that enrolled participants from 1981 through 1998. Cox regression estimated multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CI) for associations of the age at menarche with risk of each cancer in each cohort and random-effects meta-analysis was used to generate summary estimates for each cancer. Over a median 10 years of follow-up, 60,968 women were diagnosed with a first primary incident cancer. Inverse linear associations were observed for seven of 19 cancers studied. Each additional year in the age at menarche was associated with reduced risks of endometrial cancer (HR:0.91, CI:0.89-0.94), liver cancer (HR:0.92, CI:0.85-0.99), melanoma (HR:0.95, CI:0.93-0.98), bladder cancer (HR:0.96, CI:0.93-0.99), and cancers of the colon (HR:0.97, CI:0.96-0.99), lung (HR:0.98, CI:0.96-0.99), and breast (HR: 0.98, 95% CI:0.93-0.99). All but one of these associations remained statistically significant following adjustment for baseline body mass index (BMI). Similarities in the observed associations between menarche and seven cancers suggest shared underlying causes rooted early in life. We propose as a testable hypothesis that early exposure to sex hormones increases mid-life cancer risks by altering functional capacities of stem cells with roles in systemic energy balance and tissue homeostasis.
INTRODUCTION
Menarche, defined as the first menstrual period, signals the initiation of monthly hormonal cycles and the reproductive lifespan. It is also the closing event of female puberty, a phase of accelerated growth, weight gain, and secondary sexual development (1).
In Western Europe and the United States, compiled records suggest that average ages at menarche declined by as much as 5 y between 1840 and 1940 (2, 3) and still vary substantially across population subgroups (3). More recent studies have documented similar declining trends in many other countries including developing nations (4). Historical and geographic variations in menarcheal age and its stereotypical alteration in response to modern life suggest a role for environmental factors experienced early in life (5, 6).
Early menarche has long been recognized as a risk factor for breast and endometrial cancers (7–9) and so it has been conceived narrowly as a risk factor for reproductive cancers with effects mediated through the lifetime duration of estrogen exposure. Recently, epidemiologic studies have demonstrated that women who experience early menarche are also at greater risks of cardiovascular diseases (10), type 2 diabetes (11), and premature mortality of all causes (10), suggesting broader consequences for women’s health than previously appreciated.
The full range of cancer risks associated with the age at menarche is not known. Multiple adequately-powered prospective studies have been done to test associations of menarche with the risks of cancers of the lung (12, 13), pancreas (14), colon or rectum (15, 16), ovary (17), and stomach (18), but the findings for these sites are inconsistent. Menarche has been studied as a risk factor for other cancers sporadically or in small studies only, including those of the liver (19), skin (20), brain (21), bladder (22), thyroid (23), and kidney (24). Since cancers other than breast and endometrial cancers constitute 71% of incident cancers among women globally (25), clarifying age at menarche’s association with risk of other cancers is essential for understanding its role in cancer overall. A broader understanding of associations across cancer types may also provide hints about biological mechanisms. If, for example, age at menarche is associated with many different cancer types (not just estrogen-driven cancers), then we might infer multiple pathways or a shared non-estrogen-mediated mechanism.
In order to cast a wide net, we have tested for associations of 19 site-specific cancer risks with the age at menarche in a large consortial dataset that includes 536,450 women enrolled in nine prospective cohorts in the U.S. and Europe. In secondary analyses, we assessed BMI at baseline and in young adulthood as potential confounders, and then tested whether associations differed by birth cohort, height, adiposity, parity, smoking history, or postmenopausal hormone use.
MATERIALS AND METHODS
Study Design and Population:
We used a pooled data resource developed originally through the National Cancer Institute Cohort Consortium for studies of obesity and mortality (26), and of leisure-time physical activity with cancer risks (27). Of the 10 cohorts in the physical activity project that included women, nine agreed to participate in the current analysis, including six from the U.S. and three from Europe. The present study includes the 536,450 women (>18 years of age) who had available data on age at menarche and attained height, who accrued at least one year of follow-up time, and who reported no history of any malignant cancer at baseline (Supplemental Table 1).
Assessment of age at menarche and covariates:
Participants in each study completed questionnaires at the start of study follow-up that inquired about age at menarche, demographics, and other participant characteristics. For the five cohorts that did not assess key variables on their baseline questionnaire but collected the data later, we redefined baseline as the date of the later questionnaire.
Cohorts assessed age at menarche by asking participants to report their age when they had their first menstrual period (see Supplementary Table 2). Participants wrote responses directly in six cohorts, and chose from pre-coded responses, e.g. age 10 to 11 years, in three cohorts. For statistical analyses, we assigned the age at menarche as the midpoint of pre-coded response options, e.g. age 11 for the category of 10 to 11 years.
We included in our models covariates that prior studies identified as major cancer risk factors29, though we acknowledge our list is not exhaustive. Covariates were harmonized as follows: age (continuous), smoking status (never, former, current), alcohol consumption (0, 0.1-14.9, 15.0-29.9, ≥30.0 g/d), race (black, white, other), education (less than high school, high school graduate, post–high school training, some college, college degree or greater), postmenopausal hormone therapy use (ever, never), oral contraceptive use (ever, never), age at menopause (premenopausal, 40-44, 45-49, 50-54, >55 years), parity (0, 1, 2, ≥3 children), height (continuous), and physical activity level (continuous). In select models (described below), we also adjusted for body mass index (BMI). Body mass index (BMI) was calculated for each participant as her self-reported weight in kilograms divided by the square of the self-reported height in meters and categorized as follows: <18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, 35.0-39.9, ≥40.0 kg/m2.
Cancer ascertainment:
Incident first primary cancers were identified by individual participating studies through follow-up questionnaires with subsequent review of medical records (28,29), cancer registry linkages (12, 30, 31) or both (23, 32–34). Cancer types were defined using the Surveillance Epidemiology and End Results site recode and the International Classification of Diseases for Oncology, Third Edition (for specific ICD codes used, see Supplemental Table 3) (35). As in our prior paper (27), we selected for analysis those cancer types that had at least 300 cases across studies, namely cancers of the bladder, brain, breast, colon, endometrium, stomach (non-cardia only), head and neck, kidney, liver, lung, ovary, pancreas, rectum, skin (melanoma), small intestine, and thyroid, as well as lymphocytic leukemia, myeloma, myeloid leukemia, and non-Hodgkin lymphoma (case numbers in Supplemental Table 4). For each cancer, only cohorts with at least 15 cases were included in analyses. Follow-up time was calculated from the date of questionnaire response to the date of first incident cancer diagnosis, death, or the end of follow-up within each study, whichever came first.
Statistical Methods:
To corroborate reports of declining menarcheal age over the 20th century, we examined the relationship of birth year with age at menarche in general linear models that adjusted for study cohort. Each birth year from 1905 to 1962 was modeled relative to pre-1905 birth years, and we calculated the mean ages at menarche and 95% confidence intervals (CIs) using least squares means.
Cox proportional hazards models were used to estimate covariate-adjusted hazard ratios (HR) and 95% CIs for each association of age at menarche with a site-specific cancer risk, using study time as the underlying time-metric. When modeling risks of ovarian and endometrial cancers, we excluded women who reported, at baseline, previous oophorectomy or hysterectomy; those with missing/unknown status for these outcomes (1.1% and 1.6%, of all participants, respectively) were included as though they had reported no history of these procedures at baseline. HRs were estimated for each additional year in the age at menarche, modeled as a continuous variable.
In our initial models, we used restricted cubic splines (stratified by cohort) and likelihood ratio tests to confirm that associations of menarche and cancer risks were consistent with linearity. Thereafter, our primary analyses evaluated age at menarche and cancer relationships as linear functions in each cohort and used random-effects meta-analysis to generate summary risk estimates across studies (36). Multiple imputation procedures were used to accommodate missing covariate data within each cohort (37), with the overall proportions of missing data as follows: age (0%), smoking status (1.7%), alcohol consumption (2.2%), race (1.6%), education (3.3%), postmenopausal hormone therapy use (0.6%), oral contraceptive use (0.5%), age at menopause (1.6%), parity (0.6%), height (0%), and physical activity level (3.1%). For models that adjusted for BMI, BMI had a missingness of (1.3%). We considered p<0.05 to be statistically significant, but also calculated the false discovery rate (38) for our primary findings to account for the increased type I error rate stemming from multiple testing. Statistical heterogeneity was evaluated using Cochran’s Q (39). We calculated the population attributable risk to estimate the percentage of cases that, theoretically, would be prevented among study participants if age at menarche occurred one year later, assuming causal relationships between age at menarche and cancer (40).
In order to evaluate anthropometric measures as confounders and/or mediators of the observed associations, we ran models that additionally adjusted for baseline BMI and, in six cohorts with available data, adjusted instead for BMI in young adulthood. In exploratory analyses, we also evaluated multiplicative effect modification by the median birth year (before 1934; in or after 1934), median height (<162.5 cm; ≥162.5 cm), by baseline BMI (<25 kg/m2; ≥25 kg/m2), smoking status (current; never smokers), postmenopausal hormone therapy (ever-; or never-users), country (USA v. Sweden) and follow-up time (<5 y follow up; ≥5 y follow up) using the Wald test for homogeneity. The tests for effect modification were based on dichotomous categories to minimize multiple testing, and median splits were used for these categories (where possible) to maximize statistical power. Interactions were declared if p<0.01.
We conducted several sensitivity analyses to evaluate whether results were robust. We tested models that used age instead of person-years as the underlying time metric and adjusted for birth year instead of age. To evaluate the influence of outliers, we reran analyses after excluding the 4% of participants whose age at menarche was 9 or younger or 17 or older and compared results. We tested the proportional hazards assumption using an interaction term for continuous age at menarche with follow-up time and used a Wald test to assess its statistical significance.
Statistical analyses were carried out using SAS 9.4 (Cary, North Carolina).
RESULTS
Study participants included pre- and postmenopausal women, with an overall median age of 60 years (range: 31-93 years) at study enrollment (Table 1); 95% of participants were White. In a pooled sample, later age at menarche was associated with older age at baseline, lower levels of education, never use of oral contraceptives, greater attained height, and lower baseline BMI (Supplementary Table 5). Age at menarche was also higher among participants born early in the 20th century compared to those born later (Figure 1). For example, participants born in 1905 had a mean menarcheal age of 13.4 years while those born in 1960 had a mean menarcheal age of 12.4 years. Thus, age at menarche appeared to decline by one year over the 55 year span we studied, or by 0.18 years per study decade. Selected characteristics of study participants are described by cohort in Supplementary table 6.
Table 1.
Selected participant characteristics according to cohort study
| Cohort* | Participants | Year of birth, median (range) | Age at menarche, median (IQR), y | Study baseline | Age at study baseline, median (range), y | Follow-up, median (maximum), y |
|---|---|---|---|---|---|---|
| AARP | 198,244 | 1934 (1925-45) | 13 (11-13) | 1995-97 | 62 (50-71) | 11 (11) |
| BCDDP | 38,511 | 1927 (1895-1948) | 13 (12-14) | 1987-89 | 60 (39-93) | 9 (11) |
| CPS-II | 80,821 | 1930 (1903-52) | 13 (12-13) | 1992-93 | 63 (40-91) | 14 (17) |
| IWHS | 37,537 | 1924 (1915-33) | 13 (12-14) | 1986 | 61 (52-70) | 20 (20) |
| PLCO | 29,211 | 1934 (1919-46) | 13 (13-15) | 1993-2003 | 62 (52-77) | 9 (13) |
| SMC | 32,716 | 1937 (1914-49) | 13 (12-14) | 1998 | 60 (47-83) | 10 (10) |
| USRT | 49,374 | 1950 (1905-66) | 12 (12-13) | 1994-98 | 45 (31-88) | 9 (12) |
| WHS | 39,334 | 1941 (1904-55) | 12 (12-13) | 1993-96 | 52 (39-90) | 17 (18) |
| WLH | 30,702 | 1952 (1942-62) | 13 (12-14) | 2003-04 | 51 (40-62) | 7 (7) |
| Total | 536,450 | 1934 (1895-1966) | 13 (11-14) | 1986-2004 | 60 (31-93) | 11 (20) |
Abbreviations: AARP, National Institutes of Health–AARP Diet and Health Study; BCDDP, Breast Cancer Detection and Demonstration Project; CPS II, Cancer Prevention Study II; IWHS, Iowa Women’s Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SMC, Swedish Mammography Cohort; USRT, US Radiologic Technologists Cohort; WHS, Women’s Health Study; WLH, Women’s Lifestyle and Health Study; IQR, interquartile range.
Figure 1. Mean age at menarche (95% confidence intervals) by year of birth and adjusted for cohort, in a pooled sample of 536,450 middle-aged women drawn from 9 cohort studies.
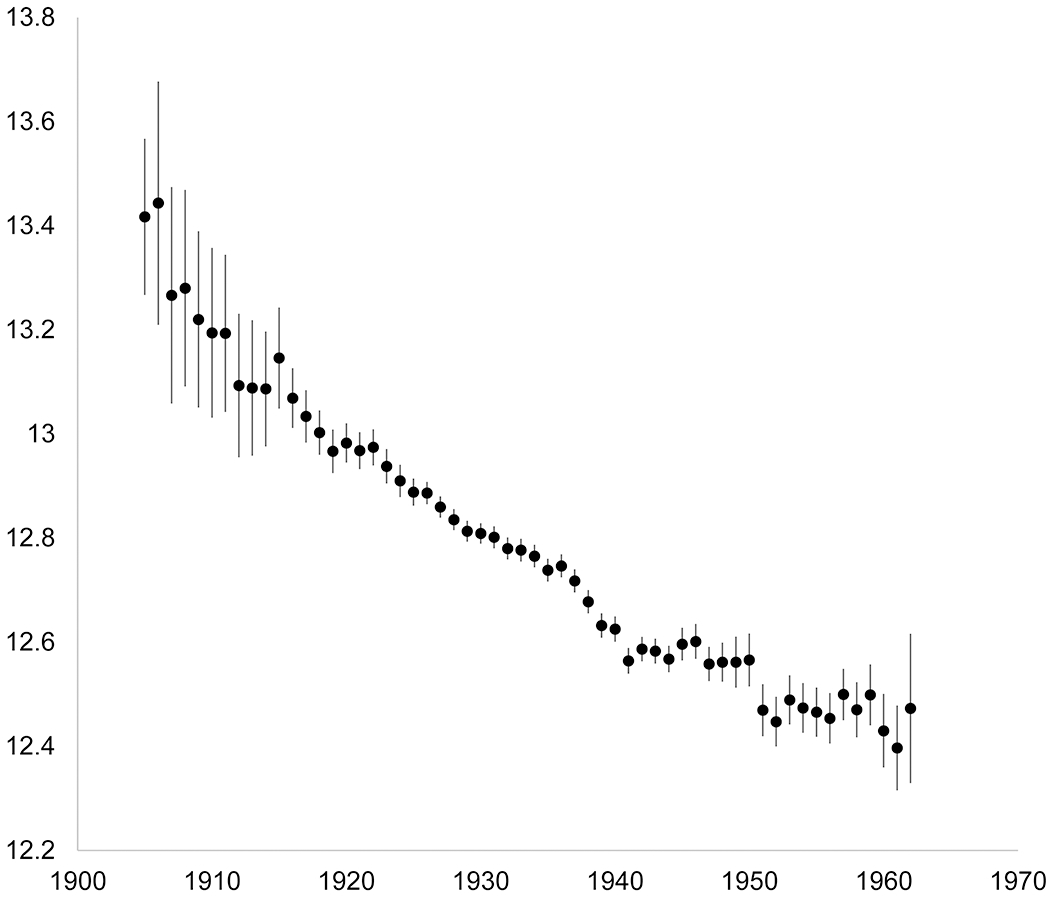
Data markers indicate the mean, and error bars, the 95% confidence intervals of the estimated mean.
Over the course of follow-up (median 10.5 years), we identified 60,968 incident cancers (occurring at a median age at diagnosis of 68.8 years; further details in Supplementary Table 7). In initial models, we used splines to evaluate the shape of the dose-response associations between age at menarche and cancer risk (See Figures 2 and 3 for cancers with statistically significant linear associations, and Supplemental Figure 1 for all other cancers). The associations were generally consistent with linearity, except for melanoma, for which the association was null at the youngest menarcheal ages but strongly inverse after age 12 y (Pnon-linearity=0.01). Because this association was still approximately linear, and because all other site-specific cancer risks were best modeled as linear functions, we based primary analyses on linear models.
Figure 2. Splines describing associations of cancer risks in middle-aged women with the age at menarche (y).
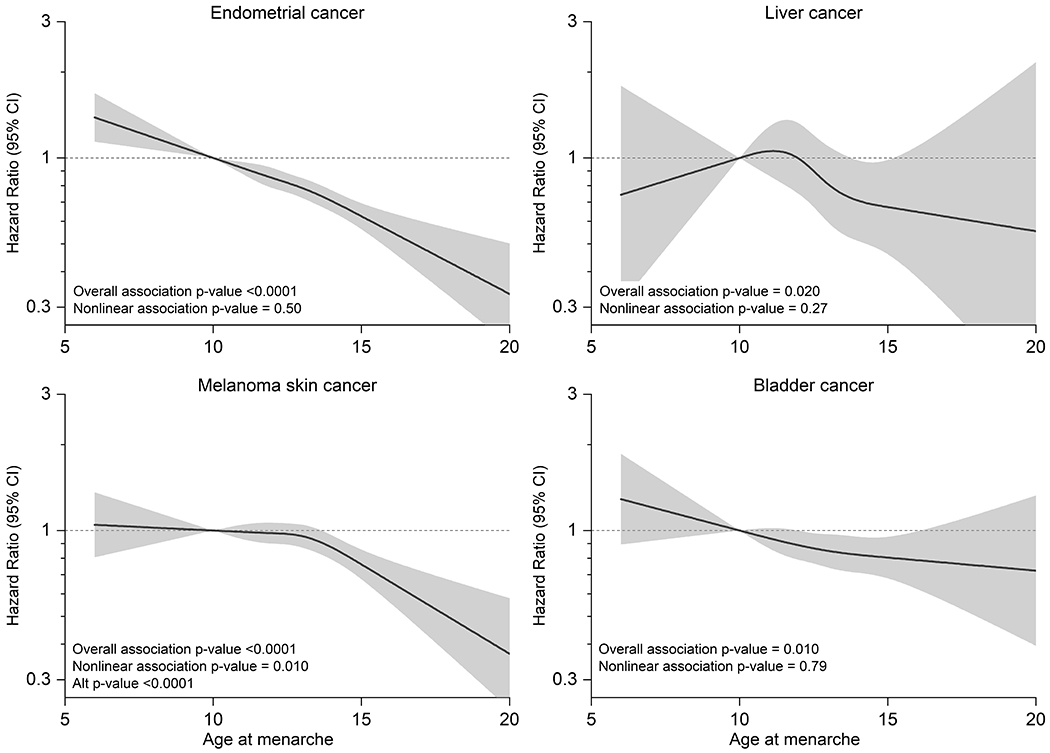
Non-linear associations of selected site-specific cancer risks with the age at menarche among middle-aged women drawn from 9 cohorts: A) endometrial cancer, B) liver cancer, C) bladder cancer and D) melanoma. Separate models were fit for each cancer type using cubic restricted splines in samples stratified by cohort. Models adjusted for baseline age, smoking, alcohol, race/ethnicity, education, and hormone replacement therapy, oral contraceptive use, age at menopause, parity and height.
Figure 3. Splines describing associations of cancer risks in middle-aged women with the age at menarche (y).
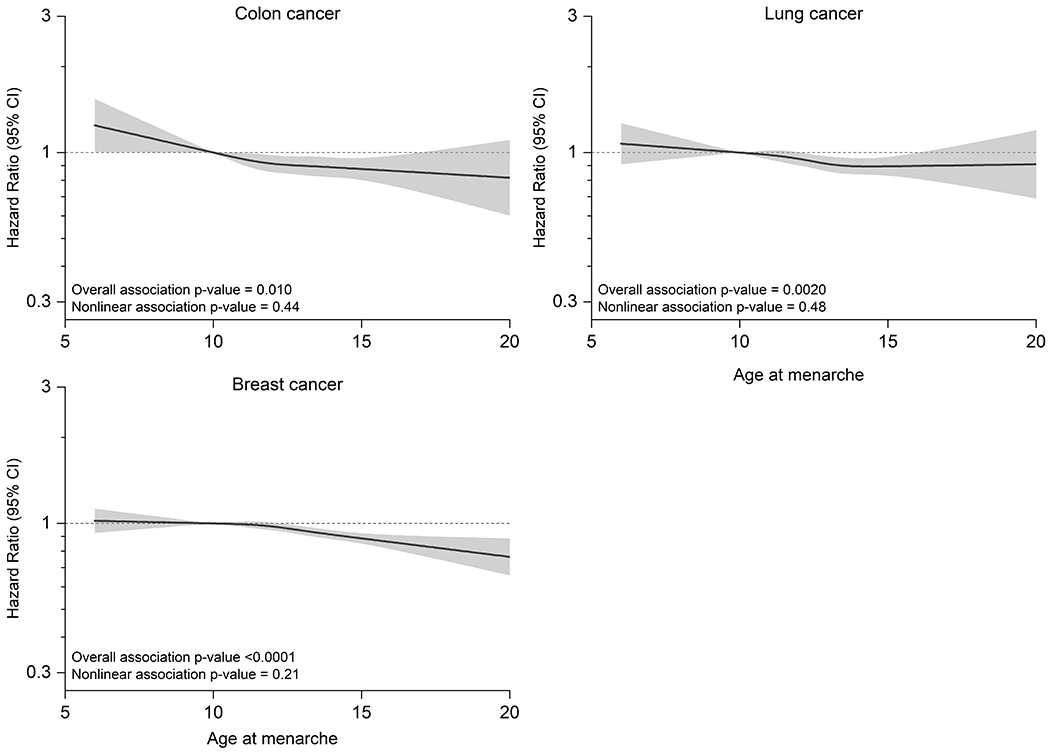
Non-linear associations of selected site-specific cancer risks with the age at menarche among middle-aged women drawn from 9 cohorts: A) lung cancer, B) colon cancer and C) breast cancer. Separate models were fit for each cancer type using cubic restricted splines in samples stratified by cohort. Models adjusted for baseline age, smoking, alcohol, race/ethnicity, education, and hormone replacement therapy, oral contraceptive use, age at menopause, parity and height.
In our primary analyses, we observed statistically significant inverse associations of age at menarche with the risks of 7 out of 19 cancers (Table 1 and Figure 4). Each additional year in the age at menarche was associated with a small but statistically significant reduction in the risks of endometrial cancer (HR:0.91, 95% CI:0.89-0.94), liver cancer (HR:0.92, 95% CI:0.85-0.99), melanoma skin cancer (HR:0.95, 95% CI:0.93-0.98), bladder cancer (HR:0.96, 95% CI:0.93-0.99), and cancers of the colon (HR:0.97, 95% CI:0.96-0.99), breast (HR: 0.98, 95% CI:0.93-0.99), and lung (HR:0.98, 95% CI:0.96-0.99) (Figure 3). Over the 19 cancers, the estimated false-discovery rate is 5%. This low rate suggests that few, if any, of our primary findings are due to chance. If the observed associations are assumed to be causal, cases attributable to the one-year shift in the age at menarche observed in our sample across birth years would represent 9.9% of endometrial cancer cases, 8.7% of liver cancer cases, 5.3% of melanoma cases, 4.2% of bladder cancer cases, 3.1% of colon cancer cases, 2.0% of lung cancer cases, and 2.0% of breast cancer cases, accounting for a total of 1,545 cancer diagnoses in a population of 536,450 middle-aged women per ~10-11 y of follow-up.
Figure 4. Summary estimates for multivariable-adjusted relative risks of 19 site-specific cancers for each additional year in the age at menarche among middle-aged women drawn from 9 cohorts.
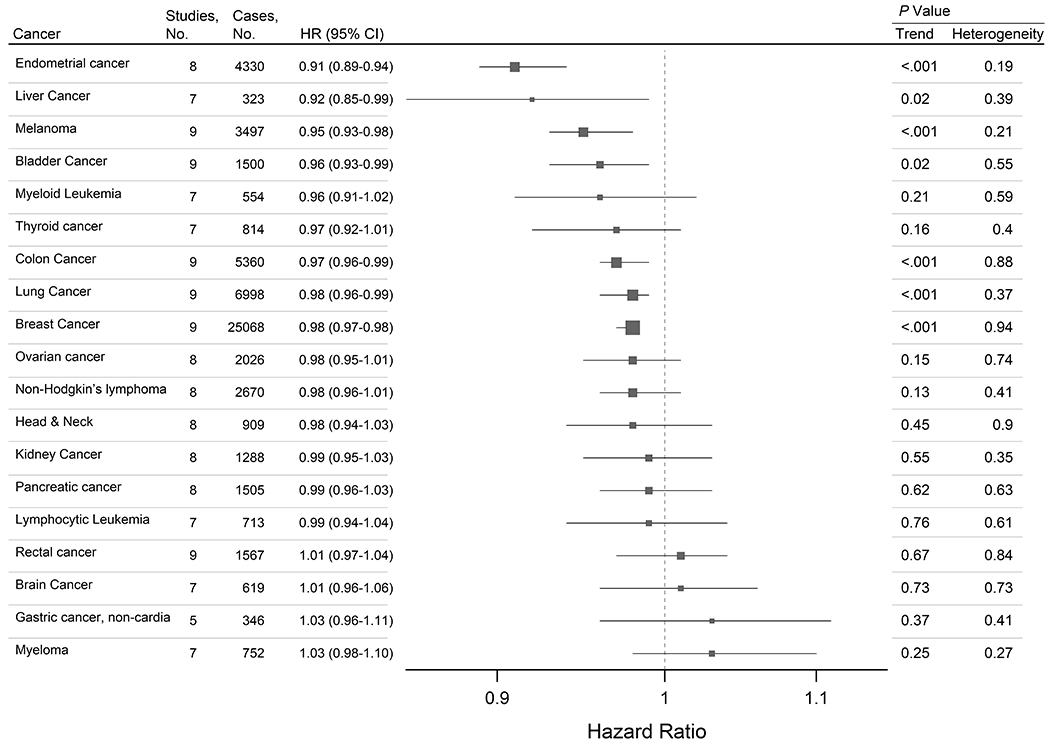
This forest plot describes summary estimates for multivariable-adjusted relative risks of site-specific cancers per additional year in the age at menarche among middle aged women drawn from 9 prospective cohorts. Cancers are ordered by effect size. Cox proportional hazard models adjusted for baseline age, smoking, alcohol, race/ethnicity, education, and hormone replacement therapy, oral contraceptive use, age at menopause, parity and height.
When models were additionally adjusted for baseline BMI, the summary HR were altered by <5% and remained statistically significant for each of these cancer sites, except liver (Table 2). Adjustment for BMI in young adulthood altered summary relative risks by less than 1% (Supplemental table 8).
Table 2.
Summary estimates for relative risks of site-specific cancers associated with the age at menarche, in multivariable models without, and with, additional adjustment for body mass index (BMI)
| Model 1* | Model 2† | Percent difference in HR | |||||
|---|---|---|---|---|---|---|---|
| HR | Ptrend | Phet | HR | Ptrend | Phet | ||
| Endometrial cancer | 0.91 (0.89-0.94) | <.001 | 0.19 | 0.95 (0.92-0.98) | <.001 | 0.16 | 4.0% |
| Liver cancer | 0.92 (0.85-0.99) | 0.02 | 0.39 | 0.94 (0.87-1.01) | 0.07 | 0.40 | 2.3% |
| Melanoma | 0.95 (0.93-0.98) | <.001 | 0.21 | 0.95 (0.93-0.99) | 0.002 | 0.25 | 0.0% |
| Bladder cancer | 0.96 (0.93-0.99) | 0.02 | 0.55 | 0.96 (0.93-1.00) | 0.03 | 0.49 | 0.4% |
| Myeloid Leukemia | 0.96 (0.91-1.02) | 0.21 | 0.59 | 0.98 (0.93-1.04) | 0.56 | 0.46 | 1.9% |
| Thyroid cancer | 0.97 (0.92-1.01) | 0.16 | 0.40 | 0.97 (0.93-1.02) | 0.18 | 0.50 | 0.3% |
| Colon cancer | 0.97 (0.96-0.99) | <.001 | 0.88 | 0.98 (0.96-1.00) | 0.04 | 0.76 | 0.6% |
| Lung cancer | 0.98 (0.96-0.99) | <.001 | 0.37 | 0.97 (0.95-0.99) | <.001 | 0.37 | −0.6% |
| Breast cancer | 0.98 (0.97-0.98) | <.001 | 0.94 | 0.98 (0.97-0.99) | <.001 | 0.91 | 0.6% |
| Ovarian cancer | 0.98 (0.95-1.01) | 0.15 | 0.74 | 0.98 (0.95-1.01) | 0.17 | 0.70 | 0.1% |
| Non-Hodgkin’s lymphoma | 0.98 (0.96-1.01) | 0.13 | 0.41 | 0.99 (0.96-1.01) | 0.26 | 0.52 | 0.5% |
| Head & Neck | 0.98 (0.94-1.03) | 0.45 | 0.90 | 0.98 (0.94-1.02) | 0.37 | 0.79 | −0.3% |
| Kidney cancer | 0.99 (0.95-1.03) | 0.55 | 0.35 | 1.01 (0.97-1.06) | 0.57 | 0.22 | 2.5% |
| Pancreatic cancer | 0.99 (0.96-1.03) | 0.62 | 0.63 | 1.00 (0.96-1.03) | 0.83 | 0.56 | 0.5% |
| Lymphocytic Leukemia | 0.99 (0.94-1.04) | 0.76 | 0.61 | 0.99 (0.95-1.05) | 0.84 | 0.53 | 0.3% |
| Rectal cancer | 1.01 (0.97-1.04) | 0.67 | 0.84 | 1.01 (0.98-1.05) | 0.50 | 0.91 | 0.4% |
| Brain cancer | 1.01 (0.96-1.06) | 0.73 | 0.73 | 1.01 (0.96-1.06) | 0.81 | 0.70 | −0.3% |
| Gastric cancer, non-cardia | 1.03 (0.96-1.11) | 0.37 | 0.41 | 1.03 (0.96-1.11) | 0.39 | 0.38 | 0.0% |
| Myeloma | 1.03 (0.98-1.10) | 0.25 | 0.27 | 1.04 (0.99-1.10) | 0.17 | 0.26 | 0.6% |
Model 1 included adjustments for baseline age, smoking, alcohol, race/ethnicity, education, and hormone replacement therapy, oral contraceptive use, age at menopause, parity and height.
Model 2 included adjustments for baseline age, smoking, alcohol, race/ethnicity, education, and hormone replacement therapy, oral contraceptive use, age at menopause, parity and height and BMI.
We tested for effect modification by selected covariates including birth year, baseline age, baseline BMI, parity, smoking status, height, use of menopausal hormones, and nation (Supplemental tables 9–16). Effect modification was not observed by baseline age, BMI, parity or country. The association of age at menarche with endometrial cancer risk was more strongly inverse in never- compared to current-users of hormone therapy (HRnever user=0.89, 95% CI=0.86-0.93 vs. HRcurrent user=0.96, 95% CI=0.93-0.99, Pinteraction= 0.01). Additionally, qualitative interactions were observed by height for ovarian cancer (HRtall=0.92, 95% CI=0.87-0.98 vs. HRshort=1.05, 95% CI=0.98-1.12, Pinteraction=0.01), and by smoking status for thyroid cancer (HRnever=0.90, 95% CI=0.85-0.95 vs. HRever=1.09, 95% CI=1.00-1.19, Pinteraction<0.001).
In sensitivity analyses, models run after excluding participants with outlying menarcheal ages yielded substantively similar results (Supplemental Table 17). Hazard ratios for a few cancers were stronger in this analysis (e.g. for liver cancer, the HR changed from 0.92 to 0.88), but the differences did not appear to be systematic across cancers.
DISCUSSION
In this pooled analysis of 536,450 middle-aged and elderly women drawn from nine cohorts in the U.S. and Europe, we observed that each additional year in the age at menarche was associated with modest but statistically significant reductions in the risks of seven cancers, including endometrial and liver cancers, melanoma skin cancers, and cancers of the bladder, colon, lung and breast. These associations showed no evidence of heterogeneity by cohort and were robust to adjustment for baseline BMI.
Our findings for the two cancers with the strongest priors, endometrial and breast cancers, are consistent with previous literature showing inverse risk associations with menarcheal age (7, 8, 9). The effect sizes observed here are similar to summary estimates from pooled or meta-analyses (7, 9). In a Mendelian randomization studies, genetic scores predicting early menarche were associated with higher risks of endometrial and breast cancers, respectively, suggesting that the consistently observed risk associations are not a result of confounding (41). In the present analysis we observed that the association of menarcheal age with endometrial cancer risk was modestly attenuated by adjustment for baseline BMI. Thus, this association is likely mediated, in part, through the association of early menarche with greater mid-life adiposity (42).
With respect to the cancer outcomes with inconsistent epidemiologic support, we observed inverse linear associations of the age at menarche with risks of colon and lung cancers, but not with risks of pancreatic, rectal, ovarian or gastric (non-cardia) cancers.
In a meta-analysis that included 11 case-control and 11 cohort studies of the association of colorectal cancer with menarcheal age, substantial heterogeneity was observed across study-specific estimates, and a non-statistically significant summary estimate was just under 1.00; findings remained null and heterogeneous whether stratified on study design, study quality, population, exposure assessment, anatomic cancer site or subsite (15). In analyses carried out in NIH-AARP, investigators observed effect modification by menopausal hormone use, with inverse associations observed in never-, but not ever-users (16). In the present study, which includes NIH-AARP, associations of menarcheal age with colon cancer did not differ by history of menopausal hormone use.
Our finding of an inverse linear association of lung cancer risk with menarcheal age contrasts with a recent meta-analysis that included more than 20 studies and observed non-significant summary estimates of association and substantial heterogeneity across studies (13). It is possible that the distribution of lung cancer subtypes influences findings—one previous study showed inverse associations of small cell and adenocarcinoma lung cancer risks with menarcheal age, and direct associations for some other subtypes (12) In the present analysis, we did not have data on lung cancer subtypes.
Among cancers that have not been frequently studied in association with reproductive factors, we observed inverse associations for cancers of the liver, skin, and bladder, but not for the remaining outcomes.
The inverse association of liver cancer risk with menarcheal age observed here is consistent with two previous studies. In a consortial analysis drawing data from 11 prospective studies (seven of which are also included in the present study) with 203 incident cases of hepatocellular carcinoma, McGlynn et al. observed a monotonic but non-significant inverse trend (OR14+ vs. <12 y=0.64, 95% CI: 0.40, 1.03, Ptrend=0.09) (43). In another retrospective study, with 218 cases of incident hepatocellular carcinoma, early menarche was strongly associated with increased risk in hepatitis B carriers (multivariable-adjusted OR<12 vs. >16 y=6.96; 95% CI, 2.52-19.18) but not in non-carriers (Pinteraction=0.005) (44). In the present study no data on viral infections was available to address this issue.
Few studies have reported on melanoma risks associated with reproductive factors and most have been underpowered or retrospective. Our finding of an inverse risk association is consistent with a previous prospective study, in which significantly reduced risk of melanoma was observed in association with each additional year in the age at menarche (RR≥15 vs. 13-14 y =0.67, 95% CI: 0.46, 0.97, ptrend=0.30) (45).
Several studies have tested associations of bladder cancer risk with menarcheal age and other reproductive factors. In the large NIH-AARP cohort, with 651 incident cases of incident bladder cancer observed among women, Daugherty et al. found reduced risk in association with late menarche and a significant trend across categories (HRage >15 vs. <10 y =0.57, 95% CI 0.39-0.84, Ptrend=0.02) (22). Other studies were smaller and had mixed findings (36, 46).
One potential explanation for our findings is confounding by year of birth, which was strongly associated with the age at menarche, and may also be associated with trends in the prevalence of cancer risk factors and thus, with cancer incidence rates. We saw little evidence of confounding in models stratified by the median year of birth and findings were similar in models adjusted for year of birth instead of baseline age.
In other stratified models, we observed a stronger inverse association between age at menarche and endometrial cancer risk among never- vs. current-users of hormone therapy. This pattern echoes that observed for BMI, which is known to be more strongly associated with endometrial cancer risk among never hormone users than current users (46). Presumably, this occurs because BMI’s estrogenic effects are blunted in hormone therapy users and it is conceivable that age at menarche’s hormonal effects are similarly blunted in this group. The qualitative interactions observed for ovarian cancer (by height) and thyroid cancer (by smoking status) are harder to explain but could reflect small case numbers and/or chance.
The seven menarche-cancer associations identified here may encompass several distinct etiologic explanations but could also be arise as a result of a common cause, either associated with, or mediated by, variations in pubertal development.
In the populations of developed nations, later menarche is associated with greater attained height and with lower BMI in childhood (47) and in midlife (48). In the present study sample made up mostly of postmenopausal women, we observed similar associations of menarcheal timing with baseline anthropometric measures. Of note, incidence of each of the seven cancers shown here to be associated with the age at menarche has previously been associated with adult BMI (49). The addition of baseline BMI to our models attenuated many of the observed associations slightly but, with the exception of liver cancer, all remained statistically significant, suggesting a BMI-independent role for menarcheal age in cancer pathogenesis. We also observed that the risk associations were not substantially attenuated by adjustment for young adult BMI, a retrospective measure more proximal to menarche, though still not its antecedent. It is worth noting that there is little evidence in the literature to support an effect of early-life BMI, independent of mid-life BMI, on any cancer risks with the notable exception of pre-menopausal breast cancer (50). A large mature prospective study with measures of many early-life factors will be required to fully address the question of whether the timing of menarche represents a cause, an intermediate, or a correlate of a true early-life cause of cancers in aging women.
Associations of breast and endometrial cancers with menarcheal age have often been framed as reflecting the duration of women’s exposure to repeated menstrual cycles, with estrogens as the causal agent, and precancerous epithelium, the target (7, 51, 52). The cancers observed to be associated with menarche in the present study are, however, heterogeneous with respect to etiologic roles for estrogen. Breast and endometrial tumors express estrogen receptors and higher circulating estrogens predict increased risk of these cancers in postmenopausal women (53, 54). Similarly, estrogen receptors are observed in bladder tumors, and at least one subtype can be promoted via an estrogen-mediated signaling pathway (55). In contrast, although hepatocellular carcinoma cells also express estrogen receptors, exposure to estrogens reduces their proliferation and survival (56). Among the seven cancers, distinct sex ratios are observed among incident cases (57). Similarly, differences in their associations with menopausal hormone use (58) and with early menopause (7,8,12,34) also suggest that estrogen exposure is not the shared causal pathway.
An emerging explanation for the effects of early-life risk factors on cardiovascular health suggests that in utero exposure to corticosteroids and metabolic stress causes epigenetically-mediated alterations in the hypothalamic-pituitary-adrenal axis associated with earlier puberty and predisposition to insulin resistance (59). Early adrenarche may contribute directly to a lifelong susceptibility to metabolic syndrome when the premature rise of adrenal androgens limits the number or proliferative capacities of mesenchymal stem cell (MSC)-derived pre-adipocytes seeded to subcutaneous fat depots (60,61). Subsequent exposures to chronically elevated insulin and inflammation could contribute directly to the development of cancers later in life (62). In addition, as women age, premature exhaustion or senescence of the MSCs found in bone marrow, which play key roles in tissue repair and homeostasis, may change tissue microenvironments in ways that permit or promote carcinogenesis (63).
Strengths of the present study include large sample size, and prospective, individual-level data, that has been harmonized and analyzed using a uniform approach. The modest effect sizes observed here suggest that power is limited for the rarer cancers. The age at menarche has previously been shown to be reported by middle-aged women with excellent reliability (64), but only modest validity compared to self-report in adolescence (65). Because the age at menarche was reported before any cancer diagnosis, errors would likely bias estimates of association towards the null. The consortial dataset did not include all known risk factors for some cancers, and so we were not able to adjust for the effects of family histories of cancer, viral infections, dietary habits or occupational exposures. Our data includes only cohorts from developed nations, and most participants were of European ancestry, perhaps limiting the generalizability of our findings.
In conclusion, we observed similar, inverse associations of seven cancers with the age at menarche. Although the effects of early menarche on site-specific cancer risks experienced by individual women are modest, these findings suggest that population-level shifts towards earlier menarche impose a cumulative burden on public health by adding to the incidence of multiple cancers. An understanding of the environmental factors that influence the timing of menarche and of the causal pathways that mediate its association with cancer risks could suggest new approaches for cancer prevention.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Age at menarche is associated with risk for seven cancers in middle-aged women, and understanding the shared underlying causal pathways across these cancers may suggest new avenues for cancer prevention.
ACKNOWLEDGEMENTS
This work, the NIH-AARP Diet and Health study, the Breast Cancer Detection Demonstration Project (BCDDP) Follow-up Study, the U.S. Radiologic Technologists (USRT) cohort study, and longitudinal follow-up of the Prostate, Lung, Colon and Ovarian Cancer Trial (PLCO) cohort were each supported by the Intramural Research Program of the National Cancer Institute (NIH), National Institutes of Health (NIH), Department of Health and Human Services (DHHS) (Z99 CA999999): S. Moore, C. Kitahara, A. Berrington de González, M. Linet, N. Freedman, L. Liao, C. Matthews, R. Stolzenberg-Solomon, R. Ziegler, R. Hoover. The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study-II cohort: M. Gaudet, A. Patel. The Iowa Women’s Health Study was supported by a grant from the NCI (R01 CA39742): K. Robien, A. Prizment. The Swedish Mammography Cohort was supported by the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare and the Swedish Cancer Foundation: A. Wolk. The WHS was supported by grants from the NCI and the National Heart, Lung, and Blood Institute (CA047988, CA182913, HL043851, HL080467, and HL099355): J. Buring, I. Lee. The Women’s Lifestyle and Health project was supported by the Swedish Cancer Society and the Swedish Research Council: A. Wolk. The funding organizations had no role in the study design, or in the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Patton GC, Viner R. Pubertal transitions in health. The Lancet. 2007;369:1130–9. [DOI] [PubMed] [Google Scholar]
- 2.Tanner JM, Eveleth PB. Worldwide variation in human growth. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- 3.McDowell MA, Brody DJ, Hughes JP. Has Age at Menarche Changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Journal of Adolescent Health. 2007;40:227–31. [DOI] [PubMed] [Google Scholar]
- 4.Parent A-S, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon J-P. The Timing of Normal Puberty and the Age Limits of Sexual Precocity: Variations around the World, Secular Trends, and Changes after Migration. Endocrine Reviews. 2003;24:668–93. [DOI] [PubMed] [Google Scholar]
- 5.Mishra GD, Cooper R, Tom SE, Kuh D. Early Life Circumstances and Their Impact on Menarche and Menopause. Women’s Health. 2009;5:175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Aloisio AA, DeRoo LA, Baird DD, Weinberg CR, Sandler DP. Prenatal and Infant Exposures and Age at Menarche: Epidemiology. 2013;24:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. The Lancet Oncology. 2012;13:1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McPherson CP, Sellers TA, Potter JD, Bostick RM, Folsom AR. Reproductive Factors and Risk of Endometrial Cancer The Iowa Women’s Health Study. American Journal of Epidemiology. 1996;143:1195–202. [DOI] [PubMed] [Google Scholar]
- 9.Gong T-T, Wang Y-L, Ma X-X. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Scientific Reports [Internet]. 2015. [cited 2019 Mar 18];5. Available from: http://www.nature.com/articles/srep14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw K-T, et al. Early Age at Menarche Associated with Cardiovascular Disease and Mortality. The Journal of Clinical Endocrinology & Metabolism. 2009;94:4953–60. [DOI] [PubMed] [Google Scholar]
- 11.Lakshman R, Forouhi N, Luben R, Bingham S, Khaw K, Wareham N, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia. 2008;51:781–6. [DOI] [PubMed] [Google Scholar]
- 12.Brinton LA, Gierach GL, Andaya A, Park Y, Schatzkin A, Hollenbeck AR, et al. Reproductive and Hormonal Factors and Lung Cancer Risk in the NIH-AARP Diet and Health Study Cohort. Cancer Epidemiology Biomarkers & Prevention. 2011;20:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Yin Z, Shen L, Wan Y, Zhou B. Menstrual Factors, Reproductive Factors and Lung Cancer Risk: A Meta-analysis. 2012;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro Silvera SA, Miller AB, Rohan TE. Hormonal and Reproductive Factors and Pancreatic Cancer Risk: A Prospective Cohort Study. Pancreas. 2005;30:369–74. [DOI] [PubMed] [Google Scholar]
- 15.Li C-Y, Song B, Wang Y-Y, Meng H, Guo S-B, Liu L-N, et al. Age at Menarche and Risk of Colorectal Cancer: A Meta-Analysis. Miao X-P, editor. PLoS ONE. 2013;8:e65645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zervoudakis A, Strickler HD, Park Y, Xue X, Hollenbeck A, Schatzkin A, et al. Reproductive History and Risk of Colorectal Cancer in Postmenopausal Women. JNCI Journal of the National Cancer Institute. 2011;103:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braem MGM, Onland-Moret NC, van den Brandt PA, Goldbohm RA, Peeters PHM, Kruitwagen RFPM, et al. Reproductive and Hormonal Factors in Association With Ovarian Cancer in the Netherlands Cohort Study. American Journal of Epidemiology. 2010;172:1181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman ND, Chow W-H, Gao Y-T, Shu X-O, Ji B-T, Yang G, et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007;56:1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong G-C, Liu Y, Chen N, Hao F-B, Wang K, Cheng J-H, et al. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: a systematic review and dose–response meta-analysis of observational studies. Human Reproduction Update. 2016;23:126–38. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher RP, Elwood JM, Hill GB, Coldman AJ, Threlfall WJ, Spinelli JJ. Reproductive factors, oral contraceptives and risk of malignant melanoma: Western Canada Melanoma Study. British Journal of Cancer. 1985;52:901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabat GC, Park Y, Hollenbeck AR, Schatzkin A, Rohan TE. Reproductive factors and exogenous hormone use and risk of adult glioma in women in the NIH-AARP Diet and Health Study. International Journal of Cancer. 2011;128:944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daugherty SE, Lacey JV, Pfeiffer RM, Park Y, Hoover RN, Silverman DT. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study: MHT and bladder cancer. International Journal of Cancer. 2013;133:462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braganza MZ, de Gonzalez AB, Schonfeld SJ, Wentzensen N, Brenner AV, Kitahara CM. Benign Breast and Gynecologic Conditions, Reproductive and Hormonal Factors, and Risk of Thyroid Cancer. Cancer Prevention Research. 2014;7:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karami S, Daugherty SE, Schonfeld SJ, Park Y, Hollenbeck AR, Grubb RL, et al. Reproductive Factors and Kidney Cancer Risk in 2 US Cohort Studies, 1993–2010. American Journal of Epidemiology. 2013;177:1368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 26.Kitahara CM, Flint AJ, Berrington de Gonzalez A, Bernstein L, Brotzman M, MacInnis RJ, et al. Association between Class III Obesity (BMI of 40–59 kg/m2) and Mortality: A Pooled Analysis of 20 Prospective Studies. Khaw K-T, editor. PLoS Medicine. 2014;11:e1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore SC, Lee I-M, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Internal Medicine. 2016;176:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SM, Moore SC, Lin J, Cook NR, Manson JE, Lee I-M, et al. Folate, Vitamin B6, Multivitamin Supplements, and Colorectal Cancer Risk in Women. American Journal of Epidemiology. 2006;163:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigurdson AJ, Doody MM, Rao RS, Freedman DM, Alexander BH, Hauptmann M, et al. Cancer incidence in the U.S. radiologic technologists health study, 1983–1998. Cancer. 2003;97:3080–9. [DOI] [PubMed] [Google Scholar]
- 30.Larsson SC, Håkansson N, Giovannucci E, Wolk A. Folate Intake and Pancreatic Cancer Incidence: A Prospective Study of Swedish Women and Men. JNCI: Journal of the National Cancer Institute. 2006;98:407–13. [DOI] [PubMed] [Google Scholar]
- 31.Roswall N, Sandin S, Adami H-O, Weiderpass E. Cohort Profile: The Swedish Women’s Lifestyle and Health cohort. International Journal of Epidemiology. 2017;46:e8–e8. [DOI] [PubMed] [Google Scholar]
- 32.Leitzmann MF, Moore SC, Peters TM, Lacey JV, Schatzkin A, Schairer C, et al. Prospective study of physical activity and risk of postmenopausal breast cancer. Breast Cancer Research [Internet]. 2008. [cited 2019 Mar 18];10. Available from: http://breast-cancer-research.biomedcentral.com/articles/10.1186/bcr2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hildebrand JS, Gapstur SM, Campbell PT, Gaudet MM, Patel AV. Recreational Physical Activity and Leisure-Time Sitting in Relation to Postmenopausal Breast Cancer Risk. Cancer Epidemiology Biomarkers & Prevention. 2013;22:1906–12. [DOI] [PubMed] [Google Scholar]
- 34.Prizment AE, Anderson KE, Harlow BL, Folsom AR. Reproductive risk factors for incident bladder cancer: Iowa Women’s Health Study. International Journal of Cancer. 2006;120:1093–8. [DOI] [PubMed] [Google Scholar]
- 35.Organization WH. International classification of diseases for oncology (ICD-O) – 3rd edition, 1st revision. 3rd ed. 2013. [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary Clinical Trials. 2015;45:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafer JL. Multiple Imputation. Analysis of Incomplete Multivariate Data. Chapman and Hall; 1997. page 104–17. [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 39.Cochran W The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 40.Rothman K, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 41.Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nature Genetics. 2017;49:834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Risch H, Lu L, Irwin ML, Mayne S, Schwartz P, et al. Joint Effect of Genotypic and Phenotypic Features of Reproductive Factors on Endometrial Cancer Risk. Scientific Reports [Internet]. 2015. [cited 2019 Mar 18];5. Available from: http://www.nature.com/articles/srep15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGlynn KA, Sahasrabuddhe VV, Campbell PT, Graubard BI, Chen J, Schwartz LM, et al. Reproductive factors, exogenous hormone use and risk of hepatocellular carcinoma among US women: results from the Liver Cancer Pooling Project. British Journal of Cancer. 2015;112:1266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu M-W, Chang H-C, Chang S-C, Liaw Y-F, Lin S-M, Liu C-J, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38:1393–400. [DOI] [PubMed] [Google Scholar]
- 45.Kvaskoff M, Bijon A, Mesrine S, Boutron-Ruault M-C, Clavel-Chapelon F. Cutaneous Melanoma and Endogenous Hormonal Factors: A Large French Prospective Study. American Journal of Epidemiology. 2011;173:1192–202. [DOI] [PubMed] [Google Scholar]
- 46.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body Mass Index, Hormone Replacement Therapy, and Endometrial Cancer Risk: A Meta-Analysis. Cancer Epidemiology Biomarkers & Prevention. 2010;19:3119–30. [DOI] [PubMed] [Google Scholar]
- 47.Biro FM, Pajak A, Wolff MS, Pinney SM, Windham GC, Galvez MP, et al. Age of Menarche in a Longitudinal US Cohort. Journal of Pediatric and Adolescent Gynecology. Elsevier; 2018;31:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallicchio L, Flaws JA, Smith RL. Age at menarche, androgen concentrations, and midlife obesity: findings from the Midlife Womenʼs Health Study. Menopause. 2016;23:1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi EK, Park HB, Lee KH, Park JH, Eisenhut M, van der Vliet HJ, et al. Body mass index and 20 specific cancers: re-analyses of dose–response meta-analyses of observational studies. Annals of Oncology. 2018;29:749–57. [DOI] [PubMed] [Google Scholar]
- 50.Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Research [Internet]. 2005. [cited 2020 Aug 26];7. Available from: http://breast-cancer-research.biomedcentral.com/articles/10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. “Hormonal” risk factors, “breast tissue age” and the age-incidence of breast cancer. Nature. 1983;303:767–70. [DOI] [PubMed] [Google Scholar]
- 52.Yang HP, Murphy KR, Pfeiffer RM, George N, Garcia-Closas M, Lissowska J, et al. Lifetime Number of Ovulatory Cycles and Risks of Ovarian and Endometrial Cancer Among Postmenopausal Women. American Journal of Epidemiology. 2016;183:800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endogenous Hormones and Breast Cancer Collaborative Group. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. British Journal of Cancer. 2011;105:709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinton LA, Trabert B, Anderson GL, Falk RT, Felix AS, Fuhrman BJ, et al. Serum Estrogens and Estrogen Metabolites and Endometrial Cancer Risk among Postmenopausal Women. Cancer Epidemiology Biomarkers & Prevention. 2016;25:1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godoy G, Gakis G, Smith CL, Fahmy O. Effects of Androgen and Estrogen Receptor Signaling Pathways on Bladder Cancer Initiation and Progression. Bladder Cancer. 2016;2:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Li Z. Interplay of estrogen receptors and FOXA factors in the liver cancer. Molecular and Cellular Endocrinology. 2015;418:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, et al. Sex Disparities in Cancer Incidence by Period and Age. Cancer Epidemiology Biomarkers & Prevention. 2009;18:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chlebowski RT, Anderson GL. Menopausal hormone therapy and cancer: Changing clinical observations of target site specificity. Steroids. 2014;90:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reynolds RM. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis – 2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38:1–11. [DOI] [PubMed] [Google Scholar]
- 60.Gupta V, Bhasin S, Guo W, Singh R, Miki R, Chauhan P, et al. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Molecular and Cellular Endocrinology. 2008;296:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansilla E, Díaz Aquino V, Zambón D, Marin GH, Mártire K, Roque G, et al. Could Metabolic Syndrome, Lipodystrophy, and Aging Be Mesenchymal Stem Cell Exhaustion Syndromes? Stem Cells International. 2011;2011:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollak MN. Insulin, insulin-like growth factors, insulin resistance, and neoplasia. Am J Clin Nutr. 2007;86:820S–2S. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed ASI, Sheng MH, Wasnik S, Baylink DJ, Lau K-HW. Effect of aging on stem cells. World Journal of Experimental Medicine. 2017;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lundblad MW, Jacobsen BK. The reproducibility of self-reported age at menarche: The Tromsø Study. BMC Women’s Health [Internet]. 2017. [cited 2019 Mar 18];17. Available from: http://bmcwomenshealth.biomedcentral.com/articles/10.1186/s12905-017-0420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth MEJ, et al. Validity of age at menarche self-reported in adulthood. Journal of Epidemiology & Community Health. 2006;60:993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


