Abstract
Background & Aims:
Pregnant women may transmit their metabolic phenotypes to their offspring, enhancing the risk for nonalcoholic fatty liver disease (NAFLD); however, the molecular mechanisms remain unclear.
Methods:
Prior to pregnancy female mice were fed either a maternal normal-fat diet (NF-group, “no effectors”), or a maternal high-fat diet (HF-group, “persistent effectors”), or were transitioned from a HF to a NF diet before pregnancy (H9N-group, “effectors removal”), followed by pregnancy and lactation, and then offspring were fed high-fat diets after weaning. Offspring livers were analyzed by functional studies, as well as next-generation sequencing for gene expression profiles and DNA methylation changes.
Results:
The HF, but not the H9N offspring, displayed glucose intolerance and hepatic steatosis. The HF offspring also displayed a disruption of lipid homeostasis associated with an altered methionine cycle and abnormal one-carbon metabolism that caused DNA hypermethylation and L-carnitine depletion associated with deactivated AMPK signaling and decreased expression of PPAR-α and genes for fatty acid oxidation. These changes were not present in H9N offspring. In addition, we identified maternal HF diet-induced genes involved in one-carbon metabolism that were associated with DNA methylation modifications in HF offspring. Importantly, the DNA methylation modifications and their associated gene expression changes were reversed in H9N offspring livers.
Conclusions:
Our results demonstrate for the first time that maternal HF diet disrupted the methionine cycle and one-carbon metabolism in offspring livers which further altered lipid homeostasis. CpG islands of specific genes involved in one-carbon metabolism modified by different maternal diets were identified.
Keywords: obesity, methionine, SAM transferase, fatty acid oxidation, DNA methylation
Lay Summary
To understand how maternal highfat (HF) diet may enhance the risk for nonalcoholic fatty liver disease (NAFLD) and to explore a potential dietary strategy to prevent such detrimental effects, we reported that maternal HF diet disrupted one-carbon metabolism in offspring, possibly due to altered DNA methylation of one-carbon metabolism genes. Disrupted one-carbon metabolism further broke lipid homeostasis in offspring livers via repressing fatty acid oxidation involving L-carnitine depletion. Importantly, an early maternal diet intervention prevented offspring NAFLD via restoring one-carbon metabolism to normal diet levels.
1. Introduction
To date, the rapid rise in obesity and obesity-associated diseases worldwide has had a major negative impact on human health and healthcare resources. Non-alcoholic fatty liver disease (NAFLD), which is regarded as the hepatic manifestation of the metabolic syndrome associated with obesity, hyperinsulinemia, and peripheral insulin resistance, affects 10 to 24% of the general population in various countries, and the prevalence of NAFLD has been reported to be as high as 75% in obese people. In recent years, changes in diet composition and decreases in physical activity have resulted in the number of NAFLD patients increasing and incidence shifting to a younger age (1). It is now well established that in-utero and early-life exposure to under- or overnutrition can disrupt normal development and growth, changing the phenotypes of offspring to increase susceptibility to future diseases (2). In humans, infants exposed to overnutrition during gestation experience increased risks of obesity, diabetes and other complications, including NAFLD (3). In murine models, exposure to a high-fat diet (HFD) in-utero worsens the NAFLD phenotype in offspring mice exposed to a Western-style HFD during adulthood (4, 5). Using a Japanese macaque non-human primate (NHP) model, McCurdy et al. reported steatosis and increased hepatic oxidative stress in fetuses from mothers fed with an HFD during the third trimester (6). These studies suggest that overnutrition in utero “primes” the metabolic capacity of the liver, potentiating postnatal metabolic liver disease in offspring.
A growing body of animal studies have provided evidence that maternal and paternal diet effects during the prenatal, neonatal, or pubertal stages of development can cause epigenetic changes that are associated with obesity, metabolic syndrome, and fatty liver diseases in the offspring (7). Epigenetic regulation, including DNA and histone methylation, is dependent on the one-carbon metabolic pathway, which includes the methionine cycle, for supply of methyl group donors. Deficiencies in nutrients involved in one-carbon metabolism and transfer, such as folic acid, vitamin B6, vitamin B12, choline and methionine, can alter epigenetic profiles, suggesting a key role for one-carbon metabolism in the regulation of DNA methylation (8, 9). However, the mechanisms underlying how maternal dietary modulation affects offspring one-carbon metabolism and epigenetic profiles remain unclear.
Previously, we have reported that an early maternal transition from an HF diet to an NF diet before pregnancy is sufficient to prevent or attenuate the observed “reprograming” effect, where the maternal HF diet promotes offspring obesity and NAFLD (10–12). Therefore, we used this model, together with the maternal HF diet mouse model, in the present study to explore the molecular mechanisms underlying these effects and to identify the molecular pathways affected by maternal HF diets that prime the offspring liver for later development of NAFLD.
2. Materials and Methods
Experimental design (Table 1)
Table 1.
Study Design for maternal diet transition from HF to NF diet before pregnancy.
| Maternal Diet | Offspring Diet | |||
|---|---|---|---|---|
| Pre-pregnancy | Transition | Pregnancy/Lactation | After Weaning | |
| REF (n=15) | NF | NA | NF | NF |
| NF (n=15) | NF | NA | NF | HF |
| HF (n=15) | HF | NA | HF | HF |
| H9N (n=15) | HF | NF | NF (9 weeks) |
HF |
Diet description:
REF Rodent diet: normal chow diet (4% kcal. from fat)
NF Rodent diet: 10% kcal from fat.
HF Rodent diet: 60% kcal from fat.
Four-week-old female mice of mixed background (B6/129/SvEv) were selected for this study and were fed either a normal fat diet (NF group, 10% kcal from fat, n=5 litters) or a high fat diet (60% kcal from fat) for 12 weeks. The female mice on the HF diet either continued to be on an HF diet throughout gestation and lactation (HF group, n=5 litters) or were transitioned to a NF diet for 9 weeks (H9N group, n=5 litters) prior to pregnancy. The H9N group was maintained on the NF diet throughout gestation and lactation. We have previously evaluated different transition periods from a maternal NF to HF diet including H1N (HF + 1 Week transition), H5N (HF + 5 Weeks) and H9N (HF+9W). Both H1N and H5N diet caused more severe obesity and NAFLD phenotypes in offspring compared to HF diet, however, the H9N offspring were glucose tolerance and did not develop NAFLD induced by postnatal HF diet for 12-week (10–13).
Offspring mice from all of the groups were fed a HF diet for 12 weeks after weaning, to promote weight gain prior to sacrifice. A separate group, born from the breeders who adhered to the normal chow diet (CD), was continuously fed the CD diet for 12 weeks and was used as a reference control group (the REF group, n=3 litters). Mouse experiments were completed according to a protocol that was reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University, in compliance with the USA Public Health Service Policy on Humane Care and Use of Laboratory Animals.
3. Results
3.1. The maternal HF diet promoted body weight gain, glucose intolerance and NAFLD in offspring, while the H9N diet did not result in glucose intolerance and NAFLD.
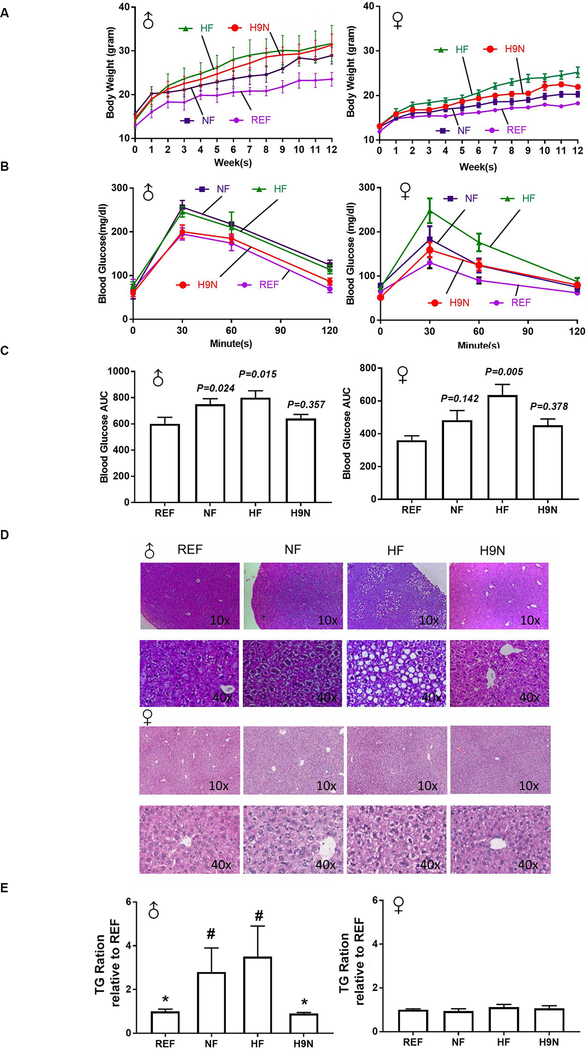
Both male and female offspring had similar body weights at weaning, regardless of the type of maternal diet intervention used. The male REF offspring slowly gained body weight on a postnatal NF diet and maintained the lowest body weights during the experimental period. Beginning week 6, the male HF offspring were heavier than the male REF offspring, and the male HF offspring maintained higher body weights through the 12-week experimental period (Figure 1A). However, comparing male pups of mothers exposed to the NF, HF or H9N diet there were no differences in body weight (Figure 1A). Female offspring on the postweaning HF diet (NF, HF and H9N groups) grew faster and gained more body weight than the female REF offspring. By repeated measurements, the body weight gain throughout the experimental period, from the highest to the lowest, was ranked as follows: HF>H9N>NF>REF.
Figure 1. The maternal HF diet promoted offspring body weight gain, glucose intolerance and NAFLD, while the H9N diet did not result in offspring glucose intolerance and NAFLD.
1A. Body weights of male (♂) and female (♀) offspring were recorded weekly, after weaning, for 12 weeks. 1B. IPGTT was measured at the end of week 12 in male (♂) and female offspring (♀). 1C. The areas under the curve (AUCs) were calculated for the IPGTT results in male (♂) and female offspring (♀). 1D. H&E staining was performed on the liver tissue of male (♂) and female (♀) offspring. 1E-F. Hepatic TG concentrations were determined in male (♂) and female (♀) offspring. Data are mean ± SE, n=15. * indicates P<0.05 vs. the NF group. # indicates P<0.05 vs. the REF group.
We performed the intraperitoneal injected glucose tolerance test (IPGTT) to examine offspring glucose tolerance at week 12 post-weaning. All offspring displayed peak glucose levels 30 minutes after the glucose challenge, which gradually returned to baseline over the next 90 minutes. For male offspring of mothers exposed to HF and NF diets, we observed a significantly increased area under the curve (AUC), indicating that they experienced glucose intolerance (Figure 1B and C); female offspring only had a significantly increased AUC for the HF offspring (Figure 1C). Both the male and female H9N offspring displayed similar patterns of changes in glucose levels and similar AUC values as those observed for the REF offspring, suggesting that they were glucose tolerant (Figure 1B and C).
We next evaluated whether different maternal diet interventions could differentially promote NAFLD in offspring fed with a postweaning obesogenic diet. Sex-related changes in the liver were observed (Figure 1D). For males, the NF offspring displayed very mild signs of hepatocyte lipid accumulation: the nuclei remained centrally located and appeared to be indented by small fat droplets. The HF offspring showed obvious lipid accumulation in hepatocytes, with widely distributed ballooning cells. In contrast to the HF offspring, the H9N offspring displayed overall normal hepatocytes. For female offspring, no obvious fatty liver signs were observed, except in the HF offspring, whose hepatocytes showed degeneration with a very similar phenotype as that observed in the NF male offspring. The histology observations were consistent with our hepatic triglyceride (TG) measurements (Figure 1E).
3.2. RNA-Seq analysis identified significant changes in biological processes involved in lipid and glucose metabolism and one-carbon metabolism associated with different maternal diets
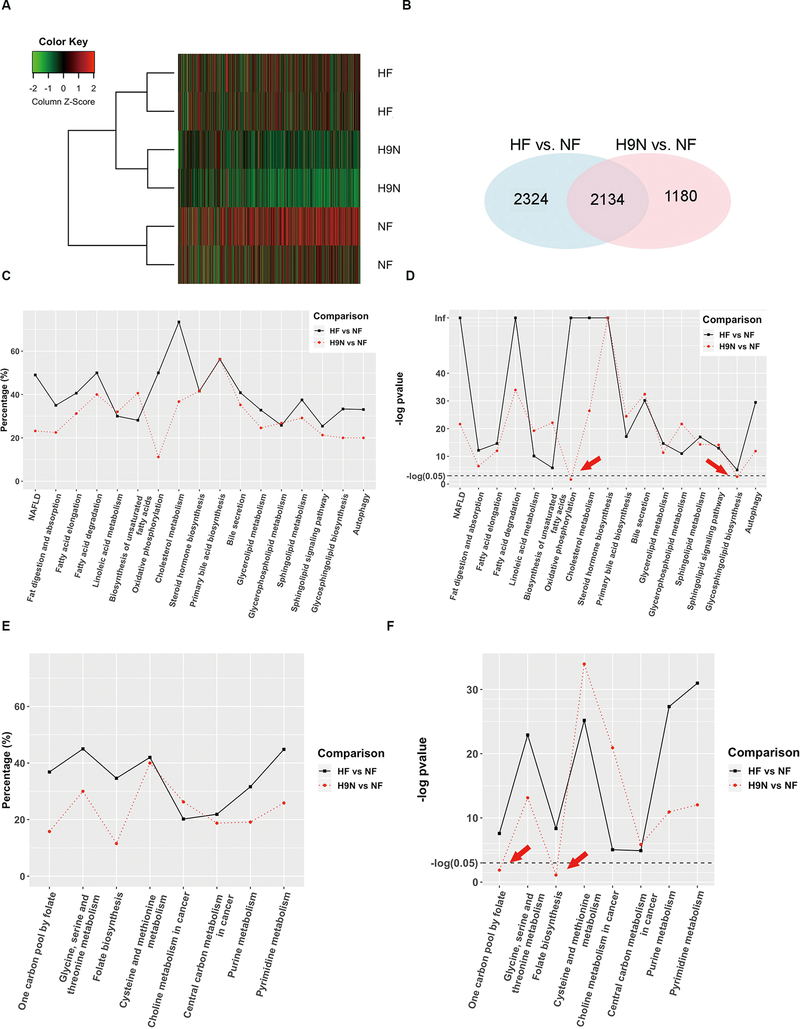
To determine the molecular mechanisms that may contribute to the different phenotypes observed in the HF and H9N offspring and to identify important genes and signaling pathways potentially contributing to these differences, we performed RNA-seq analysis on NF, HF, and H9N livers. For each sample, 20–25 million short reads were generated, using an Illumina HiSeq 2000, and > 90% of short reads were mapped to the mouse reference genome GRCm38. Hierarchical clustering analysis showed that HF offspring were clustered with H9N offspring, which were distinct from the NF offspring cluster (Figure 2A). We identified 4,458 and 3,314 differentially expressed genes (DEGs) for HF and H9N offspring, respectively, when compared with NF offspring, using a false discovery rate of 0.05 as the cutoff. A total of 2,134 DEGs overlapped between HF and H9N offspring, which accounted for about 47.6% and 63.4% of their DEGs, respectively (Figure 2B).
Figure 2. RNA-Seq analysis identified significant changes in lipid and glucose metabolism and one-carbon metabolism associated with the different maternal diet interventions.
2A. RNA-seq analysis, by an Illumina HiSeq 2000, was performed on NF, HF and H9N offspring livers. Hierarchical clustering analysis was performed on 2,000 genes. 2B. Differentially expressed genes (DEGs) identified in HF and H9N offspring compared with NF offspring, using a false discovery rate of 0.05 as the cutoff. Overlapping DEGs between the HF and H9N offspring when compared with NF offspring are shown in the Venn diagram. 2C-D. Multiple pathways were identified that are involved in lipid and fatty acid metabolism (C and D) associated with maternal diet intervention (E and F). In HF offspring, more than 25% of the genes involved in various lipid metabolism pathways were identified as DEGs, with five pathways having DEGs encompassing 50% or more of the genes (C). In general, H9N offspring had fewer DEGs associated with most of the signaling pathways. Compared with the HF offspring, the H9N offspring had 50% fewer DEGs for the oxidative phosphorylation and the cholesterol metabolism KEGG pathways (C). However, the H9N offspring had more DEGs associated with the pathway for biosynthesis of unsaturated fatty acids than the HF offspring. Two pathways, oxidative phosphorylation and glycosphingolipid biosynthesis, were not significantly associated with the H9N diet (D, indicated by red arrow). 2E-F. Multiple pathways were identified that are involved in folate and methionine metabolism associated with maternal diet intervention (E and F). Most pathways, other than two cancer-related pathways, had over 25% of genes differentially expressed in HF offspring. When we compared DEGs in HF and H9N mice, three pathways (the one carbon pool by folate pathway, the glycine, serine and threonine metabolism pathway and the folate biosynthesis pathway) contained 50% fewer DEGs in H9N offspring than in HF offspring. While all of these pathways were associated with the HF diet, the one carbon pool by folate and the folate biosynthesis pathways were not significantly associated with the H9N diet (F, indicated by red arrow).
In addition, gene ontology (GO) analysis identified distinct KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways associated with the HF and H9N diets. Specifically, we identified multiple pathways involved in lipid and fatty acid metabolism (Figure 2C and D), insulin activity and glucose metabolism (Suppl. Fig. 1), and folate and methionine metabolism (Figure 2E and F). While all of these signaling pathways in lipid and fatty acid metabolism were associated with the HF diet, two pathways, oxidative phosphorylation and glycosphingolipid biosynthesis, were not significantly associated with the H9N diet (Figure 2D, P>0.05, indicated by red arrow). Similarly, while all of the pathways in folate and methionine metabolism were associated with the HF diet, the one carbon pool by folate and the folate biosynthesis pathways were not significantly associated with the H9N diet (Figure 2E and F, indicated by red arrow). These results suggest effects of the different maternal diet strategies on folate and methionine cycles, as well as on lipid metabolism in the offspring liver.
3.3. The maternal HF diet disrupted the expression level of genes involved in folic acid metabolism, while the H9N diet had no effect.
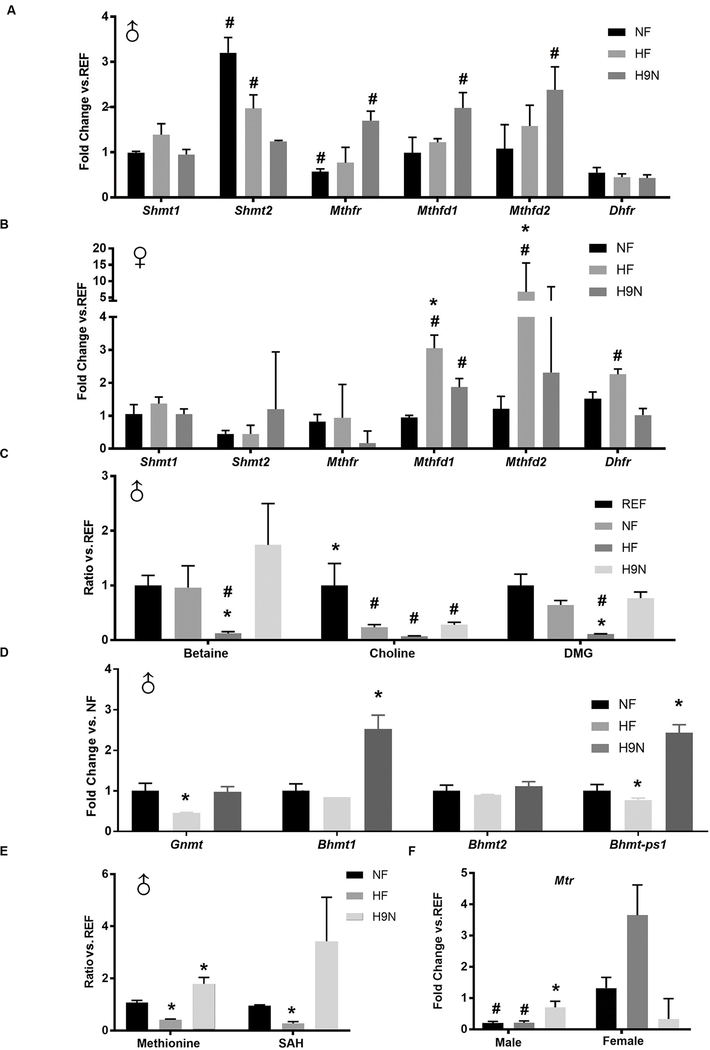
Based on our GO analysis suggesting an association between maternal diet and one-carbon metabolic processes, we examined whether folic acid metabolism was disrupted by the maternal HF diet but not the H9N diet. In male offspring, the Shmt2 gene, which is involved in the reverse reaction that converts THF to CH2-THF and reduces one-carbon processes(14), had significantly increased expression levels in NF and HF offspring livers compared to REF offspring livers, while expression was unchanged in H9N offspring (Figure 3A). The Mthfr gene encoding methylenetetrahydrofolate reductase, which positively regulates the delivery of one carbon groups from folate (14), had significantly decreased expression levels in NF offspring livers and significantly increased expression levels in H9N livers. The expression levels of two other genes, Mthfd1 and Mthfd2, were higher in the H9N offspring livers than in REF offspring livers (Figure 3A). In contrast to what we observed in the male offspring, the NF female offspring had similar expression levels for all genes as those observed for the REF female offspring. The expression levels of Mthfd1, Mthfd2 and Dhfr were significantly increased in the female HF offspring livers compared with those for the REF and NF offspring livers (Figure 3B). The H9N diet enhanced the expression level of Mthfd1 in the female offspring. These results suggest that the maternal diets had a sex-dependent effect on folic acid metabolism.
Figure 3. The maternal HF diet disrupted folic acid metabolism in offspring, while offspring of H9N diet mothers had normal folate metabolism.
3A-B. Gene expression levels of Shmt1, Shmt2, Mthfr, Mthfd1, Mthfd2, and Dhfr were measured by real-time PCR in male (A) and female (B) offspring livers. 3C. Betaine, choline and dimethylglycine (DMG) concentrations were detected via HPLC analysis. 3D. The expression levels of genes encoding methyltransferases for betaine metabolites and methyl group delivery were measured by real-time PCR in male offspring livers. 3E. Methionine and SAH contents were detected via high-resolution UPLC-MS/MS. 3F. Gene expression levels of Mtr were measured by real-time PCR in male and female offspring livers. Data are mean ± SE, n=5. * indicates P<0.05 vs. NF group. # indicates P<0.05 vs. the REF group.
3.4. The maternal HF diet disrupted the methionine cycle, while the H9N diet had no effect.
We next assessed whether the NF, HF and H9N diets differentially affected the methionine cycle in male offspring livers using HPLC analysis to detect the levels of methionine metabolites. Decreased hepatic levels of choline were observed in the NF offspring livers (Figure 3C). The HF offspring livers had significantly reduced levels of betaine, choline and dimethylglycine (DMG). Although the H9N offspring still displayed lower choline levels in the liver, the betaine and DMG levels were restored to normal (Figure 3C). We further measured the offspring hepatic expression of several genes that encode methyltransferases for betaine metabolites and methyl group delivery (Figure 3D). Significantly decreased expression levels of Gnmt and Bhmt-ps1 were observed in the HF offspring compared with the NF offspring. However, the expression levels of Gnmt and Bhmt2 in the H9N offspring were similar to those in the NF offspring, and the Bhmt1 and Bhmt-ps1 expression levels were increased in the H9N offspring compared with the NF offspring.
Methionine is formed when homocysteine receives a methyl group from 5-CH3-THF. Compared with the NF offspring liver, the methionine level was remarkably reduced in the HF offspring liver and was increased in the H9N offspring liver (Figure 3E). Consistent with these findings, the S-adenosyl-L-homocysteine (SAH) level was also reduced in HF offspring livers but not in H9N offspring livers (Figure 3E). Furthermore, Mtr, which encodes methionine synthase, was expressed at significantly reduced levels in both the NF and HF male offspring livers but not in the H9N male offspring livers (Figure 3F). Unlike in the males, the female offspring livers did not have changes in Mtr expression regardless of maternal diet (Figure 3F). These results suggest a disruption of the methionine cycle in the livers of HF males, but not females, and the H9N diet seemed to rescue this disruption.
3.5. The maternal HF diet decreased hepatic L-carnitine levels, and was associated with reduced fatty acid oxidation, while the H9N diet had no effect.
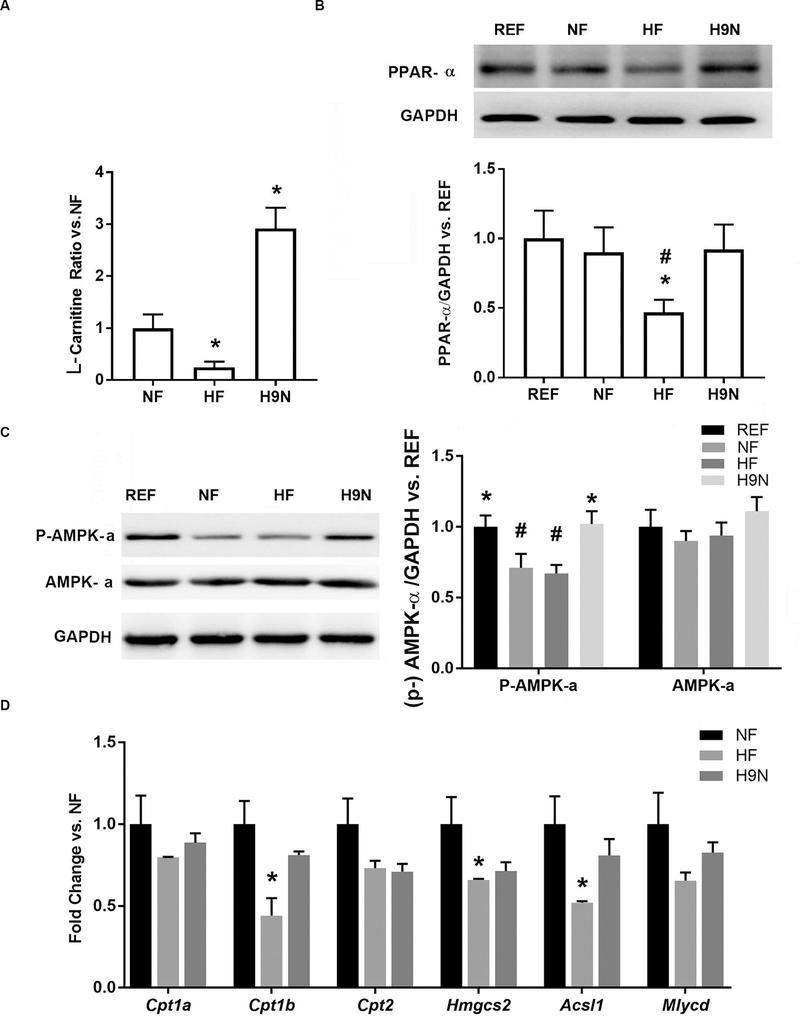
L-carnitine is primarily synthesized in the liver and one of the key steps involves the transfer of a methyl group from S-adenosyl-L-methionine (SAM) to lysine via the methionine cycle. L-carnitine is required for mitochondrial β-oxidation of long-chain fatty acids during energy production. Compared with NF offspring livers, we found significantly decreased levels of L-carnitine in the livers of HF offspring, while its content was increased in H9N offspring livers (Figure 4A).
Figure 4. The maternal HF diet disrupted the methionine cycle and decreased the production of L-carnitine in offspring, while the H9N diet did not result in such effects.
4A. L-carnitine concentrations in liver samples were detected via high-resolution UPLC-MS/MS. 4B. PPAR-α expression in male offspring livers was detected by Western blot. 4C. AMPK-α expression and phospho-AMPK-α (Thr172) levels were detected by Western blot in livers of male offspring. 4D. The expression of key genes involved in fatty acid oxidation were measured via real-time PCR. Data are mean ± SE, n=5. * indicates P<0.05 vs. the NF group. # indicates P<0.05 vs. the REF group.
PPAR-α is known to be an essential positive regulator of fatty acid oxidation (15). We found that the PPAR-α expression level was significantly decreased in HF offspring livers, whereas H9N offspring livers had expression equivalent to REF (Figure 4B). In addition, a significantly decreased level of AMPK-α phosphorylation was observed in the NF and HF offspring, but not in H9N offspring, compared with REF offspring (Figure 4C), suggesting an inhibition of AMPK signaling in NF and HF offspring. We consistently observed significantly decreased hepatic expression levels of key genes involved in fatty acid oxidation (including Cpt1b, Hmgcs2 and Acsl1) in the HF offspring when compared with the NF offspring. The expression levels of these genes were unchanged in the H9N offspring (Figure 4D). These results suggest the inhibition of fatty acid oxidation in HF offspring livers, which was not observed in the livers of H9N offspring.
3.6. The maternal HF diet resulted in genomic hypermethylation in offspring livers, while the H9N diet had no effect.
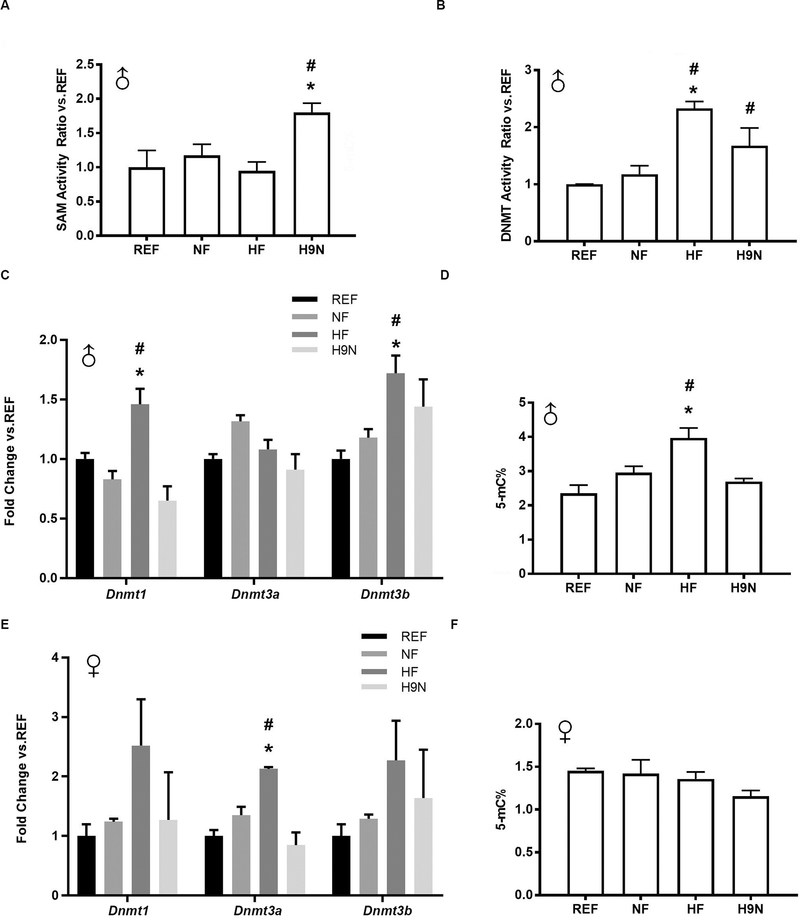
We next determined whether the disrupted methionine cycles observed in HF offspring livers were also associated with changes in DNA methylation and whether these would be corrected in H9N offspring livers. In males, hepatic SAM-dependent methyltransferase activity was unchanged in NF and HF offspring, while it was increased in H9N offspring (Figure 5A). We further measured the enzymatic activity of DNMT, which was significantly increased in HF offspring livers compared with that in NF and REF offspring livers. The DNMT activity of H9N offspring livers was higher than that of REF offspring livers but was similar to that of NF offspring livers (Figure 5B). In addition, HF offspring showed significantly increased hepatic expression levels of Dnmt1 and Dnmt3b, while their expression levels in the H9N offspring were not significantly different from REF (Figure 5C). These results were also consistent with the elevated methylation level of liver genomic DNA observed in the HF offspring. The NF and H9N offspring had similar levels of genomic DNA methylation as the REF offspring (Figure 5D).
Figure 5. The maternal HF diet resulted in genomic hypermethylation in offspring livers, and was associated with the overactivation of DNA methyl transferase (DNMT), while the H9N diet had no significant effect.
5A. The enzymatic activity of SAM dependent methyltransferases was determined in male offspring. 5B. The enzymatic activity of DNMT was determined in male offspring. 5C. Hepatic gene expression levels of Dnmt1, Dnmt3a and Dnmt3b in male offspring were measured by real-time PCR. 5D. Genomic DNA methylation levels were determined in the male offspring livers. 5E. Hepatic gene expression levels of Dnmt1, Dnmt3a and Dnmt3b in female offspring were measured by real-time PCR. 5F. Genomic DNA methylation levels were determined in female offspring livers.Data are presented as the mean ± SE, n=5. * indicates P<0.05 vs. NF group. # indicates P<0.05 vs. the REF group.
Unlike in the male offspring, the female HF offspring demonstrated overexpression of DNMT3a activity (Figure 5E). However, the genomic DNA methylation levels in the female offspring did not differ among the groups (Figure 5F). Consequently, it appears that a maternal HF diet elevates DNA methyltransferase expression and activity in male but not female offspring, resulting in a global increase in DNA methylation in male offspring livers.
3.7. Genes involved in one-carbon cycling and metabolism, whose hepatic expression was associated with DNA modification, were identified in the HF offspring
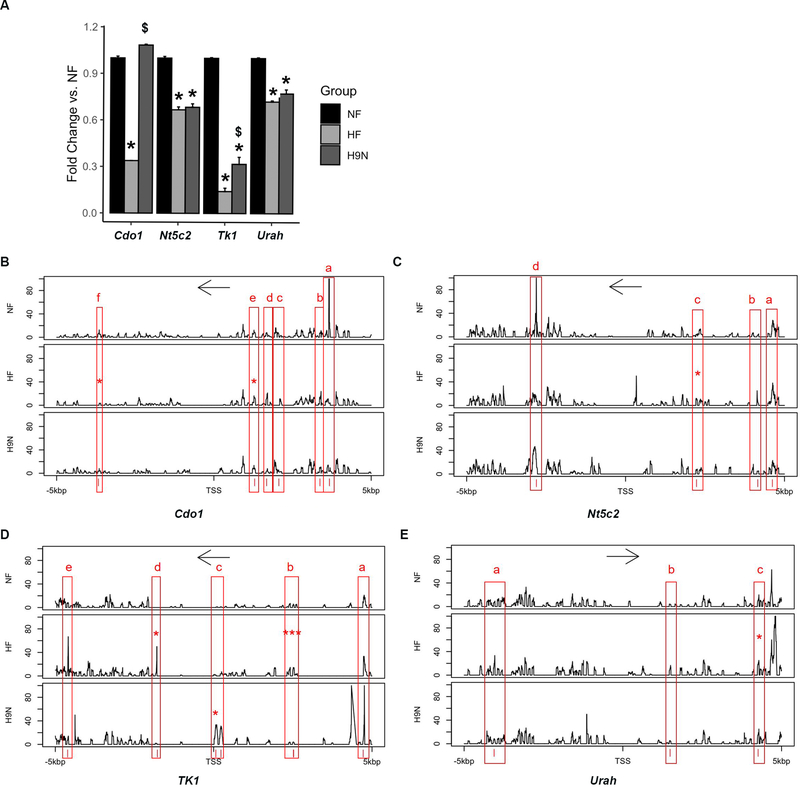
DNA methylation levels of specific genomic regions that regulate gene expression are critical for understanding how maternal diet affects offspring metabolism via epigenetic changes. We first performed Realtime-PCR to validate the expression changes of DEGs identified by RNA-seq analysis (above), in particular genes involved in one-carbon metabolism that differed compared between the HF group and the NF group. Validation of four important genes, Cdo, Nt5c2, Tk1 and Urah, confirmed that the expression was significantly lower in HF offspring than in NF offspring, suggesting an impact of maternal HF diet (Figure 6A). Among them, Cdo1 and Tk1, had their diminished expression levels at least partially reversed in H9N offspring, while the expression of Nt5c and Urah in H9N offspring livers remained at a similar level as in HF offspring livers.
Figure 6. Genes involved in one-carbon metabolism, whose hepatic expression was associated with DNA modification, had altered expression in the HF offspring.
6A. Expression of Cdo1, Nt5c2, Tk1 and Urah in offspring livers was measured by realtime-PCR. Data are presented as the mean ± SE, n=5. * indicates P < 0.05 vs. NF group. # indicates P < 0.05 vs. the REF group. $ indicates P < 0.05 vs. the HF group. 6B-E. DNA methylation pattern of a 10 kbp genomic region extending from 5 kbp upstream of the TSS to 5 kbp downstream of the TSS was analyzed by next generation sequencing. The y-axis indicates % of methylation at each DNA nucleotide. Only those DNA methylation changes more than 20% were considered potential methylation pattern changes. The x-axis indicates the location of each DNA nucleotide around the TSS. * indicates P<0.05 and *** indicates P<0.001 vs. NF group.
Next generation sequencing was performed on these four genes to uncover if a maternal HF diet resulted in DNA methylation profile modifications and if a maternal H9N diet reversed these changes. Within the 10 kbp genomic region that covers the 5kbp upstream and 5kbp downstream of the TSS sites, DNA methylation pattern changes were carefully analyzed among the NF, HF and H9N groups. The HF offspring displayed distinct DNA methylation patterns in several genomic regions of the four genes; however DNA methylation patterns in these regions were not different between NF and H9N offspring (Figure 6B–E). These include six regions for Cdo1, four regions for Nt5c2, five regions for Tk1 and three regions for Urah.
The DNA methylation pattern differences were further analyzed to elucidate if the DNA methylation level changes in these CpG islands were statistically significant (see methods) (Table 2). For the Cdo gene on chromosome 18, one hypermethylated CpG island and one hypomethylated island in the maternal HF diet offspring were identified as being significantly altered compared to NF. For Tk1, there were four CpG islands, all hypermethylated by the maternal HF diet. Among these four islands, the ones located around 117828612 and 117828617 significantly increased methylation levels by 71% and 57%, respectively. Importantly, all these CpG islands with methylation changes induced by a maternal HF diet were totally restored to baseline levels by a maternal H9N diet. We also identified one CpG site on Tk1 with a moderate amount of hypermethylation (about 20%) in maternal H9N diet offspring. For both Nt5c2 and Urah, one methylation region was identified to be hypermethylated by more than 50% in maternal HF diet offspring.
Table2.
CpG methylation sites of genes involved in one-carbon metabolism identified by Next Generation Sequencing
| Pathway | Gene | Chromesome | Position | Meth. (H9N) (%) | Meth. Diff (HF & NF) (%) | P value (HF vs. NF) | Meth. Diff (H9N & NF) (%) | P value (H9N & NF) | Dist. to TSS |
|---|---|---|---|---|---|---|---|---|---|
| Cysteine and methionine metabolism,Glycine, serine and threoninee metabolism | Cdo1 | chr18 | 46732020 | 2.17 | −23.08 | 0.182 | −22.83 | 0.198 | 3660 |
| Cdo1 | chr18 | 46730023 | 100.00 | 55.00 | 0.221 | 75.00 | 0.224 | 1680 | |
| Cdo1 | chr18 | 46729638 | 75.00 | 36.11 | 0.023 | 11.11 | 0.836 | 1295 | |
| Cdo1 | chr18 | 46724673 | 33.33 | −58.93 | 0.001* | −25.60 | 0.266 | −3669 | |
| Pyrimidine metabolism | Nt5c2 | chr19 | 47019773 | 25.00 | 50.00 | 0.135 | −25.00 | 0.774 | 4619 |
| Nt5c2 | chr19 | 47019314 | 13.89 | 29.17 | 0.186 | 9.72 | 0.410 | 4158 | |
| Nt5c2 | chr19 | 47017390 | 60.00 | 66.67 | 0.047* | 26.67 | 0.425 | 2236 | |
| Nt5c2 | chr19 | 47012351 | 22.22 | −20.30 | 0.125 | −10.28 | 0.499 | −2762 | |
| Tk1 | chr11 | 117828617 | 0.00 | 58.33 | 0.005* | 0.00 | 1.000 | 2525 | |
| Tk1 | chr11 | 117828612 | 0.00 | 70.83 | 0.002* | 0.00 | 1.000 | 2520 | |
| Tk1 | chr11 | 117828613 | 9.09 | 20.80 | 0.012* | 1.39 | 0.698 | 2516 | |
| Tk1 | chr11 | 117826327 | 40.09 | 1.88 | 0.304 | 39.21 | 0.109 | 203 | |
| Tk1 | chr11 | 117826164 | 20.83 | 0.07 | 0.854 | 20.25 | 0.001* | 38 | |
| Tk1 | chr11 | 117824318 | 0.77 | 20.00 | 0.027* | 0.77 | 0.336 | −1731 | |
| Purine metabolism | Urah | chr7 | 140830964 | 50.00 | 75.64 | 0.115 | 25.64 | 0.877 | −4050 |
| Urah | chr7 | 140836508 | 50.00 | 58.33 | 0.094 # | 8.33 | 0.864 | 1489 | |
| Urah | chr7 | 140839285 | 75.00 | 45.83 | 0.020* | 20.83 | 0.594 | 4267 |
Note:
P<0.05
P<0.1
One-carbon metabolism is reported to strongly regulated by the circadian rhythm (16). Thus, we examined whether the disrupted one-carbon metabolism in offspring is correlated with an abnormal circadian regulation at molecular level, indicating an effect of maternal diet intervention on offspring circadian regulation. The results identified that important genes involved in hepatic circadian regulation were associated with DNA methylation changes by maternal diet HF diet, but not the H9N diet (Suppl. Fig. 2 and Suppl. Table 3).
4. Discussion
Previous studies have suggested that early life exposure to overnutrition, especially in utero, increases the risk for obesity and related metabolic diseases, such as NAFLD [15]. Scientists have reported an increased accumulation of liver triglycerides, increased hepatic oxidative stress and increased levels of apoptosis in offspring exposed to overnutrition in an attempt to understand how obesity risk is transferred from the mother to the next generation (5, 10, 11, 17–19). However, this working model still leaves a gap in our understanding of how a maternal HF diet disrupts lipid homeostasis. Our data support a novel mechanism, where the maternal HF diet results in disrupted one carbon metabolic processing and shifts the subsequent methylation process in the offspring, priming the fatty acid metabolism of the liver for the later development of NAFLD. In humans, increased BMI in childbearing-age women was associated with lower serum folate and vitamin B12 levels (20–23). Specifically, obese women had lower serum folate and higher RBC folate levels, suggesting a folate storage trap and a potentially lower level in peripheral tissues such as in the liver (22). This is further explained upon consideration that obesity and pregnancy interact to significantly affect the body distribution of folate, which may reduce the amount of folate available to the developing embryo (24). Although the studies on human maternal folic acid levels had a major focus on neural tube defects (NTDs), the association between maternal obesity and other offspring health problems should not be ignored. In an HF-diet-induced obese baboon model, both the mother and the offspring on HF diets had higher levels of circulating homocysteine and lower levels of circulating vitamin B-12 and betaine (25). Although an enhanced ratio of APOB/APOA1 levels in the blood was reported, it is unknown whether the homocysteine trap in offspring baboons causes NAFLD (25). Our mouse model not only showed that a maternal HF diet caused a methyl trap in the liver, but also supported the idea that the methyl trap potentiates the response of the offspring liver to a postweaning HF diet. This finding is supported by the altered expression of genes involved in folic acid metabolism and the decreased levels of intermediate metabolites in the methionine cycle observed in HF offspring. More importantly, an early maternal transition to an NF diet before pregnancy (H9N diet) allowed the readjustment of one-carbon metabolism and the return of the methionine cycle to normal, which restored SAM-dependent methyltransferase activity in subsequent offspring.
Our results also demonstrated that the disrupted methionine cycle in offspring livers directly disrupted lipid homeostasis. Previous studies have suggested a link between mitochondrial dysfunction and methylation processes within the one-carbon metabolism cycle (26–28). In our study, the lower levels of SAM and SAH in HF offspring livers suggest an overall inhibition of methyl group transfer to substrates. Specifically, a depletion of L-carnitine was observed in the HF offspring liver, which was associated with inhibited fatty acid oxidation. Following a 9-week transition from a HF diet to a NF diet before pregnancy, the L-carnitine and creatinine levels were replenished in offspring, which rescued the mitochondrial function of breaking down fatty acids via β-oxidation, as demonstrated by the normalized expression of PPAR-α and AMPK signaling activity. Thus, our study suggests a novel mechanistic model, where a maternal HF diet resulted in a disrupted methionine cycle, which further reduced the production of L-carnitine for β-oxidation.
The demographic shift toward a more obese phenotype in just one or two generations is unlikely to have a major genetic contribution and is instead likely to be primarily attributable to environmental or epigenetic mechanisms. Recent discoveries have implicated one-carbon metabolism as essential for supplying methyl donors for DNA methylation. In our study, we detected global DNA hypermethylation in HF offspring livers consistent with previous reports (29, 30); however, H9N offspring avoided these changes. These results suggested an association between maternal diet and DNA methylation in offspring livers. We further identified four one-carbon metabolic genes, Cdo1, Nt5c2, Tk1 and Urah, with CpG islands altered by a maternal HF diet, whose DNA methylation changes were concurrent with their gene expression changes. Importantly, the changes in DNA methylation of Cdo1 and Tk1 made by a maternal HF diet, along with their associated gene expression changes, were reversed by a maternal H9N diet. Cdo1 encodes cysteine dioxygenase which regulates cellular cysteine levels and is one of the most highly regulated dietary-responsive metabolic enzymes (31). Cdo1-null mice have worse metabolic syndrome phenotypes when exposed to a HFD (32). Tk1 encodes thymidine kinase 1, an enzyme with a role in DNA precursor synthesis (33) that is a biomarker for liver cirrohosis, hepatocellular carcinoma, and end or late stage NAFLD (34). Thus, the DNA methylation and associated expression changes of these genes suggest an interaction between one-carbon metabolism and lipid metabolism. Together, our data provide evidence to support that a maternal HF diet “reprograms” one-carbon metabolism in offspring through epigenetic regulation. Our data also support that the epigenetic modifications caused by maternal diet are reversible through maternal dietary modification. Future studies will focus on identifying more specific genes and their CpG islands modified by a maternal diet that “primes” offspring for obesity and its related complications. One limitation of our study is that the results did not disclose whether the changes in offspring DNA methylation was an effector or a cause of disrupted one-carbon metabolism. This might be answered by future studies to elucidate whether similar DNA methylation modifications occur in mothers exposed to a HF diet and whether this epigenetic trait is heritable.
Circadian rhythms are critical system that regulates the temporal expression of metabolism-related genes in 24-hour intervals (35), thus to fulfill the coordinated regulation of metabolites and metabolic enzymes(36). An elegant previous study has demonstrated that maternal obesity interacts with postweaning HF diet to disrupt the canonical metabolic rhythmicity gene expression. This effect is possibly through methylation regulation of Arntl and Per2 in the liver of offspring, which further contributes to the development of NAFLD (37). This study suggests that disordered circadian rhythms during critical developmental periods may be responsible for the onset of chronic liver disease in adulthood. In our study, both the maternal HF diet, although not sufficient to induce maternal obesity (10, 11), and the maternal H9N diet changed the gene expression profile of both the circadian rhythm and circadian entrainment pathways, in offspring livers exposed to postnatal HF diet. Specifically, gene expression changes of Arntl, Clock, Cry1, Per1, Per2 and Per3 in HF offspring were correlated with DNA methylation modification at CpG Islands. The results confirmed that a maternal HF diet reprograms offspring circadian rhythms. However, although most DNA modifications of the genes were restored to control levels by the H9N diet, this DNA methylation recovery was not correlated with gene expression changes, indicating DNA methylation modification did not directly contribute to expression of circadian genes in H9N livers. The energy and nutrient sensors AMPK and mTOR influence the clock machinery which further finely adjusts metabolism (38–40). Specifically, AMPK activity is required for core clock regulation through circadian transcription enhancement by downregulating circadian repressor proteins (40). In our study, a maternal HF diet deactivated AMPK signaling which was correlated with a disrupted circadian rhythm and lipid metabolism. However, the H9N diet recovered the AMPK activity level and lipid metabolism to control levels, but the expression change of circadian genes was higher, suggesting that the AMPK pathway may not play a direct role in circadian regulation in our animal model. It should be noted that one-carbon metabolism is strongly regulated by the circadian rhythm (16). Thus, the maternal HF diet–induced disruption in the methionine cycle might be partially attributed to the disruption in cellular circadian rhythms. However, this is not the case in the H9N liver, because H9N offspring had normal methionine metabolism despite having more disrupted expression of genes involved in circadian rhythms. Taken together, the disparities between the HF and H9N groups suggest that disrupted expression of genes involved in circadian rhythms may not be responsible for the reprogramed metabolism in offspring. Nonetheless, consistent with others, our results showed that maternal HF diet reprogrammed the circadian rhythms associated with the disrupted one-carbon metabolism and lipid metabolism. It is possible that maternal HF diet is an effective stimulus to reprogram the cellular clocks in offspring livers at least partially through epigenetic regulation, which was not reversed but further aggravated by a maternal diet transition to a NF diet for 9-weeks (H9N diet). Future studies with direct data about maternal diet intervention on offspring circadian rhythms are required to test this hypothesis.
Finally, our study suggests that the sex-specific phenotype of NAFLD in offspring mice exposed to maternal overnutrition in utero could be due to male offspring being more sensitive to methionine cycle disruption. We previously reported that more severe hepatic steatosis was only observed in male, but not female, offspring exposed to overnutrition in utero (10, 11), consistent with the reports of others (41, 42). In our current study, the male HF offspring displayed disrupted one-carbon metabolism and methionine cycles, which were associated with exacerbated hepatic steatosis induced by a postweaning HF diet, while the female HF offspring displayed no or very mild changes.
In summary, our results support a working model where a maternal HF-diet leads to disrupted one-carbon metabolism in the offspring via epigenetic shifts and changes in metabolic gene expression. Methionine cycle dysfunction causes abnormal methyl-group delivery to its substrate, which further affects lipid metabolism and thus increases the risk for offspring to develop NAFLD. This study adds important knowledge to our understanding of how maternal diets affect offspring health status. It will promote the development of effective diet intervention strategies and policies to prevent obesity and reduce the risk of adverse events, improving health outcomes for mothers and children.
Supplementary Material
Acknowledgments
We thank Ms. Lauren Lawless for proofreading the manuscript.
Financial support
This project was supported by grants from the National Institutes of Health (NIDDK 1R01DK112368-01 to Drs. Xie and Zhang) and the USDA National Institute of Food and Agriculture, ([Hatch] project [1010406] to Dr. Xie).
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- HFD
high-fat diet
- NFD
normal fat diet
- IPGTT
intraperitoneal injected glucose tolerance test
- AUC
area under the curve
- TG
triglyceride
- DEGs
differentially expressed genes
- GO
gene ontology
- DMG
dimethylglycine
- SAH
S-adenosyl-L-homocysteine
- SAM
S-adenosyl-L-methionine
Footnotes
Conflict of Interests
The authors declare no Conflict of Interests.
References
- 1.Charlton M Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2(12):1048–58. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. The developmental origins of chronic adult disease. Acta paediatrica. 2004;93(446):26–33. [DOI] [PubMed] [Google Scholar]
- 3.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Reviews in obstetrics & gynecology. 2008;1(4):170–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Bayol SA, Simbi BH, Fowkes RC, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic Fatty liver disease in rat offspring. Endocrinology. 2010;151(4):1451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce KD, Cagampang FR, Argenton M, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50(6):1796–808. [DOI] [PubMed] [Google Scholar]
- 6.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119(2):323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aagaard-Tillery KM, Grove K, Bishop J, et al. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. Journal of molecular endocrinology. 2008;41(2):91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chango A, Pogribny IP. Considering maternal dietary modulators for epigenetic regulation and programming of the fetal epigenome. Nutrients. 2015;7(4):2748–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HS. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients. 2015;7(11):9492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Q, Olson P, Rasmussen D, et al. A short-term transition from a high-fat diet to a normal-fat diet before pregnancy exacerbates female mouse offspring obesity. Int J Obes (Lond). 2016;40(4):564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Fu Q, Zhou Y, et al. A long-term maternal diet intervention is necessary to avoid the obesogenic effect of maternal high-fat diet in the offspring. J Nutr Biochem. 2018;62:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summerfield M, Zhou Y, Zhou T, et al. A long-term maternal diet transition from high-fat diet to normal fat diet during pre-pregnancy avoids adipose tissue inflammation in next generation. PLoS One. 2018;13(12):e0209053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Peng H, Xu H, et al. Maternal diet intervention before pregnancy primes offspring lipid metabolism in liver. Lab Invest. 2020;100(4):553–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stover PJ. One-carbon metabolism-genome interactions in folate-associated pathologies. J Nutr. 2009;139(12):2402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brumbaugh DE, Friedman JE. Developmental origins of nonalcoholic fatty liver disease. Pediatr Res. 2014;75(1–2):140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnaiah SY, Wu G, Altman BJ, et al. Clock Regulation of Metabolites Reveals Coupling between Transcription and Metabolism. Cell Metab. 2017;25(5):1206. [DOI] [PubMed] [Google Scholar]
- 17.Borengasser SJ, Lau F, Kang P, et al. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One. 2011;6(8):e24068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon MV, Buchner DA, Hester J, et al. Maternal nutrition induces pervasive gene expression changes but no detectable DNA methylation differences in the liver of adult offspring. PLoS One. 2014;9(3):e90335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musial B, Vaughan OR, Fernandez-Twinn DS, et al. A Western-style obesogenic diet alters maternal metabolic physiology with consequences for fetal nutrient acquisition in mice. J Physiol. 2017;595(14):4875–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129(19):4613–25. [DOI] [PubMed] [Google Scholar]
- 21.Mojtabai R Body mass index and serum folate in childbearing age women. Eur J Epidemiol. 2004;19(11):1029–36. [DOI] [PubMed] [Google Scholar]
- 22.Tinker SC, Hamner HC, Berry RJ, Bailey LB, Pfeiffer CM. Does obesity modify the association of supplemental folic acid with folate status among nonpregnant women of childbearing age in the United States? Birth Defects Res A Clin Mol Teratol. 2012;94(10):749–55. [DOI] [PubMed] [Google Scholar]
- 23.O’Malley EG, Reynolds CME, Cawley S, Woodside JV, Molloy AM, Turner MJ. Folate and vitamin B12 levels in early pregnancy and maternal obesity. Eur J Obstet Gynecol Reprod Biol. 2018;231:80–4. [DOI] [PubMed] [Google Scholar]
- 24.da Silva VR, Hausman DB, Kauwell GP, et al. Obesity affects short-term folate pharmacokinetics in women of childbearing age. Int J Obes (Lond). 2013;37(12):1608–10. [DOI] [PubMed] [Google Scholar]
- 25.Nathanielsz PW, Yan J, Green R, Nijland M, et al. Maternal obesity disrupts the methionine cycle in baboon pregnancy. Physiol Rep. 2015;3(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aissa AF, Tryndyak V, de Conti A, et al. Effect of methionine-deficient and methionine-supplemented diets on the hepatic one-carbon and lipid metabolism in mice. Mol Nutr Food Res. 2014;58(7):1502–12. [DOI] [PubMed] [Google Scholar]
- 27.Berge RK, Bjorndal B, Strand E, et al. Tetradecylthiopropionic acid induces hepatic mitochondrial dysfunction and steatosis, accompanied by increased plasma homocysteine in mice. Lipids Health Dis. 2016;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbrugghe A, Bakovic M. Peculiarities of one-carbon metabolism in the strict carnivorous cat and the role in feline hepatic lipidosis. Nutrients. 2013;5(7):2811–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attig L, Vige A, Gabory A, et al. Dietary alleviation of maternal obesity and diabetes: increased resistance to diet-induced obesity transcriptional and epigenetic signatures. PLoS One. 2013;8(6):e66816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wankhade UD, Zhong Y, Kang P, et al. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS One. 2017;12(4):e0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stipanuk MH, Ueki I, Dominy JE Jr., Simmons CR, Hirschberger LL. Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids. 2009;37(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niewiadomski J, Zhou JQ, Roman HB, et al. Effects of a block in cysteine catabolism on energy balance and fat metabolism in mice. Ann N Y Acad Sci. 2016;1363:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, He E, Skog S. The proliferation marker thymidine kinase 1 in clinical use. Mol Clin Oncol. 2013;1(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iimuro Y, Fujimoto J. Strategy of gene therapy for liver cirrohosis and hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10(1):45–7. [DOI] [PubMed] [Google Scholar]
- 35.Panda S Circadian physiology of metabolism. Science. 2016;354(6315):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel VR, Eckel-Mahan K, Sassone-Corsi P, Baldi P. CircadiOmics: integrating circadian genomics, transcriptomics, proteomics and metabolomics. Nat Methods. 2012;9(8):772–3. [DOI] [PubMed] [Google Scholar]
- 37.Mouralidarane A, Soeda J, Sugden D, et al. Maternal obesity programs offspring non-alcoholic fatty liver disease through disruption of 24-h rhythms in mice. Int J Obes (Lond). 2015;39(9):1339–48. [DOI] [PubMed] [Google Scholar]
- 38.Jordan SD, Lamia KA. AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol. 2013;366(2):163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walton ZE, Patel CH, Brooks RC, et al. Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell. 2018;174(1):72–87 e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamia KA, Sachdeva UM, DiTacchio L, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miranda RA, De Almeida MM, Rocha C, et al. Maternal high-fat diet consumption induces sex-dependent alterations of the endocannabinoid system and redox homeostasis in liver of adult rat offspring. Sci Rep. 2018;8(1):14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokomizo H, Inoguchi T, Sonoda N, et al. Maternal high-fat diet induces insulin resistance and deterioration of pancreatic beta-cell function in adult offspring with sex differences in mice. Am J Physiol Endocrinol Metab. 2014;306(10):E1163–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.