Abstract
Orofacial pain, including temporomandibular joint disorders pain, trigeminal neuralgia, dental pain, and debilitating headaches, affects millions of Americans each year with significant population health impact. Despite the existence of a large body of information on the subject, the molecular underpinnings of orofacial pain remain elusive. Two decades of research has identified that transient receptor potential (TRP) ion channels play a crucial role in pathological pain. A number of TRP ion channels are clearly expressed in the trigeminal sensory system and have critical functions in the transduction and pathogenesis of orofacial pain. Although there are many similarities, the orofacial sensory system shows some distinct peripheral and central pain processing and different sensitivities from the spinal sensory system. Relative to the extensive review on TRPs in spinally-mediated pain, the summary of TRPs in trigeminally-mediated pain has not been well-documented. This review focuses on the current experimental evidence involving TRP ion channels, particularly TRPV1, TRPA1, TRPV4, and TRPM8 in orofacial pain and discusses their possible cellular and molecular mechanisms.
Keywords: TRP ion channels, Temporomandibular joint pain, Trigeminal neuropathic pain, Dental pain, Trigeminal ganglion sensory neurons
Introduction
Orofacial pain refers to pain associated with the jaw, mouth, face, head, and neck. The prevalence of pain in the orofacial region has been reported to be 17-26%, of which 7-11% is chronic [1]. Orofacial pain, including temporomandibular joint disorders (TMJD) pain, trigeminal neuralgia, dental pain, and headache, represents a major ongoing medical and social problem. The trigeminal nerve (the fifth cranial nerve) provides the principal sensory innervation of orofacial region [2]. Many trigeminal primary afferents terminate in the orofacial tissues as free nerve endings and function as nociceptors since they can be activated by noxious stimuli. Orofacial pain, derived from many unique target tissues, such as the meninges, cornea, tooth pulp, oral/nasal mucosa, and temporomandibular joint, has some unique physiologic characteristics compared to the spinal nociceptive system, which makes it more difficult to be diagnosed and treated [3]. Given that current treatments for orofacial pain are limited and have significant side effects, a deeper understanding of orofacial pain mechanisms is critical for the development of safer and more effective new therapeutics.
Numerous molecules have been implicated in contributing to orofacial pain sensations, but no family of transduction proteins has been studied as extensively as the transient receptor potential (TRP) ion channels in the past two decades. The TRP family is made up of ion channel proteins that function as non-selective cation-permeable channels, virtually all of which conduct Ca2+. The TRP superfamily can mainly be divided into six subfamilies: vanilloid (TRPV), ankyrin (TRPA), melastatin (TRPM), canonical (TRPC), polycystin (TRPP), and mucolipin (TRPML). TRP ion channels act as molecular sensors of multiple stimuli, including but not limited to temperature, mechanical stimulation, osmolarity, chemical agents, and pH, transforming them into electrical signals and directing them to the central nervous system (CNS) [4,5]. Two decades after the description of TRPV1 as the first family member with a postulated and later verified link to pain, the evidence has become increasingly robust that a number of TRP channels express in the sensory system and function in pathophysiological pain, including orofacial pain.
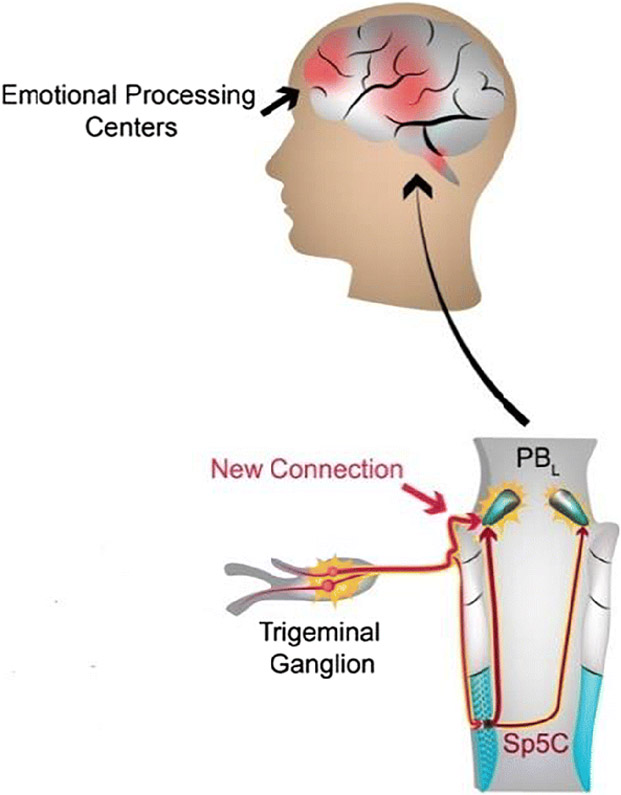
The trigeminal sensory system has some distinct characteristics from the spinal sensory system under either physiological or pathological conditions [6,7]. For instance, we recently discovered that a subset of trigeminal ganglion (TG) sensory neurons has a direct monosynaptic input to the parabrachial nucleus, bypassing the second-order neurons in the medullary dorsal horn [8]. However, this is not the case for the dorsal root ganglion (DRG) nociceptive neurons. This novel monosynaptic circuit may provide a neural substrate for heightened craniofacial affective pain [8] (Fig. 1). Moreover, peripheral nerve injury induced sprouting of sympathetic nerve fibers in DRG after spinal nerve injury, but no such sprouting was found in TG after trigeminal nerve injury [9]. Recent reviews characterized differences between the trigeminal and spinal afferent systems under basal conditions and after various forms of injury [7]. Interestingly, transcriptome analyses showed that, despite being overwhelmingly similar, a number of genes are differentially expressed between DRG and TG neurons [10]. Furthermore, the expression levels of a certain number of TRPs were different in TGs compared to DRGs [11]. Collectively, these studies indicate that the trigeminal system has some unique features that may contribute to distinct response patterns to tissue injury, and the mechanisms of orofacial pain are unique and should not be considered identical to those of spinal pain.
Fig. 1.
A schematic depiction of the novel emotional pain circuit that emanates from trigeminal ganglion afferents, as recently described [8]. Previous understanding indicates that the pain-relaying impulse projects to the trigeminal spinal nucleus caudalis (Sp5C) where it is synaptically relayed to the PBL. The hitherto unknown direct plug-in provides a direct connection to the PBL, from where it is relayed to emotional processing centers of the brain.
Accumulating evidence has indicated that TRP ion channels play a crucial role in orofacial pain. In this review, we summarize the current progresses and elaborate on relevant highlights related to the TRPV, TRPA, and TRPM subfamilies and the roles of their members in TMJD pain, trigeminal neuralgia and dental pain. A summary of TRPs in migraine is not included here because it has been extensively reviewed elsewhere [12-14].
TRPs in TMJD pain
TMJD pain is the most common form of orofacial pain, involving the masticatory muscles, temporomandibular joint (TMJ) and associated structures, and affecting approximately 10 million individuals each year in the US. TMJD is primarily characterized by mechanical pain, jaw function-induced pain and spontaneous pain in the TMJ and/or muscles of mastication. The incessancy and severity of TMJD pain emotionally wears down patients and can be exacerbated by jaw movements accompanying food intake, chewing, and even speaking or laughing [15,16]. Many lines of evidence suggest that TRPs contribute to TMJD pain (also see Table 1).
Table 1.
TRPs in TMJD pain.
| Localization in relevant tissues | Dysregulation in relevant tissues | Pain regulation | |
|---|---|---|---|
| TRPV1 | TG neurons innervating the TMJ, masseter muscle, synovial lining cells of joint of rats [17, 18, 20, 21] Synovial tissues of human TMJ [19] |
Upregulated in cultured rat synoviocytes by LPS [24], in rat TG and hippocampus after CFA injection into TMJ [25, 26], and in TG after CFA injection into rat masseter muscle [28] or mouse TMJ [22] Unchanged in amygdala, prefrontal cortex, and thalamus after CFA injection into rat TMJ [25] |
Capsaicin injection into the masseter muscle causes pain in rats [20] or human [29] Inhibition or knockout of TRPV1 attenuates mechanical hyperalgesia, spontaneous pain, and bite force reduction-caused by CFA injection into masseter muscle in rats [28, 31, 32] Ablation of TRPV1-expressing TG afferents attenuates spontaneous pain and bite force reduction-caused by CFA injection into masseter muscle in rats [32] Inhibition of TRPV1 in hippocampus attenuates mechanical allodynia-caused by CFA injection into TMJ [25] or masseter muscle [26] in rats |
| TRPV4 | TG neurons innervating mouse TMJ [22] and fibroblast-like synoviocytes of rat TMJ [23] | Upregulated in TG neurons after CFA injection into mouse TMJ [22] Downregulated in TG neurons after CFA injection into rat masseter muscle [28] |
Intraarticular injection of TRPV4 agonists into TMJ causes mechanical allodynia in rats [23] Inhibition or knockout of TRPV4 attenuates bite force reduction-caused by CFA injection into mouse TMJ [22] |
| TRPA1 | TG neurons innervating masseter muscle of rats [20] | Upregulated in astrocytes of trigeminal caudal nucleus after CFA injection into rat TMJ [27] and in TG after CFA injection into mouse TMJ [22] or rat masseter muscle [28] | Mustard oil injection into the masseter muscle causes nocifensive pain and mechanical hyperalgesia in rats [20] Inhibition of TRPA1 attenuates mechanical hyperalgesia caused by CFA injection into masseter muscle in rats [30] Inhibition or knockout of TRPA1 attenuates spontaneous pain-caused by CFA injection into masseter muscle in rats [31] Inhibition of TRPA1 does not attenuate bite force reduction-caused by CFA injection into masseter muscle in rats [31] |
| Other TRPs | TRPV2, TRPV3, TRPM2, TRPM3, TRPM8 in mouse TG [22] TRPV2, TRPV3, TRPM2, TRPM3, TRPM8, TRPC3, TRPC4, TRPC6, and TRPC7 in mouse or rat TG [28] |
TRPM3 and TRPM8 are upregulated, but TRPV2, TRPV3, TRPM2, TRPC3, TRPC4, TRPC6 and TRPC7 remained unchanged in TG after CFA injection into rat masseter muscle [28] TRPM3 and TRPM8 remain unchanged in TG after CFA injection into mouse TMJ [22] |
Unknown |
Expression of TRPs in TMJ-associated tissues under pathophysiological conditions
Immunohistochemical analysis revealed the presence of TRPV1 in the nerves innervating the TMJ, masseter muscle, and synovial lining cells of the joint in rats [17,18]. TRPV1-expressing nerves were also distributed in the synovial membrane of the joint capsule and provided branches to the joint compartment. Double labeling showed that many of these TRPV1-immunoreactive nerves are peptidergic [17]. In line with the finding from rodents, TRPV1 was also observed in the region of the posterior disk attachment of synovial tissues of human TMJ [19]. TRPV1 and TRPA1 were expressed in small- to medium-sized TG sensory neurons that are putative nociceptors. In rat TG, approximately 24% of masseter muscle afferents expressed TRPV1 [20]. In support of this finding, a new study also found that a small subset of TRPV1-expressing TG neurons innervate the masseter muscles [21]. TRPA1 was highly colocalized with TRPV1 and expressed in about 10% of masseter afferents [20]. Our recent study revealed that 34% of total TG neurons express TRPV4. Neural tracing with fast blue injected into the TMJ resulted in labeling of 6.5% of all TG neurons, and ≈50% of those expressing TRPV4, suggesting that the TMJ is innervated robustly by TRPV4-expressing TG neurons [22]. In addition, TRPV4 immunoreactivity was detected in the synovium and condylar cartilage of rat TMJ. In fibroblast-like synoviocytes, activation of TRPV4 with the selective agonists 4α-PDD or GSK1016790A caused Ca2+ influx [23].
Both in vivo and in vitro studies have demonstrated that TRPV1 can be upregulated under inflammatory conditions. For instance, TRPV1 was upregulated by lipopolysaccharide (LPS) in cultured rat synoviocytes [24]. Real-time PCR and immunoblotting assays detected significantly increased TRPV1 expression in the TGs and hippocampus, but not in the amygdala, prefrontal cortex, and thalamus after complete Freund’s adjuvant (CFA) injection into the TMJ [25,26]. Immunoreactivity for TRPA1 was consistently observed in the somata and process of astrocytes of the trigeminal caudal nucleus and was weaker than that in presumed nociceptive primary afferent terminals, but increased significantly in the fine process of astrocytes in rats with experimental TMJ inflammation caused by CFA [27]. RNA sequencing assay identified that mRNAs for TRPA1, TRPV1, TRPM3, and TRPM8 are upregulated, but TRPV2, TRPV3, TRPM2, TRPC3, TRPC4, TRPC6 and TRPC7 remain unchanged, and TRPV4 is downregulated in TGs following the masseter muscle inflammation caused by CFA in rats [28]. Our study also found that mRNAs for TRPV1 and TRPA1 are upregulated, but TRPV2, TRPV3, and TRPM2 remain unchanged in TG after CFA injection into mouse TMJ. In contrast, we and others found that TRPM3 and TRPM8 genes are not altered and TRPV4 at both gene and protein levels is increased after TMJ inflammation [22,23]. These discrepancies might be due to differences in species (rat vs mouse), pain models (masseter muscle inflammation vs TMJ inflammation), and examination approaches. Interestingly, we also found that TMJ inflammation-mediated upregulation of TRPV1 and TRPA1 mRNAs is dependent on TRPV4 because their increases are absent in TRPV4 KO mice [22].
Regulation of TMJ pain by TRPs
While TMJD has multifactorial etiologies and covers a broad range of conditions, a substantial subset of patients with TMJD pain is caused by masticatory muscle injury or joint inflammation. Injection of capsaicin or mustard oil, the agonists of TRPV1 and TRPA1, respectively, into the masseter muscle produced nocifensive responses and subsequent mechanical hyperalgesia in rats [20]. Activation of TRPV1-nociceptive muscle afferent fibers by capsaicin injection into the masseter muscle also caused pain in healthy human subjects [29]. These animal and human studies provide compelling evidence that these channels, expressed in muscle afferents, might contribute to masseter muscle pain. In support of this, using a rat model of masseter muscle inflammation caused by CFA, Chung and Ro’s groups demonstrated that: 1) mechanical hyperalgesia of masseter muscle was attenuated by blockade of TRPV1 or TRPA1 [28,30]; 2) genetic knockout or pharmacological inhibition of TRPV1 or TRPA1 attenuated spontaneous pain of the masseter muscle. Furthermore, dual blockade of TRPV1 and TRPA1 produced a greater effect of reducing spontaneous pain, indicating TRPV1 and TRPA1 co-contribute to the spontaneous pain [31,32]; 3) chemical ablation of TRPV1-expressing primary afferents using resiniferatoxin (RTX) injected into trigeminal subnucleus caudalis or chemogenetic inhibition of TRPV1-lineage masseter afferent terminals attenuated spontaneous pain [32]. TRPV1 in the CNS is also involved in masseter muscle pain. Simonic-Kocijan et al. found that injection of TRPV1 antagonist 5-iodoresiniferatoxin into the hippocampus significantly attenuated mechanical allodynia [26]. Unilateral injection of CFA-induced allodynia on the contralateral side was also attenuated by 5-iodoresiniferatoxin, further pointing to the involvement of TRPV1 in higher neural centers. Moreover, intrahippocampal injection of TRPV1 antagonists capsazepine or 5'-iodo-resiniferatoxin into the CA1 region of the hippocampus significantly attenuated allodynia of inflamed TMJ in both non-ovariectomized and ovariectomized rats that received estradiol replacement [25]. These results suggest that hippocampal TRPV1 can modulate central TMJ pain processing, and estradiol may contribute to the sexual dimorphism of TMJD pain through upregulation of TRPV1 expression in the hippocampus. Intraarticular injection into the TMJ with the TRPV4 selective agonists 4α-PDD or GSK1016790A not only induced joint inflammation but also caused mechanical allodynia [23].
TMJD is known for its mastication-associated pain. Clinical research indicates that TMJD patients have significantly reduced bite force vs. healthy controls, and this reduction correlated with pain severity [33-35]. Based on this background, we and others developed and validated a method of measuring bite force in mice as a clinically relevant metric of TMJD masticatory pain [22,32,36]. TMJ or masseter muscle injuries resulted in an appreciable reduction of bite force, yielding a metrical readout of masticatory pain. Using this novel method, we found that knockout or inhibition of TRPV4 significantly attenuate the bite force reduction caused by CFA injection into mouse TMJ. As a result of TMJ-inflammation, wild-type (WT) mice exhibited significant up-regulation of phosphorylated ERK in TMJ-innervating trigeminal sensory neurons, which is absent in TRPV4 KO mice. Mice with genetically-impaired MEK/ERK phosphorylation in neurons showed a similar resistance to reduction of bite force as TRPV4 KO mice. These results indicate that TRPV4 is necessary for masticatory sensitization in TMJ-inflammation and likely functions up-stream of MEK/ERK phosphorylation in trigeminal ganglion sensory neurons in-vivo. Therefore, TRPV4 represents a pro-nociceptive target in TMJ masticatory pain [22]. Although ablation of TRPV1-expressing afferents significantly attenuated the reduction of bite force following masseter inflammation, inhibition of TRPV1 only modestly suppressed it and inhibition of TRPA1 did not significantly affect it in mice [31,32]. Considering both TRPV1 and TRPA1 are upregulated following masseter muscle inflammation [28] and the functional interaction between TRPV1 and TRPA1 contributes to pathological pain [37], further studies are needed to clarify the discrepancies in regulating masticatory pain between these two channels.
TRPs in trigeminal neuralgia
Trigeminal neuralgia is a chronic pain condition that is defined as “a disorder characterized by recurrent unilateral brief electric shock-like pain, abrupt in onset and termination, limited to the distribution of one or more divisions of the trigeminal nerve and triggered by innocuous stimuli” [38,39]. Trigeminal neuralgia affects 4 to 13 per 100000 people annually with women being more affected than men. Trigeminal neuralgia is neuropathic in nature and most cases of trigeminal neuralgia are associated with nerve compression. Accumulating evidence demonstrates that TRPs contribute to trigeminal nerve injury-associated pain (also see Table 2).
Table 2.
TRPs in trigeminal neuropathic pain.
| Localization in relevant tissues | Dysregulation in relevant tissues | Pain regulation | |
|---|---|---|---|
| TRPV1 | TG neurons [40-46], cervical spinal cord and medulla in rats [41] | Upregulated in TG [40, 41], cervical spinal cord and medulla [41], and trigeminal spinal nucleus [42] after IoN-CCI in rats Upregulated in regenerated myelinated large-sized TG neurons after IAN in rats [44, 45] Upregulated in in larger-sized neurons but downregulated in small- to medium-sized neurons after pIONL in rats [43] |
Inhibition of TRPV1 suppresses thermal pain, but not mechanical pain, induced by pIONL in rats [43] Inhibition of TRPV1 or ablation of TRPV1-expressing TG afferents attenuates mechanical hyperalgesia induced by IoN-CCI in mice [52] Silencing of TRPV1-expressing TG afferents alleviates mechanical hyperalgesia induced by IAN in rats [45, 54] Ablation of TRPV1-expressing TG afferents attenuates heat and cold hyperalgesia induced by IoN-CCI in rats [57] Ablation of TRPV1-expressing TG afferents attenuates mechanical hyperalgesia, secondary hyperalgesia, and ongoing pain induced by IoN-CCI in mice [58] Topical capsaicin application relieves self-reported pain in postherpetic neuralgia patients [59, 60] |
| TRPV2 | TG neurons in rats [50] | Upregulated in TG neurons after pIONL in rats [50] | Unknown |
| TRPV4 | TG neurons and trigeminal spinal nucleus in rats or mice [22, 50] | Upregulated in TG neurons after pIONL in rats [50] | Inhibition of TRPV4 attenuates pIONL-induced mechanical allodynia in rats [50] |
| TRPA1 | TG neurons, trigeminal spinal nucleus, cervical spinal cord, and medulla in rats [41, 42,49] | Upregulated in TG, cervical spinal cord, medulla [41] and trigeminal spinal nucleus [42] after IoN-CCI in rats Unchanged in infraorbital nerve or TG after IoN-CCI in rats [50] |
Inhibition of TRPA1 attenuates mechanical allodynia induced by IoN-CCI in rats [41] Knockout or inhibition of TRPA1 attenuates spontaneous pain, mechanical, cold and chemical hypersensitive pain behaviors-induced by IoN-CCI [49] |
| TRPM3 | In trigeminal spinal subnucleus caudalis in rats [51] | Upregulated in trigeminal spinal subnucleus caudalis after IoN-CCI in rats [51] | Unknown |
| TRPM8 | TRPM8 in TG [40] and trigeminal spinal subnucleus caudalis in rats [42] | Upregulated in TG [40] and trigeminal spinal subnucleus caudalis [42] after IoN-CCI in rats | Subcutaneous injection of menthol into rat cheek augments cold pain-induced by IoN-CCI [64] Inhibition of TRPM8 with capsazepine reduces cold pain-induced by IoN-CCI [64] |
Expression of TRPs in trigeminal sensory system under pathophysiological conditions
In a preclinical pain model of chronic constriction injury of the infraorbital nerve (IoN-CCI), an increase of TRPV1-expressing TG neurons was detected 2 weeks post-surgery [40]. Moreover, TRPV1 mRNA was also upregulated in TG, cervical spinal cord, and the medulla in rats with IoN-CCI [41]. Using Western blotting, Wu et al. found TRPV1 is increased in the spinal trigeminal nucleus in rats 14 and 28 days after IoN-CCI [42].
Different from the IoN-CCI model, the total of TRPV1-positive TG neurons remained unchanged, but TRPV1 in large-diameter neurons was increased and in medium- and small-diameter cells was decreased strikingly 28 days after partial infraorbital nerve ligation (pIONL) in rats, indicating there might be a phenotypic change of TRPV1 [43]. The phenotypic change of TRPV1 was also found in another pain model with an increased expression in regenerated myelinated large-sized TG neurons 3-4 weeks after inferior alveolar nerve (IAN) transection in rats [44,45]. In a similar model of inferior alveolar nerve and mental nerve transection, TRPV1 expression in TG sensory neurons was found to be upregulated 3 days after nerve injury and returned to normal level after 60 days. Interestingly, this upregulation occurred in uninjured neurons but not in injured ones [46]. Collectively, the phenotypic change of TRPV1 might be involved in processing of pain hypersensitivity after nerve injury, as previous studies have implicated [47,48].
An upregulation of TRPA1 was found in trigeminal spinal nucleus 14-28 days after IoN-CCI in rats [42]. This finding is supported by another study showing an increased TRPA1 gene expression in the TGs, cervical spinal cord, and medulla in the same model [41]. In contrast, Trevisan et al. found that TRPA1 expression is not altered in the infraorbital nerve or TG at day 10 after IoN-CCI in mice [49]. These discrepancies might be due to difference in species, rat vs. mouse, but further studies are needed to clarify it.
An increase of TRPV2 and TRPV4 in TG sensory neurons which innervate the whisker pad skin was detected after pIONL in rats [50]. In addition, IoN-CCI caused an increase of TRPM3 and TRPM8 in the trigeminal spinal subnucleus caudalis [42,51] and TRPM8 in TGs [40] of rats.
Regulation of trigeminal neuropathic pain by TRPs
Urano and colleagues showed that thermal pain, but not mechanical pain, induced by pIONL is suppressed by subcutaneous injection of TRPV1 antagonist capsazepine [43]. In contrast, another study showed that TRPV1 and TRPV1+ afferents are clearly involved in mechanical hyperalgesia following IoN-CCI in mice. Injection of TRPV1 antagonist AMG9810 into the trigeminal subnucleus caudalis or systemic treatment with RTX effectively attenuated mechanical hyperalgesia following IoN-CCI [52]. QX-314 is a permanently positively charged sodium channel blocker that is unable to readily cross the cell membrane in a passive manner. However, QX-314 can enter into the neurons when TRPV1 ion channels are activated and opened, thereby blocking nociceptive sodium channels and producing a long-lasting, pain-specific local anesthesia [53]. Subcutaneous co-application of QX-314 with TRPV1 agonist capsaicin into the skin area ipsilateral to the inferior alveolar nerve alleviated mechanical hyperalgesia 3 and 4 weeks after nerve transection, indicating that silencing of TRPV1-expressing neurons can be an approach for treatment of trigeminal neuropathic pain [45,54].
Elimination of the TRPV1-expressing neurons, via long-lasting activation of TRPV1 with its agonists at either the nerve ending or the perikarya, implicates the algesic profile of these neurons and their potential as pharmacological targets [55,56]. RTX injection into the TG selectively ablated TRPV1-expressing neurons and reduced heat and cold hyperalgesia induced by IoN-CCI in rats [57]. In mice with IoN-CCI, Wang et al. found that a single local injection of capsaicin into facial skin causes attenuation of mechanical hyperalgesia, secondary hyperalgesia in extraterritorial mandibular skin, and ongoing pain. Furthermore, preventing capsaicin-induced ablation of afferent terminals by co-administration of capsaicin with MDL28170, an inhibitor of calpain, abolished capsaicin-induced mechanical analgesic effect. These results suggest that TRPV1+ TG afferents contribute to the maintenance of trigeminal neuropathic pain [58]. In humans, it has also been reported that topical capsaicin application relieves self-reported pain by >30% for about 3 months in ~40–45% of postherpetic neuralgia patients [59,60]. Thus, targeting TRPV1+ TG afferents might be a promising approach for treating trigeminal neuropathic pain.
Numerous clinical studies have shown that botulinum toxin type A (BoNT-A) are effective in treating trigeminal neuralgia [61,62], but its underlying mechanisms remain elusive. Wu et al. found peripheral application of BoNT-A significantly reduces mechanical allodynia-caused by IoN-CCI in rats [42]. In the trigeminal subnucleus caudalis region, TRPA1, TRPV1, TRPV2 and TRPM8 were increased after IoN-CCI, whereas peripheral application of BoNT-A significantly lowered the increased expression of TRPA1, TRPV1 and TRPV2, but not TRPM8, at day 7 after BoNT-A treatment. These results implicate BoNT-A may act on the trigeminal subnucleus caudalis via axonal transport and attenuates pain through suppressing upregulation of TRPA1, TRPV1, and TRPV2-caused central sensitization [42]. The mechanisms by which BoNT-A regulates the expression of TRPs are unclear. It has been demonstrated that BoNT-A inhibits TRPV1 trafficking to the plasma membrane in TG neurons [63], which might lead to a reduced axonal transport of TRPV1 to the trigeminal subnucleus caudalis. In addition, whether this working hypothesis holds true for the altered expressions of other TRPs, e.g. TRPA1 and TRPV2, in trigeminal subnucleus caudalis by BoNT-A also awaits further clarification.
A single treatment of ADM12, a TRPA1 selective antagonist, significantly reduced the mechanical allodynia induced by IoN-CCI in rats. ADM12 was able to abolish the increase of TRPA1, calcitonin gene-related peptide (CGRP), substance P (SP), and cytokines gene expression in the TG, cervical spinal cord, and medulla induced by IoN-CCI [41]. Both genetic deletion and selective pharmacological blockade (HC-030031 and A-967079) of TRPA1 abrogated spontaneous pain and mechanical, cold and chemical pain behaviors-induced by IoN-CCI [49]. IoN-CCI caused the release of chemokine CCL2 from the infraorbital nerve, which subsequently stimulates monocyte/macrophage recruitment and activation to generate oxidative stress (reactive oxygen species, ROS) and lipid peroxidation (4-HNE) byproducts. Furthermore, the increased 4-HNE in the perineural tissue was mainly localized within and around TRPA1-expressing nerve bundles. The close proximity of 4-HNE and TRPA1 channels supports the possibility that oxidative stress byproducts are the mediators that initiate and maintain the ongoing TRPA1-dependent pain-caused by IoN-CCI [49].
Insulin-like growth factor (IGF-1), released by macrophages accumulating in the injured infraorbital nerve, binds to TRPV2, which subsequently increases TRPV4 expression in TRPV2-positive small-medium sized TG neurons. Antagonism of TRPV4 in the whisker pad skin suppressed pIONL-induced mechanical allodynia, suggesting changes in the characteristics of TRPV4 channels via IGF-1-TRPV2 signaling may be a potential therapeutic target for the treatment of orofacial neuropathic pain [50].
With an orofacial operant test, Zuo et al. found IoN-CCI profoundly induces cold allodynia and hyperalgesia in rats. Subcutaneous administration of menthol, an agonist of TRPM8, into the cheek further increased cold pain-induced by IoN-CCI. In contrast, inhibition of TRPM8 with capsazepine significantly reduced cold pain. These behavioral data suggest that TRPM8 may play a role in cold allodynia and hyperalgesia following chronic trigeminal nerve injury [64]. Of note, however, capsazepine is a non-selective antagonist of TRPM8, and it also inhibits hyperpolarization-activated cyclic nucleotide-gated 1 (HCN1) [65], which might contribute to trigeminal cold sensitivity [66]. Further studies are needed to clarify the role of TRPM8 in IoN-CCI-caused cold pain.
TRPs in dental pain
Dental pain or odontogenic pain initiates from the teeth or their supporting tissues. The most common cause of dental pain is dental caries. The teeth have a distinctive nociceptive mechanism by which they detect noxious stimuli in inflammatory conditions or when dentin is exposed. Three potential mechanisms underlying dentinal hypersensitivity have been proposed [67]: the first is the neural theory, in which nerve endings that penetrate dentinal tubules directly respond to external stimuli; the second is the hydrodynamic theory, wherein fluid movements within the dentinal tubules are detected by nerve endings near the dentin; and the third is the odontoblast transducer theory, which suggests odontoblasts themselves may serve as pain transducers. For all three hypothesized mechanisms, the activation of dental primary afferents is critical for delivering dental nociception to the CNS. Elucidation of these transduction mechanisms is crucial for the development of therapeutic strategies of dental pain. Numerous studies implicate that TRPs play a critical role in dental pain (also see Table 3).
Table 3.
TRPs in dental pain.
| Localization in relevant tissues | Dysregulation in relevant tissues | Pain regulation | |
|---|---|---|---|
| TRPV1 | Dental primary afferent neurons of rats [68-72] Dental pulp nerve fibers of human permanent teeth [73-75] Odontoblasts of rat and human teeth [77,78] |
Upregulated in dental pulp nerve fibers of human carious teeth [75] Upregulated in TG after LPS injection into mouse dentine [69] Upregulated in the orthodontic force-stimulated pulps of rat molars [98] |
Ablation of TRPV1-expressing TG afferents or knockout of TRPV1attenuates spontaneous pain and bite force-evoked pain in a mouse model of orthodontic tooth movement [99] Inhibition of TRPV1 attenuates mechanical allodynia and thermal hyperalgesia in a mouse model of CFA injection into the molar [100] Inhibition of TRPV1 reduces spontaneous pain but does not affect mechanical allodynia in a rat model of intraoral wire-induced mucositis [101] |
| TRPV2 | Dental primary afferents of rats [70,79,80], mouse and rat [81] and human [82] odontoblasts | Unknown | Unknown |
| TRPV3 | human odontoblasts [82] | Unknown | Unknown |
| TRPV4 | Mouse and rat odontoblasts [81] Human dental pulp nerves [83] |
Upregulated in dental pulp nerves of human teeth with pulpitis [83] | Inhibition of TRPV4 attenuates mechanical allodynia in a rat model of intraoral wire-induced mucositis [101] |
| TRPA1 | Dental primary afferent neurons of mice [84] Dental pulp nerve fibers and odontoblast-like cells of human [85] Not expressed in odontoblasts or their processes of human healthy permanent teeth [89]. Not expressed in rat odontoblasts [90] |
Upregulated in TG in response to experimental exposure of the dental pulp of rats [86] Upregulated in the myelinated nerve fibers of human teeth with pulpitis [85] Upregulated in odontoblasts and their process in human carious teeth [87,88] |
Inhibition of TRPA1 attenuates orthodontic spontaneous pain in a rat model of loaded orthodontic force to teeth [102] |
| TRPM7 | Rat odontoblasts [94,95] | Unknown | Unknown |
| TRPM8 | Dental primary afferent neurons of rat [71] and mice [84], rat [92] and human odontoblasts [89, 91], pulpal nerves of human teeth [93] | Downregulated in dental pulp nerves of human teeth with pulpitis [93] | Unknown |
Expression of TRPs in dental sensory system under pathophysiological conditions
A number of TRP ion channels have been detected in dental primary afferents. In rats, it was reported that approximately 8% of dental primary afferent neurons express TRPV1 [68], which is close to the 10% reported by Chung and colleagues [69]. However, a much higher expression of 21-34%, 45%, and 85%, was also reported [70-72]. Chung et al. observed that TRPV1 expression in TGs is upregulated by LPS injection into the dentine [69]. TRPV1 immunoreactivity was also detected in the dental pulp nerve fibers of human permanent teeth [73-75], and it was significantly upregulated in carious teeth compared to non-carious teeth [75]. Furthermore, TRPV1 tended to be increased in painful carious teeth compared to non-painful ones, although the difference did not achieve significance [75]. Odontoblasts are organized as a single layer of specialized cells responsible for dentine formation and presumably play a role in tooth pain transmission. It has been recognized that they can also function as sensory cells that mediate the early steps of mechanical, thermal, and chemical dental sensitivity [76]. TRPV1 was detected in odontoblasts of rat and human teeth [77,78]. Stimulation of odontoblasts by capsaicin elicited Ca2+ signal and inward current, which can be attenuated by TRPV1 antagonist capsazepine [77].
Other TRPV ion channels have also been detected in dental primary afferent neurons. For instance, TRPV2 was found in the dental pulp nerves and in the cell bodies of the dental primary afferents located in the TG of rats [70,79,80]. Approximately 37% of the dental primary afferent neurons retrogradely traced in the TG expressed TRPV2 [79], which is close to the 32-51% reported by other groups [70]. Both TRPV2 and TRPV4 were localized in rat odontoblasts [81]. Furthermore, inward currents and increased Ca2+ influx in cultured mouse odontoblast lineage cells caused by hypotonicity can be inhibited by TRPV1, TRPV2 or TRPV4 antagonists [81], suggesting that these channels may act as mechanoreceptors in odontoblasts. Using some complementary approaches, TRPV1, TRPV2 and TRPV3 were detected in native human odontoblasts as well as in cultured odontoblast-like cells from human healthy teeth [82]. In addition, TRPV4 was found in human dental pulp nerves, and its expression was upregulated in dental pulp nerves of human teeth with pulpitis [83].
TRPA1 was detected in about 19% of dental primary afferents in mice [84] and in a large number of axons that branch extensively in human dental pulp [85]. Moreover, TRPA1 upregulation was found in rat TG in response to experimental exposure of the dental pulp [86] and in the myelinated nerve fibers of human teeth with pulpitis [85], implicating its pathological role in tooth pain. TRPA1 expression was also reported in human odontoblast-like cells derived from the dental pulp of permanent teeth. Blockade of TRPA1 reduced the hypotonic solution-induced Ca2+ influx, suggesting that it may function as a mechanoreceptor in odontoblasts [81]. Furthermore, TRPA1 immunoreactivity in odontoblasts and their process was increased in carious teeth compared to caries-free human third molar teeth [87,88]. In contrast, Tazawa et al. did not find TRPA1 expression in odontoblasts or their processes in extracted healthy caries-free human permanent teeth [89]. Yeon et al. also did not observe TRPA1 expression in acutely isolated adult rat odontoblasts [90]. Further studies are required to clarify this inconsistent finding.
Park et al. observed that 13% of dental primary afferents express TRPM8 in rats [71], but only 5.7% was found in mice [84]. In addition, TRPM8 was detected in human odontoblasts extracted from permanent teeth [89,91]. Although Tsumura et al. reported that TRPM8 in rat odontoblasts plays a role in detecting low-temperature stimulation of the dentin surface [92], another study showed that application of cold stimuli or TRPM8 agonist menthol did not increase the intra-odontoblastic Ca2+ concentration [90]. TRPM8 in pulpal nerves was decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia relative to healthy samples [93], suggesting that TRPM8 may not be involved in cold-mediated noxious pulpal pain mechanisms. Further studies are needed to clarify the functional role of TRPM8 in cold transduction in pulps.
TRPM7 was detected in acutely isolated rat odontoblasts at both gene and protein levels [94,95]. Specific activation of TRPM7 with an agonist naltriben or hypotonic solution-induced membrane stretch caused Ca2+ influx of odontoblasts, which can be blocked by TRPM7 inhibitor FTY720, indicating TRPM7 may act as a mechanical transducer in odontoblasts [95]. Moreover, TRPM7 in odontoblasts might play a role in dentine mineralization by regulating intracellular Mg2+ and alkaline phosphatase activity [96,97].
Regulation of dental pain by TRPs
Orthodontic force application increased TRPV1 in the force-stimulated pulps of rat molars, indicating a possible role of TRPV1 in orthodontic pain [98]. In support of this, a recent study determined the contribution of TRPV1 and TRPV1-expressing afferents to orthodontic pain in a mouse model of tooth movement. Chemical ablation of TRPV1-expressing afferents through injection of RTX into the TG or knockout of TRPV1 significantly attenuated spontaneous pain and bite force-evoked pain, suggesting TRPV1 and TRPV1-expressing TG nociceptors constitute a primary pathway mediating orthodontic pain behaviors [99]. CFA injection into the upper first molar (M1TP) caused a significant decrease in the head withdrawal threshold in response to mechanical and heat stimuli applied to the ipsilateral facial skin, which was significantly attenuated after administration of TRPV1 antagonist into the M1TP [100]. In a rat model of intraoral wire-induced mucositis, inhibition of TRPV1 with its selective inhibitor SB-366791, dramatically reduced spontaneous nociceptive behavior, assessed by mouth rubbing, but it did not affect mechanical allodynia, evaluated by head withdrawal with von Frey filaments [101]. In contrast, with the same pain model, mechanical allodynia was attenuated by inhibition of RN-1734, a selective TRPV4 antagonist [101].
Dental pulp following orthodontic force activates and sensitizes TRPA1 on nociceptive fibers, resulting in orthodontic pain, as assessed by facial rubbing and wiping, which can be attenuated by blockade of TRPA1 with HC-030031 [102]. Many patients undergoing peroxide based whitening procedures complain of bleaching sensitivity (BS) arising in the treated teeth. For BS, pain can occur in healthy intact teeth without any provoking stimulus. TRPA1 can be activated by a variety of oxidizer compounds, including hydrogen peroxide. It is hypothesized that direct activation of intradental nerve activity via TRPA1 might contribute to BS pain [103]. Noxious cold stimulation of dental pulp afferents produces an intense pain. Although TRPA1-expressing TG neurons highly innervate dental pulp, noxious cold stimulation of the tooth induced an overexpression of c-Fos in the trigeminal nucleus in a TRPA1-independent manner. This result indicates that TRPA1 may not contribute to the cold nociception of dental pulp, but further studies are needed to elucidate it [84].
Despite ample evidence demonstrates that TRPV2, TRPV4, TRPM7, and TRPM8 are distributed in the sensory systems of the teeth and some of them are dysregulated under pathological conditions (see above section ‘Expression of TRPs in dental sensory system under pathophysiological conditions’), surprisingly, their direct contributions to orofacial pain behaviors are largely unknown.
Closing remarks and future perspectives
In summary, we have reviewed a large body of evidence on TRPs in orofacial pain, mainly focused on their functional expression in the trigeminal sensory system under pathophysiological conditions and their contributions to pain behaviors. Orofacial pain is one of the most debilitating human diseases and represents a social and economic burden for our society. Considering the current therapies often have unsatisfactory efficacy or substantial adverse effects, more effective treatments for orofacial pain are high priority. Summarizing the findings of TRPs in orofacial pain may provide a better understanding of this debilitating disease and a rational for the development of new analgesics. Although studies conducted over the past two decades strongly indicate that TRPs are potential targets for treating orofacial pain, some important questions remain open to be discussed and resolved.
First, enriched expression of TRPV1 in the trigeminal sensory system and its involvements in orofacial pain suggest that pharmacologically targeting TRPV1 might be a novel therapeutic strategy for managing orofacial pain. Pharmaceutical companies have developed TRPV1 antagonists that can potentially be used for relieving pain, including orofacial pain. A few TRPV1 antagonists have been advanced into human clinical trials for dental pain [104-106]. Unfortunately, it has been proven to be unsatisfactory due to side effects, such as hyperthermia and prevention of protective noxious heat sensation [107-109]. In one clinical trial, the analgesic effect of the first-generation TRPV1 antagonist AMG 517 was tested following extraction of a third molar tooth. However, the drug increased core body temperature, resulting in termination of the trial [107]. Another TRPV1 antagonist AZD1386 was effective at relieving molar extraction pain, but its analgesic effect was short [106]. Some second-generation TRPV1 antagonists, such as SB-705498 [83], have negligible impact on body temperature in both animal experiments and clinical trials [110-113], but the clinical data on pain relief remains unknown. Moreover, ablation of TRPV1+ sensory afferents is also a promising approach to treating pain [114]. For instance, topical application of 8% capsaicin produced a long-term pain reduction in patients with post-herpetic neuralgia [115], leading to an approval by US Food and Drug Administration. However, further clinical studies are needed to support this approach for orofacial pain treatment.
Second, due to the side effects of TRP antagonists, clinical studies get delayed or stuck. The new modes of reducing the activity of TRP channels like TRPV1 and TRPA1 must be considered if they are to be used as suitable pharmacological pain targets. TRPA1 and TRPV1 interacting proteins might make for valid targets, as an alternative to direct inhibition of each channel, because TRPA1-TRPV1 interactions have been shown to be relevant for pathological pain based on the concerted interplay of these two “pain TRPs” [37]. TMEM100, a two-transmembrane protein highly conserved in vertebrates, was recently identified as a novel adaptor to govern the functional and physical interaction between TRPA1 and TRPV1 in sensory neurons [116]. Of note, mice with conditional knockout of TMEM100 in sensory neurons or subcutaneous injection of a TMEM100-inhibitory peptide had reduced mechanical allodynia evoked by CFA injection into the hind-paw or by nerve injury caused by systemic paclitaxel treatment, opening the door for targeting TMEM100 as a novel alternative approach [116]. Given that the inhibitory peptide was injected subcutaneously, these results also suggest that TMEM100 could serve as a peripheral-topical target for reducing pain while avoiding unwanted side-effects of systemic treatments. Importantly, mechanosensitivity and thermal sensitivity (both heat and cold) were not affected by genetic deletion of TMEM100 or injection of the inhibitory peptide in non-injured mice. These data collectively point toward TMEM100 as a valid new target for attenuating pathologic pain, a concept supported by available data on loss-of-function of TMEM100 in DRG-mediated pain. Interestingly, TMEM100 gene was upregulated in rat TG following inflammation of the masseter muscle [28]. However, whether TMEM100 regulates the interaction between TRPA1 and TRPV1 in trigeminal sensory neurons and whether TMEM100 is crucial for orofacial pain await experimental clarification. In addition, there are studies that suggest some TRP channels co-contribute to pain or have a synergistic effect in regulating pain [22,117-119], and it is of great interest in the development of potent dual antagonists targeting receptor-receptor interaction devoid of side effects. For example, the co-contribution of TRPV4 and TRPA1 was noted in TMJ pain and formalin-caused trigeminal irritant pain [22,117]. Recently, a compound 16-8 which can simultaneously inhibit TRPV4 and TRPA1 was developed. More importantly, the compound lacked gross systemic toxicity and produced a more robust effect on trigeminal irritant pain than that of TRPV4 inhibitor GSK205 at the same dose or an equivalent effect to TRPA1 inhibitor A967079 at a lower dose [120].
Third, more women report orofacial pain than men [121]. TMJD is about 1.5-2 times, trigeminal neuralgia is about 1.5-1.7 times, and migraine is about 3 times more prevalent in women than in men, and women appear to suffer some forms of orofacial pain more severely than men [38,122,123]. Yet the majority of preclinical studies of TRPs in orofacial pain as summarized above were performed on males. Future work should consider the hypothesis in both sexes because it is invalid to assume that data obtained in male subjects will generalize to females. Interestingly, a recent transcriptomic study with the RNA-seq assay revealed that there are 963 sex-dependent differential expression of genes in TG, including TRPV4 with a significantly higher expression in female vs. male mice [124]. Although there were no significant differences in the expression of TRPM8+ TG neurons between sexes, TRPM8+ TG neurons derived from female mice demonstrated distinct differences from male mice in their responses to the TRPM8 agonist menthol and differences in voltage gated sodium and potassium currents [125]. It has been suggested that TRP channels could be potential targets for sex-related differences in migraine pain [126], but whether TRPs in other forms of orofacial pain are also sex-dependent needs further elucidation.
Fourth, humans generally rank head and facial pain as much more severe and emotionally draining than body pain [127-132]. However, the in-depth neural mechanisms underlying the affective component of orofacial pain remains largely unknown. Distress, anxiety, fear, depression, and disrupted sleep are emotional dimensions of pain that are not directly resulting from neural activation of canonical sensory afferents via the spino–thalamic tract that feeds into cortical somatosensory circuits. Instead, these sensory-related afferent signals are relayed by the less-studied and less well-understood affective pain pathway [133-135]. It processes nociceptive afferent information from second-order relay neurons to the lateral parabrachial nucleus (PBL) which in turn projects to emotional and instinctive control centers in the brain. We recently discovered that a subset of TG sensory neurons directly projects to the lateral parabrachial nucleus (PBL), which then projects to several key emotion- and instinct-related centers in the brain [8]. The PBL was found critical for relaying the primary TG afferents that subsequently evoke the unpleasantness of pain. Surprisingly, this was not the case for afferents of the DRG, i.e. DRG sensory neurons did not provide direct monosynaptic input to the PBL neurons. In addition, we discovered that TRPV1+ afferents were a major source of TG inputs to PBL (TG→PBL) and provided a monosynaptic excitatory afferent signal to PBL-nociceptive neurons. We also found that selective activation of TRPV1+ afferent terminals in PBL induced robust aversive behaviors, whereas their silencing significantly attenuated trigeminally-mediated acute irritant pain [8]. These findings suggest that this novel circuit TG→PBL might provide a neural substrate for heightened craniofacial affective pain. Whether and how the TG→PBL direct connection contributes to the affective component of chronic orofacial pain is under investigation in our lab.
Lastly, while many TRP channels have been strongly suggested to contribute to orofacial pain, there are still some inconsistent findings either in functional expression or pain regulation as described above. More studies are needed to fully understand their roles and mechanisms in orofacial pain.
Acknowledgments
The authors acknowledge the support by National Institutes of Health Grant DE027454 to YC.
Footnotes
Conflict of interest
The authors of the manuscript declare that there are no conflicts of interest.
Availability of data and material
Not applicable to this study as no dataset or material was generated during the current study.
Consent to participate
Not applicable to this study.
Consent for Publication
Not applicable to this study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Macfarlane TV, Blinkhorn AS, Davies RM, Ryan P, Worthington HV, Macfarlane GJ (2002) Orofacial pain: just another chronic pain? Results from a population-based survey. Pain 99 (3):453–458. doi: 10.1016/s0304-3959(02)00181-1 [DOI] [PubMed] [Google Scholar]
- 2.Chichorro JG, Porreca F, Sessle B (2017) Mechanisms of craniofacial pain. Cephalalgia 37 (7):613–626. doi: 10.1177/0333102417704187 [DOI] [PubMed] [Google Scholar]
- 3.Benoliel R, Sharav Y (2010) Chronic orofacial pain. Curr Pain Headache Rep 14 (1):33–40. doi: 10.1007/s11916-009-0085-y [DOI] [PubMed] [Google Scholar]
- 4.González-Ramírez R, Chen Y, Liedtke WB, Morales-Lázaro SL (2017) TRP Channels and Pain. In: Emir TLR (ed) Neurobiology of TRP Channels. CRC Press/Taylor & Francis© 2018 by Taylor & Francis Group, LLC., Boca Raton (FL), pp 125–147. doi: 10.4324/9781315152837-8 [DOI] [PubMed] [Google Scholar]
- 5.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB (2018) Regulation of Pain and Itch by TRP Channels. Neurosci Bull 34 (1):120–142. doi: 10.1007/s12264-017-0200-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bereiter DA, Hargreaves KM, Hu JW (2008) 5.32 - Trigeminal Mechanisms of Nociception: Peripheral and Brainstem Organization. In: Masland RH, Albright TD, Albright TD et al. (eds) The Senses: A Comprehensive Reference. Academic Press, New York, pp 435–460. doi: 10.1016/B978-012370880-9.00174-2 [DOI] [Google Scholar]
- 7.Hargreaves KM (2011) Orofacial pain. Pain 152 (3 Suppl):S25–32. doi: 10.1016/j.pain.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S, Han BX, Ryu D, Yin H, Liedtke W, Wang F (2017) A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat Neurosci 20 (12):1734–1743. doi: 10.1038/s41593-017-0012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bongenhielm U, Boissonade FM, Westermark A, Robinson PP, Fried K (1999) Sympathetic nerve sprouting fails to occur in the trigeminal ganglion after peripheral nerve injury in the rat. Pain 82 (3):283–288. doi: 10.1016/s0304-3959(99)00064-0 [DOI] [PubMed] [Google Scholar]
- 10.Lopes DM, Denk F, McMahon SB (2017) The Molecular Fingerprint of Dorsal Root and Trigeminal Ganglion Neurons. Front Mol Neurosci 10:304. doi: 10.3389/fnmol.2017.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandewauw I, Owsianik G, Voets T (2013) Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci 14:21. doi: 10.1186/1471-2202-14-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benemei S, Dussor G (2019) TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals (Basel) 12 (2). doi: 10.3390/ph12020054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F (2014) Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci 5 (11):1085–1096. doi: 10.1021/cn500083e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benemei S, De Cesaris F, Fusi C, Rossi E, Lupi C, Geppetti P (2013) TRPA1 and other TRP channels in migraine. J Headache Pain 14 (1):71. doi: 10.1186/1129-2377-14-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aroucha JM, Ximenes RC, Vasconcelos FM, Nery MW, Sougey EB (2014) Temporomandibular disorders and eating disorders: a literature review. Trends Psychiatry Psychother 36 (1):11–15. doi: 10.1590/2237-6089-2013-0006 [DOI] [PubMed] [Google Scholar]
- 16.Eaves ER, Nichter M, Ritenbaugh C, Sutherland E, Dworkin SF (2015) Works of Illness and the Challenges of Social Risk and the Specter of Pain in the Lived Experience of TMD. Med Anthropol Q 29 (2): 157–177. doi: 10.1111/maq.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioi H, Kido MA, Zhang JQ, Yamaza T, Nakata S, Nakasima A, Tanaka T (2006) Capsaicin receptor expression in the rat temporomandibular joint. Cell Tissue Res 325 (1):47–54. doi: 10.1007/s00441-006-0183-7 [DOI] [PubMed] [Google Scholar]
- 18.Lund JP, Sadeghi S, Athanassiadis T, Caram Salas N, Auclair F, Thivierge B, Arsenault I, Rompré P, Westberg KG, Kolta A (2010) Assessment of the potential role of muscle spindle mechanoreceptor afferents in chronic muscle pain in the rat masseter muscle. PLoS One 5 (6):e11131. doi: 10.1371/journal.pone.0011131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato J, Segami N, Yoshitake Y, Kaneyama K, Abe A, Yoshimura H, Fujimura K (2005) Expression of capsaicin receptor TRPV-1 in synovial tissues of patients with symptomatic internal derangement of the temporomandibular joint and joint pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100 (6):674–681. doi: 10.1016/j.tripleo.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 20.Ro JY, Lee JS, Zhang Y (2009) Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 144 (3):270–277. doi: 10.1016/j.pain.2009.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benbow T, Ranjbar Ekbatan M, Cairns BE (2020) α(1) adrenergic receptor activation has a dynamic effect on masticatory muscle afferent fibers. Neuropharmacology 175:108197. doi: 10.1016/j.neuropharm.2020.108197 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C, Gereau RWt, Taylor AB, Wang F, Guilak F, Liedtke W (2013) Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain 154 (8):1295–1304. doi: 10.1016/j.pain.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denadai-Souza A, Martin L, de Paula MA, de Avellar MC, Muscará MN, Vergnolle N, Cenac N (2012) Role of transient receptor potential vanilloid 4 in rat joint inflammation. Arthritis Rheum 64 (6):1848–1858. doi: 10.1002/art.34345 [DOI] [PubMed] [Google Scholar]
- 24.Wu YW, Hao T, Kou XX, Gan YH, Ma XC (2015) Synovial TRPV1 is upregulated by 17-β-estradiol and involved in allodynia of inflamed temporomandibular joints in female rats. Arch Oral Biol 60 (9):1310–1318. doi: 10.1016/j.archoralbio.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 25.Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC (2010) 17-Beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. J Neurosci 30 (26):8710–8719. doi: 10.1523/jneurosci.6323-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonic-Kocijan S, Zhao X, Liu W, Wu Y, Uhac I, Wang K (2013) TRPV1 channel-mediated bilateral allodynia induced by unilateral masseter muscle inflammation in rats. Mol Pain 9:68. doi: 10.1186/1744-8069-9-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SM, Cho YS, Kim TH, Jin MU, Ahn DK, Noguchi K, Bae YC (2012) An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J Chem Neuroanat 45 (1-2):45–49. doi: 10.1016/j.jchemneu.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Chung MK, Park J, Asgar J, Ro JY (2016) Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol Pain 12. doi: 10.1177/1744806916668526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Arendt-Nielsen L, Svensson P (2002) Capsaicin-induced muscle pain alters the excitability of the human jaw-stretch reflex. J Dent Res 81 (9):650–654. doi: 10.1177/154405910208100915 [DOI] [PubMed] [Google Scholar]
- 30.Asgar J, Zhang Y, Saloman JL, Wang S, Chung MK, Ro JY (2015) The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience 310:206–215. doi: 10.1016/j.neuroscience.2015.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Brigoli B, Lim J, Karley A, Chung MK (2018) Roles of TRPV1 and TRPA1 in Spontaneous Pain from Inflamed Masseter Muscle. Neuroscience 384:290–299. doi: 10.1016/j.neuroscience.2018.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Lim J, Joseph J, Wang S, Wei F, Ro JY, Chung MK (2017) Spontaneous and Bite-Evoked Muscle Pain Are Mediated by a Common Nociceptive Pathway With Differential Contribution by TRPV1. J Pain 18 (11):1333–1345. doi: 10.1016/j.jpain.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kogawa EM, Calderon PS, Lauris JR, Araujo CR, Conti PC (2006) Evaluation of maximal bite force in temporomandibular disorders patients. J Oral Rehabil 33 (8):559–565. doi: 10.1111/j.1365-2842.2006.01619.x [DOI] [PubMed] [Google Scholar]
- 34.Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle BJ, Arendt-Nielsen L, Svensson P (2008) Effect of peripheral NMDA receptor blockade with ketamine on chronic myofascial pain in temporomandibular disorder patients: a randomized, double-blinded, placebo-controlled trial. J Orofac Pain 22 (2):122–130 [PubMed] [Google Scholar]
- 35.Pereira LJ, Steenks MH, de Wijer A, Speksnijder CM, van der Bilt A (2009) Masticatory function in subacute TMD patients before and after treatment. J Oral Rehabil 36 (6):391–402. doi: 10.1111/j.1365-2842.2008.01920.x [DOI] [PubMed] [Google Scholar]
- 36.Guo W, Zou S, Mohammad Z, Wang S, Yang J, Li H, Dubner R, Wei F, Chung MK, Ro JY, Ren K (2019) Voluntary biting behavior as a functional measure of orofacial pain in mice. Physiol Behav 204:129–139. doi: 10.1016/j.physbeh.2019.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akopian AN (2011) Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr Pharm Biotechnol 12 (1):89–94. doi: 10.2174/138920111793937952 [DOI] [PubMed] [Google Scholar]
- 38.Jones MR, Urits I, Ehrhardt KP, Cefalu JN, Kendrick JB, Park DJ, Cornett EM, Kaye AD, Viswanath O (2019) A Comprehensive Review of Trigeminal Neuralgia. Curr Pain Headache Rep 23 (10):74. doi: 10.1007/s11916-019-0810-0 [DOI] [PubMed] [Google Scholar]
- 39.International Classification of Orofacial Pain, 1st edition (ICOP) (2020). Cephalalgia 40 (2):129–221. doi: 10.1177/0333102419893823 [DOI] [PubMed] [Google Scholar]
- 40.Rossi HL, Jenkins AC, Kaufman J, Bhattacharyya I, Caudle RM, Neubert JK (2012) Characterization of bilateral trigeminal constriction injury using an operant facial pain assay. Neuroscience 224:294–306. doi: 10.1016/j.neuroscience.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demartini C, Greco R, Zanaboni AM, Francesconi O, Nativi C, Tassorelli C, Deseure K (2018) Antagonism of Transient Receptor Potential Ankyrin Type-1 Channels as a Potential Target for the Treatment of Trigeminal Neuropathic Pain: Study in an Animal Model. Int J Mol Sci 19 (11). doi: 10.3390/ijms19113320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C, Xie N, Lian Y, Xu H, Chen C, Zheng Y, Chen Y, Zhang H (2016) Central antinociceptive activity of peripherally applied botulinum toxin type A in lab rat model of trigeminal neuralgia. Springerplus 5:431. doi: 10.1186/s40064-016-2071-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urano H, Ara T, Fujinami Y, Hiraoka BY (2012) Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain. Int J Med Sci 9 (8):690–697. doi: 10.7150/ijms.4706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuboi Y, Honda K, Bae YC, Shinoda M, Kondo M, Katagiri A, Echizenya S, Kamakura S, Lee J, Iwata K (2015) Morphological and functional changes in regenerated primary afferent fibres following mental and inferior alveolar nerve transection. Eur J Pain 19 (9):1258–1266. doi: 10.1002/ejp.650 [DOI] [PubMed] [Google Scholar]
- 45.Zakir HM, Mostafeezur RM, Suzuki A, Hitomi S, Suzuki I, Maeda T, Seo K, Yamada Y, Yamamura K, Lev S, Binshtok AM, Iwata K, Kitagawa J (2012) Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation. PLoS One 7 (9):e44023. doi: 10.1371/journal.pone.0044023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HY, Park CK, Cho IH, Jung SJ, Kim JS, Oh SB (2008) Differential Changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain 9 (3):280–288. doi: 10.1016/j.jpain.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 47.Neumann S, Doubell TP, Leslie T, Woolf CJ (1996) Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384 (6607):360–364. doi: 10.1038/384360a0 [DOI] [PubMed] [Google Scholar]
- 48.Schäfers M, Geis C, Svensson CI, Luo ZD, Sommer C (2003) Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci 17 (4):791–804. doi: 10.1046/j.1460-9568.2003.02504.x [DOI] [PubMed] [Google Scholar]
- 49.Trevisan G, Benemei S, Materazzi S, De Logu F, De Siena G, Fusi C, Fortes Rossato M, Coppi E, Marone IM, Ferreira J, Geppetti P, Nassini R (2016) TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 139 (Pt 5):1361–1377. doi: 10.1093/brain/aww038 [DOI] [PubMed] [Google Scholar]
- 50.Sugawara S, Shinoda M, Hayashi Y, Saito H, Asano S, Kubo A, Shibuta I, Furukawa A, Toyofuku A, Iwata K (2019) Increase in IGF-1 Expression in the Injured Infraorbital Nerve and Possible Implications for Orofacial Neuropathic Pain. Int J Mol Sci 20 (24). doi: 10.3390/ijms20246360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Su Q, Lian Y, Chen Y (2019) Botulinum toxin type A reduces the expression of transient receptor potential melastatin 3 and transient receptor potential vanilloid type 4 in the trigeminal subnucleus caudalis of a rat model of trigeminal neuralgia. Neuroreport 30 (10):735–740. doi: 10.1097/wnr.0000000000001268 [DOI] [PubMed] [Google Scholar]
- 52.Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X (2014) Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81 (4):873–887. doi: 10.1016/j.neuron.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Binshtok AM, Bean BP, Woolf CJ (2007) Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449 (7162):607–610. doi: 10.1038/nature06191 [DOI] [PubMed] [Google Scholar]
- 54.Zakir HM, Masuda Y, Kitagawa J (2020) A novel approach for detection of functional expression of TRPV1 channels on regenerated neurons following nerve injury. J Oral Sci 62 (2):136–139. doi: 10.2334/josnusd.19-0356 [DOI] [PubMed] [Google Scholar]
- 55.Mishra SK, Hoon MA (2010) Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci 43 (1):157–163. doi: 10.1016/j.mcn.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50 (2):277–289. doi: 10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 57.Cruz LS, Kopruszinski CM, Chichorro JG (2014) Intraganglionar resiniferatoxin prevents orofacial inflammatory and neuropathic hyperalgesia. Behav Pharmacol 25 (2):112–118. doi: 10.1097/fbp.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Bian C, Yang J, Arora V, Gao Y, Wei F, Chung MK (2020) Ablation of TRPV1+ Afferent Terminals by Capsaicin Mediates Long-Lasting Analgesia for Trigeminal Neuropathic Pain. eNeuro 7 (3). doi: 10.1523/eneuro.0118-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mou J, Paillard F, Turnbull B, Trudeau J, Stoker M, Katz NP (2013) Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain 154 (9): 1632–1639. doi: 10.1016/j.pain.2013.04.044 [DOI] [PubMed] [Google Scholar]
- 60.Treede RD, Wagner T, Kern KU, Husstedt IW, Arendt G, Birklein F, Cegla T, Freynhagen R, Gockel HH, Heskamp ML, Jager H, Joppich R, Maier C, Leffler A, Nagelein HH, Rolke R, Seddigh S, Sommer C, Stander S, Wasner G, Baron R (2013) Mechanism- and experience-based strategies to optimize treatment response to the capsaicin 8% cutaneous patch in patients with localized neuropathic pain. Curr Med Res Opin 29 (5):527–538. doi: 10.1185/03007995.2013.781019 [DOI] [PubMed] [Google Scholar]
- 61.Morra ME, Elgebaly A, Elmaraezy A, Khalil AM, Altibi AM, Vu TL, Mostafa MR, Huy NT, Hirayama K (2016) Therapeutic efficacy and safety of Botulinum Toxin A Therapy in Trigeminal Neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain 17 (1):63. doi: 10.1186/s10194-016-0651-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubis A, Juodzbalys G (2020) The Use of Botulinum Toxin A in the Management of Trigeminal Neuralgia: a Systematic Literature Review. J Oral Maxillofac Res 11 (2):e2. doi: 10.5037/jomr.2020.11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, Kuroi T, Ebine T, Koizumi K, Suzuki N (2012) Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis 48 (3):367–378. doi: 10.1016/j.nbd.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 64.Zuo X, Ling JX, Xu GY, Gu JG (2013) Operant behavioral responses to orofacial cold stimuli in rats with chronic constrictive trigeminal nerve injury: effects of menthol and capsazepine. Mol Pain 9:28. doi: 10.1186/1744-8069-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gill CH, Randall A, Bates SA, Hill K, Owen D, Larkman PM, Cairns W, Yusaf SP, Murdock PR, Strijbos PJ, Powell AJ, Benham CD, Davies CH (2004) Characterization of the human HCN1 channel and its inhibition by capsazepine. Br J Pharmacol 143 (3):411–421. doi: 10.1038/sj.bjp.0705945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orio P, Madrid R, de la Peña E, Parra A, Meseguer V, Bayliss DA, Belmonte C, Viana F (2009) Characteristics and physiological role of hyperpolarization activated currents in mouse cold thermoreceptors. J Physiol 587 (Pt 9):1961–1976. doi: 10.1113/jphysiol.2008.165738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung G, Jung SJ, Oh SB (2013) Cellular and molecular mechanisms of dental nociception. J Dent Res 92 (11):948–955. doi: 10.1177/0022034513501877 [DOI] [PubMed] [Google Scholar]
- 68.Ichikawa H, Sugimoto T (2001) VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res 890 (1):184–188. doi: 10.1016/s0006-8993(00)03253-4 [DOI] [PubMed] [Google Scholar]
- 69.Chung MK, Lee J, Duraes G, Ro JY (2011) Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in trigeminal ganglia. J Dent Res 90 (9): 1103–1107. doi: 10.1177/0022034511413284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stenholm E, Bongenhielm U, Ahlquist M, Fried K (2002) VR1- and VRL-1-like immunoreactivity in normal and injured trigeminal dental primary sensory neurons of the rat. Acta Odontol Scand 60 (2):72–79. doi: 10.1080/000163502753509455 [DOI] [PubMed] [Google Scholar]
- 71.Park CK, Kim MS, Fang Z, Li HY, Jung SJ, Choi SY, Lee SJ, Park K, Kim JS, Oh SB (2006) Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem 281 (25): 17304–17311. doi: 10.1074/jbc.M511072200 [DOI] [PubMed] [Google Scholar]
- 72.Kim HY, Chung G, Jo HJ, Kim YS, Bae YC, Jung SJ, Kim JS, Oh SB (2011) Characterization of dental nociceptive neurons. J Dent Res 90 (6):771–776. doi: 10.1177/0022034511399906 [DOI] [PubMed] [Google Scholar]
- 73.Kim YS, Kim YJ, Paik SK, Cho YS, Kwon TG, Ahn DK, Kim SK, Yoshida A, Bae YC (2009) Expression of metabotropic glutamate receptor mGluR5 in human dental pulp. J Endod 35 (5):690–694. doi: 10.1016/j.joen.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 74.Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P (2003) Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain 17 (3):245–250 [PubMed] [Google Scholar]
- 75.Morgan CR, Rodd HD, Clayton N, Davis JB, Boissonade FM (2005) Vanilloid receptor 1 expression in human tooth pulp in relation to caries and pain. J Orofac Pain 19 (3):248–260 [PubMed] [Google Scholar]
- 76.Solé-Magdalena A, Martínez-Alonso M, Coronado CA, Junquera LM, Cobo J, Vega JA (2018) Molecular basis of dental sensitivity: The odontoblasts are multisensory cells and express multifunctional ion channels. Ann Anat 215:20–29. doi: 10.1016/j.aanat.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 77.Okumura R, Shima K, Muramatsu T, Nakagawa K, Shimono M, Suzuki T, Magloire H, Shibukawa Y (2005) The odontoblast as a sensory receptor cell? The expression of TRPV1 (VR-1) channels. Arch Histol Cytol 68 (4):251–257. doi: 10.1679/aohc.68.251 [DOI] [PubMed] [Google Scholar]
- 78.Tsumura M, Sobhan U, Muramatsu T, Sato M, Ichikawa H, Sahara Y, Tazaki M, Shibukawa Y (2012) TRPV1-mediated calcium signal couples with cannabinoid receptors and sodium-calcium exchangers in rat odontoblasts. Cell Calcium 52 (2): 124–136. doi: 10.1016/j.ceca.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 79.Ichikawa H, Sugimoto T (2000) Vanilloid receptor 1-like receptor-immunoreactive primary sensory neurons in the rat trigeminal nervous system. Neuroscience 101 (3):719–725. doi: 10.1016/s0306-4522(00)00427-9 [DOI] [PubMed] [Google Scholar]
- 80.Gibbs JL, Melnyk JL, Basbaum AI (2011) Differential TRPV1 and TRPV2 channel expression in dental pulp. J Dent Res 90 (6):765–770. doi: 10.1177/0022034511402206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sato M, Sobhan U, Tsumura M, Kuroda H, Soya M, Masamura A, Nishiyama A, Katakura A, Ichinohe T, Tazaki M, Shibukawa Y (2013) Hypotonic-induced stretching of plasma membrane activates transient receptor potential vanilloid channels and sodium-calcium exchangers in mouse odontoblasts. J Endod 39 (6):779–787. doi: 10.1016/j.joen.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 82.Wen W, Que K, Zang C, Wen J, Sun G, Zhao Z, Li Y (2017) Expression and distribution of three transient receptor potential vanilloid(TRPV) channel proteins in human odontoblast-like cells. J Mol Histol 48 (5-6):367–377. doi: 10.1007/s10735-017-9735-2 [DOI] [PubMed] [Google Scholar]
- 83.Bakri MM, Yahya F, Munawar KMM, Kitagawa J, Hossain MZ (2018) Transient receptor potential vanilloid 4 (TRPV4) expression on the nerve fibers of human dental pulp is upregulated under inflammatory condition. Arch Oral Biol 89:94–98. doi: 10.1016/j.archoralbio.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 84.Michot B, Lee CS, Gibbs JL (2018) TRPM8 and TRPA1 do not contribute to dental pulp sensitivity to cold. Sci Rep 8 (1):13198. doi: 10.1038/s41598-018-31487-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim YS, Jung HK, Kwon TK, Kim CS, Cho JH, Ahn DK, Bae YC (2012) Expression of transient Receptor potential ankyrin 1 in human dental pulp. J Endod 38 (8):1087–1092. doi: 10.1016/j.joen.2012.04.024 [DOI] [PubMed] [Google Scholar]
- 86.Haas ET, Rowland K, Gautam M (2011) Tooth injury increases expression of the cold sensitive TRP channel TRPA1 in trigeminal neurons. Arch Oral Biol 56 (12):1604–1609. doi: 10.1016/j.archoralbio.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 87.El Karim I, McCrudden MT, Linden GJ, Abdullah H, Curtis TM, McGahon M, About I, Irwin C, Lundy FT (2015) TNF-α-induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast-like cells. Am J Pathol 185 (11):2994–3002. doi: 10.1016/j.ajpath.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 88.El Karim IA, McCrudden MT, McGahon MK, Curtis TM, Jeanneau C, Giraud T, Irwin CR, Linden GJ, Lundy FT, About I (2016) Biodentine Reduces Tumor Necrosis Factor Alpha-induced TRPA1 Expression in Odontoblastlike Cells. J Endod 42 (4):589–595. doi: 10.1016/j.joen.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 89.Tazawa K, Ikeda H, Kawashima N, Okiji T (2017) Transient receptor potential melastatin (TRPM) 8 is expressed in freshly isolated native human odontoblasts. Arch Oral Biol 75:55–61. doi: 10.1016/j.archoralbio.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 90.Yeon KY, Chung G, Shin MS, Jung SJ, Kim JS, Oh SB (2009) Adult rat odontoblasts lack noxious thermal sensitivity. J Dent Res 88 (4):328–332. doi: 10.1177/0022034509334100 [DOI] [PubMed] [Google Scholar]
- 91.El Karim IA, Linden GJ, Curtis TM, About I, McGahon MK, Irwin CR, Lundy FT (2011) Human odontoblasts express functional thermo-sensitive TRP channels: implications for dentin sensitivity. Pain 152 (10):2211–2223. doi: 10.1016/j.pain.2010.10.016 [DOI] [PubMed] [Google Scholar]
- 92.Tsumura M, Sobhan U, Sato M, Shimada M, Nishiyama A, Kawaguchi A, Soya M, Kuroda H, Tazaki M, Shibukawa Y (2013) Functional expression of TRPM8 and TRPA1 channels in rat odontoblasts. PLoS One 8 (12):e82233. doi: 10.1371/journal.pone.0082233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alvarado LT, Perry GM, Hargreaves KM, Henry MA (2007) TRPM8 Axonal expression is decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia. J Endod 33 (10):1167–1171. doi: 10.1016/j.joen.2007.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kwon M, Baek SH, Park CK, Chung G, Oh SB (2014) Single-cell RT-PCR and immunocytochemical detection of mechanosensitive transient receptor potential channels in acutely isolated rat odontoblasts. Arch Oral Biol 59 (12):1266–1271. doi: 10.1016/j.archoralbio.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 95.Won J, Vang H, Kim JH, Lee PR, Kang Y, Oh SB (2018) TRPM7 Mediates Mechanosensitivity in Adult Rat Odontoblasts. J Dent Res 97 (9):1039–1046. doi: 10.1177/0022034518759947 [DOI] [PubMed] [Google Scholar]
- 96.Nakano Y, Le MH, Abduweli D, Ho SP, Ryazanova LV, Hu Z, Ryazanov AG, Den Besten PK, Zhang Y (2016) A Critical Role of TRPM7 As an Ion Channel Protein in Mediating the Mineralization of the Craniofacial Hard Tissues. Front Physiol 7:258. doi: 10.3389/fphys.2016.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Won J, Kim JH, Oh SB (2018) Molecular expression of Mg(2+) regulator TRPM7 and CNNM4 in rat odontoblasts. Arch Oral Biol 96:182–188. doi: 10.1016/j.archoralbio.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 98.Ohkura M, Ohkura N, Yoshiba N, Yoshiba K, Ida-Yonemochi H, Ohshima H, Saito I, Okiji T (2018) Orthodontic force application upregulated pain-associated prostaglandin-I(2)/PGI(2)-receptor/TRPV1 pathway-related gene expression in rat molars. Odontology 106 (1):2–10. doi: 10.1007/s10266-017-0309-2 [DOI] [PubMed] [Google Scholar]
- 99.Wang S, Kim M, Ali Z, Ong K, Pae EK, Chung MK (2019) TRPV1 and TRPV1-Expressing Nociceptors Mediate Orofacial Pain Behaviors in a Mouse Model of Orthodontic Tooth Movement. Front Physiol 10:1207. doi: 10.3389/fphys.2019.01207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watase T, Shimizu K, Komiya H, Ohara K, Iwata K, Ogiso B (2018) Involvement of transient receptor potential vanilloid 1 channel expression in orofacial cutaneous hypersensitivity following tooth pulp inflammation. J Oral Sci 60 (1):8–13. doi: 10.2334/josnusd.16-0854 [DOI] [PubMed] [Google Scholar]
- 101.Ito M, Ono K, Hitomi S, Nodai T, Sago T, Yamaguchi K, Harano N, Gunnjigake K, Hosokawa R, Kawamoto T, Inenaga K (2017) Prostanoid-dependent spontaneous pain and PAR(2)-dependent mechanical allodynia following oral mucosal trauma: involvement of TRPV1, TRPA1 and TRPV4. Mol Pain 13:1744806917704138. doi: 10.1177/1744806917704138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morii A, Miyamura Y, Sago MI, Mizuhara M, Shikayama T, Naniwa M, Hitomi S, Ujihara I, Kuroishi KN, Gunjigake KK, Shiga M, Morimoto Y, Kawamoto T, Ono K (2020) Orthodontic force-induced oxidative stress in the periodontal tissue and dental pulp elicits nociception via activation/sensitization of TRPA1 on nociceptive fibers. Free Radic Biol Med 147:175–186. doi: 10.1016/j.freeradbiomed.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 103.Markowitz K (2010) Pretty painful: why does tooth bleaching hurt? Med Hypotheses 74 (5):835–840. doi: 10.1016/j.mehy.2009.11.044 [DOI] [PubMed] [Google Scholar]
- 104.Gunthorpe MJ, Chizh BA (2009) Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today 14 (1-2):56–67. doi: 10.1016/j.drudis.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 105.Mickle AD, Shepherd AJ, Mohapatra DP (2016) Nociceptive TRP Channels: Sensory Detectors and Transducers in Multiple Pain Pathologies. Pharmaceuticals (Basel) 9 (4). doi: 10.3390/ph9040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quiding H, Jonzon B, Svensson O, Webster L, Reimfelt A, Karin A, Karlsten R, Segerdahl M (2013) TRPV1 antagonistic analgesic effect: a randomized study of AZD1386 in pain after third molar extraction. Pain 154 (6):808–812. doi: 10.1016/j.pain.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 107.Gavva NR, Bannon AW, Surapaneni S, Hovland DN Jr., Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC (2007) The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci 27 (13):3366–3374. doi: 10.1523/jneurosci.4833-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang H, Siemens J (2015) TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature (Austin) 2 (2):178–187. doi: 10.1080/23328940.2015.1040604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moran MM, Szallasi A (2018) Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol 175 (12):2185–2203. doi: 10.1111/bph.14044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gomtsyan A, McDonald HA, Schmidt RG, Daanen JF, Voight EA, Segreti JA, Puttfarcken PS, Reilly RM, Kort ME, Dart MJ, Kym PR (2015) TRPV1 ligands with hyperthermic, hypothermic and no temperature effects in rats. Temperature (Austin) 2 (2):297–301. doi: 10.1080/23328940.2015.1046013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voight EA, Gomtsyan AR, Daanen JF, Perner RJ, Schmidt RG, Bayburt EK, DiDomenico S, McDonald HA, Puttfarcken PS, Chen J, Neelands TR, Bianchi BR, Han P, Reilly RM, Franklin PH, Segreti JA, Nelson RA, Su Z, King AJ, Polakowski JS, Baker SJ, Gauvin DM, Lewis LR, Mikusa JP, Joshi SK, Faltynek CR, Kym PR, Kort ME (2014) Discovery of (R)-1-(7-chloro-2,2-bis(fluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl)urea (A-1165442): a temperature-neutral transient receptor potential vanilloid-1 (TRPV1) antagonist with analgesic efficacy. J Med Chem 57 (17):7412–7424. doi: 10.1021/jm500916t [DOI] [PubMed] [Google Scholar]
- 112.Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, Lee D, Louis JC, Magal E, Manning BH, Rubino J, Surapaneni S, Tamayo N, Wang T, Wang J, Wang J, Wang W, Youngblood B, Zhang M, Zhu D, Norman MH, Gavva NR (2008) Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther 326 (1):218–229. doi: 10.1124/jpet.107.132233 [DOI] [PubMed] [Google Scholar]
- 113.Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM, Appleby JM (2007) The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 132 (1-2):132–141. doi: 10.1016/j.pain.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 114.Chung MK, Campbell JN (2016) Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations. Pharmaceuticals (Basel) 9 (4). doi: 10.3390/ph9040066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yong YL, Tan LT, Ming LC, Chan KG, Lee LH, Goh BH, Khan TM (2016) The Effectiveness and Safety of Topical Capsaicin in Postherpetic Neuralgia: A Systematic Review and Meta-analysis. Front Pharmacol 7:538. doi: 10.3389/fphar.2016.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo GC, Wang Y, Geng Y, Zhang J, Guan Y, Akopian AN, Dong X (2015) Tmem100 Is a Regulator of TRPA1-TRPV1 Complex and Contributes to Persistent Pain. Neuron 85 (4):833–846. doi: 10.1016/j.neuron.2014.12.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Y, Kanju P, Fang Q, Lee SH, Parekh PK, Lee W, Moore C, Brenner D, Gereau RWt, Wang F, Liedtke W (2014) TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 155 (12):2662–2672. doi: 10.1016/j.pain.2014.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vermeulen W, De Man JG, De Schepper HU, Bult H, Moreels TG, Pelckmans PA, De Winter BY (2013) Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol 698 (1-3):404–412. doi: 10.1016/j.ejphar.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 119.Schwartz ES, Christianson JA, Chen X, La JH, Davis BM, Albers KM, Gebhart GF (2011) Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology 140 (4): 1283–1291.e1281-1282. doi: 10.1053/j.gastro.2010.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanju P, Chen Y, Lee W, Yeo M, Lee SH, Romac J, Shahid R, Fan P, Gooden DM, Simon SA, Spasojevic I, Mook RA, Liddle RA, Guilak F, Liedtke WB (2016) Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci Rep 6:26894. doi: 10.1038/srep26894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Häggman-Henrikson B, Liv P, Ilgunas A, Visscher CM, Lobbezoo F, Durham J, Lövgren A (2020) Increasing gender differences in the prevalence and chronification of orofacial pain in the population. Pain. doi: 10.1097/j.pain.0000000000001872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Halpern LR, Levine M, Dodson TB (2007) Sexual dimorphism and temporomandibular disorders (TMD). Oral Maxillofac Surg Clin North Am 19 (2):267–277, viii. doi: 10.1016/j.coms.2007.01.012 [DOI] [PubMed] [Google Scholar]
- 123.Cairns BE (2007) The influence of gender and sex steroids on craniofacial nociception. Headache 47 (2):319–324. doi: 10.1111/j.1526-4610.2006.00708.x [DOI] [PubMed] [Google Scholar]
- 124.Mecklenburg J, Zou Y, Wangzhou A, Garcia D, Lai Z, Tumanov AV, Dussor G, Price TJ, Akopian AN (2020) Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep 10 (1):15278. doi: 10.1038/s41598-020-72285-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Caudle RM, Caudle SL, Jenkins AC, Ahn AH, Neubert JK (2017) Sex differences in mouse Transient Receptor Potential Cation Channel, Subfamily M, Member 8 expressing trigeminal ganglion neurons. PLoS One 12 (5):e0176753. doi: 10.1371/journal.pone.0176753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Artero-Morales M, González-Rodríguez S, Ferrer-Montiel A (2018) TRP Channels as Potential Targets for Sex-Related Differences in Migraine Pain. Front Mol Biosci 5:73. doi: 10.3389/fmolb.2018.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zakrzewska JM, Wu J, Mon-Williams M, Phillips N, Pavitt SH (2017) Evaluating the impact of trigeminal neuralgia. Pain 158 (6): 1166–1174. doi: 10.1097/j.pain.0000000000000853 [DOI] [PubMed] [Google Scholar]
- 128.Giannakopoulos NN, Keller L, Rammelsberg P, Kronmüller KT, Schmitter M (2010) Anxiety and depression in patients with chronic temporomandibular pain and in controls. J Dent 38 (5):369–376. doi: 10.1016/j.jdent.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 129.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W (2013) Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 14 (12 Suppl):T75–90. doi: 10.1016/j.jpain.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith JG, Elias LA, Yilmaz Z, Barker S, Shah K, Shah S, Renton T (2013) The psychosocial and affective burden of posttraumatic neuropathy following injuries to the trigeminal nerve. J Orofac Pain 27 (4):293–303. doi: 10.11607/jop.1056 [DOI] [PubMed] [Google Scholar]
- 131.Schmidt K, Schunke O, Forkmann K, Bingel U (2015) Enhanced short-term sensitization of facial compared with limb heat pain. J Pain 16 (8):781–790. doi: 10.1016/j.jpain.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 132.Schmidt K, Forkmann K, Sinke C, Gratz M, Bitz A, Bingel U (2016) The differential effect of trigeminal vs. peripheral pain stimulation on visual processing and memory encoding is influenced by pain-related fear. Neuroimage 134:386–395. doi: 10.1016/j.neuroimage.2016.03.026 [DOI] [PubMed] [Google Scholar]
- 133.Hunt SP, Mantyh PW (2001) The molecular dynamics of pain control. Nat Rev Neurosci 2 (2):83–91. doi: 10.1038/35053509 [DOI] [PubMed] [Google Scholar]
- 134.Craig AD (1995) Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361 (2):225–248. doi: 10.1002/cne.903610204 [DOI] [PubMed] [Google Scholar]
- 135.Gauriau C, Bernard JF (2002) Pain pathways and parabrachial circuits in the rat. Exp Physiol 87 (2):251–258. doi: 10.1113/eph8702357 [DOI] [PubMed] [Google Scholar]