Abstract
A subset of stem-like tumor-propagating cells in GBM (GSC) underlies tumor propagation, therapeutic resistance and tumor recurrence. Immune evasion is critical for GSCs to carry out these functions. However, the molecular mechanisms employed by GSCs to escape anti-tumor immunity remain largely unknown. The reprogramming transcription factors Oct4 and Sox2 function as core multipotency factors and play an essential role in the formation and maintenance of GSCs, but the roles of these transcription factors in GSC immune escape have not been well explored. Here we examine how Oct4/Sox2 co-expression contributes to the immunosuppressive phenotype of GSCs. Combined transcription profiling and functional studies of Oct4/Sox2 co-expressing GSCs and differentiated GBM cells demonstrated that Oct4 and Sox2 cooperatively induce an immunosuppressive transcriptome consisting of multiple immunosuppressive checkpoints (i.e., PD-L1, CD70, A2aR, TDO) and dysregulation of cytokines and chemokines that are associated with an immunosuppressive tumor microenvironment. Mechanistically, induction and function of BRD/H3k27Ac-dependent immunosuppressive genes played a role in the immunosuppressive phenotype of GSCs. Pan-BET bromodomain inhibitors (e.g., JQ1) and shBRD4 constructs significantly inhibited the immunosuppressive transcriptome and immunosuppressive biological responses induced by Oct4/Sox2. Our findings identify targetable mechanisms by which tumor-propagating GSCs contribute to the immunosuppressive microenvironment in GBM.
Significance:
This report identifies mechanisms by which the reprogramming transcription factors Oct4 and Sox2 function to drive the immunomodulatory transcriptome of GSC and contribute to the immunosuppressive microenvironment in GBM.
Keywords: Oct4/Sox2, immunosuppressive transcriptome, BRD, GSCs
Introduction
Glioblastoma (GBM) is one of the deadliest cancers. It is well established that GBM contains stem-like cell subsets (GSCs) that efficiently propagate tumor xenografts and contribute to therapeutic resistance and tumor recurrence (1). More effective GBM treatment including emerging immunotherapeutics will have to deplete these tumor-propagating GSCs. The capacity for GSCs to efficiently carry out these oncogenic functions suggests their endowment with special mechanisms for escaping innate and adaptive anti-tumor immunity (2).
GSCs have been shown to suppress the adaptive immune system, specifically T cell responses (2). The higher expression of immunosuppressive checkpoint ligands (e.g. PD-L1, PD-L2) and immune-inhibitory molecules including certain metabolic pathways (e.g. IDO, A2aR) and lower levels of the co-stimulatory molecules (e.g., CD80/CD86) were observed in GSCs compared with non-GSCs (3,4). These immunosuppressive molecules lead to the suppression of T cell proliferation and cytokine activity and thereby impair the adaptive immunity response. GSCs also have been shown to recruit immunosuppressive cells such as anti-inflammatory M2 macrophages, regulatory T-cells (Tregs), and myeloid-derived suppressor cells (MDSC) by modulating cytokine/chemokine expression, and thus suppress innate immunity (5). However, the molecular mechanisms that regulate GSCs-intrinsic innate and adaptive immunomodulatory properties remain elusive.
Specific transcriptional networks play an essential role in sustaining the growth and self-renewal of embryonic stem (ES) cells and neoplastic stem-like cells. We and others have found that reprogramming transcription factors (TFs) Oct4 and Sox2 are overexpressed in GBM and their expression plays an essential role in maintaining glioma cell stemness and the tumor propagating potential of glioma tumor cells (6). However, the functional relationships between Oct4/Sox2 expression and the immunomodulatory pathways by which GSCs suppress anti-tumor immunity remain unknown. In this study, we specifically address this question using isogenic patient-derived GBM neurospheres +/− transgenic Oct4/Sox2 expression, and GBM cell differentiation conditions that down-regulate endogenous Oct4/Sox2. Comprehensive unbiased screening of immune checkpoints and cytokine/chemokine expression profiles shows that Oct4/Sox2 co-expression induces multiple inhibitory immune checkpoint molecules (i.e., PD-L1, A2aR,CD70, TDO), and modulates cytokine/chemokine profiles (i.e., up-regulation of SPP1, IL8, CXCL5, CXCL3 CCL20 and down-regulation of CCL5, CXCL9, CXCL10) associated with an immunosuppressive tumor microenvironment. Our in vitro and in vivo results demonstrate that Oct4/Sox2 co-expressing GSCs inhibit the function and tumor infiltration of CD8 T-cells, and induce the expansion of immune suppressive M2 macrophages and FOXP3+ T-regulatory cells. Furthermore, we show that these immune suppressive responses to Oct4/Sox2 co-expression are driven by BRD4/H3K27Ac-dependent transcriptional events. Pan-BET inhibitors significantly inhibited induction of the immunosuppressive transcriptome by Oct4/Sox2 and attenuated the GSC immunosuppressive phenotypes.
Our findings, for the first time show that the Oct4 and Sox2 transcription factors can drive GBM growth by inducing adaptive and innate immune suppressive effects in addition to their capacity to induce GBM cells to express stem-like tumor-propagating phenotypes.
Materials and Methods
Cell culture and treatment
The GBM neurosphere lines (GBM1A and GBM1B) were originally established by Dr. Vescovi and co-workers (7) and characterized by us (6). Low-passage GBM-derived neurospheres separately isolated from human May0–16, 22, 39, and 59 xenografts, and JL72508 and JL72108 xenografts (6). All other low-passage GBM patient-derived neurospheres were isolated and characterized by Dr. Quiñones-Hinojosa and his co-workers. GBM 1A and 1B neurospheres expressing transgenic Oct4/Sox2 were established as previously described (8). All neurosphere lines are cultured in serum-free neurosphere medium containing EGF/FGF and forced differentiation was performed as previously described (6). Human monocyte cells THP-1 (ATCC Cat# TIB-202) and Jurkat T (ATCC Cat# TIB-152) were original purchased from ATCC. Mouse GL261 luciferase positive cells were original purchased from Caliper Life Sciences. THP-1 and Jurkat T were cultured in RPMI-1640 medium supplemented with 10% Fetal Bovine Serum and 1% penicillin/streptomycin. Jurkat T cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA, Sigma) and 2 μg/mL phytohemagglutinin (PHA, Sigma). THP-1 monocytes were differentiated into M0 macrophages by incubation with 150 nM PMA for 24 hours (9). Mouse glioma GL261 cells were cultured in DMEM (high glucose) medium with 10% FBS and 1% penicillin/streptomycin. All cell lines are free from mycoplasma and all cell lines except low-passage GBM patient-derived neuropheres are authenticated with short tandem repeat profiling. DNA methylation inhibitor (5-Aza) and EZH2 inhibitors (CPI-1205 and EPZ-6438) were purchased from Sigma-Aldrich. JQ1(+)/JQ1(−) and other epigenetic compounds were kindly provided by Structural Genomics Consortium (University of Toronto). The concentration of each compound is listed in the Supplementary Table 1.
Lentiviral vector and cell infection
Dox-inducible mOct4/mSox2 co-expression vector was constructed from the plasmid containing mouse Oct4 and Sox2 (Addgene) by high fidelity PCR and cloned into pTRIPZ using Agel and MluI. Lentiviral packaging followed a second generation lentivirus packaging protocol using psPAX and pMD2.G vectors (Addgene). Infected cells were selected with puromycin (1 μg/ml) for stable GL261 cells expressing Oct4/Sox2.
Patient dataset analysis
The correlations between CCL5, CXCL10, CXCL9, CXCL11 and CD8A or GZMB, as well as the correlations between SPP1, CCL20, CXCL5, IL6 and CD68 were analyzed using GlioVis (https://gliovis.bioinfo.cnio.es) and the Cancer Genome Atlas (TCGA). The datasets were exported directly from GlioVis.
CD8 T cells Isolation, activation and tumor cell killing assay
Peripheral blood mononuclear cells (PBMCs) were prepared from healthy donor blood (JHU) by centrifugation on a Ficoll-Hypaque density gradient (Sigma-Aldrich). CD8 T cells were purified using CD8 negative microbeads (BioLegend) according to the manufacturer’s direction, and stimulated with anti-CD3/anti-CD28-coated beads (Invitrogen) in the presence of 20 IU/ml rhIL-2 following the manufacturer’s instructions. For CD8 T cell-mediated tumor cell killing assay, Oct4/Sox2 expressing GBM neurosphere cells or control neurosphere cells were plated on the laminin-coated 96-well plates overnight prior to co-culture with the activated CD8+ T cells at a 1:5 tumor cell: CD8+ T cell ratio for 48hrs. Dead GBM cells and non-adherent T cells were removed by washing with PBS, the remaining viable adherent GBM cells were photographed and then quantified by cell counting kit-8 (CCK-8) assay.
T cell migration assays
Briefly, 1×105 CD8+ T cells or Jurkat T cells were resuspended in 300 μL RPMI 0%FBS medium and placed in the upper chamber of the wells. 600 μL CM from Oct4/Sox2 expressing GBM neurospheres or control were placed in the lower chamber. After incubation for 6 hrs at 37°C in 5% CO2, the cells on the upper membrane surface were mechanically removed. Cells that had migrated to the lower side of the membrane were fixed and stained with DAPI. Migrated cells were photographed and then counted under a microscope in five randomly chosen fields.
T-cell apoptosis, IL2 production assays
2X105 Oct4/Sox2 expressing GBM neurospheres or control neurospheres, and GBM1A neurospheres or enforce differentiated GBM1A cells were separately plated on the laminin coated 12-well plates in GBM medium for 48 hrs. Cells were washed to remove floating cells and then co-cultured with 2 × 104 activated Jurkat cells in GBM medium overnight. Jurkat cells and co-culture supernatants were collected. Jurkat cell apoptosis was measured using the Caspase-Glo 3/7 assay (Promega). Human IL2 levels in co-culture supernatants were measured by ELISA (BioLegend). To evaluate effects of PD-L1 inhibition and JQ1 treatment, Oct4/Sox2 expressing GBM neurospheres or control were pretreated with 20 mg/mL purified anti-PD-L1 antibody (29E.2A3, BioLegend) for 24 or 200 nM JQ1 for 72 hrs prior to co-culture with activated Jurkat cells.
ELISA of SPP1
GBM1A and 1B neurosphere expressing transgenic Oct4/Sox2 or control (0.5×106/12 well) were cultured for 24 hrs. Supernatants were collected without any floating cells. The amount of SPP1 protein in the supernatant was determined following the manufacturer’s instructions using a human SPP1 ELISA (R&D systems).
Quantitative reverse transcription PCR (qRT-PCR)
The qRT-PCR was performed as described before(6). Forward and reverse primers are listed in Supplementary Table 2.
Chromatin immunoprecipitation(ChIP)-PCR
The putative super-enhancer was identified as a region proximal to the gene promoter with high levels of H3K27Ac in human brain tumor cell lines reported by the Encyclopedia of DNA Elements (ENCODE) in the UCSC genome browser (http://genome.ucsc.edu/ENCODE/index.html). Chromatin immunoprecipitation was performed using the MAGnify Chromatin Immunoprecipitation System (Life Technologies Corporation) (11). Briefly, DNAs from Oct4/Sox2 expressing GBM1A neurospheres and control neurospheres (1.5×107) were crosslinked using 4% paraformaldehyde, and chromatin was isolated and fragmented by sonication. DNA fragments (~300 bp) were incubated with anti-H3K27Ac (Cell Signaling), anti-BRD4 (BETHYL Laboratories), or IgG antibody overnight at 4 °C. Precipitation of DNA fragments complexed with H3K27Ac at the immune molecules enhancer region was quantified using quantitative PCR. Forward and reverse primer sequences are listed in Supplementary Table 2.
Immunoblotting, Immunohistochemistry and Immunofluorescence
Western blotting was performed using quantitative Western blot system (LICOR Bioscience, Lincoln, NE, USA) (6). The primary antibodies are listed in Supplementary Table 3. For immunofluorescence staining, Oct4/Sox2 expressing GBM neurospheres or control neurospheres were collected by cytospin onto glass slides. Cells and frozen tumor sections were immunostained with primary antibodies against PD-L1 or SPP1 antibody (Cell Signaling); or Iba-1 (Wako, ThermoFisher); Arginase-1 (Cell Signaling Technology). Secondary antibodies were conjugated with Alexa Fluor®647. Coverslips were treated with Vectashield antifade solution containing 4′6-diamidino-2-phenylindole (Vector Laboratories). For immunohistochemistry, frozen tumor sections were immunostained with primary antibodies against PD-L1, CD8, FOXP3 (Cell Signaling, Danvers, MA, USA) as previously described (10). Positive staining cells were manually counted or quantified by ImageJ (NIH).
Xenograft and GL261 mouse models
All animal protocols were approved by the Johns Hopkins Animal Care and Use Committee. Oct4/Sox2 expressing GBM1A and control neurospheres (1 × 104 / 2 μl PBS), and dox-inducible mOct4/Sox2 expressing GL261 cells (1 × 105 / 2 μl PBS) were stereotactically implanted into the 8-week-old female immunodeficient mice (NCI, Frederick, MD) and C57BL/6j mice (Jackson Laboratory), respectively. After 6 weeks for GBM neurospheres and 27 days for GL261, the animals were sacrificed and the brains were removed and sectioned and used for IHE staining.
Statistical analysis
All experiments were performed in triplicates and repeated at least twice in each cell model (N ≥6). Results are expressed as means ± standard error of mean (SEM). Significance of differences was determined using GraphPad Prism software (GraphPad Prism 7). Means were compared using analysis of one-way ANOVA. Post-hoc tests included either Student’s t-test, Dunnet’s test or Tukey’s Honest Significant Difference (HSD) as required by experimental design. Significant difference from the corresponding control is indicated as *, **, or *** for p<0.05, 0.01 or 0.001 respectively.
Results
Oct4 and Sox2 co-expression induces multiple intrinsic immune checkpoint molecules
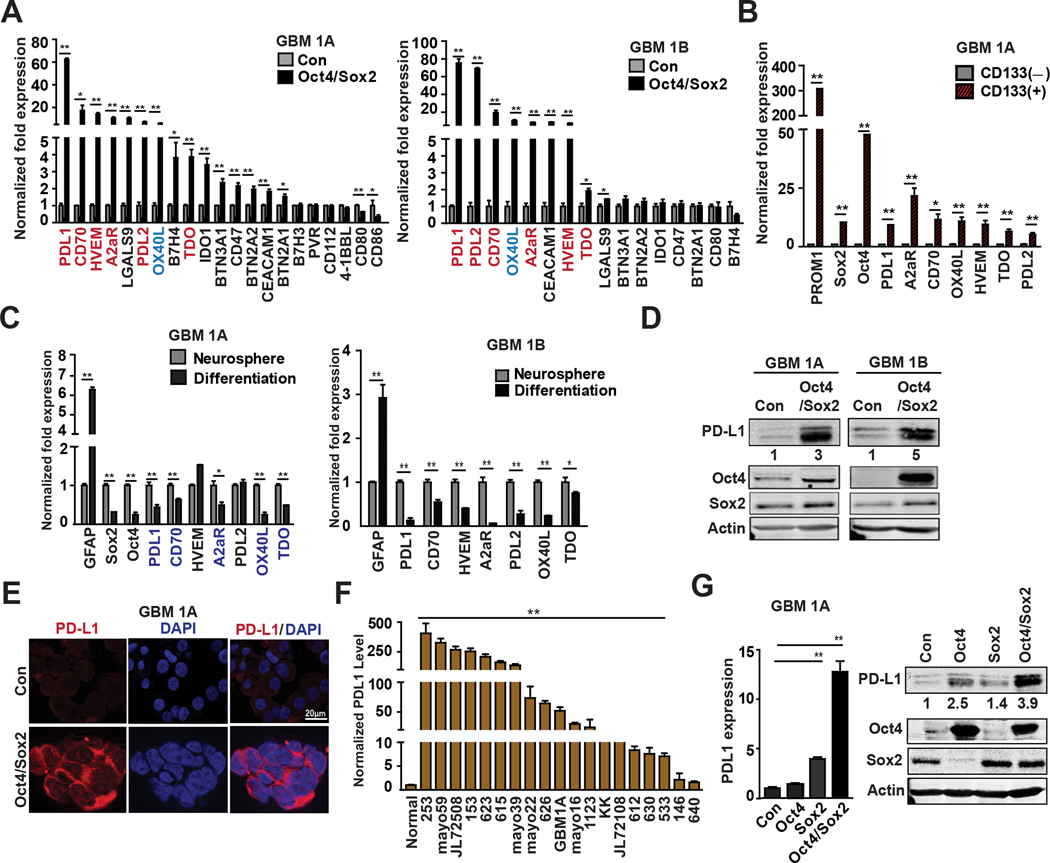
Immune checkpoint pathways and other immune regulatory molecules play important roles in tumor immune resistance mechanisms. To determine if Oct4 and Sox2 expression regulates GBM cell expression of these immune-inhibitory molecules, we performed a comprehensive screen of 21 immune checkpoint molecules using qRT-PCR. Statistically significant expression changes >2-fold (increase or decrease) in two biological replicate human GBM neurosphere models (i.e. GBM1A and GBM1B) was required to identify candidates for further study. Based on these criteria, six inhibitory immune molecules PD-L1, PD-L2, CD70, HVEM, A2aR and TDO and one co-stimulatory molecule OX40L were significantly up-regulated (2~75-fold) in GBM neurospheres expressing transgenic Oct4/Sox2 versus control parental GBM neurospheres (Fig 1A). We previously showed that Oct4 and Sox2 expression is enriched in GSC sub-populations (e.g., CD133+) (8). Separation of CD133+ and CD133− neurosphere cells by flow cytometry (Supplementary Fig 1A) followed by qRT-PCR analysis showed that the CD133+ GSCs over-expressed the inhibitory immune molecules found to be induced by Oct4/Sox2 (Fig 1B). Conversely, forcing neurosphere differentiation, a condition that down-regulates Oct4 and Sox2, resulted in the concurrent down-regulation of PD-L1, A2aR, OX40L, CD70 and TDO in both biological replicates examined (Fig 1C). PD-L1 mRNA was most prominently up-regulated (62~75-fold) by Oct4/Sox2 in our experimental biological replicates and hence the focus of more detailed study. Western blot and Immune fluorescence confirmed constitutive PD-L1 induction by Oct4/Sox2 in GBM neurospheres (Fig 1D–E). PD-L1 was also found to be over-expressed in 17 of 19 primary low-passage patient-derived GBM neurospheres compared with normal neural stem cells (Fig 1F). qRT-PCR and immunoblot analyses showed that PD-L1 was most highly increased in response to Oct4 and Sox2 co-expression compared with neurospheres expressing either Oct4 or Sox2 alone, indicating that Sox2 and Oct4 cooperate to induce PD-L1 expression (Fig 1G). Consistent with these in vitro findings, immunohistochemistry staining showed a substantial increase in PD-L1 expression within orthotopic xenografts derived from neurospheres expressing transgenic Oct4/Sox2 compared with xenografts -derived from parental controls (Supplementary Fig 1B).
Figure 1. Oct4/Sox2 Co-expression induces multiple inhibitory immune checkpoint molecules.
(A) Expression quantification by qRT-PCR of 21 immune checkpoint molecules in two GBM neurosphere lines expressing +/− transgenic Oct4/Sox. Inhibitory immune checkpoint molecules were labeled red and co-stimulatory ligand molecules were labeled blue; (B) qRT-PCR results showing induction of inhibitory immune checkpoint molecules by Oct4/Sox2 enriched in CD133+ cell fractions compared with CD133− cell fractions; (C) qRT-PCR showing down-regulation of inhibitory immune checkpoint molecules after forcing GBM neurosphere differentiation; (D) Immunoblots showing PD-L1 expression in Oct4/Sox2 expressing GBM neurospheres compared with control neurospheres; (E) Immunofluorescence staining of PD-L1 membrane distribution (red) in GBM neurospheres expressing +/− transgenic Oct4/Sox2; (F) qRT-PCR analysis measuring PD-L1 expression in 19 low-passage primary GBM patient-derived neurospheres and two normal neural stem cells. (G) qRT-PCR (left panel) and immunoblots (right panel) showing PD-L1 expression in neurospheres expressing control, or Oct4 alone, or Sox2 alone or Oct4/Sox2; Data represents mean ± SEM. *P<0.05, **P<0.01.
Oct4 and Sox2 co-expression regulate cytokine/chemokine profiles that facilitate an immunosuppressive tumor microenvironment
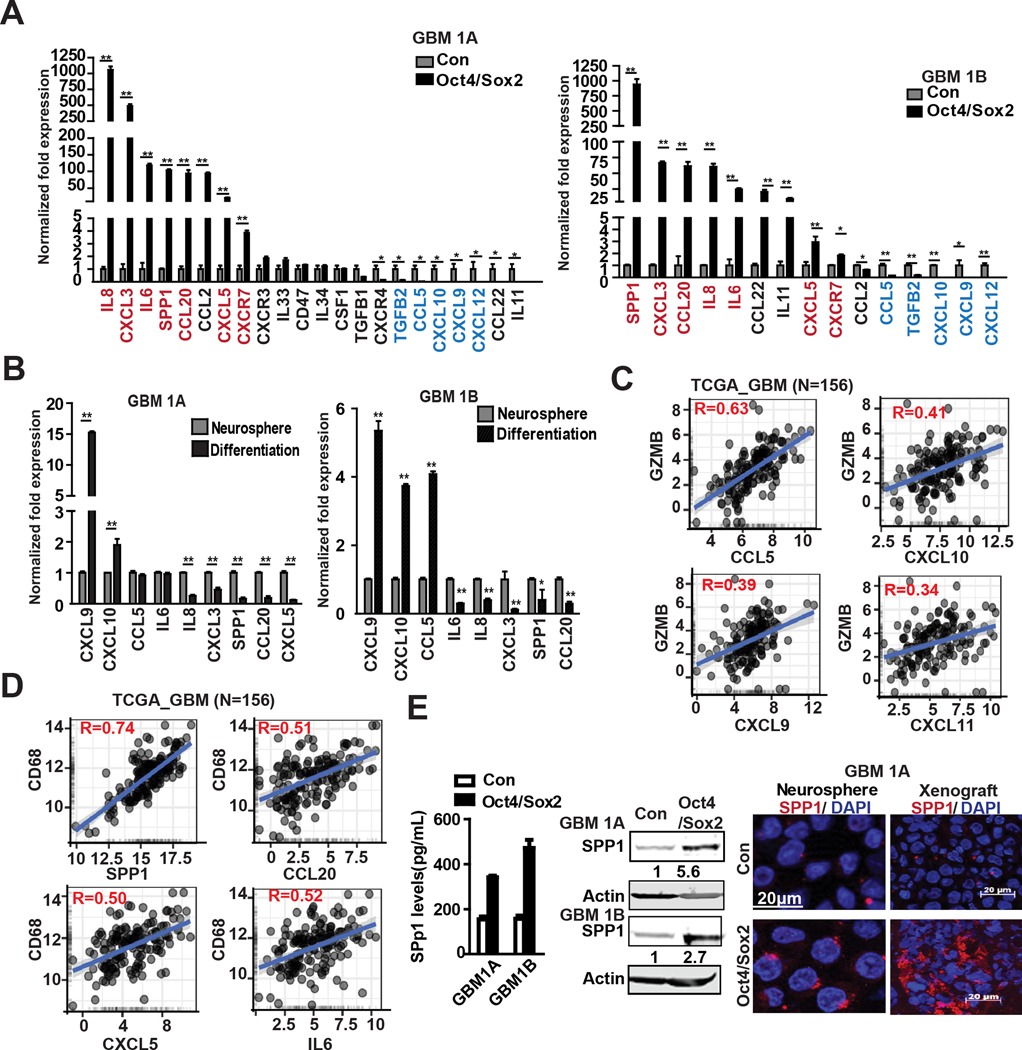
We also examined the effects of Oct4/Sox2 on GBM neurosphere expression of cytokines and chemokines that regulate effectors of innate immunity and immune cell infiltration and functions with the tumor microenvironment. Based upon an comprehensive qRT-PCR screen of 22 cytokines/chemokines, Oct4/Sox2 co-expression was found to significantly inhibit the expression of CCL5, and the Th1-type chemokines CXCL9, CXCL10 and CXCL11 that function to attract effector CD8+ T cells (Fig 2A) (12). Oct4/Sox2 concurrently induced multiple chemokines and cytokines (e.g., SPP, IL8, CXCL3, CXCL5, CCL20, IL6) known to facilitate expansion of M2 macrophages, myeloid derived suppressor cells (MDSCs) and regulatory T cells (Tregs), and promote tumor-associated angiogenesis and metastasis (Fig 2A) (13,14). Conversely, forced neurosphere differentiation induced CXCL9 and CXCL10 and repressed SPP1, IL8, CXCL3, CCL20 and CXCL5 expression (Fig 2B).
Figure 2. Co-expressing Oct4/Sox2 alters cytokine/chemokine profiles that facilitate an immunosuppressive tumor microenvironment.
(A-B) Expression quantification by qRT-PCR of 22 cytokines & chemokines in GBM neurospheres expressing +/− transgenic Oct4/Sox2 (A), and GBM neurospheres vs their differentiated cells (B); (C-D) The correlations between GZMB ( marker of CD8+ T cells) and CCL5, CXCL10, CXCL9 and CXCL11, and CD68 (macrophage marker) and SPP1, CCL20, CXCL5 and IL6 were analyzed in TCGA clinical samples. (http://gliovis.bioinfo.cnio.es); (E) ELISA (left panel), immunoblot (middle panel) and immunofluorescence (right panel) showing SPP1 induction by Oct4/Sox2; Data represents mean ± SEM. *P<0.05, **P<0.01.
We queried the TCGA database for associations between these cytokine/chemokines and known markers of CD8+ T cells (i.e. CD8A, GZMB) and macrophages (i.e. CD68) in clinical GBM specimens. CCL5, CXCL10, CXCL9, CXCL11 expression levels were found to positively correlate with CD8A and GZMB expression (R=0.63, R=0.41, R=0.39, R=0.34, respectively) (Fig 2C and Supplementary Fig 2A) and SPP1, CCL20, CXCL5, IL6 expression levels positively correlate with CD68 expression (R=0.74, R=0.51, R=0.5, R= 0.52, respectively) (Fig 2D). SPP1 was one of the cytokines most highly up-regulated by Oct4/Sox2 and TCGA database analysis revealed significantly higher SPP1 expression in clinical GBM compared with non-neoplastic tissue (Supplementary Fig 2B, left panel). ELISA of neurosphere cell conditioned medium, western blotting of neurosphere cell protein, immunofluorescence of dissociated neurosphere cells and neurosphere-derived orthotopic tumor xenografts further confirmed SPP1 induction by Oct4/Sox2 (Figure 2E). Analysis of TCGA data revealed a significant positive correlations between SPP1 and the M2 macrophage markers CD163 and IL10 (R=0.56 and R=0.6, respectively) in clinical GBM samples (Supplementary Fig 2B, right panel).
Collectively, these results show that Oct4/Sox2 co-expression induces an immunosuppressive cytokine/chemokine profile in GBM cells.
Oct4/Sox2-expressing GSCs suppress T cell infiltration and function.
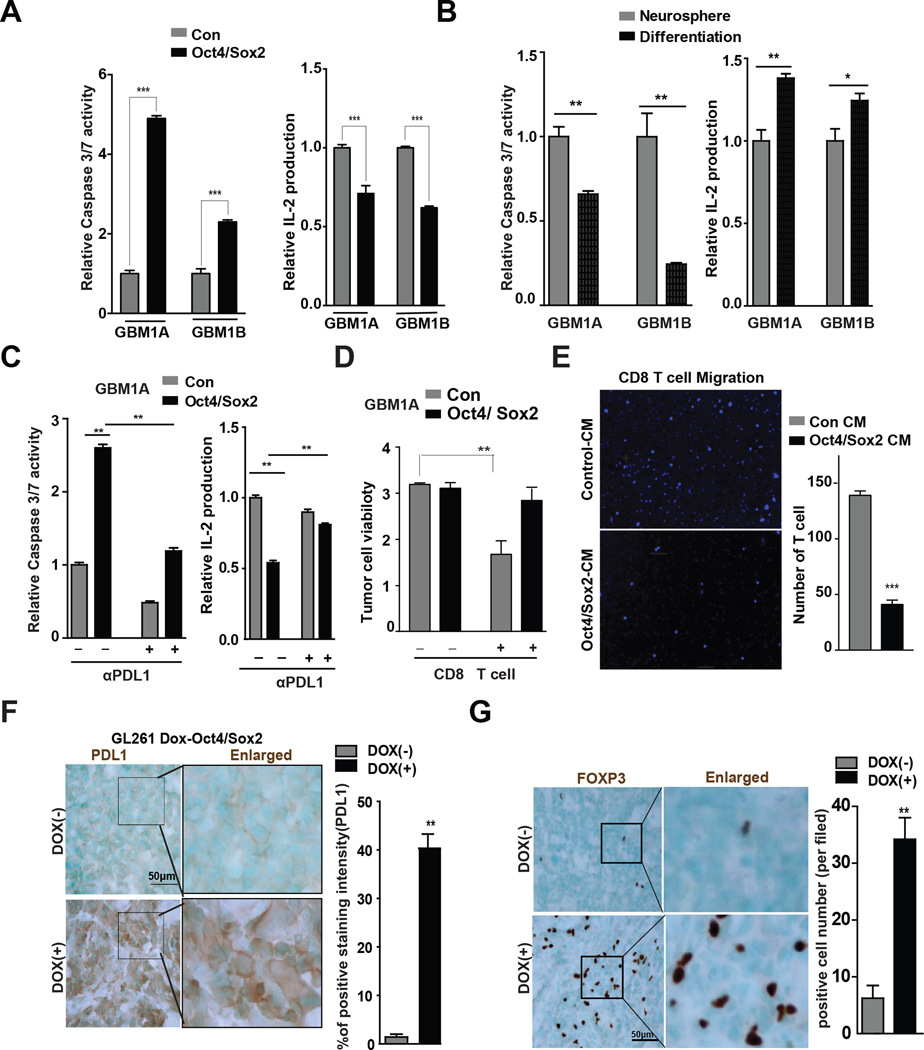
The immunosuppressive transcriptome induced by Oct4/Sox2 expressing GSCs predicted their capacity to induce T cell dysfunction. This was examined in vitro and in vivo. Jurkat cell apoptosis as determined by caspase-3/7 activation was increased 2~4-fold when Jurkat cells were co-cultured with GBM neurospheres expressing transgenic Oct4/Sox2 vs their control parental neurospheres (Fig 3A, left panel). Jurkat cell activation as measured by IL2 production was suppressed 30%~38% in co-cultures with Oct4/Sox2 expressing GBM neurospheres vs parental controls (Fig 3A, right panel). Conversely, Jurkat cell apoptosis was decreased 34%~75% and IL2 production increased 1.2–1.4-fold when Jurkat cells were co-cultured with differentiated GBM cells vs their undifferentiated controls (Fig. 3B), consistent with the down-regulation of Oct4/Sox2 and reversal of the immune-suppressive transcriptome in response to forced differentiation. The Jurkat T cell response to Oct4/Sox2-expressing neurospheres was partially reversed by neutralizing PD-L1 antibody (Fig 3C). To further determine if Oct4/Sox2 co-expression induces T cell apoptosis and promotes GSC immune evasion, activated human CD8+ T cells were cultured with GBM neurosphere cells expressing transgenic Oct4/Sox2 or their parental control neurospheres. CCK-8 assay showed enhanced survival of Oct4/Sox2 co-expressing neurosphere cells compared with control cells (Figure 3D). Transwell migration assays were used to determine if Oct4/Sox2 co-expressing GSCs inhibits T cell invasion. Condition medium (CM) from GBM neurospheres expressing transgenic Oct4/Sox2 reduced the transwell invasion of both Jurkat cells and CD8+ human T cells compared with CM from their control parental neurospheres (Fig 3E and Supplementary Fig 3A).
Figure 3. Oct4/Sox2 co-expression suppresses T cell function and infiltration.
(A) GBM neurospheres expressing +/− transgenic Oct4/Sox2 were co-cultured with the activated Jurkat T cells. Jurkat T cell apoptosis was detected by caspase 3/7 activity (left panel). IL2 production was detected by IL2 ELISA Kit (right panel). Oct4/Sox2 enhanced Jurkat cell apoptosis and diminished IL2 production; (B) GBM neurospheres 1A and 1B were forced differentiation for 7 days. Neurospheres and their differentiated cells were separately co-cultured with the activated Jurkat T cells. Jurkat T cell apoptosis and IL2 production were detected; (C) Pretreated GBM neurospheres expressing +/− transgenic Oct4/Sox2 with +/− anti-PD-L1 antibody for 2 hrs prior to co-culture with activated Jurkat T cells. Jurkat T cell apoptosis and IL2 production were detected; and the effects of Oct4/Sox2 on Jurkat T cell apoptosis and IL2 production were attenuated by anti-PDL1 antibody; (D) GBM neurosphere cells expressing +/− transgenic Oct4/Sox2 were plated as monolayers on laminin-coated substrate and then co-cultured with activated CD8+ T cells for 48 hours. The remaining adherent tumor cells were quantified by CCK-8 assay; (E) Migration of activated CD8+ T cells measured by transwell assay. Oct4/Sox2 expressing GBM neurospheres inhibit T cell migration; (F-G) IHC staining and quantification of PD-L1 expression and FOXP3+ Treg cells (brown) in orthotopic GL261 tumors +/− Dox-induced mOct4/Sox2 expression (n=8 random fields per group).
Orthotopic tumors were established in immune-competent C57BL/6j mice using murine GL261 glioma cells engineered to express dox-inducible murine Oct4 and Sox2. Immunoblot analysis confirmed Oct4 and Sox2 induction by doxycycline treatment with concurrent PD-L1 induction in GL261 cells expressing transgenic Oct4/Sox2. Dox-induction of Oct4/Sox2 co-expression also promoted tumor growth consistent with our previously published results (8). We now show that tumors harvested from Dox-treated animals have increased PD-L1 mRNA and protein expression by qRT-PCR and western blot analyses, respectively (Supplementary Fig 3B–C). Immunohistochemistry showed elevated PD-L1 expression (Fig 3F), fewer tumor-associated CD8+ T cells (Supplementary Fig 3D) and increased tumor-infiltrating FOXP3+ Treg cells in the Dox-treated tumors compared to no Dox-treated control tumors (Fig 3G). Together, these in vitro and in vivo results reinforce T cells dysfunction induced by Oct4/Sox2.
Oct4/Sox2 expressing GSCs induce pro-tumorigenic, anti-inflammatory M2-like macrophages
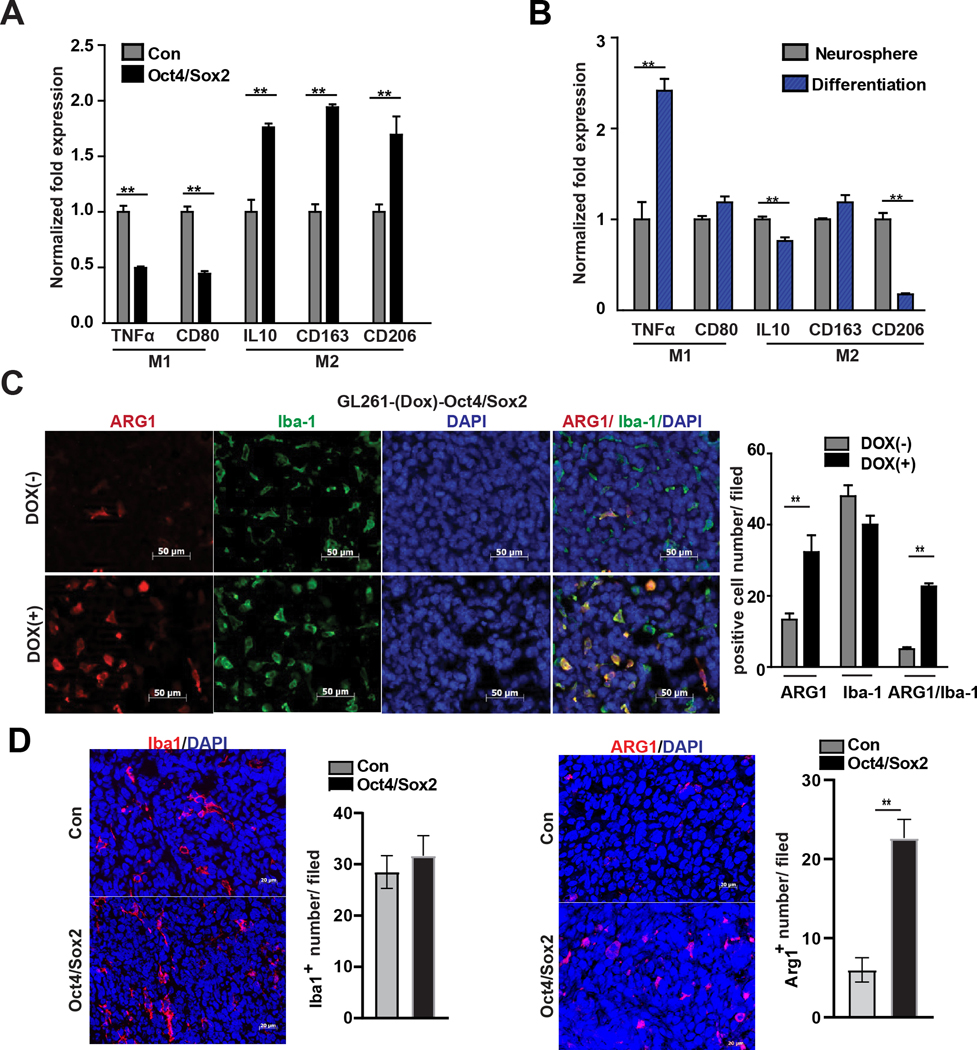
The above results identify multiple cytokines & chemokines that are induced by Oct4/Sox2 and positively associated with oncogenic tumor-associated M2-like macrophages. To assess more directly the influence of Oct4/Sox2 co-expressing GSCs on tumor-associated macrophages (TAMs), THP-1 monocytes were differentiated into M0 macrophages using PMA (phorbol 12-myristate 13-acetate) and then co-cultured with either GBM neurospheres expressing transgenic Oct4/Sox2 or their parental GBM neurospheres as control. qRT-PCR analysis showed a significant increase in expression of M2 markers (i.e., CD206, CD163, IL10) and a significant decrease in expression of M1 markers (TNFα, CD80) in response to the Oct4/Sox2 expressing cells (Fig 4A). Conversely, a significant decrease in expression of M2 markers (i.e., CD206, IL10) and a significant increase in M1 marker expression (TNFα) occurred when M0 macrophages were co-cultured with neurosphere cells after their forced differentiated (Fig 4B). Consistent with these in vitro findings, orthotopic GL261-(Dox)-Oct4/Sox2 gliomas treated with doxycycline contained many more M2 marker-expressing TAMs (~4.6-fold more Arg1+/Iba1+ cells) compared with control tumors (Fig 4C). Similarly, orthotopic tumor xenografts derived from GBM1A neurospheres expressing transgenic Oct4/Sox2 contained more Arg+ M2-like macrophages compared with tumor xenografts derived from control GBM1A neurospheres (Fig 4D). Together, these findings demonstrate that Oct4/Sox2 expressing GSCs promote the formation of M2-like macrophages within the tumor microenvironment.
Figure 4: Oct4/Sox2 promotes pro-tumorigenic M2-like macrophages.
(A-B) qRT-PCR measuring expression of M1 and M2 polarization markers in THP-1-derived macrophages cultured in condition medium obtained from GBM neurospheres +/− transgenic Oct4/Sox2 (A), and GBM1A neurospheres vs forced differentiated neurospheres (B); (C) Immunofluorescence staining and quantification of Iba1+ (green) and M2 marker Arg1+ (red) in orthotopic tumor derived from GL261 +/− transgenic Oct4/Sox2 expressing cells (n=8 random fields per group); (D) Immunofluorescence staining and quantification of Iba1+ (red) and M2 marker Arg1+ (red) in orthotopic tumor xenograft derived from GBM neurospheres +/− transgenic Oct4/Sox2 (n=5 random fields per group). Data represents mean ± SEM. *P<0.05, **P<0.01.
BET-bromodomain inhibitors suppress the immunosuppressive transcriptome and phenotype of Oct4/Sox2-expressing GSCs
A panel of 13 small-molecule inhibitors chosen for their capacity to target epigenetic regulators was screened for their capacity to inhibit PD-L1 induction by Oct4/Sox2. The Bromodomain and Extra Terminal (BET) protein inhibitor JQ1 (200nM) most potently significantly inhibited PD-L1 mRNA induction (~74% inhibition) by Oct4/Sox2 in human GBM neurospheres (Fig 5A). PD-L1 expression inhibition by JQ1 was equally effective at 200nM, 500nM and 1,000nM (Supplementary Fig 4A). JQ1 (200nM) also significantly inhibited the induction of additional immunosuppressive checkpoints (i.e., PD-L2, CD70, HVEM, A2aR) (Fig. 5B and Supplementary Fig. 4B) and chemokine/cytokines (i.e., SPP1, IL8, IL6, CXCL3 and CXCL5) (Fig 5C and Supplementary Fig 4C). JQ1 inhibition of PD-L1 and SPP1 protein induction by Oct4/Sox2 was further validated by western blotting (Fig 5D). qRT-PCR and western blotting also showed OTX-015, another potent BET inhibitor inhibition of PD-L1 and SPP1 induction by Oct4/Sox2 (Supplementary Fig 4D–F).
Figure 5. Pan-BET bromodomain inhibitors attenuate immunosuppressive transcriptome and immunosuppressive responses to Oct4/Sox2.
(A )qRT-PCR analysis measuring PD-L1 expression in Oct4/Sox2 expressing GBM1B neurospheres treated with the indicated epigenetic inhibitors for 72 hrs; (B-C) qRT-PCR analysis measuring the expression of inhibitory immune checkpoint molecules, cytokines and chemokines in neurospheres +/− transgenic Oct4/Sox2 expression treated with (+)/(−)-JQ1 for 72 hrs; (D) Immunoblot showing inhibition of PD-L1 and SPP1 induction by Oct4/Sox2 in GBM neurospheres +/− transgenic Oct4/Sox2 expression treated with (+)/(−)-JQ1. (E-F) GBM neurosphere lines +/− transgenic Oct4/Sox2 expression were pretreated with (+)/(−)-JQ1 prior to co-culture with the activated Jurkat T cells. Jurkat T cell apoptosis and IL2 production were detected by caspase 3/7 activity and IL2 ELISA Kit, respectively; (G) GBM neurospheres +/− transgenic Oct4/Sox2 expression were treated (+)/(−)-JQ1 prior to co-culture with THP-1-derived macrophages. Expression of M1 and M2 polarization markers was measured by qRT-PCR. (−)-JQ1 represents inactive small molecule control for (+)-JQ1; Data represents mean ± SEM. *P<0.05, **P<0.01.
Consistent with JQ1’s capacity to inhibit the induction of checkpoints and cytokine/chemokines, pretreating Oct4/Sox2 expressing neurosphere cells with JQ1 significantly reversed their inhibition of Jurkat cell apoptosis and IL-2 production within co-cultures (Fig 5E–F). Similarly, treating Oct4/Sox2 expressing neurospheres with JQ1 also inhibited the capacity of Oct4/Sox2 expressing GBM neurospheres to induce M2 maker expression (IL-10, CD163 and CD206) by THP-1 M0 macrophages (Fig 5G).
Together, these results indicate that BET proteins function to regulate Oct4/Sox2- driven immunosuppressive transcriptome and immunosuppressive phenotypes of GSCs.
BRD-dependent H3K27AC regulates the Oct4/Sox2-driven immunosuppressive transcriptome
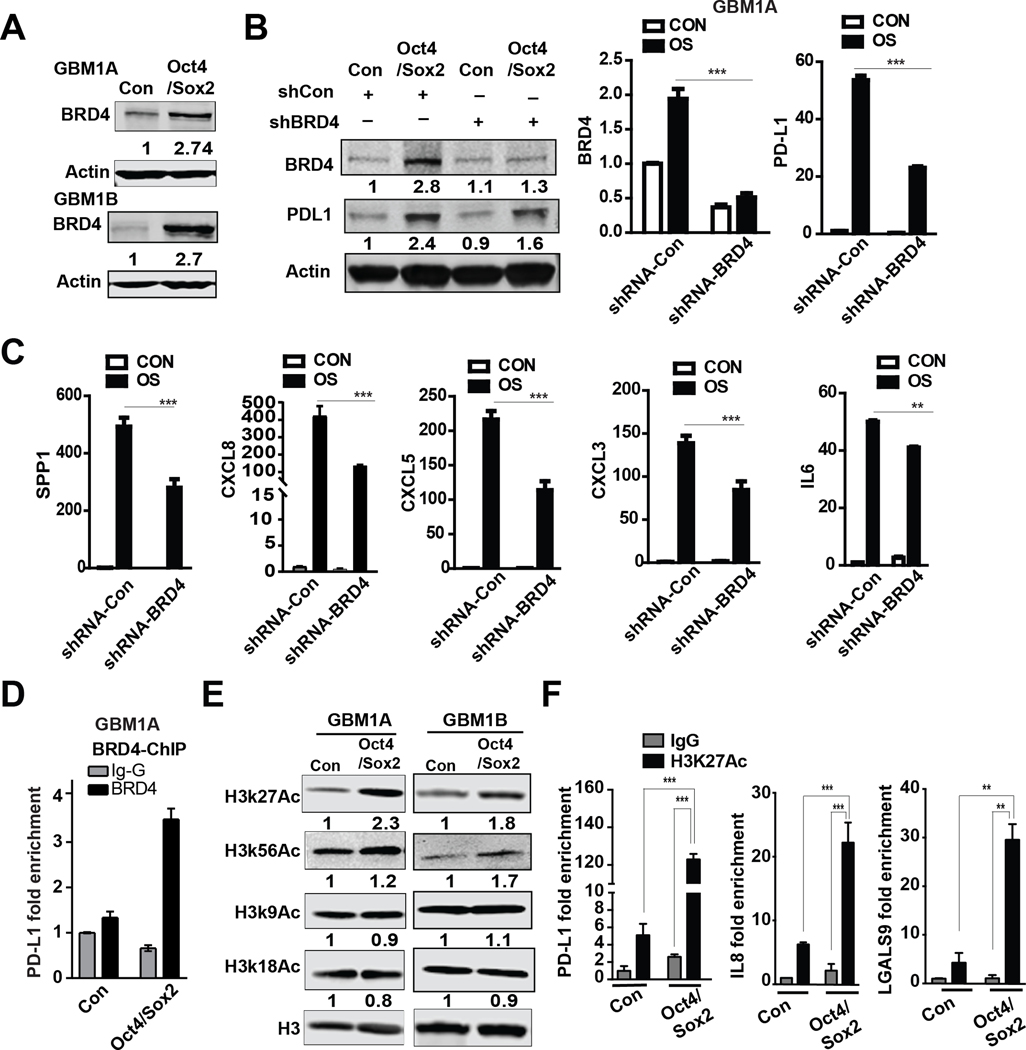
The BET protein BRD4 has been implicated in regulating PD-L1 transcription in ovarian carcinoma (15). We asked if Oct4/Sox2 expression enhances BRD family member expression as a potential mechanism for PD-L1 induction in GSCs. qRT-PCR analysis revealed induction of BRD3 and BRD4 and either no or minimal induction of either BRD1 or BRD2 by Oct4 and Sox2 co-expression (Supplementary Fig 5, left panel). Western blot analysis revealed an increase in BRD4 protein in two biological replicate human GBM neurosphere lines while BRD3 protein increased in only one of the two replicate lines in response to Oct4/Sox2 co-expression compared to isogenic controls (Fig 6A and Supplementary Fig 5, right panel). BRD4 expression inhibition using a validated shRNA-BRD4 (Fig 6B) inhibited induction of the immunosuppressive transcriptome by Oct4/Sox2 co-expression determined by qRT-PCR (Fig 6C). Western blotting further confirmed that PD-L1 induction by Oct4/Sox2 expression was inhibited by BRD4 knockdown (Fig 6B). ChIP-PCR analysis using BRD4 Ab showed that BRD4 binding to the PD-L1 enhancer region was enriched ~5.3-fold in neurospheres expressing transgenic Oct4/Sox2 (Fig 6D).
Figure 6. Oct4/Sox2 promotes enrichment of H3K27Ac at the putative super-enhancer region of immunosuppressive molecules.
(A) Immunoblotting showing higher expression of BRD4 in GBM neurospheres expressing transgenic Oct4/Sox2 compared with control GBM neurospheres; (B) Immunoblot and qRT-PCR analysis showing inhibition of BRD4 and PD-L1 expression by shRNA-BRD4 vs shRNA-control in GBM neurospheres +/− transgenic Oct4/Sox2 transfected; (C) qRT-PCR analysis of cytokine and chemokine expression in neurospheres +/− transgenic Oct4/Sox2 expression transfected with shRNA-BRD4 or shRNA-control; (D) ChIP-qPCR measuring BRD4 binding at the putative super-enhancer region of PD-L1 in neurospheres +/− transgenic Oct4/Sox2 expression; (E) Immunoblot showing increased H3K27Ac in GBM neurospheres expressing transgenic Oct4/Sox2 compared with control GBM neurospheres; (F) ChIP-qPCR showing enrichment of H3K27Ac at the putative super-enhancer regions of PD-L1, IL8 and LGALS9 in neurospheres expressing transgenic Oct4/Sox2 compared to control neurospheres. Data represents mean ± SEM. *P<0.05, **P<0.01.
Super-enhancers correlate with higher levels of occupancy of both H3K27Ac and BRD4 (16) and BRD4 is an epigenetic reader that enhances gene transcription by binding to histone acetylation marks especially H3K27Ac (17). Histone H3 acetylation (e.g. H3K27Ac, H3K56Ac, H3K9Ac, H3K18Ac) was examined and western blot analysis revealed a ~2-fold increase in global H3K27Ac levels in neurospheres expressing transgenic Oct4/Sox2 (Fig 6E). We performed ChIP-PCR using H3K27Ac mAb to determine if the putative super-enhancers for select checkpoint and cytokine/chemokine genes (i.e., PD-L1, IL8 and LGALS9) were enriched for H3K27Ac marks in Oct4/Sox2 expressing GBM1A neurospheres compared to their parental controls. H3K27Ac was enriched ~27-fold in the PD-L1 super-enhancer region, ~10-fold in the IL8 super-enhancer and ~7-fold in the LGALS9 in neurospheres expression transgenic Oct4/Sox2 (Fig 6F). These results combined with the JQ1 findings described earlier support a mechanism by which Oct4/Sox2 co-expression induces an immune suppressive transcriptome by increasing super-enhancer H3K27 acetylation and BRD-dependent transcription.
Discussion
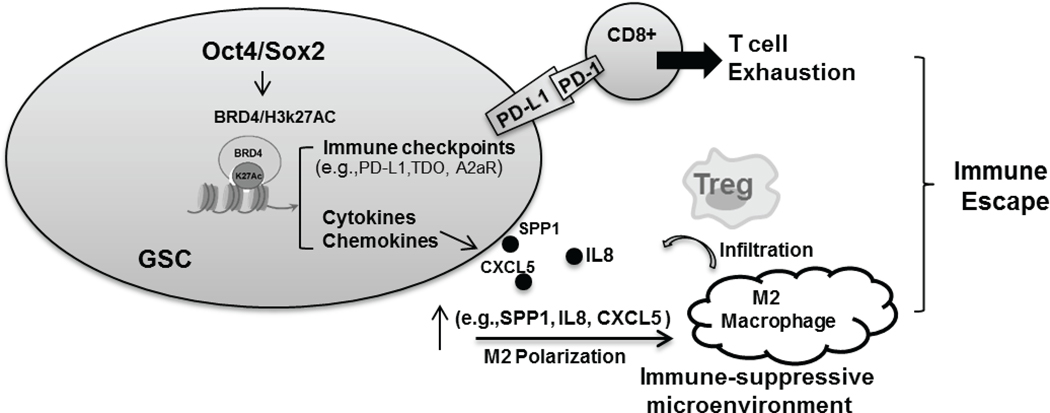
GBM is characterized as an immunologically “cold” tumor with loss of effector CD8+ cells and expansion of M2 macrophages, myeloid derived suppressor cells (MDSCs) and regulatory T cells (Tregs) (18). These features explain at least in part the poor efficacy of immunotherapy in GBM. An increasing number of genomic, epigenomic, transcriptomic profiles and experimental analysis have suggested negative associations between the cancer stemness phenotype and cytotoxic T cell responses and anticancer immunity (19,20). However, the molecular mechanisms responsible for immunomodulating features of CSCs remain poorly understood. Prior work has demonstrated that reprogramming transcription factors Oct4 and Sox2 function as drivers of cancer stemness phenotype and play critical roles in CSC fate determination (6,21). The functional relationships between Oct4/Sox2 expression and cytotoxic T cell responses and anticancer immunity of CSCs remain largely unexplored. A few recent reports have linked Sox2 alone or Oct4 alone with PD-L1 expression through transcriptional or post-transcriptional mechanisms (22,23). For the first time we now demonstrate that Oct4 and Sox2 drive GSC immunosuppressive phenotypes by inducing a BRD4/H3k27Ac-dependent immunosuppressive transcriptome consisting of multiple immunosuppressive checkpoint molecules and cytokine & chemokine profiles that are associated with T-cell apoptosis, Treg infiltration and M2 macrophage polarization, consistent with an oncogenic, immune-suppressive tumor microenvironment (Fig 7). Our findings indicate that understanding and ultimately reversing immunosuppressive transcriptome of GSCs will contribute to inhibit immunosuppressive microenvironment of GBM.
Figure 7. Pathways and cellular responses used by glioma-propagating stem cells (GSCs) to escape anti-tumor immunity.
Oct4/Sox2 co-expression drives BRD4/H3k27Ac-dependent immunosuppressive transcriptome leading to GSC adaptive and innate immune evasion.
Multiple immune-checkpoint molecules such as PD-L1, TDO, A2AR and CD70 were over-expressed in various tumor cells including GBM. Their activities in tumor microenvironment play immunosuppressive roles and contribute to tumor cell immune escape and malignancy (24–26). Several immune checkpoint inhibitors have been developed for clinical trials and have resulted in remarkable clinical responses in multiple cancer types (27,28). Unfortunately, the response to immune checkpoint blockade in GBM is low with limited clinical efficacy (29). We found that multiple immune inhibitory checkpoints are simultaneously induced by Oct4/Sox2 co-expression and are enriched in GSCs, indicating that targeting any single immune checkpoint (e.g., by anti-PD1, anti-PD-L1, or A2AR antagonist therapy) may be insufficient to overcome GSC immune escape. This highlights the impact of identifying upstream mediators of multiple effectors of immune resistance as revealed here.
Tumor infiltration of CD8+ T cells is required to achieve meaningful therapeutic responses to immunotherapy (30,31). However, clinical GBM contain very few T cells. The chemokines CCL5, CXCL9 and CXCL10 are known to promote CD8+ T cell invasion (12,32). Oct4/Sox2 co-expression was found to significantly down-regulate CCL5 and CXCL9/CXCL10 expression by GSCs. This finding is consistent with the sparse number of CD8+ T cells within experimental Oct4/Sox2-expressing orthotopic GBM. The potential to stimulate T cell tumor infiltration by reestablishing CCL5 and CXCL9/CXCL10 expression in GSCs is supported by a recent findings showing that CCL5 and CXCL9 cooperatively recruit T cells into ovarian tumors and that CCL5hiCXCL9hi tumors are immunoreactive and respond to checkpoint blockade (12). Notably, it has been reported that cancer cells negatively regulate CCL5, CXCL9 and CXCL10 expression by epigenetic silencing mechanisms such as DNA methylation (33) that we have reported to be induced by Oct4/Sox2 and up-regulated in GSCs (8). This raises the possibility that DNMT-dependent epigenetic mechanisms might be involved in the down-regulation of CCL5, CXCL9 and CXCL10 by Oct4/Sox2. Contrasting the loss of tumor-infiltrating CD8+ T cells, many more Foxp3+ T cells were observed in Oct4/Sox2 expressing orthotopic tumors compared with control tumors. This is consistent with a previous study showing that Sox2+ breast cancer cells promote Treg recruitment (34) and the association between Oct4/Sox2 expression and Treg infiltration in aggressive glioma (35).
Tumor-associated macrophages (TAM) are promising targets for therapeutic intervention due to their abundance in GBM and their oncogenic immunosuppressive phenotypes (36). Glioma cells secreted multiple cytokines & chemokines (e.g. CSF-1, CCL-2, IL-4, IL-6, IL-10 and periostin) that recruit macrophages and their subsequent polarization to oncogenic M2-like subsets (5,37,38). Loss of phosphatase and tensin homolog (PTEN) in GBM cells increase macrophage infiltration via a YAP1-LOX-β integrin-PYK2 axis (39). NF1 deficiency in GBM cells is associated with an increase in macrophages infiltration (40). Here, we show that Oct4/Sox2 expressing GSCs promoted M2 TAM polarization and up-regulate SPP1, CCL20, CXCL5 and IL6, cytokines/chemokines that are highly expressed in GBM and positively associated with macrophage infiltration (41,42). Wei et al has linked high expression of SPP1 (also called osteopontin) to macrophage M2 polarization in glioma supporting a comparable influence on the induction of M2 macrophage polarization by Oct4/Sox2-expressing GSCs (41). Future studies employing genetic deletion and /or antibody-mediated depletion will be necessary to determine how each cytokine/chemokine alone or in combination affect macrophage recruitment and polarization.
Oct4, Sox2 and BRD4 are overexpressed in glial malignancy and correlate with tumor grade (43–45). Kaplan-Meier survival analysis from TCGA shows that high co-expression of Oct4, Sox2 and BRD4 is associated with a poor survival (Supplementary Fig 6). In this study, we found that BRD4 expression level is higher in Oct4/Sox2 co-expressing GBM neurospheres compared with control neurospheres. Several putative Oct4/Sox2 binding sites are present within the 2,000 bps upstream of the human BRD4 transcriptional start site (http://alggen.lsi.upc.es/). Moreover, we previously found that miRNAs (e.g. miR200a, miR-29, miR-296–5p) predicated to target BRD4 are down-regulated by Oct4/Sox2 co-expression (8). Thus, Oct4/Sox2 co-expression may up-regulate BRD4 expression through both transcriptional and post-transcriptional mechanisms.
BET family proteins act upon super enhancers to regulate gene transcription including drivers of cancer initiation and progression (46). A role for BET proteins in immune escape of systemic cancer has been described but their roles in the immune escape of cancer stem cells and brain cancer remain largely unknown (47). We now show that the BRD4 BET protein and super enhancer histone mark H3K27Ac are up-regulated globally and specifically at the super enhancers of immune-suppressive genes (i.e. PD-L1, IL8, LGALS9) induced by Oct4/Sox2 in GSCs. While BRD4 has been shown to regulate PD-L1 expression in other contexts (15), we further implicate BRD-dependent mechanisms in the immunosuppressive GSC phenotype by showing that the pan-BET inhibitors JQ1 and OTX01533 (BRD2, BRD3, BRD4 inhibitor) repress the immunosuppressive transcriptome and biological immunosuppressive responses induced by Oct4/Sox2, and inhibition of BRD4 partially inhibits immunosuppressive transcriptome induction by Oct4/Sox2. Other mechanisms are likely to contribute to GSC immune escape. ChIP data from squamous carcinoma revealed that CXCL5 is a direct genomic target of Sox2 (48), and Oct4 binds to the first intron of SPP1 gene and regulates SPP1 expression (49). Alternatively, the miR-34 family targets PD-L1 and A2aR by binding to their 3’UTR (50), and down-regulation of miR-34 family expression was observed in Oct4/Sox2 expressing GBM neurospheres (8). Understanding how these multiple mechanisms (i.e., transcriptional and post-transcriptional mechanisms) contribute to Oct4/sox2-mediated immunosuppressive transcriptome should guide novel combinatorial therapies toward the goal of enhancing immunotherapeutics.
Supplementary Material
Acknowledgements
This work was supported by the China Scholarship Council and the United States NIH grants NS096754 and NS073611 (JL), and the Mayo Clinic Professorship, the Mayo Clinic Clinician Investigator award, the Florida Department of Health Cancer Research Chair Fund (AQH), as well as the National Institutes of Health R43CA221490, R01CA200399, R01CA195503, R01CA216855 (AQH). The SGC is a registered charity (number 1097737) that receives funds from AbbVie, Bayer Pharma AG, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada through the Ontario Genomics Institute [OGI-055], Innovative Medicines Initiative (EU/EFPIA) [ULTRA-DD grant no. 115766], Janssen, Merck KGaA, Darmstadt, Germany, MSD, Novartis Pharma AG, Pfizer, São Paulo Research Foundation-FAPESP, Takeda, and Wellcome.
Footnotes
Conflict of Interest: The authors disclose no potential conflicts of interest.
References
- 1.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396–401 [DOI] [PubMed] [Google Scholar]
- 2.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clinical cancer research : an official journal of the American Association for Cancer Research 2010;16:461–73 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2010;16:800–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell 2015;27:450–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol 2015;17:170–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Li A, Glas M, Lal B, Ying M, Sang Y, et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proceedings of the National Academy of Sciences of the United States of America 2011;108:9951–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer research 2004;64:7011–21 [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Bertoni H, Lal B, Li A, Caplan M, Guerrero-Cazares H, Eberhart CG, et al. DNMT-dependent suppression of microRNA regulates the induction of GBM tumor-propagating phenotype by Oct4 and Sox2. Oncogene 2015;34:3994–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015;15:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lal B, Xia S, Abounader R, Laterra J. Targeting the c-Met pathway potentiates glioblastoma responses to gamma-radiation. Clinical cancer research : an official journal of the American Association for Cancer Research 2005;11:4479–86 [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Bertoni H, Lal B, Michelson N, Guerrero-Cazares H, Quinones-Hinojosa A, Li Y, et al. Epigenetic modulation of a miR-296–5p:HMGA1 axis regulates Sox2 expression and glioblastoma stem cells. Oncogene 2016;35:4903–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer cell 2019;35:885–900 e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braune J, Weyer U, Hobusch C, Mauer J, Bruning JC, Bechmann I, et al. IL-6 Regulates M2 Polarization and Local Proliferation of Adipose Tissue Macrophages in Obesity. J Immunol 2017;198:2927–34 [DOI] [PubMed] [Google Scholar]
- 14.Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clinical cancer research : an official journal of the American Association for Cancer Research 2016;22:3924–36 [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Bengsch F, Svoronos N, Rutkowski MR, Bitler BG, Allegrezza MJ, et al. BET Bromodomain Inhibition Promotes Anti-tumor Immunity by Suppressing PD-L1 Expression. Cell reports 2016;16:2829–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013;153:320–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Prakash C, Sum C, Gong Y, Li Y, Kwok JJ, et al. Bromodomain-containing protein 4 (BRD4) regulates RNA polymerase II serine 2 phosphorylation in human CD4+ T cells. J Biol Chem 2012;287:43137–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alessio A, Proietti G, Sica G, Scicchitano BM. Pathological and Molecular Features of Glioblastoma and Its Peritumoral Tissue. Cancers (Basel) 2019;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018;173:338–54 e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miranda A, Hamilton PT, Zhang AW, Pattnaik S, Becht E, Mezheyeuski A, et al. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proceedings of the National Academy of Sciences of the United States of America 2019;116:9020–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rath P, Lal B, Ajala O, Li Y, Xia S, Kim J, et al. In Vivo c-Met Pathway Inhibition Depletes Human Glioma Xenografts of Tumor-Propagating Stem-Like Cells. Translational oncology 2013;6:104–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong F, Cheng X, Sun S, Zhou J. Transcriptional activation of PD-L1 by Sox2 contributes to the proliferation of hepatocellular carcinoma cells. Oncol Rep 2017;37:3061–7 [DOI] [PubMed] [Google Scholar]
- 23.Dong P, Xiong Y, Yu J, Chen L, Tao T, Yi S, et al. Control of PD-L1 expression by miR-140/142/340/383 and oncogenic activation of the OCT4-miR-18a pathway in cervical cancer. Oncogene 2018;37:5257–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu JM, Xia W, Hsu YH, Chan LC, Yu WH, Cha JH, et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nature communications 2018;9:1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, Yang L, Shi L, Song H, Shi P, Yang T, et al. Prognostic Impact of Adenosine Receptor 2 (A2aR) and Programmed Cell Death Ligand 1 (PD-L1) Expression in Colorectal Cancer. Biomed Res Int 2019;2019:8014627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozawa Y, Yamamuro S, Sano E, Tatsuoka J, Hanashima Y, Yoshimura S, et al. Indoleamine 2,3-dioxygenase 1 is highly expressed in glioma stem cells. Biochem Biophys Res Commun 2020;524:723–9 [DOI] [PubMed] [Google Scholar]
- 27.Xue S, Hu M, Iyer V, Yu J. Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. Journal of hematology & oncology 2017;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silence K, Dreier T, Moshir M, Ulrichts P, Gabriels SM, Saunders M, et al. ARGX-110, a highly potent antibody targeting CD70, eliminates tumors via both enhanced ADCC and immune checkpoint blockade. MAbs 2014;6:523–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 2017;8:91779–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–30 [DOI] [PubMed] [Google Scholar]
- 32.Ochiai E, Sa Q, Brogli M, Kudo T, Wang X, Dubey JP, et al. CXCL9 is important for recruiting immune T cells into the brain and inducing an accumulation of the T cells to the areas of tachyzoite proliferation to prevent reactivation of chronic cerebral infection with Toxoplasma gondii. The American journal of pathology 2015;185:314–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015;527:249–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Dong X, Qi P, Ye Y, Shen W, Leng L, et al. Sox2 Communicates with Tregs Through CCL1 to Promote the Stemness Property of Breast Cancer Cells. Stem cells 2017;35:2351–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu L, Yang C, Gao Q, Long Y, Ge H, DeLeon G, et al. CD4+ and Perivascular Foxp3+ T Cells in Glioma Correlate with Angiogenesis and Tumor Progression. Frontiers in immunology 2017;8:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 2016;19:20–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vakilian A, Khorramdelazad H, Heidari P, Sheikh Rezaei Z, Hassanshahi G. CCL2/CCR2 signaling pathway in glioblastoma multiforme. Neurochem Int 2017;103:1–7 [DOI] [PubMed] [Google Scholar]
- 38.Nusblat LM, Carroll MJ, Roth CM. Crosstalk between M2 macrophages and glioma stem cells. Cell Oncol (Dordr) 2017;40:471–82 [DOI] [PubMed] [Google Scholar]
- 39.Chen P, Zhao D, Li J, Liang X, Li J, Chang A, et al. Symbiotic Macrophage-Glioma Cell Interactions Reveal Synthetic Lethality in PTEN-Null Glioma. Cancer cell 2019;35:868–84 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer cell 2017;32:42–56 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M, et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. The Journal of clinical investigation 2019;129:137–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai Z, Wu J, Chen F, Cheng Q, Zhang M, Wang Y, et al. CXCL5 promotes the proliferation and migration of glioma cells in autocrine- and paracrine-dependent manners. Oncol Rep 2016;36:3303–10 [DOI] [PubMed] [Google Scholar]
- 43.Krogh Petersen J, Jensen P, Dahl Sorensen M, Winther Kristensen B. Expression and Prognostic Value of Oct-4 in Astrocytic Brain Tumors. PLoS One 2016;11:e0169129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Annovazzi L, Mellai M, Caldera V, Valente G, Schiffer D. SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics Proteomics 2011;8:139–47 [PubMed] [Google Scholar]
- 45.Pastori C, Daniel M, Penas C, Volmar CH, Johnstone AL, Brothers SP, et al. BET bromodomain proteins are required for glioblastoma cell proliferation. Epigenetics 2014;9:611–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White ME, Fenger JM, Carson WE 3rd. Emerging roles of and therapeutic strategies targeting BRD4 in cancer. Cell Immunol 2019;337:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrieu GP, Shafran JS, Smith CL, Belkina AC, Casey AN, Jafari N, et al. BET protein targeting suppresses the PD-1/PD-L1 pathway in triple-negative breast cancer and elicits anti-tumor immune response. Cancer Lett 2019;465:45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mollaoglu G, Jones A, Wait SJ, Mukhopadhyay A, Jeong S, Arya R, et al. The Lineage-Defining Transcription Factors SOX2 and NKX2–1 Determine Lung Cancer Cell Fate and Shape the Tumor Immune Microenvironment. Immunity 2018;49:764–79 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kijewska M, Kocyk M, Kloss M, Stepniak K, Korwek Z, Polakowska R, et al. The embryonic type of SPP1 transcriptional regulation is re-activated in glioblastoma. Oncotarget 2017;8:16340–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Wang L. miR-34a attenuates glioma cells progression and chemoresistance via targeting PD-L1. Biotechnol Lett 2017;39:1485–92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.