Abstract
Sporadic angiosarcomas (ASs) are aggressive vascular sarcomas whose rarity and genomic complexity present significant obstacles in deciphering the pathogenic significance of individual genetic alterations. Numerous fusion genes have been identified across multiple types of cancers, but their existence and significance remain unclear in sporadic ASs. In this study, we leveraged RNA sequencing data from thirteen human ASs and 76 spontaneous canine hemangiosarcomas (HSAs) to identify fusion genes associated with spontaneous vascular malignancies. Ten novel protein-coding fusion genes, including TEX2-PECAM1 and ATP8A2-FLT1, were identified in seven of the thirteen human tumors, with two tumors showing mutations of TP53. HRAS and NRAS mutations were found in ASs without fusions or TP53 mutations. We found fifteen novel protein-coding fusion genes including MYO16-PTK2, GABRA3-FLT1, and AKT3-XPNPEP1 in eleven of the 76 canine HSAs; these fusion genes were seen exclusively in tumors of the angiogenic molecular subtype that contained recurrent mutations in TP53, PIK3CA, PIK3R1, and NRAS. In particular, fusion genes and mutations of TP53 co-occurred in tumors with higher frequency than expected by random chance, and they enriched gene signatures predicting activation of angiogenic pathways. Comparative transcriptomic analysis of human ASs and canine HSAs identified shared molecular signatures associated with activation of PI3K/AKT/mTOR pathways. Our data suggest that genome instability induced by TP53 mutations might create a predisposition for fusion events that may contribute to tumor progression by promoting selection and/or enhancing fitness through activation of convergent angiogenic pathways in this vascular malignancy.
Keywords: Angiosarcoma, chromosome translocation, fusion gene, hemangiosarcoma, TP53
Introduction
Sarcomas are diverse tumors that arise from cells of mesenchymal origin in soft tissues such as blood and lymphatic vessels, fat, bone, cartilage, muscle, and connective tissues. The heterogeneity of sarcomas has provided an impetus for developing molecular approaches to classify these tumors (1,2), leading to their categorization into genomically simple and genomically complex sarcomas (1,3). Angiosarcomas (ASs) are rare, highly aggressive, genomically complex sarcomas of blood vessel-forming cells (3,4). The five-year survival rate of AS is approximately 40% (5-7), but half of patients have metastatic or unresectable disease with a median overall survival of less than 6 months (8). The events that drive progression are incompletely understood; previous studies have identified recurrent mutations of RAS, PTPRB, PLCG1, KDR (kinase insert domain receptor, also known as VEGFR2), TP53, PIK3CA, and FLT4 (VEGFR3) in human ASs (9-12). MYC gene amplification and alterations in the TP53, CDKN2, NF-κB/IL-6, PIK3CA/AKT/mTOR pathways have also been reported (13); however, these studies represent a small case series, precluding definitive conclusions regarding pathogenic mechanisms that contribute to the genetic cause and to the progression of the disease.
Hemangiosarcoma (HSA) is a malignant vascular tumor that is common in dogs with an estimated tens of thousands of cases diagnosed each year (14-16). Canine HSA shares clinical and morphological features with human AS, as well as aspects of its mutational landscape (17-20). We previously documented three molecular subtypes of HSA, characterized by angiogenic, inflammatory, and adipogenic transcriptomic signatures (21). These gene expression signatures are conserved in HSA progenitor cells that show multipotency and self-renewal (21). Nevertheless, the transcriptional state of these HSA progenitor cells seems to be somewhat malleable, regulated by immune and metabolic reprogramming (22). Mutations in genes that regulate genomic integrity, such as TP53, can alter the intrinsic transcriptional program of tumor cells; however, genomic instability in the tumor can create even more dramatic changes by modulating transcriptional programs of heterotypic stromal cells in the tumor tissue, as well as in the composition of the niche (23,24).
Chromosome translocations and the resulting fusion genes are important contributors to the pathogenesis of cancer, particularly in sarcomas and hematopoietic malignancies (25). However, the nature and frequency of these events in canine HSA and human AS remains unclear. Here, we used next generation RNA sequencing (RNA-Seq) data to identify fusion genes in thirteen human ASs and 76 visceral HSAs originating from 74 dogs, and we investigated the relationship of these fusions to the mutational landscape of the tumor. We identified ten novel protein-coding fusion genes including TEX2-PECAM1 and ATP8A2-FLT1 in seven of thirteen human ASs, and two of the fusion-detected tumors showed mutations of TP53 (R248Q and P250L). In canine HSAs, we found novel protein-coding fusion genes in a subset of the tumors of the angiogenic subtype. These fusion genes co-occurred with TP53 mutations and were associated with gene enrichment for activated angiogenic pathways in the tumors. Our data suggest that genomic instability induced by mutations of TP53 creates a permissive environment for fusion genes, with selection for angiogenic molecular programs in malignant vasoformative tumors. Our data also demonstrate that human AS and canine HSA maintain molecular programs that activate convergent signaling pathways to establish angiogenic phenotypes despite their genomic complexity.
Materials and Methods
Human tissue samples
Snap frozen and formalin fixed paraffin embedded (FFPE) tissues for human biospecimens were obtained from the University of Minnesota Biological Materials Procurement Network (UMN BioNet) and from the Cooperative Human Tissue Network (CHTN) under their standardized patient consent protocols. The demographic characteristics of human patients from whom we obtained ASs (n = 13) and normal tissue samples (n = 6) are summarized in Supplementary Table S1.
Dog tissue samples
Seventy-six snap frozen and FFPE tissue samples were obtained from 74 dogs with HSAs. Frozen and FFPE tissues samples from 10 dogs with splenic hematomas, which are benign lesions with enlarged vascular spaces lined by endothelial cells, were used as controls. Samples were obtained as part of medically necessary diagnostic procedures and were used for research with owner consent. The origin of these samples was reported previously (14,21,26-28), or they were collected from dogs with HSA or with splenic hematomas at the Veterinary Medical Center, University of Minnesota. Procedures involving animal use were done with approval and under the supervision of the University of Minnesota Animal Care and Use Committee (protocols 1110A06186, 1507-32804A, 0802A27363, 1101A94713, 1312-31131A, and 1702-34548A). The demographic characteristics of dogs (n = 74) from whom we acquired HSA and non-malignant splenic hematomas (n = 10) are summarized in Supplementary Table S2.
Histological assessment
FFPE sections (4 μm) were stained with hematoxylin and eosin (H&E) and examined by veterinary pathologists to assign a histological diagnosis of canine HSA. Solid, capillary, cavernous, or mixed histological subtypes were assigned using accepted criteria (29); mitotic index (MI) was calculated per 1,000 cells in 5-10 random fields under 400X magnification (30). H&E slides were further reviewed for tumor content by two board-certified medical pathologists (ML and PM) (31), with the percent of sample containing viable nucleated cells corresponding to tumor recorded in a range of 0 to > 90% based on the planar surface of the sections. Diagnostic and histopathology reports of human tissues were provided by the specimen providers, the UMN BioNet and the CHTN.
RNA isolation and generation of RNA-Seq libraries
Total RNA was isolated from tissue samples using the TriPure Isolation Reagent (Roche Applied Science, Indianapolis, IN, USA). The RNeasy Mini Kit (Qiagen, Valencia, CA, USA) was used for clean-up according to the manufacturer's instructions. RNA-Seq from 74 canine HSA tissues is published (17,21,32,33) and an additional data set was generated from two canine HSA tissues and from 10 non-malignant splenic hematoma tissues. Total RNA was also extracted from 13 human AS tissues and from six normal tissues. Two μg of total RNA from each sample were quantified and assessed for quality; RNA-Seq libraries were generated as described (21) using the TruSeq RNA sample preparation kit (Illumina Inc., San Diego, CA). Sequencing was performed using HiSeq 2000 or 2500 systems (Illumina Inc.). Each sample was sequenced to a targeted depth of 20 – 80 million paired-end reads with mate-pair distance of 50 bp. Primary analysis and demultiplexing were performed using CASAVA software version 1.8.2 (Illumina Inc.) to verify the quality of the sequence data. The end result of the CASAVA workflow was demultiplexed into FASTQ files for analysis. Bioanalyzer quality control and RNA-Seq were performed at the University of Minnesota Genomics Center (UMGC) or at the Broad Institute.
Bioinformatics analysis
The original FASTQ files prepared from thirteen human ASs and six non-malignant tissues were mapped to the human reference genome (GRCh38). The FASTQ files generated from 76 canine HSAs and ten non-malignant splenic hematomas were mapped to the dog reference genome (Canfam3.1). Sequencing quality was assessed by FastQC. The deFuse algorithm (34) was used to identify putative fusion events. To discriminate true fusion candidates from artifacts, we included fusion events with exon boundaries in both fusion partners and excluded events created from adjacent genes that showed breakpoint homology (>1). We also filtered highly recurrent fusion events that were found at implausible frequencies across tumor and non-malignant tissue samples (35) and transcription-induced chimeras. The split sequences of the fusion genes were validated by de novo assembly using Trinity (36). TranscriptsToOrfs and deFuse-Trinity tools verified the deFuse fusion predictions with Trinity-assembled transcripts and open reading frames. TopHat2 was used to generate BAM files, and the Integrative Genomics Viewer (IGV 2.3; Broad Institute, Cambridge, MA) was used to visualize the mate pair sequences of fusion genes. A protein translation tool in Expert Protein Analysis System (ExPASy; SIB Swiss Institute of Bioinformatics, Lausanne, Switzerland) was used to determine in-frame fusion proteins. Tumor purity and microenvironment scores were assessed using the bioinformatics tools ESTIMATE (37) and xCell (38).
Reverse transcription polymerase chain reaction (RT-PCR) and Sanger sequencing
RT-PCR was performed to validate fusion transcripts identified by deFuse (39). Briefly, cDNA was synthesized using SuperScript® VILO cDNA Synthesis Kit and Master Mix (Invitrogen). PCR amplification was performed using a conventional thermocycler with HotStarTaq DNA polymerase (Qiagen) or using a LightCycler® 96 (Roche Applied Science, Indianapolis, IN, USA) with FastStart SYBR Green Master Mix (Roche Applied Science) for quantitative real-time RT-PCR (40). PCR primer pairs used for fusion gene amplification are presented in the Results section. GAPDH was used as a control for RNA integrity and for the RT-PCR reactions. The forward and reverse primer sequences for GAPDH were 5’-GGA GTC CAC TGG CGT CTT CAC-3’ and 5’-GAG GCA TTG CTG ATG ATC TTG AGG-3’, respectively. Relative mRNA values were expressed as delta-Ct values normalized to GAPDH. Sanger sequencing was performed at the UMGC.
Fluorescence in situ hybridization (FISH)
FISH was performed to detect MYO16-PTK2 and GABRA3-FLT1 fusion genes by designing FISH probes derived from the genome-anchored canine CHORI-82 bacterial artificial chromosome (BAC) library (41). Single locus probes were used for proximal MYO16 at dog chromosome (CFA) 22:57,565,917-57,750,789 (clone 183H20), distal MYO16 at CFA 22:57,750,801-57,967,880 (clone 385H13), and PTK2 at CFA 13:35,302,679-35,483,060 (clone 451H13) with distinct fluorescent tags. For GABRA3-FLT1 fusion, break-apart FISH probes were used for proximal FLT1 at CFA 25:11,057,892-11,263,935 (clone 363B20) and distal FLT1 at CFA 25:11,274,078-11,471,538 (clone 235H9). The PureLink® HiPure Plasmid Maxiprep Kit (Invitrogen) was used for BAC DNA extraction. For preparation and hybridization of FISH probes, BAC DNA probes were labeled by Nick Translation Kit (Abbott Molecular) using Green-500 dUTP, Orange-552 dUTP and Aqua-431 dUTP (Enzo Life Science). Labeled DNA was precipitated in COT-1 DNA, salmon sperm DNA, sodium acetate and 95% ethanol, then dried and resuspended in 50% formamide hybridization buffer. The Red-proximal MYO16, Green-distal MYO16 and Aqua-PTK2 probes were combined into one 3-color FISH probe for MYO16-PTK2 fusion. The Red-proximal FLT1 and Green-distal FLT1 break-apart probes were applied for the split FLT1 gene.
FFPE sections (4 μm) were processed according to the Dako IQFISH protocol; probes were applied to the slide and hybridized for 24 hours at 37°C in a humidified chamber. After hybridization, slides were washed and counterstained with DAPI. Fluorescent signals were visualized on an Olympus BX61 microscope workstation (Applied Spectral Imaging, Vista, CA) with DAPI, FITC, Texas Red and Aqua filter sets. FISH images were captured using an interferometer-based CCD cooled camera (ASI) and FISHView ASI software. A total of 200 interphase cells were examined for each sample. Non-malignant canine spleen tissues were used as controls for the FISH experiment.
Validation of somatic mutations using RNA-Seq data
A pipeline was developed to identify the bases present at locations defined as somatic mutations in the Tumor-Normal exome calls (17). Briefly, RNA-Seq data were mapped using the STAR-Mapper (42) with STAR-FUSION mapping settings (43) to the Canfam3.1 or GRCh38 genome. BAM files generated by STAR were sorted and indexed using Samtools (44). Starting from a file containing somatic mutation locations and a file containing a list of BAM file locations, the pipeline uses Samtools (44) functions to identify the bases present at each location at each file and then reports whether a variant is found at that location. For inclusion within the variant file, at least one sample must have at least 3 reads that support the variant and that represent greater than 10% of all reads at that location.
Gene expression profiling
CLC Bio Genomics Workbench 10 (CLC Bio, Aarhus, Denmark) and DESeq2 R packages were used to quantify gene expression and differential gene expression analysis as described (45). Briefly, paired-end RNA-Seq data with mate-pair distance of 50 bp in FASTQ format were imported, and sequencing quality was determined. Transcriptomics analysis was then performed to generate the expression level of each gene presented as total reads by mapping the sequencing reads to Canfam3.1 or GRCh38. Heatmaps and hierarchical clustering based on average linkage were visualized using Cluster 3.0, Morpheus (https://clue.io/morpheus), or R packages. GO Enrichment Analysis (46-48) or Ingenuity® Pathway Analysis software version 8.6 (Qiagen, Redwood City, CA) were used to define biological functions, canonical pathways, and upstream regulators associated with differently expressed genes (DEGs) between groups using Benjamini-Hochberg multiple testing corrections to evaluate significance. For gene expression profiling, unsupervised PCA and hierarchical clustering were performed to define subtypes of canine HSAs as described previously (21). Gene expression data of human sarcomas in The Cancer Genome Atlas (TCGA) database were also compared with our data sets.
TMA generation and immunohistochemistry (IHC)
Canine TMA blocks were generated from 45 HSA tissues, including 32 tumors used for RNA-Seq and eight non-tumor tissues (six splenic hematomas and two non-malignant liver samples). Tissue cores of 1-mm diameter in quadruplicate from each sample were assembled in random order in four TMA blocks. One TMA block with mouse tissues was generated for staining controls. Immunostaining with CD31, Vimentin and Pan-Cytokeratin antibodies was evaluated to support tumor content estimates. A human TMA block was generated from ten AS tissues and six non-malignant tissues (submandibular gland, skin, breast adipose tissue, thigh skeletal muscle, spleen, and lung).
Unstained TMA sections (4 μm) were de-paraffinized and rehydrated using standard methods for IHC. All of the immunohistochemical assays, including validation for antibodies, were performed and optimized at the UMN BioNet Histology Laboratory or the Veterinary Diagnostic Laboratory at the University of Minnesota. Antibodies used for IHC are summarized in Supplementary Table S3. The immunostaining score assigned to each case was a semiquantitative assessment derived from the product of two integers, ranging from 0 to 3 and from 1 to 3, that respectively reflect the percentage of positive cells in a sample and the intensity of staining at high power magnification (400X) as described previously with some modifications (49). The percentage of positive cells was scored from 0 to 3+, where 0 reflected specific staining in <1% of the cells, 1+ reflected specific staining in >1% and <25% of the cells, 2+ reflected specific staining in 25–75% of the cells, and 3+ reflected specific staining in >75% of the cells. The intensity was assessed as weak (intensity score 1), moderate (intensity score 2), or strong (intensity score 3). Immunostaining results were scored (ranging from 0 to 9) by multiplying the percentage of positive cells (score 0-3) by the intensity (score 1-3).
Statistical analysis
Chi-square or Fisher’s exact test, was performed for contingency tables analysis. Continuous values were analyzed by Welch’s (Heteroscedastic) T-test or Mann-Whitney U test. The statistical tests were two-tailed. Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA). P-values are reported without inference of significance, consistent with the American Statistical Association’s Statement on Statistical Significance and P-Values (50).
Results
Novel protein-coding fusion genes are identified in human ASs and canine HSAs
Putative fusion gene events were identified from RNA-Seq data as paired-end sequence reads that mapped connecting two distant genes (Supplementary Fig. S1). We identified novel in-frame protein-coding fusion transcripts for ten fusion events in 7 of 13 (53.8%) human ASs (Fig. 1A; Table 1). Two of the fusions were inter-chromosomal events and eight were intra-chromosomal events. The fusions included TEX2-PECAM1, which contained the gene that encodes CD31, and ATP8A2-FLT1, a kinase fusion gene that encodes the vascular endothelial growth factor receptor 1 (VEGFR1). None of the ten fusion events was seen in more than one tumor, and three of the seven fusion-positive tumors contained two distinct fusion events each.
Figure 1.
Identification of novel putative protein-coding fusion transcripts in human AS and canine HSA. The Circos plots visualize fusion genes identified in human ASs (A, n = 13) and canine HSA (B, n = 76). Bar graphs show DNA copy number alterations for each fusion partner gene using publicly available Exome-sequencing data in an independent dataset of human AS (C, n = 36; data retrieved from Ref (12)) and using oaCGH in a larger HSA dataset (D, n = 123; data retrieved from Ref (19)).
Table 1.
Putative fusion genes identified in transcriptomic data of human ASs
| Patient ID | Gene 1 | Gene 2 | Putative fusion gene |
Gene 1 chromosome |
Gene 2 chromosome |
Gene 1 fusion location |
Gene 2 fusion location |
Fusion Type |
Gene 1 Ensembl ID |
Gene 2 Ensembl ID |
Genomic break position in gene 1 |
Genomic break position in gene 2 |
Somatic variants |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Patient 2 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Patient 3 | VKORC1L1 | STEAP1B | VKORC1L1-STEAP1B | 7 | 7 | coding | coding | Intra-chromosomal | ENSG00000196715 | ENSG00000105889 | 65873565 | 22419836 | - |
| PPP1R13B | ATP5MPL | PPP1R13B-ATP5MPL | 14 | 14 | coding | coding | Intra-chromosomal | ENSG00000088808 | ENSG00000156411 | 103847299 | 103915189 | ||
| Patient 4 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Patient 5 | - | - | - | - | - | - | - | - | - | - | - | - | HRAS |
| Patient 6 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Patient 7 | SMURF1 | TMEM139 | SMURF1-TMEM139 | 7 | 7 | coding | intron | Intra-chromosomal | ENSG00000198742 | ENSG00000178826 | 99143726 | 143286424 | TP53 |
| AGO2 | TRAPPC9 | AGO2-TRAPPC9 | 8 | 8 | coding | coding | Intra-chromosomal | ENSG00000123908 | ENSG00000167632 | 140635485 | 140451383 | ||
| Patient 8 | - | - | - | - | - | - | - | - | - | - | - | - | NRAS |
| Patient 9 | PEMT | ANKRD6 | PEMT-ANKRD6 | 17 | 6 | coding | utr5p | Inter-chromosomal | ENSG00000133027 | ENSG00000135299 | 17577027 | 89433375 | - |
| CPD | NSRP1 | CPD-NSRP1 | 17 | 17 | coding | coding | Intra-chromosomal | ENSG00000108582 | ENSG00000126653 | 30461311 | 30172542 | ||
| Patient 10 | SCLT1 | NIPBL | SCLT1-NIPBL | 4 | 5 | coding | coding | Inter-chromosomal | ENSG00000151466 | ENSG00000164190 | 128888679 | 37057186 | - |
| Patient 11 | TEX2 | PECAM1 | TEX2-PECAM1 | 17 | 17 | utr5p | utr5p | Intra-chromosomal | ENSG00000136478 | ENSG00000261371 | 64263168 | 64390763 | - |
| Patient 12 | ATP8A2 | FLT1 | ATP8A2-FLT1 | 13 | 13 | coding | coding | Intra-chromosomal | ENSG00000132932 | ENSG00000102755 | 25968679 | 28357685 | - |
| Patient 13 | IRF9 | THTPA | IRF9-THTPA | 14 | 14 | coding | coding | Intra-chromosomal | ENSG00000213928 | ENSG00000259431 | 24164134 | 23558695 | TP53 |
In canine HSA, we found fifteen novel protein-coding fusion genes in eleven of 76 tumors (14.5%) (Fig. 1B; Table 2). Ten of the fusions were inter-chromosomal events and five were intra-chromosomal events. None of the fifteen fusion events was seen in more than one tumor, and four of the eleven fusion-positive tumors involved two distinct fusion genes each. One fusion partner in four of the translocations encoded either a protein kinase or a protein phosphatase associated with angiogenic signaling (MYO16-PTK2, GABRA3-FLT1, AKT3-XPNPEP1, and PTPRB-NOL10). Eight of the fusion genes were associated with kinase signaling or kinase binding activity, such as PI3 kinase signaling, a MAP kinase, a receptor tyrosine kinase, a protein serine/threonine kinase, and an NAD+ kinase. Gene ontology annotations of the fusion partners for every translocation are described in Supplementary Table S4 for human AS and Supplementary Table S5 for canine HSA. Fusion genes were not present in any of the human non-malignant tissues (n = 6) or canine hematomas (n = 10) examined. Conserved driver translocations such as BCR-ABL and MYC-IGH that are present in both human and canine chronic myelogenous leukemia and Burkitt lymphoma, respectively (51), were not identified in human AS and canine HSA. All the fusion events identified in the seven human ASs and eleven canine HSAs involved different gene pairs, with the exception of the FLT1 gene, which created fusions with a different partner gene in one case from each species.
Table 2.
Putative fusion genes identified in transcriptomic data of canine HSAs
| Dog sample ID |
Gene 1 | Gene 2 | Putative fusion gene |
Gene 1 chromosome |
Gene 2 chromosome |
Gene 1 fusion location |
Gene 2 fusion location |
Fusion type | Gene 1 Ensembl ID | Gene 2 Ensembl ID | Genomic break position in gene 1 |
Genomic break position in gene 2 |
Somatic variants | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHAD-B7 | PREX2 | LPCAT1 | PREX2-LPCAT1 | 29 | 34 | coding | coding | Inter-chromosomal | ENSCAFG00000007620 | ENSCAFG00000010491 | 17653781 | 11166324 | TP53 | - |
| DHSA-1204 | AP4E1 | BAIAP2 | AP4E1-BAIAP2 | 30 | 9 | coding | coding | Inter-chromosomal | ENSCAFG00000015318 | ENSCAFG00000005700 | 16748493 | 968509 | TP53 | - |
| NOL10 | PTPRB | NOL10-PTPRB | 17 | 10 | coding | coding | Inter-chromosomal | ENSCAFG00000003435 | ENSCAFG00000000446 | 7515966 | 12372230 | |||
| DHSA-0906 | MYO16 | PTK2 | MYO16-PTK2 | 22 | 13 | coding | coding | Inter-chromosomal | ENSCAFG00000006050 | ENSCAFG00000001217 | 57750807 | 35397284 | TP53 | - |
| ATP9A | SNX5 | ATP9A-SNX5 | 24 | 24 | coding | coding | Intra-chromosomal | ENSCAFG00000011659 | ENSCAFG00000005463 | 37856900 | 5096627 | |||
| DHSA1101 | GABRA3 | FLT1 | GABRA3-FLT1 | X | 25 | utr5p | coding | Inter-chromosomal | ENSCAFG00000019161 | ENSCAFG00000006701 | 120379631 | 11232377 | TP53 | - |
| DHSA1407 | ANKH | ATG16L1 | ANKH-ATG16L1 | 4 | 25 | coding | coding | Inter-chromosomal | ENSCAFG00000014290 | ENSCAFG00000011752 | 88259666 | 44761299 | - | - |
| DHSA1416 | LAMB1 | CBLB | LAMB1-CBLB | 18 | 33 | coding | coding | Inter-chromosomal | ENSCAFG00000025057 | ENSCAFG00000009793 | 12675054 | 11259031 | TP53 | PIK3CA |
| DHSA1513 | PIK3AP1 | REV3L | PIK3AP1-REV3L | 28 | 12 | coding | coding | Inter-chromosomal | ENSCAFG00000008880 | ENSCAFG00000003942 | 10085967 | 67953124 | TP53 | PIK3CA |
| AKT3 | XPNPEP1 | AKT3-XPNPEP1 | 7 | 28 | coding | coding | Inter-chromosomal | ENSCAFG00000015806 | ENSCAFG00000010661 | 34778111 | 21321899 | |||
| DHSA-1015 | MRPS35 | CACNA1C | MRPS35-CACNA1C | 27 | 27 | coding | coding | Intra-chromosomal | ENSCAFG00000010963 | ENSCAFG00000016051 | 20003244 | 44487698 | - | PIK3CA |
| CCDC172 | ABLIM1 | CCDC172-ABLIM1 | 28 | 28 | coding | coding | Intra-chromosomal | ENSCAFG00000011803 | ENSCAFG00000011513 | 26980198 | 25377079 | |||
| DHSA-0803 | COPS5 | NCOA2 | COPS5-NCOA2 | 29 | 29 | utr5p | utr5p | Intra-chromosomal | ENSCAFG00000007386 | ENSCAFG00000007775 | 16578975 | 19448341 | TP53 | PIK3CA |
| DHSA-0805 | MSN | LUC7L2 | MSN-LUC7L2 | X | 16 | coding | coding | Inter-chromosomal | ENSCAFG00000016607 | ENSCAFG00000004069 | 50748329 | 9413652 | TP53 | PIK3CA |
| DHSA-1113 | RANBP3L | ARL15 | RANBP3L-ARL15 | 4 | 4 | coding | coding | Intra-chromosomal | ENSCAFG00000018711 | ENSCAFG00000018394 | 72325076 | 61484049 | - | - |
To determine whether the non-tumor components in the tumor tissue affected the detection of fusion genes, we quantified tumor content histologically and bioinformatically in canine HSAs (Supplementary Fig. S2A - E). Seventy of the 76 HSA samples were histologically evaluated, and the tumor content was not different between HSA samples with fusion events (n = 11) and those without fusion events (n = 59). We used two independent bioinformatic tools, xCell and ESTIMATE, to predict stromal and immune cell components, and these algorithms generated consistent output scores (Pearson’s R = 0.84; R2 = 0.71; P < 0.00001) that showed the presence of fusion genes was not associated with tumor purity. The detection of fusion genes was also independent of sequencing depth (Supplementary Fig. S2F). To rule out artifacts from the computational process, we validated the presence of the inter-chromosomal fusion gene, SCLT1-NIPBL, in the original human AS sample where it was identified, in two additional samples where it was undetectable based on sequencing data, and in a non-malignant tissue sample. The fusion transcript was detectable by quantitative real time RT-PCR amplification; PCR primer pairs were designed to amplify putative split sequences (up to 200 base pairs) involving the breakpoints identified by deFuse (Supplementary Fig. S3A). We confirmed that the junction sequences between the two genes producing the new fusion event were amplified by PCR (Supplementary Fig. S3B). Four representative fusion transcripts found in canine HSAs (MYO16-PTK2; AKT3-XPNPEP1; AP4E1-BAIAP2; NOL10-PTPRB) were also detected, but only in the respective cases where they were identified in the sequencing data (Supplementary Fig. S3C - E). Each PCR amplification product was verified by Sanger sequencing. We then used RT-PCR to evaluate RNA-Seq data from 63 canine tissue samples (53 HSAs and 10 hematomas) for the presence of these four fusion transcripts. The results were consistent between RNA-Seq and PCR, as we found neither false-positive nor false-negative events in the samples tested (Supplementary Table S6).
Fusion genes are associated with DNA copy number variations
We then determined if any of the fusion partner genes identified in our analysis were associated with DNA copy number alterations. Publicly available whole Exome-sequencing data generated from an independent data set of 36 human patients with ASs was used (12). Copy number variations were found in twelve of the twenty (60%) fusion partner genes: nine genes were amplified, and five genes were deleted (Fig. 1C; Supplementary Fig. S4). TEX2 (39%), STEAP1B (25%) and PECAM1 (25%) were the top three genes where copy number gains occurred most frequently. For canine HSA, we used oligonucleotide array comparative genomic hybridization (oaCGH) in a larger HSA dataset (n = 123) (19). Copy number gains were observed in 29 of the 30 (96.7%) fusion partners, and copy number losses were observed in 27 of the 30 (90.0%) fusion partners. Protein kinase-encoding genes, PTK2, FLT1, and AKT3 revealed a higher frequency of copy number gain: 15.5% gain vs 0.8% loss for PTK2; 4.9% gain vs 0.8% loss for FLT1; and 4.1% gain vs 0.8% loss for AKT3, suggesting that copy number alterations leading to dysregulation of downstream kinase signaling contribute at least partly to the angiogenic program in a subset of canine HSAs (Fig. 1D).
Chromosomal translocations resulting in fusion genes are detectable in canine HSAs
We performed FISH to confirm that fusion genes were generated by chromosome translocations. We chose representative inter-chromosomal fusions, MYO16-PTK2 and GABRA3-FLT1 for cytogenetic validation because PTK2 and VEGFR1 are key molecules that regulate pathogenic signaling in vascular cancers, including canine HSA (52). Fig. 2A illustrates the predicted structure of MYO16-PTK2 inter-chromosomal fusion between CFA 22 and CFA 13, based on deFuse and Sanger sequencing data (Fig. 2B). The predicted fusion gene comprises exons 1-32 of MYO16 (CFA 22) and exons 12-31 of PTK2 (CFA 13), with the putative junction joining MYO16 exon 32 and PTK2 exon 12. Breakage occurs between exons 32 and 33 of MYO16 at CFA 22:57,750,807 bp, and between exons 11 and 12 of PTK2 at CFA 13:35,397,284 bp. Independent FISH probes identifying the association between proximal and distal MYO16 and the breakpoint of PTK2 (Fig. 2C) confirmed the presence of the MYO16-PTK2 fusion gene between CFA 22 and 13 in archival FFPE samples from the same dog tumor. The MYO16-PTK2 fusion was identified by deFuse and RT-PCR (Fig. 2D). The t(CFA 13;CFA 22) translocation was present in interphase nuclei of 16.8% of the tumor cells, with a smaller subpopulation showing amplification of the fusion (Fig. 2E).
Figure 2.
Validation of fusion genes in canine HSA. A, MYO16-PTK2 fusion gene track and visualization of the breakpoint in UCSC Genome Browser (Canfam3.1). B, Sanger sequencing result of the PCR product for MYO16-PTK2 fusion gene. C, Schematic illustration of the putative MYO16-PTK2 fusion gene and designed FISH probes. D, Detection of the MYO16-PTK2 fusion gene on primary canine HSA tissue by FISH. In wild type cells an association between BAC clones 183H20 (red) and 385H13 (green) can be appreciated, both showing independent localization from 451H13 (aqua). A portion of tumor cells shows a breakage within 451H13, with one half of that signal associating with 183H20, and independent of the localization of 385H13 indicating the existence of the MYO16-PTK2 fusion at the genomic level. E, Arrows indicate the amplification of the MYO16-PTK2 fusion gene. F, Detection of the GABRA3-FLT1 fusion gene by FISH. The GABRA3-FLT1 fusion is identified by break-apart FISH probes for proximal FLT1 (clone 363B20; red) at CFA 25 and distal FLT1 at CFA 25 (clone 235H9; green). Split FLT1 genes indicate the fusion event identified by single color signal (white arrows). Dual colors represent the intact FLT1 gene (grey arrows). G, DNA amplification of FLT1 gene.
We used break-apart FISH to validate the presence of the GABRA3-FLT1 fusion gene (Fig. 2F). Split FLT1 probes were found in 36.7% of tumor cells in archival FFPE samples from the dog tumor in which the GABRA3-FLT1 fusion was identified by deFuse and RT-PCR. Interestingly, in this tumor the intact FLT1 gene showed consistent amplification (up to four copies), suggesting Flt-1 (also known as VEGFR1) activation in this tumor might have occurred through multiple mechanisms (Fig. 2G). We next used FISH analysis to assess recurrence of the MYO16-PTK2 fusion in a tissue microarray (TMA) comprised of 45 visceral HSAs and eight non-malignant tissues (six spleens; two livers). The MYO16-PTK2 fusion was once again present in the sample from the canine tumor in which it was discovered, but it was not seen in any other sample on the TMA. We also performed FISH to detect the ATP8A2-FLT1 fusion in human AS using a break-apart FLT1 probe, but the fusion was undetectable in our FFPE sample. This might have been due to the small number of tumor cells that were likely to contain the fusion event in a heterogeneous clonal population. Since none of the fusion transcripts identified in our cohorts of human and canine tumors were recurrent, we sought to determine if the fusion events were associated with other genetic and molecular programs.
Fusion genes and somatic variants in human ASs enrich angiogenic gene signatures
To examine genomic aberrations associated with the fusion genes, we determined somatic variations and gene expression profiles using RNA-Seq data. In human ASs, TP53 mutations (R248Q and P250L) were observed in two of thirteen human ASs, which also had fusion genes (SMURF1-TMEM139 and AGO2-TRAPPC9 in one tumor, IRF9-THTPA in the other tumor). NRAS (Q61L; n = 1) or HRAS (Q61L; n = 1) mutations were also detected, and both were present in tumors that did not have fusion genes or TP53 mutations (Fig. 3A; Table 1). The RNA-Seq data did not provide evidence of mutations in PIK3CA, PTEN, or KRAS in this group of thirteen ASs.
Figure 3.
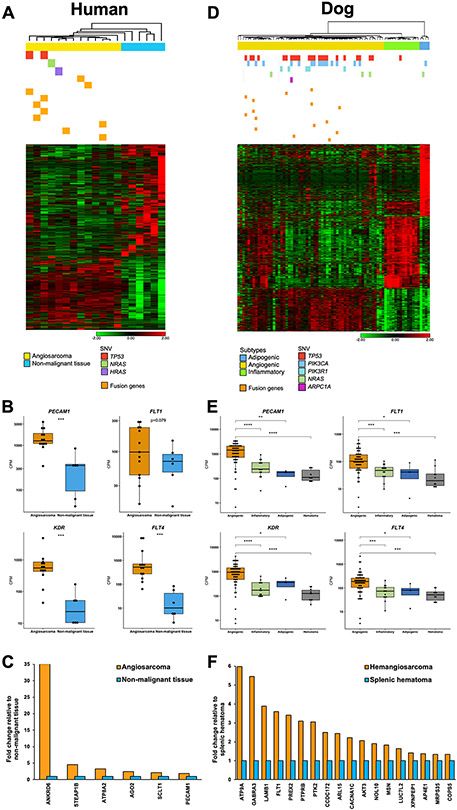
Fusion genes, mutations of somatic variants, and molecular subtypes of human AS (A-C) and canine HSA (D-F). A, 1,237 differentially expressed genes were identified between human ASs (n = 13) and non-malignant tissue samples (n = 6) (False Discovery Rate or FDR P < 0.05): 490 genes were upregulated and 747 genes were downregulated in ASs. Ten fusion genes are marked as yellow bars in seven AS samples. Somatic variations in TP53 (n = 2), NRAS (n = 1), and HRAS (n = 1) were found in four ASs. B, Box plots show gene expression of PECAM1, FLT1, KDR, and FLT4 representing vasculogenic and angiogenic functions in human ASs and non-malignant tissues (two-tailed Mann-Whitney test). ***, P < 0.001. C, Bar graphs show the relative expression of genes in human ASs normalized to the expression of non-malignant tissues. Six of 20 genes where the P-value was less than 0.05 are displayed (two-tailed Welch’s T-test). D, Heatmap illustrates 1,477 significant differentially expressed genes among three subtypes of canine HSA (n = 76) (FDR P < 0.001; Fold change > 3). Somatic variant analysis identified mutations in TP53 (n = 24), PIK3CA (n = 16), PIK3R1 (n = 5), NRAS (n = 4), and ARPC1A (n = 1). Grey bars indicate unavailable somatic variants data. Fifteen fusion genes are marked as yellow bars in 11 HSA samples. E, Box plots display gene expression of PECAM1, FLT1, KDR, and FLT4 representing vasculogenic and angiogenic functions in subtypes of HSA and non-malignant hematomas (two-tailed Mann-Whitney test). ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05. F, Bar graphs show the relative expression of genes in canine HSA normalized to the expression of hematomas. Eighteen of 30 genes where the P-value is less than 0.05 are displayed (two-tailed Welch’s T-test). Heatmaps (A and D) show unsupervised hierarchical clustering (average linkage). Heatmap colors display mean-centered fold change expression following log2 transformation. Upregulated genes are presented in red and downregulated genes are shown in green.
We established transcriptomic profiles of human ASs to identify molecular traits that regulate global gene expression. We identified 1,237 differentially expressed genes between ASs (n = 13) and non-malignant controls (N = 6) (FDR P-value < 0.05): 490 genes were upregulated and 747 genes were downregulated in ASs. Biological functions and pathway analysis revealed that upregulated genes in ASs were associated with cancer, angiogenesis, vasculogenesis, and development of vasculature (P < 0.00001) (Supplementary Table S7). Additionally, we performed cell type enrichment analysis using the xCell tool (38) to predict relative populations of cellular components that comprised the AS tissues (Supplementary Fig. S5). We compared the cell type signature of AS with that of sarcomas (n = 263) from the TCGA database, which did not include AS. The results showed that gene signatures associated with endothelial cells and activated dendritic cells were highly enriched in ASs, while other sarcomas in the TCGA revealed gene enrichment of smooth muscle cells. Gene expression profiles of non-malignant samples indicated distinct tissue-specific patterns of submandibular gland, skin, breast adipose tissue, thigh skeletal muscle, spleen, and lung. The ASs showed upregulation of key angiogenic genes such as PECAM1 (CD31), FLT1 (VEGFR1), KDR (VEGFR2), and FLT4 (VEGFR3) compared to non-malignant tissues (Fig. 3B). In addition, 6 of 20 (30.0%) fusion partners (including PECAM1) showed a higher level of expression in the tumors compared to non-malignant tissues (P < 0.05; fold change in a range from 1.9 to 35.0) (Fig. 3C). Collectively, our data showed enriched angiogenic molecular programs were present in human ASs. Furthermore, both tumors with TP53 mutations also harbored fusion genes.
Fusion genes that co-occur with mutations of TP53 are present exclusively in angiogenic canine HSAs
In canine HSAs, TP53 (n = 24/74; 32.4%), PIK3CA (n = 16/74; 21.6%), PIK3R1 (n = 5/74; 6.8%), and NRAS (n = 4/74; 5.4%) transcripts showed recurrent mutations that were consistent with those identified using tumor:normal Exome-sequencing (17). We found associations between mutations of TP53 (TP53mt) and PIK3CA (PIK3CAmt) and fusion genes (Fusion+) (Supplementary Table S8). Specifically, TP53mt commonly co-occurred with PIK3CAmt (P = 0.004) and with fusion genes (P = 0.004); and Fusion+ tumors were seen in the tumors with TP53mt or PIK3CAmt (P = 0.005) more frequently than would be expected by random chance. When fusion genes co-occurred with PIK3CAmt, they invariably co-occurred with mutations of TP53 (P = 0.037), and they were not associated with PIK3CAmt alone (P = 0.324).
Next, we sought to determine if fusion genes were preferentially associated with specific molecular subtypes of canine HSA. We previously defined distinct angiogenic, inflammatory, and adipogenic molecular subtypes of canine HSA (21). To further validate this classification, we applied unsupervised principal component analysis (PCA) and hierarchical clustering to identify distinct groups in the sample cohort from this study (Supplementary Fig. S6). Our results show that the three molecular subtypes (58 angiogenic, 14 inflammatory, and 4 adipogenic) were reproducibly identified in the current dataset, as illustrated in the heatmap of 1,477 DEGs (FDR P < 0.001; fold change > ∣3∣) shown in Fig. 3D. Interestingly, fusion genes were present only in tumors of the angiogenic HSA subtype (P = 0.046). Likewise, TP53 mutations were identified in 23 of 56 (41.1%) angiogenic HSAs, and in one of 18 tumors from the two other molecular subtypes (P = 0.008). The somatic variants of TP53, PIK3CA, PIK3R1, and NRAS were found in 33 of 56 (58.9%) angiogenic HSAs, and in 3 of 18 (16.7%) tumors from the two other subtypes (P = 0.002). The angiogenic subtype of HSA also showed upregulation of PECAM1 (CD31), FLT1 (VEGFR1), KDR (VEGFR2), and FLT4 (VEGFR3) compared to the other two HSA subtypes and to non-malignant hematomas (Fig. 3E). We found that five of 32 dogs (16%) with angiogenic HSA lived longer than five months, while four of 10 dogs (40%) with inflammatory HSA survived longer than that (Supplementary Fig. S7). Eighteen of 30 (60.0%) fusion partners, including protein kinase-encoding genes such as FLT1, PTK2, and AKT3, showed higher levels of expression in HSAs compared to non-malignant controls (P < 0.05; fold change in a range from 1.3 to 6.0) (Fig. 3F). Neither breed, sex, neuter status, age, nor affected organs were associated with the presence of fusion genes (Supplementary Fig. S8). There was also no association between the fusion events and histological subtype or mitotic index (Supplementary Table S9).
Next, we analyzed DEGs (FDR P < 0.05; fold change > ∣2∣) and gene pathways to examine gene signatures enriched in HSAs that had both fusion genes and TP53 mutations. We classified tumors according to their mutations as summarized in Supplementary Table S10. Co-occurrence of fusion genes with TP53 mutations (i.e., TP53mt/Fusion+/PIK3CAwt or PF tumors) was associated with angiogenic and vascular signaling with enrichment of genes in pathways such as PI3K, VEGF, and PDGF (Supplementary Table S11 - S13). Thirteen genes that were commonly enriched in PF tumors were associated with activation of WNT3A as an upstream regulator (Supplementary Fig. S9). Fig. 4 illustrates a model integrating the data from these findings to highlight potential pathogenetic contributions of fusion genes and recurrent mutations in canine HSA.
Figure 4.
Hypothetical model for angiogenic pathogenesis of canine HSA.
Human ASs and canine HSAs establish molecular programs that activate convergent signaling pathways
To determine if the genetic and molecular features of human AS and canine HSAs contributed to the activation of functional pathways, we performed IHC in eleven human AS tissues and in 44 canine HSAs (Fig. 5; Supplementary Tables S14 and S15). First, we evaluated the effects of TP53 mutation on the presence and location of p53, phospho-p53 (Ser15), and phospho-p53 (Ser20) (Fig. 5A and B). In human ASs, nuclear expression of p53 was found in all of eleven (100%) tumors, showing various levels of expression. Nuclear expression of phospho-p53 (Ser15) was seen in seven of eleven (63.6%) tumors, showing low expression in five of seven (71%) tumors (IHC score ≤ 3). Nuclear and cytoplasmic expression of phospho-p53 (Ser20) protein was detected in all eleven (100%) tumors, and seven of those showed high expression (IHC score ≥ 7). In canine HSAs, p53 protein was localized to the nucleus in 34 of 44 (77%) tumors with various levels of expression. Immunoreactivity of phospho-p53 (Ser15) was observed in the nuclei of tumor cells in 38 of 40 (95%) cases, with 34 (90%) showing low or medium expression (IHC score ≤ 6). Nuclear and cytoplasmic expression of phospho-p53 (Ser20) was seen in all of 40 HSAs (100%), with 32 tumors (80%) showing high levels of expression. These data revealed that patterns of p53 and activated p53 were comparable in human ASs and canine HSAs; especially, p53 was strongly phosphorylated at residue Ser20 in tumors from both species. We found no association between phosphorylated p53 and TP53 mutations or fusion genes (Supplementary Fig. S10), suggesting that DNA damage and cellular stress are widespread among these tumors, and they are likely to activate p53-mediated repair mechanisms independent of these genetic alterations.
Figure 5.
Immunohistochemical expression of p53 and AKT proteins in human AS and canine HSA. A, Bar graphs show the number of cases (y-axis) plotted as a function of IHC scores (x-axis) for staining with anti-p53, anti-phosphorylated (p)-p53 (Ser15), and anti-p-p53 (Ser20) antibodies in human ASs (Left panel) and canine HSAs (Right panel). B, Representative photomicrographs show IHC staining of p53, p-p53 (Ser15), and p-p53 (Ser20) in human AS (Left panel) and canine HSA tissues (Right panel). Bar graphs (C) for IHC scores of AKT and p-AKT (Thr308) proteins and representative photomicrographs (D) are also displayed for human ASs and canine HSAs. H&E = hematoxylin and eosin stain. IHC staining (Horseradish peroxidase and hematoxylin counterstain). 200X magnification.
The PI3K/AKT/mTOR signaling pathway is important for regulation of angiogenic, vascular, and energetic functions. To assess whether PI3K mutations resulted in higher levels of downstream pathway activation, we evaluated expression of AKT and phospho-AKT proteins in human ASs and canine HSAs (Fig. 5C and D). Expression of nuclear and cytoplasmic AKT protein was observed in all eleven human ASs (100%) with eight of eleven (73%) tumors showing medium or high expression. Expression of phospho-AKT (Thr308) was evaluated in eight tumors; all of them (100%) showed weak or medium levels of expression. Similarly, AKT protein was detectable in the nucleus and cytoplasm of all forty (100%) canine HSAs with 37 (93%) showing medium or high expression. Phospho-AKT (Thr308) was detected in the nuclei and cytoplasm of all evaluable 39 (100%) canine HSAs, with 34 (87%) expressing medium or high level of the protein. Strong AKT immunoreactivity was also seen in scattered stromal cells in both human AS and canine HSA. Neither mutations of TP53, PIK3CA, PIK3R1 nor the presence of fusion genes were associated with expression of AKT and phospho-AKT in human or canine tumors (Supplementary Fig. S11). Since PIK3CA and PIK3R1 mutations were undetectable in this set of eleven human ASs, we examined expression of mTOR and phospho-mTOR (Ser2448) proteins as surrogates to confirm activation of their downstream pathways in these tumors. mTOR protein was observed in the nuclei and cytoplasm of all eleven tumors (IHC score ≥ 6), and it was not associated with the presence of TP53 mutations or fusions (Supplementary Fig. S12A and B). However, nuclear and cytoplasmic expression of phospho-mTOR was higher in ASs that had TP53 mutations or that had fusion genes than it was in tumors without one of these genetic changes (Supplementary Fig. S12C and D). In summary, the immunostaining data suggest that human AS and canine HSA have comparable activation of the p53 and PI3K/AKT/mTOR pathways, and these events are largely independent of their mutational states. Our results further suggest that these vasoformative tumors from both species activate convergent signaling pathways that contribute to their final architecture and organization with predictable enrichment of angiogenic gene signatures.
Discussion
For this study, our objective was to identify novel fusion genes in human ASs and spontaneous canine HSAs. We showed that novel protein-coding fusion genes were identified in approximately 50% of human ASs of which two had TP53 mutations. In canine HSAs, protein-coding fusion genes were detectable in ~15% of tumors, and those were associated with p53 deficiency and enrichment of angiogenic gene signatures. Our data suggest that convergent molecular mechanisms associated with p53 inactivation and enhanced PI3K/AKT/mTOR signaling pathways are operational in genomically complex human ASs and canine HSAs.
In the past decade, advances in next-generation sequencing and bioinformatics have enabled genome-wide identification of unbiased cancer-associated fusion transcripts in a variety of tumor types. Previous studies have reported 7,887 fusion transcripts identified across thirteen tumor types in TCGA datasets and 9,928 fusion genes with a 3% recurrence rate in the Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer (53-55). These findings illustrate the complexity of the cancer-associated fusion gene landscape, showing a relatively high rate of fusions with low recurrence, possibly arising from catastrophic chromosome rearrangements by chromothripsis (56) and chromoplexy (57). Despite this relatively high frequency of fusion genes, a solution to define their pathogenic significance remains elusive. One key finding from this work was that protein-coding fusion genes co-occurred with mutations of TP53 in the angiogenic molecular subtype of canine HSA, suggesting that genomic instability might create a predisposition for translocations and the resultant fusion genes and that, in turn, these fusion genes create unique transcriptional programs that promote angiogenic phenotypes in these p53-deficient backgrounds.
Specifically, kinase fusion genes involving FLT1, PTK2, and AKT3 can activate key convergent gene pathways associated with blood vessel formation and remodeling (58), and they represent potential therapeutic targets for kinase inhibitors (35,59). When we consider that sarcomas have the highest frequency of kinase fusions in TCGA datasets (35), but that they also have extremely low recurrence, a more rational approach might be to develop agents that target these convergent angiogenic pathways instead of the products from the individual fusion genes themselves (60).
The two fusion genes that we confirmed by genomic structural evaluation in canine HSAs were present in approximately 20 - 40% of cells in the tumor, both genes showing chaotic amplification. Several explanations could account for these observations. One is that histology and bioinformatics assays overestimated tumor content and tumor purity. Another is that fusions are epiphenomena arising from chaotic genomes with no influence on selection. A third, which we believe is most likely, is that translocation and the resulting fusion events occur stochastically in genomically unstable cells late in the course of tumor evolution. However, the enrichment of fusion genes and angiogenic transcriptional programs suggests that these traits endow tumor cells with selective growth and/or survival advantages that contribute to tumor progression by promoting proangiogenic environments. It is worth noting that the selective pressures in the AS milieu favor not only fusion-positive clones, but also fusion-negative clones, as the establishment of a proangiogenic environment could improve survival of all the subpopulations within the tumor. Indeed, a similar mechanism might be operative in alveolar rhabdomyosarcomas, where PAX3-FOXO1A fusions are necessary for tumor initiation but have no effect on tumor recurrence (61,62). Further work will be necessary to distinguish which among these non-mutually exclusive possibilities are operative, and to better understand the role of fusion genes in tumor evolution of human AS and canine HSA and, potentially, in promoting clonal heterogeneity through the creation of a permissive niche.
Fusion genes have been reported in human ASs (10,63-65). For instance, one study found a CIC-LEUTX fusion in one of 120 (0.8%) FFPE ASs examined (10); another found a CEP85L-ROS1 fusion in one of 34 (3.0%) ASs examined (63); and a third found an EWSR1-ATF1 fusion in one case of AS (64). A NUP160-SLC43A3 fusion has also been reported in the ISO-HAS AS cell line (65). However, none of these fusion genes has been identified recurrently in subsequent studies of AS samples. While these observations are consistent with our stochastic hypothesis, we cannot completely exclude the possibility that fusion genes in human ASs, or for that matter in canine HSAs, are non-pathogenic passenger aberrations.
A larger case series will be required to define the fusion gene landscape in human AS, but canine HSA provides potential insights for what might be expected. Mutations of TP53 are largely mutually exclusive of mutations in KDR, PIK3CA, and RAS gene family in human AS, and the mutational patterns seem to be associated with the location of the primary tumor (10,12,17,66,67). We see a similar pattern emerge in a subset of canine HSA, and from our data we propose a model that can be used as a foundation to test mechanistic links between the mutational and transcriptional landscapes in malignant vascular tumors (Fig. 4) and determine their roles in tumor progression. In this model, inflammatory HSAs harbor no mutations of PIK3CA, and only rarely of TP53, maintaining sufficient genomic stability that disfavors formation of fusion genes. Furthermore, the transcriptional programs in these inflammatory HSAs are weakly angiogenic, and their permissive inflammatory environments restrain growth and metastasis. Conversely, angiogenic HSAs harbor frequent mutations of PIK3CA and TP53, and fusion events. Mutations of PIK3CA in p53-proficient backgrounds promote pro-angiogenic environments, while in p53-deficient backgrounds, these mutations promote altered chromatin regulation and immunomodulatory transcriptional programs. Finally, mutations of TP53 enable genomic instability with formation of fusion genes. These events are stochastic, but fusion genes that promote pro-angiogenic transcriptional programs can enhance or even supplant the effects of PIK3CA mutations and create environments that accelerate tumor growth and metastatic propensity.
Molecular distinctions among human ASs could be driven by their clinical phenotype and potential therapeutic responses (9,68,69). For instance, a subset of ASs harbor gene amplifications of MYC and FLT4 which frequently co-occur in tumors associated with ultraviolet (UV) irradiation- or therapeutic radiation. Mutational signatures associated with UV exposure and high mutational burden might predict more favorable immunotherapeutic responses in AS patients, as they do in patients diagnosed with malignant melanoma; however, supportive clinical trials to test this premise are limited, and the use of immune checkpoint inhibitors in human AS patients thus far has yielded mixed results (70,71). The mutational signatures in canine HSA are largely confined to the “aging” (cellular replication) signature (17), and total mutational burden is relatively low (17,72), so this condition is unlikely to provide a model to address the utility of immunotherapy in this context. However, canine HSA could provide a suitable model to address other treatments, whether pharmacologic or immunologic, directed at the molecular programs that drive progression and maintenance of the tumors in both species (33). Such validation studies could alter the paradigms for diagnosis and treatment of human AS and canine HSA, as well as of other aggressive, genomically complex sarcomas that affect humans and dogs alike.
Supplementary Material
Implications statement:
This study shows that, while drive events of malignant vasoformative tumors of humans and dogs include diverse mutations and stochastic rearrangements that create novel fusion genes, convergent transcriptional programs govern the highly conserved morphological organization and biological behavior of these tumors in both species.
Acknowledgements
The authors would like to acknowledge Dr. Corrie Painter for reviewing the manuscript and providing feedback. The authors acknowledge Mitzi Lewellen for assistance with inventory, database management, and editorial assistance. The authors would also like to thank Lauren Mills for processing of the next generation sequencing data and Dr. Douglas Yee, Director of Masonic Cancer Center, for assisting with the collection of human tissues. Human biospecimens were obtained from the UMN BioNet and from the CHTN. Tissue samples were provided by the CHTN which is funded by the National Cancer Institute (NCI). Other investigators may have received specimens form the same subjects. This work was partially supported by grants 1R03CA191713-01 (J.F. Modiano, A.L. Sarver, J.H. Kim) and R37CA218570 (E.K. Karlsson) from the NCI of the National Institutes of Health (NIH), grants #422 (J.F. Modiano) and 1889-G (J.F. Modiano, M. Breen, K. Lindblad-Toh) from the AKC Canine Health Foundation, grant JHK15MN-004 (J.H. Kim) from the National Canine Cancer Foundation, grant D10-501 (J.F. Modiano, M. Breen, K. Lindblad-Toh) from Morris Animal Foundation, and a grant from Swedish Cancerfonden (K. Lindblad-Toh). This work was also supported by an NIH NCI R50 grant, CA211249 (A.L. Sarver). The NIH Comprehensive Cancer Center Support Grant to the Masonic Cancer Center, University of Minnesota (P30 CA077598) provided support for the cytogenetic analyses performed in the Cytogenomics Shared Resource. K. Megquier is supported by the NCI of the NIH under Award Number F32CA247088. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. M. Breen is supported in part by the Oscar J. Fletcher Distinguished Professorship in Comparative Oncology Genetics at North Carolina State University. K. Lindblad-Toh is supported by a Distinguished Professor award from the Swedish Research Council. J.F. Modiano is supported by the Alvin and June Perlman Chair in Animal Oncology. The UMGC (http://genomics.umn.edu) supported for generation of genomic sequencing data libraries, and the Minnesota Supercomputing Institute (MSI) at the University of Minnesota (http://www.msi.umn.edu) provided computational resources that contributed to the results in this study. The authors gratefully acknowledge donations to the Animal Cancer Care and Research Program of the University of Minnesota that helped support this project.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Data availability
RNA-Seq gene expression data generated from human sarcomas are available from the TCGA Research Network (https://www.cancer.gov/tcga). Exome sequencing data from human angiosarcomas are available from The Angiosarcoma Project (https://ascproject.org), a project of Count Me In (https://joincountmein.org/). RNA-Seq data from human AS tissues are available through the Gene Expression Omnibus (GEO; http://www.ncbi.nlm. nih.gov/geo; accession number GSE163359). RNA-Seq data from canine HSA tissues are published (17,21,32,33) and available through the GEO (accession number GSE95183) and the NCBI Sequence Read Archive (accession number PRJNA562916). All other data generated from this study are available upon request to the corresponding author.
References
- 1.Taylor BS, Barretina J, Maki RG, Antonescu CR, Singer S, Ladanyi M. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer 2011;11(8):541–57 doi 10.1038/nrc3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuville A, Ranchere-Vince D, Dei Tos AP, Montesco MC, Hostein I, Toffolatti L, et al. Impact of molecular analysis on the final sarcoma diagnosis: a study on 763 cases collected during a European epidemiological study. Am J Surg Pathol 2013;37(8):1259–68 doi 10.1097/PAS.0b013e31828f51b9. [DOI] [PubMed] [Google Scholar]
- 3.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer 2003;3(9):685–94 doi 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 4.Italiano A, Chen CL, Thomas R, Breen M, Bonnet F, Sevenet N, et al. Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer 2012;118(23):5878–87 doi 10.1002/cncr.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buehler D, Rice SR, Moody JS, Rush P, Hafez GR, Attia S, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol 2014;37(5):473–9 doi 10.1097/COC.0b013e31827e4e7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD, et al. Angiosarcoma: clinical and molecular insights. Ann Surg 2010;251(6):1098–106 doi 10.1097/SLA.0b013e3181dbb75a. [DOI] [PubMed] [Google Scholar]
- 7.Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol 2007;18(12):2030–6 doi 10.1093/annonc/mdm381. [DOI] [PubMed] [Google Scholar]
- 8.Smrke A, Hamm J, Karvat A, Simmons C, Srikanthan A. A retrospective review of 145 patients with angiosarcoma: Radiation therapy, extent of resection and chemotherapy are important predictors of survival. Molecular and Clinical Oncology. Molecular and Clinical Oncology 2020;13:179–85 doi 10.3892/mco.2020.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behjati S, Tarpey PS, Sheldon H, Martincorena I, Van Loo P, Gundem G, et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet 2014;46(4):376–9 doi 10.1038/ng.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SC, Zhang L, Sung YS, Chen CL, Kao YC, Agaram NP, et al. Recurrent CIC Gene Abnormalities in Angiosarcomas: A Molecular Study of 120 Cases With Concurrent Investigation of PLCG1, KDR, MYC, and FLT4 Gene Alterations. Am J Surg Pathol 2016;40(5):645–55 doi 10.1097/PAS.0000000000000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonescu CR, Yoshida A, Guo T, Chang NE, Zhang L, Agaram NP, et al. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res 2009;69(18):7175–9 doi 10.1158/0008-5472.CAN-09-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Painter CA, Jain E, Tomson BN, Dunphy M, Stoddard RE, Thomas BS, et al. The Angiosarcoma Project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat Med 2020;26(2):181–7 doi 10.1038/s41591-019-0749-z. [DOI] [PubMed] [Google Scholar]
- 13.Antonescu C. Malignant vascular tumors--an update. Mod Pathol 2014;27 Suppl 1:S30–8 doi 10.1038/modpathol.2013.176. [DOI] [PubMed] [Google Scholar]
- 14.Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GR, Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol 2006;34(7):870–8. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SM, Storer RD, Criswell KA, Doerrer NG, Dellarco VL, Pegg DG, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci 2009;111(1):4–18 doi 10.1093/toxsci/kfp131. [DOI] [PubMed] [Google Scholar]
- 16.Mullin C, Clifford CA. Histiocytic Sarcoma and Hemangiosarcoma Update. Vet Clin North Am Small Anim Pract 2019;49(5):855–79 doi 10.1016/j.cvsm.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Megquier K, Turner-Maier J, Swofford R, Kim JH, Sarver AL, Wang C, et al. Comparative Genomics Reveals Shared Mutational Landscape in Canine Hemangiosarcoma and Human Angiosarcoma. Mol Cancer Res 2019;17(12):2410–21 doi 10.1158/1541-7786.MCR-19-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Wu M, Durham AC, Radaelli E, Mason NJ, Xu X, et al. Molecular subtypes in canine hemangiosarcoma reveal similarities with human angiosarcoma. PLoS One 2020;15(3):e0229728 doi 10.1371/journal.pone.0229728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas R, Borst L, Rotroff D, Motsinger-Reif A, Lindblad-Toh K, Modiano JF, et al. Genomic profiling reveals extensive heterogeneity in somatic DNA copy number aberrations of canine hemangiosarcoma. Chromosome Res 2014;22(3):305–19 doi 10.1007/s10577-014-9406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen NJ, Nickoloff BJ, Dykema KJ, Boguslawski EA, Krivochenitser RI, Froman RE, et al. Pharmacologic inhibition of MEK signaling prevents growth of canine hemangiosarcoma. Mol Cancer Ther 2013;12(9):1701–14 doi 10.1158/1535-7163.MCT-12-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorden BH, Kim JH, Sarver AL, Frantz AM, Breen M, Lindblad-Toh K, et al. Identification of three molecular and functional subtypes in canine hemangiosarcoma through gene expression profiling and progenitor cell characterization. Am J Pathol 2014;184(4):985–95 doi 10.1016/j.ajpath.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Frantz AM, Sarver AL, Gorden Klukas BH, Lewellen M, O'Brien TD, et al. Modulation of fatty acid metabolism and immune suppression are features of in vitro tumour sphere formation in ontogenetically distinct dog cancers. Vet Comp Oncol 2018;16(1):E176–E84 doi 10.1111/vco.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell 2005;123(6):1001–11 doi 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein I, Marcel V, Olivier M, Oren M, Rotter V, Hainaut P. Understanding wild-type and mutant p53 activities in human cancer: new landmarks on the way to targeted therapies. Cancer Gene Ther 2011;18(1):2–11 doi 10.1038/cgt.2010.63. [DOI] [PubMed] [Google Scholar]
- 25.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer 2007;7(4):233–45 doi 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 26.Fosmire SP, Dickerson EB, Scott AM, Bianco SR, Pettengill MJ, Meylemans H, et al. Canine malignant hemangiosarcoma as a model of primitive angiogenic endothelium. Lab Invest 2004;84(5):562–72 doi 10.1038/labinvest.3700080. [DOI] [PubMed] [Google Scholar]
- 27.Dickerson EB, Thomas R, Fosmire SP, Lamerato-Kozicki AR, Bianco SR, Wojcieszyn JW, et al. Mutations of phosphatase and tensin homolog deleted from chromosome 10 in canine hemangiosarcoma. Vet Pathol 2005;42(5):618–32 doi 10.1354/vp.42-5-618. [DOI] [PubMed] [Google Scholar]
- 28.Tamburini BA, Trapp S, Phang TL, Schappa JT, Hunter LE, Modiano JF. Gene expression profiles of sporadic canine hemangiosarcoma are uniquely associated with breed. PLoS One 2009;4(5):e5549 doi 10.1371/journal.pone.0005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J-H, Graef A, Dickerson E, Modiano J. Pathobiology of Hemangiosarcoma in Dogs: Research Advances and Future Perspectives. Veterinary Sciences 2015;2(4):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudolph P, Peters J, Lorenz D, Schmidt D, Parwaresch R. Correlation between mitotic and Ki-67 labeling indices in paraffin-embedded carcinoma specimens. Hum Pathol 1998;29(11):1216–22. [DOI] [PubMed] [Google Scholar]
- 31.Tschida BR, Temiz NA, Kuka TP, Lee LA, Riordan JD, Tierrablanca CA, et al. Sleeping Beauty Insertional Mutagenesis in Mice Identifies Drivers of Steatosis-Associated Hepatic Tumors. Cancer Res 2017;77(23):6576–88 doi 10.1158/0008-5472.CAN-17-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonomura N, Elvers I, Thomas R, Megquier K, Turner-Maier J, Howald C, et al. Genome-wide association study identifies shared risk loci common to two malignancies in golden retrievers. PLoS Genet 2015;11(2):e1004922 doi 10.1371/journal.pgen.1004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borgatti A, Koopmeiners JS, Sarver AL, Winter AL, Stuebner K, Todhunter D, et al. Safe and Effective Sarcoma Therapy through Bispecific Targeting of EGFR and uPAR. Mol Cancer Ther 2017;16(5):956–65 doi 10.1158/1535-7163.MCT-16-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McPherson A, Hormozdiari F, Zayed A, Giuliany R, Ha G, Sun MG, et al. deFuse: an algorithm for gene fusion discovery in tumor RNA-Seq data. PLoS Comput Biol 2011;7(5):e1001138 doi 10.1371/journal.pcbi.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun 2014;5:4846 doi 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 2013;8(8):1494–512 doi 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013;4:2612 doi 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol 2017;18(1):220 doi 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JH, Frantz AM, Anderson KL, Graef AJ, Scott MC, Robinson S, et al. Interleukin-8 promotes canine hemangiosarcoma growth by regulating the tumor microenvironment. Experimental cell research 2014;323(1):155–64 doi 10.1016/j.yexcr.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson KL, Snyder KM, Ito D, Lins DC, Mills LJ, Weiskopf K, et al. Evolutionarily conserved resistance to phagocytosis observed in melanoma cells is insensitive to upregulation of pro-phagocytic signals and to CD47 blockade. Melanoma Res 2020;30(2):147–58 doi 10.1097/CMR.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas R, Duke SE, Karlsson EK, Evans A, Ellis P, Lindblad-Toh K, et al. A genome assembly-integrated dog 1 Mb BAC microarray: a cytogenetic resource for canine cancer studies and comparative genomic analysis. Cytogenet Genome Res 2008;122(2):110–21 doi 10.1159/000163088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29(1):15–21 doi 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas B, Dobin A, Stransky N, Li B, Yang X, Tickle T, et al. 2017. STAR-Fusion: Fast and Accurate Fusion Transcript Detection from RNA-Seq. bioRxiv <https://www.biorxiv.org/content/early/2017/03/24/120295>. [Google Scholar]
- 44.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009;25(16):2078–9 doi 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian X, Khammanivong A, Song JM, Teferi F, Upadhyaya P, Dickerson E, et al. RNA-sequencing studies identify genes differentially regulated during inflammation-driven lung tumorigenesis and targeted by chemopreventive agents. Inflamm Res 2015;64(5):343–61 doi 10.1007/s00011-015-0815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000;25(1):25–9 doi 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Gene Ontology C. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res 2019;47(D1):D330–D8 doi 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 2019;47(D1):D419–D26 doi 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez AM, Graef AJ, LeVine DN, Cohen IR, Modiano JF, Kim JH. Association of Sphingosine-1-phosphate (S1P)/S1P Receptor-1 Pathway with Cell Proliferation and Survival in Canine Hemangiosarcoma. J Vet Intern Med 2015;29(4):1088–97 doi 10.1111/jvim.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasserstein Ronald L., Lazar NA. The ASA's Statement on p-Values: Context, Process, and Purpose. The American Statistician 2016;70(2):129–33 doi 10.1080/00031305.2016.1154108. [DOI] [Google Scholar]
- 51.Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans--man and his best friend share more than companionship. Chromosome Res 2008;16(1):145–54 doi 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 52.Marley K, Maier CS, Helfand SC. Phosphotyrosine enrichment identifies focal adhesion kinase and other tyrosine kinases for targeting in canine hemangiosarcoma. Vet Comp Oncol 2012;10(3):214–22 doi 10.1111/j.1476-5829.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- 53.Mertens F, Johansson B, Fioretos T, Mitelman F. The emerging complexity of gene fusions in cancer. Nat Rev Cancer 2015;15(6):371–81 doi 10.1038/nrc3947. [DOI] [PubMed] [Google Scholar]
- 54.Yoshihara K, Wang Q, Torres-Garcia W, Zheng S, Vegesna R, Kim H, et al. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene 2015;34(37):4845–54 doi 10.1038/onc.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitelman F, Johansson B, Mertens F. 2020. Mitelman Database Chromosome Aberrations and Gene Fusions in Cancer. <https://mitelmandatabase.isb-cgc.org/>.
- 56.Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011;144(1):27–40 doi 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson ND, de Borja R, Young MD, Fuligni F, Rosic A, Roberts ND, et al. Rearrangement bursts generate canonical gene fusions in bone and soft tissue tumors. Science 2018;361(6405) doi 10.1126/science.aam8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee HK, Chauhan SK, Kay E, Dana R. Flt-1 regulates vascular endothelial cell migration via a protein tyrosine kinase-7-dependent pathway. Blood 2011;117(21):5762–71 doi 10.1182/blood-2010-09-306928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim P, Jia P, Zhao Z. Kinase impact assessment in the landscape of fusion genes that retain kinase domains: a pan-cancer study. Brief Bioinform 2018;19(3):450–60 doi 10.1093/bib/bbw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Medves S, Demoulin JB. Tyrosine kinase gene fusions in cancer: translating mechanisms into targeted therapies. J Cell Mol Med 2012;16(2):237–48 doi 10.1111/j.1582-4934.2011.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandey PR, Chatterjee B, Olanich ME, Khan J, Miettinen MM, Hewitt SM, et al. PAX3-FOXO1 is essential for tumour initiation and maintenance but not recurrence in a human myoblast model of rhabdomyosarcoma. J Pathol 2017;241(5):626–37 doi 10.1002/path.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selfe JL, Shipley J. Fusion gene addiction: can tumours be forced to give up the habit? J Pathol 2017;242(3):263–6 doi 10.1002/path.4902. [DOI] [PubMed] [Google Scholar]
- 63.Giacomini CP, Sun S, Varma S, Shain AH, Giacomini MM, Balagtas J, et al. Breakpoint analysis of transcriptional and genomic profiles uncovers novel gene fusions spanning multiple human cancer types. PLoS Genet 2013;9(4):e1003464 doi 10.1371/journal.pgen.1003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gru AA, Becker N, Pfeifer JD. Angiosarcoma of the parotid gland with a t(12;22) translocation creating a EWSR1-ATF1 fusion: a diagnostic dilemma. J Clin Pathol 2013;66(5):452–4 doi 10.1136/jclinpath-2012-201433. [DOI] [PubMed] [Google Scholar]
- 65.Shimozono N, Jinnin M, Masuzawa M, Masuzawa M, Wang Z, Hirano A, et al. NUP160-SLC43A3 is a novel recurrent fusion oncogene in angiosarcoma. Cancer Res 2015;75(21):4458–65 doi 10.1158/0008-5472.CAN-15-0418. [DOI] [PubMed] [Google Scholar]
- 66.Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer 2011;50(1):25–33 doi 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cornejo KM, Deng A, Wu H, Cosar EF, Khan A, St Cyr M, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol 2015;46(6):868–75 doi 10.1016/j.humpath.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 68.Beca F, Krings G, Chen YY, Hosfield EM, Vohra P, Sibley RK, et al. Primary mammary angiosarcomas harbor frequent mutations in KDR and PIK3CA and show evidence of distinct pathogenesis. Mod Pathol 2020;33(8):1518–26 doi 10.1038/s41379-020-0511-6. [DOI] [PubMed] [Google Scholar]
- 69.Chan JY, Lim JQ, Yeong J, Ravi V, Guan P, Boot A, et al. Multiomic analysis and immunoprofiling reveal distinct subtypes of human angiosarcoma. J Clin Invest 2020;130(11):5833–46 doi 10.1172/JCI139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Florou V, Rosenberg AE, Wieder E, Komanduri KV, Kolonias D, Uduman M, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer 2019;7(1):213 doi 10.1186/s40425-019-0689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dancsok AR, Gao D, Lee AF, Steigen SE, Blay JY, Thomas DM, et al. Tumor-associated macrophages and macrophage-related immune checkpoint expression in sarcomas. Oncoimmunology 2020;9(1):1747340 doi 10.1080/2162402X.2020.1747340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang G, Wu M, Maloneyhuss MA, Wojcik J, Durham AC, Mason NJ, et al. Actionable mutations in canine hemangiosarcoma. PLoS One 2017;12(11):e0188667 doi 10.1371/journal.pone.0188667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq gene expression data generated from human sarcomas are available from the TCGA Research Network (https://www.cancer.gov/tcga). Exome sequencing data from human angiosarcomas are available from The Angiosarcoma Project (https://ascproject.org), a project of Count Me In (https://joincountmein.org/). RNA-Seq data from human AS tissues are available through the Gene Expression Omnibus (GEO; http://www.ncbi.nlm. nih.gov/geo; accession number GSE163359). RNA-Seq data from canine HSA tissues are published (17,21,32,33) and available through the GEO (accession number GSE95183) and the NCBI Sequence Read Archive (accession number PRJNA562916). All other data generated from this study are available upon request to the corresponding author.