Abstract
Methylmercury (MeHg) is a persistent environmental neurotoxicant that may cause adverse neurodevelopmental effects. Previous studies showed that developmental MeHg exposure caused damage to brain functions that were unmasked after a silent period of years or decades. However, the underlying mechanisms of the latent neurotoxicity associated with MeHg exposure from earlier developmental stages have yet to be fully understood. Herein, we established a Caenorhabditis elegans (C. elegans) model of developmental MeHg latent toxicity. Synchronized L1 stage worms were exposed to MeHg (0, 0.05, 0.5 and 5 μM) for 48 h. Swimming moving speeds at adulthood were analyzed in worms exposed to MeHg exposure at early larvae stages. Worms developmentally exposed to MeHg had a significant decline in swimming moving speed on day 10 adult stage, but not on day 1 or 5 adult stage, even though the mercury level in the worms exposed to 0.05 or 0.5 μM MeHg were below the quantification limit on day 10 adult. Day 10 adult worms treated with MeHg showed a significant decrease in bending angle and bending frequency during swimming. Furthermore, their reduced moving speeds tended to increase during the 300-second swimming experiment. Dopamine signaling is known to be involved in the regulation of worms’ moving speed. Accordingly, the moving speed of worms with cat-2 (mammalian tyrosine hydroxylase homolog) mutation or dat-1 deletion were assayed on day 10 adult. The cat-2 mutant worms did not show a decline in moving speeds, body bends or bending angles during swimming on day 10 adult stage. Analyses of moving speeds of worms with dat-1 deletion showed that the moving speeds were further reduced after MeHg exposure. However, the effects of MeHg and dat-1 deletion were not synergistic, as the interaction between these parameters did not attain statistical significance. Altogether, our results suggest that developmental MeHg exposure reduced moving speed, and this latent toxicity was less pronounced in the context of deficient production of dopamine synthesis. Tyrosine hydroxylase plays an important role in regulating dopamine-mediated modulation of neurobehavioral functions. These findings uncovered a pivotal role of dopamine and its metabolism in the latent neurotoxic effects of MeHg.
Keywords: methylmercury, C. elegans, behavior, dopamine, tyrosine hydroxylase
Introduction
Methylmercury (MeHg) is a potent neurotoxicant and persistent organic form of mercury in the environment. Environmental sources of human exposure to MeHg come from consumption of fish that contains variable levels of MeHg. The acute toxicity of MeHg and thresholds for clinical symptoms were documented in follow-up studies during a MeHg poisoning outbreak in Iraq (Amin-zaki et al., 1978; Amin-Zaki et al., 1979; Bakir et al., 1973). However, the toxicity or biochemical effects of MeHg in humans at the upper range level of average background mercury contents have yet to be characterized.
A follow-up study on the outcomes of acute MeHg poisoning in Iraq showed that children had mild or moderate MeHg poisoning steadily recovered to normal functions of the brain. However, all the children still suffered a residual generalized hyperreflexia 2 years post exposure (Amin-zaki et al., 1978). Generally, the recovery rate of neurological signs was closely related with the severity of clinical stage of MeHg poisoning and level of blood mercury concentration (Amin-zaki et al., 1978). The neurologic signs and brain tissue damage of severe MeHg poisoning could last for decades as shown in the case study of a family accidentally exposed to MeHg by consumption of pork containing MeHg (Davis et al., 1994). In addition to the long-lasting effects of acute exposure, delayed MeHg neurotoxicity was observed in 13-year old monkeys with developmental MeHg exposure from birth to 7 years of age (Rice, 1996). Silent latency periods in MeHg poisoning were also noted in late onset Minamata disease cases (Igata, 1993). However, the Seychelles child cohort study did not show a positive link between prenatal and recent postnatal MeHg exposure and adult cognitive functions (van Wijngaarden et al., 2017).
In non-lethal moderate MeHg exposure, studies in primates and observations in humans suggested that there was a long latent period for the manifestation of chronic MeHg toxicity, and that the toxic endpoints of MeHg were masked for years or decades after cessation of exposure (Weiss et al., 2002). A recent follow-up study in surviving patients with congenital Minamata disease in Japan, showed that these patients had an accelerated decline in motor and cognitive function in their 50 to 60 years of age (Yorifuji et al., 2018). These studies suggested there was an age-dependent window during which the output of MeHg effects became apparent. It was hypothesized that the vulnerability to MeHg effects is dependent on the capacity of the brain to maintain homeostasis, for example vis-à-vis reactive oxygen species (ROS) generation, which declines over the aging process (Weiss et al., 2002). Overall, the mechanisms underlying latent effects of MeHg are much less clear.
The Caenorhabditis elegans (C. elegans) model system has several advantages for the study of latent biological, especially neurological, effects of developmental exposures on adult animals. To characterize latent toxicity of MeHg, the short life-span model organism is ideal for such purpose, allowing very short exposures (0-2 days) that cover the entire developmental period. Also C. elegans has a relatively short life span of around 25 days, thus latent effects can be studied within a single month of time. Further, the invariable neuron lineages during development allows precision in determination of the cellular systems targeted and responsible for latent phenotypes. The neurological system employs all major classes of neurotransmitters that are commonly found in mammals (Pereira et al., 2015). Our previous studies showed that the response of C. elegans to various levels of MeHg was comparable to humans (Caito and Aschner, 2016; Caito et al., 2013; Ke, Tao et al., 2020a). Notably, the dopaminergic (DAergic) system of C. elegans was an important target of MeHg, which caused morphological and functional alterations in DAergic neurons (Caito and Aschner, 2016; Ke, T. et al., 2020). In addition, the expression of the dopamine transporter dat-1 was repressed by developmental MeHg exposure (Ke, T. et al., 2020). These findings corroborated that the functional changes in DAergic neurons induced by MeHg could be mediated by the regulation of metabolism of dopamine, as reported in other studies (Tiernan et al., 2013; Tiernan et al., 2015).
Dopamine signaling regulates specific C. elegans behavioral outputs including swimming, locomotion, mechanosensory, defecation and egg-laying (McDonald et al., 2006). Genes that regulate dopamine metabolism modulate C. elegans behavior by altering dopamine-mediated signaling (Chase et al., 2004; Kindt et al., 2007). dat-1 regulates extracellular dopamine levels by transporting dopamine back into intracellular pool, establishing a feedback loop for the control of spillover of dopamine signaling (Carvelli et al., 2008). Dopamine antagonizes the stimulatory effect of acetylcholine signaling on muscle contraction (Correa et al., 2012). When worms encounter liquid environment, a steady rate of swimming movement is generated by contraction of body wall muscles. The importance of dopamine signaling in the swimming behavior was illustrated in worms with a null deletion of dat-1. These mutants gradually decreased their moving speed during swimming, supporting an inverse relationship between dopamine signaling and swimming speed (McDonald et al., 2007). Further investigations showed that over stimulation of the DOP-3 receptor by excess of dopamine in the synaptic cleft was the underlying mechanism of the inability to generate sustained swimming behavior in the dat-1 deletion strain (McDonald et al., 2007). The C. elegans homolog of the rate-limiting enzyme for dopamine synthesis, tyrosine hydroxylase, is cat-2. The involvement of dopamine signaling in swimming behaviors was also demonstrated in the studies in a cat-2 mutant strain, which had a diminished level of dopamine (McDonald et al., 2007; Smith et al., 2019). Worms with the null point mutation in cat-2 are dopamine deficiency; thereby their moving speed during swimming did not decease gradually (McDonald et al., 2007).
Herein, we established a developmental MeHg exposure model with C. elegans. The exposure started at the earliest larvae stage and ended at the last larvae stage, and the swimming behavior was analyzed during adulthood. By analyzing the behavior phenotypes of genetic modulation of dopamine signaling and developmental MeHg exposure, we reported that the latent alterations in swimming behavior upon MeHg exposure is modulated by dopamine signaling.
Materials and Methods
C. elegans strains and maintenance
The following C. elegans strains were used in the current study: the wild type N2 strain, and the cat-2 (e1112) null mutant CB1112 strain and dat-1 deletion RM2702 strain. All the strains were obtained from the Caenorhabditis Genetic Center (University of Minnesota). Worms were cultured on the standard nematode growth medium (NGM) plates seeded with OP50 food. Worms were maintained in a 20°C incubator. Synchronized larvae stage 1 (L1) worms were harvested from newly hatched eggs prepared by bleaching of adult stage gravid worms.
MeHg exposure
The chemical MeHgCl (Sigma-Aldrich) was dissolved in ddH2O to make 40× stock solutions, which was stored in a −20°C freezer. Before treatment, the stock MeHg solution was fully thawed at room temperature. Synchronized L1 stage worms were washed with NGM buffer (3 g NaCl, 2.5 g peptone, 975 ml H2O, 1 ml cholesterol (5 mg/ml in ethanol), 1 ml nystatin, 1ml 1M CaCl2, 1ml 1M MgSO4, 25ml 1M pH6 KPO4). The number of worms in a 10 μl sample of NGM buffer was counted under a stereo microscope (stemi 2000, Zeiss), which was adjusted to the concentration of 300 worms/87.5 μl by adding adequate volume of NGM buffer. To feed worms in NGM buffer, a 10× stock solution of overnight cultured OP50 (OD600=2.060 ± 0.008) was made by centrifugation and washing with NGM buffer. Lastly, 300 worms were treated in 100 μl NGM buffer (87.5 μl worms, 10 μl OP50 food and 2.5 μl MeHg) for 48 h in a sterile 96 well plate (costar, corning) on a shaker at 200 rpm and 20 °C.
Swimming recording
Following MeHg treatment, worms were washed with M9 buffer for 3 times. They were transferred to OP50 seeded 60 mm NGM plates. Worms exposed to 5 μM MeHg had a significant developmental delay (Ke, Tao et al., 2020b). However, a proportion of the worms (20%-30%) in the 5 μM MeHg group could reach to late L4 stage post treatment. For all groups, Late L4 stage worms were sampled. Twenty four hours (h) later, worms reached early adult stage, and were designated as day 1 adults. They were picked each day into fresh, OP50 seeded, 35 mm plates for the swimming experiment with day 5 and day 10 adult worms. In day 10 adult worms, only healthy worms without vulva protrusion, bacterial infection or locomotion defect were selected for the experiments.
Prior to the swimming experiments, plates with worms were blindly coded and placed on the bench at the room temperature of 18-22 °C for 5 min. A 60 mm NGM plate without OP50 was used as assay plate which was filled with 5 ml M9 buffer. The assay plate was placed at room temperature for 5 min. Individual worms were gently picked into the M9 buffer of the assay plate. The M9 buffer could immediately trigger worms to swim. To record the swimming speed of worms, a digital camera (DFK 72AUC02, USB 2.0 color industrial camera) controlled by the software (IC Capture 2.4, the Imaging Source) was mounted on the light path of a stereo microscope (stemi 2000, Zeiss). The assay plate was placed on the stage of the microscope. The magnification knob of the microscope was adjusted to ensure that the whole worm body was clearly imaged, and a desired magnification level was obtained for image processing by the WormLab software (MicroBrightField Inc.) (Roussel et al., 2014). Based on our experience, a higher magnification level ensures a more reliable tracking of worms. However, the assay plate should be constantly re-centered when worms were swimming. As outlined in the Wormlab software, re-centering of worms does not interfere with the analysis. After recording, worms were picked out of the M9 buffer. We found that the swimming speed of worms did not change in used M9 buffer compared with new M9 buffer. However, if a large clot of OP50 is present in the M9 buffer, it is highly likely that worms will start to eat the bacteria with their heads sticking towards the bacterial clot. To avoid this, any visible clot was removed and the M9 buffer was displaced with fresh buffer after three batches of tracking. A 10 cm ruler with a minimal scale of 1 mm was used as the reference for the magnification during swimming experiments. After tracking, worms were picked out. Each worm was recorded for 300s (5 min). 6-9 worms in each treatment group were analyzed and repeated three times. The key parameters for swimming experiment were identical for each recording, which included magnification level, exposure time, and No. of frames per second (fps).
Mercury measurement
For mercury measurement, 3,000 worms in 1,000 μl NGM buffer were treated in a 24-well plate. Following treatment, worms were equally allocated to three 100 mm NGM plates with 100 μM FUDR (5-Fluoro-2′-deoxyuridine). Each treatment had three repeats. When worms reached specific chronological ages (day 1, 5 and 10 adult stages), they were harvested for whole-worm protein extraction. Worms pellets were treated with lysis buffer (50 mM pH 8.0 Tris HCI, 0.5 mM NaCI, 4 mM EDTA and 1 %(v/v) NP-40) and sonication at 20 Hz for 5 s twice for each sample. The protein concentration was determined with BCA method (ThermoFisher scientific). The samples containing three repeats were analyzed with single wavelength atomic absorption specific to mercury (254nm), with the quantification limit of 0.5 ng (DMA-80, Milestone).
Statistical analysis
The swimming speed data was automatically calculated by the software WormLab based on the frame by frame comparison of the moving distance of the worm body’s center point. Sometimes, the software mistakenly identified a worm’s head as tail or vice versa; however, this affected only the direction of speed, but not the absolute values of speed. To exclude this effect, all speed data were converted to absolute values before statistical analysis. To compare the average speed for the 5 min (300 s) recording period, a 5-s period data was extracted and combined from 6-9 recordings. The analyses of moving speed, body bends and bending angles were performed with one-way ANOVA and Tukey’s multiple comparisons test, whereas a t-test for a two-sample comparison was adopted after the Shapiro-Wilk test for normality. To analyze the effect of the cat-2 and dat-1 genes, two-way ANOVA was performed (GraphPad 8.4.3). Data were expressed as mean ± SD. P<0.05 was designated as the significant level.
Results
To investigate the latent effects of MeHg exposure on adult worms following exposures at early developmental stages, we established a new C. elegans exposure model by treating worms at the young larvae stages. In this model, synchronized L1 stage worms were treated with MeHg for 48 h, and their swimming speeds were assayed when on days 1, 5 and 10 adult stages (Fig. 1).
Figure 1.
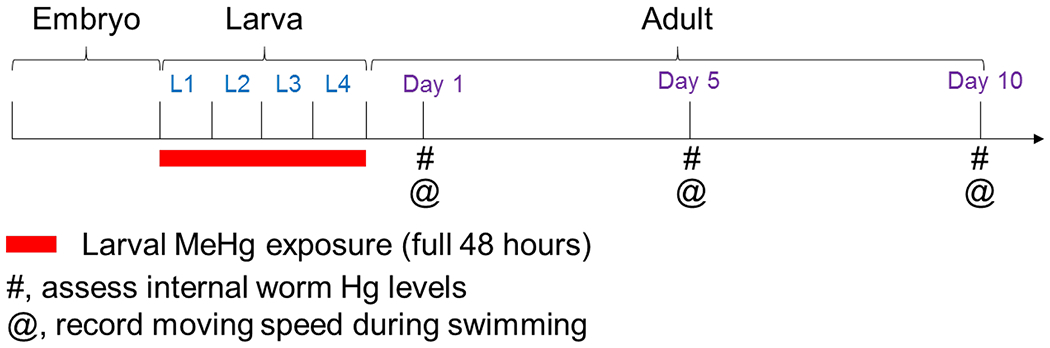
Timeline of study design. Representative figure shows that the 48-h MeHg exposure spans across four larva stages (L1-L4). 24 h post exposure, worms were on day 1 adult stage. Mercury measurements and swimming assessment were carried out in worms on days 1, 5 and 10 adult stages.
To determine the intra-worm mercury contents following larvae stage MeHg exposure, day 1, 5 and 10 adult stage worms were collected for the determination of mercury levels using an atomic absorption method. Worms with 0 or 0.05 μM MeHg had mercury levels below the 0.5 ng limit of detection. However, in worms treated with 5 μM MeHg, mercury levels on day 1 adult were significantly higher than on days 5 and 10 adult stages. The mercury level in day 5 adult had no significant difference as compared with day 10 adult following 5 μM MeHg exposure. In worms with 0.5 μM MeHg, the body mercury level was detectable only on day 1 adult stage, which was significantly lower than worms of day 1 adult stage following 5 μM MeHg exposure (Table 1).
Table 1.
Intra-worm mercury levels (ng Hg/mg protein) in adult stage worms following developmental MeHg exposure.
| Adult stage |
|||
|---|---|---|---|
| MeHg, μM | Day 1 | Day 5 | Day 10 |
| 0 | b.q. | b.q. | b.q. |
| 0.05 | b.q. | b.q. | b.q. |
| 0.5 | 0.397# | b.q. | b.q. |
| 5 | 31.020 | 6.775* | 2.827* |
The mercury contents in adult stage worms were measured with a single wavelength atomic absorption specific to mercury, “b.d.” denotes mercury content below the quantification limit of 0.5 ng Hg. Analyses were performed with one-way ANOVA and Tukey’s multiple comparisons test.
p<0.05 compared with day 1 adult stage.
p<0.001 compared with day 1 adult worms with 5 μM MeHg.
Multiple comparisons of swimming speeds of worms with different MeHg doses showed that day 10 adult worms showed a significant decline in swimming speeds (Fig. 2c). Specifically, in a 5-min swimming experiment, worms treated with MeHg showed a significantly slower swimming speed during the early, middle and late phases of swimming, as compared to control worms. However, the swimming speeds among worms treated with different doses of MeHg were statistically indistinguishable (Fig. 2). This absence of a dose-dependency is also consistent with reports on the latent effects of mercury in humans and other experimental model systems (Lam et al., 2013; Weiss et al., 2002).
Figure 2.
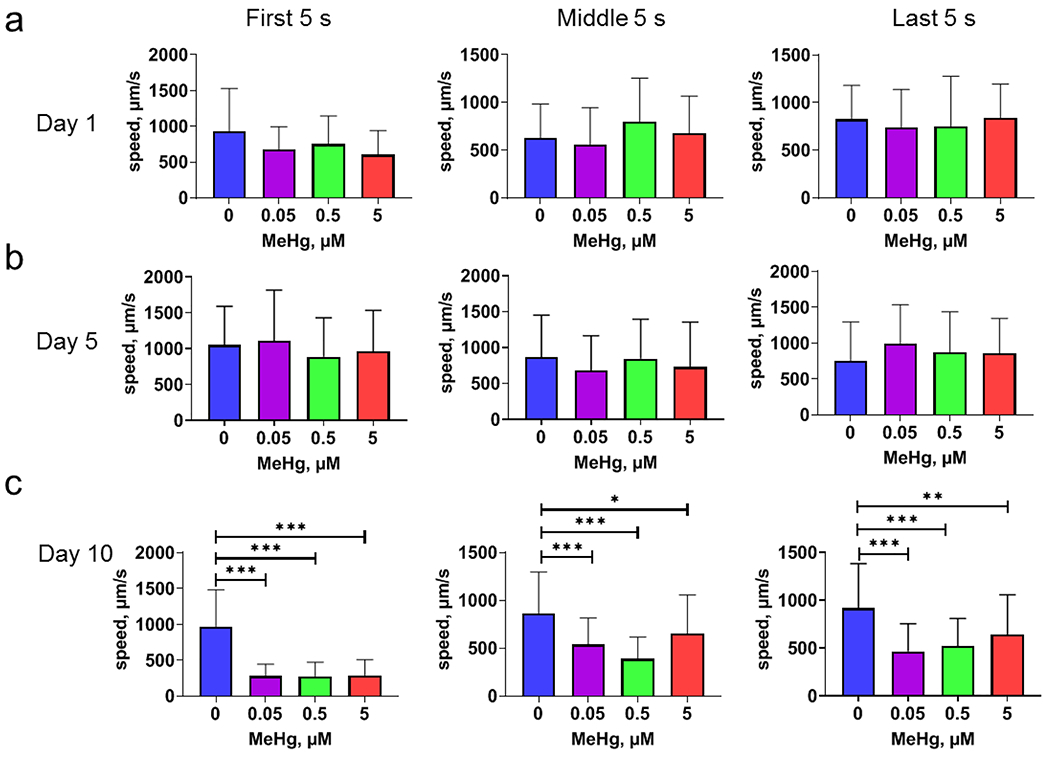
Developmental MeHg exposure is associated with time-dependent decline in swimming speed. Synchronized L1 stage N2 worms were exposed with various concentrations of MeHg for 48 h. Following exposure, adult stage worms were recorded for swimming speed. a, swimming speed of day 1 adult worms during a 300 s recording period. Left panel: the average swimming speed during 0-5 s. Middle panel: the average swimming speed during 150-155 s. Right panel: the average swimming speed during 295-300 s. b, swimming speed of day 5 adult worms. c. swimming speed on day 10 adult worms. For comparison, all speed data was converted to absolute values before analysis. Analyses were made with one-way ANOVA and Tukey’s multiple comparisons test. * p<0.05; ** p<0.01; *** p<0.001.
During the 5-min swimming experiment, the control day 10 adult worms did not show a significant change in the early phase compared with the late phase swimming speed (Fig. 3a). However, in worms treated with MeHg, the swimming speeds trended higher during the 5-min swimming experiment. There was a significant increase in the swimming speed at the last phase compared to the first phase of swimming (Fig. 3). For day 1 and 5 adult worms, their swimming speeds did not change over time (Fig. S1).
Figure 3.
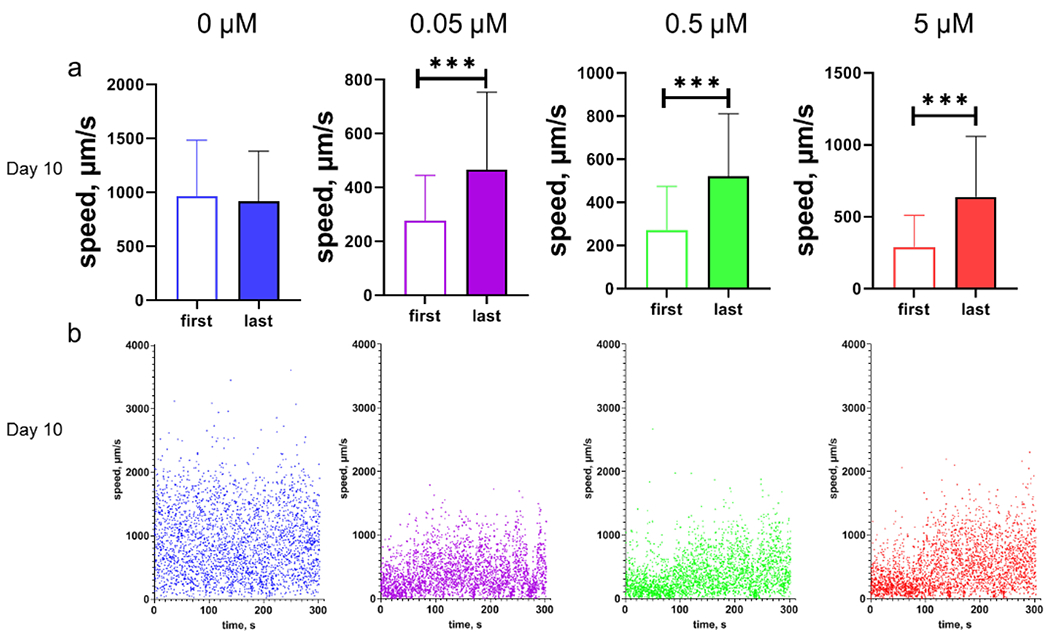
The swimming speeds of day 10 adult worms with MeHg increase with time. a, swimming speeds of worms during the first (the first 5 seconds) phase and last (the last 5 seconds) phase of swimming. The dataset is part from Fig. 2 c. b, representatives of swimming speed of day 10 adult worms with developmental MeHg exposure. Each dot represents moving speed of one worm at every 0.1s interval. The parameters used to calculate swimming speed were recorded with the software WormLab for worms swimming in M9 buffer. Swimming speed was calculated as the moving distance of the center point of longitudinal axis of the worm’s body per time unit (0.1 s). All speed data were converted to absolute values. Analyses in a were performed with t-test. *** p<0.001
The gene cat-2 is the C. elegans homolog of mammalian gene for tyrosine hydroxylase, the rate-limiting enzyme for in vivo dopamine synthesis (Sulston et al., 1975). The CB1112 strain harbors a single mutation of cat-2, and they have a diminished level of dopamine (Sulston et al., 1975). The worms of the CB1112 strain with MeHg did not slow down during a 5-min swimming experiment compared to the control worms (Fig. 4a and S2). Two-way ANOVA analysis shows that there was a significant interactive effect between MeHg and the cat-2 mutation (Fig. 4d).
Figure 4.
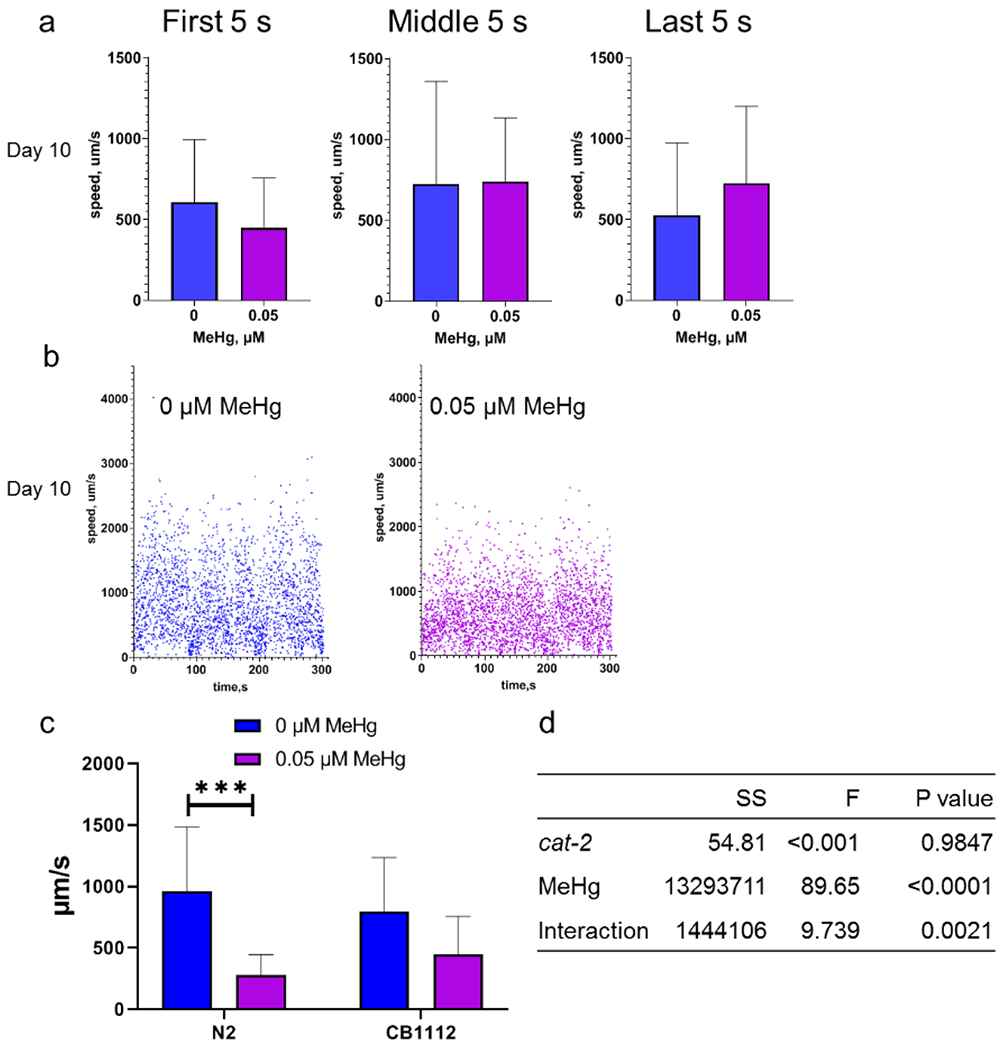
The time-dependent decline in swimming speed is modified with the cat-2 mutation. a, the average swimming speed of day 10 adult worms of the CB1112 strain. Left panel: the average swimming speed during 0-5 s. Middle panel: the average swimming speed during 150-155 s. Right panel: the average swimming speed during 295-300 s. b, representatives of swimming speed of day 10 adult worms during 0-300 s. c, moving speeds of the cat-2 mutant and N2 worms during the 15 s of swimming (the first 5 s, middle 5 s and last 5 s) on day 10 adult stage. d, statistical analysis by two-way ANOVA analysis of swimming data in c.
To further investigate whether the effect of MeHg on the swimming speed was modified by the dopamine transporter: dat-1, the dat-1 partial deletion strain (RM2702) was evaluated for swimming speeds following MeHg exposure. Day 10 adult worms of the RM2702 strain had a significant decline in swimming speeds after MeHg exposure (Fig. 5b and S3). Particularly, in worms absent MeHg exposure, swimming speeds gradually slowed down. However, in MeHg-exposed worms, swimming speeds were unchanged over time. Two-way ANOVA analysis showed that both the dat-1 deletion and MeHg had a significant effect on swimming speeds, however their effects were not synergistic as the interactive term did not attain significance (Fig. 5e). Gene expression analyses of the day 10 adult N2 worms did not reveal a consistent pattern in the expression of dat-1 or cat-2 (Fig. S4).
Figure 5.
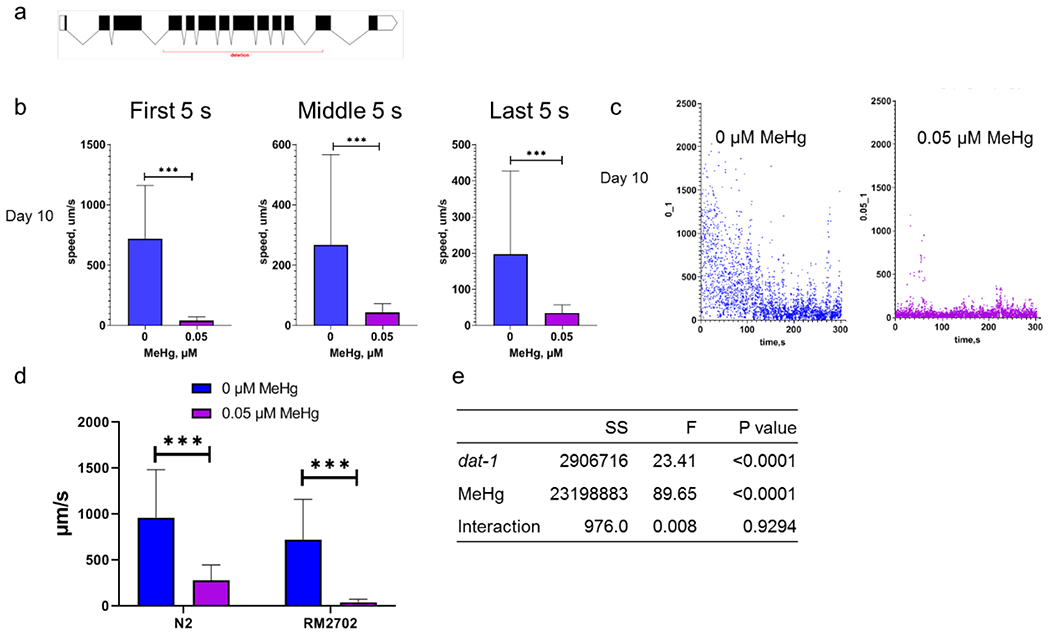
The decline in swimming speed by MeHg is not modified with the dat-1 deletion. a, schematic representation of the genomic region with dat-1 deletion (red underline). b, the average swimming speed of day 10 adult worms of the dat-1 mutant strain RM2702. Left panel: the average swimming speed during 0-5 s. Middle panel: the average swimming speed during 150-155 s. Right panel: the average swimming speed during 295-300 s. c. representatives of swimming speed of day 10 adult worms during 0-300 s. d, moving speeds of the dat-1 mutant and N2 worms during the 15 s of swimming (the first 5 s, middle 5 s and last 5 s) on day 10 adult stage. e, statistics of two-way ANOVA analysis of swimming data in d.
The swimming motion of C. elegans in liquid is propelled by a rhythmic bending of their body along the head-to-tail axis. Analysis of body bends shows that, in the wild type N2 worms, the average number of body bends were significantly reduced on day 10 adult stage following MeHg exposure at young larvae stages (Fig. 6 a). Bending angle during swimming was defined as the angle between the midpoint-head segment and the midpoint-tail segment. In addition, the average bend angle of body bends during swimming was also decreased in worms treated with MeHg (Fig. 6b and 6c). In the CB1112 strain, worms with MeHg did not show a significant decline in average body bends. Unexpectedly, the body bends were not decreased in worms of the RM2702 strain treated with MeHg (Fig. 7), although they showed a significant reduction in swimming speed (Fig. 5).
Figure 6.
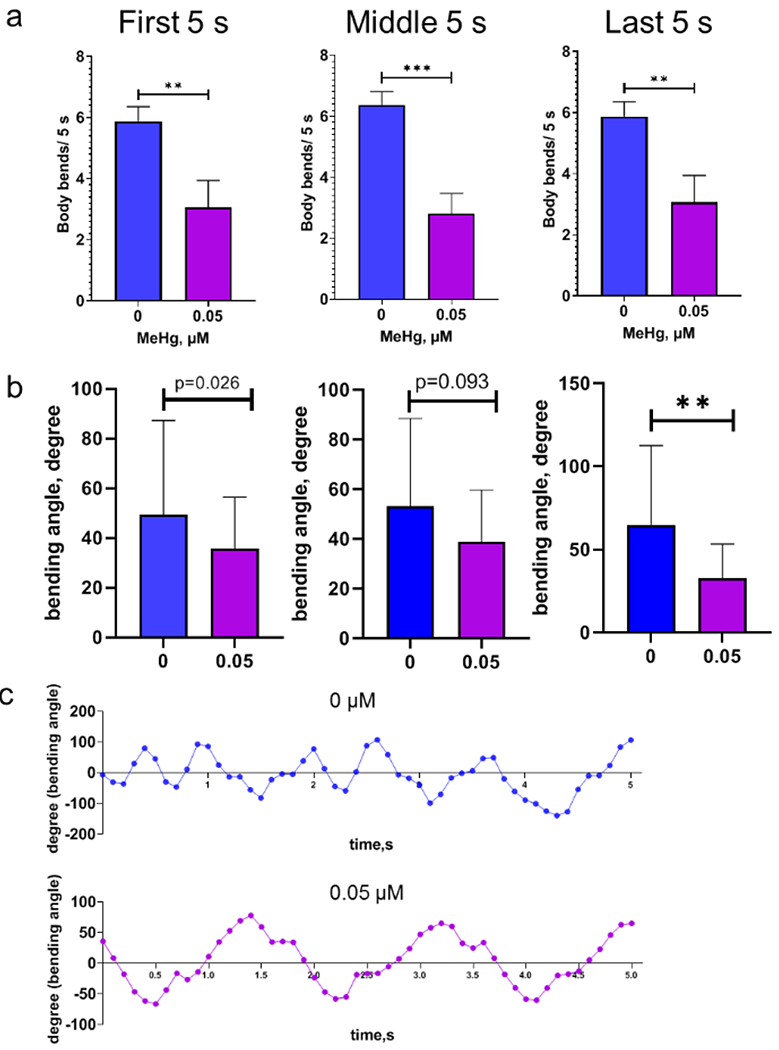
Body bends during swimming is reduced in the N2 wild type worms with developmental MeHg exposure. Day 10 adult worms of the wild type strain were recorded for body bends during a 300-s period. a, average body bends of worms during 0-5 s (first 5 s), 150-155 s (middle 5 s) and 295-300 s (last 5 s) of the swimming period. b. average bending angles of worms during the swimming period. c, representative of bend angles of worms with 0 or 0.05 μM MeHg. Analyses in a and b were performed with t-test. ** p<0.01; *** p<0.001.
Figure 7.
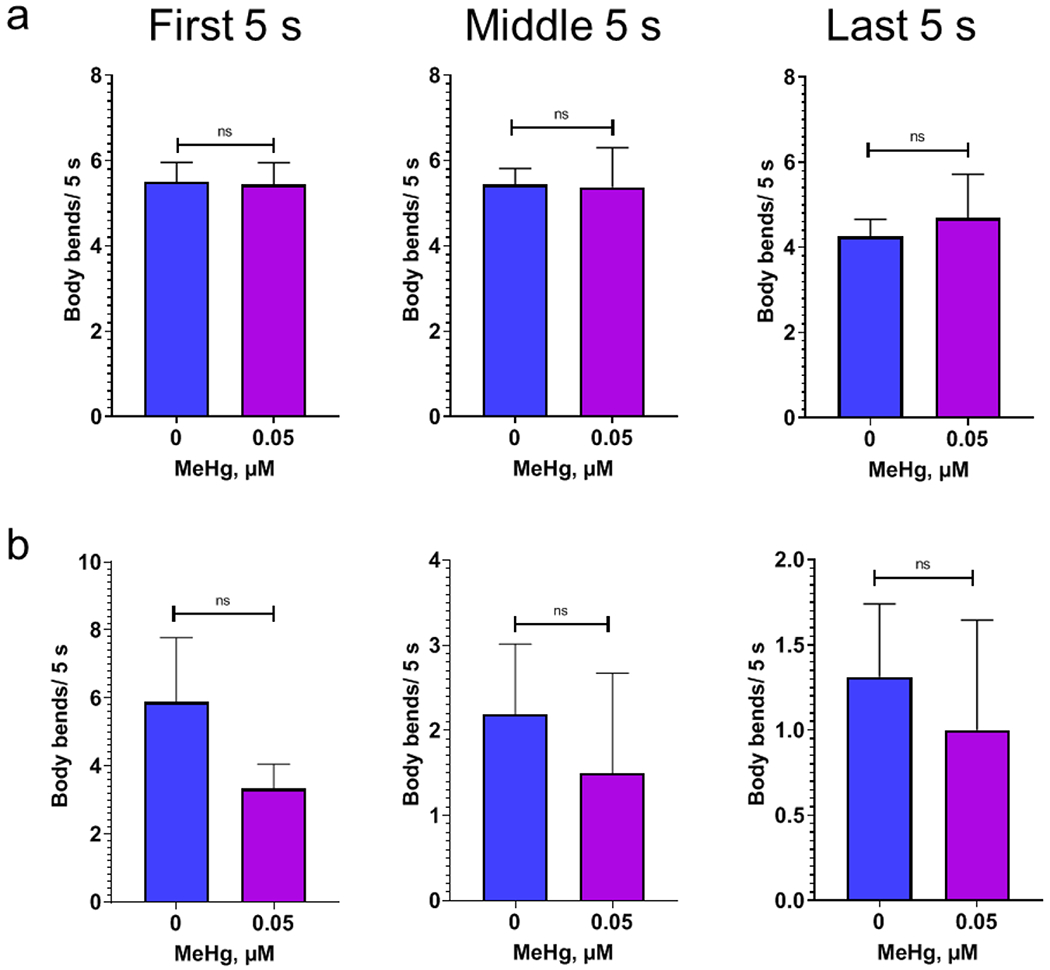
Body bends during swimming is not reduced in the worms with the cat-2 mutation or dat-1 deletion. a, average body bends of the worms with mutant cat-2 (CB1112) during 0-5 s (first 5 s), 150-155 s (middle 5 s) and 295-300 s (last 5 s) of the swimming period. b, average body bends of the worms with dat-1 deletion (CB1112) during the periods as shown in a. Analysis was performed with t-test.
In the RM2702 strain, the average bending angle significantly decreased after MeHg exposure (Fig. 8a), and the maximum bending angle dropped from ~50 degrees in the control worms to ~5 degrees in the worms with MeHg (Fig. S5). In the CB1112 strain, the average bending angle was not significantly decreased (Fig. 8b and S5). The parameters of swimming movement in the N2, CB1112 and RM2702 worms were summarized in table 2.
Figure 8.
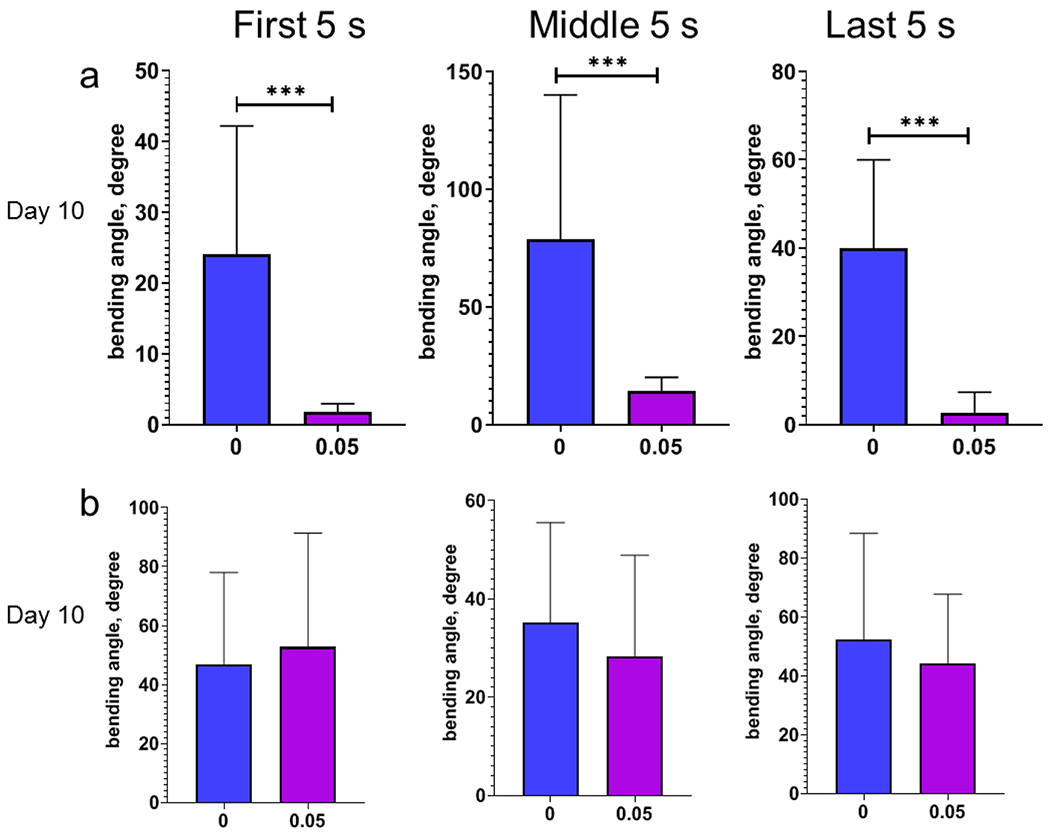
Bending angles of the worms with dat-1 deletion during swimming is reduced following developmental MeHg exposure. a, average bending angles of the day 10 adult worms of the RM2702 strain with the dat-1 deletion during the swimming period. b, average bending angles of the day 10 adult worms of the CB1112 strain with the cat-2 mutation during the swimming period.
Table 2.
Summary of swimming parameters of the worms in the swimming experiments.
| N2 | CB1112 | RM2702 | |
|---|---|---|---|
| Moving speed | decrease | no change | decrease |
| Body bend | decrease | no change | no change |
| Bending angle | decrease | no change | decrease |
Discussion
With longitudinal tracking of swimming behavior of C. elegans with developmental MeHg exposure, we showed, for the first time, detection of latent MeHg-induced neurotoxicity on day 10 adult worms, which was absent at earlier time points (Fig. 2). Both bending frequency and bending angle during swimming was reduced in these day 10 adult worms exposed to MeHg in the larval stages (Fig. 6 and 8). The effects of MeHg on the moving speed were modified by a null point mutation in cat-2, but independent of dat-1. The cat-2 mutant worms did not exhibit decreased moving speeds on day 10 adult stage. Our results established a necessary role for cat-2 in the latent effects of MeHg, that became apparent after nearly a half lifetime window (10 days) following exposure, supporting the long-term latency theory for chronic MeHg toxicity (Weiss et al., 2002).
Our results suggest that the effects of developmental MeHg exposure manifested in day 10 adult worms (Fig. 2), which was long after internal mercury levels dropped below detection for at least two of the doses (Table 1). Furthermore, even at the highest dose of 5 μM, where levels remained detectable, the magnitude of the toxic effect was not stronger, thus supporting an interpretation that it was the earlier developmental levels that led to the later behavior effects. In a previous study, it showed that the internal mercury level of worms exposed to 5 μM was 2-3 ng Hg/μg protein (Wyatt et al., 2016). Our results showed that the mercury level in day 1 adult 24 h post the 48-h MeHg exposure (5 μM) was 0.031 ng Hg/μg protein (table 1), suggesting most of mercury was removed during the 24 h post treatment. We had previously shown that worms exposed to MeHg with live bacteria had more severe damages than worms exposed with dead bacteria (Ke, Tao et al., 2020b), as live bacteria probably concentrated MeHg and biomagnified mercury in worms. Therefore, the internal mercury dose in the worms immediately after MeHg exposure should be higher in our study than Wyatt’s, as the worms were exposed to MeHg with live bacteria. In Wyatt’s study, worms were exposed to MeHg with dead bacteria killed by UV (Wyatt et al., 2016).
Previous studies showed that monkeys that exposed to MeHg from birth to 7 years of age showed apparent slowness and clumsiness in their motions when they reached 13 years of age (Rice, 1996). In these monkeys, the steady-state blood mercury concentration was 0.7 ppm at 7 years of age, which can be converted to the mercury level at 3.5 ng Hg/mg protein (Aschner, 2012). Based on our results (Table 1), it can be inferred that the mercury levels far below 3.5 ng Hg/mg protein could cause neurobehavior damages that manifest in older age. Our results are also consistent with the mouse study showed that mice with in utero MeHg exposure exhibited abnormal swimming movements and posture at later developmental stage (Spyker, 1975). In the mouse study, pregnant mice were injected with 8 ppm MeHg dicyandiamide, followed by locomotion and swimming tests in the offspring at 30-day-old. During the 10-min swimming test, it showed that the mice in the MeHg-treated group had a high frequency of incoordination and impaired swimming ability such as: immobility in the water with all legs extended for 2 min; floating in a vertical position with head above water; or swimming with legs askew (Spyker et al., 1972).
Our observations are also consistent with recent observations that congenital Minamata disease patients experienced accelerated decline of brain function at 50- or 60-years-of-age (Yorifuji et al., 2018). In addition, animal studies with gestational exposure to MeHg showed that MeHg induced neurobehavioral abnormities were unmasked during aging (Newland and Rasmussen, 2000; Newland et al., 2004). Furthermore, in the Iraq outbreak, the length of latency period does not correlate with blood mercury levels (Bakir et al., 1973). It was proposed that the considerable redundancy in brain functions determines that the long-term adverse effects of MeHg to the brain only emerge when the compensatory capacity is exhausted (Weiss et al., 2002). Consistent with this hypothesis, our data suggests that MeHg unmasks a developmentally vulnerable target that leads to a latent toxicity that manifests only later in adult life stages.
Aged worms showed a marked decrease in locomotion rates (Newell Stamper et al., 2018). On day 10 adult stage, nearly half the worms died due to bacterial infection, and the remaining worms available for swimming experiment could have variable degrees of muscle degeneration, as reported in an earlier study (Herndon et al., 2002). However, the swimming moving speeds in day 10 adult worms did not significantly decline (Fig. 2). This might be related to the fact that only healthy worms that did not have locomotion defects on agar plates were selected for the swimming experiments. In day 10 adult N2 worms, the moving speeds in the late swimming phase tended to increase compared to the early phase (Fig. 3), suggesting that there was a feedback loop, possibly mediated by increased acetylcholine signaling, to mitigate the effects of MeHg in wild type animals. However, overstimulation of acetylcholine signaling might have caused paralysis in worms (Ghosh and Emmons, 2008). Further investigations into the possible role of acetylcholine in the swimming behavior are warranted.
Our previously published work has shown that developmental MeHg exposure resulted in a significant decrease in the expression of the transgenic construct dat-1p::mCherry, suggesting the transcriptional activity of dat-1 was repressed in worms with MeHg (Ke, T. et al., 2020). An earlier study on the isolated membrane fraction of rat striatum described that MeHg inhibited the interaction of DAT-1 and dopamine, impeding uptake of extracellular dopamine via DAT-1 (Bonnet et al., 1994). However, our results failed to show whether the latent effects of MeHg on the moving speed were dat-1-dependent (Fig. 5). In addition, dopamine metabolism is also an established target for MeHg. An in vitro study with undifferentiated pheochromocytoma (PC12) cells demonstrated that the threshold level of MeHg for the induction of dopamine release was around 2 μM. Furthermore, increased dopamine release resulted from increased levels of dopamine synthesis and vesicular exocytosis by MeHg (Tiernan et al., 2013). Furthermore, in the same model, MeHg was shown to inhibit the dopamine metabolizing enzyme aldehyde dehydrogenase (ALDH) by depleting the ALDH cofactor NAD(+) (Tiernan et al., 2015). Our results are consistent with these observations, corroborating that dopamine metabolism modifies MeHg’s effects on the slowing down of swimming speed (Fig. 4), supporting a critical role for dopamine metabolism in the latent effects of MeHg.
The predicted product of cat-2 is the C. elegans homolog of tyrosine hydroxylase, which is based on the facts that the defect in the basal slowing response of the cat-2 (e1112) mutant can be rescued by dopamine pretreatment (Sawin et al., 2000) and the mutant worms had a diminished level of dopamine in vivo (Sulston et al., 1975). The tyrosine hydroxylase inhibitor α-methyltyrosine completely abolished dopamine release by 2 μM MeHg in PC12 cells (Tiernan et al., 2013). Furthermore, the effect of d-amphetamine, a dopamine release stimulator, was potentiated in adult rats with developmental MeHg exposure (Rasmussen and Newland, 2001). In addition, the expression of dopamine receptors showed a time-dependent decline during adulthood (Seeman et al., 1987; Volkow et al., 2000). Analogous to the function of mammalian dopamine receptors, the C. elegans D1-like receptor dop-1 antagonizes the locomotion-slowing effect of the D2-like receptor dop-3 (Chase et al., 2004). Based on the current study, we speculated that tyrosine hydroxylase could be a target of MeHg or metabolized inorganic mercury species in vivo (Friberg and Mottet, 1989), and that the effects could emerge only when the ratio of the dopamine receptors (D1/D2) deceased in older adults (Seeman et al., 1987), favoring a dominant slowing effect of D2-receptors on locomotion. Structural analysis showed that tyrosine hydroxylases form homotetramers with a-helical basket in the C-terminal region holding the catalytic iron (Goodwill et al., 1997). The effects are likely attributed to a direct stimulatory action on the active catalytic domain of tyrosine hydroxylase, though further in vitro analysis of catalytic activity of tyrosine hydroxylase with different mercury species may resolve the molecular basis of these modulating effects.
The neurotoxicity of MeHg in C. elegans mimics several important features inherent to MeHg-exposed humans, including growth retardation and neurobehavioral abnormities (Caito and Aschner, 2016; Ke and Aschner, 2019). Notably, our results highlight the importance of dopamine metabolism in latent alterations in neurobehaviors following developmental MeHg exposure, at exceedingly low doses (0.05 μM) that do not invoke overt antioxidant responses (Ke and Aschner, 2019). We demonstrated that the effects of MeHg on moving speeds can be suppressed by a cat-2 mutation. Further investigations on the contribution of altered tyrosine hydroxylase structure and function in mediating MeHg-induced neurotoxicity are warranted.
Supplementary Material
Highlights.
Developmental MeHg exposure reduces swimming moving speeds at later adult stage in C. elegans.
The C. elegans homolog of tyrosine hydroxylase modifies the effect of MeHg on swimming moving speeds.
Acknowledgments
This work was supported by the National Institutes of Health to MA and ABB (NIEHS R01ES007331). Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary data
References
- Amin-zaki L, Majeed MA, Clarkson TW, Greenwood MR, 1978. Methylmercury poisoning in Iraqi children: clinical observations over two years. Br Med J 1(6113), 613–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Zaki L, Majeed MA, Elhassani SB, Clarkson TW, Greenwood MR, Doherty RA, 1979. Prenatal methylmercury poisoning. Clinical observations over five years. American journal of diseases of children (1960) 133(2), 172–177. [PubMed] [Google Scholar]
- Aschner M, 2012. Considerations on methylmercury (MeHg) treatments in in vitro studies. Neurotoxicology 33(3), 512–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA, 1973. Methylmercury poisoning in Iraq. Science 181(4096), 230–241. [DOI] [PubMed] [Google Scholar]
- Bonnet JJ, Benmansour S, Amejdki-Chab N, Costentin J, 1994. Effect of CH3HgCl and several transition metals on the dopamine neuronal carrier; peculiar behaviour of Zn2+. Eur J Pharmacol 266(1), 87–97. [DOI] [PubMed] [Google Scholar]
- Caito SW, Aschner M, 2016. NAD+ Supplementation Attenuates Methylmercury Dopaminergic and Mitochondrial Toxicity in Caenorhabditis Elegans. Toxicol Sci 151(1), 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito SW, Zhang Y, Aschner M, 2013. Involvement of AAT transporters in methylmercury toxicity in Caenorhabditis elegans. Biochem Biophys Res Commun 435(4), 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Blakely RD, DeFelice LJ, 2008. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci U S A 105(37), 14192–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR, 2004. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci 7(10), 1096–1103. [DOI] [PubMed] [Google Scholar]
- Correa P, LeBoeuf B, García LR, 2012. C. elegans dopaminergic D2-like receptors delimit recurrent cholinergic-mediated motor programs during a goal-oriented behavior. PLoS Genet 8(11), e1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Kornfeld M, Mooney HS, Fiedler KJ, Haaland KY, Orrison WW, Cernichiari E, Clarkson TW, 1994. Methylmercury poisoning: long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann Neurol 35(6), 680–688. [DOI] [PubMed] [Google Scholar]
- Friberg L, Mottet NK, 1989. Accumulation of methylmercury and inorganic mercury in the brain. Biol Trace Elem Res 21, 201–206. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Emmons SW, 2008. Episodic swimming behavior in the nematode C. elegans. J Exp Biol 211(Pt 23), 3703–3711. [DOI] [PubMed] [Google Scholar]
- Goodwill KE, Sabatier C, Marks C, Raag R, Fitzpatrick PF, Stevens RC, 1997. Crystal structure of tyrosine hydroxylase at 2.3 A and its implications for inherited neurodegenerative diseases. Nat Struct Biol 4(7), 578–585. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M, 2002. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419(6909), 808–814. [DOI] [PubMed] [Google Scholar]
- Igata A, 1993. Epidemiological and clinical features of Minamata disease. Environ Res 63(1), 157–169. [DOI] [PubMed] [Google Scholar]
- Ke T, Aschner M, 2019. Bacteria affect Caenorhabditis elegans responses to MeHg toxicity. Neurotoxicology 75, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke T, Bornhorst J, Schwerdtle T, Santamaría A, Soare FAA, Rocha JB, Farina M, Bowman AB, Aschner MJNR, 2020a. Therapeutic Efficacy of the N, N′ Bis-(2-Mercaptoethyl) Isophthalamide Chelator for Methylmercury Intoxication in Caenorhabditis elegans. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke T, Tsatsakis A, Santamaría A, Antunes Soare FA, Tinkov AA, Docea AO, Skalny A, Bowman AB, Aschner M, 2020. Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. Neurotoxicology 77, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke T, Tsatsakis A, Santamaría A, Soare FAA, Tinkov AA, Docea AO, Skalny A, Bowman AB, Aschner MJN, 2020b. Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR, 2007. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55(4), 662–676. [DOI] [PubMed] [Google Scholar]
- Lam HS, Kwok KM, Chan PH, So HK, Li AM, Ng PC, Fok TF, 2013. Long term neurocognitive impact of low dose prenatal methylmercury exposure in Hong Kong. Environ Int 54, 59–64. [DOI] [PubMed] [Google Scholar]
- McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD, 2007. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci 27(51), 14216–14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PW, Jessen T, Field JR, Blakely RD, 2006. Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol 26(4–6), 593–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell Stamper BL, Cypser JR, Kechris K, Kitzenberg DA, Tedesco PM, Johnson TE, 2018. Movement decline across lifespan of Caenorhabditis elegans mutants in the insulin/insulin-like signaling pathway. Aging Cell 17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Rasmussen EB, 2000. Aging unmasks adverse effects of gestational exposure to methylmercury in rats. Neurotoxicol Teratol 22(6), 819–828. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA, Langston JL, 2004. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol 26(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Pereira L, Kratsios P, Serrano-Saiz E, Sheftel H, Mayo AE, Hall DH, White JG, LeBoeuf B, Garcia LR, Alon U, Hobert O, 2015. A cellular and regulatory map of the cholinergic nervous system of C. elegans. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, Newland MC, 2001. Developmental exposure to methylmercury alters behavioral sensitivity to D-amphetamine and pentobarbital in adult rats. Neurotoxicol Teratol 23(1), 45–55. [DOI] [PubMed] [Google Scholar]
- Rice DC, 1996. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicology 17(3-4), 583–596. [PubMed] [Google Scholar]
- Roussel N, Sprenger J, Tappan SJ, Glaser JR, 2014. Robust tracking and quantification of C. elegans body shape and locomotion through coiling, entanglement, and omega bends. Worm 3(4), e982437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR, 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26(3), 619–631. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jellinger K, Watanabe S, et al. , 1987. Human brain dopamine receptors in children and aging adults. Synapse 1(5), 399–404. [DOI] [PubMed] [Google Scholar]
- Smith LL, Ryde IT, Hartman JH, Romersi RF, Markovich Z, Meyer JN, 2019. Strengths and limitations of morphological and behavioral analyses in detecting dopaminergic deficiency in Caenorhabditis elegans. Neurotoxicology 74, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyker JM, 1975. Assessing the impact of low level chemicals on development: behavioral and latent effects. Fed Proc 34(9), 1835–1844. [PubMed] [Google Scholar]
- Spyker JM, Sparber SB, Goldberg AM, 1972. Subtle consequences of methylmercury exposure: behavioral deviations in offspring of treated mothers. Science 177(4049), 621–623. [DOI] [PubMed] [Google Scholar]
- Sulston J, Dew M, Brenner S, 1975. Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163(2), 215–226. [DOI] [PubMed] [Google Scholar]
- Tiernan CT, Edwin EA, Goudreau JL, Atchison WD, Lookingland KJ, 2013. The role of de novo catecholamine synthesis in mediating methylmercury-induced vesicular dopamine release from rat pheochromocytoma (PC12) cells. Toxicol Sci 133(1), 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiernan CT, Edwin EA, Hawong HY, Rios-Cabanillas M, Goudreau JL, Atchison WD, Lookingland KJ, 2015. Methylmercury impairs canonical dopamine metabolism in rat undifferentiated pheochromocytoma (PC12) cells by indirect inhibition of aldehyde dehydrogenase. Toxicol Sci 144(2), 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijngaarden E, Thurston SW, Myers GJ, Harrington D, Cory-Slechta DA, Strain JJ, Watson GE, Zareba G, Love T, Henderson J, Shamlaye CF, Davidson PW, 2017. Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24years. Neurotoxicol Teratol 59, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, Felder C, Gatley SJ, Ding YS, Hitzemann R, Pappas N, 2000. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry 157(1), 75–80. [DOI] [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W, 2002. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect 110 Suppl 5, 851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt LH, Diringer SE, Rogers LA, Hsu-Kim H, Pan WK, Meyer JN, 2016. Antagonistic Growth Effects of Mercury and Selenium in Caenorhabditis elegans Are Chemical-Species-Dependent and Do Not Depend on Internal Hg/Se Ratios. Environ Sci Technol 50(6), 3256–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Takaoka S, Grandjean P, 2018. Accelerated functional losses in ageing congenital Minamata disease patients. Neurotoxicol Teratol 69, 49–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


