Abstract
BACKGROUND
Variable definitions and an incomplete understanding of the gradient of reverse cardiac remodeling following continuous flow left ventricular assist device (LVAD) implantation has limited the field of myocardial plasticity. We evaluated the continuum of left ventricular remodeling by serial echocardiographic imaging to define three stages of reverse cardiac remodeling following LVAD.
METHODS
The study enrolled consecutive LVAD patients across four study sites. A blinded echocardiographer evaluated the degree of structural (left ventricular internal diastolic diameter, LVIDd) and functional (left ventricular ejection fraction, LVEF) change after LVAD. Patients experiencing an improvement in LVEF ≥40% and LVIDd ≤6.0cm and were termed Responders, absolute change (Δ) in LVEF of ≥5% and LVEF <40%, were termed Partial Responders, and the remaining patients with no significant improvement in LVEF, Non-Responders.
RESULTS
Among 358 LVAD patients, 34 (10%) were Responders, 112 (31%) Partial Responders, and the remaining 212 (59%) were Non-Responders. The use of guideline-directed medical therapy for heart failure was higher in Partial Responders and Responders. Structural changes (LVIDd) followed a different pattern with significant improvements even in patients that had minimal LVEF improvement. With mechanical unloading the median reduction in LVIDd was: −0.6cm (IQR: −1.1 to −0.1cm, Non-Responders), −1.1cm (IQR: −1.8 to −0.4cm, Partial Responders), and −1.9cm (IQR: −2.9 to −1.1cm, Responders). Similarly, the median Δ in LVEF was: −2% (IQR: −6% to 1%), 9% (IQR: 6% to 14%), and 27% (IQR: 23% to 33%), respectively.
CONCLUSIONS
Reverse cardiac remodeling associated with durable LVAD support is not an all or none phenomenon and manifests in a continuous spectrum. Defining three stages across this continuum can inform clinical management, facilitate the field of myocardial plasticity, and improve the design of future investigations.
Keywords: Heart failure, myocardial recovery, reverse remodeling, left ventricular assist device
INTRODUCTION
Left ventricular assist device (LVAD) therapy reduces intraventricular filling pressures and decreases myocardial work by unloading the left ventricle (LV) which improves cardiac output, patient symptoms, and long-term survival. The improvement in LV function and structure with mechanical unloading was first described in 1994,1 and the phenome of sufficient reverse remodeling that permits device explantation for myocardial remission or recovery has been recapitulated by many others.1–13 In the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), significant reverse remodeling (LVEF ≥ 40%) was seen in ~10% of combined ischemic and non-ischemic cardiomyopathy (NICM) patients at one-year.2, 3 However, a critical limitation in the LVAD recovery field has been the dichotomous approach to assessing reverse remodeling, that defines a Responder by whether or not the patient achieves sufficient significant reverse remodeling to permit device explantation. Our current conceptual framework fails to describe the continuum of reverse remodeling that can occur after LVAD implant.7, 14–16 This limits the ability to study earlier stages of reverse remodeling and characterize the associated molecular processes and clinical management strategies that promote reverse remodeling.
Studies in heart failure with reduced ejection fraction (HFrEF), report reverse cardiac remodeling in 29–56% of patients treated with neurohormonal or cardiac resynchronization therapy.17 These studies also suggest that a partial improvement in LVEF is associated with reduced ventricular arrhythmias and improved patient survival.18–20 Specifically, in the seminal study by Lowes and Bristow, changes in LVEF of ≥ 5% associated with beta-blocker therapy were found to define meaningful reverse cardiac remodeling and altered myocardial gene expression in a favorable manner.21 Further, a 5% change in LVEF reduces the odds of death 5-fold compared to patients with no change in LVEF.22
We hypothesized that similar to HFrEF and many other biological processes, reverse cardiac remodeling with an LVAD does not manifest as an all or none phenomenon. Therefore, descriptors of the stages of recovery are needed to better characterize this continuum. Better descriptors of the stages of functional and structural changes associated with mechanical unloading will inform study design of future clinical and translational investigations that seek to understand and manipulate these events, as well as the use of adjunctive therapies to promote reverse remodeling.23–31 Our research proposes a framework for staging reverse cardiac remodeling with mechanical unloading.
METHODS
Data Sharing
The data, analytical methods, and study materials will be made available to other researchers for the purposes of reproducing results or replicating procedures. Please contact the corresponding author.
Population
Four study sites participated in this analysis and included all patients implanted with a continuous-flow LVAD from 2009 to 2017 and followed through 2018. Patients were prospectively consented and enrolled at sites 1–3, the Utah Transplantation Affiliated Hospitals (U.T.A.H.) Cardiac Transplant program (University of Utah Health Sciences Center, Intermountain Medical Center, Salt Lake VA Medical Center), and retrospectively reviewed at site 4, the Inova Heart and Vascular Institute (IHVI). Consecutive patients with HFrEF who required an LVAD were included. The study was approved by all involved institutional review boards as part of U.T.A.H. where subjects were prospectively consented, and a waiver of consent was granted at Inova where data was retrospectively reviewed.
Duration of HFrEF was defined as the time from symptom onset to LVAD implantation. To target patients with chronic, advanced cardiomyopathy we excluded patients with confirmed acute myocarditis or symptoms for less than 3 months (i.e., acute HF patients that may have been considered as more prone to cardiac recovery). Additional exclusion criteria were age < 18 years, infiltrative or restrictive cardiomyopathies, and heart failure with preserved ejection fraction. Demographics, comorbidities, laboratory data, and vital signs were collected within 24 hours prior to LVAD implantation.
LVAD and pharmacological management
Patients in this study were managed according to institutional protocols for blood pressure management and LVAD speed titration,7, 32 which were consistent with the standard HF and LVAD guideline directed medical therapy.33, 34 Neurohormonal therapy for heart failure, heart rate, and mean arterial pressure (i.e. Doppler opening pressure) for LVAD patients was recorded in the final 3 months of LVAD support.
Echocardiography
Echocardiographic examinations were performed by the echocardiography laboratories of the participating institutions and digitally stored. The echocardiograms were performed within 1-month preceding LVAD implantation and then 1, 2, 3, 6, 9, and 12 months subsequently based on institutional protocol. A requirement for study inclusion was the presence of a pre-LVAD and at least one echocardiogram ≥ 3-month post-LVAD implant. Each echocardiogram was reviewed by a blinded cardiologist at each institution (MAS and OWP) who was unaware of each patient’s responder status. Echocardiography was performed according to guidelines from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.7, 35 To assess change in LV function and structure the last available echocardiographic LVEF and LVIDd was analyzed within a year of LVAD implant. The LVIDd was also indexed to the body-surface area. The left ventricular end-diastolic volume index (LVEDVi) and left ventricular end-systolic volume index (LVESVi) at follow-up echocardiogram were calculated when suitable images were available for volumetric analysis. The degree of mitral regurgitation was quantitated and classified as none, trivial, mild, moderate, and severe.
Responder Definitions
Following at least 3 months of mechanical patients achieving an LVEF to ≥ 40% and an LVIDd ≤ 6.0 cm were termed “Responders” based on prior publications.2–4, 7 The remaining patients were categorized by change in LVEF: “Partial Responders”: absolute LVEF improvement of ≥5% and LVEF <40%,19, 21 ,22 and “Non-Responders”: <5% change in LVEF or no improvement.
Statistical Analysis
Data were summarized using descriptive statistics. Categorical variables were compared with the Pearson chi-square or Fisher exact test; since most continuous variables appear to be non-normally distributed based on the Shapiro-Wilk test, they were presented with the median (interquartile range, IQR) and compared with the Wilcoxon rank-sum test, or the Kruskal-Wallis test. The slope of the change in LVEF over the change in LVIDd (i.e., ΔLVEF (%)/ ΔLVIDd (cm)) was used to compare the response to mechanical unloading between responder groups. A Pearson correlation was performed to understand the relationship between the LVIDd and LVEDVi and LVESVi at the final echocardiographic follow-up. A two-sided p-value of < 0.05 was considered statistically significant. All analyses were performed with R (R Core Team, Vienna, Austria).36
RESULTS
LVAD Patient Characteristics
After application of our exclusion criteria, there were 358 continuous-flow LVAD patients with baseline and repeat echocardiographic measurements 3 to 12 months following LVAD implantation. Of these, 231 (65%) were from sites 1–3 (UTAH), and 127 (35%) were from site 4 (INOVA). Overall, 1,349 echocardiograms were re-read by a blinded cardiologist as part of this analysis with an average of 4 echocardiograms per patient (1 pre-LVAD and 3 after LVAD implant). Baseline characteristics were similar between the study sites except that patients from Inova were more likely to have a non-ischemic rather than ischemic cardiomyopathy, be of African American race, and to be classified as INTERMACS Profile 3 rather than 4 (Table 1). The median time from HF diagnosis to LVAD implantation was 60 months (IQR: 24 to 120 months). Baseline echocardiographic measurements between the groups were similar as were the proportion of centrifugal-flow LVADs implanted (38%). The baseline LVEF for the entire cohort was 19% (IQR: 14% to 23%), with a LVIDd of 6.8 cm (IQR: 6.2 to 7.5 cm).
Table 1.
Patient Characteristics Prior to LVAD Implantation by Study Site
| Attribute | Sites 1–3 (U.T.A.H.) (n=231, 65%) | Site 4 (INOVA) (n=127, 35%) | p-value |
|---|---|---|---|
| Age (years), median (IQR) | 60 (51, 68) | 57 (50, 64) | 0.42 |
| Male | 196 (85%) | 103 (84%) | 0.78 |
| Ischemic Cardiomyopathy | 99 (43%) | 38 (32%) | 0.02 |
| Hypertension | 110 (48%) | 72 (57%) | 0.03 |
| Diabetes Mellitus | 81 (35%) | 48 (38%) | 0.61 |
| Smoking History | 116 (50%) | 53 (42%) | 0.12 |
| Atrial Fibrillation | 98 (42%) | 52 (41%) | 0.79 |
| Pre-Implantation LVEF (%) | 18 (15, 23) | 19 (14, 24) | 0.65 |
| Pre-Implantation LVIDd (cm) | 6.7 (6.1, 7.4) | 6.9 (6.3, 7.6) | 0.04 |
| INTERMACS Profile | 0.03 | ||
| 1 | 11 (5%) | 14 (11%) | |
| 2 | 41 (18%) | 29 (23%) | |
| 3 | 101 (44%) | 65 (51%) | |
| 4 | 55 (24%) | 12 (9%) | |
| 5–7 | 23 (10%) | 7 (8%) | |
| LVAD Type | 0.01 | ||
| HeartMate II | 122 (53%) | 81 (64%) | |
| HVAD | 86 (37%) | 46 (36%) | |
| Jarvik 2000 | 18 (8%) | 0 (0%) | |
| VentrAssist | 3 (1%) | 0 (0%) | |
| Levacor | 2 (1%) | 0 (0%) |
INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; LVIDd = left ventricular internal end-diastolic dimension. U.T.A.H.: Utah Transplant Affiliated Hospitals Cardiac Transplant Program: University of Utah Health & School of Medicine, Intermountain Medical Center & Salt Lake VA Medical Center
Of the 358 LVADs implanted, 203 (57%) were HeartMate II (Abbott Laboratories, Abbott Park IL), 132 (37%) were HVAD (Medtronic, Minneapolis MN), 18 (5%) were Jarvik 2000 (Jarvik Heart Inc., New York, NY), 3 (1%) were VentrAssist, and 2 (1%) were Levacor. There were no HeartMate 3 (Abbott Laboratories) devices included in this study.
Response to Mechanical Unloading and Effect of Turndown Studies
Individual response to mechanical unloading varied significantly between the baseline and follow-up echocardiograms, suggesting a gradient of reverse cardiac remodeling after LVAD (Figure 1). At sites 1–3, all patients were tested with prospective LVAD turndown studies for 30 minutes at the lowest manufacturer recommended LVAD speed setting. During the LVAD turndown study, the change from optimal LVAD support to minimal LVAD support in LVEF was a 0% (IQR: 0 to 2%) and LVIDd 0.13cm (IQR: −0.12 to 0.44cm). This was consistent with prior studies that also showed small changes between pre- and post-turndown LVAD studies.7, 37 For the purpose of these analyses we used values before the LVAD turndown study was performed as this was not routinely performed at site 4. Over one-year follow-up, the median overall change in LVIDd was a decrease of 0.7 cm (IQR: 0.2 to 1.6cm) with an increase in LVEF of 3% (IQR: −3% to 10%, Supplemental Figure I).
Figure 1. Echocardiographic Measurements at Baseline and After LVAD Implantation.

Visual depiction of change in each patient’s left ventricular cavity size and ejection fraction at baseline before LVAD and at follow-up. The blue circles represent the intersection of the patient LVEF and LVIDd at baseline before LVAD and the orange triangles represent the values at follow-up while on LVAD support. The LVIDd is graphed on the x-axis and LVEF on the y-axis. Dashed lines mark left ventricular end-diastolic internal diameter (LVIDd) of 6.0 cm and left ventricular ejection fraction (LVEF) of 40%.
Staging Reverse Cardiac Remodeling with Mechanical Unloading
A majority of LVAD patients experienced changes in structure as manifest by a reduction in the LVIDd within one-month of LVAD implant and this occurred even in patients with minimal to no improvement in systolic function (Figure 1). Change in ventricular function, LVEF, was quantified before and after LVAD, and patients were divided into subgroups based on their degree of functional response to mechanical unloading. Following at least 3 months of mechanical unloading 34 patients (10%) achieved an LVEF to ≥ 40% and an LVIDd ≤ 6.0 cm and were termed “Responders.” The remaining patients were categorized by change in LVEF: “Partial Responders”: absolute LVEF improvement of ≥5% and LVEF <40% (n=112, 31%), and “Non-Responders”: <5% change in LVEF or no improvement (n=212, 59%, Figure 2).
Figure 2. Development of Stages for Myocardial Reverse Remodeling with Mechanical Unloading.
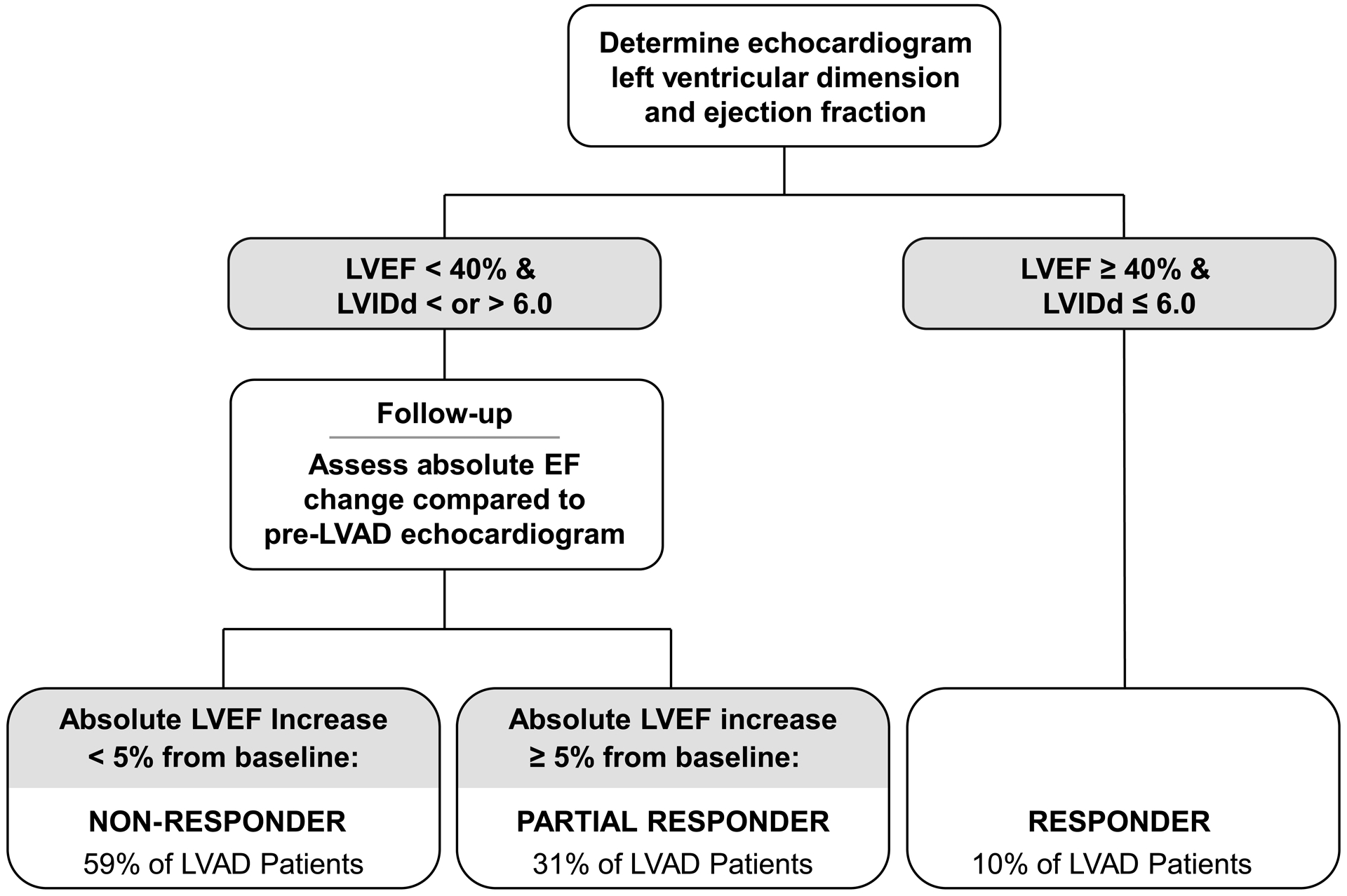
Flow diagram to assist in categorizing the stages of response to mechanical unloading using baseline and follow-up left ventricular size and function. In the general LVAD population patients are classified as: Responders, LVEF ≥ 40% & LVIDd ≤ 6.0cm (10%),2–4 Partial Responders (31%),19,22 and Non-Responders (59%), based on change in ventricular function and structure with ventricular unloading.
We calculated the slope of the change in LVEF over change in LVIDd during LVAD support (ΔLVEF %/ ΔLVIDd cm), for each responder category. Specifically, we found that Responders had a steeper slope, indicating more significant remodeling (slope = −12.6, IQR: −19.6 to −9.1) compared to Partial Responders (slope = −8.3, IQR: −15.5 to −3.4), and Non-Responders (slope = 0, IQR: −2.9 to 7.7, Figure 3). Changes in ventricular structure and function were compared between the 3 responder groups; Responders had an improvement in LVIDd of 1.9cm (IQR: 1.1 to 2.9 cm) and Non-Responders by 0.6cm (IQR: 0.1 to 1.1 cm) (p < 0.001). The median change in ventricular function was an LVEF improvement in Responders of 27% (IQR: 23 to 33%) compared to a LVEF reduction in Non-Responders of −2% (IQR: −6 to −1%) (p < 0.001, Table 2). For each patient group, reverse remodeling in terms of structure and function is noted as early as the first month after LVAD implantation and continues to evolve throughout the first year (Figure 4).
Figure 3. Slope of Reverse Cardiac Remodeling based on Responder Stage.
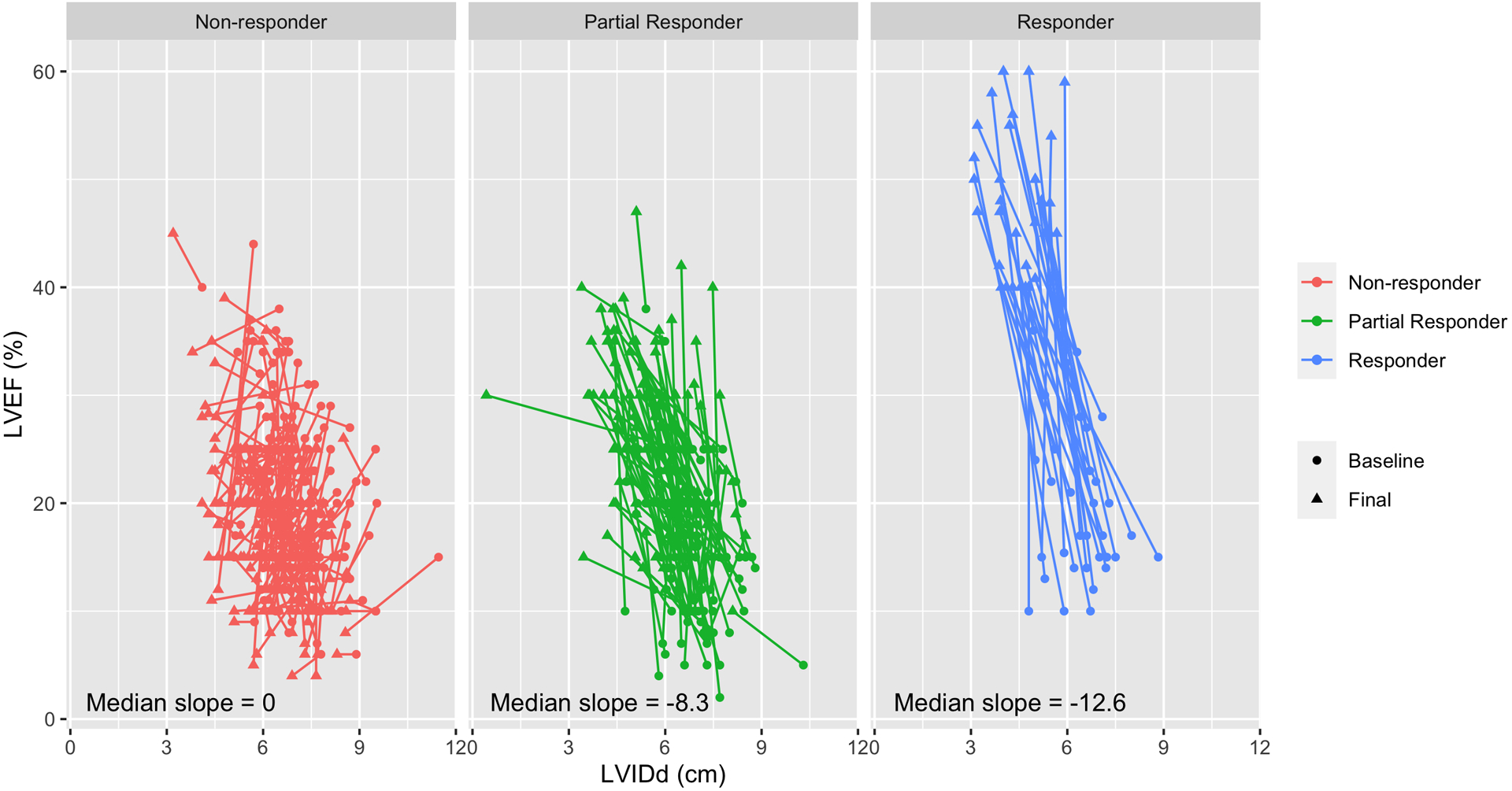
Visual depiction of the change in each patient’s left ventricular cavity size on the x-axis (LVIDd) and ejection fraction on the y-axis (LVEF) at baseline before and after mechanical unloading with an LVAD. The circle indicates the baseline LVIDd and LVEF and the arrow tip represents the final LVIDd and LVEF. Each color group represents the three responder categories. The slope of the change in LVEF over the change in LVIDd is provided. Increasing negative numbers suggest more significant reverse remodeling.
Table 2.
Comparison of Change in Left Ventricular Structure and Function with Mechanical Unloading among Responder Categories
| Echocardiographic Parameter | Non-Responder | Partial Responder | Responder | p-value (Kruskal-Wallis Rank Sum Test) |
|---|---|---|---|---|
| Baseline LVIDd (cm), median (IQR) | 6.8 (6.3, 7.6) | 6.8 (6.2, 7.3) | 6.5 (5.7, 7.0) | 0.02 |
| Follow Up LVIDd (cm) | 6.3 (5.6, 7.0) | 5.7 (4.8, 6.5) | 4.6 (3.9, 5.0) | <0.001 |
| Δ in LVIDd (cm) | −0.6 (−1.1, −0.1) | −1.1 (−1.8, −0.4) | −1.9 (−2.9, −1.1) | < 0.001 |
| Baseline LVEF (%) | 20 (15, 25) | 15 (10, 20) | 17 (15, 25) | <0.001 |
| Follow Up LVEF (%) | 16 (13, 20) | 26 (20, 30) | 47 (42, 52) | <0.001 |
| Δ in LVEF (%) | −2 (−6, −1) | 9 (6, 14) | 27 (23, 33) | < 0.001 |
LVIDd = left ventricular internal end-diastolic dimension; LVEF = left ventricular ejection fraction.
Figure 4. Time Course for Change in Left Ventricular Function and Structure after LVAD Based on Responder Stage.
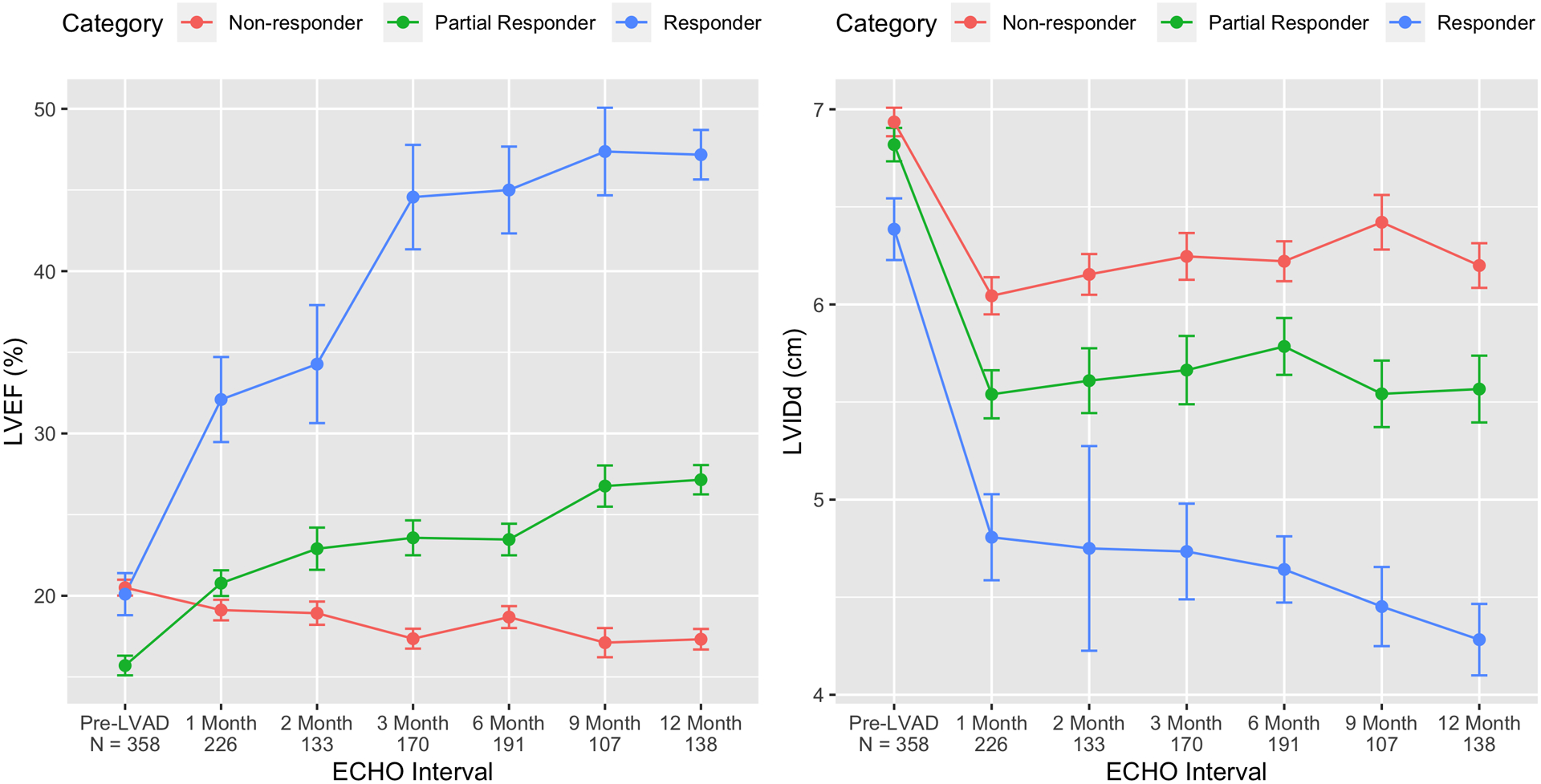
By comparing the baseline and last follow-up echocardiogram, changes in LVEF and LVIDd were used to categorize LVAD patients into three distinct groups: Responders (blue), Partial Responders (green), and Non-Responders (red). Using serial echocardiography, the change in function (LVEF) and structure (LVIDd) after LVAD implant are depicted by responder category.
In a subset of our population, we had adequate echocardiographic images to perform volumetric assessments of the LVEDVi and LVESVi at baseline and last follow-up (n=172, Supplemental Table I). Although most patients had a reduction in the LVEDVi with mechanical unloading, the magnitude was greatest in the Responder group (−87; IQR: −93 to −58 ml/m2) compared to Partial Responders (−43; IQR: −71 to −27 ml/m2), and Non-Responders (−29; IQR: −55 to −9 ml/m2, p<0.001). Similar changes were also noted for the LVESVi. Volumetric LV measurements at final follow-up were compared to the LVIDd, as well as the LVIDd indexed to the patient body-surface area. There was a high correlation between the final LVIDd and LVIDd indexed to the LVEDVi and LVESVi (R = 0.53–0.60, p < 0.001, Supplemental Figure II). The results are similar after adjusting for baseline measurements of LV size and function (R =0.48 – 0.52, p < 0.001, data not presented).
Clinical Characteristics of Responders
Clinical characteristics of patients by responder stage were compared. In comparing Non-Responders, Partial Responders and Responders, we see key statistical differences or non-significant trends across groups which are consistent with prior myocardial recovery publications. As the degree of cardiac reverse remodeling increases (i.e., Non-Responder to Partial Responder to Responder), we see that patients are younger, more likely to be female, have a shorter duration of heart failure before LVAD, a smaller baseline LVIDd, received an axial-flow device, and the proportion of NICM patients increases (Table 3). In addition, we looked at medical therapy between patients at final follow-up. Although final heart rate and blood pressure did not vary across responder categories, the proportion of patients treated with an angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB) was higher in Partial Responders and Responders (p=0.04) and the use of β-blockers also tended to be higher (p=0.11, Table 4).
Table 3.
Patient Characteristics at LVAD Implant by Reverse Remodeling Stage
| Characteristic | Non-Responder (n=212, 59%) | Partial Responder (n=112, 31%) | Responder (n=34, 10%) | p-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 59 (51, 66) | 59 (50, 67) | 56 (44, 65) | 0.23 |
| Male | 186 (89%) | 91 (80%) | 24 (71%) | 0.01 |
| Heart Failure Duration (months) | 72 (25, 132) | 60 (23, 108) | 16 (6, 44) | <0.001 |
| Ischemic Cardiomyopathy | 90 (43%) | 38 (33%) | 9 (27%) | 0.08 |
| Hypertension | 104 (50%) | 62 (54%) | 16 (47%) | 0.63 |
| Diabetes Mellitus | 84 (40%) | 36 (32%) | 9 (27%) | 0.15 |
| Smoking History | 100 (48%) | 54 (47%) | 15 (44%) | 0.93 |
| Atrial Fibrillation | 85 (41%) | 53 (47%) | 12 (35%) | 0.41 |
| Axial-flow device | 117 (56%) | 78 (68%) | 26 (77%) | 0.01 |
| Study Site 4 Patient | 71 (34%) | 42 (37%) | 14 (41%) | 0.66 |
| INTERMACS Profile | 0.84 | |||
| 1 | 16 (8%) | 7 (6%) | 2 (6%) | |
| 2 | 39 (19%) | 22 (19%) | 9 (27%) | |
| 3 | 101 (48%) | 52 (46%) | 13 (38%) | |
| 4 | 39 (19%) | 20 (18%) | 8 (24%) | |
| 5–7 | 15 (7%) | 13 (11%) | 2 (6%) |
INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; LVAD = left ventricular assist device; LVIDd = left ventricular internal end-diastolic dimension; LVEF = left ventricular ejection fraction.
Table 4.
Medical Therapy, Vital Signs, and Echocardiographic Parameters at Final Follow-up
| Characteristic | Non-Responder | Partial Responder | Responder | P value |
|---|---|---|---|---|
| Vital Signs | ||||
| Heart Rate, bpm, median (IQR) | 82 (75, 90) | 80 (73, 90) | 81 (73, 90) | 0.47 |
| Mean Arterial Pressure, mmHg | 84 (77, 90) | 84 (77, 92) | 88 (76, 94) | 0.95 |
| Guideline-Directed Medical Therapy | ||||
| ACE-I or ARB | 95 (45%) | 67 (59%) | 20 (59%) | 0.04 |
| β-blocker | 129 (61%) | 80 (70%) | 26 (76%) | 0.11 |
| MRA | 83 (40%) | 46 (40%) | 13 (38%) | 0.97 |
| Digoxin | 26 (12%) | 20 (18%) | 7 (21%) | 0.28 |
| Echocardiographic Parameters | ||||
| LVEF, % | 16 (13, 20) | 26 (20, 30) | 47 (42, 52) | <0.001 |
| LVIDd, cm | 6.3 (5.6, 7.0) | 5.7 (4.8, 6.5) | 4.6 (3.9, 5.0) | <0.001 |
| LVIDd indexed, cm/m2 | 3.0 (2.6, 3.4) | 2.7 (2.4, 3.1) | 2.2 (2.1, 2.4) | <0.001 |
| Mitral Regurgitation | 0.16 | |||
| None-Mild | 146 (90%) | 84 (90%) | 24 (89%) | |
| Moderate | 16 (10%) | 6 (7%) | 2 (7%) | |
| Severe | 0 (0%) | 3 (3%) | 1 (4%) |
ACE-I: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, MRA: mineralocorticoid receptor antagonist, LVIDd indexed: LVIDd / body surface area.
DISCUSSION
Our work describes the continuum of LV myocardial functional and structural change following LVAD-induced mechanical unloading and circulatory support using a large, cohort of advanced HFrEF patients. Reverse cardiac remodeling is a continuous phenomenon38, and we propose a framework for staging the functional and structural changes after LVAD support into three distinct categories that can be used to characterize reverse remodeling: Responder (LVEF ≥ 40%, LVIDd ≤ 6.0cm), Partial Responder (LVEF Δ ≥ 5%, LVEF<40%), and Non-Responder (LVEF Δ <5% or no improvement).
Reverse Remodeling in HFrEF
Reverse cardiac remodeling in HFrEF patients treated with neurohormonal blockade and cardiac resynchronization therapy, is associated with improvements in survival compared to patients without reverse remodeling.17–20 A meta-analysis suggests that even a 5% improvement in LVEF corresponds to a 14% reduction in mortality.22 This suggests that improvements in LVEF with a therapeutic intervention may serve as surrogate for clinical outcomes.22 The concept of staging reverse remodeling in HFrEF by absolute changes in LVEF is well-established, but has not been applied to LVAD patients. Whether an LVAD patient who is classified as a Partial Responder has improved clinical outcomes (e.g., less gastrointestinal bleeding, heart failure), similar to other HFrEF populations, will need to be validated in future studies with larger patient populations. A recent INTERMACS analysis strongly suggests that standard heart failure drug therapy/neurohormonal blockade and the associated reverse remodeling are beneficial to LVAD patients.39 In our analyses we found that the use of guideline-directed medical therapy was higher in Partial Responders and Responders than Non-Responders.
Reverse Remodeling with LVADs
One of the reasons for the reported clinical variability in reverse remodeling with LVAD studies, includes the lack of consensus definitions that can inform clinical and translational investigations. Myocardial recovery leading to device explantation is reported to occur in about 1–2% of patients based on INTERMACS analyses and other large registries.2, 3, 40 Perhaps, more importantly, close to 10% of LVAD patients demonstrate significant improvements in myocardial function and achieve an LVEF ≥ 40% on follow-up echocardiography.2, 3, 40 What proportion of patients have milder degrees (i.e. Partial Responder) of reverse cardiac remodeling has not been analyzed. These earlier responder categories may benefit from further mechanical unloading or titration of guideline-direct medical therapy (GDMT) to improve to LV structure and function and ultimately become a Responder.
The distinction between change in LV structure (LVIDd) and function (LVEF) provides a more nuanced expression of reverse remodeling that can encourage future identification of the molecular, cellular, and histological signatures that underlie each of these two components of the observed change. In our analysis we saw that all patients experienced a reduction in ventricular size with mechanical unloading and this occurred within one-month after the initiation of LVAD support (Supplemental Figure I). This can likely be attributed to reductions in left ventricular pressures that reduce LV “distension” with mechanical unloading. Changes in ventricular function on the other hand were delayed and occurred throughout the first year (Figure 4). INTERMACS and single center analyses support the assertion that improvements in LVEF trail temporally behind reductions in LV dimension.3, 37 Importantly, to contrast cardiac reverse remodeling with an LVAD to how it is conceptualized within broader HF research (e.g. medical therapy, cardiac resynchronization therapy), we performed volumetric LV measurements (i.e. LVEDVi, LVESVi) in patients who had adequate image quality. The volumetric measures were highly correlated with the LVIDd, suggesting that in LVAD patients, LVIDd can reliably be used to characterize changes in LV structure with cardiac reverse remodeling.
Response to Ventricular Unloading and Clinical Implications
We found that the prevalence of certain clinical characteristics was more commonly seen with enhanced reverse cardiac remodeling (i.e., Non-Responder to Responder). This includes a higher proportion of younger patients with a NICM, shorter duration of HF, female sex, receipt of an axial-flow device, and a smaller pre-implant LVIDd. These clinical predictors of response to mechanical unloading have previously been reported in LVAD-induced myocardial recovery studies and strengthen our proposed staging of reverse cardiac remodeling.2, 3, 5, 40, 41 Critical to our staging of recovery is the classification of Non-Responders to mechanical unloading. This patient population represents ~60% of patients receiving an LVAD and is a HFrEF population with severe adverse remodeling that seems to gradually progress despite mechanical unloading and/or adjuvant therapy. These patients will likely remain on long-term LVAD support as destination therapy (DT) and if eligible could be considered for cardiac transplantation.
Myocardial recovery with mechanical unloading is seen as a clinically rare phenomenon, that can only be achieved in a minority of patients receiving an LVAD (1–2%).2 Our multicenter data suggests that in fact 10% of all LVAD patients may be classified as Responders. Responders have sufficient phenotypic evidence of left ventricular reverse remodeling and can proceed to formal cardiac recovery testing.5, 6, 11, 37, 42–44 An additional 30% of all LVAD patients can be classified as Partial Responders. These patients demonstrate reverse left ventricular remodeling and may achieve Responder status with appropriate therapeutic interventions. By dichotomizing our definitions however, we remove this critical nuance and ignore the significant remodeling changes observed in a large part of the LVAD patient population, which could inform the myocardial plasticity and reverse remodeling field. For example, in Partial Responders with an improved LVEF, but persistently dilated LV, an LVAD-ramp study could be considered to maximize ventricular unloading and reduce mitral regurgitation in order to facilitate a further decrease in LV size.32, 45 Partial Responders with an LVIDd ≤6.0cm could benefit from continued optimization of guideline-directed medical therapy and regular echocardiographic re-testing (i.e. 3 months). These recommendations are based on prior studies and will need to be validated in large-scale prospective studies (Figure 5).
Figure 5: Conceptual Framework for Clinical Trial Design Incorporating the U-NOVA Reverse Remodeling Stages.
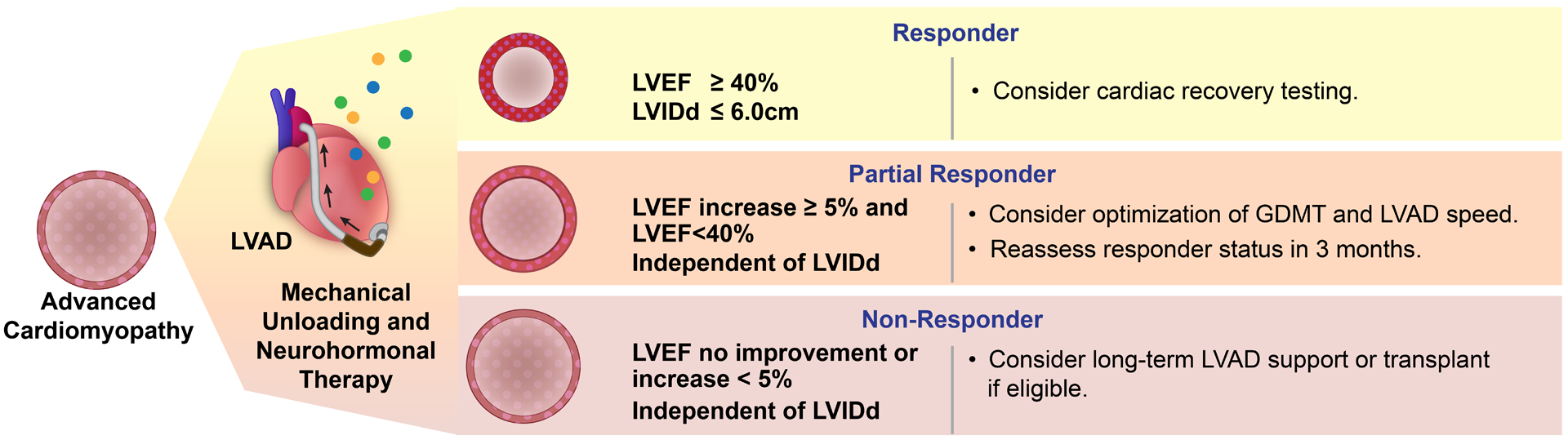
Reverse left ventricular remodeling is not an all or none phenomenon and as many other biological phenomena manifests as a continuous spectrum. We describe a new framework to characterize stages of reverse remodeling with mechanical unloading. We identified three distinct HF sub-populations and their cardiac functional and structural response to durable mechanical circulatory support. This framework for grading reverse remodeling allows for heart failure clinicians and investigators to readily recognize the stages of reverse remodeling and identify subpopulations for personalized management. This functional/structural reverse cardiac remodeling staging may also facilitate advancing the field of myocardial plasticity by informing the study design of future clinical and translational investigations. GDMT: Guideline directed medical therapy.
Study Limitations
Limitations of this study include the relatively small sample size and inclusion of data from only four study sites. Nonetheless, there was little difference in LV myocardial response between the sites suggesting generalizability of the results despite practice differences in clinical management after LVAD implantation (e.g., turn-down studies, speed titration, GDMT utilization). Additional limitations include the lack of centralized echocardiographic interpretation, the use of only one modality to assess mechanical unloading and its limitations to assess right ventricular structure and function. The cardiologists, who reviewed the echocardiograms, were however blinded to final responder status. We note that patients left ventricular function may fluctuate (up or down) during LVAD support in between the proposed stages of response, and our analysis was limited to the last available echocardiographic time point. Further, a 5% change in LVEF may be hard to detect with echocardiography, but we used the biplane Simpson’s method which has been shown to have the highest correlation to cardiac magnetic resonance imaging,46 and has been validated in previous HFrEF studies as a meaningful improvement in LVEF.22
Conclusion
Reverse cardiac remodeling with mechanical circulatory support is not an all or none phenomenon and as many other biological phenomena manifests as a continuous spectrum. We describe a framework to characterize the stages of reverse remodeling during mechanical circulatory support with an LVAD. We identified three distinct groups and characterize their left ventricular functional and structural response to durable mechanical circulatory support. This framework for grading reverse remodeling allows for clinicians and investigators to readily recognize and communicate changes in reverse cardiac remodeling and forms the basis for future clinical and translational investigations.
Supplementary Material
Clinical Perspective.
What Is New?
We describe the continuum of left ventricular myocardial functional and structural change following left ventricular assist device (LVAD) induced mechanical unloading and circulatory support in a large, multicenter, cohort of advanced heart failure patients receiving LVAD support.
Reverse cardiac remodeling is a continuous phenomenon, and we identify 3 distinct stages of response to mechanical unloading: Responder (LVEF ≥ 40%, LVIDd ≤ 6.0cm), Partial Responder (LVEF Δ ≥ 5%, LVEF<40%), and Non-Responder (LVEF Δ <5% or no improvement).
The proportion of unselected, LVAD patients achieving each responder category were: Responder (10%), Partial Responder (31%), and Non-Responder (59%).
What Are the Clinical Implications?
Use of the U-NOVA (Utah-INOVA) stages of reverse cardiac remodeling can improve delineation of the gradient of recovery seen with mechanical unloading and neurohormonal therapy for LVAD patients.
These stages can be incorporated into the design of future clinical trials investigating how reverse cardiac remodeling improves clinical response to LVAD therapy or how novel therapeutic strategies can enhance myocardial recovery rates.
Acknowledgement –
We are grateful to Diana L. Lim for assistance in creating figures 2 and 5 in the manuscript.
Sources of Funding – Inova Health System intramural seed grant (Dr. Shah), AHA / Enduring Hearts Scientist Development Grant (Dr. Shah) 17SDG33660431, NHLBI Career Development Award 1K23HL143179-01A1 (Dr. Shah), AHA Heart Failure Strategically Focused Research Network - Grant 16SFRN29020000 (Drs. Selzman, Drakos & Stehlik), NHLBI R01 Grant HL135121 (Dr. Drakos), NHLBI R01 Grant HL132067 (Dr. Drakos), Nora Eccles Treadwell Foundation (Dr. Drakos).
Disclosures
Dr. Drakos is a consultant to Abbott (i.e., Steering Committee member of Intellect-2, an Abbott funded multicenter LVAD trial). Dr. Shah receives grant support from Abbott, Merck, and Bayer, and consults for Procyrion, Ortho Clinical Diagnostics and Novartis. The remaining authors have nothing to disclose.
ABBREVIATIONS
- GDMT
guideline-directed medical therapy
- HFrEF
heart failure with reduced ejection fraction
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- LV
left ventricle
- LVAD
left ventricular assist device (continuous flow)
- LVEF
left ventricular rejection fraction
- LVEDVi
left ventricular end-diastolic volume index
- LVESVi
left ventricular end-systolic volume index
- LVIDd
left ventricular internal end-diastolic diameter
- NICM
non-ischemic cardiomyopathy
Footnotes
Supplemental Materials
Supplemental Figures I-II
Supplemental Table I
REFERENCES
- 1.Frazier OH. First use of an untethered, vented electric left ventricular assist device for long-term support. Circulation. 1994;89:2908–14. [DOI] [PubMed] [Google Scholar]
- 2.Wever-Pinzon O, Drakos SG, McKellar SH, Horne BD, Caine WT, Kfoury AG, Li DY, Fang JC, Stehlik J and Selzman CH. Cardiac Recovery During Long-Term Left Ventricular Assist Device Support. J Am Coll Cardiol. 2016;68:1540–53. [DOI] [PubMed] [Google Scholar]
- 3.Topkara VK, Garan AR, Fine B, Godier-Furnemont AF, Breskin A, Cagliostro B, Yuzefpolskaya M, Takeda K, Takayama H, Mancini DM, et al. Myocardial Recovery in Patients Receiving Contemporary Left Ventricular Assist Devices: Results From the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). Circulation Heart failure. 2016;9. PMC4943678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, Miller LW, Margulies K, McRee S, Frazier OH, et al. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–505. [DOI] [PubMed] [Google Scholar]
- 5.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A and Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–84. [DOI] [PubMed] [Google Scholar]
- 6.Birks EJ, George RS, Hedger M, Bahrami T, Wilton P, Bowles CT, Webb C, Bougard R, Amrani M, Yacoub MH, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123:381–90. [DOI] [PubMed] [Google Scholar]
- 7.Drakos SG, Wever-Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, Saidi A, Diakos NA, Stoker S, Davis ES, et al. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol. 2013;61:1985–94. 3819804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diakos NA, Selzman CH, Sachse FB, Stehlik J, Kfoury AG, Wever-Pinzon O, Catino A, Alharethi R, Reid BB, Miller DV, et al. Myocardial atrophy and chronic mechanical unloading of the failing human heart: implications for cardiac assist device-induced myocardial recovery. J Am Coll Cardiol. 2014;64:1602–12. [DOI] [PubMed] [Google Scholar]
- 9.Frazier OH, Benedict CR, Radovancevic B, Bick RJ, Capek P, Springer WE, Macris MP, Delgado R and Buja LM. Improved left ventricular function after chronic left ventricular unloading. The Annals of thoracic surgery. 1996;62:675–81; discussion 681–2. [DOI] [PubMed] [Google Scholar]
- 10.Muller J, Wallukat G, Weng YG, Dandel M, Spiegelsberger S, Semrau S, Brandes K, Theodoridis V, Loebe M, Meyer R, et al. Weaning from mechanical cardiac support in patients with idiopathic dilated cardiomyopathy. Circulation. 1997;96:542–9. [DOI] [PubMed] [Google Scholar]
- 11.Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB and Hetzer R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation. 2005;112:I37–45. [DOI] [PubMed] [Google Scholar]
- 12.Mann DL, Barger PM and Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol. 2012;60:2465–72. 3522780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birks EJ, Drakos SG, Patel SR, Lowes BD, Selzman CH, Starling RC, Trivedi J, Slaughter MS, Alturi P, Goldstein D, et al. Prospective Multicenter Study of Myocardial Recovery Using Left Ventricular Assist Devices (RESTAGE-HF [Remission from Stage D Heart Failure]): Medium-Term and Primary End Point Results. Circulation. 2020;142:2016–2028. [DOI] [PubMed] [Google Scholar]
- 14.Drakos SG and Mehra MR. Clinical myocardial recovery during long-term mechanical support in advanced heart failure: Insights into moving the field forward. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35:413–20. [DOI] [PubMed] [Google Scholar]
- 15.Kim GH, Uriel N and Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nature reviews Cardiology. 2018;15:83–96. [DOI] [PubMed] [Google Scholar]
- 16.Drakos SG, Pagani FD, Lundberg MS and Baldwin JT. Advancing the Science of Myocardial Recovery With Mechanical Circulatory Support: A Working Group of the National, Heart, Lung and Blood Institute. J Card Fail. 2017;23:416–421. [DOI] [PubMed] [Google Scholar]
- 17.Aimo A, Gaggin HK, Barison A, Emdin M and Januzzi JL Jr. Imaging, Biomarker, and Clinical Predictors of Cardiac Remodeling in Heart Failure With Reduced Ejection Fraction. JACC Heart failure. 2019;7:782–794. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox JE, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Heywood JT, Inge PJ, McBride ML, Mehra MR, O’Connor CM, et al. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: findings from IMPROVE HF. Am Heart J. 2012;163:49–56 e2. [DOI] [PubMed] [Google Scholar]
- 19.Kubanek M, Sramko M, Maluskova J, Kautznerova D, Weichet J, Lupinek P, Vrbska J, Malek I and Kautzner J. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol. 2013;61:54–63. [DOI] [PubMed] [Google Scholar]
- 20.Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A and Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;57:1468–76. [DOI] [PubMed] [Google Scholar]
- 21.Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–65. [DOI] [PubMed] [Google Scholar]
- 22.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA and Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56:392–406. PMC4523221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farris SD, Don C, Helterline D, Costa C, Plummer T, Steffes S, Mahr C, Mokadam NA and Stempien-Otero A. Cell-Specific Pathways Supporting Persistent Fibrosis in Heart Failure. J Am Coll Cardiol. 2017;70:344–354. [DOI] [PubMed] [Google Scholar]
- 24.Catino AB, Ferrin P, Wever-Pinzon J, Horne BD, Wever-Pinzon O, Kfoury AG, McCreath L, Diakos NA, McKellar S, Koliopoulou A, et al. Clinical and histopathological effects of heart failure drug therapy in advanced heart failure patients on chronic mechanical circulatory support. Eur J Heart Fail. 2018;20:164–174. [DOI] [PubMed] [Google Scholar]
- 25.Ton VK, Vunjak-Novakovic G and Topkara VK. Transcriptional patterns of reverse remodeling with left ventricular assist devices: a consistent signature. Expert review of medical devices. 2016;13:1029–1034. [DOI] [PubMed] [Google Scholar]
- 26.Margulies KB, Matiwala S, Cornejo C, Olsen H, Craven WA and Bednarik D. Mixed messages: transcription patterns in failing and recovering human myocardium. Circ Res. 2005;96:592–9. [DOI] [PubMed] [Google Scholar]
- 27.Klotz S, Burkhoff D, Garrelds IM, Boomsma F and Danser AH. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? European heart journal. 2009;30:805–12. [DOI] [PubMed] [Google Scholar]
- 28.Klotz S, Danser AH, Foronjy RF, Oz MC, Wang J, Mancini D, D’Armiento J and Burkhoff D. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–74. [DOI] [PubMed] [Google Scholar]
- 29.Klotz S, Barbone A, Reiken S, Holmes JW, Naka Y, Oz MC, Marks AR and Burkhoff D. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J Am Coll Cardiol. 2005;45:668–76. [DOI] [PubMed] [Google Scholar]
- 30.Barbone A, Holmes JW, Heerdt PM, The AH, Naka Y, Joshi N, Daines M, Marks AR, Oz MC and Burkhoff D. Comparison of right and left ventricular responses to left ventricular assist device support in patients with severe heart failure: a primary role of mechanical unloading underlying reverse remodeling. Circulation. 2001;104:670–5. [DOI] [PubMed] [Google Scholar]
- 31.Heerdt PM, Holmes JW, Cai B, Barbone A, Madigan JD, Reiken S, Lee DL, Oz MC, Marks AR and Burkhoff D. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation. 2000;102:2713–9. [DOI] [PubMed] [Google Scholar]
- 32.Shah P, Badoe N, Phillips S, Abdullah K, May CW, Nabut JL, Singh R, deFilippi C and Desai SS. Unrecognized Left Heart Failure in LVAD Recipients: The Role of Routine Invasive Hemodynamic Testing. ASAIO journal. 2018;64:183–190. [DOI] [PubMed] [Google Scholar]
- 33.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–87. [DOI] [PubMed] [Google Scholar]
- 34.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 35.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 36.R: A language and environment for statistical computing. R Core Team;2020. https://www.R-project.org/. [Google Scholar]
- 37.Wever-Pinzon J, Selzman CH, Stoddard G, Wever-Pinzon O, Catino A, Kfoury AG, Diakos NA, Reid BB, McKellar S, Bonios M, et al. Impact of Ischemic Heart Failure Etiology on Cardiac Recovery During Mechanical Unloading. J Am Coll Cardiol. 2016;68:1741–1752. [DOI] [PubMed] [Google Scholar]
- 38.Drakos SG, Kfoury AG, Stehlik J, Selzman CH, Reid BB, Terrovitis JV, Nanas JN and Li DY. Bridge to recovery: understanding the disconnect between clinical and biological outcomes. Circulation. 2012;126:230–41. PMC3714227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCullough M, Caraballo C, Ravindra NG, Miller PE, Mezzacappa C, Levin A, Gruen J, Rodwin B, Reinhardt S, van Dijk D, et al. Neurohormonal Blockade and Clinical Outcomes in Patients With Heart Failure Supported by Left Ventricular Assist Devices. JAMA Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan S, Aksut B, Wever-Pinzon OE, Rao SD, Levin AP, Garan AR, Fried JA, Takeda K, Hiroo T, Yuzefpolskaya M, et al. Incidence and predictors of myocardial recovery on long-term left ventricular assist device support: Results from the United Network for Organ Sharing database. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1624–9. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein DJ, Maybaum S, MacGillivray TE, Moore SA, Bogaev R, Farrar DJ, Frazier OH and HeartMate IICI. Young patients with nonischemic cardiomyopathy have higher likelihood of left ventricular recovery during left ventricular assist device support. J Card Fail. 2012;18:392–5. [DOI] [PubMed] [Google Scholar]
- 42.Dandel M, Weng Y, Siniawski H, Stepanenko A, Krabatsch T, Potapov E, Lehmkuhl HB, Knosalla C and Hetzer R. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: criteria for weaning from ventricular assist devices. European heart journal. 2011;32:1148–60. PMC3086897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Formica P, Murthy S, Edwards P, Goldstein D and Maybaum S. A structured 3-step approach to evaluate cardiac recovery with continuous flow circulatory support. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:1440–2. [DOI] [PubMed] [Google Scholar]
- 44.Drakos SG, Pagani FD, Lundberg MS and Baldwin JT. Advancing the Science of Myocardial Recovery With Mechanical Circulatory Support: A Working Group of the National, Heart, Lung, and Blood Institute. JACC Basic Transl Sci. 2017;2:335–340. PMC5516933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N, et al. Hemodynamic Ramp Tests in Patients With Left Ventricular Assist Devices. JACC Heart failure. 2016;4:208–17. [DOI] [PubMed] [Google Scholar]
- 46.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG and Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? European heart journal. 2000;21:1387–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


