Abstract
Psychotherapy research is increasingly targeting both psychological and neurobiological mechanisms of therapeutic change. This trend is evident in and applicable to post-traumatic stress disorder (PTSD) treatment research given the high nonresponse rate of individuals with PTSD who undergo cognitive-behavioral therapy (CBT). Functional connectivity analyses investigating disrupted brain networks across mental disorders have been employed to understand both mental disorder symptoms and therapeutic mechanisms. However, few studies have examined pre-post CBT brain changes in PTSD using functional connectivity analyses. The current study investigated a) whether brain networks commonly implicated in psychopathology (e.g., default mode network [DMN], central executive network [CEN], and salience network [SN]) changed following Cognitive Processing Therapy (CPT) for PTSD and b) whether change in these networks was associated with PTSD and/or transdiagnostic symptom change. Independent components analysis was implemented to investigate resting-state functional connectivity in DMN, CEN, and SN in 42 women with PTSD and 18 trauma-exposed controls (TEC). Results indicated decreased CEN-cerebellum connectivity in PTSD participants versus TEC prior to CPT and decreased DMN connectivity in PTSD participants after CPT. Additionally, DMN and SN connectivity was related to change in positive and negative affectivity, while exploratory analyses at a cluster threshold of pFDR < .10 indicated DMN and SN connectivity was also related to change in PTSD symptoms and rumination. These findings provide evidence for normalization of CEN connectivity with treatment and implicate the DMN and SN in clinical symptom change following CPT.
Keywords: PTSD, Cognitive Processing Therapy, functional connectivity, default mode network, central executive network, salience network
Post-traumatic stress disorder (PTSD) has received significant clinical and research attention given its lifetime prevalence rate of 6.1%, its detrimental impact on social and occupational functioning, and its high comorbidity with other mental disorders (Goldstein et al., 2016). PTSD is characterized by four clusters of symptoms arising after an individual experiences a traumatic event. Symptom clusters include intrusions (e.g., recurrent memories of the trauma, flashbacks), avoidance of trauma stimuli, alterations in arousal and reactivity (e.g., sleep difficulty, hypervigilance), and negative alterations in cognition and mood (American Psychiatric Association, 2013).
Despite the existence of empirically-supported treatments for PTSD, including several cognitive-behavioral therapies (CBT), high rates of nonresponse in individuals who undergo CBT for PTSD have prompted efforts to research mechanisms of therapeutic change (Schottenbauer et al., 2008). Neuroscience approaches have increasingly been used to study neural substrates of cognitive, affective, and behavioral variables in PTSD, as well as the neural correlates of psychotherapy mechanisms, in an attempt to understand how psychotherapy affects the PTSD brain.
1.1. Neurobiological Correlates of PTSD
The most commonly cited neuroanatomical model of PTSD focuses on altered threat detection and fear learning systems and suggests that the disorder is a result of a hyperresponsive amygdala, insula, and dorsal anterior cingulate cortex (ACC) to threat stimuli, inadequate inhibition of the amygdala by the ventromedial prefrontal cortex (VMPFC), and an inability, or resistance to, extinction and extinction recall (Shin & Liberzon, 2010; Graham & Milad, 2011). Further, altered hippocampal activity, in combination with medial prefrontal cortex (MPFC) hyporesponsivity, leads to problems with contextualizing stimuli across numerous domains (e.g., cognitive, social, and internal), which enables the individual to appropriately respond to the environment. For example, failure to interpret trauma reminders within the current spatial and temporal context leads to re-experiencing symptoms, while emotional numbing results from a failure to experience emotions consistent with the context (Liberzon & Garfinkel, 2009; Liberzon & Sripada, 2008). Despite the wealth of evidence for the neuroanatomical model in describing PTSD pathology, initial fMRI studies investigating CBT effects in the brain focusing on regions in the model have found mixed results, with inconsistent evidence that CBT decreased amygdala activation, increased prefrontal cortex activation, or affected hippocampal or insula activity (Felmingham et al., 2007; Peres et al., 2007, 2011; Aupperle et al., 2013; Fonzo et al., 2017).
Recent research has increasingly focused on network-based models of psychopathology using functional connectivity analyses, with some stating that changes in functional connectivity networks will serve as “key neurobiological outcomes” of psychotherapy treatment research (Weingarten & Strauman, 2015, p. 201). In PTSD specifically, investigating disrupted networks may serve as a better representation of the heterogeneous clinical profiles of those with PTSD than studies examining activity in isolated brain regions (Patel et al., 2012). Network-based models of psychopathology suggest that abnormal connectivity within and between functional networks across neurodegenerative and psychological disorders results in transdiagnostic symptom clusters and is a key factor in pathology (Buckholtz & Meyer-Lindenberg, 2012; Menon, 2011). A tripartite model of psychopathology and aberrant cognitive and affective networks was first proposed by Menon (2011). The first aberrant network implicated is the central executive network (CEN), a frontoparietal system including the lateral prefrontal cortex, dorsal ACC, and posterior parietal cortices which is activated during decision-making and working memory tasks and is critical for attention allocation (Menon, 2011; Buckholtz & Meyer-Lindenberg, 2012). The second aberrant network is the salience network (SN), a corticolimbic system including lateral and medial PFC, ACC, amygdala, substantia nigra, and insula that is responsible for processing salient environmental and internal information (including emotion) and is thus prone to influencing negative affective states (Menon, 2011; Buckholtz & Meyer-Lindenberg, 2012). The third aberrant network is the default mode network (DMN), which is commonly activated during resting states or self-referential processes (Buckner et al., 2008; Buckner & DiNicola, 2019; Andrews-Hanna et al., 2014) and comprises the lateral parietal lobes, posterior cingulate, and MPFC (Menon, 2011; Buckholtz & Meyer-Lindenberg, 2012; Fornito and Bullmore, 2012).
Currently, many researchers conceptualize PTSD network dysfunction in terms of emotion-generating and modulating networks. Disrupted amygdala-frontal networks have been demonstrated in PTSD in both task and resting states (Duval et al., 2015; MacNamara et al., 2016). The authors suggest that these patterns reflect impaired emotion regulation and threat processing (MacNamara et al., 2016) or are an interaction of threat processing and emotion regulation circuits (Duval et al., 2015). Other research indicates that PTSD is associated with altered large-scale network connectivity within the networks of Menon’s tripartite model (2011), such that re-experiencing, dissociation, and avoidance symptoms result from dysfunctional DMN connectivity, while altered CEN connectivity is implicated in reduced prefrontal inhibition and attention/memory problems (Akiki et al., 2017). Finally, disrupted SN connectivity leads to hyperarousal symptoms via increased sensitivity to threat detection. Taken together, PTSD is conceptualized as consisting of a hyperactive SN and hypoactive CEN and DMN (Akiki et al., 2017). Further, there is evidence for aberrant between network connectivity in PTSD. For example, network-based models of PTSD suggest that there is impaired top-down regulation of the SN by the CEN (Akiki et al., 2017) as well as increased connectivity between the DMN and SN (MacNamara et al., 2016).
1.2. Mechanisms of CBT in PTSD Using Functional Connectivity Analyses
While these models speak to the etiology of PTSD, CBT’s effects on brain networks are less clearly defined. If one hypothesizes that CBT ‘normalizes’ the etiological processes of PTSD, the SN and CEN, which comprise oft-cited prefrontal-limbic regions, might be implicated first (Weingarten & Strauman; 2015). However, CBT may also modulate networks responsible for transdiagnostic symptoms inherent to anxiety and stress, such as impaired concentration (Frewen et al., 2008). In this case, transdiagnostic symptoms of anxiety and stress disorders would be affected by CBT (Buckholtz & Meyer-Lindenberg, 2012; Williams, 2017; MacNamara et al., 2016).
Few studies have examined brain network changes pre-post CBT in PTSD using functional connectivity analyses. Studies examining changes following Prolonged Exposure (PE) document increased frontopolar cortex connectivity with VMPFC during a reappraisal task after treatment (Fonzo et al., 2017) as well as increased rostral ACC connectivity with VMPFC during an extinction recall task after treatment (Helpman et al., 2016). A third study demonstrated increased resting-state connectivity between specific amygdala nuclei and orbitofrontal cortex as well as between hippocampus and MPFC in PTSD participants after PE (Zhu et al., 2018). The authors concluded that PE enhanced attention toward emotion regulation processes (Fonzo et al., 2017) and enabled PTSD participants to better evaluate threat and process emotional information (Zhu et al., 2018), providing evidence that PE modified disrupted emotion- and threat-processing networks in PTSD.
Studies examining other CBT interventions found that combat veterans with PTSD exhibited increased connectivity between the posterior cingulate in the DMN and prefrontal regions (DLPFC, ACC) following group mindfulness-based exposure therapy. Furthermore, posterior cingulate-DLPFC connectivity was associated with avoidance and hyperarousal symptom improvement (King et al., 2016). The single study investigating functional connectivity changes after Cognitive Processing Therapy (CPT) demonstrated that participants showed increased executive network connectivity during a symptom provocation task after CPT but no change in SN connectivity (Abdallah et al., 2019). Notably, the authors found no evidence of change in network functional connectivity during their resting-state scans.
1.3. Aims and Hypotheses
Network-based models of psychopathology using functional connectivity analyses may generate new hypotheses about how therapeutic processes may engender their effects in individuals with PTSD. Though this knowledge would be greatly beneficial to both psychotherapy and PTSD literatures, there is a dearth of research analyzing brain changes after CBT in PTSD using functional connectivity methods, with only one study to date examining functional connectivity changes after CPT in a PTSD sample (Abdallah et al., 2019). Further, no studies have investigated all three networks of the tri-partite model or examined whether network changes with treatment are associated with transdiagnostic symptoms of clinical disorders. The current study will address these gaps in the literature by utilizing data-driven independent components analyses to examine resting-state functional connectivity changes in the DMN, CEN, and SN following CPT. These networks were selected based on their empirical support in healthy and psychiatric samples (Menon, 2011), as well as in PTSD (Akiki et al., 2017; MacNamara et al., 2016).
Our study aims are twofold. First, we will investigate whether resting-state functional connectivity in DMN, CEN, and SN is significantly different between PTSD participants before and after CPT compared to trauma-exposed controls (TEC). We hypothesize that PTSD participants will exhibit altered functional connectivity compared to TEC before CPT, but will exhibit no differences in functional connectivity compared to TEC after CPT. Second, we will investigate whether resting-state functional connectivity in DMN, CEN, and SN changes following CPT in PTSD treatment completers, and whether change in resting-state functional connectivity in DMN, CEN, and SN following CPT is related to change in PTSD and/or transdiagnostic symptoms of clinical disorders (e.g., rumination, positive and negative affectivity). We hypothesize change in functional connectivity will be correlated with change in PTSD symptoms, rumination, positive affectivity, and negative affectivity.
2. Method
2.1. Participants
Data collected for a larger study in women with PTSD as a result of interpersonal violence were used in the present research. The intent-to-treat sample (ITT) included 42 women aged 18-55 with a DSM-IV-TR diagnosis of PTSD (American Psychiatric Association, 2000) resulting from interpersonal violence and 18 TEC. DSM-IV-TR criteria for PTSD were used as the study began recruitment prior to the publication of DSM-5 criteria; of note, research has shown that a majority of individuals meeting DSM-IV-TR criteria for PTSD also meet DSM-5 criteria (Kilpatrick et al., 2013). Sixteen women in the treatment group discontinued treatment, leaving 26 women with pre- and post-CPT data (e.g., PTSD treatment completers). Exclusion criteria included: diagnosed neurological disorders, current substance abuse disorders, schizophrenia/psychotic disorder, bipolar, or obsessive-compulsive disorder. Additionally, participants were excluded if they displayed active suicidality as determined by the investigator, were taking psychotropic drugs (e.g., beta blockers, antipsychotics, antidepressants), or had ever experienced a loss of consciousness greater than five minutes. All participants provided written informed consent in accordance with criteria established by the University’s Human Subjects Committee.
2.2. Clinical Measures
2.2.1. Life Events Checklist
This checklist is administered as part of the CAPS and is used to determine whether Criterion A for a PTSD diagnosis has been met. It contains items such as “physical assault,” “sexual assault,” and “other unwanted or uncomfortable sexual experience.” Participants endorsed events that have happened to them personally, that they have witnessed happening to someone else, or that they have learned happened to a close other.
2.2.2. Clinician-Administered PTSD Scale (CAPS)
The CAPS is a 30-item structured interview that corresponds to the DSM-IV criteria for PTSD (Blake et al., 1995). The scoring criteria consider a PTSD symptom present if the frequency of the CAPS item is rated as 1 or higher and the intensity is rated at a 2 or higher. Previous studies have reported high test-retest reliability (r = .90-.98) and internal consistency (α = .94) of the overall severity score (Weathers & Litz, 1994). Additionally, severity scores for each symptom cluster displayed test-retest coefficients between .77-.96 and alpha coefficients between .85-.87 (Weathers & Litz, 1994). For the current analysis, PTSD diagnosis was based on cutoff scores > 45 on the CAPS.
2.2.3. Ruminative Thought Style Questionnaire (RTS)
This is a 20-item assessment measuring an individual’s tendency to ruminate (Brinker & Dozois, 2009). Participants rate how well each statement describes them on a Likert scale from 1 to 7. Examples of items include “I find my mind often goes over things again and again,” “If I have an important event coming up, I can’t stop thinking about it,” and “I tend to replay past events as I would have liked them to happen” (Brinker & Dozois, 2009). The measure exhibits high internal consistency (α = .87) and test-retest reliability (r = .80) (Brinker & Dozois, 2009).
2.2.4. Positive and Negative Affect Schedule-Expanded (PANAS-X)
This 60-item assessment measures positive and negative affect, which are factors that contribute largely to mood states (Watson & Clark, 1999). Participants are provided a list of 60 words or phrases that describe emotional states and asked to rate on a Likert scale of 1 (“not at all”) to 5 (“extremely”) how much they have felt that way in the last few weeks (Watson & Clark, 1999). Sample items include cheerful, lonely, nervous, ashamed, frightened, irritable, and distressed (Watson & Clark, 1999). Both the Positive Affect and Negative Affect scales of the PANAS-X display high internal consistency (ranging from .83 - .90) across student, nonclinical adult, and psychiatric samples (Watson & Clark, 1999). Further, the Positive Affect and Negative Affect scales are not highly correlated with each other, suggesting they are measuring independent constructs. Finally, both scales exhibit moderate test-retest reliability (.39 and .43 for Positive and Negative Affect, respectively) (Watson & Clark, 1999).
2.3. Procedure
All participants completed a baseline assessment in which they were administered the CAPS, Life Events Checklist, RTS, and PANAS-X. On a second day, all participants underwent structural and resting-state fMRI scans, which lasted approximately 1.5 hours. Subsequently, participants diagnosed with PTSD received a standard course of CPT, a “strongly recommended” therapy for PTSD according to the American Psychological Association Clinical Practice Guideline for PTSD (American Psychological Association, 2017). CPT is a 12-session manualized treatment designed to target maladaptive beliefs and emotions following a traumatic event (Resick & Schnicke, 1992). After completing treatment, PTSD participants completed a follow-up assessment of the measures collected at baseline and underwent a second resting-state fMRI scan which lasted approximately 1.5 hours. In sum, PTSD participants who completed CPT underwent two fMRI scans. TEC completed one fMRI scan.
2.4. fMRI Data Acquisition
fMRI images at both Time 1 and Time 2 were collected on a Siemens 3T TrioTim MRI scanner (Erlangen, Germany). The protocol included localizer images, a high-resolution, magnetization prepared rapid gradient echo (MPRAGE) structural image, and a series of functional images. The structural images were acquired with 1×1×1mm3 resolution using a sagittal 3-D T1-weighted sequence with repetition time (TR) of 2.4 s, time-to-echo (TE) of 3.13 ms, flip angle=8 °, and inversion time (TI) of 1000 ms. Two 7 minute, 35 second functional resting-state image acquisitions were collected using an asymmetric spin-echo planar sequence TR=2.2 s, TE=27 ms, flip angle=90° and field of view (FOV) of 384 cm. One acquisition consisted of 36 transverse slices, 4 mm thick (no gap), and with an in-plane resolution of 4×4 mm.
2.5. Functional Connectivity Analysis
Functional connectivity analyses were conducted using the group-ICA approach within the CONN toolbox (release 18.a; Whitfield-Gabrieli & Nieto-Castanon, 2012). Imaging data underwent spatial preprocessing using standard methods in the Statistical Parametric Mapping software package (SPM12) (Friston et al., 2007), which included realignment, slice-timing correction, coregistration, segmentation, normalization, and smoothing (using a 6-mm FWHM Gaussian kernel) (Whitfield-Gabrieli & Nieto-Castanon, 2012; Vergara et al., 2017). Data then underwent denoising, which included regression of subject motion (e.g., realignment parameters) from the voxel-level time series, followed by linear detrending, despiking, and band-pass filtering at .008-.09 Hz.
CONN implements spatial group-ICA analyses using methods previously described by Calhoun (Calhoun et al., 2001; 2009). As such, CONN conducted variance normalization preconditioning and concatenated the BOLD signal along the temporal dimension (Whitfield-Gabrieli & Nieto-Castanon, 2017). All neuroimaging data (e.g., from both time points and all groups) was entered simultaneously into the group-ICA to ensure comparisons between the same components could be made. After preprocessing the fMRI data (as described above), the number of independent components (e.g., functional connectivity networks) to be estimated was determined by the minimum description length technique as implemented in the GIFT toolbox (GIFT Documentation Team, 2017). This technique resulted in an estimated 194 components in the data. However, review of previous papers using ICA in PTSD samples indicated no other study had analyzed more than 44 components; therefore, 100 components were determined to be sufficient for this analysis (St. Jacques et al., 2013; Shang et al., 2014; Rabellino et al., 2015; Tursich et al., 2015; Zhang et al., 2015; Reuveni et al., 2016). Subsequently, CONN utilized the G1 fastICA algorithm to identify 100 independent spatial components (e.g., functional connectivity networks) whose z-scores were correlated in their time course. In this analysis, z-scores indicate the correlation between activation in each voxel (e.g., the BOLD signal) and the time course of the entire network. Finally, GICA3 back-projection was used to recreate individual subject spatial maps to be used in the second-level analyses (Whitfield-Gabrieli & Nieto-Castanon, 2017).
After these steps, the spatial maps for the 100 independent components were correlated with template maps for the networks of interest for this study (DMN, CEN, and SN) within CONN. A high correlation between a spatial map identified in the current dataset and a template map within CONN indicated the presence of that network within the dataset. In this dataset, the two components with the strongest correlation with the DMN, CEN, and SN template maps were selected for further analysis (see Figure 1). Spatial maps that were composed of regions within white matter or cerebrospinal fluid were identified as noise and discarded from second-level analyses. This method for selecting functional networks for second-level analysis has been utilized in Zhang et al. (2015), Liao et al. (2010), and Hoekzema et al. (2014).
Figure 1. Spatial Pattern of Network Components.
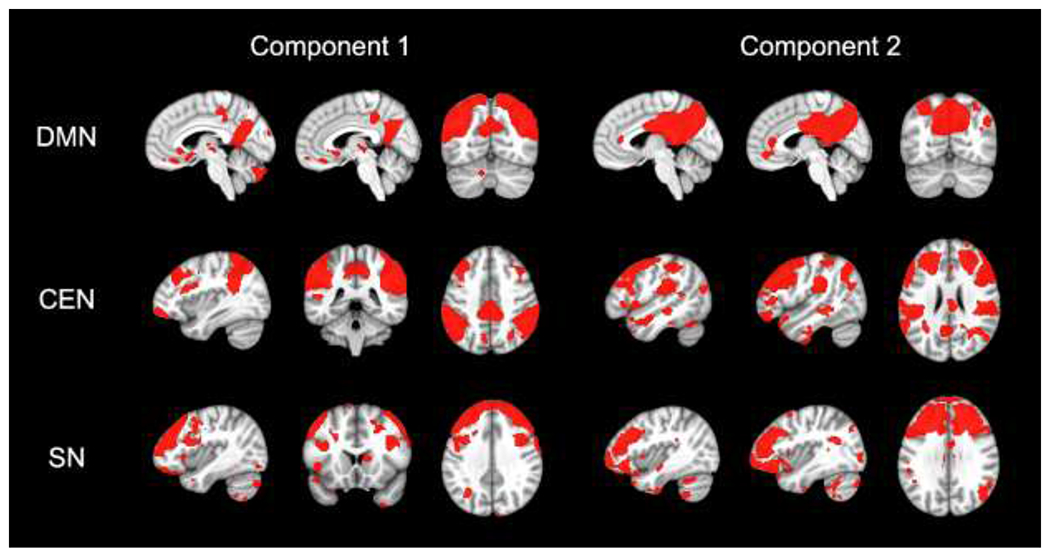
Note. Component maps illustrate the spatial distribution for the three networks of interest from the independent component analysis. The two components with the highest correlations with the DMN, CEN, and SN templates are shown. For visualization purposes, component maps were thresholded at p-uncorrected < .001 and z > 1. All component maps are displayed on the MNI-152 template. DMN = default mode network; CEN = central executive network; SN = salience network.
2.6. Second-Level Analyses
All second-level analyses were conducted within the CONN toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012) or in SPSS (IBM SPSS Statistics for Windows, Version 24.0). Two components comprising each network were entered into second-level analyses at the same time. We utilized voxel-level thresholds of p < .001 and cluster-level FDR-corrected thresholds of p < .05 to detect significant differences in brain activation between groups and across time. For our second aim, symptom change scores were calculated by subtracting the score at Time 2 from the score at Time 1. Change scores were used as the dependent variable while Time 1 scores were entered as a covariate of no interest. Given the smaller sample size for our second aim, we also considered results that survived a more liberal cluster-level FDR-corrected threshold of p < .10.
Finally, Pearson’s correlation coefficient was used to determine the relation between length of time in treatment (in weeks) and change in PTSD and transdiagnostic symptoms (e.g., rumination, positive affectivity, negative affectivity) in the treatment completer sample.
3. Results
3.1. Demographic Characteristics
See Table 1 for a summary of the demographic characteristics of the whole sample and each experimental group. Independent samples t-tests indicated that the ITT group did not significantly differ from TEC on age (t(58) = −0.26, p = .79) or education level (t(58) = 0.80, p = .43). Similarly, the PTSD treatment completer group did not significantly differ from TEC on age (t(42) = 0.53, p = .60) or education level (t(42) = 1.05, p = .31). An independent samples t-test indicated that CAPS scores at Time 1 were not significantly different between participants who completed treatment and those that dropped out of treatment (t(40) = −0.34, p = .74).
Table 1.
Demographic Characteristics
| PTSD | ||||
|---|---|---|---|---|
| Variable | Total Sample | Treatment Completers | ITT | TEC |
| n | 60 | 26 | 42 | 18 |
| Age (SD) | 31.38 (9.67) | 33.62 (11.09) | 31.17 (9.71) | 31.89 (9.84) |
| Education (SD) | 15.16 (2.57) | 15.62 (1.63) | 15.38 (1.89) | 14.64 (3.73) |
| CAPS T1 | 66.92 (17.71) | 67.64 (17.35) | ||
| CAPS T2 | 23.75 (20.89) | |||
| Ethnicity (%) | ||||
| Caucasian | 37 (61.70) | 16 (61.50) | 25 (59.50) | 12 (66.70) |
| African American | 14 (23.30) | 6 (23.10) | 11 (26.20) | 3 (16.70 |
| Hispanic | 2 (3.30) | 1 (3.80) | 2 (4.80) | 0 |
| Other | 5 (8.30) | 2 (7.70) | 2 (4.80) | 3 (16.70) |
| Not reported | 2 (3.30) | 1 (3.80) | 2 (4.80) | 0 |
Note. ITT= Intent-to-treat sample of participants with PTSD. TEC= Trauma-exposed controls. CAPS= Clinician-Administered PTSD scale.
3.2. Preliminary Analyses of Treatment Effects
Preliminary analyses were conducted to determine whether there were relationships between time in treatment and symptom change scores. Time in treatment was not significantly correlated with change in CAPS (r(22) = .11, p = .62), rumination (r(22) = −.15, p = .50), positive affectivity (r(22) = −.36, p = .09), or negative affectivity (r(22) = .05, p = .81) scores. This indicates that change in PTSD and transdiagnostic symptoms was not simply due to passage of time. Notably, a paired-samples t-test found that CAPS scores at Time 1 were significantly different from CAPS scores at Time 2 (t(23) = 12.18, p = .00), indicating that CPT resulted in a reduction of PTSD symptoms.
3.3. Differences in Resting-State Network Connectivity in PTSD vs TEC
3.3.1. Time 1
At Time 1, the ITT sample of PTSD participants showed decreased connectivity between CEN and a region in the cerebellum (MNI coordinates [x, y, z]: 4, −38, −16; k = 302) compared to TEC. Similarly, the PTSD treatment completer sample showed decreased connectivity between CEN and a similar cerebellar region (MNI coordinates [x, y, z]: −4, −48, −16; k = 242). Neither the ITT nor the PTSD treatment completer samples exhibited significant differences in DMN or SN functional connectivity compared to TEC.
3.3.2. Time 2
At Time 2, the PTSD treatment completer sample showed decreased connectivity compared to TEC between DMN and several regions in the temporal and occipital lobes (see Table 2 and Figure 2). There were no significant differences in CEN or SN functional connectivity between treatment completers at Time 2 and TEC.
Table 2.
DMN Functional Connectivity at Time 2 in PTSD Treatment Completers vs TEC at Time 1
| Cluster Region | MNI Coordinates (x, y, z) | k | pFDR |
|---|---|---|---|
| L Posterior Superior and Middle Temporal Gyrus |
−52, −26, −2 | 1011 | < .05 |
| R Posterior Superior and Middle Temporal Gyrus |
44, −26, −14 | 835 | <.05 |
| L and R Precentral Gyrus | −10, −22, 54 | 572 | <.05 |
| L Lateral Occipital Cortex | −22, −86, 4 | 362 | <.05 |
Note. TEC= trauma-exposed controls. DMN= default mode network. L= left. R= right. k= cluster size
Figure 2. DMN Connectivity Differentiating PTSD Treatment Completers at Time 2 from TEC.
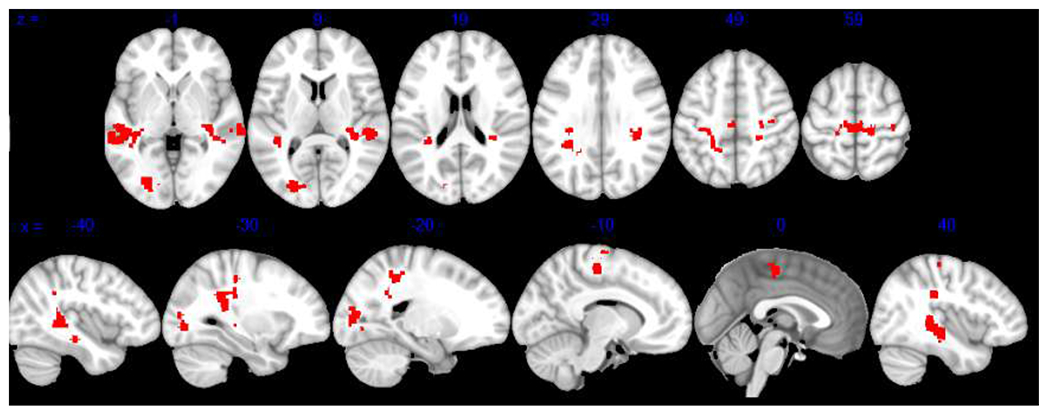
Note. Results show significant differences in DMN connectivity between PTSD and TEC participants at pFDR < .05. Findings are displayed on the MNI-152 template. DMN = default mode network; TEC = trauma-exposed controls.
3.4. Altered Resting-State Network Connectivity in PTSD Treatment Completers
3.4.1. Time 1 vs Time 2
PTSD treatment completers did not exhibit significant differences in DMN, CEN, or SN functional connectivity between Time 1 and Time 2.
3.4.2. Association with Clinical Measures
3.4.2.1. PTSD Symptoms.
PTSD symptom change was not related to change in functional connectivity in any network from Time 1 to Time 2 at a threshold of pFDR < .05. However, when using a more liberal cluster threshold of pFDR < .10, connectivity between DMN and two cerebellar regions was correlated with PTSD symptom change, such that individuals with greater PTSD symptom reduction showed increased anticorrelations from Time 1 to Time 2 (see Table 3 and Figure 3).
Table 3.
Functional Connectivity Time 1 to Time 2 and Clinical Symptom Change
| Clinical Variable | Network | Cluster Region | MNI Coordinates (x,y,z) | k | pFDR |
|---|---|---|---|---|---|
| PTSD Symptoms | DMN | Cerebellum | −34, −56, −50 | 89 | < .10 |
| Cerebellum | −20, −48, −46 | 82 | < .10 | ||
| Rumination | SN | Brain Stem | 22, −36, −34 | 74 | < .10 |
| Positive Affectivity |
DMN | L Middle Temporal Gyrus R Lateral Occipital Cortex & Angular Gyrus |
−58, −60, 00 | 170 | < .05 |
| SN | L Frontal Pole | −28, 50, −6 | 86 | < .10 | |
| R Frontal Pole | 20, 52, −8 | 64 | < .10 | ||
| Negative Affectivity |
DMN | Cerebellum | −20, −80, −34 | 129 | < .05 |
| 24, −70, −56 | 88 | < .10 | |||
| −30, −52, −40 | 87 | < .10 | |||
| −12, −70, −36 | 83 | < .10 | |||
| R Putamen | 22, 22, 04 | 73 | < .10 |
Note. DMN= default mode network. SN= salience network. L= left. R= right. k= cluster size
Figure 3. Pre-Post DMN Connectivity Associated with Clinical Symptom Change.
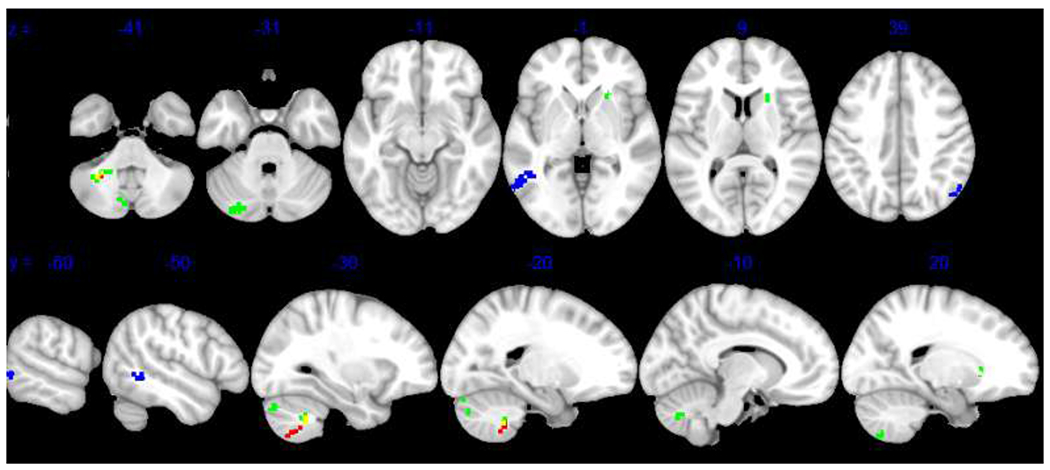
Note. Results reveal significant relationships between pre-post DMN connectivity and change in PTSD symptoms (red), positive affectivity (blue), and negative affectivity (green) at pFDR < .05 or pFDR < .10. Yellow represents overlap in PTSD symptom and negative affectivity clusters. Results are displayed on the MNI-152 template. DMN = default mode network.
3.4.2.2. Rumination.
Change in rumination was not related to change in functional connectivity in any network from Time 1 to Time 2 at a threshold of pFDR < .05. However, at pFDR < .10, connectivity between SN and the brain stem was correlated with rumination symptom change, such that individuals with greater reduction in rumination showed stronger positive connectivity from Time 1 to Time 2 (see Table 3 and Figure 4).
Figure 4. Pre-Post SN Connectivity Associated with Clinical Symptom Change.
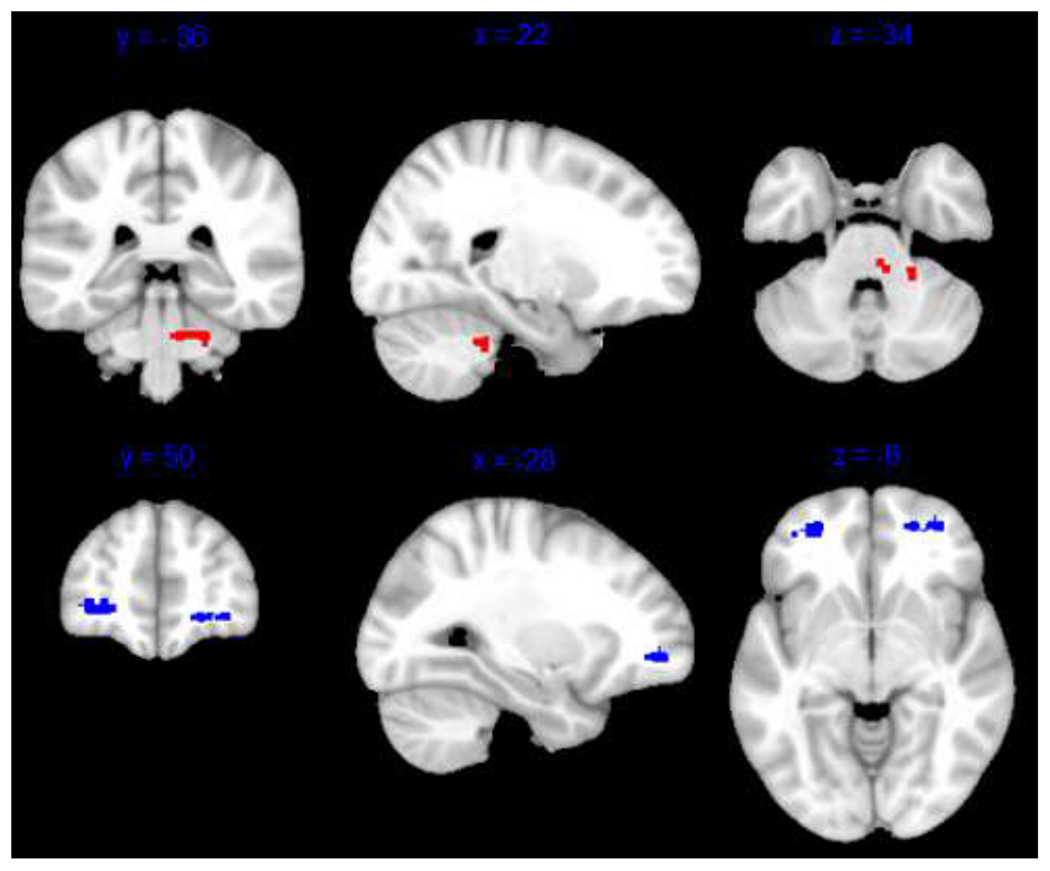
Note. Results show significant relationships between pre-post SN connectivity and change in rumination (red) and positive affectivity (blue) at pFDR < .10. Results are displayed on the MNI-152 template. SN = salience network.
3.4.2.3. Positive Affectivity.
Change in positive affectivity was related to connectivity between DMN and left middle temporal gyrus and right lateral occipital cortex, such that individuals with greater increase in positive affectivity showed a) stronger anticorrelations between DMN-left middle temporal gyrus and b) stronger positive connectivity between DMN-right lateral occipital cortex from Time 1 to Time 2 (see Table 3 and Figure 3). Additionally, at a cluster threshold of pFDR < .10, change in positive affectivity was related to higher connectivity between SN and regions in the left and right frontal pole, such that individuals with greater increase in positive affectivity showed stronger positive SN-frontal pole connectivity (see Table 3 and Figure 4).
3.4.2.4. Negative Affectivity.
Change in negative affectivity was related to DMN-cerebellum connectivity, such that individuals with greater reduction in negative affectivity showed stronger DMN-cerebellar anticorrelation from Time 1 to Time 2. Additional cerebellum clusters were found at pFDR <.10 (see Table 3 and Figure 3).
4. Discussion
4.1. PTSD vs TEC
Our results show that an ITT sample of individuals with PTSD exhibit decreased connectivity between CEN and cerebellum compared to TEC that is not evident after a course of CPT, suggesting this connectivity in PTSD ‘normalized’ to the level of TEC following treatment. Abdallah et al. (2019) also found increased executive network connectivity during a symptom provocation task after CPT. These results align with research indicating that PTSD is characterized by a hypoactive CEN and suggests that CPT may normalize this hypoactivity. A normalized CEN may represent increased prefrontal inhibition, a process suggested to be disrupted in PTSD specifically (Akiki et al., 2017; Duval et al., 2015; MacNamara et al., 2016) as well as modified by CBT generally (Roffman et al., 2005; Porto et al., 2009; Sheynin & Liberzon, 2017; Brooks & Stein, 2015; Straube, 2016; Lueken & Hahn, 2016). Our results also indicate that after CPT, PTSD treatment completers exhibit decreased DMN connectivity with clusters in visual and somatomotor networks compared to TEC that is not evident prior to treatment, suggesting this connectivity is a ‘compensatory’ change with treatment. Though DMN connectivity is typically described as hypoactive in PTSD, based on decreased connectivity of MPFC and posterior cingulate cortex seeds (Bluhm et al., 2009; Shang et al., 2014; DiGangi et al., 2016), Shang et al. (2014) have documented altered visual and somatomotor network connectivity in PTSD compared to healthy controls and suggested that altered connectivity in both networks was related to hyperarousal symptoms. More recently, Zhu et al. (2020) reported that DMN-visual network connectivity was positively correlated with PTSD symptoms and contributed significantly to correct classification of PTSD compared to TEC using multivariate pattern analysis. Our results provide further evidence for the role of DMN connectivity with these networks in PTSD.
4.2. PTSD Treatment Completers and Symptom Change
Though we did not find evidence of DMN, SN, or CEN change after CPT within the PTSD treatment completer group, connectivity between these networks and other regions was related to both PTSD and transdiagnostic symptom change. DMN connectivity was related to change in positive and negative affectivity. Specifically, DMN connectivity with temporal and occipital regions was associated with greater increases in positive affectivity, while DMN-cerebellum connectivity was associated with greater reduction in negative affectivity. When using liberal cluster thresholds at pFDR < 0.10, DMN-cerebellum connectivity was also related to greater reduction in PTSD symptoms. DMN connectivity has been shown to relate to improvement in PTSD symptoms in King et al. (2016) and Akiki et al. (2017) posited that re-experiencing, dissociation, and avoidance symptoms are related to DMN connectivity. We replicate and extend these findings by demonstrating a novel relation between DMN connectivity and positive and negative affectivity. Finally, at liberal cluster thresholds of pFDR < 0.10, SN-brain stem connectivity was related to greater reduction in rumination and SN-frontal pole connectivity was related to greater increases in positive affectivity. Fonzo et al. (2017) also demonstrated increased SN-frontopolar cortex connectivity during cognitive reappraisal in individuals who had completed prolonged exposure compared to a wait-list group, suggesting that SN-frontal pole connectivity in PTSD treatment completers is affected by cognitive behavioral therapies and related to a transdiagnostic symptom (positive affectivity) targeted by therapy.
4.3. Role of Cerebellum
Our results indicated that decreased cerebellar connectivity with CEN differentiated PTSD participants from TEC pre-treatment, while cerebellar connectivity with DMN correlated with several types of clinical symptom change. These findings are notable given that the cerebellum has been increasingly recognized in PTSD, potentially due to its role in conditioning and extinction via its connections to limbic and prefrontal regions (Carletto & Borsato, 2017). Previous research has found that PTSD groups show cerebellar hypoactivation (Koch et al., 2016), weaker cerebellar connectivity (Holmes et al., 2018) and differential connectivity of widespread cerebellar regions compared to healthy controls (Rabellino et al., 2018). Additionally, several groups report that cerebellar connectivity is related to PTSD symptom severity (Holmes et al., 2018; Rabellino et al., 2018). Abdallah et al. (2019) also documented weaker cerebellar connectivity in PTSD participants compared to TEC during a symptom provocation task, but not a resting-state scan, both before and after CPT. Our results suggest that decreased resting-state cerebellar connectivity may be normalized by CPT, and that cerebellar connectivity is related to reduction in PTSD symptoms (at pFDR < 0.10) and negative affectivity (at pFDR < .05).
4.4. Synthesis of Results in the Literature
There is little research investigating brain changes related to CPT specifically. When examining all studies investigating change in functional connectivity pre- and post-CBT for PTSD (n = 5), three studies showed changes following Prolonged Exposure (one during a reappraisal task [Fonzo et al., 2017], one during an extinction recall task [Helpman et al., 2016], and one in a resting-state scan [Zhu et al., 2018]); one study showed resting-state changes following a mindfulness-based exposure intervention (King et al., 2016); and one showed changes after both CPT and a control treatment during symptom provocation (Abdallah et al., 2019). As such, there is some evidence for resting-state change after exposure-based interventions and some evidence for change after CPT in a symptom provocation scan, but no published evidence for resting-state change after CPT. This study is the first to our knowledge to demonstrate changes in both CEN and DMN resting-state connectivity after a course of CPT, as well as the first to document the relation between change in transdiagnostic symptoms in PTSD and network connectivity changes following treatment.
4.5. Limitations & Future Directions
This study had several limitations. First, we utilized a template-matching approach to identify networks of interest within our data. Though this was a procedure used by several other groups (Zhang et al., 2015; Liao et al., 2010; Hoekzema et al., 2014) and the correlations between our networks and the templates were similar to those obtained in other studies, our network-template correlations were moderate in size (versus large), indicating that our identified DMN, SN, and CEN did not overlap entirely with all the brain regions located within the template networks. For example, the DMN network components only included posterior brain regions. Lack of precision in matching networks or regions of interest used in other studies is not a problem unique to our study but is instead common throughout neuroimaging research (Hong et al., 2019). Nonetheless, it could lead to problems replicating effects. Second, given the lack of research investigating brain network change after CPT for PTSD and the exploratory nature of the current study, we elected not to perform multiple comparisons corrections for our between- and within-groups analyses, and we reported certain results at a cluster threshold of pFDR < .10 (Althouse, 2016; Rothman, 1990). As such, these findings would require replication before determining their robustness. Future analyses may additionally examine whether there are changes between networks pre- to post-CPT.
4.6. Conclusion
This study implemented a data-driven functional connectivity analysis method to examine large-scale brain networks implicated in PTSD pathology. Our results provide evidence for both normalization and compensatory processes in PTSD participants who completed an evidence-based treatment for PTSD. Additionally, our findings contribute to the growing literature documenting the role of the cerebellum in PTSD and implicate the DMN and SN in both PTSD and transdiagnostic symptom change with treatment. Notably, this is the first study that implemented an ICA method in pursuit of this research question, and is only the second study to examine neurobiological changes after a course of CPT. As such, our findings provide a novel contribution to research literature related to both PTSD neurobiology and psychotherapy process.
Funding:
MIMH 1K23 MH090366-01. (Steven E. Bruce, PI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- Abdallah CG, Averill CL, Ramage AE, Averill LA, Alkin E, Nemati S, Krystal JH, Roache JD, Resick PA, Young-McCaughan S, Peterson AL, Fox P, & the STRONG STAR Consortium. (2019). Reduced salience and enhanced central executive connectivity following PTSD treatment. Chronic Stress, 3. 10.1177/2470547019838971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki TJ, Averill CL, & Abdallah CG (2017). A network-based neurobiological model of PTSD: Evidence from structural and functional neuroimaging studies. Current Psychiatry Reports, 19(11), 81. 10.1007/s11920-017-0840-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althouse AD (2016). Adjust for multiple comparisons? It’s not that simple. The Annals of Thoracic Surgery, 101(5), 1644–1645. 10.1016/j.athoracsur.2015.11.024 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). American Psychiatric Association Publishing. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association Publishing. [Google Scholar]
- American Psychological Association. (2017). Clinical practice guideline for the treatment of PTSD in adults, http://www.apa.org/ptsd-guideline/ptsd.pdf
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29–52. 10.Ill1/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Simmons AN, Flagan T, Thorp SR, Norman SB, Paulus MP, Stein MB (2013). Neural responses during emotional processing before and after cognitive trauma therapy for battered women. Psychiatry Research, 214(1), 48–55. 10.1016/j.pscychresns.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, & Mechelli A (2014). The effects of psychotherapy on brain function: A systematic and critical review. Progress in Neurobiology, 114, 1–14. 10.1016/j.pneurobio.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2007). Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences, 11(7), 307–316. 10.1016/j.tics.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1007/bf02105408 [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, … & Lanius RA (2009). Alterations in default network connectivity in posttraumatic stress disorder related to early life trauma. Journal Psychiatry Neuroscience, 34(3), 187–194. [PMC free article] [PubMed] [Google Scholar]
- Brinker JK, & Dozois DJA (2009). Ruminative thought style and depressed mood. Journal of Clinical Psychology, 65( 1), 1–19. 10.1002/jclp.20542 [DOI] [PubMed] [Google Scholar]
- Brooks SJ, & Stein DJ (2015). A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues in Clinical Neuroscience, 17(3), 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, & Meyer-Lindenberg A (2012). Psychopathology and the human connectome: Toward a transdiagnostic model of risk for mental illness. Neuron, 74(6), 990–1004. 10.1016/j.neuron.2012.06.002 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The Brain’s Default Network. Annals of the New York Academy of Sciences, 1124(1), 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buckner RL, & DiNicola LM (2019). The brain’s default network: Updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience, 20(10), 593–608. 10.1038/s41583-019-0212-7 [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, & Pekar JJ (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151. 10.1002/hbm.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Hansen JC, Larsen J, & Pekar JJ (2003, April 1-4). ICA of fMRI: An overview. [Conference presentation]. Fourth International Symposium on Independent Component Analysis and Blind Signal Separation, Nara, Japan. [Google Scholar]
- Calhoun VD, Liu J, & Adali T (2009). A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage, 45(1), S163–S172. 10.1016/j.neuroimage.2008.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletto S, & Borsato T (2017). Neurobiological correlates of post-traumatic stress disorder: A focus on cerebellum role. European Journal of Trauma & Dissociation, 1(3), 153–157. doi: 10.1016/j.ejtd.2017.03.012 [DOI] [Google Scholar]
- Casey B, Glatt C, & Lee F (2015). Treating the developing versus developed brain: Translating preclinical mouse and human studies. Neuron, 86(6), 1358–1368. 10.1016/j.neuron.2015.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, & Beck AT (2010). Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends in Cognitive Sciences, 14(9), 418–424. 10.1016/j.tics.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine, 11(1), 126–126. 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raedt R (2006). Does neuroscience hold promise for the further development of behavior therapy? The case of emotional change after exposure in anxiety and depression. Scandinavian Journal of Psychology, 47(3), 225–236. 10.1111/j.1467-9450.2006.00511.x [DOI] [PubMed] [Google Scholar]
- DiGangi JA, Tadayyon A, Fitzgerald DA, Rabinak CA, Kennedy A, Klumpp H, …Phan KL (2016). Reduced default mode network connectivity following combat trauma. Neuroscience Letters, 615, 37–43. 10.1016/j.neulet.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval E, Javanbakht A, & Liberzon I (2015). Neural circuits in anxiety and stress disorders: A focused review. Therapeutics and Clinical Risk Management, 11, 115–126. 10.2147/TCRM.S48528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman J, Linnman C, Van Dijk KRA, & Milad MR. (2016). Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology, 63, 34–42. 10.1016/j.psyneuen.2015.09.012 [DOI] [PubMed] [Google Scholar]
- Etkin A (2014). Neuroimaging and the future of personalized treatment in psychiatry. Depression and Anxiety, 31(11), 899–901. 10.1002/da.22325 [DOI] [PubMed] [Google Scholar]
- Etkin A, Pittenger C, Polan HJ, & Kandel ER (2005). Toward a neurobiology of psychotherapy: Basic science and clinical applications. The Journal of Neuropsychiatry and Clinical Neurosciences, 17(2), 145–158. 10.1176/jnp.17.2.145 [DOI] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, & Bryant R (2007). Changes in anterior cingulate and amygdala after cognitive behavior therapy of Posttraumatic Stress Disorder. Psychological Science, 18(2), 127–129. 10.1111/j.1467-9280.2007.01860.x [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, Weiss ME, Thompson AL, Zack SE, Mills-Finnerty CE, Rosenberg BM, Edelstein R, Wright RN, Kole CA, Lindley SE, Arnow BA, Jo B, Gross JJ, Rothbaum BO, & Etkin A (2017). Selective effects of psychotherapy on frontopolar cortical function in PTSD. American Journal of Psychiatry, 174(12), 1175–1184. 10.n76/appi.ajp.2017.16091073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, & Bullmore ET (2012). Connectomic intermediate phenotypes for psychiatric disorders. Frontiers in Psychiatry, 3, 32. 10.3389/fpsyt.2012.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JC, & Price RB (2014). Psychotherapy and neuroimaging. Focus (Am Psychiatr Publ)., 12(3), 290–298. 10.1176/appi.focus.12.3.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, & Lanius RA (2008). Neuroimaging studies of psychological interventions for mood and anxiety disorders: Empirical and methodological review. Clinical Psychology Review, 28(2), 228–246. 10.1016/j.cpr.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel S, Nichols T, & Penny W (Eds.). (2007). Statistical Parametric Mapping: The analysis of functional brain images. Elsevier/Academic Press. [Google Scholar]
- Friston K (2012). Ten ironic rules for non-statistical reviewers. Neuroimage, 61(4), 1300–1310. 10.1016/j.neuroimage.2012.04.018 [DOI] [PubMed] [Google Scholar]
- GIFT Documentation Team. (2017). Group ICA/IVA of fMRI toolbox (GIFT) manual. http://mialab.mrn.org/software/gift/docs/v4.0b_gica_manual.pdf
- Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, Pickering RP, Ruan WJ, Huang B, & Grant BF (2016). The epidemiology of DSM-5 Posttraumatic Stress Disorder in the United States: Results from the national epidemiologic survey on alcohol and related conditions-III. Social Psychiatry and Psychiatric Epidemiology, 51(8), 1137– 1148. 10.1007/s00127-016-1208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, & Milad MR (2011). The study of fear extinction: Implications for anxiety disorders. American Journal of Psychiatry, 168(12), 1255–1265. 10.1176/appi.ajp.2011.11040557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, & Phelps EA (2010). Changing fear: The neurocircuitry of emotion regulation. Neuropsychopharmacology, 35(1), 136–146. 10.1038/npp.2009.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman L, Marin M, Papini S, Zhu X, Sullivan GM, Schneier F, Neria M, Shvil E, Malaga Aragon MJ, Markowitz JC, Lindquist MA, Wager T, Milad M, & Neria Y (2016). Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study. Neuroimage: Clinical, 12, 715–723. 10.1016/j.nicl.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos- Quiroga JA, Richarte Fernández V, Bosch R, Soliva JC, Rovira M, Bulbena A, Tobena A, Casas M, Vilarroya O (2014). An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Human Brain Mapping, 35(4), 1261–1272. 10.1002/hbm.22250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG (2008). Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorders. Clinical Psychology Review, 28(2), 199–210. 10.1016/j.cpr.2007.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Scheinost D, DellaGioia N, Davis MT, Matuskey D, Pietrzak RH, … Esterlis I (2018). Cerebellar and prefrontal cortical alterations in PTSD: Structural and functional evidence. Chronic Stress (Thousand Oaks, Calif.), 2, 1–11. doi: 10.1177/2470547018786390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Yoo Y, Han J, Wager TD, & Woo C (2019). False-positive neuroimaging: Undisclosed flexibility in testing spatial hypotheses allows presenting anything as a replicated finding. Neuroimage, 195, 384–395. 10.1016/j.neuroimage.2019.03.070 [DOI] [PubMed] [Google Scholar]
- Hupé J (2015). Statistical inferences under the null hypothesis: Common mistakes and pitfalls in neuroimaging studies. Frontiers in Neuroscience, 9, 18. 10.3389/fnins.2015.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2007). Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology, 3(1), 1–27. 10.1146/annurev.clinpsy.3.022806.091432 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, & Zaslavsky AM (2005). Prevalence and treatment of mental disorders, 1990 to 2003. The New England Journal of Medicine, 352(24), 2515–2523. 10.1056/NEJMsa043266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, & Cahill LF (2006). Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage, 30(2), 452–461. 10.1016/j.neuroimage.2005.09.065 [DOI] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Angstadt M, Kessler D, Welsh R, & Liberzon I (2016). Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for Posttraumatic Stress Disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depression and Anxiety, 33(4), 289–299. 10.1002/da.22481 [DOI] [PubMed] [Google Scholar]
- Klein C (2010). Images are not the evidence in neuroimaging. The British Journal for the Philosophy of Science, 61(2), 265–278. 10.1093/bjps/axp035 [DOI] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, & Olff M (2016). Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depression and Anxiety, 33(7), 592–605. doi: 10.1002/da.22478 [DOI] [PubMed] [Google Scholar]
- Kumari V (2006). Do psychotherapies produce neurobiological effects? Acta Neuropsychiatrica, 18(2), 61–70. 10.1111/j.1601-5215.2006.00127.x [DOI] [PubMed] [Google Scholar]
- LeDoux JE, & Pine DS (2016). Using neuroscience to help understand fear and anxiety: A two-system framework. American Journal of Psychiatry, 173(11), 1083–1093. 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, Ding J, Duan X, Qiu C, Lui S, Gong Q, & Zhang W (2010). Selective aberrant functional connectivity of resting state networks in Social Anxiety Disorder. Neuroimage, 52(4), 1549–1558. 10.1016/j.neuroimage.2010.05.010 [DOI] [PubMed] [Google Scholar]
- Liberzon I, & Martis B (2006). Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences, 1071(1), 87–109. 10.1196/annals.1364.009 [DOI] [PubMed] [Google Scholar]
- Liberzon I, & Sripada CS (2008). The functional neuroanatomy of PTSD: A critical review. Progress in Brain Research, 167, 151–168. 10.1016/S0079-6123(07)67011-3 [DOI] [PubMed] [Google Scholar]
- Liberzon I & Garfinkel SN (2009). Functional neuroimaging in Post-traumatic Stress Disorder. In Shiromani PJ, Keane TM, & LeDoux JE (Eds.), Post-traumatic stress disorder: Basic science and clinical practice (pp. 297–317). Humana Press. [Google Scholar]
- Linden D (2006). How psychotherapy changes the brain - the contribution of functional neuroimaging. Molecular Psychiatry, 11(6), 528–538. 10.1038/sj.mp.4001816 [DOI] [PubMed] [Google Scholar]
- Lipka J, Hoffmann M, Miltner WHR, & Straube T (2014). Effects of cognitive-behavioral therapy on brain responses to subliminal and supraliminal threat and their functional significance in specific phobia. Biological Psychiatry, 76(11), 869–877. 10.1016/j.biopsych.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Lohmann G, Stelzer J, Lacosse E, Kumar VJ, Mueller K, Kuehn E, Grodd W, & Scheffler K (2018). LISA improves statistical analysis for fMRI. Nature Communications, 9(1), 4014–9. 10.1038/s41467-018-06304-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, & Hahn T (2016). Functional neuroimaging of psychotherapeutic processes in anxiety and depression: From mechanisms to predictions. Current Opinion in Psychiatry, 29(1), 25–31. 10.1097/YCO.0000000000000218 [DOI] [PubMed] [Google Scholar]
- MacNamara A, DiGangi J, & Phan KL (2016). Aberrant spontaneous and task-dependent functional connections in the anxious brain. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(3), 278–287. 10.1016/j.bpsc.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Messina I, Sambin M, Palmieri A, & Viviani R (2013). Neural correlates of psychotherapy in anxiety and depression: A meta-analysis. Plos One, 8(9), e74657. 10.1371/journal.pone.0074657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I, Sambin M, Beschoner P, & Viviani R (2016). Changing views of emotion regulation and neurobiological models of the mechanism of action of psychotherapy. Cognitive, Affective, & Behavioral Neuroscience, 16(4), 571–587. 10.3758/s13415-016-0440-5 [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, & Girard TA (2012). Neurocircuitry models of Posttraumatic Stress Disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 36(9), 2130–2142. 10.1016/j.neubiorev.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Peres JFP, Newberg AB, Mercante JP, Simao M, Albuquerque VE, Peres MJP, & Nasello AG (2007). Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: A SPECT study. Psychological Medicine, 37(10), 1481. 10.1017/S003329170700997X [DOI] [PubMed] [Google Scholar]
- Peres JFP, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, Moreira-Almeida A, & Lederman H (2011). Police officers under attack: Resilience implications of an fMRI study. Journal of Psychiatric Research, 45(6), 727–734. 10.1016/j.jpsychires.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Porto PR, Ventura P, Volchan E, Figueira I, Oliveira L, & Mari J (2009). Does cognitive behavioral therapy change the brain? A systematic review of neuroimaging in anxiety disorders. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(2), 114–125. 10.1176/jnp.2009.21.2.114 [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Wright JA, & Velicer WF (2008). Evaluating theories of health behavior change: A hierarchy of criteria applied to the transtheoretical model. Applied Psychology an International Review, 57(4), 561–588. 10.1111/j.1464-0597.2008.00345.x [DOI] [Google Scholar]
- Rabellino D, Tursich M, Frewen PA, Daniels JK, Densmore M, Théberge J, & Lanius RA (2015). Intrinsic connectivity networks in post- traumatic stress disorder during sub- and supraliminal processing of threat- related stimuli. Acta Psychiatrica Scandinavica, 132(5), 365–378. 10.1111/acps.12418 [DOI] [PubMed] [Google Scholar]
- Rabellino D, Densmore M, Théberge J, McKinnon MC, & Lanius RA (2018). The cerebellum after trauma: Resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Human Brain Mapping, 39(8), 3354–3374. doi: 10.1002/hbm.24081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, & Phelps EA (2006). Neurocircuitry models of Posttraumatic Stress Disorder and extinction: Human neuroimaging research—Past, present, and future. Biological Psychiatry, 60(4), 376–382. 10.1016/j.biopsych.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Resick PA, & Schnicke MK (1992). Cognitive processing therapy for sexual assault victims. Journal of Consulting and Clinical Psychology, 60(5), 748–756. doi: 10.1037/0022-006X.60.5.748 [DOI] [PubMed] [Google Scholar]
- Reuveni T, Bonne O, Giesser R, Shragai T, Lazarovits G, Isserles M, Schreiber S, Bick AS, & Levin N (2016). Anatomical and functional connectivity in the default mode network of post- traumatic stress disorder patients after civilian and military- related trauma. Human Brain Mapping, 37(2), 589–599. 10.1002/hbm.23051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman JL, Marci CD, Glick DM, Dougherty DD, & Rauch SL (2005). Neuroimaging and the functional neuroanatomy of psychotherapy. Psychological Medicine, 35(10), 1385–1398. 10.1017/S0033291705005064 [DOI] [PubMed] [Google Scholar]
- Rothman KJ (1990). No adjustments are needed for multiple comparisons. Epidemiology, 1(1), 43–46. [PubMed] [Google Scholar]
- Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, & Gray SH (2008). Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry, 71(2), 134–168. 10.1521/psyc.2008.71.2.134 [DOI] [PubMed] [Google Scholar]
- Shang J, Lui S, Meng Y, Zhu H, Qiu C, Gong Q, Liao W, & Zhang W (2014). Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: A resting-state fMRI study. PloS One, 9(5), e96834. 10.1371/journal.pone.0096834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin J, & Liberzon I (2017). Circuit dysregulation and circuit-based treatments in Posttraumatic Stress Disorder. Neuroscience Letters, 649, 133–138. 10.1016/j.neulet.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, & Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169–191. 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvil E, Rusch HL, Sullivan GM, & Neria Y (2013). Neural, psychophysiological, and behavioral markers of fear processing in PTSD: A review of the literature. Current Psychiatry Reports, 15(5), 1–10. 10.1007/s11920-013-0358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Kragel PA, & Rubin DC (2013). Neural networks supporting autobiographical memory retrieval in posttraumatic stress disorder. Cognitive, Affective, & Behavioral Neuroscience, 13(3), 554–566. 10.3758/s13415-013-0157-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T (2016). Effects of psychotherapy on brain activation patterns in anxiety disorders. Zeitschrift Fur Psychologie, 224(2), 62–70. 10.1027/2151-2604/a000240 [DOI] [Google Scholar]
- Tursich M, Ros T, Frewen PA, Kluetsch RC, Calhoun VD, & Lanius RA (2015). Distinct intrinsic network connectivity patterns of post- traumatic stress disorder symptom clusters. Acta Psychiatrica Scandinavica, 132(1), 29–38. 10.1111/acps.12387 [DOI] [PubMed] [Google Scholar]
- Vergara VM, Mayer AR, Damaraju E, Hutchison K, & Calhoun VD (2017). The effect of preprocessing pipelines in subject classification and detection of abnormal resting state functional network connectivity using group ICA. Neuroimage, 145(Pt B), 365–376. 10.1016/j.neuroimage.2016.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D & Clark LA (1999). The PANAS-X: Manual for the positive and negative affect schedule - Expanded form. Iowa Research Online: The University of Iowa’s Institutional Repository, 10.17077/48vt-m4t2 [DOI] [Google Scholar]
- Weathers FW, & Litz BT (1994). Psychometric properties of the clinician-administered PTSD scale, CAPS-1. PTSD Research Quarterly, 5(2), 2–6. 10.1037/pas0000486 [DOI] [Google Scholar]
- Weingarten CP, & Strauman TJ (2015). Neuroimaging for psychotherapy research: Current trends. Psychotherapy Research, 25(2), 185–29. 10.1080/10503307.2014.883088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Patil KR, Hoffstaedter F, Nostro A, Yeo BTT, & Eickhoff SB (2019). Sex classification by resting state brain connectivity. Cerebral Cortex, 10.1093/cercor/bhz129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S & Nieto-Castanon A (2017). Conn functional connectivity SPM toolbox 2017. Neuroimaging Tools and Resources Collaboratory. http://www.nitrc.org/projects/conn [Google Scholar]
- Whitfield-Gabrieli S (n.d.). Artifact detection and OA manual. SWG Imaging, http://web.mit.edu/swg/art/art.pdf [Google Scholar]
- Williams LM (2017). Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: A theoretical review of the evidence and future directions for clinical translation. Depression and Anxiety, 34(1), 9–24. 10.1002/da.22556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu F, Chen H, Li M, Duan X, Xie B, & Chen H (2015). Intranetwork and internetwork functional connectivity alterations in Post-traumatic Stress Disorder. Journal of Affective Disorders, 187, 114–121. 10.1016/j.jad.2015.08.043 [DOI] [PubMed] [Google Scholar]
- Zhu X, Suarez-Jimenez B, Lazarov A, Helpman L, Papini S, Lowell A, … Neria Y (2018). Exposure-based therapy changes amygdala and hippocampus resting-state functional connectivity in patients with PTSD. Depression and Anxiety, 35(10), 974–984. doi: 10.1002/da.22816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Yuan M, Qiu C, Ren Z, Li Y, Wang J, … Zhang Y (2020). Multivariate classification of earthquake survivors with post- traumatic stress disorder based on large-scale brain networks. Acta Psychiatrica Scandinavica, 141(3), 285–298. doi: 10.1111/acps.13150 [DOI] [PubMed] [Google Scholar]


