Abstract
Individuals with schizophrenia exhibit widespread cortical thinning associated with illness severity and deficits in cognition. However, intact cortical thickness (CTh) may serve as a protective factor. The current study sought to examine changes in CTh in response to auditory targeted cognitive training (TCT) in individuals with recent onset schizophrenia. Participants underwent MRI scanning and a cognitive assessment before and after being randomly assigned to 40 hours of either TCT (N=21) or a computer games control condition (CG; N=22) over 16 weeks. Groups did not differ at baseline on demographic variables or measures of CTh. At the level of group averages, neither group showed significant pre-post changes in CTh in any brain region. However, changes in CTh related to individual differences in treatment outcome, as improved global cognition in the TCT group corresponded to reduced cortical thinning in frontal, temporal, parietal, and occipital lobes. These relationships were not observed in the CG group. The current findings suggest that TCT may be neuroprotective in early schizophrenia, such that individuals who improved in response to training also showed a reduction in cortical thinning that may be otherwise hastened due to age and illness.
Keywords: Early Schizophrenia, Cognitive Training, Cortical Thickness, Cognition, Structural MRI
1. Introduction
Schizophrenia is characterized by widespread cortical thinning that appears to be present at the onset of the illness and progressive over its course (Cannon et al. 2015; Haijma et al. 2013). Cortical thinning has appeared most pronounced in frontal and temporal regions in first episode psychosis (Schultz et al. 2010) as well as across a large sample (N=4,474) of individuals with a lifetime diagnosis of schizophrenia (Van Erp et al. 2018), though other studies have also observed reductions in occipital and parietal regions (Sprooten et al. 2013). While the pathophysiological mechanisms driving reduced cortical thickness (CTh) in schizophrenia remain unclear, it does appear to correspond to aspects of clinical severity (Xiao et al. 2015), and may offer important insights about the relationship between cognitive dysfunction and functional outcomes (Tully et al. 2014).
In healthy adults, the relationship between CTh and aspects of cognition have been previously described. Greater CTh has been generally observed to correspond to higher intelligence quotient (IQ) (Schnack et al. 2015), though this relationship is developmentally non-linear, with higher achieving children having comparatively lower CTh in their youth (Shaw et al. 2006). In patients with schizophrenia, varying patterns have been observed relating aspects of cognitive functioning to CTh, primarily in frontal and temporal regions. In a chronic schizophrenia sample, verbal learning was found to positively correlate with CTh in the right temporal and frontal gyrus, while verbal IQ positively correlated with bilateral frontal and temporal sub-regions (Hartberg et al. 2010). Similar findings were observed in a different study of chronic schizophrenia patients, showing positive relationships between verbal working memory and CTh in the right temporal lobe, and verbal processing and CTh in bilateral frontal regions (Ehrlich et al. 2012).
Many of these observations overlap with similar relationships found in the healthy controls of these studies, though schizophrenia patients are well understood to exhibit deficits across most all cognitive domains (Heinrichs and Zakzanis 1998). Interestingly, in a study that compared neuropsychologically impaired patients with schizophrenia with those who appeared to be more neuropsychologically spared, the unaffected patients showed less cortical thinning compared to those exhibiting cognitive deficits (Cobia, Csernansky, and Wang 2011). This may suggest that increased cortical thickness could serve as a protective factor in schizophrenia or may be neuroplastic in response to treatments that target neuropsychological deficits.
Animal findings have demonstrated that CTh is neuroplastic in response to environmental factors. Rats were found to have increased cortical thickness after 138 days in an enriched environment (i.e., a cage with toys) compared to a group of rats in a non-enriched environment (Diamond et al. 1985). Similarly, longitudinal studies of meditating humans have established that experience-dependent plasticity may result in structural changes in specific cortical areas (Tang, Hölzel, and Posner 2015). In patients with schizophrenia, previous work has established that cognitive training interventions can influence both structural and functional plasticity (Penadés et al. 2013; Ramsay and MacDonald 2015), and may be neuroprotective to progressive structural dysfunction found to be associated with cognitive decline in the illness (Eack et al. 2010). In a study that examined the long-term effects of a cognitive enhancement intervention targeting both cognition and social functioning, patients with schizophrenia in the active intervention showed gray matter preservation in the hippocampus, and gray matter increases in the amygdala compared to patients receiving treatment as usual (Eack et al. 2010). In a diffusion tensor imaging study examining a paper and pencil cognitive remediation approach, schizophrenia patients who received the active treatment showed increased white matter connectivity in the corpus callosum that corresponded to increased functional activation on a working memory task (Penadés et al. 2013). In the same subjects, preserved prefrontal and temporal CTh was predictive of response to the cognitive remediation intervention (Penadés et al. 2016), but whether such interventions influence CTh by way of neuroplasticity or neuroprotection remains unclear.
In our own laboratory, changes in global cognition following 40 hours of targeted cognitive training (TCT) of the auditory and working memory system were found to coincide with changes in thalamic volume in patients with recent onset schizophrenia (SZ)(Ramsay, Fryer, et al. 2018). These findings suggest that TCT may drive cognitive improvements by preserving or even enhancing the morphometric structure of the thalamus, which is well understood to have rich cortical connections essential to sensory processing and subsequent cognitive functioning (Sherman 2016). While TCT is hypothesized to drive neuroplasticity in distributed frontal and temporal brain regions (Vinogradov, Fisher, and de Villers-Sidani 2012), and has demonstrated functional plasticity in these regions (Dale et al. 2016; Ramsay et al. 2020), whether such an intervention directly affects cortical thickness remains unclear. The current study assessed CTh in patients with early schizophrenia (SZ) who were randomized to undergo 40 hours of either TCT or a computer games control condition (CG). We hypothesized that improved global cognition would coincide with increases in CTh in frontal and temporal regions in response to TCT. We also sought to extend our previous finding in this sample (Ramsay, Fryer, et al. 2018) to determine whether changes in CTh corresponded to previously observed changes in thalamic volume. We hypothesized that changes in frontal and temporal CTh would correlate with changes in left thalamic volume.
2. Methods
2.1. Participants
Participants (N=44) were included from a larger clinical trial examining remote cognitive training in patients with recent onset SZ (Fisher et al. 2015) (Clinicaltrials.gov NTC00694889). Participants were recruited from the University of California, San Francisco Early Psychosis Clinic and other community clinics. Participants met the following inclusion/exclusion criteria: (a) diagnosis of schizophrenia, schizoaffective disorder, or schizophreniform disorder confirmed using the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer, Gibbon, & Williams, 1997); (b) onset of first psychotic episode within the last 5 years (M=1.70; SD=1.30); (c) good general physical health; (d) age between 14–36 years old; (e) fluent and proficient in English; (f) IQ of 70 or greater; (g) no known neurological disorder; (h) no substance dependence in the past year; (i) no MRI contraindications. Additionally, all participants were outpatients (with no prior hospitalization in the last 3 months) on stable doses of medication (with no changes 1 month prior to participation). All participants 18 and older provided informed consent, while participants under 18 years old provided assent in addition to parent or legal guardian consent. Consenting and baseline assessment procedures were conducted prior to random assignment (stratified by IQ, gender, and symptom severity) to one of the two training conditions. The Institutional Review Board at the University of California, San Francisco approved all study procedures.
2.2. Training Procedure
The targeted cognitive training (TCT) procedure is described in detail elsewhere (Fisher et al. 2015). Briefly, participants completed either the TCT (N=22) or computer games (CG; N=22) control intervention remotely on a laptop computer provided by the research staff. Participants were asked to complete 40 hours of training over the course of 8 weeks (1 hour/day, 5 days/week). Study staff contacted participants 1–2 times per week and as needed to discuss their progress. Check-in appointments were also conducted after every 10 training sessions, and participants were paid $5 for each completed hour, an additional $20 for 10 completed sessions, and $30 after completing the full 40 hours of training.
TCT was developed and provided free of charge by Posit Science, Inc. The computerized training exercises were designed to improve the speed and accuracy of early auditory processing as well as engage auditory and verbal working memory (Fisher et al. 2009). The training exercises were adaptive and sought to maintain 80–85% accuracy across trials. In each session, participants completed 4 to 6 exercises over the course of one hour. Early in the intervention, exercises focused on basic sensory processes (i.e. sound sweeps), before progressing onto more complicated tasks of auditory working memory (i.e. verbal list learning). Compliance was monitored remotely via electronic data upload.
The CG control condition was designed to be matched to the TCT on the basis of computer exposure, contact with research personnel, monetary incentives, with non-specific engagement of attention and motivation. Participants had access to 16 different commercially available computer games (i.e. Hangman, Dominoes, Checkers), and played 4–5 different games per training session. Participants in the TCT group completed an average of 36.48 (SD=7.21) hours of training, and an average of 39.82 (SD=.59) hours in the CG group (t=−2.12; p=.047). The total number of days it took to complete the training (and subsequent time between scans) did not differ between the TCT (M=128.90; SD=53.31) and CG (M=142.00; SD=57.85) conditions (t=−.76; p=.45).
2.3. Assessment Procedures
Cognitive assessments were conducted by study staff blind to group assignment, and all participants were paid $20 for each completed assessment. A battery of MATRICS-recommended measures (Nuechterlein et al. 2008) (Trail Making Test Part A; Animal Fluency; Letter-Number Span; Wechsler Memory Scale-III Spatial Span; Hopkins Verbal Learning Test-Revised; Brief Visuospatial Memory Test-Revised; Delis-Kaplan Executive Functioning System Tower Test) were used to assess cognition. All subtests were converted to age-normed Z-scores before calculating Global Cognition summary Z-scores. One participant was subsequently removed due to observed insufficient effort during the follow-up assessments (2.87 SDs below the mean), which caused the data point to be a statistical outlier. Therefore, the final sample included N=21 in the TCT condition and N=22 in the CG condition.
2.4. Neuroimaging Procedures and Pre-processing
Structural MRIs were collected at baseline, and following the intervention (mean of 19.58 weeks, SD of 7.93 weeks). Whole brain volumetric magnetization-prepared rapid gradient echo (MPRAGE) scans were acquired with TR=2300ms, TE=2.95ms, TI=900ms, Flip Angle=9 degrees, Base Resolution=256, Slice Thickness=1.2mm, and anterior to posterior collection in the sagittal plane. Cortical segmentation was performed using FreeSurfer (v5.1), a software program used to process and analyze brain MRI images (Fischl, Sereno, and Dale 1999; Fischl and Dale 2000, Segonne, Pacheco, and Fischl 2007). FreeSurfer carries out Talairach transformations, atlas registration, white and gray matter segmentation, tessellation of gray and white matter boundaries, and places borders around gray/white matter and gray/cerebrospinal fluid boundaries based on an intensity gradient. Following this step, each image was manually inspected for segmentation errors and was corrected to ensure that the white/gray matter boundaries were accurately delineated. This process was applied separately to data from each time-point in order to remove the possibility of a temporal bias.
Next, images were passed through the Freesurfer longitudinal volumetric pipeline (Reuter et al. 2012), where a within-subject template (base) was created by averaging the two time-points together in order to estimate the average anatomy of the subject across time based on the intensity median of each voxel. The base was then manually checked for registration and overlay errors. Each time-point was then processed longitudinally, and automatically compared to the base. Each longitudinal time-point was manually checked for overlay, registration, and boundary errors. Individual sub-lobar regions of interest (ROIs) based on parcellation data from the Desikan-Killiany atlas (Desikan et al. 2006) were grouped by the four major lobes in each hemisphere: frontal, temporal, parietal, and occipital.
2.5. Planned Analyses
Though our primary hypotheses regarded the frontal and temporal cortices, we expanded our analysis strategy to include parietal and occipital cortices for thoroughness. First, we used linear models to regress out the effect of age and gender on CTh by residualizing each Desikan sub-lobar ROI individually. These residualized individual sub-lobar ROIs were grouped into each of the four major brain lobes in each hemisphere by taking their average. To assess whether either condition showed significant change in response to the intervention, we performed a repeated-measures ANOVA to examine change in CTh (Time 1 to Time 2) between the TCT and CG groups. We also followed up within groups using one-sample t-tests to determine whether either group showed significant change from baseline.
Next, we sought to assess whether the relationship between change in CTh and change in global cognition differed between groups. We used linear regression models to examine whether change in CTh of each lobe (N=8) was predictive of change in global cognition and formally compared the regression slopes between the TCT and CG groups by examining the group by change in CTh interaction. We used a false-discovery rate (FDR) correction to control for multiple tests. To account for potential outliers driving the observed effects, we also conducted linear models using Winsorized data that replaced extreme values (in the 5%-quantile) with the nearest value. These Winsorized tests also used FDR to control for multiple comparisons and are reported alongside the primary results. We also examined relationships with medication status by performing correlations between change in CTh and baseline chlorpromazine equivalents (CPZ). Following between groups tests at the lobe level, we conducted post-hoc tests that followed up on the constituent sob-lobar ROIs for each region, by performing linear regression models predicting change in global cognition to identify ROIs driving any lobar effects (uncorrected p-values).
Last, we examined the relationship between change in CTh in the lobar regions and change in left thalamic volume (age, gender, and intracranial volume were also regressed out of this measure), which was previously shown to relate to change in global cognition in the same participants (Ramsay, Fryer, et al. 2018). We used the Sobel test for mediation in the TCT group alone to determine whether change in lobar CTh mediated the effects of the relationship between change in left thalamic volume predicting change in global cognition.
3. Results
3.1. Group Differences
Forty-three participants were randomized to either TCT (N=21) or CG (N=22) and completed 20–40 hours of training as well as pre- and post-training MRI scans (See Figure 1: CONSORT Diagram). The two groups did not differ on baseline factors related to gender, age, duration of illness, education, estimated IQ, baseline global cognition, chlorpromazine (CPZ) equivalence, or symptoms (all p’s>.13; See Table 1). Additionally, no lobar regions differed between groups in either hemisphere at baseline (all p’s>.47). In the parent clinical trial, participants in the TCT group showed a significant change in global cognition compared to the CG group (Fisher et al. 2015). In the subset of participants in the current study, change in global cognition between groups did not statistically differ (t=1.85; p=.07; d=.57), though we did observe a significant increase in global cognition in the TCT group alone (t=2.47; p=.02), and no statistical change in the CG group (t=.07; p=.94). We then performed repeated-measures ANOVAs to examine change in CTh between the TCT and CG groups. No lobar ROIs in either hemisphere showed a group by time interaction effect after controlling for age and gender (all p’s>.40; See Supplementary Table 1). Additionally, neither group independently showed significant change from baseline using paired t-tests (all p’s>.10).
Figure 1.
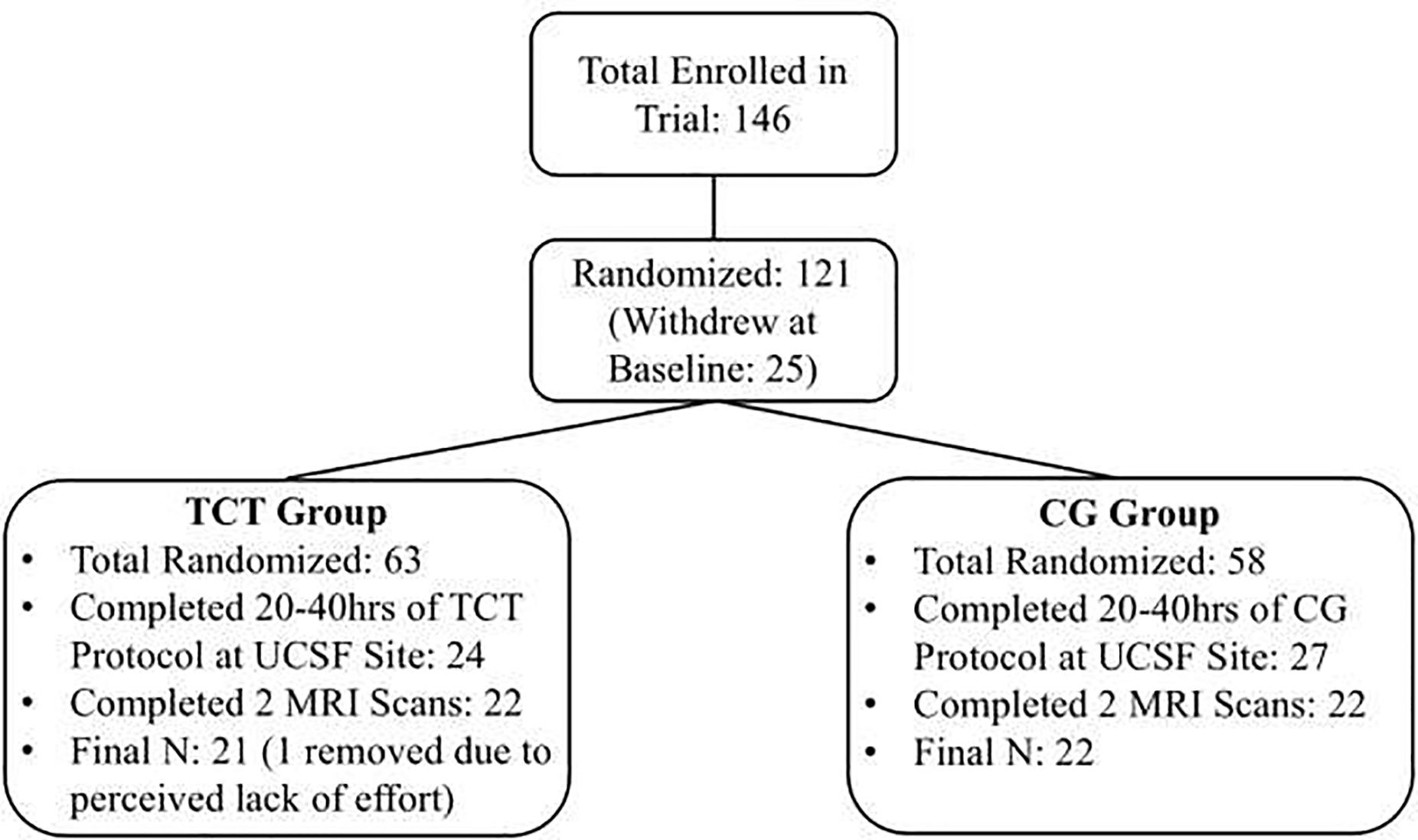
CONSORT Diagram
Figure 1 Note: While the parent clinical trial was conducted at multiple sites, the current imaging data was only collected from the University of California San Francisco (UCSF). Because imaging data was not collected as part of this site’s protocol until after the cognitive training trial was already underway, not all participants who were randomized had the opportunity to undergo an MRI scan.
Table 1.
Demographics
| TCT (N=21; Male=13) | CG (N=22; Male=16) | |||||
|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | t | p |
| Age | 22.79 | 4.57 | 20.87 | 3.58 | 1.53 | 0.13 |
| Duration of Illness | 1.68 | 1.25 | 1.71 | 1.38 | –0.08 | 0.94 |
| Education | 12.76 | 1.89 | 12.55 | 3.21 | 0.26 | 0.79 |
| Verbal IQ | 106.05 | 14.2 | 103.5 | 15.33 | 0.57 | 0.57 |
| Global Cognition (Z) | –1.00 | 0.82 | –0.77 | 0.70 | –0.98 | 0.33 |
| CPZ Equivalence | 361.11 | 456.56 | 387.08 | 346.47 | –0.16 | 0.87 |
| PANSS Total | 59.19 | 9.71 | 65.71 | 18.82 | –1.41 | 0.17 |
| PANSS Positive | 11.9 | 3.43 | 13.24 | 4.76 | –1.04 | 0.31 |
| PANSS Negative | 16.43 | 6.52 | 17.67 | 7.17 | –0.59 | 0.56 |
| PANSS General | 30.86 | 5.32 | 34.81 | 10.83 | –1.50 | 0.14 |
3.2. Frontal Cortex Change in Cortical Thickness
Next, we used linear models to examine the relationship between change in global cognition (Time 2 - Time 1) and change in CTh (Time 2 - Time 1) in both TCT and CG in each lobe (left and right) individually. We followed up on these relationships by examining post-hoc individual group correlations, and sub-lobar ROIs that may be driving the observed effects. In the left frontal cortex, we observed a between groups slope difference (t=2.42; FDR-p=.04; Winsorized-p=.048; Figure 2A), driven by a significant positive correlation between change in global cognition and change in CTh in the TCT group (r=.50; p=.02), and no significant relationship in CG group (r=−.18; p=.42). Changes in CTh did not significantly correlate with medication status in either group (p’s>.06). Sub-lobar regions driving the between group slope difference (uncorrected p<.05) included left superior frontal gyrus, left rostral middle frontal gyrus, left pars triangularis and orbitalis, and left lateral orbitofrontal cortex (see Table 2C). The right frontal cortex also showed a strong between groups slope difference (t=3.29; FDR-p=.008; Winsorized-p=.01; Figure 2B) with a positive correlation in the TCT group (r=.59; p=.004) and a non-significant negative relationship in the CG group (r=−.29; p=.18). Again, change in CTh did not correlate with medication dose in either group (p’s>.12). Sub-lobar regions contributing to the right frontal effect (uncorrected p<.05) included right frontal pole, right superior frontal gyrus, right rostral middle frontal gyrus, right pars opercularis, triangularis, and orbitalis, and right pre- and paracentral gyrus (see Table 2D).
Figure 2.
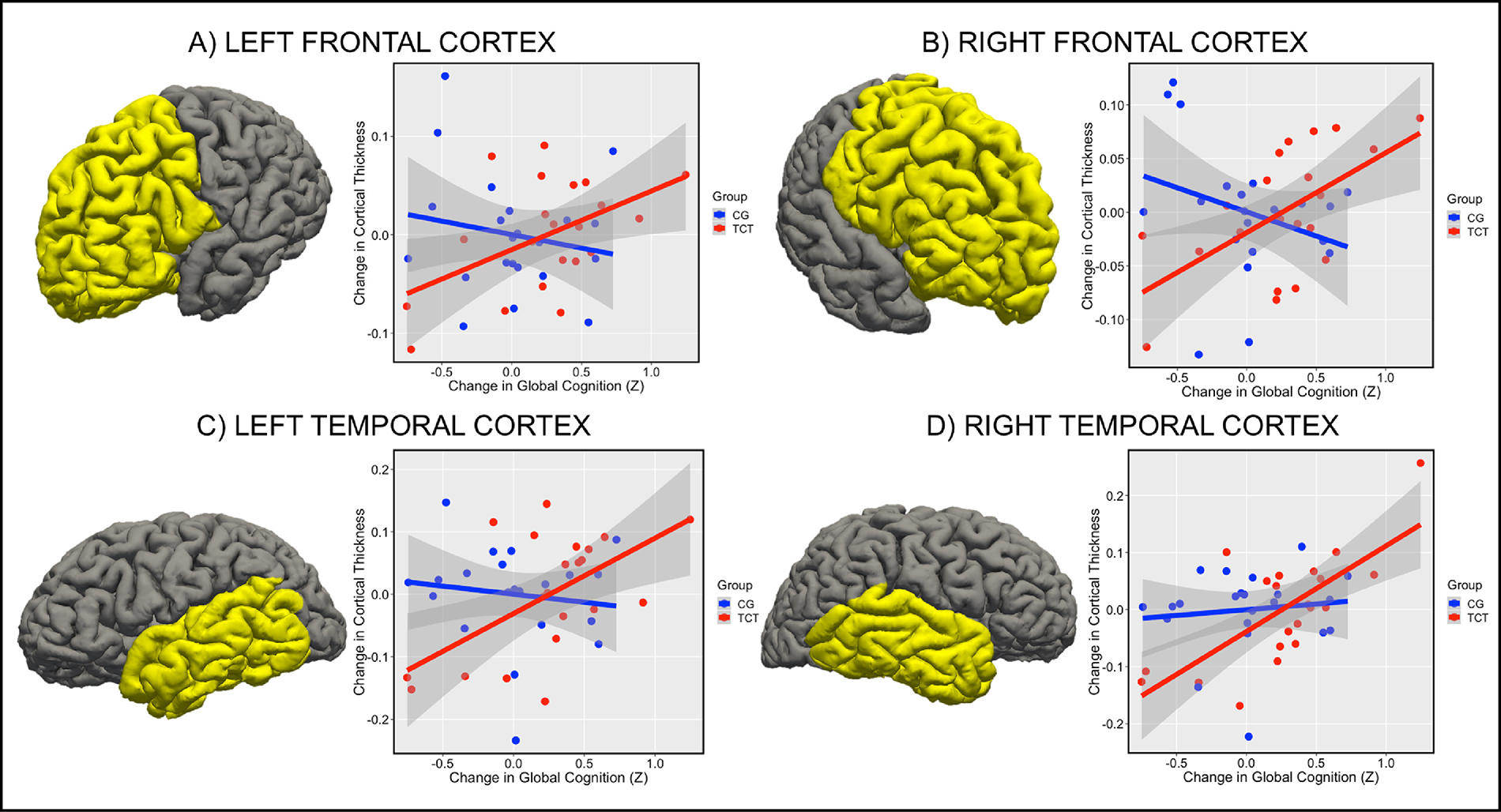
Figure 2 Note: Changes in frontal and temporal cortex predict changes in global cognition in TCT but not CG. (A) Slope difference between TCT and CG in left frontal cortex (t=2.42; FDR-p=.04; Winsorized-p=.048), (B) right frontal cortex (t=3.29; FDR-p=.008; Winsorized-p=.01), (C) left temporal cortex (t=2.42; FDR- p=.04; Winsorized-p=.048), and (D) right temporal cortex (t=1.93; FDR- p=.07; Winsorized-p=.11).
Table 2.
Relationships Between Change in Cortical Thickness and Change in Cognition
| Slopes Difference | TCT Correlation | CG Correlation | ||||
|---|---|---|---|---|---|---|
| Region of Interest | t | p/FDR-p/Winsorized-p | r | p | r | p |
| Left Frontal | 2.42 | 0.02/.04/.048 | 0.5 | 0.02 | −0.18 | 0.42 |
| Left Superior Frontal Gyrus | 2.46 | 0.02 | 0.43 | 0.049 | −0.28 | 0.2 |
| Left Rostral Middle Frontal Gyrus | 2.06 | 0.04 | 0.37 | 0.1 | −0.25 | 0.27 |
| Left Caudal Middle Frontal Gyrus | 1.13 | 0.26 | 0.12 | 0.59 | −0.25 | 0.26 |
| Left Pars Opercularis | 1.39 | 0.17 | 0.4 | 0.07 | 0.02 | 0.95 |
| Left Pars Triangularis | 2.66 | 0.01 | 0.65 | 0.001 | −0.13 | 0.56 |
| Left Pars Orbitalis | 2.74 | 0.01 | 0.61 | 0.003 | −0.05 | 0.82 |
| Left Lateral Orbitofrontal Cortex | 2.09 | 0.04 | 0.41 | 0.06 | −0.22 | 0.33 |
| Left Medial Orbitofrontal Cortex | 0.76 | 0.45 | 0.21 | 0.36 | −0.03 | 0.9 |
| Left Precentral Gyrus | 1.72 | 0.09 | 0.23 | 0.31 | −0.31 | 0.16 |
| Left Paracentral Gyrus | 0.94 | 0.35 | 0.06 | 0.78 | −0.24 | 0.28 |
| Left Frontal Pole | 1.71 | 0.09 | 0.31 | 0.17 | −0.24 | 0.28 |
| Left Rostral Anterior Cingulate Cortex | 0.37 | 0.71 | 0.04 | 0.86 | 0.18 | 0.42 |
| Left Caudal Anterior Cingulate Cortex | −0.32 | 0.75 | 0.05 | 0.85 | 0.12 | 0.58 |
| Right Frontal | 3.29 | 0.002/.008/.01 | 0.59 | 0.005 | −0.3 | 0.18 |
| Right Superior Frontal Gyrus | 2.92 | 0.01 | 0.33 | 0.15 | −0.52 | 0.01 |
| Right Rostral Middle Frontal Gyrus | 2.85 | 0.01 | 0.46 | 0.04 | −0.37 | 0.09 |
| Right Caudal Middle Frontal Gyrus | 1.9 | 0.07 | 0.27 | 0.23 | −0.31 | 0.16 |
| Right Pars Opercularis | 2.54 | 0.02 | 0.62 | 0.003 | −0.13 | 0.58 |
| Right Pars Triangularis | 2.13 | 0.04 | 0.49 | 0.02 | −0.11 | 0.63 |
| Right Pars Orbitalis | 2.12 | 0.04 | 0.53 | 0.01 | −0.09 | 0.7 |
| Right Lateral Orbitofrontal Cortex | 1.99 | 0.05 | 0.46 | 0.04 | −0.08 | 0.71 |
| Right Medial Orbitofrontal Cortex | 1.33 | 0.19 | 0.18 | 0.44 | −0.25 | 0.26 |
| Right Precentral Gyrus | 2.14 | 0.04 | 0.42 | 0.06 | −0.25 | 0.25 |
| Right Paracentral Gyrus | 2.46 | 0.02 | 0.36 | 0.11 | −0.38 | 0.08 |
| Right Frontal Pole | 2.21 | 0.03 | 0.55 | 0.01 | −0.02 | 0.92 |
| Right Rostral Anterior Cingulate Cortex | 1.74 | 0.09 | 0.11 | 0.63 | −0.44 | 0.04 |
| Right Caudal Anterior Cingulate Cortex | −0.38 | 0.7 | −0.12 | 0.61 | −0.002 | 0.99 |
| Left Temporal | 2.38 | 0.02/.04/.048 | 0.58 | 0.006 | −0.13 | 0.55 |
| Left Temporal Pole | 2.29 | 0.03 | 0.38 | 0.09 | −0.33 | 0.14 |
| Left Inferior Temporal Gyrus | 2.17 | 0.04 | 0.45 | 0.04 | −0.16 | 0.47 |
| Left Middle Temporal Gyrus | 3.02 | 0.004 | 0.64 | 0.002 | −0.16 | 0.46 |
| Left Superior Temporal Gyrus | 1.74 | 0.09 | 0.31 | 0.17 | −0.2 | 0.37 |
| Left Banks of Superior Temporal Sulcus | 2.26 | 0.03 | 0.42 | 0.06 | −0.25 | 0.27 |
| Left Fusiform Gyrus | 2.26 | 0.03 | 0.31 | 0.17 | −0.37 | 0.08 |
| Left Entorhinal Cortex | 0.6 | 0.55 | 0.61 | 0.003 | 0.41 | 0.06 |
| Left Transvrse Temporal Cortex | 1.56 | 0.13 | 0.3 | 0.19 | −0.21 | 0.35 |
| Left Parahippocampal Cortex | 1.51 | 0.14 | 0.45 | 0.04 | −0.01 | 0.95 |
| Right Temporal | 1.93 | 0.06/.07/.11 | 0.72 | 0.0002 | 0.11 | 0.61 |
| Right Temporal Pole | 1.16 | 0.25 | 0.39 | 0.08 | 0.004 | 0.99 |
| Right Inferior Temporal Gyrus | 2.11 | 0.04 | 0.62 | 0.002 | 0.03 | 0.91 |
| Right Middle Temporal Gyrus | 2.92 | 0.03 | 0.72 | 0.0002 | 0.19 | 0.41 |
| Right Superior Temporal Gyrus | 1.28 | 0.21 | 0.63 | 0.002 | 0.07 | 0.76 |
| Right Banks of Superior Temporal Sulcus | 2.2 | 0.03 | 0.65 | 0.001 | 0.21 | 0.36 |
| Right Fusiform Gyrus | 2.05 | 0.046 | 0.58 | 0.005 | −0.11 | 0.61 |
| Right Entorhinal Cortex | 1.55 | 0.13 | 0.63 | 0.002 | 0.13 | 0.57 |
| Right Transvrse Temporal Cortex | 1.14 | 0.26 | 0.36 | 0.11 | 0.09 | 0.69 |
| Right Parahippocampal Cortex | 0.92 | 0.36 | 0.66 | 0.001 | 0.12 | 0.59 |
| Left Parietal | 2.24 | 0.03/.04/.08 | 0.34 | 0.14 | −0.34 | 0.12 |
| Left Superior Parietal | 1.89 | 0.07 | 0.22 | 0.34 | −0.37 | 0.09 |
| Left Inferior Parietal | 0.34 | 0.73 | −0.11 | 0.63 | −0.25 | 0.25 |
| Left Supramarginal Gyrus | 3.04 | 0.004 | 0.48 | 0.03 | −0.38 | 0.08 |
| Left Postcentral Gyrus | 3.19 | 0.002 | 0.4 | 0.07 | −0.52 | 0.01 |
| Left Precuneus | 1.88 | 0.07 | 0.25 | 0.28 | −0.34 | 0.12 |
| Left Posterior Cinguate Cortex | 0.16 | 0.87 | 0.3 | 0.19 | 0.21 | 0.34 |
| Left Isthmus Cingulate Cortex | 2.1 | 0.04 | 0.43 | 0.05 | 0.27 | 0.22 |
| Right Parietal | 1.61 | 0.12/.12/.11 | 0.46 | 0.04 | −0.004 | 0.99 |
| Right Superior Parietal | 1.95 | 0.06 | 0.33 | 0.14 | −0.26 | 0.24 |
| Right Inferior Parietal | 2.03 | 0.049 | 0.61 | 0.004 | 0.06 | 0.8 |
| Right Supramarginal Gyrus | 0.98 | 0.33 | 0.36 | 0.11 | 0.14 | 0.55 |
| Right Postcentral Gyrus | 2.14 | 0.44 | 0.48 | 0.03 | −0.15 | 0.5 |
| Right Precuneus | 1 | 0.32 | 0.32 | 0.16 | 0.03 | 0.89 |
| Right Posterior Cinguate Cortex | 1.5 | 0.14 | 0.28 | 0.21 | −0.18 | 0.42 |
| Right Isthmus Cingulate Cortex | −0.21 | 0.84 | 0.2 | 0.39 | 0.31 | 0.16 |
| Left Occipital | 2.33 | 0.03/.04/.048 | 0.38 | 0.09 | −0.31 | 0.16 |
| Left Lateral Occipital | 1.04 | 0.31 | 0.13 | 0.59 | −0.22 | 0.33 |
| Left Lingual Gyrus | 2.28 | 0.03 | 0.43 | 0.05 | −0.19 | 0.39 |
| Left Cuneus | 2.17 | 0.04 | 0.35 | 0.12 | −0.29 | 0.19 |
| Left Paricalcarine | 1.42 | 0.16 | 0.14 | 0.55 | −0.34 | 0.12 |
| Right Occipital | 3.45 | 0.001/.008/.008 | 0.65 | 0.001 | −0.22 | 0.33 |
| Right Lateral Occipital | 3.07 | 0.004 | 0.71 | 0.0003 | −0.004 | 0.99 |
| Right Lingual Gyrus | 2.01 | 0.05 | 0.52 | 0.02 | −0.07 | 0.77 |
| Right Cuneus | 2.45 | 0.02 | 0.43 | 0.05 | −0.27 | 0.22 |
| Right Paricalcarine | 2.51 | 0.02 | 0.38 | 0.09 | −0.37 | 0.09 |
3.3. Temporal Cortex Change in Cortical Thickness
We observed a between groups slope difference in the left temporal cortex (t=2.38; FDR-p=.04; Winsorized-p=.048; Figure 2C) characterized by a positive correlation in the TCT condition (r=.58; p=.006) and no significant relationship in the CG condition (r=−.13; p=.55). Change in CTh did not relate to medication status in either group (p’s>.31). Next, we examined the sub-lobar regions in the left temporal lobe individually. Regions that showed a significant (p<.05 uncorrected) slope difference between groups included the left temporal pole, left inferior temporal gyrus, left middle temporal gyrus, left banks of the superior temporal sulcus, and left fusiform gyrus (See Table 2A). We did not observe a significant group difference in slopes in the right temporal cortex (t=1.93; FDR-p=.07; Winsorized-p=.11; Figure 2D), though we did observe a strong positive correlation in TCT (r=.72 p=.0002) and no relationship in CG (r=.11; p=.61). Again, change in CTh did not relate to medication status in either group (p’s>.31). Sub-lobar regions showing a significant slope difference (p<.05 uncorrected) included right middle and inferior temporal gyrus, and the right banks of the superior temporal sulcus (see Table 2B).
3.4. Parietal Cortex Change in Cortical Thickness
Slope differences in the left parietal cortex (t=2.24; FDR-p=.04; Winsorized-p=.08; Figure 3A) were driven by a non-significant positive relationship in the TCT group (r=.34; p=.14) and a non-significant negative one in the CG group (r=−.34; p=.12). Neither group showed a significant relationship between change in CTh and medication dose (p’s>.16). Sub-lobar regions driving the difference in slopes (p<.05 uncorrected) included the left supramarginal gyrus, left postcentral gyrus, and left isthmus of the cingulate cortex (see Table 2E). The right parietal cortex did not show a significant slope difference (t=1.61; FDR-p=.12; Winsorized-p=.11; Figure 3B), though the TCT group showed a significant positive relationship (r=.46; p=.04), while the CG group did not (r=−.004; p=.99). While change in CTh in the TCT group did not relate to medication dose (p=.38), we did observe a significant relationship in the CG group, where higher CPZ equivalents were related to cortical thinning (r=−.66; p=.006). The right post central gyrus was the only sub-lobar region that showed a significant slope difference effect (p<.05 uncorrected; See Table 2F).
Figure 3.
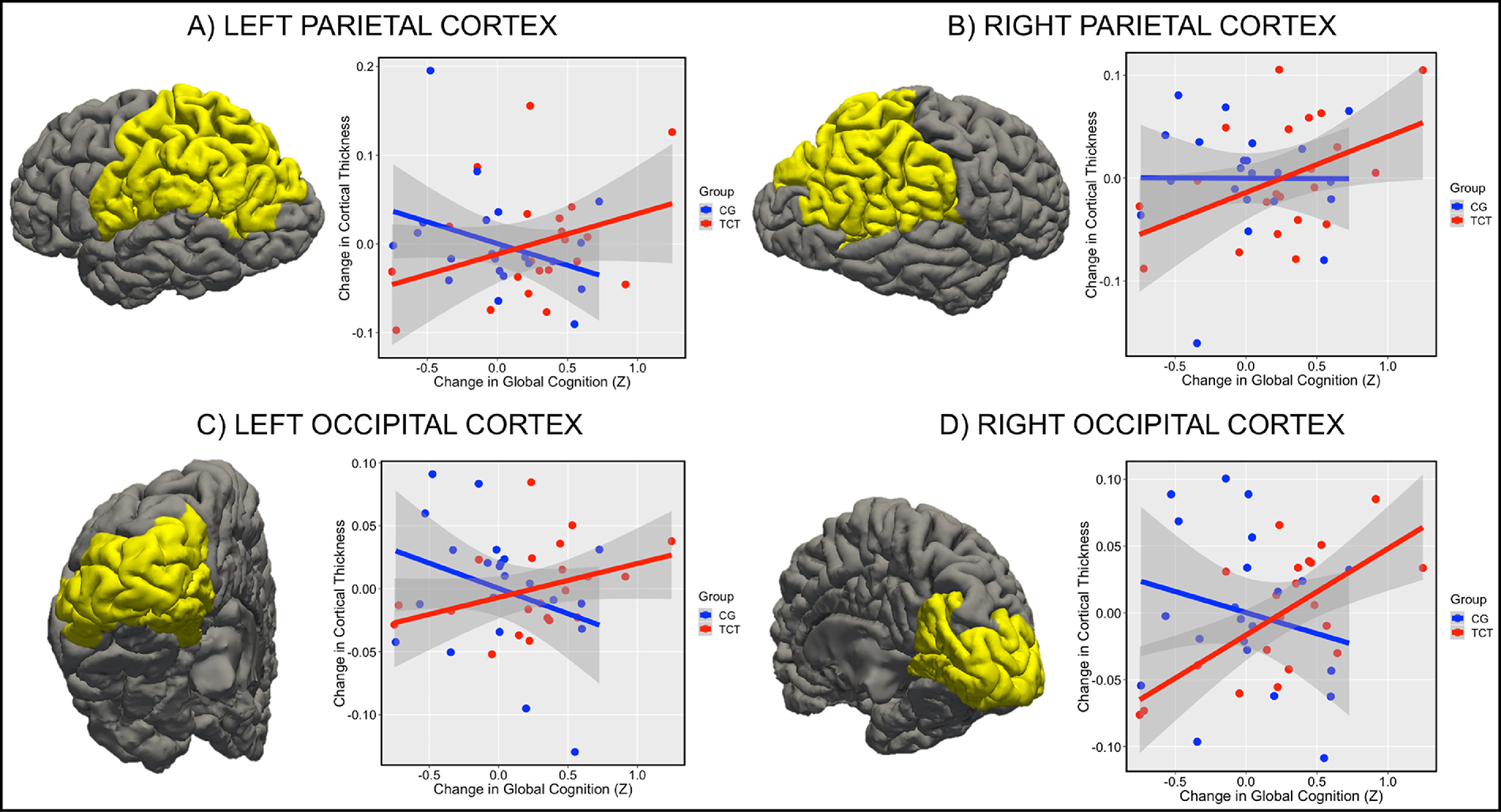
Figure 3 Note: Changes in parietal and occipital cortex predict changes in global cognition in TCT but not CG. (A) Slope difference between TCT and CG in left parietal cortex (t=2.24; FDR-p=.04; Winsorized-p=.08), (B) right parietal cortex (t=1.61; FDR-p=.12; Winsorized-p=.11), (C) left occipital cortex (t=2.33; FDR-p=.04; Winsorized-p=.048), and (D) right occipital cortex (t=3.45; FDR-p=.008; Winsorized-p=.008).
3.5. Occipital Cortex Change in Cortical Thickness
Finally, we examined the left occipital cortex, which showed a significant between groups slope difference (t=2.33; FDR-p=.04; Winsorized-p=.048; Figure 3C) driven by a non-significant positive relationship in the TCT group (r=.38; p=.09), and a non-significant negative relationship in the CG group (r=−.31; p=.16). Change in CTh did not relate to medication dose in the TCT group (p=.22) but was negatively correlated in the CG group (r=−.81; p=.0001). The left lingual gyrus was the only sub-lobar region showing a between group slope difference (p<.05 uncorrected; see Table 2G). There was also a significant between groups slope difference in the right occipital cortex (t=3.45; FDR-p=.008; Winsorized-p=.008; Figure 3D) characterized by a strong positive relationship in the TCT group (r=.65; p=.001), and no relationship in the CG group (r=−.22; p=.33). Again, change in CTh showed no relationship to medication dose in the TCT group (p=.94), and was negatively correlated in the CG group (r=−.74; p=.001). The right cuneus and right pericalcarine were significant sub-lobar regions contributing to the slope difference effect (p<.05 uncorrected; see Table 2H).
3.6. Relationship Between Change in Cortical Thickness Across Lobes
We followed up these analyses by examining correlation matrices measuring change in CTh between each possible lobe in both groups. In the TCT group, all possible correlations between lobes except for one (right frontal and right occipital lobe) were found to be significant after FDR-correction (p<.05; see Figure 4A), suggesting that changes in CTh in specific regions broadly correlated with changes in most other regions as well. A similar but more restricted pattern was observed in the CG group after FDR-correction (p<.05; see Figure 4B), indicating that regional changes positively correlated, but in general, not as strongly as in the TCT group. Differences between the two correlation matrices were also found to be significant (χ2=124; p=.000005)
Figure 4.
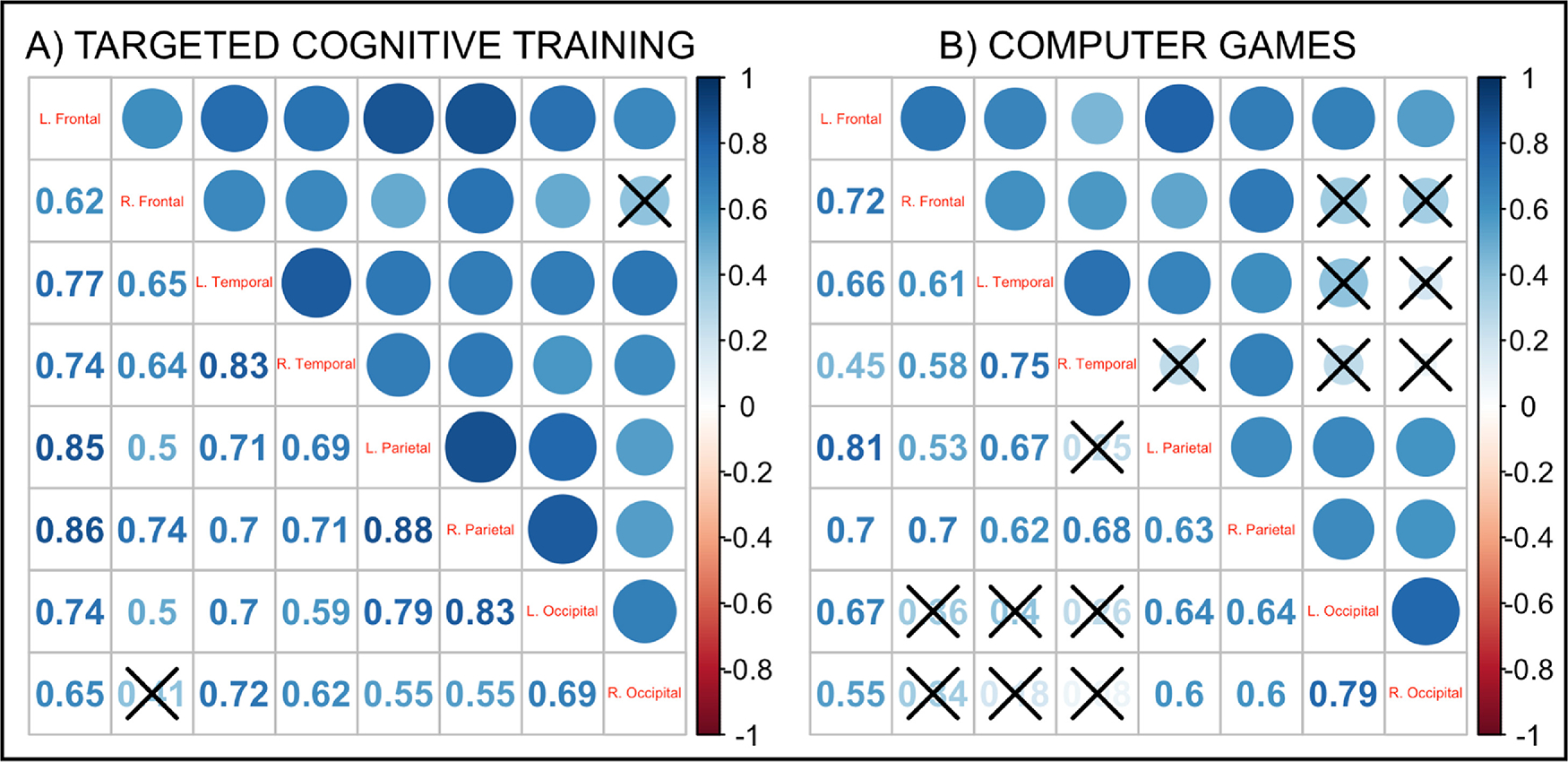
Figure 4 Note: Correlation matrices of change in cortical thickness. (A) Change in cortical thickness correlation matrix in the targeted cognitive training (TCT) group showed a significant positive correlation for all correlation pairs (FDR-corrected p<.05) except for the right frontal and right occipital cortex (indicated by ‘X’). (B) Change in cortical thickness correlation matrix in the computer games (CG) group showed positive correlations in most correlation pairs (FDR-corrected p<.05; insignificant correlations indicated by ‘X’).
3.7. Relationship Between Change in Cortical Thickness and Thalamic Volume
Last we assessed the relationship between change in CTh and change in left thalamic volume in the TCT group alone, as our previous findings in the same sample of participants demonstrated a correlation between change in left thalamic volume and change in global cognition (in the current subset of TCT participants: r=.56; p=.008)(Ramsay, Fryer, et al. 2018). First, we examined the relationship between change in CTh in all four cortices in both the left and right hemispheres with change in left thalamic volume in the TCT group. Three regions showed a significant relationship surviving FDR-correction, including the left temporal cortex (r=.59; p=.005; FDR-p=.013), the right temporal cortex (r=.62; p=.003; FDR-p=.013), and the right occipital cortex (r=.60; p=.004; FDR-p=.013). We followed up on these three regions to assess whether change in CTh in the TCT group mediated the relationship between change in thalamic volume and change in global cognition using the Sobel test. Change in CTh in the right temporal cortex was found to mediate the relationship between change in left thalamic volume and change in global cognition following TCT (Z=2.22; p=.03). Neither of the other mediation models were found to be significant (p’s>.13).
4. Discussion
The current study examined the relationship between cortical thickness (CTh) and response to targeted cognitive training (TCT) in patients with recent onset schizophrenia (SZ). While we did not observe significant changes in mean CTh in response to TCT in any region, individual differences in increased CTh in response to training were predictive of increases in global cognition. Based on previous work, our initial hypotheses were localized to frontal and temporal brain areas consistent with training designed to drive functional plasticity in these regions (Adcock et al., 2009). Instead, we observed widespread effects throughout the brain in frontal, temporal, parietal, and occipital regions. These effects were not observed in the computer games (CG) control group (though it is noteworthy that this group did show significant negative relationships between change in CTh and medication dose in parietal and occipital regions). These findings also persisted when controlling for outliers using a Winsorizing procedure, though the effect in the left parietal cortex was no found to no longer be significant. Additionally, changes in CTh in each region positively correlated with changes in all other regions, though this effect was found to be much more robust and widespread in the TCT group. The current results offer support for the hypothesis that response to TCT may be neuroprotective, such that individuals with SZ who showed improvements in global cognition also showed widespread sustained or increased CTh over the ~4-month assessment period.
In typically developing individuals, cortical thinning occurs rapidly late in adolescence and into early adulthood (Tamnes et al. 2010), before a more steady age-related decline in CTh which is observed into aging adulthood (Storsve et al. 2014). However, individuals with schizophrenia are found to have “accelerated” brain aging, with progressive changes related to age emerging more rapidly than in controls (Schnack et al. 2016). These differences are shown to be present in the early years of the illness (such that schizophrenia participants appeared to have gray matter structure closer to that of healthy controls 3 years older) and are found to decline at an augmented rate (1.36 years/year) over time. This means that the participants in the current study were not only likely to have already been experiencing accelerated cortical thinning at baseline, but also were likely to have continued accelerated CTh reductions over the brief course of the study. Though subtle, the current findings suggest that individuals who responded to TCT may have had slowed cortical thinning that coincided with improvements in global cognition. However, future work using longitudinal assessment will be necessary to determine if TCT explicitly slows accelerated brain aging in SZ.
The current findings also contextualize our previous results in the same participants that demonstrated changes in left thalamic volume were also predictive of changes in global cognition following TCT (Ramsay, Fryer, et al. 2018). Here, we find that change in left thalamic volume strongly correlates with change in CTh in bilateral temporal cortex, as well as the right occipital cortex, suggesting that TCT may drive changes in thalamocortical structure. This is consistent with previous findings that have demonstrated changes in thalamocortical functional connectivity in response to cognitive training (Ramsay et al. 2020; Ramsay, Nienow, and MacDonald 2017). It also offers a holistic explanation of neural response to TCT, such that training does not appear to impact neuroplasticity in specific cortical or subcortical regions, but instead may evoke neuroprotection in a more general manner.
Taken with previous work examining behavioral and neurobiological responses to TCT, this study offers support for the notion that variations in outcome may be related to heterogeneity in treatment response. It is therefore crucial to identify markers of early treatment response that could offer insights into treatment personalization or go-no-go decisions for practitioners seeking to quickly pivot to a different intervention for patients who do not appear to be responding. Early response to TCT captured in electrophysiological measurement has offered insights into long-term treatment outcomes (Tarasenko et al. 2016; Perez et al. 2019; Hochberger et al. 2019), as has aspects of baseline cognitive and demographic profiles (Ramsay, Ma, et al. 2018). These approaches will need to be examined in coordination with measures of cortical thickness and brain morphometry to better understand how neurophysiological and behavioral measures may predict not only cognitive changes but structural plasticity and neuroprotection as well.
We did not observe overall changes in CTh in response to TCT in the current study, and it is unclear whether this was due to an insufficient dose of training, insufficient power, a limited time window for follow-up (~4 months), or the possibility that TCT alone is simply insufficient to evoke neuro-restorative structural changes in SZ. This finding was broadly consistent with a study examining changes in CTh in response to exercise in individuals with SZ and healthy controls. Scheewe and colleagues (2013) did not observe significant changes in CTh or brain volume but did identify a relationship between improvements in cardiorespiratory fitness and increased CTh in frontal and temporal regions (Scheewe et al. 2013). This has also been found to be the case in hippocampal response to aerobic exercise in SZ, where exercise has largely conferred a neuroprotective rather than a neurorestorative response (Firth et al. 2018). However, recent findings indicate that aerobic exercise can offer temporary neuroplastic response, where CTh in the entorhinal cortex was increased after 6 weeks of exercise but normalized after 12 weeks (Takahashi et al. 2020), suggesting that changes in CTh may be state-dependent or quick to return to baseline levels.
A major limitation of the current study is the small sample size. Though not inconsistent with previous studies of this nature, sample sizes near 20 in either group arguably raise concerns about the number of regressions performed in our analysis, and ultimately reflect that the current findings are preliminary and warrant replication. It is likely that large-scale multi-site studies will be necessary to adequately power a study examining structural plasticity in response to TCT. Another limitation is that the current study was conducted on young individuals early on in their illness, which may be indicative of less brain volume loss and cortical thinning than in patients of an older age. Future studies will need to examine samples stratified across age groups to determine whether neuroprotective benefits are unique to an early psychosis population, or similarly present in chronic phases of the illness. Finally, we cannot rule out the effects of medication, as previous findings demonstrate that antipsychotic dose coincides with cortical thinning (Roiz-Santianez et al, 2012). Here we did not observe differences in CPZ equivalents between groups at baseline, though we did observe relationships between medication dose and cortical thinning in parietal and occipital regions in the control group. It is possible that antipsychotic medications were contributing to cortical thinning in these regions, and in part driving a between group effect. Future work will be required to more systematically examine the effects of antipsychotic medications on cortical thickness, and how this may interact with response to cognitive training interventions.
The current findings offer support for a neuroprotective rather than a neuro-restorative effect of targeted cognitive training in early schizophrenia. Participants who showed increased global cognitive scores following training had corresponding increases (or lack of decline) in cortical thickness across widespread regions, while this relationship was not demonstrated in the computer games control condition. Future work will be required to not only replicate these effects, but also understand the specific mechanisms that may drive a neuroprotective response.
Supplementary Material
Highlights.
Response to cognitive training coincided with changes in cortical thickness.
Change in cortical thickness correlated across regions following training.
Cortical thickness changes mediated thalamic volume and global cognition relationships.
Acknowledgement
Funding was provided by the Stanley Medical Research Institute (06TAF-972) and the National Institute of Mental Health (MH076989). ISR was funded by the Wells Family Trust and the National Institute of Mental Health (K01 MH117451). Posit Science Inc. supplied the training software used in this study free of charge.
Role of funding
The current study was funded by the Stanley Medical Research Institute (06TAF-972) and the National Institute of Mental Health (MH076989). ISR was funded by the Wells Family Trust and the National Institute of Mental Health (K01 MH117451).
Footnotes
Declaration of competing interest
SV has served as a paid consultant to Posit Science Inc. In the past three years, DHM has received compensation as a consultant for Boehringer-Ingelheim, Amgen, and Hoffmann-LaRoche. The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Cannon Tyrone D., Chung Yoonho, He George, Sun Daqiang, Jacobson Aron, van Erp Theo G. M., McEwen Sarah, et al. 2015. “Progressive Reduction in Cortical Thickness as Psychosis Develops: A Multisite Longitudinal Neuroimaging Study of Youth at Elevated Clinical Risk.” Biological Psychiatry 77 (2): 147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobia Derin J., Csernansky John G., and Wang Lei. 2011. “Cortical Thickness in Neuropsychologically near-Normal Schizophrenia.” Schizophrenia Research 133 (1–3): 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale Corby L., Brown Ethan G., Fisher Melissa, Herman Alexander B., Dowling Anne F., Hinkley Leighton B., Subramaniam Karuna, Nagarajan Srikantan S., and Vinogradov Sophia. 2016. “Auditory Cortical Plasticity Drives Training-Induced Cognitive Changes in Schizophrenia.” Schizophrenia Bulletin 42 (1): 220–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan Rahul S., Ségonne Florent, Fischl Bruce, Quinn Brian T., Dickerson Bradford C., Blacker Deborah, Buckner Randy L., et al. 2006. “An Automated Labeling System for Subdividing the Human Cerebral Cortex on MRI Scans into Gyral Based Regions of Interest.” NeuroImage 31 (3): 968–80. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Johnson RE, Protti AM, Ott C, and Kajisa L. 1985. “Plasticity in the 904-Day-Old Male Rat Cerebral Cortex.” Experimental Neurology 87 (2): 309–17. [DOI] [PubMed] [Google Scholar]
- Eack Shaun M., Hogarty Gerard E., Cho Raymond Y., Prasad Konasale M. R., Greenwald Deborah P., Hogarty Susan S., and Keshavan Matcheri S.. 2010. “Neuroprotective Effects of Cognitive Enhancement Therapy against Gray Matter Loss in Early Schizophrenia: Results from a 2-Year Randomized Controlled Trial.” Archives of General Psychiatry 67 (7): 674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich Stefan, Brauns Stefan, Yendiki Anastasia, Ho Beng-Choon, Calhoun Vince, Schulz S. Charles, Gollub Randy L., and Sponheim Scott R.. 2012. “Associations of Cortical Thickness and Cognition in Patients with Schizophrenia and Healthy Controls.” Schizophrenia Bulletin 38 (5): 1050–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth Joseph, Stubbs Brendon, Vancampfort Davy, Schuch Felipe, Lagopoulos Jim, Rosenbaum Simon, and Ward Philip B.. 2018. “Effect of Aerobic Exercise on Hippocampal Volume in Humans: A Systematic Review and Meta-Analysis.” NeuroImage 166 (February): 230–38. [DOI] [PubMed] [Google Scholar]
- Fischl B, and Dale AM. 2000. “Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images.” Proceedings of the National Academy of Sciences of the United States of America 97 (20): 11050–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, and Dale AM. 1999. “Cortical Surface-Based Analysis. II: Inflation, Flattening, and a Surface-Based Coordinate System.” NeuroImage 9 (2): 195–207. [DOI] [PubMed] [Google Scholar]
- Fisher Melissa, Holland Christine, Merzenich Michael M., and Vinogradov Sophia. 2009. “Using Neuroplasticity-Based Auditory Training to Improve Verbal Memory in Schizophrenia.” The American Journal of Psychiatry 166 (7): 805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Melissa, Loewy Rachel, Carter Cameron, Lee Ashley, Ragland J. Daniel, Niendam Tara, Schlosser Danielle, Pham Lien, Miskovich Tara, and Vinogradov Sophia. 2015. “Neuroplasticity-Based Auditory Training via Laptop Computer Improves Cognition in Young Individuals with Recent Onset Schizophrenia.” Schizophrenia Bulletin 41 (1): 250–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijma Sander V., Van Haren Neeltje, Cahn Wiepke, Cédric P, Koolschijn MP, Hilleke E, Pol Hulshoff, and Kahn René S.. 2013. “Brain Volumes in Schizophrenia: A Meta-Analysis in over 18 000 Subjects.” Schizophrenia Bulletin 39 (5): 1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg Cecilie Bhandari, Lawyer Glenn, Nyman Håkan, Jönsson Erik G., Haukvik Unn K., Saetre Peter, Bjerkan Petr S., Andreassen Ole A., Hall Håkan, and Agartz Ingrid. 2010. “Investigating Relationships between Cortical Thickness and Cognitive Performance in Patients with Schizophrenia and Healthy Adults.” Psychiatry Research 182 (2): 123–33. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, and Zakzanis KK. 1998. “Neurocognitive Deficit in Schizophrenia: A Quantitative Review of the Evidence.” Neuropsychology 12 (3): 426–45. [DOI] [PubMed] [Google Scholar]
- Hochberger William C., Joshi Yash B., Thomas Michael L., Zhang Wendy, Bismark Andrew W., Treichler Emily B. H., Tarasenko Melissa, et al. 2019. “Neurophysiologic Measures of Target Engagement Predict Response to Auditory-Based Cognitive Training in Treatment Refractory Schizophrenia.” Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 44 (3): 606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein Keith H., Green Michael F., Kern Robert S., Baade Lyle E., Barch Deanna M., Cohen Jonathan D., Essock Susan, et al. 2008. “The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity.” The American Journal of Psychiatry 165 (2): 203–13. [DOI] [PubMed] [Google Scholar]
- Penadés Rafael, Pujol Nuria, Catalán Rosa, Masana Guillem, García-Rizo Clemente, Bargalló Nuria, González-Rodríguez Alexandre, Vidal-Piñeiro Dídac, Bernardo Miquel, and Junqué Carme. 2016. “Cortical Thickness in Regions of Frontal and Temporal Lobes Is Associated with Responsiveness to Cognitive Remediation Therapy in Schizophrenia.” Schizophrenia Research 171 (1–3): 110–16. [DOI] [PubMed] [Google Scholar]
- Penadés Rafael, Pujol Nuria, Catalán Rosa, Massana Guillem, Rametti Giuseppina, García-Rizo Clemente, Bargalló Nuria, Gastó Cristóbal, Bernardo Miquel, and Junqué Carme. 2013. “Brain Effects of Cognitive Remediation Therapy in Schizophrenia: A Structural and Functional Neuroimaging Study.” Biological Psychiatry 73 (10): 1015–23. [DOI] [PubMed] [Google Scholar]
- Perez Veronica B., Miyakoshi Makoto, Makeig Scott D., and Light Gregory A.. 2019. “Mismatch Negativity Reveals Plasticity in Cortical Dynamics after 1-Hour of Auditory Training Exercises.” International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology 145 (November): 40–47. [DOI] [PubMed] [Google Scholar]
- Ramsay Ian S., Fryer Susanna, Boos Alison, Roach Brian J., Fisher Melissa, Loewy Rachel, Vinogradov Sophia, and Mathalon Daniel H.. 2018. “Response to Targeted Cognitive Training Correlates with Change in Thalamic Volume in a Randomized Trial for Early Schizophrenia.” Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 43 (3): 590–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay Ian S., and MacDonald Angus W. 3rd. 2015. “Brain Correlates of Cognitive Remediation in Schizophrenia: Activation Likelihood Analysis Shows Preliminary Evidence of Neural Target Engagement.” Schizophrenia Bulletin 41 (6): 1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay Ian S., Ma Sisi, Fisher Melissa, Loewy Rachel L., Ragland J. Daniel, Niendam Tara, Carter Cameron S., and Sophia Vinogradov. 2018. “Model Selection and Prediction of Outcomes in Recent Onset Schizophrenia Patients Who Undergo Cognitive Training.” Schizophrenia Research. Cognition 11 (March): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay Ian S., Nienow Tasha M., and MacDonald Angus W. 3rd. 2017. “Increases in Intrinsic Thalamocortical Connectivity and Overall Cognition Following Cognitive Remediation in Chronic Schizophrenia.” Biological Psychiatry. Cognitive Neuroscience and Neuroimaging 2 (4): 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay Ian S., Roach Brian J., Fryer Susanna, Fisher Melissa, Loewy Rachel, Ford Judith M., Vinogradov Sophia, and Mathalon Daniel H.. 2020. “Increased Global Cognition Correlates with Increased Thalamo-Temporal Connectivity in Response to Targeted Cognitive Training for Recent Onset Schizophrenia.” Schizophrenia Research, January. 10.1016/j.schres.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter Martin, Schmansky Nicholas J., Rosas H. Diana, and Bruce Fischl. 2012. “Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis.” NeuroImage 61 (4): 1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiz-Santiáñez R, Tordesillas-Gutiérrez D, de la Foz VOG, Ayesa-Arriola R, Gutiérrez A, Tabarés-Seisdedos R, … & Crespo-Facorro B (2012). Effect of antipsychotic drugs on cortical thickness. A randomized controlled one-year follow-up study of haloperidol, risperidone and olanzapine. Schizophrenia research, 141(1), 22–28. [DOI] [PubMed] [Google Scholar]
- Scheewe Thomas W., van Haren Neeltje E. M., Sarkisyan Gayane, Schnack Hugo G., Brouwer Rachel M., de Glint Maria, Hulshoff Pol Hilleke E., Backx Frank J. G., Kahn René S., and Cahn Wiepke. 2013. “Exercise Therapy, Cardiorespiratory Fitness and Their Effect on Brain Volumes: A Randomised Controlled Trial in Patients with Schizophrenia and Healthy Controls.” European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology 23 (7): 675–85.22981376 [Google Scholar]
- Schnack Hugo G., van Haren Neeltje E. M., Brouwer Rachel M., Alan Evans, Sarah Durston, Boomsma Dorret I., Kahn René S., and Hulshoff Pol Hilleke E.. 2015. “Changes in Thickness and Surface Area of the Human Cortex and Their Relationship with Intelligence.” Cerebral Cortex 25 (6): 1608–17. [DOI] [PubMed] [Google Scholar]
- Schnack Hugo G., van Haren Neeltje E. M., Mireille Nieuwenhuis, Hulshoff Pol Hilleke E., Wiepke Cahn, and Kahn René S.. 2016. “Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study.” The American Journal of Psychiatry 173 (6): 607–16. [DOI] [PubMed] [Google Scholar]
- Schultz , C. Christoph, Koch Kathrin, Wagner Gerd, Roebel Martin, Schachtzabel Claudia, Gaser Christian, Nenadic Igor, Reichenbach Jürgen R., Sauer Heinrich, and Schlösser Ralf G. M.. 2010. “Reduced Cortical Thickness in First Episode Schizophrenia.” Schizophrenia Research 116 (2–3): 204–9. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, and Fischl B “Geometrically accurate topology-correction of cortical surfaces using nonseparating loops.” IEEE Transactions on Medical Imaging 26 (4): 518–29. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, and Giedd J. 2006. “Intellectual Ability and Cortical Development in Children and Adolescents.” Nature 440 (7084): 676–79. [DOI] [PubMed] [Google Scholar]
- Sherman S. Murray. 2016. “Thalamus Plays a Central Role in Ongoing Cortical Functioning.” Nature Neuroscience 19 (4): 533–41. [DOI] [PubMed] [Google Scholar]
- Sprooten Emma, Papmeyer Martina, Smyth Annya M., Vincenz Daniel, Honold Sibylle, Conlon Guy A., William T, Moorhead J, et al. 2013. “Cortical Thickness in First-Episode Schizophrenia Patients and Individuals at High Familial Risk: A Cross-Sectional Comparison.” Schizophrenia Research 151 (1–3): 259–64. [DOI] [PubMed] [Google Scholar]
- Storsve Andreas B., Fjell Anders M., Tamnes Christian K., Westlye Lars T., Overbye Knut, Aasland Hilde W., and Walhovd Kristine B.. 2014. “Differential Longitudinal Changes in Cortical Thickness, Surface Area and Volume across the Adult Life Span: Regions of Accelerating and Decelerating Change.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 34 (25): 8488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Shun, Keeser Daniel, Rauchmann Boris-Stephan, Schneider-Axmann Thomas, Keller-Varady Katriona, Maurus Isabel, Dechent Peter, et al. 2020. “Effect of Aerobic Exercise Combined with Cognitive Remediation on Cortical Thickness and Prediction of Social Adaptation in Patients with Schizophrenia.” Schizophrenia Research 216 (February): 397–407. [DOI] [PubMed] [Google Scholar]
- Tamnes Christian K., Ostby Ylva, Fjell Anders M., Westlye Lars T., Due-Tønnessen Paulina, and Walhovd Kristine B.. 2010. “Brain Maturation in Adolescence and Young Adulthood: Regional Age-Related Changes in Cortical Thickness and White Matter Volume and Microstructure.” Cerebral Cortex 20 (3): 534–48. [DOI] [PubMed] [Google Scholar]
- Tang Yi-Yuan, Hölzel Britta K., and Posner Michael I.. 2015. “The Neuroscience of Mindfulness Meditation.” Nature Reviews. Neuroscience 16 (4): 213–25. [DOI] [PubMed] [Google Scholar]
- Tarasenko Melissa, Perez Veronica B., Pianka Sean T., Vinogradov Sophia, Braff David L., Swerdlow Neal R., and Light Gregory A.. 2016. “Measuring the Capacity for Auditory System Plasticity: An Examination of Performance Gains during Initial Exposure to Auditory-Targeted Cognitive Training in Schizophrenia.” Schizophrenia Research 172 (1): 123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully Laura M., Lincoln Sarah Hope, Liyanage-Don Nadia, and Hooker Christine I.. 2014. “Impaired Cognitive Control Mediates the Relationship between Cortical Thickness of the Superior Frontal Gyrus and Role Functioning in Schizophrenia.” Schizophrenia Research 152 (2–3): 358–64. [DOI] [PubMed] [Google Scholar]
- Erp Van, Theo GM, Walton Esther, Hibar Derrek P., Schmaal Lianne, Jiang Wenhao, Glahn David C., Pearlson Godfrey D., et al. 2018. “Cortical Brain Abnormalities in 4474 Individuals with Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium.” Biological Psychiatry 84 (9): 644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov Sophia, Fisher Melissa, and de Villers-Sidani Etienne. 2012. “Cognitive Training for Impaired Neural Systems in Neuropsychiatric Illness.” Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 37 (1): 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Yuan, Lui Su, Deng Wei, Yao Li, Zhang Wenjing, Li Shiguang, Wu Min, et al. 2015. “Altered Cortical Thickness Related to Clinical Severity but Not the Untreated Disease Duration in Schizophrenia.” Schizophrenia Bulletin 41 (1): 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


