Abstract
Background -
Sudden cardiac death (SCD) studies report higher incidence in men and Blacks but presume cardiac cause. We sought to identify sex and race differences in rates and causes of presumed SCDs in a prospective postmortem study in San Francisco County.
Methods -
All incident presumed SCDs meeting World Health Organization definition ages 18–90 were autopsied via active surveillance of consecutive out-of-hospital deaths in the POstmortem Systematic InvesTigation of Sudden Cardiac Death (POST SCD) Study (2/1/2011 – 3/1/2014). Autopsy-defined sudden arrhythmic deaths (SADs) had no extra-cardiac cause or acute heart failure.
Results -
Among 541 presumed SCDs, 525 (97%) were autopsied; 362 (69%) were male, 110 Asian (21%), 81 Black (15%), 40 Hispanic (8%), 279 White (53%), and 15 Other Race (3%). Adjusted for age and race, women had more non-cardiac causes of presumed SCD, including pulmonary emboli (8% vs. 2%) and neurologic causes (10% vs. 3%, both p<0.01). Of autopsy-defined SAD, men had 3-fold higher rates while women had more primary electrical disease (4% vs. 2%, p=0.02) and non-ischemic causes (53% vs. 39%, p<0.01). Age-adjusted incidence rate ratios were higher for Black women (2.55, p<0.01), and lower for Asian and Hispanic men (0.51 for both, p<0.05) than their White counterparts. Myocardial infarction without obstructive coronary arteries was more common among SADs in Asians than Whites (7% vs. 1%; adjusted p<0.05). Sudden neurologic deaths were more common in Asians, endocrine causes more common in Blacks, and gastrointestinal causes more common in Hispanics than Whites (adjusted p all <0.05).
Conclusions -
In this countywide postmortem study of presumed SCDs, women had more non-ischemic and non-cardiac causes. Black women had higher rates of autopsy-defined SAD than White women while Asian and Hispanic men had lower rates than White men. These findings have implications for risk stratification and prevention of sudden mortality in women and minority populations.
Keywords: epidemiology, cardiac arrest, arrhythmia, autopsy
Journal Subject Terms: Sudden Cardiac Death, Epidemiology, Race and Ethnicity
Introduction
Sudden cardiac death (SCD) is a major public health concern and may account for between 300,000 and 450,000 deaths per year in the United States alone.1 Prior studies have demonstrated substantial sex and racial differences in the incidence rates of SCD, with rates reportedly higher in men and Blacks.1–4 Moreover, these differences persist after adjusting for sociodemographic and cardiovascular risk factors, suggesting differences in the underlying pathophysiology and phenotype of SCD.5 However, almost all studies rely on medical or emergency medical service (EMS) records and death certificates to infer cardiac cause,6 which leads to significant misclassification of non-cardiac (e.g., occult overdose, pulmonary embolism, hemorrhage) and non-arrhythmic (e.g., tamponade, acute heart failure) causes masquerading as SCDs that cannot be excluded without full autopsy. Thus whether sex and race affect the incidence of true sudden arrhythmic death (SAD) – the only type of sudden death potentially rescuable by defibrillator – is unknown.7
Recently we conducted the 3-year San Francisco POstmortem Systematic InvesTigation of Sudden Cardiac Death (POST SCD) Study to identify and autopsy nearly every incident World Health Organization (WHO)-defined (presumed) SCDs countywide ages 18–90 years via active surveillance of consecutive out of hospital deaths, and found that only half (55.8%) of SCDs defined by these conventional criteria were SAD after autopsy evaluation with the remainder having an easily identifiable non-arrhythmic cause of death.8 In this POST SCD analysis, we sought to further characterize differences in premortem characteristics, incidence rates, autopsy-defined causes, and circumstances of sudden death by sex and race to evaluate whether standard definitions of SCD may differentially misrepresent women and minority populations.
Methods
The data that support the findings of this study are available from the corresponding author upon approval of reasonable request. The UCSF IRB approved all aspects of this study with additional IRB approval obtained from 10 San Francisco county hospitals and 3 EMS agencies.
Study Population and Definition of Sudden Cardiac Death
A detailed description of the methods used in the POST SCD investigation has been previously reported8 and is briefly summarized here. The study population included all residents of San Francisco County, California (population: 805,235). Between February 1, 2011 to March 1, 2014, all presumed out of hospital cardiac arrests (OHCA) were captured as consistent with the Cardiac Arrest Registry to Enhance Survival definition. OHCA deaths were defined as those patients who died in the field or ED if witnessed and/or active resuscitation was performed, or unwitnessed sudden unexpected deaths if the victim was last observed alive and symptom free within 24 hours as defined by the WHO.9 WHO-defined (presumed) SCDs were defined as sudden unexpected death either within 1 hour of symptom onset (event witnessed), or within 24 hours of having been observed alive and symptom free (unwitnessed).10 Subjects otherwise meeting WHO criteria who had recent admission for myocardial infarction were included. Patients were excluded if they had a terminal illness, end stage renal disease on hemodialysis, or an alternative identifiable non-cardiac cause of sudden death, including trauma, violent death, suicide, and overt drug overdose. Consistent with the initial reporting of incidence in POST SCD, we provided estimated weighted and age-adjusted incidence rate ratios for autopsy-defined SAD using 2011 San Francisco census data as a proportion of the overall set of 630 initially identified WHO-defined SCDs that included 89 physician-attended deaths with recent medical care, thus not under medical examiner jurisdiction and ineligible for autopsy.8 These 89 attended deaths were subsequently adjudicated to be non-sudden and thus excluded as presumed SCDs, arriving at the final cohort of 541 presumed SCDs, 525 (97%) of which were autopsied. Race was ascertained from prior medical records and EMS runsheets which were obtained from all hospitals involved for 94% (492/525) of all SCDs.8
Postmortem Investigation and Adjudication of Sudden Cardiac Deaths and Sudden Arrhythmic Deaths
All unexpected out of hospital deaths including OHCA deaths must be reported to the Medical Examiner by California state law and were referred for detailed autopsy as previously described.8 A multidisciplinary committee comprised of two electrophysiologists, the assistant medical examiner of San Francisco County, a cardiac pathologist, and a neurologist reviewed EMS and comprehensive medical records of all OHCA deaths to determine those that met sudden death criteria as WHO-defined (presumed) SCDs. The cause of death was classified as SAD (considered potentially rescuable with an implantable cardioverter-defibrillator [ICD]) or non-SAD and adjudicated with an underlying primary cause of death. Nearly half of the presumed SCDs had an obvious non-arrhythmic cause of death identified on comprehensive autopsy evaluation (e.g., tamponade, intracranial or gastrointestinal hemorrhage, pulmonary embolism, acute renal failure with markedly elevated postmortem serum creatinine, occult overdose with lethal serum levels). Autopsy-defined SAD was defined as those presumed SCDs without an obvious non-arrhythmic (e.g., acute myocardial infarction with wall rupture and tamponade, acute heart failure with pulmonary edema) or extra-cardiac (e.g., intracranial or gastrointestinal hemorrhage, pulmonary embolism, acute renal failure with markedly elevated postmortem vitreous creatinine, occult overdose with lethal serum levels) cause of death and potentially rescuable with a defibrillator. Adjudication of primary cause of SADs was determined by expert panel review/consensus opinion based on pre-specified criteria for certainty of diagnosis after consideration of gross pathology and cardiac histopathology findings, and absence of lethal toxicology. Full details of the methods and adjudication protocols for the original POST SCD study have been previously published.8
Statistical Analysis
Continuous variables are presented as means with standard deviations, and categorical variables are presented as totals with percentages. Comparisons were made using unequal-variance t and Fischer’s exact tests when appropriate with two-tailed p<0.05 considered statistically significant. Sex differences in SCD and SAD causes of death were adjusted for age and race, and racial differences were adjusted for age and sex using logistic models. Weighted incidence of SAD as a proportion of presumed SCD by race and sex was estimated by inverse weighting, based on a logistic model, to make the 525 presumed SCDs with autopsy data representative of the overall set of 630 WHO-defined SCDs. Age-adjusted incidence rate ratios (IRR) were subsequently calculated to make comparisons across sex and race. All calculations were performed using Stata Version 14.2 (StataCorp LP, College Park, TX).
Results
We identified 541 unattended presumed SCDs over 37 months of which 525 (97%) were autopsied; 362 were male (69.0%), 110 Asian (21.0%), 81 Black (15.4%), 40 Hispanic (7.6%), 279 White (53.1%), and 15 Other Race (2.9%), representative of the diverse San Francisco County population (Table 1).
Table 1.
Pre-Mortem Conditions for WHO-Defined (Presumed) SCDs by Sex and Race.
| WHO-Defined (Presumed) SCDs (525) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Race | |||||||||
| No. (%) | Female | Male | p* | Asian | p* | Black | p* | Hispanic | p* | White |
| 163 (31) | 362 (69) | – | 110 (21) | – | 81 (15) | – | 40 (8) | – | 279 (53) | |
| Medical Records Unobtainable | 8 (5) | 25 (7) | NS | 8 (7) | NS | 5 (6) | NS | 2 (5) | NS | 18 (6) |
| Confirmed No Medical History | 8 (5) | 16 (4) | NS | 9 (8) | NS | 2 (2) | NS | 3 (8) | NS | 10 (4) |
| Age (years) | 66.9 ± 15.1 | 60.9 ± 13.8 | <0.01 | 67.3 ± 15.1 | <0.01 | 60.3 ± 14.5 | NS | 59.3 ± 16.2 | NS | 62.3 ± 13.7 |
| History of: | ||||||||||
| Hypertension | 100 (61) | 190 (53) | NS | 64 (58) | NS | 57 (70) | <0.01 | 21 (53) | NS | 138 (49) |
| Diabetes Mellitus | 42 (26) | 75 (21) | NS | 33 (30) | <0.01 | 22 (27) | <0.05 | 13 (33) | <0.01 | 44 (16) |
| Dyslipidemia | 50 (31) | 107 (30) | NS | 43 (39) | <0.05 | 23 (28) | NS | 9 (23) | NS | 79 (28) |
| Myocardial Infarction | 25 (15) | 51 (14) | NS | 11 (10) | NS | 16 (20) | NS | 8 (20) | NS | 38 (14) |
| Congestive Heart Failure | 20 (12) | 48 (13) | NS | 13 (12) | NS | 18 (22) | <0.01 | 4 (10) | NS | 30 (11) |
| Chronic Kidney Disease (non-ESRD) | 24 (15) | 34 (9) | NS | 12 (11) | NS | 21 (26) | <0.01 | 4 (10) | NS | 18 (6) |
| Seizure Disorder | 13 (8) | 26 (7) | NS | 2 (1) | <0.05 | 8 (10) | NS | 6 (15) | NS | 21 (8) |
| Cerebrovascular Accident | 17 (10) | 16 (4) | <0.01 | 10 (9) | <0.05 | 8 (10) | 0.01 | 4 (10) | <0.05 | 9 (3) |
| Psychiatric Disorder† | 58 (36) | 85 (23) | <0.01 | 16 (15) | <0.01 | 30 (37) | NS | 11 (28) | NS | 83 (30) |
| Chronic Obstructive Pulmonary Disease | 27 (17) | 37 (10) | <0.05 | 13 (12) | NS | 15 (19) | NS | 5 (13) | NS | 31 (11) |
| Non-Metastatic Cancer | 23 (14) | 40 (11) | NS | 7 (6) | <0.05 | 11 (14) | NS | 3 (8) | NS | 42 (15) |
| Tobacco Use | 54 (33) | 157 (43) | <0.05 | 31 (28) | <0.01 | 37 (46) | NS | 20 (50) | NS | 119 (43) |
| Excess Alcohol Use | 16 (10) | 106 (30) | <0.01 | 11 (10) | <0.01 | 21 (26) | NS | 14 (35) | NS | 74 (27) |
| Illicit Drug Use | 21 (13) | 58 (16) | NS | 1 (1) | <0.01 | 26 (32) | <0.01 | 9 (23) | NS | 43 (15) |
ESRD indicates end-stage renal disease; SCD, sudden cardiac death
p-value comparing Female vs. Male and comparing Asian, Black, and Hispanic vs. White
Includes prior diagnosis of Depression, Anxiety, Schizophrenia, Bipolar Disorder, PTSD, Mood Disorders, Psychosis, Borderline Personality Disorder, Obsessive Compulsive Disorder, Insomnia, and other psychiatric conditions not otherwise specified
Premortem Characteristics of Presumed SCDs by Sex and Race
For presumed SCDs, women were older (66.9 ± 15.1 vs. 60.9 ± 13.8), more likely to have had prior cerebrovascular accident (CVA), psychiatric disorder, or chronic obstructive pulmonary disease (COPD), and less likely to have had prior tobacco or excess alcohol use as compared to men (p<0.05 for all, Table 1). While the mean ages of Black and Hispanic presumed SCDs were similar to that of White cases, the mean age of Asian presumed SCDs was 5 years older. As compared to reference Whites, Asian, Black, and Hispanic presumed SCDs had more diabetes and prior CVA; Black presumed SCDs also had more hypertension, congestive heart failure, and chronic kidney disease (p<0.05 for all, Table 1). Ejection fraction as measured by echocardiogram performed as part of clinical practice was available in 14% (71/525) of presumed SCDs and was similar by sex (59 ± 11% in women vs. 52 ± 20% in men) and race (59 ± 18% in Asians vs. 50 ± 22% in Blacks vs. 52 ± 21% in Hispanics vs. 55 ± 14% in Whites, p>0.05). Mean interval from echocardiogram to death was 2.63 2.95 years.
Autopsy-Defined Causes of SAD and Presumed SCDs by Sex and Race
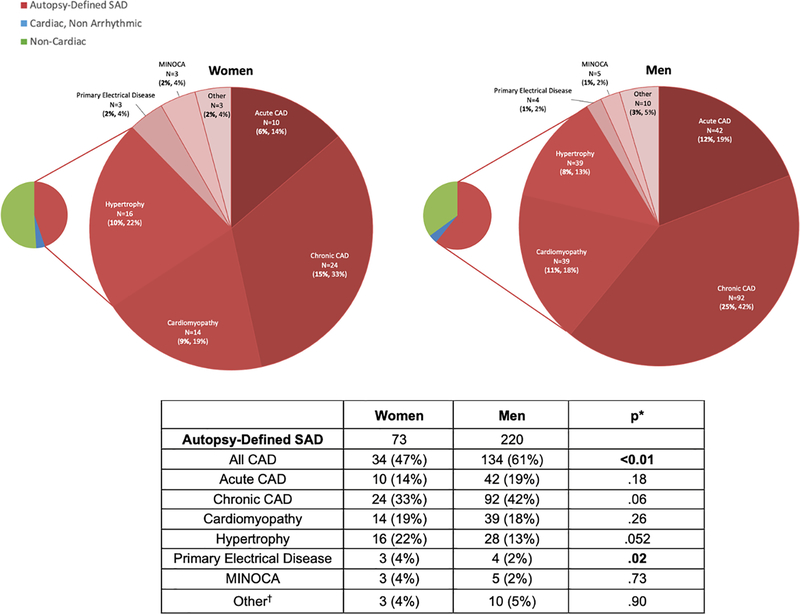
Adjusted for age and race, the proportion of presumed SCDs attributable to SAD was higher in men compared to women (61% vs. 45%; p<0.01, Table 2). Women were conversely more likely than men to have non-cardiac causes of sudden death found on autopsy (51% vs. 35%; p<0.01), in particular more pulmonary emboli (8% vs. 2%; p<0.01) and sudden neurologic deaths (10% vs. 3%; p<0.01), which included acute CVA, intracranial hemorrhage, and sudden unexpected death in epilepsy (SUDEP). Among SADs, women were less likely to have ischemic causes than men (47% vs. 61%; p<0.01), and more likely to have primary electrical disease (4% vs. 2%; p=0.02), defined as arrhythmic cause without structural heart disease (Figure 1). We also found a trend towards more SAD due to hypertrophy in women (22% vs. 13%; p=0.052).
Table 2.
Autopsy-Defined Causes of Presumed SCDs in Women vs. Men.
| Women (163) | Men (362) | p* | |
|---|---|---|---|
| Autopsy-Defined SAD | 73 (45%) | 220 (61%) | <0.01 |
| All CAD | 34 (21%) | 134 (37%) | <0.01 |
| Acute CAD | 10 (6%) | 42 (12%) | .02 |
| Chronic CAD | 24 (15%) | 92 (25%) | <0.01 |
| Cardiomyopathy | 14 (9%) | 39 (11%) | NS |
| Hypertrophy | 16 (10%) | 28 (8%) | NS |
| Primary Electrical Disease | 3 (2%) | 4 (1%) | NS |
| MINOCA | 3 (2%) | 5 (1%) | NS |
| Other† | 3 (2%) | 10 (3%) | NS |
| Cardiac, Non-Arrhythmic | 7 (4%) | 15 (4%) | NS |
| Acute MI w/ Rupture | 5 (3%) | 7 (2%) | NS |
| Acute MI w/ Pump Failure | 1 (1%) | 3 (1%) | NS |
| Chronic Heart Failure | 0 (0%) | 5 (1%) | NS |
| Pericarditis | 1 (1%) | 0 (0%) | NS |
| Non-Cardiac | 83 (51%) | 127 (35%) | <0.01 |
| Acute Renal Failure | 4 (2%) | 2 (1%) | NS |
| Aortic Dissection | 5 (3%) | 9 (2%) | NS |
| Aspiration/Asphyxia | 1 (1%) | 4 (1%) | NS |
| Occult Overdose | 26 (16%) | 45 (12%) | NS |
| GI Hemorrhage/Other GI‡ | 2 (1%) | 13 (4%) | NS |
| Hypo/Hyperglycemia/DKA | 3 (2%) | 6 (2%) | NS |
| Infection | 7 (4%) | 16 (4%) | NS |
| Neurologic | 17 (10%) | 12 (3%) | <0.01 |
| Pulmonary Embolism | 13 (8%) | 6 (2%) | <0.01 |
| Other Non-Cardiac§ | 5 (3%) | 14 (4%) | NS |
CAD indicates coronary artery disease; DKA, diabetic ketoacidosis; GI, gastrointestinal; MI, myocardial infarction; MINOCA, myocardial infarction without obstructive coronary arteries; SAD, sudden arrhythmic death; SCD, sudden cardiac death
Autopsy etiologies of presumed SCDs by sex after review of comprehensive medical records, EMS run sheets, and comprehensive autopsy data including post-mortem chemistry and toxicology. Percentages are presented in relation to the total number of presumed SCDs. Autopsy-defined SADs accounted for 61% of presumed SCDs in men and 45% in women (p <0.01). Cardiomyopathy (CM) included non-ischemic/dilated, drug/alcohol-induced, non-compaction CM, stress CM, arrhythomogenic right ventricular dysplasia (ARVD), HIV-CM, and amyloidosis. Hypertrophy as determined by histology included hypertensive heart disease, hypertrophic cardiomyopathy (HCM), and unspecified.
Adjusted for age and race
Other cardiac arrhythmic causes included acquired long QT syndrome (LQTS), bicuspid aortic valve, cardiac implantable external device (CIED) concern and/or failure, mitral valve prolapse, and critical aortic stenosis (AS).
Other GI causes included incarcerated/strangulated hernia, bowel obstruction, hepatorenal failure/pancreatitis, and liver failure.
Other non-cardiac causes included acute alcohol withdrawal, disseminated cancer, hypothermia, liver failure, other hemorrhage/trauma, end-stage chronic obstructive pulmonary disease (COPD), obstructive sleep apnea (OSA), aortic aneurysm rupture, renal artery dissection, iliac artery dissection, and pulmonary artery dissection
Figure 1.
Autopsy-Defined Causes of SAD in Women vs. Men. Autopsy etiologies of SAD by sex after review of comprehensive medical records, EMS run sheets, and comprehensive autopsy data including post-mortem chemistry and toxicology. First % is of total presumed SCDs and second % is of cause of death category. CAD accounted for 61% of SAD in men and 47% in women (p <0.05).
CAD indicates coronary artery disease; MINOCA, myocardial infarction without obstructive coronary arteries; SAD, sudden arrhythmic death
*Adjusted for age and race
†Other cardiac arrhythmic causes included acquired long QT syndrome (LQTS), bicuspid aortic valve, cardiac implantable external device (CIED) concern and/or failure, mitral valve prolapse, and critical aortic stenosis (AS).
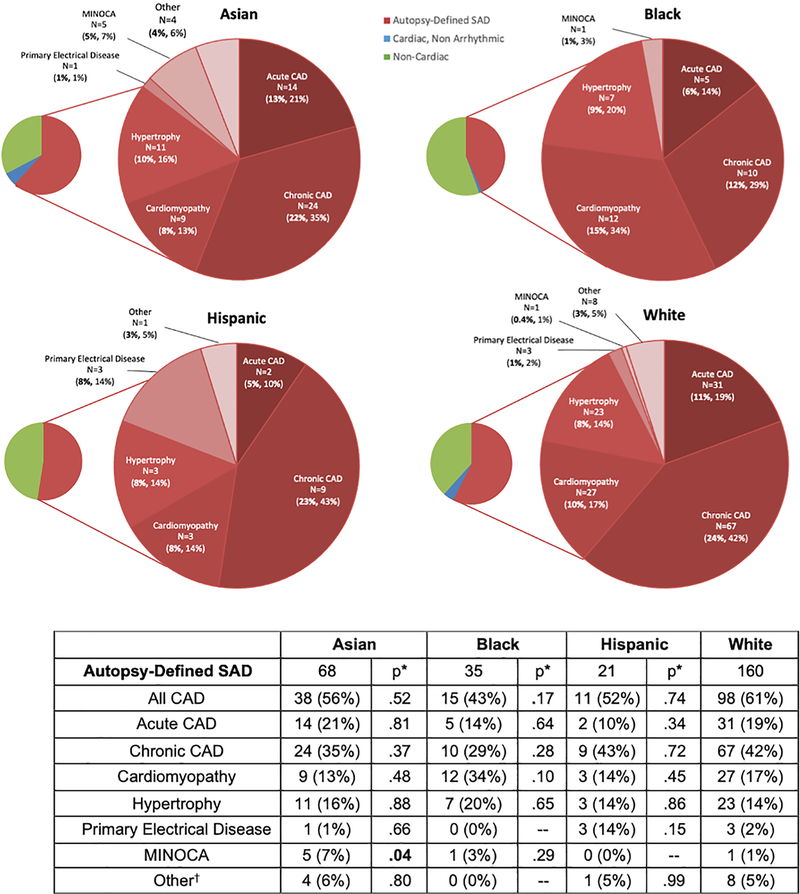
Adjusted for age and sex, the proportion of presumed SCDs due to non-cardiac causes was similar for Asians and Hispanics, but higher for Blacks (56% vs. 39%; p=0.04). Blacks had the lowest proportion of presumed SCDs attributed to chronic CAD and the most due to glycemic emergencies (p<0.05, Table 3). Among presumed SCDs, Asians had the highest proportion of sudden neurologic deaths and the fewest occult overdoses (p<0.05), while Hispanic cases had the highest proportion due to gastrointestinal causes (p<0.05). Of the overdose deaths, opiates were found to be the most common culprit. The distribution of SAD causes was similar for Hispanics and Blacks, while Asians had a higher proportion due to myocardial infarction without obstructive CAD (MINOCA) as compared to Whites (7% vs. 1%; adjusted p<0.05, Figure 2), defined by histologic evidence of myocardial infarction without significant coronary stenoses on autopsy.
Table 3.
Autopsy-Defined Causes of Presumed SCDs by Race.
| Asian (N=110) | Black (81) | Hispanic (40) | White (279) | ||||
|---|---|---|---|---|---|---|---|
| Autopsy-Defined SAD | 68 (62%) | p* = NS | 35 (43%) | p* = NS | 21 (53%) | p* = NS | 160 (57%) |
| All CAD | 38 (35%) | NS | 15 (19%) | .03 | 11 (28%) | NS | 98 (35%) |
| Acute CAD | 14 (13%) | NS | 5 (6%) | NS | 2 (5%) | NS | 31 (11%) |
| Chronic CAD | 24 (22%) | NS | 10 (12%) | NS | 9 (23%) | NS | 67 (24%) |
| Cardiomyopathy | 9 (8%) | NS | 12 (15%) | NS | 3 (8%) | NS | 27 (10%) |
| Hypertrophy | 11 (10%) | NS | 7 (9%) | NS | 3 (8%) | NS | 23 (8%) |
| Primary Electrical Disease | 1 (1%) | NS | 0 (0%) | NS | 3 (8%) | .03 | 3 (1%) |
| MINOCA | 5 (5%) | .04 | 1 (1%) | NS | 0 (0%) | NS | 1 (0.4%) |
| Other† | 4 (4%) | NS | 0 (0%) | NS | 1 (3%) | NS | 8 (3%) |
| Cardiac, Non-Arrhythmic | 6 (5%) | NS | 1 (1%) | NS | 0 (0%) | NS | 12 (4%) |
| Acute MI w/ Rupture | 6 (5%) | NS | 0 (0%) | NS | 0 (0%) | NS | 6 (2%) |
| Acute MI w/ Pump Failure | 0 (0%) | NS | 1 (1%) | NS | 0 (0%) | NS | 1 (0.4%) |
| Chronic Heart Failure | 0 (0%) | NS | 0 (0%) | NS | 0 (0%) | NS | 4 (1%) |
| Pericarditis | 0 (0%) | NS | 0 (0%) | NS | 0 (0%) | NS | 1 (0.4%) |
| Non-Cardiac | 36 (33%) | NS | 45 (56%) | .04 | 19 (47%) | NS | 107 (39%) |
| Acute Renal Failure | 0 (0%) | NS | 1 (1%) | NS | 1 (3%) | NS | 3 (1%) |
| Aortic Dissection | 4 (4%) | NS | 0 (0%) | NS | 2 (5%) | NS | 8 (3%) |
| Aspiration/Asphyxia | 2 (2%) | NS | 0 (0%) | NS | 1 (3%) | NS | 2 (1%) |
| Occult Overdose | 5 (5%) | 0.01 | 18 (22%) | NS | 4 (10%) | NS | 44 (16%) |
| GI Hemorrhage/Other GI‡ | 2 (2%) | NS | 3 (4%) | NS | 4 (10%) | .01 | 6 (2%) |
| Hypo/Hyperglycemia/DKA | 1 (1%) | NS | 5 (6%) | .02 | 0 (0%) | NS | 3 (1%) |
| Infection | 5 (5%) | NS | 5 (6%) | NS | 3 (8%) | NS | 9 (3%) |
| Neurologic | 11 (10%) | .02 | 5 (6%) | NS | 3 (8%) | NS | 10 (4%) |
| Pulmonary Embolism | 1 (1%) | NS | 6 (7%) | NS | 0 (0%) | NS | 12 (4%) |
| Other Non-Cardiac§ | 5 (5%) | NS | 2 (2%) | NS | 1 (3%) | NS | 10 (4%) |
CAD indicates coronary artery disease; DKA, diabetic ketoacidosis; GI, gastrointestinal; MI, myocardial infarction; MINOCA, myocardial infarction without obstructive coronary arteries; SAD, sudden arrhythmic death; SCD, sudden cardiac death
Autopsy etiologies of SAD and presumed SCDs by race after review of comprehensive medical records, EMS run sheets, and systematic autopsy data including postmortem chemistry and toxicology. All p-values are for comparison to White subjects. Cardiomyopathy (CM) included non-ischemic/dilated, drug/alcohol-induced, non-compaction CM, stress CM, ARVD, HIV-CM, and amyloidosis. Hypertrophy as determined by histology included hypertensive heart disease, hypertrophic cardiomyopathy (HCM), and unspecified.
Adjusted for age and sex vs. reference White
Other cardiac arrhythmic causes included acquired long QT syndrome (LQTS), bicuspid aortic valve, MI with non-obstructive coronary arteries (MINOCA), cardiac implantable external device (CIED) concern and/or failure, mitral valve prolapse, and critical aortic stenosis (AS).
Other GI causes included incarcerated/strangulated hernia, bowel obstruction, hepatorenal failure/pancreatitis, and liver failure.
Other non-cardiac causes included acute alcohol withdrawal, disseminated cancer, hypothermia, liver failure, other hemorrhage/trauma, end-stage chronic obstructive pulmonary disease (COPD), obstructive sleep apnea (OSA), aortic aneurysm rupture, renal artery dissection, iliac artery dissection, and pulmonary artery dissection.
Figure 2.
Autopsy-Defined Causes of SAD by Race. Autopsy etiologies of SAD by race after review of comprehensive medical records, EMS run sheets, and comprehensive autopsy data including post-mortem chemistry and toxicology. First % is of total presumed SCDs and second % is of cause of death category. MINOCA accounted for 3% of SAD in Asians and Blacks and 0% in Whites (p=0.03). CAD accounted for 43% in Blacks and 61% in Whites (p<0.05) while cardiomyopathy accounted for 34% of SAD in Blacks and 17% in Whites (p=0.02). Primary electrical disease accounted for 14% of SAD in Hispanics and 2% in Whites (p<0.01).
CAD indicates coronary artery disease; MINOCA, myocardial infarction without obstructive coronary arteries; SAD, sudden arrhythmic death
*Adjusted for age and sex vs. reference White
†Other cardiac arrhythmic causes included acquired long QT syndrome (LQTS), bicuspid aortic valve, cardiac implantable external device (CIED) concern and/or failure, mitral valve prolapse, and critical aortic stenosis (AS).
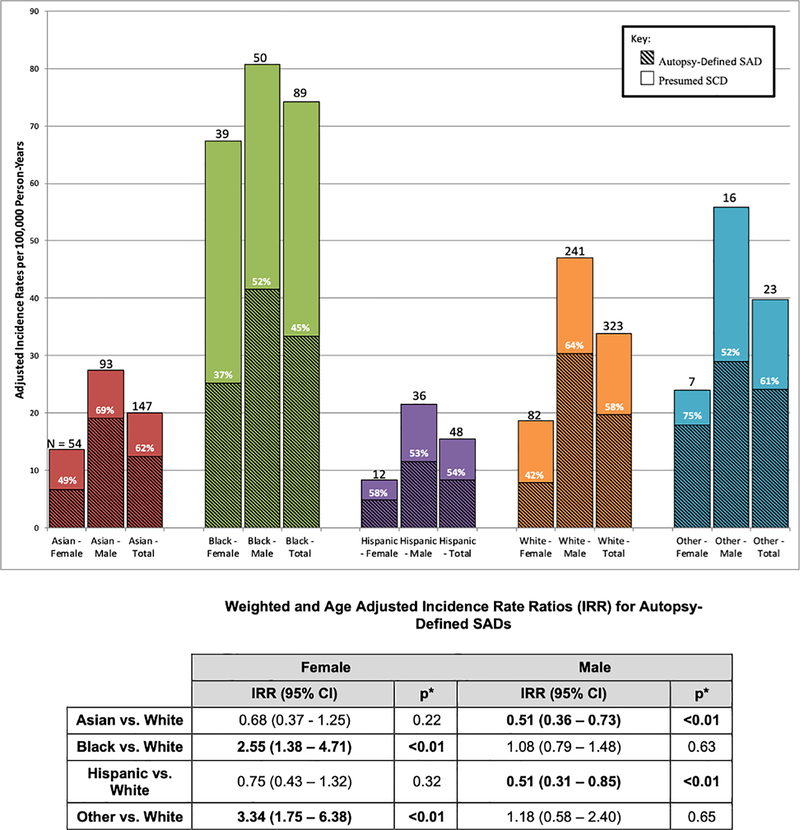
Incidence of Autopsy-Defined SAD by Sex and Race
Incidence rates of presumed SCD and SAD were over 2- and 3-fold higher in men vs. women, respectively (p<0.01). While Asian and Hispanic men had the lowest incidence rate ratio (IRR) for SAD as compared to White men (IRR 0.51 for both, p<0.01), IRR for SAD was similar for Asian, Hispanic, and White women (Figure 3). Among women, Blacks had a higher rate of SAD (IRR 2.55, p<0.01) than Whites, while Black men had similar rates compared to White men (IRR 1.08, p=0.63).
Figure 3.
Rates of Presumed SCD and Autopsy-Defined SAD per 100,000 Person-Years by Sex and Race. Adjusted incidence rates per 100,000 person-years for all presumed SCDs in San Francisco County from 1 February 2011 to 1 March 2014 with weighted incidence rates for autopsy-defined SAD. Sex- and race-specific incidence rate ratios (IRR) for all weighted autopsy-defined SADs are shown. Other race includes American Indian/Alaskan Natives, Native Hawaiians, and other Pacific Islanders. Autopsy-defined SAD accounted for 57.1% of all presumed SCDs. Incidence rate ratios for autopsy-defined SAD were over 3-fold higher in men vs. women, highest in black females, and lowest in Asian and Hispanic males (all p<0.01).
Characteristics of Autopsy-Defined SADs by Sex and Race
Female victims of SAD were older than male victims (70.6 ± 13.4 vs. 61.7 ± 14.4, p<0.01), more likely to have prior history of CVA (11% vs. 5%; p<0.05), and less likely to have prior substance use history (Table 4). As compared to White SADs, Asian cases were less likely to have prior substance use history, while Black SADs were more likely to have history of heart failure (31% vs. 14%; p=0.02), chronic kidney disease (31% vs. 7%; p<0.01), and CVA (14% vs. 3%; p<0.01). Hispanic SADs were more likely to have prior history of diabetes (43% vs. 19%; p=0.01, Table 4).
Table 4.
Pre-Mortem Conditions in Autopsy-Defined SADs by Sex and Race.
| Autopsy-Defined SAD (293) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Race | |||||||||
| No. (%) | Female | Male | p* | Asian | p* | Black | p* | Hispanic | p* | White |
| 73 (25) | 220 (75) | – | 68 (23) | – | 35 (12) | – | 21 (7) | – | 160 (55) | |
| Medical Records Unobtainable | 2 (3) | 13 (6) | NS | 5 (7) | NS | 2 (6) | NS | 2 (10) | NS | 6 (4) |
| Confirmed No Medical History | 5 (7) | 10 (5) | NS | 6 (9) | NS | 1 (3) | NS | 0 (0) | NS | 8 (5) |
| Age (years) | 70.6 ± 13.4 | 61.7 ± 14.4 | <0.01 | 66.5 ± 16.0 | NS | 61.0 ± 16.8 | NS | 60.6 ± 18.8 | NS | 64.3 ± 12.7 |
| Female | – | – | – | 21 (31) | 0.04 | 13 (37) | 0.02 | 6 (29) | NS | 30 (19) |
| History of: | ||||||||||
| Hypertension | 46 (63) | 129 (59) | NS | 40 (59) | NS | 25 (71) | NS | 11 (52) | NS | 94 (59) |
| Diabetes Mellitus | 21 (29) | 51 (23) | NS | 19 (28) | NS | 10 (29) | NS | 9 (43) | 0.01 | 31 (19) |
| Dyslipidemia | 23 (32) | 85 (39) | NS | 29 (43) | NS | 13 (37) | NS | 6 (29) | NS | 58 (36) |
| Myocardial Infarction | 12 (16) | 37 (17) | NS | 6 (9) | NS | 9 (26) | NS | 5 (24) | NS | 27 (17) |
| Congestive Heart Failure | 11 (15) | 36 (16) | NS | 8 (12) | NS | 11 (31) | 0.02 | 3 (14) | NS | 23 (14) |
| Chronic Kidney Disease (non ESRD) | 11 (15) | 22 (10) | NS | 6 (9) | NS | 11 (31) | <0.01 | 3 (14) | NS | 11 (7) |
| Seizure Disorder | 3 (4) | 11 (5) | NS | 2 (3) | NS | 2 (6) | NS | 2 (10) | NS | 7 (4) |
| Cerebrovascular accident | 8 (11) | 10 (5) | <0.05 | 5 (7) | NS | 5 (14) | <0.01 | 2 (10) | NS | 5 (3) |
| Psychiatric Disorder† | 19 (26) | 42 (19) | NS | 9 (13) | NS | 10 (29) | NS | 5 (24) | NS | 37 (23) |
| Chronic Obstructive Pulmonary Disease | 11 (15) | 21 (10) | NS | 7 (10) | NS | 5 (14) | NS | 3 (14) | NS | 17 (11) |
| Non-Metastatic Cancer | 15 (21) | 25 (11) | <0.05 | 6 (9) | NS | 6 (17) | NS | 2 (10) | NS | 26 (16) |
| Tobacco Use | 19 (26) | 96 (44) | <0.01 | 19 (28) | <0.05 | 14 (40) | NS | 12 (57) | NS | 67 (42) |
| Excess Alcohol Use | 2 (3) | 55 (25) | <0.01 | 7 (10) | 0.03 | 6 (17) | NS | 6 (29) | NS | 36 (23) |
| Illicit Drug Use | 1 (1) | 26 (12) | <0.01 | 1 (1) | 0.02 | 8 (23) | 0.04 | 2 (10) | NS | 16 (10) |
ESRD indicates end-stage renal disease; SAD, sudden arrhythmic death
p-value Female vs. Male, and Asian, Black, and Hispanic vs. White
Includes prior diagnosis of Depression, Anxiety, Schizophrenia, Bipolar Disorder, PTSD, Mood Disorders, Psychosis, Borderline Personality Disorder, Obsessive Compulsive Disorder, Insomnia, and other psychiatric condition not otherwise specified
Presenting Rhythms
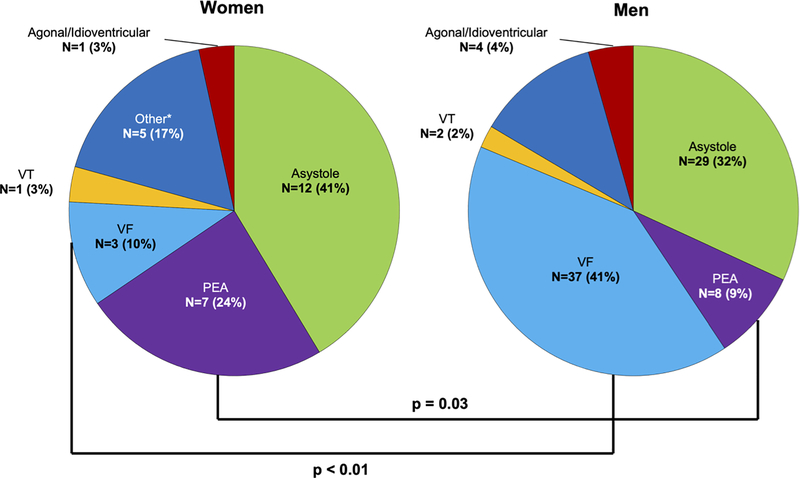
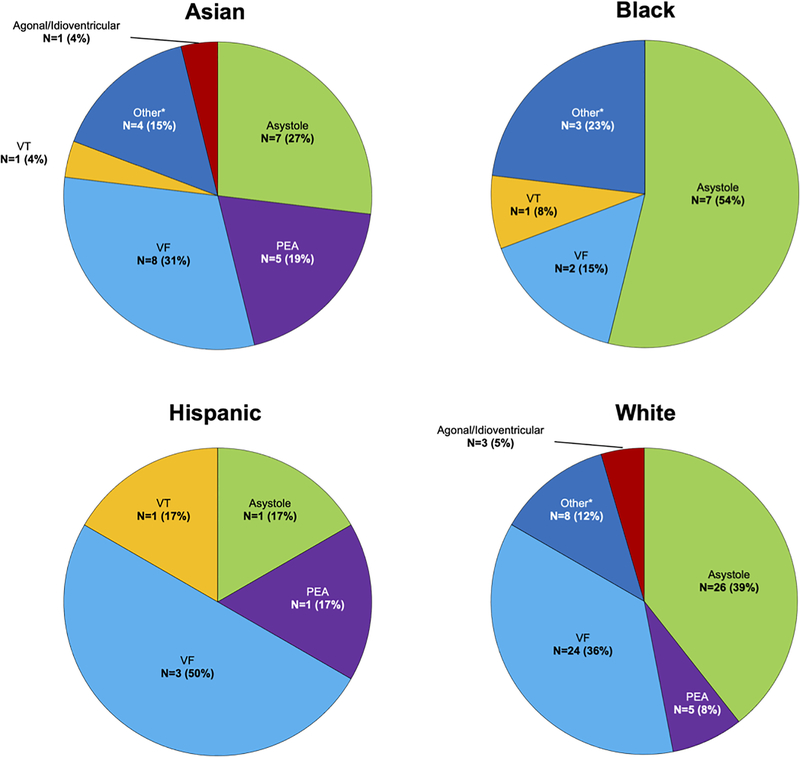
One quarter of presumed SCDs were witnessed (120 of 525, 23%), with a trend toward fewer witnessed cases in women (29/163 [18%] vs. 91/362 [25%]; p=0.06). Despite similar EMS response times (in minutes) for all witnessed arrests (6.4 ± 4.0 in women vs. 6.0 ± 3.8 in men, p=NS), an initial rhythm of pulseless electrical activity (PEA, 24% vs. 9%; p=0.03) or sinus bradycardia (14% vs. 1%; p<0.01) was more common in women while men were more likely to be found in ventricular tachycardia/ventricular fibrillation (VT/VF) (43% vs. 14%; p<0.01, Figure 4). Of the witnessed arrests with initial presenting rhythm of PEA or sinus bradycardia, there was no significant difference, between women and men, in the proportion that were classified as arrhythmic (28% vs. 11%, p>0.05) or in EMS response times. We found no significant racial differences in EMS response time, proportion of witnessed deaths, or initial presenting rhythms.
Figure 4.
Initial Rhythms at EMS Arrival for Witnessed WHO-defined SCD by Sex and Race. Initial rhythms at EMS arrival for witnessed WHO-defined SCD (N = 120) by sex and race. An initial rhythm of pulseless electrical activity (PEA, 24% vs. 9%; p=0.03) was more common in women while men were more likely to be found in ventricular tachycardia/ventricular fibrillation (VT/VF) (43% vs. 14%; p<0.01). There were no significant racial differences in initial presenting rhythms.
EMS indicates emergency medical service; PEA, pulseless electrical activity; SCD, sudden cardiac death, VF, ventricular fibrillation; VT, ventricular tachycardia; WHO, World Health Organization
*Normal sinus rhythm (ST-elevation myocardial infarction), sinus bradycardia/tachycardia (ST-elevation and Non ST-elevation myocardial infarction), atrial fibrillation/flutter (ST-elevation myocardial infarction)
Discussion
In this systematic postmortem investigation of all deaths attributed to out-of-hospital cardiac arrest and presumed SCD in an entire diverse metro area to exclude non-cardiac causes misclassified as presumed SCD, the rate of autopsy-defined SAD was more than 3-fold higher in men than women overall, but greater than 2-fold higher in Black vs. White women. Women had fewer ischemic causes of SAD, while Asians had higher rates of MINOCA suggesting phenotypic variation in arrhythmic risk. Among sudden deaths overall, we also found significant differences in the prevalence of underlying causes by sex and race, with more non-cardiac causes in women as compared to men, and more sudden neurologic deaths in Asians, endocrine causes in Blacks, and gastrointestinal causes in Hispanics as compared to Whites. Our findings demonstrate the poor accuracy of currently accepted definitions of SCD for actual arrhythmic cause, particularly in women, with implications for risk stratification and prevention of sudden mortality specific to women and minority populations.
It has been reported that men have higher rates of SCD than women, with most causes attributed to CAD,11 while women are less likely to have structural heart disease as manifest by less severe left ventricular dysfunction or CAD prior to presumed SCD.12,13 However, these studies were based on review of medical records and death certificate data which presume arrhythmic cause, and determining the differential risk of true SAD in women is important to identify patients rescuable by ICD therapy.6,14 In POST SCD, we found that there is significant misclassification of presumed SCD in women, who had more non-cardiac causes than men, including sudden neurologic deaths (10% of presumed SCDs) and fatal pulmonary emboli (8% of presumed SCDs). Consistent with this, women were more likely to present with PEA and sinus bradycardia despite comparable EMS response times and a similar proportion of witnessed cases as compared to men. While the reported mortality rates for pulmonary embolism are decreasing in the U.S., a substantial number of sudden deaths attributable to pulmonary emboli are likely missed without postmortem data as available in POST SCD.15 Women also have higher lifetime risk of stroke than men and we have previously reported the under-recognition of sudden neurologic death as an underlying cause of presumed SCDs.16,17 Within our refined population of true SAD, women appeared phenotypically distinct from men with a lower proportion of ischemic causes and a greater proportion due to primary electrical disease, consistent with a female preponderance of congenital long QT syndrome.18 These findings highlight potential sex differences in mechanisms that underlie overall sudden death risk and challenge the assumed role of CAD in the majority of SADs. By including systematic toxicology, our findings expand upon the results of a recent Finnish autopsy study which reported lower rates of SCD and more non-ischemic causes of death in women19 but did not report postmortem toxicology as a means to rule out occult overdose which may be positive in more than half of SCD victims.20 Taken together, our findings indicate that focusing on modification of traditional CAD risk factors may not have as significant an impact on sudden death in women, which may also require attention to non-ischemic causes of SAD such as primary electrical disease, along with cerebrovascular and thrombotic/embolic disease risk.
Recent data demonstrate higher presumed SCD risk in Blacks compared to Whites, particularly among women, and not fully explained by socioeconomic class or traditional cardiovascular risk factors.5,21 POST SCD confirmed significant racial differences in rates of presumed SCDs and SADs, which were highest in Blacks and lowest in Hispanics, yet the proportion of sudden deaths attributable to arrhythmic causes was lowest in both populations.8 Therefore, our results suggest that at least some of the previously reported racial differences are due to miclassification.5,21 As such, public health resources may not currently address the areas of greatest disparity. Prior evidence suggests higher rates of OHCA in the Black population with lower rates of survival to hospital discharge.2–4,22–25 While one reason for this disparity may be higher rates of cardiac co-morbidities in the Black population, it may also be explained by our finding that Blacks had the lowest proportion of presumed SCDs attributed to SAD and the largest proportion (more than half) due to non-cardiac causes which are not rescuable by ICD and may not be as amenable to traditional recuscitative strategies. Rates of diabetes are highest in the Black population with correspondingly poor control of disease and Blacks had the highest proportion of glycemic emergencies as the cause of sudden death.26 Taken together, these findings suggest the importance of targeted primary prevention interventions beyond arrhythmic risk, including attention to more aggressive glycemic control in diabetics to impact overall sudden mortality in the Black population.
Even after accounting for misclassification, the overall incidence of SAD was higher in Blacks. In addition, we found a compound effect of sex and race: while Black women had a significantly higher incidence of SAD as compared to White women, SAD rates were similar for Black and White men. Within the refined SAD population, Blacks had a trend towards fewer ischemic and more cardiomyopathy causes than Whites. Overall, this highlights the need for further attention to risk factor modification and prevention strategies targeting non-ischemic causes of SAD to address the disparity in Black populations.
Limited data are available for incidence of SCD in other minority groups in the U.S. It has been reported that Hispanic Americans have lower rates of presumed SCD than Whites.4 Our autopsy data confirm this finding, as Hispanic men had the lowest incidence of SAD. Hispanic victims of presumed SCD also had significantly higher gastrointestinal causes of sudden death, including gastrointestinal hemorrhage and liver failure, again highlighting the differential impact of non-cardiac causes on sudden mortality.
While other studies have reported lower incidence rates of presumed SCD in homogenous Asian countries, no studies have examined rates of SCD and SAD in Asian populations in the United States.27,28 In POST SCD, Asians overall had lower incidence of SAD as compared to Whites but higher rates of MINOCA. This finding is consistent with higher rates of MINOCA in non-Whites and specifically higher rates of inducible vasospasm in Asians,29–31 challenging the assumption that this condition rarely causes SCD.32,33 Stratified by gender, while Asian and White women had similar rates of SAD, these rates were nearly 2-fold lower among Asian men as compared to White men. Asians had the lowest rate of occult overdose but the highest rate of sudden neurologic death among all ethnic groups.17 Notably, more than half of Asian sudden neurologic deaths were on anti-coagulation medications (6 of 11 subjects). Asian populations have a higher age-adjusted incidence of stroke, a lower mean age of stroke onset, and a higher proportion of intracranial hemorrhage independent of cardiovascular risk factors.17,28,34,35 These observations have led to studies examining whether modification of the CHA2DS2-VASc score in Asians with atrial fibrillation to support anti-coagulation at younger ages or at lower scores is warranted.36–38 However, an increase in anti-coagulation among Asians to prevent ischemic stroke may be accompanied by higher rates of sudden death from intracranial hemorrhage.39,40 Our findings are consistent with these studies and support more careful monitoring of anti-coagulation therapy in Asians as these causes may be misclassified as presumed SCD and not adequately captured in traditional safety surveillance.
Limitations
As previously noted, the validity of SCD studies is dependent on their specificity for actual cardiac causes. While we were reliably able to exclude non-arrhythmic causes by systematic autopsy for nearly every WHO-defined SCD and use rigorous pre-specified criteria to determine underlying causes of SAD, the precise cause of death remained subject to some degree of uncertainty. However, this level of uncertainty is a significant improvement compared to studies lacking post-mortem data. Moreover, our findings are comparable to other recent autopsy-based SCD studies19, with the added innovation of comprehensive toxicology analysis to exclude occult overdose as a cause of non-cardiac sudden death. Furthermore, although the diverse nature of San Francisco County allowed for the novel examination of ethnicity-specific rates and causes of community sudden death, our findings may not be fully generalizable to other populations. A recent publication found that clinical autopsy rates nationally are declining, and significantly higher in Blacks than Whites which may reflect underlying health disparities, “mistrust” in the healthcare system, or less aggressive premortem care translating into a less established diagnosis for cause of death.41,42 In the POST SCD study however, autopsy data was available for 97% of all subjects, providing an unbiased picture of the current epidemiology and burden of sudden death in an urban setting. Since women had a non-significant trend towards more unwitnessed deaths compared to men and these deaths are inherently more heterogenous, these deaths may have contributed to the differential misclassification observed between sexes. However, since misclassification was due to neurologic death and pulmonary embolism, causes which have been established to differentially impact women, this is unlikely to account for all of the differences we observed. We would also like to emphasize that this is not a study of biologic risk underlying sex and race. As an epidemiologic study, we documented causes of death within populations and identified disparities in disease states that may be targeted for intervention. Finally, genetic testing was not available for this study but is the subject of ongoing investigation in POST SCD.
Conclusion
In this analysis of race- and sex-specific data from our countywide postmortem study of all incident sudden deaths, we found significant differences in misclassification as more than than half of these deaths presumed cardiac in women and Blacks had non-arrhythmic causes not rescuable by an defibrillator. Women had more fatal pulmonary emboli and sudden neurologic deaths misclassified as SCD while Asians had the most neurologic causes, Blacks the most glycemic emergencies, and Hispanics the most gastrointestinal causes. Within the refined population of SAD, women had fewer ischemic causes and more normal heart primary electrical disease, while Asians had higher rates of lethal MINOCA. These findings have implications for risk stratification and prevention of sudden mortality specific to women and minority populations and suggest the importance of primary prevention interventions beyond atherosclerosis risk to better address disparities in sudden death.
What is Known?
Prior studies have demonstrated substantial sex and racial differences in sudden cardiac death (SCD) incidence, with rates reportedly higher in men and Blacks.
Almost all studies rely on medical or emergency medical service records and death certificates to infer cardiac cause, yet non-cardiac and non-arrhythmic causes not rescuable with implantable cardioverter-defibrillators cannot be excluded without full autopsy.
What the Study Adds?
Among presumed SCDs, women were more likely to experience non-arrhythmic sudden death due to pulmonary emboli and neurologic causes and present with an initial rhythm of pulseless electrical activity, while men had 3-fold higher rates of autopsy-defined sudden arrhythmic death (SAD) and were more likely to present with ventricular tachycardia or fibrillation.
Among SADs, women had fewer ischemic causes and more causes due to normal heart primary electrical disease, and Black women had the highest age-adjusted incidence of SAD compared to White women.
Adjusted for age and sex, among presumed SCDs, Asians had higher rates of myocardial infarction with non-obstructive coronary arteries (MINOCA) and sudden neurologic death, Blacks had fewer ischemic causes, and Hispanics had more primary electrical disease than reference Whites.
Acknowledgments:
We are indebted to Nikolas Lemos, Ph.D. for expert review of all toxicological analyses in the POST SCD study. We thank Annie Bedigian and Joanne Probert for assistance with figures and tables. We are grateful for the efforts of all forensic investigators in the Office of the Chief Medical Examiner and EMS personnel in San Francisco County. Author Contributions: Zian H. Tseng contributed to study conception and design, analysis and interpretation of data, acquisition of study funding, and drafting of the manuscript. Satvik Ramakrishna and James W. Salazar contributed to analysis and interpretation of the data and drafting of the manuscript. Eric Vittinghoff contributed to analysis of data and critical revision of the manuscript. Jeffrey Olgin contributed to study conception and design, analysis and interpretation of data, and critical revision of the manuscript. Ellen Moffatt contributed to study conception and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript.
Sources of Funding:
This study was funded by NIH R01 HL102090 (National Heart, Lung, and Blood Institute to ZHT). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosures: Dr. Tseng reports grants from NIH/NHLBI and CDC during the conduct of the study, and personal fees from Biotronik, outside the submitted work. Dr. Olgin reports grants from NIH/NIBIB/NHLBI and ZOLL, outside the submitted work. Dr. Salazar reports receiving grants from the National Heart, Lung, and Blood Institute (Award No. R38HL143581). No other disclosures were reported.
Nonstandard Abbreviations and Acronyms
- SCD
Sudden Cardiac Death
- SAD
Sudden Arrhythmic Death
- MINOCA
Myocardial infarction in the absence of obstructive coronary artery disease
- OHCA
Out-of-hospital cardiac arrest
- SUDEP
Sudden unexpected death in epilepsy
References:
- 1.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. [DOI] [PubMed] [Google Scholar]
- 2.Steinhaus DA, Vittinghoff E, Moffatt E, Hart AP, Ursell P, Tseng ZH. Characteristics of sudden arrhythmic death in a diverse, urban community. Am Heart J. 2012;163:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinier K, Nichols GA, Huertas-Vazquez A, Uy-Evanado A, Teodorescu C, Stecker EC, Gunson K, Jui J, Chugh SS. Distinctive Clinical Profile of Blacks Versus Whites Presenting With Sudden Cardiac Arrest. Circulation. 2015;132:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillum RF. Sudden cardiac death in Hispanic Americans and African Americans. Am J Public Health. 1997;87:1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deo R, Safford MM, Khodneva YA, Jannat-Khah DP, Brown TM, Judd SE, McClellan WM, Rhodes JD, Shlipak MG, Soliman EZ, et al. Differences in Risk of Sudden Cardiac Death Between Blacks and Whites. J Am Coll Cardiol. 2018;72:2431–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iribarren C, Crow RS, Hannan PJ, Jacobs DR, Luepker RV. Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. Am J Cardiol. 1998;82:50–53. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e190–e252. [DOI] [PubMed] [Google Scholar]
- 8.Tseng ZH, Olgin JE, Vittinghoff E, Ursell PC, Kim AS, Sporer K, Yeh C, Colburn B, Clark NM, Khan R, et al. Prospective Countywide Surveillance and Autopsy Characterization of Sudden Cardiac Death: POST SCD Study. Circulation. 2018;137:2689–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNally B, Robb R, Mehta M, Vellano K, Valderrama AL, Yoon PW, Sasson C, Crouch A, Perez AB, Merritt R, et al. Out-of-hospital cardiac arrest surveillance --- Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005--December 31, 2010. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002. 2011;60:1–19. [PubMed] [Google Scholar]
- 10.Sudden cardiac death. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1985;726:5–25. [PubMed] [Google Scholar]
- 11.Kannel WB, Wilson PW, D’Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. [DOI] [PubMed] [Google Scholar]
- 12.Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study). J Am Coll Cardiol. 2009;54:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chugh SS, Chung K, Zheng Z-J, John B, Titus JL. Cardiac pathologic findings reveal a high rate of sudden cardiac death of undetermined etiology in younger women. Am Heart J. 2003;146:635–639. [DOI] [PubMed] [Google Scholar]
- 14.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. [DOI] [PubMed] [Google Scholar]
- 15.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 17.Kim AS, Moffatt E, Ursell PC, Devinsky O, Olgin J, Tseng ZH. Sudden neurologic death masquerading as out-of-hospital sudden cardiac death. Neurology. 2016;87:1669–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imboden M, Swan H, Denjoy I, Van Langen IM, Latinen-Forsblom PJ, Napolitano C, Fressart V, Breithardt G, Berthet M, Priori S, et al. Female predominance and transmission distortion in the long-QT syndrome. N Engl J Med. 2006;355:2744–2751. [DOI] [PubMed] [Google Scholar]
- 19.Haukilahti MAE, Holmström L, Vähätalo J, Kenttä T, Tikkanen J, Pakanen L, Kortelainen M-L, Perkiömäki J, Huikuri H, Myerburg RJ, et al. Sudden Cardiac Death in Women. Circulation. 2019;139:1012–1021. [DOI] [PubMed] [Google Scholar]
- 20.Bjune T, Risgaard B, Kruckow L, Glinge C, Ingemann-Hansen O, Leth PM, Linnet K, Banner J, Winkel BG, Tfelt-Hansen J. Post-mortem toxicology in young sudden cardiac death victims: a nationwide cohort study. Europace. 2018;20:614–621. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D, Post WS, Blasco-Colmenares E, Cheng A, Zhang Y, Deo R, Pastor-Barriuso R, Michos ED, Sotoodehnia N, Guallar E. Racial Differences in Sudden Cardiac Death. Circulation. 2019;139:1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, Barrett J. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993;329:600–606. [DOI] [PubMed] [Google Scholar]
- 25.Cowie MR, Fahrenbruch CE, Cobb LA, Hallstrom AP. Out-of-hospital cardiac arrest: racial differences in outcome in Seattle. Am J Public Health. 1993;83:955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Willis M, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y-N, Chang S-S, Wang L-M, Chi H-T, Ueng K-C, Tsai C-F, Phan C-S, Lu L-H, Hii C-H, Chung Y-T, et al. Prehospital Predictors of Initial Shockable Rhythm in Out-of-Hospital Cardiac Arrest: Findings From the Taichung Sudden Unexpected Death Registry (THUNDER). Mayo Clin Proc. 2017;92:347–359. [DOI] [PubMed] [Google Scholar]
- 28.Tsai C-F, Thomas B, Sudlow CLM. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e891–e908. [DOI] [PubMed] [Google Scholar]
- 30.Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, et al. Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. J Am Heart Assoc. 2018;7:e009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 32.Ciliberti G, Finocchiaro G, Papadakis M, Westaby JD, Sharma S, Sheppard MN. Myocardial Infarction With Nonobstructed Coronary Arteries and Sudden Cardiac Death: A Clinical and Pathological Perspective. Circ Arrhythm Electrophysiol. 2020;13:e008302. [DOI] [PubMed] [Google Scholar]
- 33.Kosmas N, Manolis AS, Dagres N, Iliodromitis EK. Myocardial infarction or acute coronary syndrome with non-obstructive coronary arteries and sudden cardiac death: a missing connection. Europace. 2020;22:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feigin V, Carter K, Hackett M, Barber PA, McNaughton H, Dyall L, Chen M, Anderson C, Auckland Regional Community Stroke Study Group. Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002–2003. Lancet Neurol. 2006;5:130–139. [DOI] [PubMed] [Google Scholar]
- 35.Khan NA, McAlister FA, Pilote L, Palepu A, Quan H, Hill MD, Fang J, Kapral MK. Temporal trends in stroke incidence in South Asian, Chinese and white patients: A population based analysis. PloS One. 2017;12:e0175556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao T-F, Liu C-J, Wang K-L, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F, Tuan T-C, Chen T-J, Lip GYH, et al. Using the CHA2DS2-VASc score for refining stroke risk stratification in “low-risk” Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:1658–1665. [DOI] [PubMed] [Google Scholar]
- 37.Siu C-W. One more “C” for CHA2DS2-VASc score? J Am Coll Cardiol. 2015;65:1602–1603. [DOI] [PubMed] [Google Scholar]
- 38.Chao T-F, Lip GYH, Liu C-J, Tuan T-C, Chen S-J, Wang K-L, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F, et al. Validation of a Modified CHA2DS2-VASc Score for Stroke Risk Stratification in Asian Patients With Atrial Fibrillation: A Nationwide Cohort Study. Stroke. 2016;47:2462–2469. [DOI] [PubMed] [Google Scholar]
- 39.Shen AY-J, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. [DOI] [PubMed] [Google Scholar]
- 40.Cha M-J, Choi E-K, Han K-D, Lee S-R, Lim W-H, Oh S, Lip GYH. Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Asian Patients With Atrial Fibrillation. Stroke. 2017;48:3040–3048. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A, Premnath N, Kuo P-L, Sedhom R, Brawley OW, Chino F. Assessment of Racial Differences in Rates of Autopsy in the US, 2008–2017. JAMA Intern Med. 2020;180:1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salazar JW, Tseng ZH. Preserving Access to the Invaluable Clinical Autopsy. JAMA Intern Med. 2020;180:1124–1125. [DOI] [PubMed] [Google Scholar]