Abstract
Parkinson’s disease (PD) is characterized by motor symptoms, but non-motor symptoms also significantly impair daily functioning and reduce quality of life. Anxiety is prevalent and debilitating in PD, but remains understudied and under-treated. Most affective research in PD focuses on depression rather than anxiety, and as such, there are no evidence-based treatments for anxiety in this population. Cognitive-behavioral therapy (CBT) has shown promise for treating depression in PD and may be efficacious for anxiety. This exploratory study implemented a multiple-baseline single-case experimental design to evaluate the utility and feasibility of CBT for individuals with PD who also met criteria for a DSM-5 anxiety disorder (N=9). Participants were randomized to a 2-, 4-, or 6- week baseline phase, followed by 12 CBT sessions, and two post-treatment assessments (immediately post-treatment and 6-week follow-up). Multiple outcome measures of anxiety and depression were administered weekly during baseline and intervention. Weekly CBT sessions were conducted in-person (N=5) or via secure videoconferencing (N=4). At post-treatment, seven of nine participants showed significant reductions in anxiety and/or depression, with changes functionally related to treatment and most improvements maintained at 6-week follow-up. Effects of CBT on secondary outcomes varied across participants, with preliminary evidence for reduction in fear of falling. Adherence and retention were high, as were treatment satisfaction and acceptability. The findings of this pilot study provide preliminary evidence for the utility of CBT as a feasible treatment for anxiety and comorbid depressive symptoms in PD and highlight the potential of telehealth interventions for mood in this population.
Keywords: Parkinson’s disease, anxiety, depression, mood, CBT
Anxiety is a prevalent and distressing non-motor symptom of Parkinson’s disease (PD) but it remains understudied and inadequately treated (Chen & Marsh, 2014; N. Dissanayaka et al., 2015). The prevalence of anxiety disorders in PD is approximately 31%, with significant anxiety symptoms occurring in up to 26% of individuals with the disease (Broen, Narayen, Kuijf, Dissanayaka, & Leentjens, 2016). Common anxiety disorders in PD include generalized anxiety disorder, social phobia, anxiety not otherwise specified, and specific phobia (Broen et al., 2016). There is also evidence of anxiety symptoms specific to PD, such as distress, worry, and embarrassment related to motor symptoms (N. N. Dissanayaka et al., 2016). Anxiety frequently co-occurs with other non-motor symptoms (M. A. Menza, Robertson-Hoffman, & Bonapace, 1993; Naismith & Lewis, 2011; Reynolds, Hanna, Neargarder, & Cronin-Golomb, 2017) and compromises quality of life, even early in the disease course (Duncan et al., 2014; Hanna & Cronin-Golomb, 2012).
Despite this prevalence and negative impact of anxiety in PD, there is a lack of evidence-based strategies to guide treatment (N. Dissanayaka et al., 2015). There have been a small number of uncontrolled and pilot studies for the treatment of anxiety in PD, but no randomized controlled trials for anxiety disorders specifically (Schrag, Sauerbier, & Chaudhuri, 2015; Seppi et al., 2011; Troeung, Egan, & Gasson, 2013), as affective research in PD often focuses on depression (N. N. Dissanayaka et al., 2014; Yang, Sajatovic, & Walter, 2012). Selective serotonin reuptake inhibitors (SSRIs) are often the standard pharmacologic treatment for anxiety in PD despite limited research on their efficacy (Chen & Marsh, 2014; B. Connolly & Fox, 2014; N. Dissanayaka et al., 2015); across two depression trials, SSRIs and tricyclic antidepressants have yielded secondary improvements on anxiety in PD, though side effects include bradykinesia, erectile dysfunction, orthostatic hypotension, and cardiac arrhythmias (Devos et al., 2008; M. Menza et al., 2009; Troeung et al., 2013). Benzodiazepines may not be recommended due to potential for increased falls and cognitive dysfunction (Chen & Marsh, 2014; B. Connolly & Fox, 2014; N. Dissanayaka et al., 2015). Psychotropic drugs, in general, may exacerbate PD symptoms and/or interact with PD medications (B. S. Connolly & Fox, 2012). Overall, there remains a limited evidence base to guide the pharmacologic treatment of anxiety in PD (Pontone et al., 2013; Seppi et al., 2011).
Non-pharmacological treatments are encouraging alternatives, with particular promise for cognitive-behavioral therapy (CBT) (Armento et al., 2012; Egan, Laidlaw, & Starkstein, 2015; Pachana et al., 2013), which has reduced symptoms of depression and anxiety in PD (Yang et al., 2012). In single-case, pilot, and uncontrolled studies, individual and group CBT have also shown treatment potential for individuals with PD and diagnosed anxiety and/or depression (Calleo et al., 2015; Feeney, Egan, & Gasson, 2005; Heinrichs, Hoffman, & Hofmann, 2001; Mohlman et al., 2010; Veazey, Cook, Stanley, Lai, & Kunik, 2009). In a waitlist-controlled trial, group CBT reduced depression and anxiety among 18 participants with PD and comorbid diagnoses of depression and/or anxiety (Troeung, Egan, & Gasson, 2014). Six sessions of manualized CBT reduced anxiety and caregiver burden in 12 PD participants and their caregivers in an uncontrolled study (N. N. W. Dissanayaka et al., 2017); the protocol emphasized behavioral elements and was delivered to persons with PD and caregivers simultaneously. These findings on CBT for anxiety in PD are consistent with the more extensive evidence for the efficacy of CBT for depression in this population, with some trials also yielding positive secondary effects on anxiety (Dobkin, Menza, Allen, Gara, et al., 2011; Dobkin, Menza, Allen, Tiu, et al., 2011; Farabaugh et al., 2010). Compared to medications, non-pharmacological treatments in PD may face barriers to use, including mobility and transportation (Dobkin et al., 2013). Telephone-delivered CBT has shown promise for reducing anxiety and depression in PD (Dobkin, Menza, Allen, Tiu, et al., 2011; Veazey et al., 2009), as has internet-delivered CBT (Kraepelien, Svenningsson, Lindefors, & Kaldo, 2015).
The aim of the present pilot study was to examine the utility and feasibility in PD of an in-person or internet-delivered CBT protocol that has demonstrated efficacy in the treatment of anxiety and depression in non-PD populations (Farchione et al., 2012). A single-case, multiple-baseline experimental design was used to examine within-subject variability and individual responses to specific intervention components. The goal of this study was to examine utility and feasibility of CBT for anxiety in PD in order to drive future treatment development and research on this topic. It was predicted that the intervention would reduce anxiety and yield positive effects on secondary outcomes, including depression, and ultimately drive future research and treatment development for anxiety in PD.
Method
Participants
Participants were nine individuals who met the clinical criteria for mild to moderate idiopathic PD (Hoehn & Yahr stage I-II (Hoehn & Yahr, 1967); MDS-UPDRS (Goetz et al., 2008): motor score (part 3): mean [SD]=28 [12.8]), following the United Kingdom Parkinson’s Disease Society Brain Bank diagnostic criteria (Hughes, Daniel, Kilford, & Lees, 1992). Inclusion criteria were (a) anxiety disorder per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; (American Psychiatric Association, 2013)), (b) 8+ years of education, (c) psychotropic medication stability 6+ weeks prior to intake with no changes during study, (d) internet access and a webcam if doing therapy online. Exclusion criteria were (a) serious or chronic medical or neurological illness if not well-managed, other than PD, (b) history of moderate-severe head injury, (c) current or recent (past 90 days) alcohol or substance use disorder, excepting nicotine, marijuana, and caffeine, (d) current suicidal or homicidal ideation or intent, (e) Mini-Mental State Exam score <25 (Stern, Sano, Paulsen, & Mayeux, 1987), (f) corrected binocular visual acuity poorer than 20/50, (g) average “off” time >50% of the day, (h) current DSM-5 diagnosis of bipolar, schizoaffective, or substance/medication-induced disorder, schizophrenia, or principal diagnosis of specific phobia, (i) 8+ sessions of CBT within the past 5 years, (j) concurrent psychotherapy focused on anxiety or depression. Seven participants reported current use of psychotropic medications. One participant (P8) had a history of deep brain stimulation (DBS). Participant information is reported in Table 1.
Table 1.
Participant Characteristics
| Participant | Age (yrs) | Education (yrs) | Sex | Side of MotorSymptom Onset | MDS-UPDRS (motor score) | Disease Duration (yrs) | Anxiety/Depression Diagnosis (CSR) | In-person vs. Internet-delivered Tx | Baseline Length (weeks) |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 67 | 18 | F | Right | 23 | 4.4 | GAD (4) | In-person | 2 |
| P2 | 55 | 14 | M | Right | 18 | 3.5 | PD (4) | In-person | 4 |
| P3 | 65 | 16 | M | Right | 40 | 15.4 | GAD (5); DYS (5) | In-person | 6 |
| P4 | 79 | 18 | M | Bilateral | 54 | 7.9 | OTHER SPEC ANX (LSA) (6); SOC (5) | In-person | 2 |
| P5 | 59 | 16 | F | Right | 24 | 4.9 | PD (5); AG (6); GAD (4) | Internet | 4 |
| P6 | 64 | 20+ | M | Left | 31 | 10.4 | SOC (4) | Internet | 6 |
| P7 | 56 | 14 | F | Right | 21 | 1.6 | SOC (5); GAD (5) | Internet | 2 |
| P8 | 33 | 19 | F | Left | 30 | 9.0 | PD (6); AG (6); SOC (6); GAD (6); MDD (5) | In-person | 4 |
| P9 | 73 | 16 | F | Left | 11 | 2.5 | GAD (4) | Internet | 6 |
MDS-UPDRS=Unified Parkinson’s Disease Rating Scale (participants tested in “on” state); CSR = Clinical Severity Rating; GAD = generalized anxiety disorder; SOC = social anxiety disorder; LSA=limited-symptom panic attack; DYS=dysthymia; PD=panic disorder. MDD=major depressive disorder. Tx=Treatment. Only clinically significant anxiety/depression diagnoses (CSR ≥4) are reported.
Research Design
This study implemented a single-case, multiple-baseline across subjects experimental design (Barlow, Nock, & Hersen, 2009; Hayes, Barlow, & Nelson-Gray, 1999) to evaluate symptom change during treatment. Single-case experimental designs (SCEDs) have high internal validity (Barlow & Hersen, 1973) and allow for flexibility to adapt treatment to meet the needs of each individual participant (Nock, Michel, & Photos, 2007). In contrast to traditional nomothetic approaches, SCEDs focus on rigorous idiographic analysis of the individual to elucidate variability among individuals and establish functional relationships between an intervention and behavior (Barlow & Nock, 2009; Barlow et al., 2009). SCEDs are well-established and frequently utilized in behavioral and intervention research (Au et al., 2017; Bentley, Nock, Sauer-Zavala, Gorman, & Barlow, 2017; Shingleton et al., 2016), often to examine the initial efficacy of an intervention, and to inform future research, namely the implementation of group-based randomized controlled trials (Barlow & Nock, 2009; Biglan, Ary, & Wagenaar, 2000; Byiers, Reichle, & Symons, 2012). The multiple-baseline design is especially well-suited to examine the utility and feasibility of a novel intervention and establish functional relations between individual symptomatic changes and specific treatment modules (Barlow et al., 2009; Hayes et al., 1999). To control for the passage of time and other potential external confounds, participants were randomized to one of three baseline conditions (two, four, or six weeks) with three participants per condition, in accordance with sample-size recommendations (Barlow et al., 2009). In this design, each participant served as his or her own control, allowing for within-participant analysis of baseline data (e.g., control data) relative to treatment data collected during the intervention and follow-up phases. Outcome measures of anxiety and depression were assessed weekly to track symptom change.
Procedures
Participants were recruited through neurology clinics and other sources and gave written informed consent. Procedures were approved by Boston University’s Institutional Review Board. See Figure 1 for participant flow. Participants completed four primary assessments of 3–5 hours and twelve hour-long sessions of CBT. Sessions were conducted at the Center for Anxiety and Related Disorders at Boston University or through secure videoconferencing (WebEx™). For the latter, primary assessments were conducted at participants’ homes.
Figure 1.
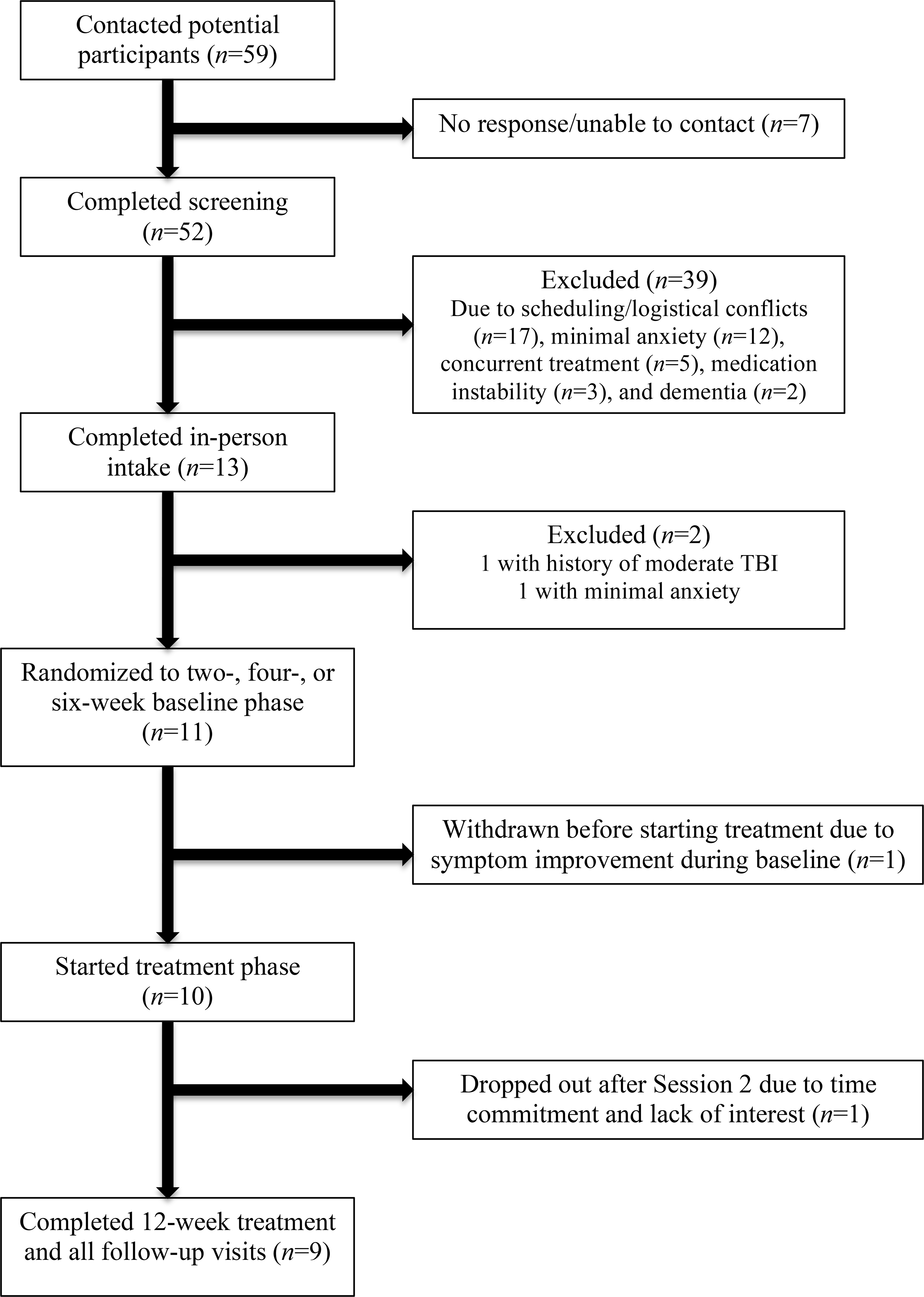
Participant Flow
Primary Assessments (intake/pre-baseline, post-baseline/pre-treatment, post-treatment, 6-week follow-up) consisted of a semi-structured diagnostic interview and self-report questionnaires. After the intake, participants were randomized to a two-, four-, or six-week baseline phase. Self-report questionnaires for secondary outcome measures were available online via Qualtrics, a secure online platform used regularly in clinical research.
Baseline Phase (2, 4, or 6 weeks):
Participants completed weekly self-report measures of anxiety and depression online. Questionnaires were reviewed weekly by the examiner to assess stability in scores and to monitor for issues of risk. Once stability was established, the second primary assessment was administered. The baseline phase was extended for three participants in the 4-week baseline condition: two because of variability in anxiety necessitating extension of the baseline phase by three weeks (P2) and one week (P5), and one (P8) because of variability in PD symptoms and scheduling conflicts (3-week extension). For these three participants, the last 4 weeks of the baseline were used for comparison to the intervention phase. One participant (P9) in the 6-week baseline condition showed variability in anxiety during the first 4 baseline weeks and failed to complete one anxiety measure (the Overall Anxiety Severity and Impairment Scale (OASIS)) during week 3; baseline was extended by one week. The last 6 weeks of the baseline condition were used for her analyses, with the exception of the OASIS, for which only 6 datapoints were collected across the 7 weeks; these six OASIS datapoints were utilized as a baseline.
Intervention Phase (12 weeks):
Participants engaged in twelve 50–60 minute weekly sessions of CBT following the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders (UP; (Barlow et al., 2011)), with five core modules to target emotional processing (present-focused awareness, cognitive flexibility, emotional avoidance and emotion-driven behaviors, awareness and tolerance of physical sensations, interoceptive and situational emotion exposures) plus modules on motivation, psychoeducation, and relapse prevention. In session, weekly homework was reviewed and progress was tracked by charting weekly anxiety and depression scores. Halfway through treatment, an optional 20–30 minute informational session was conducted with a partner or family member (in person, via WebEx™, or by phone) to provide psychoeducation about emotions and cognitive appraisal/reappraisal to facilitate practicing of these skills by the participant; 7 of 9 participated. Sessions were supervised by a licensed clinical psychologist, were audiotaped (or videotaped via WebEx™), and 12 tapes were randomly chosen for adherence review by a certified UP trainer. Adherence ratings were 85% or greater, with modal adherence of 100%.
Measures
Diagnostic interview:
Diagnostic information was obtained with the Anxiety Disorders Interview Schedule (ADIS-5; (Brown, Dinardo, & Barlow, 2013)), a semi-structured diagnostic interview for DSM-5 anxiety disorders, with clinical severity ratings assigned on a scale from 0 (no symptoms) to 8 (severe symptoms). Severity rating of 4+ (clinical significance) was required for enrollment.
Main outcome measures:
Measures of anxiety and depression were administered at all assessments and weekly during the baseline and intervention phases: Beck Anxiety Inventory (BAI; (Beck & Steer, 1993)), Overall Anxiety Severity and Impairment Scale (OASIS; (Norman, Cissell, Means-Christensen, & Stein, 2006)), State-Trait Anxiety Inventory (STAI; (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983)), Beck Depression Inventory (BDI-II; (Beck, Steer, & Brown, 1996)), Overall Depression Severity and Impairment Scale (ODSIS; (Bentley, Gallagher, Carl, & Barlow, 2014)), Geriatric Depression Scale (GDS; (Yesavage et al., 1982)). The OASIS was used as the primary outcome measure, given its applicability to various presentations of anxiety. Multiple measures of anxiety were used to capture the heterogeneous presentation of anxiety in PD (N. N. Dissanayaka et al., 2014), including somatic, state- and trait-based symptoms (BAI, STAI). Given the wide age range of participants, two measures of depression (BDI-II, GDS) were included, as depression can differ in presentation across the lifespan (Fiske, Wetherell, & Gatz, 2009). The BDI-II and GDS have been validated for use in PD, with the former better suited to capture depression severity and the latter better suited for screening across the disease course due to its limited assessment of motor symptoms (Schrag et al., 2007). The OASIS and ODSIS were included as brief, transdiagnostic measures, designed to be administered weekly as part of the UP, though these measures had not yet been used in PD.
Secondary outcome measures:
Administered at the four primary assessments were the Apathy Scale (AS; (Starkstein et al., 1992)) and Falls Self-Efficacy Scale (FES; (Tinetti, Richman, & Powell, 1990)); the Telepresence in Videoconference Scale (Bouchard & Robillard, 2000, 2006) at mid- and post-treatment; and two measures of treatment satisfaction at post-treatment: the Client Satisfaction Questionnaire (CSQ-8; (Larsen, Attkisson, Hargreaves, & Nguyen, 1979)) and a qualitative Post-treatment Feedback Form.
Data Analysis
As is standard in SCEDs, visual inspection was the primary analytic strategy (Barlow et al., 2009; Hayes et al., 1999; Kazdin, 2003). The relative change in slope between assessment points determined the effects of treatment components on symptom change. Observed changes in the slope or level of the dependent variables (weekly anxiety and depression) during phase shifts (transitions from baseline to intervention/follow-up), compared to either previous within-subject or simultaneous between-subject baseline phases, were used to determine if observed changes in symptoms were functionally related to the study phase (treatment).
To complement visual inspection, reliable change (RC) scores were computed for main and secondary outcomes as described in Au et al. (2017). For each participant, change scores were calculated for baseline (last baseline score – first baseline score), pre-post treatment (post-treatment score – last baseline score), and pre-follow-up (6-week follow-up score – last baseline score). A standard error of the difference (Sdiff) was calculated: Sdiff = sqrt[2(SE)2], where SE = SD X sqrt(1 – rxx) and SE=standard error of measurement; sqrt=square root; SD=standard deviation of the measure (from published norms or larger samples either in PD or clinical populations); rxx=reliability coefficient from published data. The small sample size of the present study dictated that SDs and internal consistency coefficients be taken from published studies with larger samples (Bentley et al., 2014; Campbell-Sills et al., 2009; Delbaere et al., 2010; Kabacoff, Segal, Hersen, & Van Hasselt, 1997; Leentjens et al., 2011; Starkstein et al., 1992; Williams et al., 2012; Yardley et al., 2005). 95% confidence intervals (CIs) around change scores were computed for each measure by multiplying Sdiff by 1.96. RC scores were used to determine if changes during the baseline and treatment phases were greater than expected by chance. Exploratory, qualitative analyses examined treatment satisfaction and acceptability.
Results
Figures 2–3 plot the scores of the weekly measures of anxiety and depression across all nine participants, displayed in panels of three participants (one from each baseline condition per panel to allow for within- and between-group visual inspection during phase shifts, e.g., baseline relative to intervention). Table 2 reports individual change scores and 95% CIs for main outcome measures of anxiety and depression during baseline, intervention, and follow-up phases.
Figure 2.
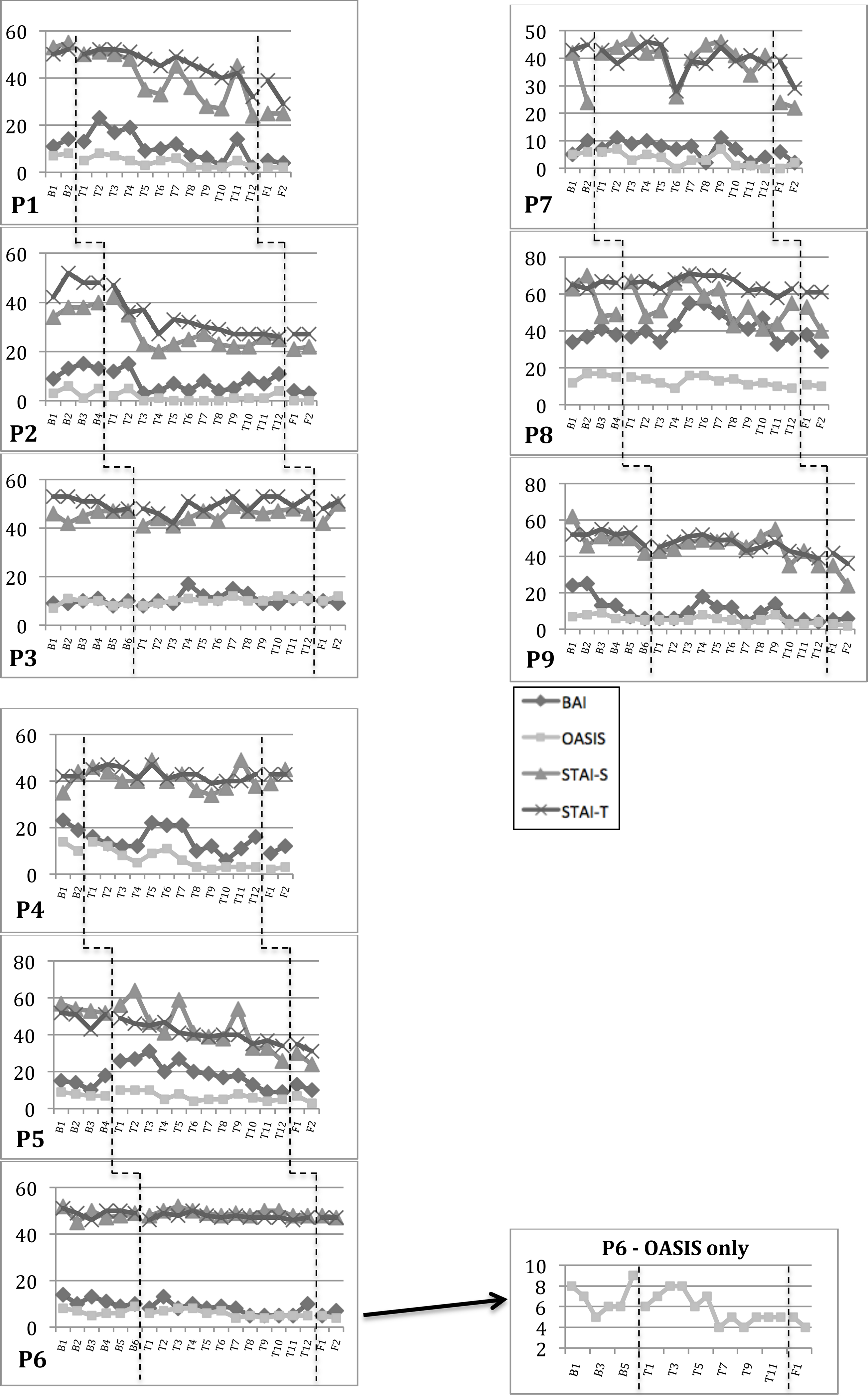
Weekly Anxiety Scores.
P2: Rapid response to treatment (weeks 2 and 3) corresponded with sessions on motivation and psychoeducation.
P5: Elevated BAI at the beginning of treatment (sessions 1–5) coincided with external stressor. BAI trended downward from sessions 3–12. P8: Initial increase in slope on BAI/STAI-S (weeks 2/3–5) coincided with increased “off” periods related to DBS reprogramming. There was a subsequent decline in BAI/STAI-S/OASIS from weeks 5–12. Throughout treatment, she reported several medically-warranted adjustments in dopaminergic dose and DBS settings, which worsened motor complications. These changes likely affected her anxiety ratings, but these changes were consistent from weeks 1–10 of the intervention phase, and the downward trend in anxiety scores began in week 5.
Figure 3.
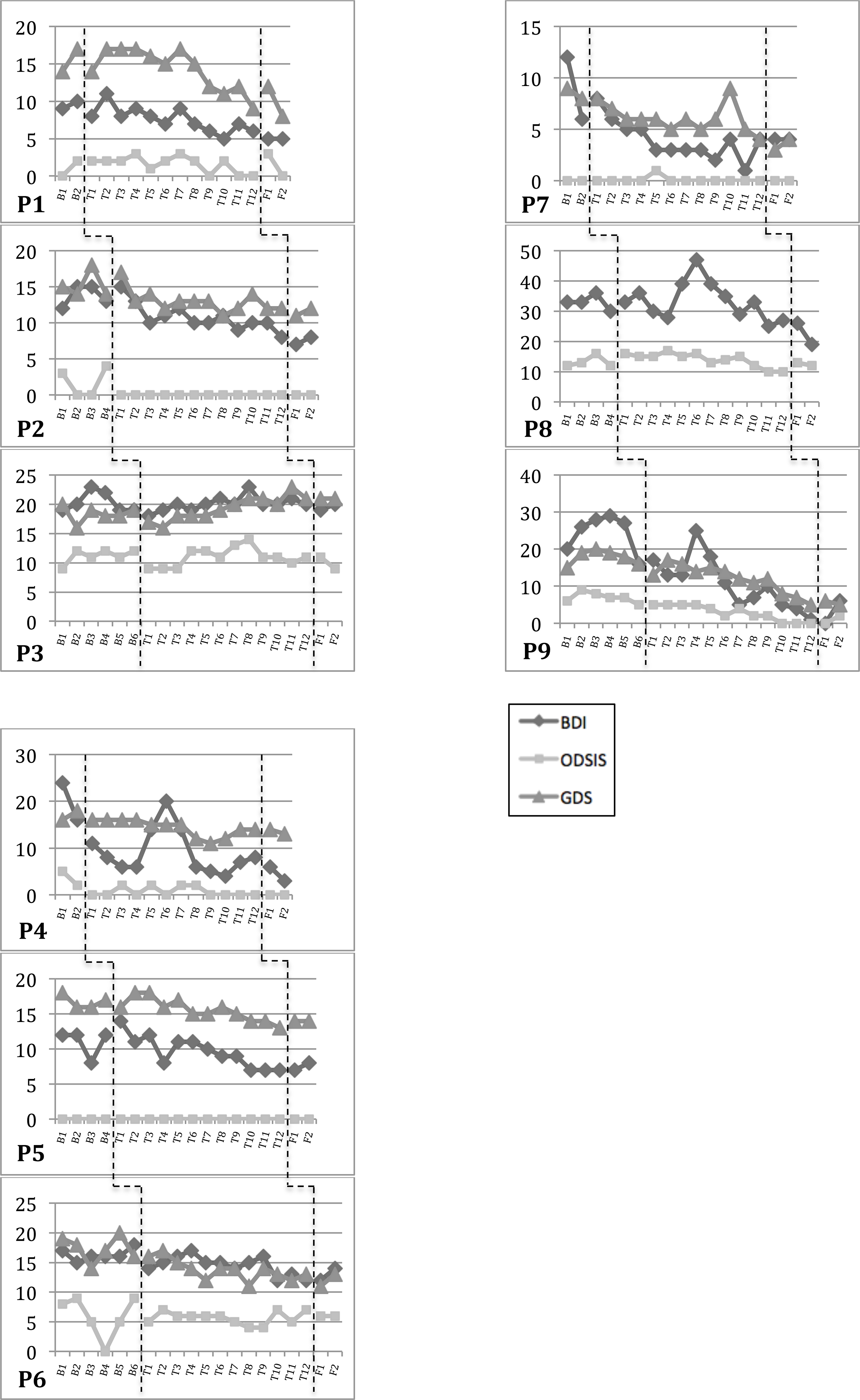
Weekly Depression Scores.
Sharp increases in depressive symptoms during treatment (e.g., weeks 5–6 for P4, week 10 for P7, weeks 5–6 for P8, week 4 for P9) corresponded with treatment components (e.g., exposures), external stressors, or poor responses to DBS/PD-medication adjustments (P8 only).
Table 2.
Anxiety and Depression Change Scores (CS) with 95% Confidence Intervals (CIs)
| ANXIETY | DEPRESSION | ||||||
|---|---|---|---|---|---|---|---|
| BAI 95% CI = CS±8.9 |
OASIS 95% CI = CS±4.5 |
STAI-S 95% CI = CS±10.5 |
STAI-T 95% CI = CS±11.7 |
BDI-II**
95% CI = CS±6.5 95% CI = CS±4.6 |
ODSIS 95% CI = CS±3.4 |
GDS**
95% CI = CS±5.3 95% CI = CS±4.1 |
|
| P1 | |||||||
| BL | 3 (−5.9, 11.9) | 1 (−3.5, 5.5) | 2 (−8.5, 12.5) | 2 (−9.7, 13.7) | 1 (−3.6, 5.6) | 2 (−1.4, 5.4) | 3 (−1.1, 7.1) |
| Pre-Post | −9 (−17.9, −0.1)* | −6 (−10.5, −1.5)* | −30 (−40.5, −19.5)* | −13 (−24.7, −1.3)* | −5 (−9.6, −0.4)* | 1 (−2.4, 4.4) | −5 (−9.1, −0.9)* |
| Pre-FU | −10 (−18.9, −1.1)* | −6 (−10.5, −1.5)* | −30 (−40.5, −19.5)* | −23 (−34.7, −11.3)* | −5 (−9.6, −0.4)* | −2 (−5.4, 1.4) | −9 (−13.1, −4.9)* |
| P2 | |||||||
| BL | 4 (−4.9, 12.9) | 2 (−2.5, 6.5) | 6 (−4.5, 16.5) | 6 (−5.7, 17.7) | 1 (−3.6, 5.6) | 1 (−2.4, 4.4) | −1 (−5.1, 3.1) |
| Pre-Post | −9 (−17.9, −0.1)* | −5 (−9.5, −0.5)* | −19 (−29.5, −8.5)* | −21 (−32.7, −9.3)* | −6 (−10.6, −1.4)* | −4 (−7.4, −0.6)* | −3 (−7.1, 1.1) |
| Pre-FU | −10 (−18.9, −1.1)* | −5 (−9.5, −0.5)* | −18 (−28.5, −7.5)* | −21 (−32.7, −9.3)* | −5 (−9.6, −0.4)* | −4 (−7.4, −0.6)* | −2 (−6.1, 2.1) |
| P3 | |||||||
| BL | 1 (−7.9, 9.9) | 2 (−2.5, 6.5) | 1 (−9.5, 11.5) | −5 (−16.7, 6.7) | 0 (−6.5, 6.5) | 3 (−0.4, 6.4) | −1 (−6.3, 4.3) |
| Pre-Post | 0 (−8.9, 8.9) | 1 (−3.5, 5.5) | −5 (−15.5, 5.5) | 0 (−11.7, 11.7) | 0 (−6.5, 6.5) | −1 (−4.4, 2.4) | 2 (−3.3, 7.3) |
| Pre-FU | −1 (−9.9, 7.9) | 3 (−1.5, 7.5) | 3 (−7.5, 13.5) | 3 (−8.7, 14.7) | 1 (−5.5, 7.5) | −3 (−6.4, 0.4) | 2 (−3.3, 7.3) |
| P4# | |||||||
| BL | −4 (−12.9, 4.9) | −4 (−8.5, 0.5) | 9 (−1.5, 19.5) | 0 (−11.7, 11.7) | −8 (−12.6, −3.4)* | −3 (−6.4, 0.4) | 2 (−2.1, 6.1) |
| Pre-Post | −10 (−18.9, −1.1)* | −8 (−12.5, −3.5)* | −5 (−15.5, 5.5) | 1 (−10.7, 12.7) | −10 (−14.6, −5.4)* | −2 (−5.4, 1.4) | −4 (−8.1, 0.1) |
| Pre-FU | −7 (−15.9, 1.9) | −7 (−11.5, −2.5)* | 1 (−9.5, 11.5) | 1 (−10.7, 12.7) | −13 (−17.6, −8.4)* | −2 (−5.4, 1.4) | −5 (−9.1, −0.9)* |
| P5 | |||||||
| BL | 3 (−5.9, 11.9) | −2 (−6.5, 2.5) | −5 (−15.5, 5.5) | −1 (−12.7, 10.7) | 0 (−4.6, 4.56) | 0 (−3.4, 3.4) | −1 (−5.1, 3.1) |
| Pre-Post | −5 (−13.9, 3.9) | 0 (−4.5, 4.5) | −22 (−32.5, −11.5)* | −16 (−27.7, −4.3)* | −5 (−9.6, −0.4)* | 0 (−3.4, 3.4) | −3 (−7.1, 1.1) |
| Pre-FU | −8 (−16.9, 0.9) | −4 (−8.5, 0.5) | −28 (−38.5, −17.5)* | −20 (−31.7, −8.3)* | −4 (−8.6, 0.6) | 0 (−3.4, 3.4) | −3 (−7.1, 1.1) |
| P6 | |||||||
| BL | −4 (−12.9, 4.9) | 1 (−3.5, 5.5) | −3 (−13.5, 7.5) | −2 (−13.7, 9.7) | 1 (−3.6, 5.6) | 1 (−2.4, 4.4) | −3 (−7.08, 1.1) |
| Pre-Post | −5 (−13.9, 3.9) | −4 (−8.5, 0.5) | −1 (−11.5, 9.5) | −2 (−13.7, 9.7) | −6 (−10.6, −1.4)* | −3 (−6.4, 0.4) | −5 (−9.08, −0.9)* |
| Pre-FU | −3 (−11.9, 5.9) | −5 (−9.5, −0.5)* | −2 (−12.5, 8.5) | −2 (−13.7, 9.7) | −4 (−8.6, 0.6) | −3 (−6.4, 0.4) | −3 (−7.08, 1.1) |
| P7^ | |||||||
| BL | 5 (−3.9, 13.9) | 1 (−3.5, 5.5) | −18 (−28.5, −7.5)* | 2 (−9.7, 13.7) | −6 (−10.6, −1.4)* | 0 (−3.4, 3.4) | −1 (−5.1, 3.1) |
| Pre-Post | −4 (−12.9, 4.9) | −6 (−10.5, −1.5)* | 0 (−10.5, 10.5) | −6 (−17.7, 5.7) | −2 (−6.6, 2.6) | 0 (−3.4, 3.4) | −5 (−9.1, −0.9)* |
| Pre-FU | −8 (−16.9, 0.9) | −4 (−8.5, 0.5) | −2 (−12.5, 8.5) | −16 (−27.7, −4.3)* | −2 (−6.6, 2.6) | 0 (−3.4, 3.4) | −4 (−8.1, 0.1) |
| P8 | |||||||
| BL | 4 (−4.9, 12.9) | 3 (−1.5, 7.5) | −14 (−24.5, −3.5)* | 1 (−10.7, 12.7) | −3 (−9.5, 3.5) | 0 (−3.4, 3.4) | NA |
| Pre-Post | 0 (−8.9, 8.9) | −4 (−8.5, 0.5) | 4 (−6.5, 14.5) | −5 (−16.7, 6.7) | −4 (−10.5, 2.5) | 1 (−2.4, 4.4) | |
| Pre-FU | −9 (−17.9, −0.1)* | −5 (−9.5, −0.5)* | −9 (−19.5, 1.5) | −5 (−16.7, 6.7) | −11 (−17.5, −4.5)* | 0 (−3.4, 3.4) | |
| P9 | |||||||
| BL | −18 (−26.9, −9.1)* | −2 (−6.5, 2.5) | −20 (−30.5, −9.5)* | −6 (−17.7, 5.7) | −4 (−8.6, 0.6) | −1 (−4.4, 2.4) | 1 (−3.1, 5.1) |
| Pre-Post | −1 (−9.9, 7.9) | −2 (−6.5, 2.5) | −7 (−17.5, 3.5) | −4 (−15.7, 7.7) | −16 (−20.6, −11.4)* | −5 (−8.4, −1.6)* | −10 (−14.1, −5.9)* |
| Pre-FU | 0 (−8.9, 8.9) | −3 (−7.5, 1.5) | −18 (−28.5, −7.5)* | −10 (−21.7, 1.7) | −10 (−14.6, −5.4)* | −3 (−6.4, 0.4) | −11 (−15.1, −6.9)* |
Improvement significant at p<.05. BL=last baseline score – first baseline score. Pre-post=post-treatment score – last baseline score. Pre-FU=6-week follow-up score – last baseline score. NA=GDS was not administered to P8 due to her younger age.
Two confidence intervals were calculated for the BDI-II and GDS: one for participants (P3, P8) who met criteria for a current depressive disorder (BDI-II=6.5; GDS=5.3), and the second for all other participants who did not meet diagnostic criteria for a current depressive disorder (BDI-II=4.6; GDS=4.1).
P4 slightly increased his parkinsonian medications to address motor symptoms during the middle of treatment.
At the follow-up, P7 reported retrospectively that she increased her psychotropic medication dose from 20mg to 30mg for “about a week” during the middle of treatment in response to increased frustration about an ongoing physical pain issue (unrelated to anxiety). She was unable to provide the exact dates when she adjusted the dose, but likely in the 1–2 weeks prior to session 9. She denied noticing any effect of the increased dose and as a result returned to the original 20mg dose shortly after making the change.
Additional notes:
Significant variability was observed during baseline on the following measures: BAI (P9); STAI-S (P7, P8, P9); BDI-II (P4, P7). The significant reduction in STAI-S at follow-up for P9 cannot be solely attributed to the intervention because there was variability during baseline; however, her post-treatment and follow-up STAI-S scores do not overlap with any of the scores during her baseline, suggesting some positive effect of the intervention. Likewise, P4 reported a significant reduction on the BDI-II at post-treatment and follow-up, but these changes cannot be fully accounted for by the intervention because of the extent of baseline variability.
Summary of Main Findings
Treatment response was defined as a statistically significant reliable change score and/or significant change in the level and/or slope of weekly anxiety and depression scores per visual inspection across phase shifts (e.g., baseline compared to intervention and/or follow-up). Overall, seven (of nine) participants showed a significant treatment response in anxiety symptoms per visual inspection (all except P3 and P8) and/or reliable change analyses (all except P3 and P9). Across participants, results from visual inspection and reliable change analyses were generally consistent, with the exception of P8 (described below). Results were similar for depressive symptoms, such that seven participants showed a treatment response per visual inspection and reliable change analyses (all except P3 and P4).
Functional Analysis of Baseline Data
Weekly anxiety and depression scores remained relatively stable for participants during baseline regardless of baseline length, except as noted in Table 2. Any significant variability during baseline precluded analysis of pre-post or pre-follow-up changes on those specific measures for a given participant due to potential threats to internal validity. For those who demonstrated a significant change in the level or slope of weekly anxiety and depression scores (P1, P2, P4, P5, P6, P7, P9), the change did not occur until the intervention and/or follow-up phases regardless of baseline condition (Figures 2–3). That is, changes in anxiety and depression among the seven responders were functionally related to the intervention, and not to external factors or the passage of time.
Functional Analyses of Anxiety Outcome Measures
During the intervention phase, visual inspection showed a reduction (change in level or slope) on at least one anxiety measure for seven participants (all except P3 and P8). Likewise, reliable change (RC) scores showed statistically significant reductions in anxiety for 7/9 participants (all except P3 and P9*) at post-treatment and/or follow-up, relative to baseline (Table 2). *We note that P9 also showed a significant decrease in anxiety per RC analysis at follow-up (STAI-S), consistent with visual inspection, but we did not qualify this RC finding as significant because she showed similar variability during baseline, thereby limiting our interpretation of subsequent significant changes. Also of note, P8 did not show clear changes in anxiety on visual inspection, but her RC scores captured slight, but significant, reductions in anxiety at follow-up on two measures (BAI, OASIS). This minor discrepancy highlights the more conservative nature of visual analyses as compared to statistical analyses for this type of experimental design.
Regarding different anxiety outcome measures, significant changes were observed on the OASIS for 6 of the 7 responders, highlighting the general applicability of this measure across different presentations of anxiety. The OASIS finding also converged with significant changes on the BAI and/or STAI for 5 participants; a sixth participant (P5) showed significant changes on both STAI indices with her BAI and OASIS change scores nearing significance. Isolated spikes in anxiety were observed for some participants during treatment, which were related to specific external factors (e.g., family stressors, physical pain, the holidays, winter weather, missed sessions, illness) or treatment components (e.g., exposures) (Figure 2). Likewise, certain treatment components were associated with greater symptom change at an individual level. Greater reduction in anxiety symptoms was observed after sessions on motivation and psychoeducation (P2; weeks 2–3), emotional awareness (P7; weeks 5–6), emotion avoidance (P4; weeks 7–8), and especially, following the sessions on exposures (P1, P5, P9; weeks 9–12).
Secondary Outcome Measures
Two participants endorsed elevated fear of falling (FES>23; (Delbaere et al., 2010)) at intake (P1, P4) with significant reduction at post-treatment and follow-up (RC: P1=−5 (−8.6, −1.5) at post-treatment and follow-up; P4=−25 (−28.6, −21.5) at post-treatment, −22 (−25.6, −18.5) at follow-up). Six endorsed elevated apathy (AS≥14; (Starkstein et al., 1992)) at intake, two (P4, P9) with reduction (RC: P4=−9 (−16.7, −1.3) at post-treatment, −10 (−17.7, −2.3) at follow-up; P9=−9 (−16.7, −1.3) at follow-up only) and one (P3) with increase at post-treatment and/or follow-up (RC=9 (1.3, 16.7) at post-treatment and follow-up). On Likert scales, all rated the intervention as “very acceptable” (4/5) or “extremely acceptable” (5/5) (mean= 4.6/5), and all were “very satisfied” (4/5) or “extremely satisfied” (5/5) (mean=4.6/5). Mean score on CSQ-8 was 30.9/32 (range=29–32). For the four internet participants, mean telepresence scores were 80.7% at mid-treatment and 80.8% at post-treatment, indicating agreement that videoconference sessions seemed natural, that participants felt present with the therapist in session, and that they were absorbed in the sessions.
Discussion
This exploratory pilot trial of individual cognitive-behavioral therapy for anxiety in PD is the first to implement a multiple-baseline, single-case experimental design using a 12-week, transdiagnostic CBT protocol. Relative to baseline, we found significant reductions in anxiety at post-treatment or 6-week follow-up for seven of the nine PD participants, all of whom met diagnostic criteria for an anxiety disorder at baseline with these changes functionally related to treatment. Seven of the nine participants also reported reduced depressive symptoms at post-treatment and/or follow-up. Levels of acceptability and satisfaction with treatment were high, regardless of treatment modality. Regarding secondary outcomes, the two participants who endorsed fear of falling at the intake reported reduced fear at post-treatment and follow-up. There were minimal, inconsistent effects on apathy across participants.
Strengths of the study included the use of a rigorous single-case experimental design, stringent inclusion criteria including presence of at least one DSM-5 anxiety disorder, use of an efficacious transdiagnostic CBT protocol, and the option of in-person or internet-delivered CBT. The Unified Protocol targets shared, underlying factors, namely emotional reactivity, biased thought processes, and avoidance behaviors, which manifest across a variety of mood and anxiety disorders (Farchione et al., 2012). This transdiagnostic approach is applicable to the treatment of anxiety disorders in PD, given the heterogeneity in their presentation (Broen et al., 2016) and frequent comorbidity with depression (Wee et al., 2016). Early sessions in the UP focus on psychoeducation and emotional awareness, which helps to characterize the clinical presentation and inform the remainder of treatment. Moreover, the flexibility of the UP allows for the therapist to tailor weekly sessions (and duration of time spent on a given module) to each individual, based on presenting complaints, in order to facilitate symptom reduction and functional improvement. A further strength of the study was in its use of multiple mood scales, in order to guide future research and also considering that performance on certain measures in PD may be influenced by individual items probing for motor as well as mood symptoms (Salazar, Le, Neargarder, & Cronin-Golomb, 2017). That said, though warranted in a pilot study, the use of multiple outcome measures is also a limitation, as it increases the potential for spurious or chance findings. In addition, our study was limited by the lack of a PD-specific measure of anxiety, such as the Parkinson Anxiety Scale (PAS; Leentjens et al., 2014), which was published after the initial design of the current study. Future studies on anxiety in PD should consider use of the PAS, perhaps in conjunction with the OASIS, given its brevity and demonstrated sensitivity to various anxiety presentations in the present study.
Contributors to treatment response may include comorbid apathy, which afflicts approximately 40% of individuals with PD (den Brok et al., 2015). One of the two nonresponders in our study reported elevated apathy throughout baseline, treatment, and follow-up. Given that CBT relies so heavily on homework compliance and out-of-session practice, apathy may be a significant barrier to treatment response, and additional sessions on motivation enhancement strategies may be required. The other non-responder had a history of DBS and frequent OFF periods. Examining the relation between anxiety and motor symptoms may inform treatments for individuals with anxiety, DBS, and motor fluctuations.
This pilot study also highlights the utility and feasibility of in-person and internet-delivered CBT, as results did not differ by treatment modality, and provides rationale for future research and non-pharmacological treatment development for anxiety in PD. The protocol was modified to allow for internet-delivered sessions due to slow and difficult recruitment, as has been reported in previous treatment trials for depression and anxiety in PD (Troeung et al., 2014). Dobkin and colleagues described several barriers to seeking mental healthcare in this population and reported high interest in telehealth among PD respondents (Dobkin et al., 2013). The four telehealth participants reported high treatment satisfaction, and showed a significant response to treatment. Qualitatively, participants reported benefit from most aspects of treatment, including writing down their experiences before and after exposures. Participants also expressed interest in greater flexibility regarding the number of treatment sessions; some individuals commented that additional sessions would have been helpful to fully integrate all skills, while others commented that fewer and/or shorter sessions would have been preferable. Lastly, based on qualitative participant feedback, future studies should consider providing participants with a workbook (rather than weekly handouts) and also strive to limit weekly self-rating forms (e.g., perhaps use only the OASIS and ODSIS).
Single-case experimental designs emphasize internal validity and allow for flexibility to tailor an intervention to the individual, but may be limited in terms of generalizability. To build upon this work, controlled studies with larger samples are needed, though additional research may first be needed to explore recruitment difficulties and associated barriers/facilitators to research and/or treatment engagement in this population. Telehealth strategies may offer one strategy to enhance recruitment, though we cannot fully comment on this approach, as telehealth approaches were not implemented until halfway through the current study. Future studies may consider use of only telehealth sessions, given observed recruitment difficulties and barriers to attending weekly sessions, particularly in urban settings, for individuals with PD. In addition, modifications to the CBT protocol that may be particularly helpful for PD are 1) additional modules on motivation or increased caregiver involvement (Kraepelien et al., 2015), especially for individuals with high baseline apathy, 2) shorter treatment course for individuals who show a rapid response, and 3) supplemental modules or adaptation of weekly handouts to optimize organization for individuals with executive dysfunction. Future work should also consider the potential of CBT in combination with pharmacotherapies (Cuijpers et al., 2014) or exercise (Merom et al., 2008) to maximize treatment response in PD.
We hope that these preliminary findings will provide the impetus for larger treatment trials with a focus on anxiety (e.g., Mulders et al., 2018), with the goal of establishing evidence-based psychological treatments for individuals with PD. Disseminating these treatments may assist individuals in accessing mental healthcare and developing long-term skills to manage anxiety across the disease course.
Acknowledgements:
We sincerely thank the individuals who participated in this study. We also thank Katy Hendron, PT, DPT, Ollie Barthelemy, MA, and Hannah Boettcher, MA, for their invaluable help with training and consultation, participant recruitment, and data collection and analysis.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Armento ME, Stanley MA, Marsh L, Kunik ME, York MK, Bush AL, & Calleo JS (2012). Cognitive behavioral therapy for depression and anxiety in Parkinson’s disease: a clinical review. Journal of Parkinson’s Disease, 2(2), 135–151. doi: 10.3233/JPD-2012-12080 [DOI] [PubMed] [Google Scholar]
- Au TM, Sauer-Zavala S, King MW, Petrocchi N, Barlow DH, & Litz BT (2017). Compassion-Based Therapy for Trauma-Related Shame and Posttraumatic Stress: Initial Evaluation Using a Multiple Baseline Design. Behavior Therapy, 48(2), 207–221. doi: 10.1016/j.beth.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Barlow DH, Farchione TJ, Fairholme CF, Ellard KK, Boisseau C, Allen LB, & Ehrenreich-May J (2011). The unified protocol for transdiagnostic treatment of emotional disorders. New York: Oxford University Press. [Google Scholar]
- Barlow DH, & Hersen M (1973). Single-case experimental designs. Uses in applied clinical research. Archives of General Psychiatry, 29(3), 319–325. [DOI] [PubMed] [Google Scholar]
- Barlow DH, & Nock MK (2009). Why can’t we be more idiographic in our research? Perspectives on Psychological Science, 4(1), 19–21. doi: 10.1111/j.1745-6924.2009.01088.x [DOI] [PubMed] [Google Scholar]
- Barlow DH, Nock MK, & Hersen M (2009). Single case experimental designs: Strategies for studying behavior change (3rd ed.). Boston, MA: Allyn & Bacon. [Google Scholar]
- Beck AT, & Steer RA (1993). Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bentley KH, Gallagher MW, Carl JR, & Barlow DH (2014). Development and validation of the overall depression severity and impairment scale. Psychological Assessment. doi: 10.1037/a0036216 [DOI] [PubMed] [Google Scholar]
- Bentley KH, Nock MK, Sauer-Zavala S, Gorman BS, & Barlow DH (2017). A functional analysis of two transdiagnostic, emotion-focused interventions on nonsuicidal self-injury. Journal of Consulting and Clinical Psychology, 85(6), 632–646. doi: 10.1037/ccp0000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglan A, Ary D, & Wagenaar AC (2000). The value of interrupted time-series experiments for community intervention research. Prevention Science, 1(1), 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard S, & Robillard G (2000, 2006). Telepresence in videoconference scale. Unpublished manuscript. Cyberpsychology Lab of UQO. [Google Scholar]
- Broen MP, Narayen NE, Kuijf ML, Dissanayaka NN, & Leentjens AF (2016). Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Movement Disorders. doi: 10.1002/mds.26643 [DOI] [PubMed] [Google Scholar]
- Brown TA, Dinardo PA, & Barlow DH (2013). Anxiety Disorders Interview for DSM-5 (ADIS-5). Unpublished assessment interview.
- Byiers BJ, Reichle J, & Symons FJ (2012). Single-subject experimental design for evidence-based practice. American Journal of Speech-Language Pathology, 21(4), 397–414. doi: 10.1044/1058-0360(2012/11-0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleo JS, Amspoker AB, Sarwar AI, Kunik ME, Jankovic J, Marsh L, … Stanley MA (2015). A pilot study of a cognitive-behavioral treatment for anxiety and depression in patients with Parkinson disease. Journal of Geriatric Psychiatry and Neurology, 28(3), 210–217. doi: 10.1177/0891988715588831 [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, … Stein MB (2009). Validation of a brief measure of anxiety-related severity and impairment: the Overall Anxiety Severity and Impairment Scale (OASIS). Journal of Affective Disorders, 112(1–3), 92–101. doi: 10.1016/j.jad.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, & Marsh L (2014). Anxiety in Parkinson’s disease: identification and management. Therapeutic Advances in Neurological Disorders, 7(1), 52–59. doi: 10.1177/1756285613495723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B, & Fox SH (2014). Treatment of cognitive, psychiatric, and affective disorders associated with Parkinson’s disease. Neurotherapeutics, 11(1), 78–91. doi: 10.1007/s13311-013-0238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly BS, & Fox SH (2012). Drug treatments for the neuropsychiatric complications of Parkinson’s disease. Expert Review of Neurotherapeutics, 12(12), 1439–1449. doi: 10.1586/ern.12.142 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Sijbrandij M, Koole SL, Andersson G, Beekman AT, & Reynolds CF 3rd. (2014). Adding psychotherapy to antidepressant medication in depression and anxiety disorders: a meta-analysis. World Psychiatry, 13(1), 56–67. doi: 10.1002/wps.20089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbaere K, Close JC, Mikolaizak AS, Sachdev PS, Brodaty H, & Lord SR (2010). The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age and Ageing, 39(2), 210–216. doi: 10.1093/ageing/afp225 [DOI] [PubMed] [Google Scholar]
- den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RM, & Richard E (2015). Apathy in Parkinson’s disease: A systematic review and meta-analysis. Movement Disorders, 30(6), 759–769. doi: 10.1002/mds.26208 [DOI] [PubMed] [Google Scholar]
- Devos D, Dujardin K, Poirot I, Moreau C, Cottencin O, Thomas P, … Defebvre L (2008). Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Movement Disorders, 23(6), 850–857. doi: 10.1002/mds.21966 [DOI] [PubMed] [Google Scholar]
- Dissanayaka N, White E, O’Sullivan JD, Marsh R, Silburn PA, Copland DA, … Byrne GJ (2015). Characteristics and treatment of anxiety disorders in Parkinson’s disease. Movement Disorders Clinical Practice, 2(2), 155–162. doi: 10.1002/mdc3.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayaka NN, O’Sullivan JD, Pachana NA, Marsh R, Silburn PA, White EX, … Byrne GJ (2016). Disease-specific anxiety symptomatology in Parkinson’s disease. International Psychogeriatrics, 28(7), 1153–1163. doi: 10.1017/S1041610215002410 [DOI] [PubMed] [Google Scholar]
- Dissanayaka NN, White E, O’Sullivan JD, Marsh R, Pachana NA, & Byrne GJ (2014). The clinical spectrum of anxiety in Parkinson’s disease. Movement Disorders, 29(8), 967–975. doi: 10.1002/mds.25937 [DOI] [PubMed] [Google Scholar]
- Dissanayaka NNW, Pye D, Mitchell LK, Byrne GJ, O’Sullivan JD, Marsh R, & Pachana NA (2017). Cognitive behavior therapy for anxiety in Parkinson’s disease: Outcomes for patients and caregivers. Clinical Gerontologist, 40(3), 159–171. doi: 10.1080/07317115.2016.1240131 [DOI] [PubMed] [Google Scholar]
- Dobkin RD, Menza M, Allen LA, Gara MA, Mark MH, Tiu J, … Friedman J (2011). Cognitive-behavioral therapy for depression in Parkinson’s disease: a randomized, controlled trial. American Journal of Psychiatry, 168(10), 1066–1074. doi: 10.1176/appi.ajp.2011.10111669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin RD, Menza M, Allen LA, Tiu J, Friedman J, Bienfait KL, … Mark MH (2011). Telephone-based cognitive-behavioral therapy for depression in Parkinson disease. Journal of Geriatric Psychiatry and Neurology, 24(4), 206–214. doi: 10.1177/0891988711422529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin RD, Rubino JT, Friedman J, Allen LA, Gara MA, & Menza M (2013). Barriers to mental health care utilization in Parkinson’s disease. Journal of Geriatric Psychiatry and Neurology, 26(2), 105–116. doi: 10.1177/0891988713481269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GW, Khoo TK, Yarnall AJ, O’Brien JT, Coleman SY, Brooks DJ, … Burn DJ (2014). Health-related quality of life in early Parkinson’s disease: the impact of nonmotor symptoms. Movement Disorders, 29(2), 195–202. doi: 10.1002/mds.25664 [DOI] [PubMed] [Google Scholar]
- Egan SJ, Laidlaw K, & Starkstein S (2015). Cognitive behaviour therapy for depression and anxiety in Parkinson’s disease. Journal of Parkinson’s Disease, 5(3), 443–451. doi: 10.3233/JPD-150542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh A, Locascio JJ, Yap L, Growdon J, Fava M, Crawford C, … Alpert JE (2010). Cognitive-behavioral therapy for patients with Parkinson’s disease and comorbid major depressive disorder. Psychosomatics, 51(2), 124–129. doi: 10.1176/appi.psy.51.2.124 [DOI] [PubMed] [Google Scholar]
- Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Thompson-Hollands J, Carl JR, … Barlow DH (2012). Unified protocol for transdiagnostic treatment of emotional disorders: a randomized controlled trial. Behavior Therapy, 43(3), 666–678. doi: 10.1016/j.beth.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney F, Egan S, & Gasson N (2005). Treatment of depression and anxiety in Parkinson’s Disease: A pilot study using group cognitive behavioural therapy. Clinical Psychologist, 9(1), 31–38. [Google Scholar]
- Fiske A, Wetherell JL, & Gatz M (2009). Depression in older adults. Annual Review of Clinical Psychology, 5, 363–389. doi: 10.1146/annurev.clinpsy.032408.153621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, … Movement Disorder Society, U. R. T. F. (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement Disorders, 23(15), 2129–2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- Hanna KK, & Cronin-Golomb A (2012). Impact of anxiety on quality of life in Parkinson’s disease. Parkinsons Disease, 2012, 640707. doi: 10.1155/2012/640707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Barlow DH, & Nelson-Gray RO (1999). The scientist practitioner: Research and accountability in the age of managed care (2nd ed.). Needham Heights, MA: Allyn and Bacon. [Google Scholar]
- Heinrichs N, Hoffman EC, & Hofmann SG (2001). Cognitive-behavioral treatment for social phobia in Parkinson’s disease: A single-case study. Cognitive and Behavioral Practice, 8(4), 328–335. doi: 10.1016/S1077-7229(01)80005-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, & Yahr MD (1967). Parkinsonism: onset, progression and mortality. Neurology, 17(5), 427–442. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, & Lees AJ (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery and Psychiatry, 55(3), 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabacoff RI, Segal DL, Hersen M, & Van Hasselt VB (1997). Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with older adult psychiatric outpatients. Journal of Anxiety Disorders, 11(1), 33–47. [DOI] [PubMed] [Google Scholar]
- Kazdin AE (2003). Research design in clinical psychology (4th ed.). Needham Heights, MA: Allyn & Bacon. [Google Scholar]
- Kraepelien M, Svenningsson P, Lindefors N, & Kaldo V (2015). Internet-based cognitive behavioral therapy for depression and anxiety in Parkinson’s disease - A pilot study. Internet Interventions, 2(1), 1–6. doi:doi: 10.1016/j.invent.2014.11.006 [DOI] [Google Scholar]
- Larsen DL, Attkisson CC, Hargreaves WA, & Nguyen TD (1979). Assessment of client/patient satisfaction: development of a general scale. Evaluation and Program Planning, 2(3), 197–207. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Dujardin K, Marsh L, Richard IH, Starkstein SE, & Martinez-Martin P (2011). Anxiety rating scales in Parkinson’s disease: a validation study of the Hamilton anxiety rating scale, the Beck anxiety inventory, and the hospital anxiety and depression scale. Movement Disorders, 26(3), 407–415. doi: 10.1002/mds.23184 [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Dujardin K, Pontone GM, Starkstein SE, Weintraub D, & Martinez-Martin P (2014). The Parkinson Anxiety Scale (PAS): development and validation of a new anxiety scale. Movement Disorders, 29(8), 1035–1043. doi: 10.1002/mds.25919 [DOI] [PubMed] [Google Scholar]
- Menza M, Dobkin RD, Marin H, Mark MH, Gara M, Buyske S, … Dicke A (2009). A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology, 72(10), 886–892. doi: 10.1212/01.wnl.0000336340.89821.b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza MA, Robertson-Hoffman DE, & Bonapace AS (1993). Parkinson’s disease and anxiety: comorbidity with depression. Biological Psychiatry, 34(7), 465–470. [DOI] [PubMed] [Google Scholar]
- Merom D, Phongsavan P, Wagner R, Chey T, Marnane C, Steel Z, … Bauman A (2008). Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. Journal of Anxiety Disorders, 22(6), 959–968. doi: 10.1016/j.janxdis.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Mohlman J, Reel DH, Chazin D, Ong D, Georgescu B, Tiu J, & Dobkin RD (2010). A novel approach to treating anxiety and enhancing executive skills in an older adult with Parkinson’s disease. Clinical Case Studies, 9(1), 74–90. doi: 10.1177/1534650109351305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders AEP, Moonen AJH, Dujardin K, Kuijf ML, Duits A, Flinois B, … Leentjens AFG (2018). Cognitive behavioural therapy for anxiety disorders in Parkinson’s disease: Design of a randomised controlled trial to assess clinical effectiveness and changes in cerebral connectivity. Journal of Psychosomatic Research, 112, 32–39. doi: 10.1016/j.jpsychores.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Naismith SL, & Lewis SJ (2011). “DASH” symptoms in patients with Parkinson’s disease: red flags for early cognitive decline. Journal of Clinical Neuroscience, 18(3), 352–355. doi: 10.1016/j.jocn.2010.07.106 [DOI] [PubMed] [Google Scholar]
- Nock MK, Michel BD, & Photos VI (2007). Single-case research designs. In McKay D (Ed.), Handbook of research methods in abnormal and clinical psychology (pp. 337–350). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Norman SB, Cissell SH, Means-Christensen AJ, & Stein MB (2006). Development and validation of an Overall Anxiety Severity And Impairment Scale (OASIS). Depression and Anxiety, 23(4), 245–249. doi: 10.1002/da.20182 [DOI] [PubMed] [Google Scholar]
- Pachana NA, Egan SJ, Laidlaw K, Dissanayaka N, Byrne GJ, Brockman S, … Starkstein S (2013). Clinical issues in the treatment of anxiety and depression in older adults with Parkinson’s disease. Movement Disorders, 28(14), 1930–1934. doi: 10.1002/mds.25689 [DOI] [PubMed] [Google Scholar]
- Pontone GM, Williams JR, Anderson KE, Chase G, Goldstein SR, Grill S, … Marsh L (2013). Pharmacologic treatment of anxiety disorders in Parkinson disease. American Journal of Geriatric Psychiatry, 21(6), 520–528. doi: 10.1016/j.jagp.2012.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GO, Hanna KK, Neargarder S, & Cronin-Golomb A (2017). The Relation of Anxiety and Cognition in Parkinson’s Disease. Neuropsychology, 31(6), 596–604. doi: 10.1037/neu0000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar RD, Le AM, Neargarder S, & Cronin-Golomb A (2017). The impact of motor symptoms on self-reported anxiety in Parkinson’s disease. Parkinsonism & Related Disorders, 38, 26–30. doi: 10.1016/j.parkreldis.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, … Goetz CG (2007). Depression rating scales in Parkinson’s disease: critique and recommendations. Movement Disorders, 22(8), 1077–1092. doi: 10.1002/mds.21333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Sauerbier A, & Chaudhuri KR (2015). New clinical trials for nonmotor manifestations of Parkinson’s disease. Movement Disorders, 30(11), 1490–1504. doi: 10.1002/mds.26415 [DOI] [PubMed] [Google Scholar]
- Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, … Sampaio C (2011). The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson’s disease. Movement Disorders, 26(Suppl 3), S42–80. doi: 10.1002/mds.23884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingleton RM, Pratt EM, Gorman B, Barlow DH, Palfai TP, & Thompson-Brenner H (2016). Motivational text message intervention for eating disorders: A single-case alternating treatment design using ecological momentary assessment. Behavior Therapy, 47(3), 325–338. doi: 10.1016/j.beth.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, & Jacobs GA (1983). Manual for the state-trait anxiety inventory. Menlo Park, CA: Mind Garden. [Google Scholar]
- Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, & Robinson RG (1992). Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. Journal of Neuropsychiatry and Clinical Neurosciences, 4(2), 134–139. [DOI] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulsen J, & Mayeux R (1987). Modified Mini-Mental State Examination: validity and reliability. Neurology, 37(Suppl 1), 179.3808297 [Google Scholar]
- Tinetti ME, Richman D, & Powell L (1990). Falls efficacy as a measure of fear of falling. Journal of Gerontology, 45(6), P239–243. [DOI] [PubMed] [Google Scholar]
- Troeung L, Egan SJ, & Gasson N (2013). A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson’s disease. PloS One, 8(11), e79510. doi: 10.1371/journal.pone.0079510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeung L, Egan SJ, & Gasson N (2014). A waitlist-controlled trial of group cognitive behavioural therapy for depression and anxiety in Parkinson’s disease. BMC Psychiatry, 14, 19. doi: 10.1186/1471-244X-14-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey C, Cook KF, Stanley M, Lai EC, & Kunik ME (2009). Telephone-administered cognitive behavioral therapy: a case study of anxiety and depression in Parkinson’s disease. Journal of Clinical Psychology in Medical Settings, 16(3), 243–253. doi: 10.1007/s10880-009-9167-6 [DOI] [PubMed] [Google Scholar]
- Wee N, Kandiah N, Acharyya S, Chander RJ, Ng A, Au WL, & Tan LC (2016). Depression and anxiety are co-morbid but dissociable in mild Parkinson’s disease: A prospective longitudinal study of patterns and predictors. Parkinsonism & Related Disorders, 23, 50–56. doi: 10.1016/j.parkreldis.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Williams JR, Hirsch ES, Anderson K, Bush AL, Goldstein SR, Grill S, … Marsh L (2012). A comparison of nine scales to detect depression in Parkinson disease: which scale to use? Neurology, 78(13), 998–1006. doi: 10.1212/WNL.0b013e31824d587f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Sajatovic M, & Walter BL (2012). Psychosocial interventions for depression and anxiety in Parkinson’s disease. Journal of Geriatric Psychiatry and Neurology, 25(2), 113–121. doi: 10.1177/0891988712445096 [DOI] [PubMed] [Google Scholar]
- Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, & Todd C (2005). Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age and Ageing, 34(6), 614–619. doi: 10.1093/ageing/afi196 [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1982). Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]


