Abstract
Purpose: Adjuvant endocrine therapy (AET) prevents recurrence after early-stage, hormone sensitive breast cancer; however, adherence to AET is suboptimal, and efficacious interventions are severely lacking. Barriers to adherence are well established; however, interventions thus far have failed to produce meaningful changes in adherence and have generally not followed guiding principles of psychosocial intervention development. The purpose of this paper is to describe the iterative development, using the National Institutes of Health Stage Model for Behavioral Intervention Development, of an evidence-based, patient-centered, telehealth intervention to enhance adherence, improve symptom management, and reduce distress for patients taking AET after breast cancer, with a focus on 1) a small open pilot study which informed modifications and refinement of the intervention based on quantitative and qualitative patient feedback about feasibility and acceptability; and 2) the underlying theoretical and empirical rationale for each component of the finalized intervention. Clinical implications and directions for future research are discussed.
Keywords: hormonal therapy, breast cancer, symptom management, adherence, distress, cognitive-behavioral intervention
Approximately 60–75% of early-stage breast malignancies are hormone sensitive (i.e., hormone receptor-positive) and treated with adjuvant endocrine therapy (AET), a critical component of recurrence prevention during survivorship (Burstein, Lacchetti, & Griggs, 2018). While breast cancer cells that are hormone receptor-positive use estrogen and/or progesterone to grow and spread, AET reduces the risk of cancer recurrence by lowering estrogen levels in the body or by blocking estrogen from acting on these breast cancer cells. Thus, AET in the form of tamoxifen (blocks estrogen receptors on breast cancer cells) or an aromatase inhibitor (AI; stops estrogen production), for example, reduces breast cancer mortality (Fisher et al., 2002), improving 15-year survival by a third and decreasing risk of recurrence and/or contralateral breast cancer by 40–50% (Davies et al., 2011). While current clinical practice guidelines recommend between 5–10 years of AET (Burstein et al., 2014), adherence (taking medication as prescribed) is remarkably poor and declines continuously each year following therapy initiation (Coombes et al., 2004; Murphy, Bartholomew, Carpentier, Bluethmann, & Vernon, 2012; Saha et al., 2017). Poor adherence is detrimental for patients after breast cancer, as adherence to AET is critical in the initial years following treatment (Davies et al., 2011) and is the single most modifiable factor that influences treatment outcomes (Sabate & De Geest, 2004). Non-adherence is associated with increased breast cancer recurrence (Markkula et al., 2012), breast cancer mortality (Yood et al., 2008), overall mortality (Hershman et al., 2011; Makubate, Donnan, Dewar, Thompson, & McCowan, 2013; McCowan et al., 2008), higher rates of physician visits and hospitalization, longer hospital stays (DiMatteo, Giordani, Lepper, & Croghan, 2002), and poorer patient-provider relationships (Jacob Arriola et al., 2014).
Researchers striving to understand the central barriers to AET adherence have identified factors at the patient, treatment, and provider/healthcare system levels (Cahir, Guinan, Dombrowski, Sharp, & Bennett, 2015; Greer et al., 2016; Moon, Moss-Morris, Hunter, Carlisle, & Hughes, 2017). Among the significant correlates of non-adherence are patient factors, including perceptions of low recurrence risk (Stanton, Petrie, & Partridge, 2014), low necessity for AET (Fink, Gurwitz, Rakowski, Guadagnoli, & Silliman, 2004), poor self-efficacy for taking medication (Stanton et al., 2014), and demographic factors such as younger age, lower income or publicly-insured, and race/ethnicity (black women are at greater risk for non-adherence to AET) (Spencer, Reeve, Troester, & Wheeler, 2020). In addition, psychological distress, such as depressive or anxiety symptoms (Bender et al., 2014), and a lack of social support (Bright & Stanton, 2018) have been associated with poor adherence to AET. Treatment factors are major barriers to adherence, including side effects and toxicities such as joint pain, hot flashes, fatigue, insomnia, and sexual dysfunction (Bender et al., 2014; Burstein et al., 2018; Lambert, Balneaves, Howard, & Gotay, 2018). Furthermore, these side effects disrupt quality of life, exacerbate distress after breast cancer, serve as reminders of the breast cancer experience, and perpetuate negative attitudes towards AET, further interfering with AET adherence. Finally, systems factors such as poorer patient-physician relationships and higher cost of medication contribute to worse adherence (Given, Spoelstra, & Grant, 2011).
Despite a strong understanding of the barriers to AET adherence, few intervention studies have addressed this significant problem, with only four completed randomized controlled trials to date (Heiney et al., 2019). Generally, these trials failed to produce meaningful changes in adherence and neglected to address the established myriad factors contributing to poor adherence (Ekinci et al., 2018; Finitsis, Vose, Mahalak, & Salner, 2018; Heiney et al., 2019). For example, studies have neither targeted the self-management of AET-related side effects, a major and modifiable contributor to poor adherence (Bright & Stanton, 2018), nor capitalized on evidence suggesting that social support may enhance adherence (Kroenke et al., 2018; Wassermann et al., 2019). In addition, investigators have generally not followed guiding principles of psychosocial intervention development, which involve several formative steps prior to efficacy testing (Rounsaville, Carroll, & Onken, 2001). Specifically, interventions should be conceptualized within existent theoretical models and empirical knowledge in order to design or adapt the treatment based on drivers of behavior that are treatment targets (Rounsaville et al., 2001). Furthermore, mixed qualitative and quantitative research methods must be employed to maximize patient-centeredness and optimize practical aspects such as the mode, timing, and duration of treatment delivery (Czajkowski et al., 2015). The intervention should then be refined by incorporating feedback from pilot and feasibility testing (Onken, Carroll, Shoham, Cuthbert, & Riddle, 2014). Studies that employ these steps towards rigorous intervention development and testing while targeting known modifiable barriers are warranted and needed to optimize adherence to AET for patients after breast cancer.
Therefore, employing the aforementioned procedures, we developed a patient-centered, evidence-based, small-group telehealth intervention for patients prescribed AET after breast cancer (see Figure 1; Stage I). First, we conducted a systematic review of the literature on existing interventions and barriers to adherence to conceptualize the intervention (Greer et al., 2016). Next, we conducted an in-depth qualitative analysis of 30 patients’ perceptions and experiences on AET, barriers and motivators for adherence, and impressions of the proposed intervention (Jacobs et al., 2019). A main finding from this study was that patients struggle with side effects and distress regardless of their level of self-reported adherence. Finally, to inform the intervention content, delivery modality, and duration, we 1) gained expert feedback from behavioral scientists and breast oncology clinicians, 2) drew from prior empirically-based treatments related to adherence and breast cancer (Antoni & Smith, 2003; Safren, Gonzalez, & Soroudi, 2007), and 3) were guided by the Health Information Technology Acceptance Model (HITAM), which incorporates the Health Belief Model and Technology Acceptance Model (Kim & Park, 2012). More specifically, the HITAM proposes that stronger health belief concerns and greater perceived ease of use and benefit will result in greater uptake by telehealth users, translating to intended behavioral changes (Kim & Park, 2012). In addition, evidence suggests that video teleconference groups are feasible with similar outcomes to in-person groups and high satisfaction from participants (Gentry, Lapid, Clark, & Rummans, 2019). The intervention, STRIDE [Symptom-Targeted Randomized Intervention for Distress and Adherence on Adjuvant Endocrine Therapy] aims to improve adherence, enhance symptom management, and reduce distress.
Fig. 1.
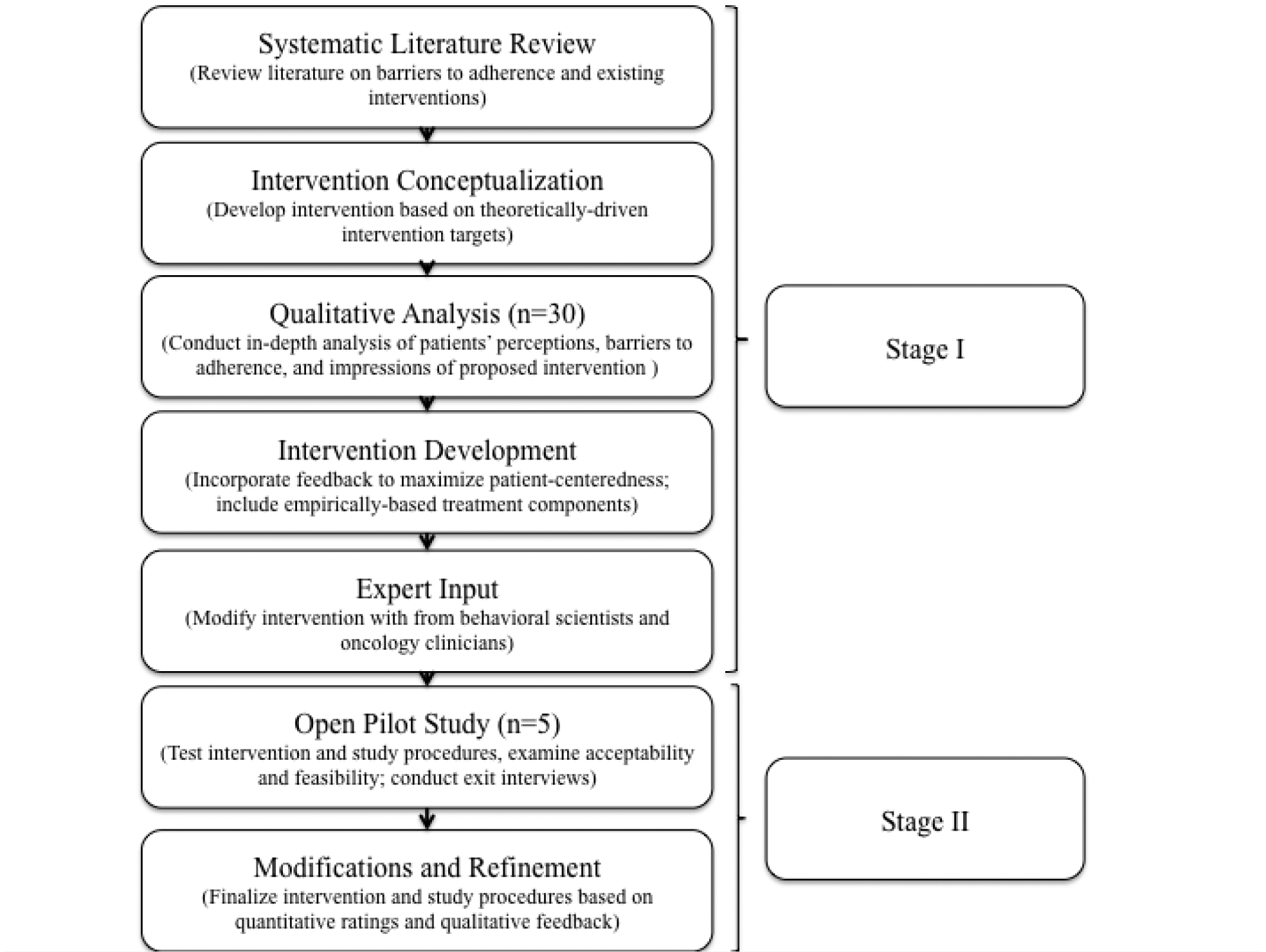
Iterative stages of intervention development
In the present study, we provide an in-depth description of Stage II: a small open pilot study designed to modify and refine the STRIDE intervention based on feedback from patients regarding acceptability and feasibility (Figure 1; Stage II). We also detail the specific modifications made to the final STRIDE intervention per the quantitative and qualitative analysis of patient feedback. Finally, we provide a detailed outline of STRIDE and the theoretical foundations supporting each intervention component.
Methods
Study Design
From 3/2019–6/2019, we conducted a small, single-group open pilot study to modify and refine the STRIDE intervention and study procedures in patients who reported distress related to AET, adherence difficulties, or side effects (Clinicaltrials.gov ID: NCT03837496). We refined STRIDE based on quantitative data and feedback from semi-structured exit interviews. The study was conducted at the Massachusetts General Hospital (MGH) Cancer Center in Boston, Massachusetts and was approved by the DF/HCC Institutional Review Board.
Study Participants
We enrolled female patients with a diagnosis of early-stage (0-IIIb) hormone-receptor positive breast cancer who a) were ≥ 21 years of age, b) were fluent in English, c) were within one week to three years of initiating AET, d) had completed primary breast cancer treatment (i.e., surgery, chemotherapy, or radiation therapy), and e) had an Eastern Cooperative Oncology Group performance status ≤ 2, indicating that they were ambulatory, capable of self-care, and up and about at least 50% of waking hours (Oken et al., 1982). Those who had discontinued AET, or had a condition that would interfere with study procedures, such as an untreated serious psychiatric disorder, co-morbid disease, or cognitive impairment, were not eligible. As the purpose was to modify and refine the intervention content and study procedures based on patient feedback, we aimed to enroll approximately five patients, consistent with prior work from our group (Greer, Park, Prigerson, & Safren, 2010; Safren et al., 2004). More specifically, our group has adopted a procedure of performing a trial run of any developed interventions with approximately five patients in order to further develop, modify, refine, and finalize intervention content, delivery, and overall study implementation. As such, this 5-person trial run maximizes patient-centeredness and optimizes delivery prior to testing feasibility and acceptability in a pilot randomized controlled trial, and is conceptually the final step in the development of the intervention. As the intention is not to draw conclusions, but rather vet the intervention a final time, we have traditionally enrolled approximately five patients to achieve this goal (Greer, Park, et al., 2010; Safren et al., 2004).
Study Procedures
Study staff queried the electronic health record (EHR) to identify patients with upcoming breast oncology follow-up appointments. In addition, oncology clinicians referred potentially eligible patients to the study staff. With permission from the treating oncology clinician, study staff privately approached patients in clinic to describe the study procedures and administer screening measures to those interested. Patients completed a screening measure assessing distress (range=0–10) related to AET (“How upset are you by having to take hormonal therapy?”), adherence difficulties (“How difficult is it for you to take your hormonal therapy medication every day?”), or side effects (“How bothered are you by symptoms or side effects?”). Patients who screened in for high distress (≥ 4/10) on any of the three items were offered participation in the study. Study staff obtained informed consent and interested and eligible patients were enrolled in the study. Enrolled patients completed baseline self-report questionnaires and were instructed to store their AET in a Medication Event Monitoring System (MEMS) Cap that electronically records the date and time of opening and closing as a proxy for taking medication. To investigate delivery of the intervention in both a small-group and individual format, we placed patients into three cohorts: two groups of two participants each and one single participant cohort. Patients received $20 at the pre- and post-intervention assessments for a total possible $40 over the course of the study.
STRIDE Intervention
The structured, manualized, group-based STRIDE intervention consisted of five weekly one-hour sessions that addressed the following: assessment and problem-solving of barriers to adherence (Figure 2), psychoeducation, cognitive-behavioral skills, relaxation training, and coping strategies for side effects. The STRIDE sessions were administered by a licensed clinical psychologist (JJ) via MGH-approved HIPAA-compliant videoconference platform. While patients could participate in-person, if preferred, all chose to participate via videoconference. The finalized intervention and corresponding theoretical rationale is described below in depth.
Fig. 2.
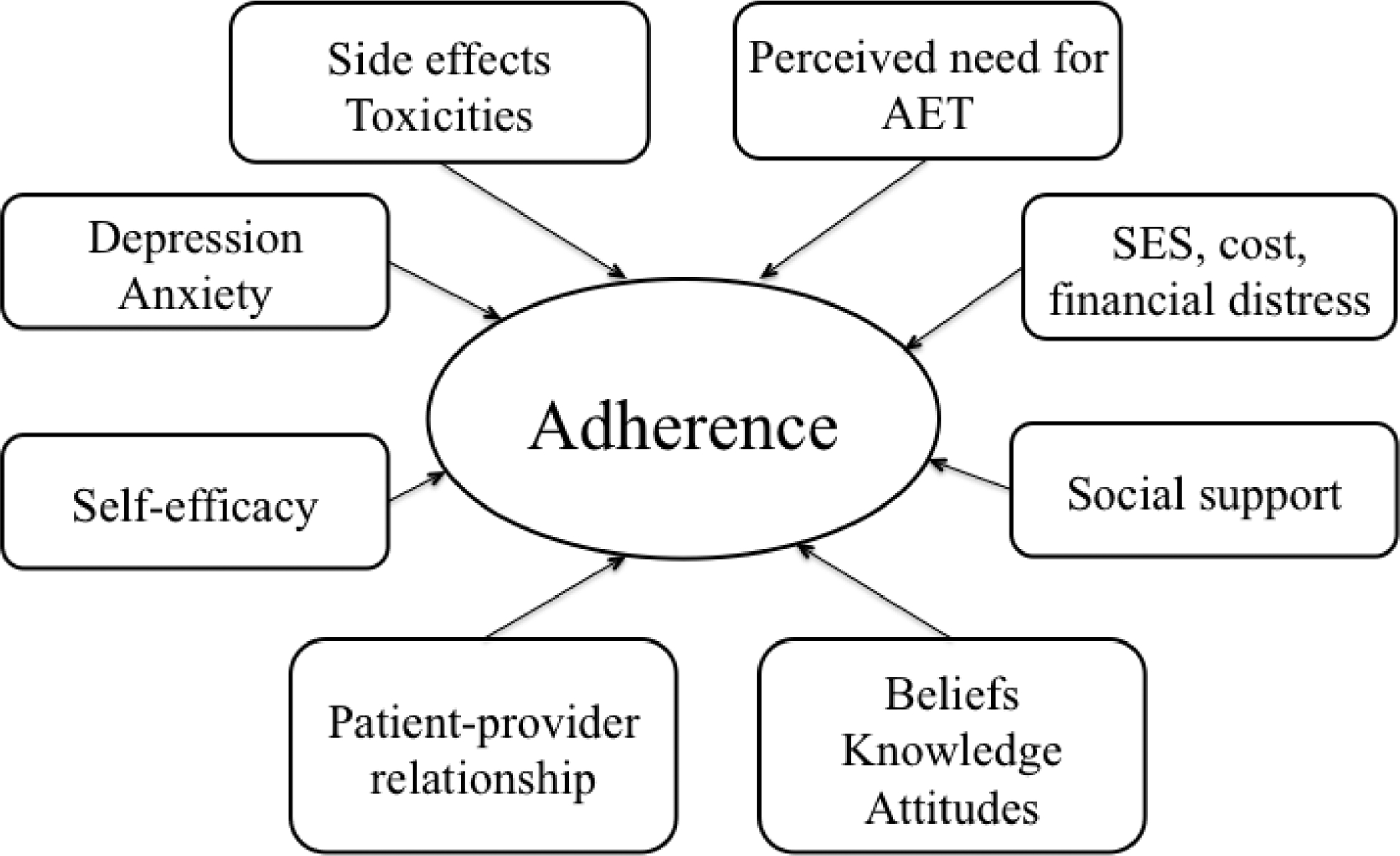
Modifiable barriers and contributors to adherence to adjuvant endocrine therapy
Patient Feedback
Feasibility.
The primary feasibility measure was patient attendance in the STRIDE sessions and completion of all study procedures. We also conducted semi-structured exit interviews (described below) to assess feasibility.
Session acceptability.
Following each session, participants rated five acceptability constructs (i.e., enjoyableness, convenience, helpfulness, usefulness, and satisfaction) on a 1–10 scale, with higher scores indicating greater acceptability. For example, patients were asked “How likely are you to use skills/information from today’s session in the future?” This survey was built into the telehealth software to appear automatically after patients exited the virtual visit. We descriptively examined the mean rating per session and for each construct across all sessions.
Overall intervention and study acceptability.
At 3-months post-baseline, participants completed a post-intervention assessment, via the HIPAA-compliant online survey tool, REDCap (Research Electronic Data Capture), which included a 5-item questionnaire to assess overall satisfaction (i.e., amount of time, length of sessions, length of study questionnaires, virtual delivery, and comfort in group/individual setting) on a scale of 1 (quite dissatisfied) to 4 (very satisfied). For example, participants were asked, “How satisfied were you with the number of sessions that you received?” In addition, patients completed the Client Satisfaction Questionnaire (CSQ) (Attkisson & Zwick, 1982), a three-item, validated measure to assess satisfaction with services provided to the patient with questions such as, “To what extent has our program met your needs?” The CSQ uses a Likert-type scale (1–4) with higher scores indicating greater satisfaction. We descriptively examined the mean of each overall satisfaction item and the mean CSQ.
Exit interviews.
Following the 3-month assessment, patients completed 15–20 minute semi-structured interviews to solicit feedback about intervention content, length of assessments, group vs. individual format, and logistical feasibility of participating in the study. Trained study staff not involved with the delivery of the intervention conducted the interviews with each study patient separately by telephone. Interviews were audio-recorded and transcribed. Two study staff collated the participants’ answers to each specific question by placing them in categories by subject (e.g., evaluation of MEMS, feedback about skills learned, evaluation of videoconference modality) and these responses summaries were reviewed by the PI and investigative team to inform any modifications to the intervention.
Results
Twenty-five patients were approached for the study and 14 stated that they had no issues with taking AET. Of the 11 eligible patients, five declined, stating that they were not interested in participating, and six signed informed consent. One of the six participants subsequently transferred her care from MGH and therefore became ineligible to participate prior to enrollment. Ultimately, a total of five patients were enrolled in the open pilot study and were an average of 57.5 years of age (SD=12.3), married, non-Hispanic white, and had been on AET for median of five months (range =1–12). Stage of disease ranged from stage 0 to stage IIB breast cancer (see Table 1 for patient characteristics). With respect to measures of feasibility, all participants completed 100% of sessions and all post-study measures.
Table 1.
Patient Sociodemographic and Treatment-related Characteristics (N=5)
| Patient Characteristic | Mean (SD) or Frequency (%) |
|---|---|
| Age (years) | 57.5 (12.3) |
| Woman | 5 (100%) |
| Race/ethnicity | |
| Non-Hispanic white | 5 (100%) |
| Education | |
| 2 years of college/AA/technical | 4 (80%) |
| College graduate | 1 (20%) |
| Annual household income | |
| $50,000-$99,999 | 3 (60%) |
| $100,000 or greater | 2 (40%) |
| Current relationship status | |
| Married or living with someone else | 5 (100%) |
| Menopausal Status | |
| Post-menopausal | 4 (80%) |
| Breast cancer stage | |
| Stage 0 | 1 (20%) |
| Stage IA | 1 (20%) |
| Stage IIA | 2 (40%) |
| Stage IIB | 1 (20%) |
| Type of adjuvant endocrine therapy | |
| Letrozole | 2 (40%) |
| Exemestane | 1 (20%) |
| Anastrozole | 1 (20%) |
| Tamoxifen | 1 (20%) |
| Months since initiating adjuvant endocrine therapy (median; range) | 5 (1–12) |
| Type of primary breast cancer treatment | |
| Surgery only | 2 (40%) |
| Surgery & Chemotherapy | 1 (20%) |
| Surgery & Radiation | 1 (20%) |
| Surgery, Chemotherapy, & Radiation | 1 (20%) |
| Baseline Adherence Rate (Electronic Medical Event Monitoring System [MEMS Caps]) | 82.86% (38.33) |
Note: SD = Standard Deviation
Quantitative acceptability results.
The sessions were found to be highly acceptable averaging 9.55 (SD=0.36) across sessions (scale potential range =1–10). Ratings for sessions four (M=9.90, SD=0.14) and five (M=9.88, SD=0.11), which focused on coping skills for individual side effects, were the highest (Figure 3a). Across sessions (Figure 3b), patients rated the intervention as highly enjoyable (M=9.59, SD=0.46), convenient (M=9.34, SD=0.46), helpful (M=9.72, SD=0.44), useful (M=9.39, SD=0.64), and satisfactory (M=9.69, SD=0.28). On the overall intervention and study acceptability measure (Figure 3c; scale potential range =1–4), participants were highly satisfied with the virtual delivery (M=3.8, SD=0.45), length of assessments (M=3.8, SD=0.45), and the group setting (M=4.0, SD=0.0). Satisfaction with the duration of each session (M=3.6, SD=0.55) and number of sessions (M=3.2, SD=0.84) was slightly lower. Finally, on the CSQ (scale potential range =1–4), 80% (4/5) of patients reported that the study met most to almost all their needs (M=3.2, SD=0.84), 100% (5/5) reported that they were mostly or very satisfied with the skills (e.g., relaxation) they received (M=3.6, SD=0.55), and 100% (5/5) would or definitely would come back to the program if they needed to seek help again (M=3.6, SD=0.55).
Fig. 3.
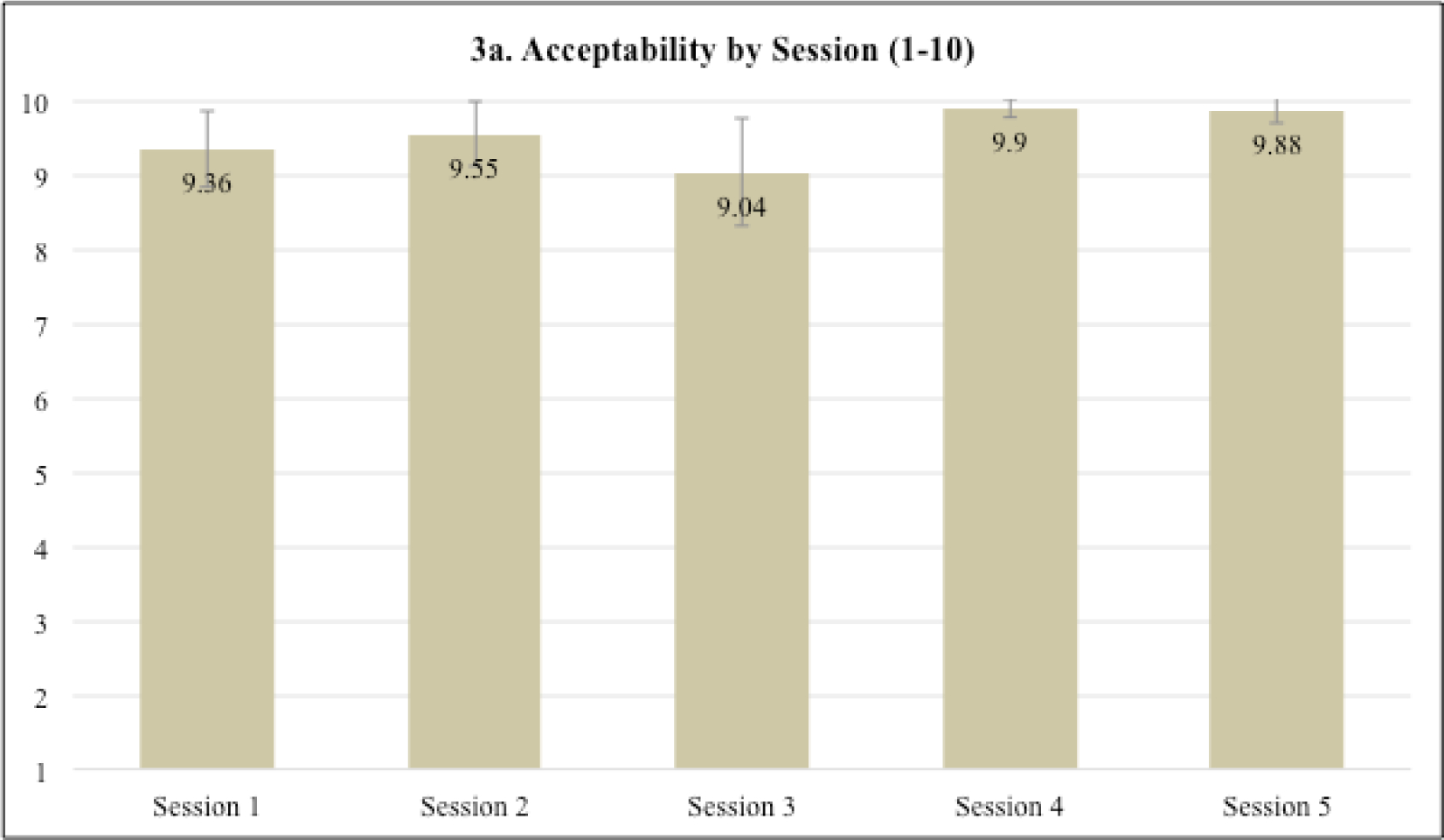
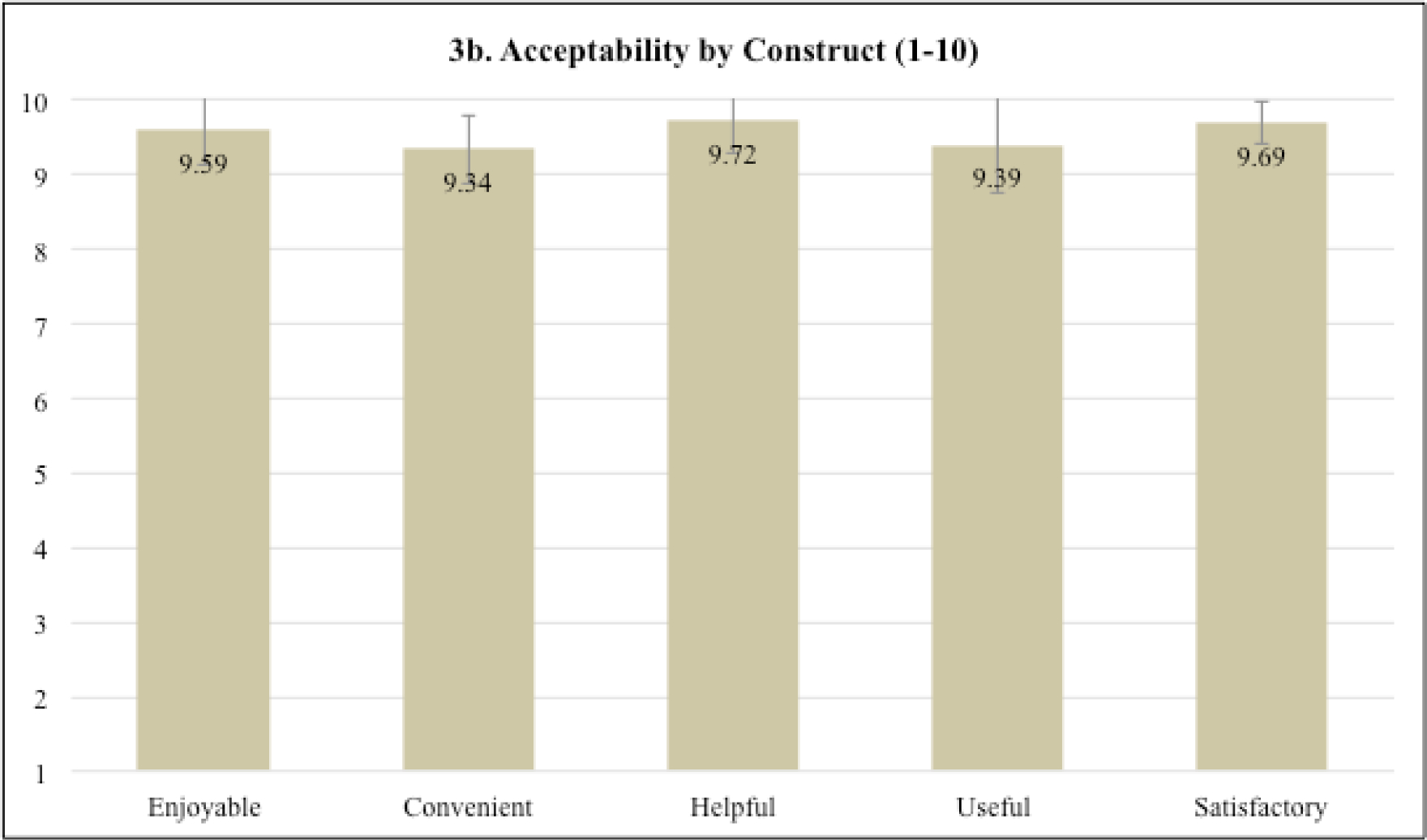
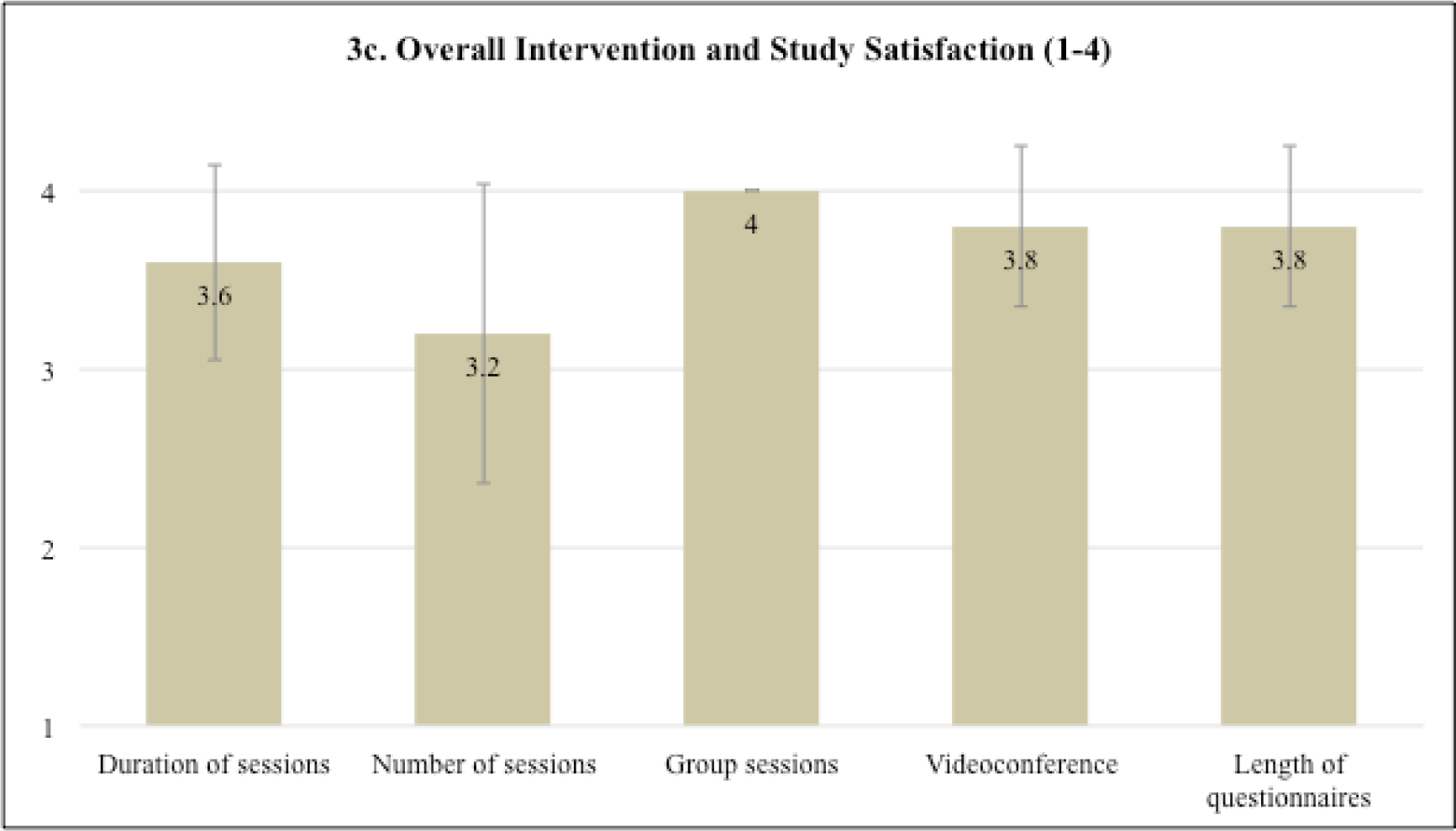
a-c Intervention session acceptability and overall intervention and study satisfaction
Exit interview results.
Patients’ feedback in the exit interviews mirrored the quantitative data. Recurrent themes are described below with illustrative quotes. First, patients described benefitting from the skills they learned and continuing to use them, especially relaxation exercises, coping strategies, and problem-solving medication-taking behaviors:
The most helpful was probably the way [you] learn to think about things, the mindful thinking and the breathing exercises. Mindfulness is the one I use all the time.
I thought the coping exercises that we did were good. The relaxation …just taking a step back was helpful…I thought it was a good mix of information and strategies.
Taking my pill…when I’m distracted, can be a problem. They were telling me how to do it in a regular routine, so I’m attempting to use that.
In addition, the feedback about benefiting from and wanting more time to focus on self-management of their individual side effects, such as hot flashes, pain, and sleep difficulties, was consistent with the fact that patients quantitatively rated sessions 4 and 5 the highest, which focused on side effect management:
In the beginning I was feeling like I wasn’t going to be able to do this [take the medication], but it made me think differently about being on the hormonal therapy. I came to the realization that maybe I will have side effects, but I will just be able to deal with them.
I think it’s nice to have a focused time that you can ask questions and get an immediate answer. The other lady mentioned something that she was going through and I realized I was going through that, too, and didn’t realize it was related [to the hormonal therapy].
It was somewhat tailored to our specific complaints, which I thought was good.
It really teaches you a lot about the medication you’re going to be on for so many years and how to deal with the aftermath of being diagnosed with cancer.
Third, consistent with the quantitative ratings for number and duration of sessions, patients expressed the desire for additional sessions:
I was kind of looking forward to them so I kind of wish there was more.
I think it should be longer, like maybe a week or two longer to touch more on the side effects, because by the time you jump in, it’s done.
Fourth, the four patients who participated in the two small groups expressed an overwhelming benefit from the social support they received from one another.
I really enjoyed [the group aspect]. I wasn’t sure how that would look and I resisted doing any kind of support group, but I really enjoyed it…I do feel like we benefitted from it.
I liked to see another person and know what was happening to me was happening to someone else as well-that I wasn’t alone…I have friends…and I try to tell them how I’m feeling with my medication and they don’t understand. I liked that in the group you were able to talk to someone about the experience…Everybody needs that.
Sharing with another person on the program was really very helpful because you have another human being that is in the same situation that you are in.
I thought it was helpful. It’s sometimes easier to talk to someone you don’t know instead of someone you do know when it’s tough like that.
Finally, there was unanimous support for the use and convenience of the virtual visit as compared to in-person visits:
I think the program was very helpful. I could not do this going into Boston once a week… it wasn’t going to happen.
I prefer to do a videoconference because of the time it would take [going to the hospital for in-person sessions].
We also solicited feedback from patients about the acceptability of adding two individual brief check-in sessions via telephone, at one and two months post-intervention, given the long-term AET regimen and need for ongoing adherence; patients were unanimously in favor of this modification. In summary, patients expressed that the relaxation exercises, ability to receive support from others, and the tailoring of coping strategies, were extremely helpful. Patients reported no issues or challenges using the MEMS Cap to store their medication, stating that “it was fine to use.” One participant reported that she was questioned about the bottle during an airport security screening, but she did not describe that this was particularly problematic. All participants found the length of the study questionnaires to be acceptable, with statements such as “ I don’t think they were too long,” and “fine; it was very thorough.” They described enthusiasm for the intervention and stated that they would strongly recommend the program to others taking AET, with one stating “yes, absolutely [I would recommend the program], because it really teaches you a lot about the medication you are going to be on for so many years.” Based on this quantitative and qualitative feedback, three key modifications were made to the intervention as detailed below.
Intervention Modifications and Refinement
First, we added a sixth session to continue focusing on side effect management, but without introducing any new content. With this additional session, self-management skills are distributed across three sessions (i.e., sessions 4, 5, and 6) instead of the two (i.e., sessions 4 and 5). We also added a “bonus” guided visual imagery exercise to the final session, which is sent via e-mail to patients, but not conducted in-session due to the feedback about content feeling rushed. Second, based on unanimous feedback about the feasibility, ease of use, and convenience of virtual visits, we chose only to offer the STRIDE intervention virtually, rather than give an option for in-person sessions. Finally, given the clear feedback about the benefits of peer support in the small group format, and to standardize the intervention, we chose to offer the STRIDE intervention in small groups of two only, rather than provide an option for individual sessions. We chose to limit group size to two given feedback about content feeling rushed and to maintain individualization of coping strategies for specific side effects. As a final step, the manual was reviewed with a breast oncologist and a nurse practitioner in breast oncology, and the overall study and procedures were presented to the MGH Breast Oncology Research Group for feedback. Based on the review, slight refinements were made to the description about the need for AET, and a strategy for side effect management was added.
Description of Finalized Intervention
The STRIDE intervention is derived from cognitive-behavioral theory and includes skills-based strategies to achieve three major goals: (a) optimize adherence to AET, (b) reduce distress, and (c) manage symptoms. It is designed to be delivered virtually in small groups of two to three patients via six weekly one-hour sessions using videoconference and two brief, follow-up check-in sessions by telephone. Specific components are informed by identified unmet needs and themes from our qualitative study with patients taking AET and are adapted from two prior related and published treatments: 1) Cognitive Behavioral Therapy for Adherence and Depression (Safren et al., 2007) which is shown to improve adherence via integration of adherence counseling and skills-based treatment for co-occurring psychiatric symptoms (Safren et al., 2016), and 2) Cognitive Behavioral Stress Management (CBSM) (Antoni, Ironson, & Schneiderman, 2007; Antoni & Smith, 2003), which is shown to reduce symptoms of depression and anxiety during and after breast cancer through combined cognitive-behavioral skills and relaxation training (Antoni, Lechner, et al., 2006; Antoni, Wimberly, et al., 2006; Gudenkauf et al., 2015; Stagl, Bouchard, et al., 2015).
STRIDE consists of four modules, including an introductory session involving psychoeducation about AET and distress, motivational interviewing, and assessment and problem-solving of adherence barriers; a second session focused on cognitive restructuring; a third session to enhance coping strategies and activity scheduling; and a final three sessions dedicated to behavioral management of specific AET-related symptoms and side effects, chosen by group members. Each session builds upon skills learned in the previous session and begins with a discussion of medication adherence and a review of skills’ practice. Relaxation training is integrated throughout the entire intervention, with five of six sessions including a new relaxation skill (i.e., diaphragmatic breathing, progressive muscle relaxation) accompanied by e-mail of an audio-recorded relaxation script for at-home practice.
Given the lengthy duration of AET and subsequent need to reinforce skills practice over the long-term (Safren et al., 2012), patients engage in a 15–30 minute, individual, semi-structured, follow-up telephone call with the therapist at one and two months after intervention completion to discuss and problem-solve ongoing challenges with adherence or symptom management.
Module 1: Understanding AET and barriers to adherence (session 1).
The primary aims of this module are to 1) elicit patients’ understanding of their need for AET; 2) identify barriers to adherence; 3) increase motivation for AET by setting goals for taking medication and problem-solving barriers; and 4) introduce and practice relaxation training. The session begins with a brief overview of CBT, the rationale for the program, and considerations for the group environment, such as confidentiality. Patients are asked to share their understanding of their need for AET and to reflect on their experiences and concerns. Murray’s Framework for Medication Adherence accounts for several factors that influence adherence behaviors, including patient, provider, treatment, and systems factors (Murray et al., 2004). Therefore, targeted questions aim to probe common factors contributing to AET adherence, such as perceived risk of breast cancer recurrence, relationship and comfort discussing treatment with the prescribing physician, negative or ambivalent beliefs about therapeutic efficacy, and symptoms or side effects that interfere with quality of life (QOL). Psychoeducation about the therapeutic mechanism for AET is provided, and intervention components include articulating goals for medication-taking, identifying barriers, and developing a back-up plan to overcome barriers and achieve the goal (Safren, Otto, & Worth, 1999). Motivational interviewing (Rollnick & Miller, 1995) is employed based on the Health Belief Model, which maintains that the health behavior (i.e., AET adherence) will be adopted if the patient perceives that (a) they have an increased risk for a condition, (b) the medication will reduce risk, (c) the benefits outweigh costs, and (d) they have self-efficacy for taking the medication (Becker, 1974; Becker, 1985; Becker & Maiman, 1975; Moore, 2010). Motivational exercises include rating self-efficacy for taking medication for the duration of the prescribed regimen as well as examining the advantages and disadvantages to continuing or stopping AET. Patients are encouraged to share medication-taking strategies with one another and brainstorm new ones to try.
The session concludes with a discussion about how the stress response worsens cancer-related symptoms and side effects and perpetuates negative or perseverative thoughts and worries (Cannon, 1929). The therapist introduces diaphragmatic breathing as a tool to engage the relaxation response, thereby slowing the stress response (Benson, 1975). An audio-recorded relaxation is e-mailed to patients after the session in a media file. Patients are asked to practice diaphragmatic breathing for 5–10 minutes once per day and to practice one strategy for routinely taking medication prior to the next session.
Module 2: Identifying and restructuring unhelpful automatic thoughts (session 2).
The primary aims of the second module are to 1) review medication adherence strategies and relaxation practice, as well as problem-solve barriers to practice; 2) teach patients to identify automatic thoughts, differentiate those that are unhelpful or biased, and understand how unhelpful automatic thoughts may trigger a cascade of negative emotions and maladaptive coping responses/behaviors; 3) help patients learn to cognitively challenge unhelpful thought patterns or thinking traps that perpetuate negative emotions and behavioral responses; 4) identify a more realistic, accurate, or balanced thought to replace an unhelpful automatic thought and alter downstream effects by neutralizing emotions and engaging in more productive behavioral coping responses.
This module is based on a cognitive model for menopausal symptoms (Hunter & Mann, 2010), which asserts that cognitive appraisal of symptoms will influence the emotional and behavioral reactions to them. Specifically, negative beliefs or catastrophic thoughts about AET-related side effects (e.g., side effects as problematic, uncontrollable, embarrassing, or AET as harmful or toxic) lead to maladaptive behavioral reactions (e.g., avoidance of activities, social isolation, skip medication dose). Furthermore, in accordance with Symptom Perception Theory (Kolk, Hanewald, Schagen, & Gijsbers van Wijk, 2003), the tendency to focus attention on bodily sensations can increase the likelihood of detecting sensations and amplify the somatic experience, perpetuating cancer-related symptoms.
CBT has been beneficial for menopausal symptoms after breast cancer (Mann et al., 2012), and the use of cognitive facilitators and self-talk about the efficacy of AET is associated with better adherence to AET (Bright, Petrie, Partridge, & Stanton, 2016). Therefore, this module draws from traditional cognitive restructuring techniques (Beck, 1995) with specific application to breast cancer and AET to enhance adherence and manage distress and symptoms. Patients are presented with an example of common automatic and negative thoughts in response to experiencing a hot flash in a public place, as well as the likely emotions, physical sensations, and behavioral reactions that perpetuate the cycle. Patients are taught a step-by-step process to break the cycle by identifying unhelpful patterns in their thinking (e.g., catastrophic thinking, jumping to conclusions), cognitively challenging the accuracy of the automatic thought (e.g., examining evidence), and identifying a more accurate thought in place of the old one (Antoni & Smith, 2003). Comprehensive descriptions of common thought distortions are provided, and patients complete exercises throughout the module to facilitate understanding. Example cognitive reframes are provided to optimize accuracy related to recurrence risk perception and clinical necessity for therapy. A thought monitoring and restructuring log is provided for practice, and patients are encouraged to continue practicing diaphragmatic breathing.
Module 3: Enhancing coping skills and mindfulness-based techniques (session 3).
The primary aims of this third module are to 1) review cognitive restructuring and relaxation practice and problem-solve barriers to skills practice; 2) explore two types of coping responses, problem-focused and emotion-focused, and identify the appropriate coping response based on the controllability of a situation; 3) employ a worry algorithm to guide coping choices in response to realistic and non-biased cancer-related worries; 4) identify and reduce unhelpful coping behaviors; 5) teach mindfulness-based techniques to manage realistic worries while remaining present-focused.
Within the foundations of stress, appraisal, and coping theory (Lazarus & Folkman, 1984), the cognitive model of adjustment to cancer (Moorey & Greer, 2002) postulates that an individual cognitively appraises the stress of cancer (e.g., breast cancer) and treatment (e.g., AET) as a threat to the self and survival, and this threat exceeds perceived coping resources. In the case of cancer, threats to survival are not just perceived, as fears of cancer recurrence are prominent and realistic. Two broad coping responses are recommended in stressful situations: emotion-oriented coping strategies to manage the emotional distress arising from an uncontrollable situation, and problem-focused coping strategies to change or solve the problem in a controllable situation (Lazarus & Folkman, 1984). Within this framework, the first part of this module teaches patients to parse the controllable and uncontrollable aspects of a stressor, and to then choose an emotion-focused coping strategy (e.g., cognitive restructuring, emotional expression, relaxation, engagement in pleasurable activities) in response to an uncontrollable event and/or a problem-focused coping strategy (e.g., goal-setting, decision making, planning and preparing, information-seeking) in response to a controllable event. A worry algorithm is presented to guide patients in determining whether the fear is realistic, and to choose the appropriate path to either resolve the problem or manage distress when no solution is possible (adapted from Greer, Graham, & Safren, 2010 (Greer, Graham, & Safren, 2010). The section culminates with a discussion of maladaptive coping behaviors that are passive or unhelpful (e.g., procrastination, avoidance, social isolation, substance use) that may resolve short-term distress, yet perpetuate the problem in the long-term. Examples are discussed to apply this logic to decisions to take AET, discuss alternative medications or side effect management with their doctor, alleviate distress related to symptoms and side effects, and more broadly manage challenges after breast cancer while re-adjusting to life post-treatment.
The second part of this module introduces mindfulness skills (Baer, 2003) to stay present-focused and cultivate acceptance of thoughts and emotions as a strategy to manage realistic fears of cancer recurrence, difficulties with AET, post-treatment symptoms, and AET-related side effects. As in Mindfulness-Based Stress Reduction (Kabat-Zinn & University of Massachusetts Medical Center/Worcester. Stress Reduction Clinic., 1990), patients are taught that mindfulness is ‘awareness that emerges through paying attention on purpose, in the present moment, and nonjudgmentally to things as they are’ (Williams, 2007). Patients learn that the urge to resist or avoid unwanted thoughts, feelings, or symptoms may increase distress and perpetuate their symptoms, rather than resolve them. Two strategies are taught to increase present focus and disrupt the ruminative cycle, a proposed driver of depression and anxiety (Kuyken et al., 2010). First, patients are introduced to concepts of mindfulness and the process of paying attention, on purpose, to informal daily activities (e.g., washing dishes, bathing, going for a walk) to increase selective attention and present-moment awareness. Second, a 6-minute guided body scan meditation is conducted to bring attention to bodily sensations and promote release of tension. Home practice is assigned, including completing the worry algorithm in response to a stressor and practicing diaphragmatic breathing or the body scan meditation.
Module 4: Managing specific side effects (sessions 4–6).
The primary aims of the final module, delivered across three sessions, are to 1) review relaxation and skills practice from prior sessions; 2) identify patients’ most distressing and interfering side effects and discuss strategies for effective communication with the healthcare team; 3) teach specific cognitive and behavioral skills to improve self-management of side effects and provide referrals to other care providers as necessary; 4) practice additional guided relaxations to increase exposure to various forms of relaxation and self-efficacy for engaging the relaxation response to manage fears of recurrence. The main content for this module focuses on side effect management, with additional cognitive-behavioral strategies and relaxation exercises in each of sessions four, five, and six.
Main content (sessions 4–6).
Patients identify the most interfering and distressing side effects. As a group, patients are encouraged to come to consensus on 3–4 side effects in which to focus the remaining three sessions. CBT is effective for helping patients manage cancer-related symptoms such as pain (Tatrow & Montgomery, 2006), fatigue (Wu et al., 2019), and menopausal symptoms such as hot flashes (Duijts et al., 2012; Mann et al., 2012), and insomnia (Espie et al., 2008). Therefore, sessions four through six each encompass personalized CBT-based strategies for managing side effects with the goal of building a comprehensive “toolkit” of helpful strategies. Although several strategies for self-management of side effects can be applied to multiple side effects, the following topics are presented to patients to choose from: a) body image, weight, hair; b) memory difficulties and concentration; c) fatigue or loss of energy; d) hot flashes; e) mood swings, sadness, or nervousness; f) joint pain, muscle aches, or headaches; g) sexuality and intimacy; and h) insomnia or sleep. Each topic begins with brief psychoeducation about the theory or mechanism for the side effect as it relates to AET. Relaxation and cognitive restructuring are offered as strategies for each side effect with pertinent explanations and examples, as these are important components of self-management of all cancer-related physical symptoms as well as emotional distress (Luebbert, Dahme, & Hasenbring, 2001; Tatrow & Montgomery, 2006). Additional skills map onto specific side effects. For example, components of CBT for Insomnia (CBT-I) are taught, such as sleep stimulus control and sleep hygiene education (Edinger & Carney, 2015), to manage sleep difficulties upon waking with night sweats or hot flashes. Time-based activity planning and pacing is taught as a strategy for both pain and fatigue management, helping patients optimally participate in daily activities based on a circumscribed amount of time to maintain physical functioning while minimizing energy expenditure (Otis, 2007). Physical activity is explored as a strategy for managing several side effects such as pain, fatigue, hot flashes, and concerns about body image (Duijts et al., 2012). Local and online resources for physical activity in cancer survivorship are offered, and patients set realistic and achievable goals for exercise.
Additional content (session 4).
The quality of the patient-healthcare provider relationship influences AET adherence (Stanton et al., 2014), and patients who are well-informed about medication side effects are more likely to adhere to AET (Heisig et al., 2015); therefore, session four begins with brief strategies for seeking information and communicating assertively and effectively with the healthcare team. This session culminates with an introduction to and practice of Progressive Muscle Relaxation to reduce anxiety and stress by blunting sympathetic nervous system arousal (Jacobson, 1938).
Additional content (session 5).
This session also includes discussion of acceptance, driven by mindfulness theory (Baer, 2003), to facilitate an enhanced awareness of thoughts and feelings rather than an attempt to change thought content that is realistic (e.g., fears of recurrence or difficulties with AET-related side effects). Patients are reminded that acceptance does not equate with approval of a situation (e.g., having to take AET daily for 5–10 years), and a 2-minute guided acceptance exercise is conducted to facilitate letting go and reducing resistance.
Additional content (session 6).
The final session introduces and reviews cognitive-behavioral techniques to manage worries and fears of breast cancer recurrence (e.g., setting aside time for worrying and using emotion-focused coping for uncontrollable stressors), based on the theory that perceived uncertainty about symptoms, treatment, and outcome is a major contributor to stress in illness (Mishel, 1984). The session concludes with patients identifying a plan to prevent a decline in adherence over time and to maintain acquisition of skills to manage distress and symptoms. A guided visual imagery is offered as a “bonus” relaxation exercise to practice after the session.
Individual Check-ins.
The therapist provides ongoing adherence counseling and discussion of skills via two 15–30-minute check-in telephone calls at one month and two months post-treatment. The therapist and patient discuss use of strategies in the patient’s “toolkit” for symptom management and review medication-taking behaviors for AET adherence. Difficulties or challenges are explored, drawing from problem-solving therapy in chronic illness (Nezu, Nezu, Friedman, Hours, & Faddis, 1997) by helping patients define the problem, generate solutions and alternatives, make decisions, and implement solutions by breaking overwhelming tasks into manageable steps.
Implementation and Training
The STRIDE intervention can be delivered in-person or virtually using HIPAA-compliant videoconference software. However, virtual delivery is preferable to optimize efficiency and accessibility given post-treatment challenges after breast cancer (Richardson, Frueh, Grubaugh, Egede, & Elhai, 2009; Stanton, 2012; Stanton & Bower, 2015). Groups of two or three patients are advisable to optimize peer support while maximizing individualization and tailoring of strategies to increase self-management of side effects. Clinicians with mental health backgrounds (e.g., social workers, psychologists, psychiatrists, nurses) can be trained to deliver the STRIDE intervention. It is preferable that clinicians are familiar with traditional CBT and have administered CBT in cancer care settings. Training includes a) reading the STRIDE manual and qualitative findings (Jacobs et al., 2019); b) attending a training to learn about the rationale and evidence-base for the intervention; and c) participating in role-plays to practice implementing the intervention. If possible, clinicians would shadow a breast oncologist during follow-up oncology visits with patients taking AET to gain firsthand insight into the concerns raised by patients receiving prescriptions for AET. Finally, therapists should receive ongoing supervision to uphold treatment integrity.
Discussion
Despite extensive literature outlining barriers to AET adherence, including side effects and related distress, interventions thus far have failed to produce meaningful improvements in adherence, have lacked a theoretical basis, and have not addressed the myriad factors contributing to poor adherence (Ekinci et al., 2018; Finitsis et al., 2018; Heiney et al., 2019). The purpose of this study was to modify and refine an evidence-based, virtual intervention for patients on AET after breast cancer, and to describe the theoretically-driven intervention components and final modifications prior to testing. Findings from the small open pilot study with five patients taking AET after breast cancer suggest feasibility and acceptability with all patients completing all sessions and reporting that the sessions were highly enjoyable, useful, convenient, helpful, and satisfying. Feasibility was further demonstrated on the overall study acceptability measure with high scores related to the virtual delivery, group setting, convenience, and assessment length. Exit interviews and high ratings on the latter sessions focused on side effect management indicated that patients found the material to be tailored to their needs and desired more time and/or sessions to address personal side effects. Subsequently, the final six-session intervention, as described, includes three sessions focused on strategies for self-management of side effects. The intervention aims to improve self-efficacy for managing AET-related side effects, lower symptoms of depression and anxiety, and optimize adherence behaviors. Patients found the group environment to be supportive and helpful in normalizing their difficulties with AET after breast cancer. Importantly, all patients noted that the virtual delivery of the intervention made participation possible while readjusting to their lives after breast cancer.
Our findings are notable given that interventions that have been developed to date have not been comprehensively based on the body of qualitative work that would inform intervention components relevant for the sample they intend to intervene upon. Thus, published qualitative studies have not directly guided intervention development, which is a crucial step for evidence-based psychosocial intervention development, per the National Institutes of Health Stage Model for Behavioral Intervention Development (Onken et al., 2014; Rounsaville et al., 2001). The STRIDE intervention was informed by the established literature, patient-centered themes from an in-depth qualitative study [38], effective empirically-based interventions for adherence (Safren et al., 2007) and stress management during breast cancer (Antoni & Smith, 2003), as well as expert input. For example, breast oncologists provided feedback on the intervention manual itself, including the description of the necessity for AET and strategies for side effect management. In addition, interventions to date have not incorporated a strong theoretical premise for intervention components and have failed to address the multitude of factors influencing adherence. However, the STRIDE intervention incorporates empirically-based components from CBSM, which has been beneficial for patients during breast cancer with sustained effects on depressive symptoms and QOL at 5-years and 11-years follow-up (Stagl, Antoni, et al., 2015; Stagl, Lechner, et al., 2015), and CBT-AD (Safren et al., 2016; Safren et al., 2012), which has improved adherence and reduced distress in chronic illness. Furthermore, STRIDE’s peer connection and support may also be an important component given evidence that adherence is better for those who simply have a peer with breast cancer (Simon, Latreille, Matte, Desjardins, & Bergeron, 2014). The focus on self-management of side effects with cognitive-behavioral techniques has been beneficial in prior studies (Mann et al., 2012) and may promote adherence to AET. Finally, our integration of patient feedback to refine the intervention optimizes patient-centeredness and feasibility.
Several limitations pertain to this open pilot study. First, while this is a first step in developing and refining an evidence-based intervention for patients taking AET after breast cancer, a larger sample size and rigorous study design are needed to examine definitively the feasibility, acceptability, and preliminary effects, and therefore, a pilot randomized controlled trial (RCT) is currently underway. In addition, the study therapist was a licensed clinical psychologist who delivered all intervention sessions and was also the lead investigator on the study. For the RCT in progress, additional licensed psychologists and unlicensed post-doctoral and pre-doctoral fellows are delivering sessions. Third, the sample was homogenous, while also skewed towards women with earlier stage disease (0-IIB); thus, future research should seek to diversify the sociodemographic representativeness of the sample in order to maximize generalizability, including collecting information on sexual orientation/identification, which may be related to satisfaction with cancer care (Fobair et al., 2001; Jabson & Kamen, 2016). Fourth, all patients had an electronic device with video capability. While such access to technology is becoming more common, with 81%, 73%, and 53% of Americans owning a Smartphone, desktop/laptop computer, and tablet computer in 2018, respectively (Anderson, 2015), a virtual delivery modality may still be insufficient for patients without access to these devices or with limited technological literacy. In addition, while participants requested additional sessions, the total of six 2-person group sessions in addition to two individual follow-ups may increase cost and time, which may be more challenging to implement in a clinical oncology setting. Future work might benefit from examining a briefer version of this intervention to evaluate effects with lower cost and time intensity. Finally, the fact that the intervention was delivered by the principal investigator may have introduced bias, positively affecting the quantitative and qualitative intervention ratings. To mitigate bias going forward, trained study therapists will administer the intervention in the aforementioned pilot RCT. Despite these limitations, all patients reported finding the sessions highly useful and helpful, describing that they benefited from learning strategies for self-management of side effects, peer support, and relaxation techniques.
In summary, patients on AET after breast cancer who participated in a patient-centered, small group-based, virtual intervention for adherence, symptom management, and distress reported high intervention acceptability, enjoyableness, and usefulness. This treatment may be a useful approach with a strong theoretical premise while also being grounded in the actual patient experience. Future research is needed to explore rigorously the feasibility, acceptability, and potential efficacy of the STRIDE intervention. In the long-term, results may have implications for breast cancer survivorship care plans and the provision of support for patients taking AET. Ultimately, improved adherence may optimize clinical outcomes for patients with breast cancer.
Supplementary Material
Acknowledgements:
We thank the study patients for their time and dedication to this research study.
Funding: This study was funded by a Career Development Award from the National Cancer Institute of the National Institutes of Health (K07CA211107; Jacobs).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of Dana-Farber / Harvard Cancer Center Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients signed informed consent with trained study staff.
Conflict of Interest: MA receives a small royalty from a book and related treatment manuals on stress management in breast cancer. JG receives royalties from Springer Humana Press, has research funding from Gaido Health/BCG Digital Ventures, and is a paid consultant from Concerto HealthAI. JP has received research funding from Pfizer, is a paid consultant for Athenex, and JP’s spouse in an employee of GlaxoSmithKline. All other authors declare that they have no conflict of interest.
References
- Anderson M (2015). Technology Device Ownership: 2015. Retrieved from http://www.pewinternet.org/2015/10/29/technology-device-ownership-2015
- Antoni MH, Ironson G, & Schneiderman N (2007). Cognitive-Behavioral Stress Management: Workbook. New York: Oxford University Press. [Google Scholar]
- Antoni MH, Lechner SC, Kazi A, Wimberly SR, Sifre T, Urcuyo KR, … Carver CS (2006). How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol, 74(6), 1143–1152. doi: 10.1037/0022-006X.74.6.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, & Smith R (2003). Stress Management Intervention for Women with Breast Cancer: Participant’s Workbook. Washington, DC: American Psychological Association. [Google Scholar]
- Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, … Carver CS (2006). Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatry, 163(10), 1791–1797. doi: 10.1176/ajp.2006.163.10.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attkisson CC, & Zwick R (1982). The Client Satisfaction Questionnaire: Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation and program planning, 5(3), 233–237. [DOI] [PubMed] [Google Scholar]
- Baer RA (2003). Mindfulness Training as a Clinical Intervention: A Conceptual and Empirical Review. Clinical Psychology: Science and Practice, 10(2), 125–143. doi: 10.1093/clipsy.bpg015 [DOI] [Google Scholar]
- Beck JS (1995). Cognitive therapy : basics and beyond. New York: Guilford Press. [Google Scholar]
- Becker MH (1974). The health belief model and personal health behavior (Vol. 2). Thorofare, NJ: Slack. [Google Scholar]
- Becker MH (1985). Patient adherence to prescribed therapies. Med Care, 23(5), 539–555. [DOI] [PubMed] [Google Scholar]
- Becker MH, & Maiman LA (1975). Sociobehavioral determinants of compliance with health and medical care recommendations. Med Care, 13(1), 10–24. [DOI] [PubMed] [Google Scholar]
- Bender CM, Gentry AL, Brufsky AM, Casillo FE, Cohen SM, Dailey MM, … Sereika SM (2014). Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum, 41(3), 274–285. doi: 10.1188/14.ONF.274-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson H (1975). The relaxation response. New York: Morrow. [Google Scholar]
- Bright EE, Petrie KJ, Partridge AH, & Stanton AL (2016). Barriers to and facilitative processes of endocrine therapy adherence among women with breast cancer. Breast Cancer Res Treat, 158(2), 243–251. doi: 10.1007/s10549-016-3871-3 [DOI] [PubMed] [Google Scholar]
- Bright EE, & Stanton AL (2018). Prospective investigation of social support, coping, and depressive symptoms: A model of adherence to endocrine therapy among women with breast cancer. J Consult Clin Psychol, 86(3), 242–253. doi: 10.1037/ccp0000272 [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Lacchetti C, & Griggs JJ (2018). Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Oncol Pract, 15(2), 106–107. doi: 10.1200/JOP.18.00617 [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, … Griggs JJ (2014). Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol, 32(21), 2255–2269. doi: 10.1200/JCO.2013.54.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir C, Guinan E, Dombrowski SU, Sharp L, & Bennett K (2015). Identifying the determinants of adjuvant hormonal therapy medication taking behaviour in women with stages I-III breast cancer: A systematic review and meta-analysis. Patient Educ Couns. doi: 10.1016/j.pec.2015.05.013 [DOI] [PubMed] [Google Scholar]
- Cannon WB (1929). Bodily changes in pain, hunger, fear and rage : an account of recent researches into the function of emotional excitement. New York: D. Appleton and Company. [Google Scholar]
- Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, … Intergroup Exemestane S (2004). A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med, 350(11), 1081–1092. doi: 10.1056/NEJMoa040331 [DOI] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, … Charlson ME (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol, 34(10), 971–982. doi: 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, … (EBCTCG), E. B. C. T. C. G. (2011). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet, 378(9793), 771–784. doi: 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR, Giordani PJ, Lepper HS, & Croghan TW (2002). Patient adherence and medical treatment outcomes: a meta-analysis. Med Care, 40(9), 794–811. doi: 10.1097/00005650-200209000-00009 [DOI] [PubMed] [Google Scholar]
- Duijts SF, van Beurden M, Oldenburg HS, Hunter MS, Kieffer JM, Stuiver MM, … Aaronson NK (2012). Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol, 30(33), 4124–4133. doi: 10.1200/JCO.2012.41.8525 [DOI] [PubMed] [Google Scholar]
- Edinger JD, & Carney CE (2015). Overcoming InsomniaA Cognitive-Behavioral Therapy Approach, Therapist Guide: Oxford University Press. [Google Scholar]
- Ekinci E, Nathoo S, Korattyil T, Vadhariya A, Zaghloul HA, Niravath PA, … Trivedi MV (2018). Interventions to improve endocrine therapy adherence in breast cancer survivors: what is the evidence? Journal of Cancer Survivorship, 1–9. [DOI] [PubMed] [Google Scholar]
- Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, … Paul J (2008). Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol, 26(28), 4651–4658. doi: 10.1200/JCO.2007.13.9006 [DOI] [PubMed] [Google Scholar]
- Finitsis DJ, Vose BA, Mahalak JG, & Salner AL (2018). Interventions to promote adherence to Endocrine Therapy among breast cancer survivors: A meta-analysis. Psychooncology. [DOI] [PubMed] [Google Scholar]
- Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, & Silliman RA (2004). Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor--positive breast cancer. J Clin Oncol, 22(16), 3309–3315. doi: 10.1200/JCO.2004.11.064 [DOI] [PubMed] [Google Scholar]
- Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, … Pisansky TM (2002). Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. Journal of clinical oncology, 20(20), 4141–4149. [DOI] [PubMed] [Google Scholar]
- Fobair P, O’Hanlan K, Koopman C, Classen C, Dimiceli S, Drooker N, … Spiegel D (2001). Comparison of lesbian and heterosexual women’s response to newly diagnosed breast cancer. Psychooncology, 10(1), 40–51. doi: [DOI] [PubMed] [Google Scholar]
- Gentry MT, Lapid MI, Clark MM, & Rummans TA (2019). Evidence for telehealth group-based treatment: A systematic review. J Telemed Telecare, 25(6), 327–342. doi: 10.1177/1357633X18775855 [DOI] [PubMed] [Google Scholar]
- Given BA, Spoelstra SL, & Grant M (2011). The challenges of oral agents as antineoplastic treatments. Semin Oncol Nurs, 27(2), 93–103. doi: 10.1016/j.soncn.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Greer JA, Amoyal N, Nisotel L, Fishbein JN, MacDonald J, Stagl J, … Pirl WF (2016). A systematic review of adherence to oral antineoplastic therapies. Oncologist, 21(3), 354–376. doi: 10.1634/theoncologist.2015-0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JA, Graham JS, & Safren SA (2010). Resolving treatment complications associated with comorbid medical conditions. In Otto M & Hofmann S (Eds.), Avoiding Treatment Failures in the Anxiety Disorders (pp. 317–346). New York: Springer. [Google Scholar]
- Greer JA, Park ER, Prigerson HG, & Safren SA (2010). Tailoring Cognitive-Behavioral Therapy to Treat Anxiety Comorbid with Advanced Cancer. J Cogn Psychother, 24(4), 294–313. doi: 10.1891/0889-8391.24.4.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudenkauf LM, Antoni MH, Stagl JM, Lechner SC, Jutagir DR, Bouchard LC, … Carver CS (2015). Brief cognitive-behavioral and relaxation training interventions for breast cancer: A randomized controlled trial. J Consult Clin Psychol, 83(4), 677–688. doi: 10.1037/ccp0000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiney SP, Parker PD, Felder TM, Adams SA, Omofuma OO, & Hulett JM (2019). A systematic review of interventions to improve adherence to endocrine therapy. Breast Cancer Res Treat, 173(3), 499–510. doi: 10.1007/s10549-018-5012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisig SR, Shedden-Mora MC, von Blanckenburg P, Schuricht F, Rief W, Albert US, & Nestoriuc Y (2015). Informing women with breast cancer about endocrine therapy: effects on knowledge and adherence. Psychooncology, 24(2), 130–137. doi: 10.1002/pon.3611 [DOI] [PubMed] [Google Scholar]
- Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, … Neugut AI (2011). Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat, 126(2), 529–537. doi: 10.1007/s10549-010-1132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MS, & Mann E (2010). A cognitive model of menopausal hot flushes and night sweats. J Psychosom Res, 69(5), 491–501. doi: 10.1016/j.jpsychores.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Jabson JM, & Kamen CS (2016). Sexual minority cancer survivors’ satisfaction with care. J Psychosoc Oncol, 34(1–2), 28–38. doi: 10.1080/07347332.2015.1118717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Arriola KR, Mason TA, Bannon KA, Holmes C, Powell CL, Horne K, & O’Regan R (2014). Modifiable risk factors for adherence to adjuvant endocrine therapy among breast cancer patients. Patient Educ Couns, 95(1), 98–103. doi: 10.1016/j.pec.2013.12.019 [DOI] [PubMed] [Google Scholar]
- Jacobs JM, Walsh EA, Park ER, Peppercorn J, Partridge A, Safren SA, … Greer JA (2019). Incorporating the Patient’s Voice in an Intervention to Promote Adherence to Adjuvant Endocrine Therapy for Breast Cancer Survivors. Paper presented at the American Psychosocial Oncology Society, Atlanta, GA. [Google Scholar]
- Jacobson E (1938). Progressive relaxation: a physiological and clinical investigation of muscular states and their significance in psychology and medical practice. Chicago, Ill.,: The University of Chicago press. [Google Scholar]
- Kabat-Zinn J, & University of Massachusetts Medical Center/Worcester. Stress Reduction Clinic. (1990). Full catastrophe living : using the wisdom of your body and mind to face stress, pain, and illness. New York, N.Y.: Delacorte Press. [Google Scholar]
- Kim J, & Park HA (2012). Development of a health information technology acceptance model using consumers’ health behavior intention. J Med Internet Res, 14(5), e133. doi: 10.2196/jmir.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk AM, Hanewald GJ, Schagen S, & Gijsbers van Wijk CM (2003). A symptom perception approach to common physical symptoms. Soc Sci Med, 57(12), 2343–2354. doi: 10.1016/s0277-9536(02)00451-3 [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Hershman DL, Gomez SL, Adams SR, Eldridge EH, Kwan ML, … Kushi LH (2018). Personal and clinical social support and adherence to adjuvant endocrine therapy among hormone receptor-positive breast cancer patients in an integrated health care system. Breast Cancer Res Treat, 170(3), 623–631. doi: 10.1007/s10549-018-4774-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyken W, Watkins E, Holden E, White K, Taylor RS, Byford S, … Dalgleish T (2010). How does mindfulness-based cognitive therapy work? Behav Res Ther, 48(11), 1105–1112. doi: 10.1016/j.brat.2010.08.003 [DOI] [PubMed] [Google Scholar]
- Lambert LK, Balneaves LG, Howard AF, & Gotay CC (2018). Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat, 167(3), 615–633. doi: 10.1007/s10549-017-4561-5 [DOI] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. New York: Springer. [Google Scholar]
- Luebbert K, Dahme B, & Hasenbring M (2001). The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology, 10(6), 490–502. doi: 10.1002/pon.537 [DOI] [PubMed] [Google Scholar]
- Makubate B, Donnan PT, Dewar JA, Thompson AM, & McCowan C (2013). Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer, 108(7), 1515–1524. doi: 10.1038/bjc.2013.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E, Smith MJ, Hellier J, Balabanovic JA, Hamed H, Grunfeld EA, & Hunter MS (2012). Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol, 13(3), 309–318. doi: 10.1016/S1470-2045(11)70364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markkula A, Hietala M, Henningson M, Ingvar C, Rose C, & Jernström H (2012). Clinical Profiles Predict Early Nonadherence to Adjuvant Endocrine Treatment in a Prospective Breast Cancer Cohort. Cancer Prevention Research. doi: 10.1158/1940-6207.capr-11-0442 [DOI] [PubMed] [Google Scholar]
- McCowan C, Shearer J, Donnan PT, Dewar JA, Crilly M, Thompson AM, & Fahey TP (2008). Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer, 99(11), 1763–1768. doi: 10.1038/sj.bjc.6604758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishel MH (1984). Perceived uncertainty and stress in illness. Res Nurs Health, 7(3), 163–171. doi: 10.1002/nur.4770070304 [DOI] [PubMed] [Google Scholar]
- Moon Z, Moss-Morris R, Hunter MS, Carlisle S, & Hughes LD (2017). Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Prefer Adherence, 11, 305–322. doi: 10.2147/PPA.S126651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S (2010). Nonadherence in patients with breast cancer receiving oral therapies. Clin J Oncol Nurs, 14(1), 41–47. doi: 10.1188/10.CJON.41-47 [DOI] [PubMed] [Google Scholar]
- Moorey S, & Greer S (2002). Cognitive behaviour therapy for people with cancer. Oxford: Oxford University Press. [Google Scholar]
- Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, & Vernon SW (2012). Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast cancer research and treatment, 134(2), 459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MD, Morrow DG, Weiner M, Clark DO, Tu W, Deer MM, … Weinberger M (2004). A conceptual framework to study medication adherence in older adults. Am J Geriatr Pharmacother, 2(1), 36–43. [DOI] [PubMed] [Google Scholar]
- Nezu AM, Nezu CM, Friedman SH, Hours ES, & Faddis S (1997). Project Genesis: Application of problem-solving therapy to individuals with cancer. The Behavior Therapist, 9(15). [Google Scholar]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, & Carbone PP (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol, 5(6), 649–655. [PubMed] [Google Scholar]
- Onken LS, Carroll KM, Shoham V, Cuthbert BN, & Riddle M (2014). Reenvisioning Clinical Science: Unifying the Discipline to Improve the Public Health. Clin Psychol Sci, 2(1), 22–34. doi: 10.1177/2167702613497932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JD (2007). Managing chronic pain : a cognitive-behavioral therapy approach. Therapist guide. Oxford; New York: Oxford University Press. [Google Scholar]
- Richardson LK, Frueh BC, Grubaugh AL, Egede L, & Elhai JD (2009). Current directions in videoconferencing tele-mental health research. Clin Psychol (New York), 16(3), 323–338. doi: 10.1111/j.1468-2850.2009.01170.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnick S, & Miller WR (1995). What is motivational interviewing? Behavioural and cognitive Psychotherapy, 23(4), 325–334. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM, & Onken LS (2001). A Stage Model of Behavioral Therapies Research: Getting Started and Moving on From Stage I. Clinical Psychology: Science and Practice, 8(2), 133–142. doi: 10.1093/clipsy.8.2.133 [DOI] [Google Scholar]
- Sabate E, & De Geest S (2004). Adherence to long-term therapies management: a call for cardiovascular nursing managers and policymakers. Prog Cardiovasc Nurs, 19(1), 28–29. [DOI] [PubMed] [Google Scholar]
- Safren S, Gonzalez J, & Soroudi N (2007). Coping with chronic illness: a cognitive-behavioral approach for adherence and depression client workbook. New York: Oxford University Press. [Google Scholar]
- Safren SA, Bedoya CA, O’Cleirigh C, Biello KB, Pinkston MM, Stein MD, … Mayer KH (2016). Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV, 3(11), e529–e538. doi: 10.1016/S2352-3018(16)30053-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Hendriksen ES, Mayer KH, Mimiaga MJ, Pickard R, & Otto MW (2004). Cognitive-Behavioral Therapy for HIV Medication Adherence and Depression. Cognitive and Behavioral Practice, 11(4), 415–424. [Google Scholar]
- Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, & Pollack MH (2012). Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J Consult Clin Psychol, 80(3), 404–415. doi: 10.1037/a0028208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Otto MW, & Worth JL (1999). Life-Steps: applying cognitive behavioral therapy to HIV medication adherence. Cogn Behav Pract(6), 332–341. [Google Scholar]
- Saha P, Regan MM, Pagani O, Francis PA, Walley BA, Ribi K, … International Breast Cancer Study, G. (2017). Treatment Efficacy, Adherence, and Quality of Life Among Women Younger Than 35 Years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. J Clin Oncol, 35(27), 3113–3122. doi: 10.1200/JCO.2016.72.0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Latreille J, Matte C, Desjardins P, & Bergeron E (2014). Adherence to adjuvant endocrine therapy in estrogen receptor-positive breast cancer patients with regular follow-up. Can J Surg, 57(1), 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JC, Reeve BB, Troester MA, & Wheeler SB (2020). Factors Associated with Endocrine Therapy Non-Adherence in Breast Cancer Survivors. Psychooncology, 29(4), 647–654. doi: 10.1002/pon.5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagl JM, Antoni MH, Lechner SC, Bouchard LC, Blomberg BB, Glück S, … Carver CS (2015). Randomized controlled trial of cognitive behavioral stress management in breast cancer: a brief report of effects on 5-year depressive symptoms. Health Psychol, 34(2), 176–180. doi: 10.1037/hea0000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagl JM, Bouchard LC, Lechner SC, Blomberg BB, Gudenkauf LM, Jutagir DR, … Antoni MH (2015). Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer: 11-year follow-up of a randomized controlled trial. Cancer, 121(11), 1873–1881. doi: 10.1002/cncr.29076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagl JM, Lechner SC, Carver CS, Bouchard LC, Gudenkauf LM, Jutagir DR, … Antoni MH (2015). A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up. Breast Cancer Res Treat, 154(2), 319–328. doi: 10.1007/s10549-015-3626-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL (2012). What happens now? Psychosocial care for cancer survivors after medical treatment completion. J Clin Oncol, 30(11), 1215–1220. doi: 10.1200/JCO.2011.39.7406 [DOI] [PubMed] [Google Scholar]
- Stanton AL, & Bower JE (2015). Psychological adjustment in breast cancer survivors. In Ganz PA (Ed.), Improving outcomes for breast cancer survivors: perspective on research challenges and opportunities. Switzerland: Springer International Publishing. [Google Scholar]
- Stanton AL, Petrie KJ, & Partridge AH (2014). Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat, 145(2), 525–534. doi: 10.1007/s10549-014-2961-3 [DOI] [PubMed] [Google Scholar]
- Tatrow K, & Montgomery GH (2006). Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med, 29(1), 17–27. doi: 10.1007/s10865-005-9036-1 [DOI] [PubMed] [Google Scholar]
- Wassermann J, Gelber SI, Rosenberg SM, Ruddy KJ, Tamimi RM, Schapira L, … Partridge AH (2019). Nonadherent behaviors among young women on adjuvant endocrine therapy for breast cancer. Cancer. doi: 10.1002/cncr.32192 [DOI] [PubMed] [Google Scholar]
- Williams JMG (2007). The mindful way through depression : freeing yourself from chronic unhappiness. New York: Guilford Press. [Google Scholar]
- Wu C, Zheng Y, Duan Y, Lai X, Cui S, Xu N, … Lu L (2019). Nonpharmacological Interventions for Cancer-Related Fatigue: A Systematic Review and Bayesian Network Meta-Analysis. Worldviews Evid Based Nurs, 16(2), 102–110. doi: 10.1111/wvn.12352 [DOI] [PubMed] [Google Scholar]
- Yood MU, Owusu C, Buist DS, Geiger AM, Field TS, Thwin SS, … Silliman RA (2008). Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg, 206(1), 66–75. doi: 10.1016/j.jamcollsurg.2007.07.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


