Abstract
Aim:
To assess the role of momentary pain on opioid craving and illicit opioid use among individuals receiving opioid agonist treatment.
Design:
Observational study using ecological momentary assessment.
Setting:
The National Institute of Drug Abuse’s Intramural Research Program in the USA.
Participants:
Fifty-six adults who qualified for opioid agonist treatment.
Measurements:
Participants completed randomly prompted assessments of pain severity, stress, negative mood, opioid craving, and illicit opioid use for a mean of 66 days (SD=27). Urine samples were collected 2–3x/week throughout.
Findings:
Close to 70% of participants reported moderate average pain severity in the past 24 hours at intake and 35% of participants reported chronic pain. There were no significant differences in percent of opioid-positive urine samples [F(1,54)=.13, p= .73] and average level of opioid craving during the study period [F(1,54)=.01, p= .91] among opioid agonist treatment only patients vs. opioid agonist treatment patients with chronic pain. However, momentary pain severity significantly predicted concurrent opioid craving (B=.02, 95% CI: .01, .03), over and above stress and negative mood. Momentary opioid craving, in turn, significantly predicted illicit opioid use that was assessed in the next moment (OR=1.88, 95% CI: .70, 5.04), while controlling for autocorrelation and the effects of pain, negative mood, and stress. Momentary opioid craving significantly mediated the prospective association between momentary pain and illicit opioid use (95% CI= .013, .177). Exploratory analysis revealed that momentary pain severity also significantly moderated the momentary association between stress and opioid craving (B=.02, 95% CI: .00, .05), such that when momentary pain severity increased, the association between the two intensified.
Conclusions:
Among people receiving opioid agonist treatment, momentary pain appears to be indirectly associated with illicit opioid use via momentary opioid craving.
Keywords: opioid agonist treatment, opioid use disorder, pain, craving, stress, negative mood
Introduction
Opioid-use disorder (OUD) is an important public health concern. In 2017, it was estimated that 2.1 million individuals in the United States met the diagnostic criteria for OUD related to prescription opioids, and about 626,000 individuals met the criteria for OUD related to heroin use (1). Both methadone and buprenorphine maintenance are effective opioid-agonist treatments (OAT) for OUD. However, it is common for patients to experience lapses during OAT, which can increase risk for progression to relapse (2–6). This underscores the importance of identifying and remediating risk factors for illicit opioid use during OAT.
One likely risk factor that generally has not been a primary target of comprehensive OAT programs is the experience of pain. Previous studies report that the vast majority of individuals in OAT reported notable pain experiences in the past week (7,8). In fact, not only do chronic pain and OUD commonly co-occur (ranging 37% to 62%) (9,10), but also, compared to patients with primary OUD, patients with co-occurring OUD and chronic pain show significantly worse psychiatric symptom profiles, greater functional impairment, and greater likelihood of using illicit substances during OAT (11,12). Some studies also show that OAT patients with co-occurring chronic pain are more likely to use illicit opioids during OAT compared to those without (11,13,14), although some other studies suggest no significant differences (15–18).
Opioid craving (i.e., the urge to experience the effects of opioids) may help elucidate the potential link between pain and illicit opioid use in OAT (19). First, opioid craving is a key symptom of OUD that predicts problematic opioid use (17,20). Although an OAT can reduce craving (21,22), it is common for OAT patients to continuously experience craving (6), which is a significant risk factor for both lapse and relapse during OAT (20,23). Second, recent studies suggest the possibility that pain can elevate opioid craving. For instance, Tsui and colleagues (17) found that OAT patients with co-occurring chronic pain exhibited higher odds of reporting opioid craving than those without chronic pain. However, to our knowledge, none of the previous studies have investigated the association between momentary pain and opioid craving in the context of OAT, over and above the effects of stress and negative mood, which are robust predictors of opioid craving (24–29). It is also an open question whether opioid craving serves as a mediator of the prospective association between pain and illicit opioid use during OAT.
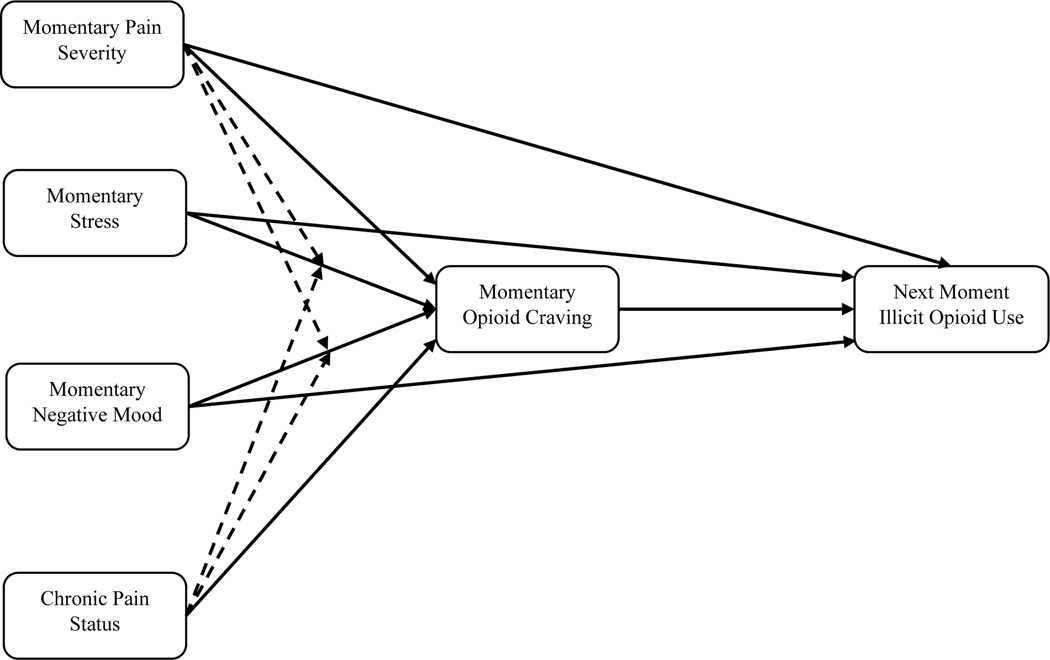
In the present study, we used ecological momentary assessment (EMA) – a data-collection approach that captures experience in real time and in real-world settings – to examine nuanced associations among pain, opioid craving, and illicit opioid use among OAT patients. First, we tested whether OUD patients with chronic pain are more likely to report opioid craving and use illicit opioids compared to those without chronic pain. Second, we examined whether opioid craving would mediate the prospective association between momentary pain severity and illicit opioid use, while controlling for the effects of momentary stress and negative mood. Third, because pain can modulate self-regulatory functions and can augment the deleterious effects of stress and negative mood (30), as an exploratory aim, we tested if momentary pain and chronic pain status would serve as moderators in the association between momentary stress/mood and opioid craving. Figure 1 summarizes the hypothetical model we tested.
Figure 1.
Visual illustration of hypothesized model. Note. Dotted lines indicate exploratory moderation effects.
Methods
Overview
This study is a secondary data analysis of a larger parent project conducted at the National Institute of Drug Abuse’s Intramural Research Program (NIDA IRP) in Baltimore, MD, USA. Hence, the primary research questions and analytical plan were not pre-registered on a publicly available platform. Consequently, the results should be considered exploratory.
Participants were 1) seeking a treatment for addiction and enrolled into an office-based outpatient treatment program at the NIDA IRP outpatient treatment research clinic called Archway, or 2) already receiving addiction treatment elsewhere in the community. In the treatment elsewhere setting, community clinics provided either methadone or buprenorphine/naloxone maintenance treatment. All the study procedures were approved by the NIDA IRB. Prior to study enrollment, all participants signed informed consent.
Participants
Participants were 56 adults who qualified for OAT to treat OUD. Fliers at local outpatient treatment facilities and newspaper advertisements were used for recruitment. Participants in the office-based outpatient treatment cohort had to meet the following inclusion criteria: 1) age between 18 and 75; 2) physical dependence on opioids and/or frank opioid withdrawal. Participants in the TE cohort had to provide documentation that they were currently receiving either methadone or buprenorphine treatment for OUD at an OAT program in the community. The treatment elsewhere participants provided consent for NIDA research staff to confirm their enrollment at other clinics.
Participants in the office-based outpatient treatment cohort were excluded if they presented with: 1) a history DSM-5 diagnosis of psychotic disorder or bipolar disorder, or ongoing Major Depressive Disorder; 2) current alcohol-use disorder or sedative-hypnotic-use disorder; 3) cognitive impairment that would preclude informed consent or valid self-report; 4) any condition that would interfere with urine collection; or 5) current medical illness (e.g., cirrhosis, nephrotic syndrome, etc.) or use of medications (e.g., glucocorticoids, etc.) that could complicate medical management or compromise participation in research. In the case of treatment elsewhere cohort, candidates were subject only to exclusion criteria 1, 3, and 4; criteria 2 and 5 were for the safe administration of OAT within the NIDA clinic.
Procedures
Participants in the office-based outpatient treatment cohort were enrolled in 30 weeks of office-based buprenorphine treatment at the NIDA Archway Clinic, with twice-weekly urine and breath samples to verify self-reported substance use. EMA was conducted for maximum 15 weeks (from weeks 3 to 18). Additional office-based outpatient treatment details can be found in the Online Supplemental Materials (pp. 1). Participants in the treatment elsewhere cohort, who were already receiving either methadone or buprenorphine treatment at a local clinic, were enrolled in our monitoring study for a maximum of 8 weeks. They visited the NIDA IRP three days per week, during which urine and breath samples and self-reported substance use were collected as described for the office-based outpatient treatment cohort. Methadone or buprenorphine dose was self-reported at the first visit.
Participants’ chronic-pain status was assessed with two items: “In the past 3 months, have you experienced any pain other than pain from opiate withdrawal?” and “Is this pain constant or does it flare up frequently?” Participants who answered yes to both items were considered to have chronic pain.
Each participant was provided with a smartphone for EMA data collection. Before being issued the smartphone, each participant had an instructional session with study staff in which the app and the items were explained. Experimenter-configurable software was used to program the smartphones to deliver fixed and random prompts and to log participant-initiated event-contingent reports of drug use. The items in the EMA assessments covered a broader range of topics (sleep, pleasurable events, locations, etc.) than we use in the current analyses. We focus here on pain, stress, negative mood, opioid craving, and illicit opioid use items that were assessed in randomly prompted entries. Note that participants received approximately 28 random and end-of-day summary prompts per week and were required to respond to at least 82% of them (that is, at least 23) within 15 minutes as a condition of continued participation. Details on procedures for attaining good EMA compliance are available in the online supplement material (pp. 2).
Measures
EMA measures
Pain Severity:
Participants were asked to rate their level of pain that is not related to opioid withdrawal in the present moment using the Numerical Rating Scale (NRS; 24) which ranges from 0 (no pain) to 10 (pain as bad as it can be).
Stress:
Participants were asked to rate the degree to which they were “feeling stress right now” on a five-point numerical scale ranging from 1 (None) to 5 (An Extreme Amount), as in prior published study from our group (32).
Negative Mood:
Negative mood was assessed with 12 adjectives (i.e., fatigued, worn out, afraid, annoyed, angry, hopeless, on edge, sad, discouraged, resentful, exhausted, and uneasy). These items were summed into a negative-mood score on the basis of a factor analyses we have described previously (32). Participants were asked to rate the intensity with which they felt each mood “just before the phone beeped,” using a scale ranging from 1 (Not at All) to 5 (Extremely).
Opioid Craving:
Participants were asked to rate the degree to which they were feeling craving for heroin or other opioids (Percocet, oxycodone, etc.) in the past 5 minutes on a five-point numerical scale ranging from 1 (Not at All) to 5 (Extremely), as in prior published study from our group (32).
Illicit opioid use:
Participants were asked to indicate whether they had used heroin, other opiates (e.g., Percocet, oxycodone, etc.), or street methadone/buprenorphine since they arrived at their present location on a binary (yes/no) question.1 If a participant endorsed any of these, we coded the entry as one of illicit opioid use.
Baseline and other person-level measures
At baseline, we assessed: 1) baseline pain severity (from Brief Pain Inventory; (33)), 2) pain interference [from Brief Pain Inventory (BPI); (33)], and 3) depressive symptoms [from the Center for Epidemiological Studies Depression (CES-D) scale; (34)] based upon well-validated measures. Cronbach’s alphas were .92, .96, and .84, respectively, indicating adequate internal consistency.
We also calculated each participant’s percentage of opioid-positive urine samples (i.e., percent of urines positive for opioids other than the one prescribed as OAT) during the study period, as well as the average opioid craving level across the EMA period. On average, participants provided 30 days (SD = 12.3) of urine samples.
Data Analytic Strategy
Prior to conducting the main analyses, we compared baseline and person-level measures for participants who did or did not meet criteria for chronic pain. We then calculated descriptive statistics of EMA variables. Note that we elected to include those without chronic pain in the main statistical analysis due to two main reasons. First, a large proportion of OAT patients who do not meet the criteria for chronic pain report varying degrees of pain experiences (7,8), and therefore, excluding them would limit generalizability and implications of current findings. Second, it was also necessary to include those without chronic pain, because testing a potential moderating effect of chronic pain status was one of our study aims.
We used a three-level multilevel modeling approach to address the nested nature of the data. When using illicit opioid use as an outcome, we used a multilevel logistic regression. All of the level-1 (moment-level) predictor variables were day-mean centered (i.e., for each participant, we subtracted the day’s mean from the random-prompt rating). Multilevel models included random intercepts (both at level-2 and level-3), along with a set of fixed effects (i.e., momentary pain, negative mood, and stress for opioid craving as an outcome model; and opioid craving, pain, negative mood, stress, and previous moment illicit opioid use for illicit opioid use as an outcome model). Random slopes at both level-2 (day-level) and level-3 (person-level) were included for pain in the craving as an outcome model. Random slopes at both level-2 (day-level) and level-3 (person-level) were included for craving in the illicit opioid use as an outcome model. A first-order autocorrelation [AR(1)] covariance structure was used to account for the autocorrelation among level-1 residuals due to the narrow time interval between adjacent random-prompt assessments. These analyses were conducted by SPSS Version 26 using the MIXED and GENLINMIXED (for the multilevel logistic regression model) commands. See the online supplement (pp. 3–5) for a detailed description of each model’s parameters.
To test mediation, we used Rmediation (35), which provides higher statistical power and better control of Type I error rates than traditional mediation analyses (i.e., the Sobel test). See the online supplement (pp. 6) for details on Rmediation.
Effect sizes were calculated by computing the pseudo-R2 (i.e., proportion of the variance reduction). However, this method is not applicable to the multilevel logistic regression because it does not provide level-1 residuals. Thus, instead, for the logistic regression, we reported odds ratios for each predictor.
Power analysis
We have conducted a multilevel model power analysis based upon the MLPowSim program (36) using 1,000 simulation data sets with α = .05. The simulation suggested that one can reach sufficient statistical power (> 80%) to detect a small level-1 (moment-level) effect when the level-3 cluster size (i.e., total sample size) is 20, level-2 cluster size is 17 (i.e., the number of days of EMA assessment), and level-1 cluster size is 3 (i.e., the number of random prompts per day). Hence, the present study, which had a sample size of 56 with an average level-2 cluster size of 66 and an average level-1 cluster size of 3, was adequately powered to detect small moment-level effects.
Results
On average random prompts were sent to participants 2.8 times per day (SD = .61). Approximately there were about 4.7-hour (SD = 1.75) difference between random prompts. Participants completed 10,326 out of 10,444 (98.9%) random prompt assessments possible across the study period. Participants in the office-based outpatient treatment group completed a mean of 299.93 random prompts during the 15-week assessment period (SD=41.29), and those in the treatment elsewhere group completed a mean of 142.12 random prompts during the 8-week assessment period (SD=37.35). We used all available random prompt data for both treatment groups rather than trying to match the length of the data because a greater number of assessments can significantly increase the precision of mean estimates (37).
Sample characteristics
Table 1 summarizes characteristics of the present sample. Approximately 35% (n = 20) of our participants had chronic pain at baseline. The most prevalent pain site (measured by the Brief Pain Inventory [BPI]) was lower back (39.3%). See the Online Supplement Table 1 (pp. 7) for details on the frequency of pain sites. It should also be noted that 50% (n = 28) of participants reported experiencing at least moderate pain (at least 4 out of 10 from the BPI average pain severity item) during the past 24 hours. This further supports our rationale for including the full sample (both individuals with and without chronic pain) in the main analyses. Except for pain severity and interference, those with only OUD vs. those with OUD and chronic pain did not differ significantly on baseline characteristics. In addition, there were no differences in percentage of opioid-positive urine samples during the study period and average level of opioid craving across the EMA period.
Table 1.
Sample Characteristics
| No Chronic Pain (N=36) | Chronic Pain (N=20) | p | |
|---|---|---|---|
| Age* | 47.92 (9.97) | 50.40 (8.12) | .35 |
| Sex (% Male)* | 72.2 | 80.0 | .52 |
| Race* | |||
| % African American | 58.3 | 40.0 | .10 |
| % Caucasian | 33.3 | 60.0 | |
| % Other | 8.3 | 0 | |
| Education* | |||
| % Some HS | 11.1 | 10.0 | .13 |
| % HS degree or GED | 72.2 | 50.0 | |
| % Some College | 16.7 | 30.0 | |
| % College Grad | 0 | 10.0 | |
| Methadone Treatment (%)* | 52.8 | 35.0 | .20 |
| Positive Urine (%)** | 40.13 (37.32) | 36.39 (39.05) | .73 |
| Opioid Craving (EMA mean score)** | 1.32 (.48) | 1.31 (.43) | .91 |
| CES-D Total Score* | 13.00 (8.56) | 12.75 (6.21) | .91 |
| BPI Pain Severity* | 1.82 (1.98) | 4.64 (2.16) | < .001 |
| BPI Pain Interference* | 2.04 (2.54) | 5.09 (2.81) | < .001 |
Note. HS = High School.
at enrollment
during the study
Descriptive statistics for momentary assessments
As shown in Table 2, participants on average reported quite low levels of momentary pain severity, stress, and opioid craving. Illicit opioid use was reported on 5.2% of random prompts. ICCs ranged from .19 to .62 at level-3 (between-person level) and ranged from .14 to .22 at level-2 (day-level), indicating there were substantial moment-level variations among EMA variables. All EMA variables were significantly correlated at the moment level, but the overall strength of correlations was small.
Table 2.
Descriptive statistics and bivariate correlations of study variables
| Variables | Pain Severity | Stress | Negative Mood | Opioid Craving | Illicit Opioid Use |
|---|---|---|---|---|---|
| 1. Pain Severity | – | .05** | .04* | .04** | .03* |
| 2. Stress | – | .35** | .07** | .04** | |
| 3. Negative Mood | – | .06** | .05** | ||
| 4. Opioid Craving | – | .06** | |||
| 5. Illicit Opioid Use | – | ||||
| Mean | 1.13 | 1.56 | 1.21 | 1.34 | 0.05 |
| SD | 2.39 | 0.91 | .37 | 0.72 | 0.22 |
| Observed Range | 0–10 | 1–5 | 1–4.75 | 1–5 | 0–1 |
| ICClevel-3 | .62 | .41 | .52 | .44 | .19 |
| ICClevel-2 | .19 | .14 | .22 | .15 | .15 |
Note. All the level-1 variables were day-mean centered so that the bivariate correlations indicate pure level-1 associations.
p < .05
p < .01
Momentary pain predicts concurrent opioid craving
The summary of the opioid craving outcome model is presented in Table 3. Momentary pain severity significantly predicted concurrent opioid craving while controlling for the effects of momentary stress and negative mood. These findings indicate that when a participant experienced higher-than-usual pain severity, he or she reported greater opioid craving over and above the effects of momentary stress and negative mood (each of which was also a significant predictor of concurrent opioid craving). The momentary pain predictor reduced moment-level (level-1) variance by 2.7% from a null model that does not include any predictors. Inclusion of both momentary stress and negative mood in addition to pain reduced a total of 5.5% moment-level variance in opioid craving.
Table 3.
Multilevel model fixed parameter estimates for concurrent opioid cravingt outcome
| Parameter | B | SE | 95% CI | t | p |
|---|---|---|---|---|---|
| Intercept | 1.31 | .06 | [1.19, 1.43] | 21.57 | <.001 |
| Momentary Pain Severityt | .02 | .01 | [.01, .04] | 2.80 | .011 |
| Momentary Stresst | .06 | .01 | [.05, .08] | 7.03 | <.001 |
| Momentary Moodt | .18 | .03 | [.13, 24] | 7.05 | <.001 |
Momentary opioid craving predicts illicit opioid use measured in the next moment
The summary of the illicit opioid use outcome model is presented in Table 4. When a participant had reported use of an illicit opioid in the previous randomly prompted entry, he or she was far less likely to report it in the next entry (OR = .01). Momentary opioid craving was significantly associated with illicit opioid use assessed in the next randomly prompted entry (OR = 1.72); for a one-unit increase in the score for momentary craving, there was a 72% increase in the odds of reported illicit opioid use at the next assessment, controlling for all other covariates. Momentary stress, negative mood, and pain severity were not significantly associated with illicit opioid use measured in the next assessment, controlling for opioid craving and previous use.
Table 4.
Multilevel logistic regression model fixed parameter estimates for lead illicit opioid uset+1 outcome
| Parameter | B | SE | OR | 95% CI | t | p |
|---|---|---|---|---|---|---|
| Intercept | −3.88 | .31 | .02 | [.01, .04] | −12.74 | <.001 |
| Illicit Opioid Uset | −5.30 | 1.00 | .01 | [.001, .04] | −5.30 | <.001 |
| Momentary Opioid Craving t | .54 | .22 | 1.72 | [1.12, 2.64] | 2.46 | .014 |
| Momentary Pain Severityt | −.10 | .05 | .90 | [.82, 1.00] | −1.94 | .053 |
| Momentary Stresst | .07 | .18 | 1.07 | [.76, 1.51] | .41 | .682 |
| Momentary Moodt | .003 | .43 | 1.00 | [.43, 2.33] | .01 | .995 |
| Time lapse | −.03 | .02 | .98 | [.95, 1.01] | −1.61 | .108 |
Rmediation revealed that there was a significant indirect effect of momentary pain severity on illicit opioid use via opioid craving (point estimate = .015 [95% CI = .003, .032]).
Exploratory moderating effects of momentary pain severity and chronic-pain status
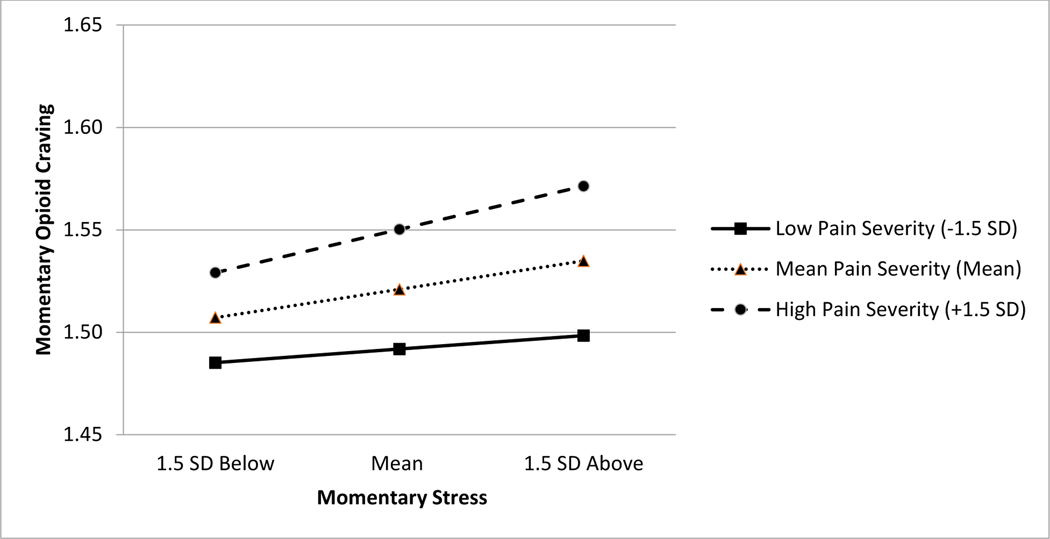
As presented in Table 5, the concurrent momentary association between stress and opioid craving was significantly moderated by momentary pain severity, but not by the person-level predictor chronic-pain status. As shown in Figure 2, simple slope analysis indicated a significant positive association between momentary stress and opioid craving when the level of momentary pain severity was 1.5 SD above the day mean (B=.08, SE=.03, p=.01, 95% CI = .02, .15). However, this association became smaller and not statistically significant when momentary pain severity was at its day mean (B=.05, SE=.03, p=.07, 95% CI = −.01, .11) and 1.5 SD below the mean (B=.03, SE=.03, p=.44, 95% CI = −.04, .09). The concurrent momentary association between negative mood and opioid craving was not significantly moderated by either momentary pain severity or chronic-pain status.
Table 5.
Multilevel model fixed parameter estimates for concurrent opioid cravingt outcome with interaction terms
| Parameter | B | SE | 95% CI | t | p |
|---|---|---|---|---|---|
| Intercept | 1.52 | .12 | [1.29, 1.76] | 12.89 | <.001 |
| Momentary Paint | .02 | .01 | [.01, .03] | 4.88 | <.001 |
| Momentary Stresst | .05 | .03 | [−.01, .11] | 1.83 | .074 |
| Momentary Negative Moodt | .16 | .06 | [.04, .27] | 2.72 | .011 |
| Chronic Pain Status (Chronic Pain) | −.01 | .13 | [−.26, .24] | −.09 | .931 |
| Sex (Female) | −.20 | .13 | [−.47, .07] | −1.48 | .144 |
| Study Cohort (TE) | −.21 | .13 | [−.48, .05] | −1.64 | .106 |
| Momentary Stresst × Momentary Pain Severityt | .02 | .01 | [.00, .04] | 1.97 | .049 |
| Momentary Stresst × Chronic Pain Status | .04 | .05 | [−.06, .13] | .76 | .451 |
| Momentary Negative Moodt × Momentary Pain Severityt | −.01 | .02 | [−.05, .04] | −.44 | .663 |
| Momentary Negative Moodt × Chronic Pain Status | .02 | .09 | [−.16, .21] | .22 | .825 |
Note. TE = treatment elsewhere in the community.
Figure 2.
Slopes and intercepts portraying the effects of momentary pain severity (−1.5 SD, mean, 1.5 SD) on the momentary relations between day-mean centered stress and illicit opioid craving. Note. The original scale of the y-axis ranges from 1 to 5. A significant positive association between momentary stress and opioid craving when the level of momentary pain severity was 1.5 SD above the day mean (B = .08, SE = .03, p = .014, 95% CI = .02, .15). The association between momentary stress and opioid craving was marginally significant at its day mean (B = .05, SE = .03, p = .074, 95% CI = −.01, .11). The association between momentary stress and opioid craving was not statistically significant when momentary pain severity was 1.5 SD below the mean (B = .03, SE = .03, p = .44, 95% CI = −.04, .09).
Findings of post-hoc analyses
Post-hoc analyses were conducted to test the robustness of the present findings. These are reported in the online supplementary material (pp. 7).
Discussion
To our knowledge, this is the first study to examine the mediating role of opioid craving in the prospective association between momentary pain severity and illicit opioid use among OAT patients. As hypothesized, momentary pain severity was significantly associated with greater opioid craving, which in turn, significantly predicted illicit opioid use assessed in the next moment. These effects were robust to controls for negative affect and stress. We also found that the momentary association between stress and opioid craving was magnified by greater momentary pain severity, but not by the person-level predictor chronic-pain status.
The role of stress and negative mood on opioid craving has been explicated in several studies of OAT patients (24,26,29). Our findings suggest pain, too, may be an important risk factor for opioid craving. In fact, this finding is consistent with a relevant recent finding: higher daily pain severity was associated with greater opioid craving among chronic-pain patients without OUD, even after controlling for negative mood (38). Also consistent with the findings of Martel et al. (38), the effect of momentary pain on opioid craving was small. Although observing small effects at the within-person level using intensive longitudinal data is quite common (39–41), whether these small moment-to-moment effects can accumulate over time and pose a clinically meaningful risk for opioid craving among OAT patients is yet to be determined.
Our exploratory analysis showed that momentary pain severity also significantly moderated the association between stress and opioid craving, such that when pain severity was elevated, the momentary association between stress and opioid craving was amplified. Our study did not probe the specific mechanisms of these associations. However, prior literature suggests a possibility: pain may interfere with cognitive inhibitory capacity and/or reward processing (42). For example, healthy adults experiencing acute pain showed increased risk-seeking responses and greater preference for immediate monetary rewards (43). Furthermore, in people with alcohol-use disorder, higher pain severity was associated with greater levels of both subjectively and objectively measured impulsivity (44). Future studies may want to determine whether links between stress, pain, and opioid craving are modulated via alterations in cognitive inhibition and/or reward processing.
As we anticipated, opioid craving appeared to be the path through which momentary pain severity led to greater likelihood of illicit opioid use. This result is in accord with findings that craving is a robust predictor of illicit opioid lapse among OAT patients (20,23). Although OAT generally reduces opioid craving (21), some patients continue to crave (6). Thus, it is important to provide additional resources for OAT patients to effectively cope with craving. Mindfulness-based interventions appear to be particularly promising in that regard, as they are effective in reducing craving, as well as pain, negative mood, and stress (45,46). For instance, Mindfulness-Based Relapse Prevention (MBRP) has been demonstrated to be effective in reducing craving and substance use in people with substance use disorders (47–49). Another mindfulness-based intervention focused on positive-emotion enhancement—Mindfulness-Oriented Recovery Enhancement (MORE)—has recently been found to reduce both craving and pain in patients on methadone maintenance (50).
Our finding that momentary pain indirectly predicted illicit opioid use specifically centered on dynamic changes in pain: our OAT patients with chronic pain were not more likely overall to use illicit opioids than those without chronic pain. While the latter finding is consistent with a number of previous studies (15–17), it is also inconsistent with some other findings (11,13,14). The reason for these inconsistencies is not clear, but sample heterogeneity across studies is a plausible contributor. For example, our study lacked verifiable diagnostic information on chronic pain, rendering the variance associated with the “chronic pain status” variable largely unexplained. As studies begin to more narrowly define and assess comorbid chronic pain in OUD, findings may become more consistent. It should also be noted that although the present study was focused on examining the momentary associations between pain and illicit opioid use, in order to develop more effective treatments for individuals with co-morbid chronic pain and OUD, future studies should examine common risk factors that are associated with the development of this co-morbidity. For instance, early life stress is a robust prospective predictor of chronic pain development and substance use disorders (51,52), and a growing body of literature suggests that deficits in reward processing may be an important mediator of these relationships (53).
Our study has limitations that need to be addressed in future studies. First, the sample size was relatively small, particularly testing for cross-level interaction (i.e., exploratory chronic pain status moderation). However, EMA was conducted for an extended period of time (i.e., an average of 66 days and 190 random prompt assessments), making the moment-level findings reliable. Second, assessment of momentary stress and craving were based upon single items so as to minimize participants’ response burden. Although these measures were used in our previously published studies, they were not thoroughly validated. To our knowledge, there are no well-validated gold-standard measures of momentary stress and craving. Hence, in order to more accurately measure individuals’ momentary experience of stress and craving, future studies should comprehensively evaluate the psychometric properties of these brief EMA-specific measures of stress and craving in the context of an EMA study. Third, the sample of this study is from a single geographical region. Hence, our findings may not be generalizable to other OAT clinics in a different geographical region, which may be associated with different social factors that differentially impact use patterns. Fourth, potential measurement reactivity (i.e., the notion that EMA can elevate self-monitoring, which in turn influences the frequency of the behavior that is under investigation (54)) was not assessed. However, the present study did not reveal any significant changes in opioid craving and illicit opioid use patterns as a function of time. In addition, a recent randomized experimental study also demonstrated that daily monitoring of substance use had either no significant measurement reactivity or had some short-term reactivity (i.e., one week) depending on the substance use outcomes (55). Fifth, the present study did not measure diagnostic information on chronic pain. Having this information may have shed more light on our null findings on chronic pain status’ main effect. For instance, it is an open empirical question whether individuals with OUD who also have highly disabling chronic primary pain (e.g., fibromyalgia) are at greater risk for experiencing opioid craving and relapse than those with chronic pain with an identifiable pathology (e.g., osteoarthritis). Lastly, it is unknown in the present study whether participants were able to clearly distinguish between pain primarily caused by opioid withdrawal and exacerbation of underlying physical pain due to opioid withdrawal. Future studies should investigate if these different types of pain can modulate the effect of pain severity on craving and illicit opioid use among OAT patients.
In conclusion, we did not find strong evidence that OAT patients with chronic pain are at a greater risk for experiencing higher opioid craving or using more illicit opioids compared to those without chronic pain. However, we found preliminary evidence that momentary pain is indirectly associated with illicit opioid use via craving among OAT patients. Further, exploratory analyses revealed that momentary pain amplified the link between stress and opioid craving. Our findings suggest that opioid craving remains an important clinical target for improving OAT outcomes. It is possible, however, that OAT outcomes may be further improved by helping patients effectively cope with changes in dynamic pain experiences in addition to opioid craving.
Supplementary Material
Acknowledgments
Source of Financial Support for the Project
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, a postdoctoral training grant awarded to CJM (F32DA049393), a career development grant awarded to PHF (K23DA035915) and an R01 awarded to PHF (R01DA48206).
Footnotes
Conflicts of Interest Declaration: The authors have no conflicts of interest in conducting this research.
If participants endorsed “yes” to a previously asked item that assesses whether they were walking from one place to another, the participants were asked to indicate illicit opioid use in the past 5 minutes instead of since they arrived at their present location.
References
- 1.Center for Behavioral Health Statistics and Quality. Results from the 2017 National Survey on drug use and health: detailed tables. Prevalence estimates, standard errors, p values, and sample sizes. 2018; [Google Scholar]
- 2.Stein MD, Cioe P, Friedmann PD. Brief report: Buprenorphine retention in primary care. J Gen Intern Med. 2005;20(11):1038–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hser Y, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi‐site trial. Addiction. 2014;109(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JD, Grossman E, DiRocco D, Gourevitch MN. Home buprenorphine/naloxone induction in primary care. J Gen Intern Med. 2009;24(2):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11(5):641–53. [DOI] [PubMed] [Google Scholar]
- 6.Northrup TF, Stotts AL, Green C, Potter JS, Marino EN, Walker R, et al. Opioid withdrawal, craving, and use during and after outpatient buprenorphine stabilization and taper: a discrete survival and growth mixture model. Addict Behav. 2015;41:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mun CJ, Beitel M, Oberleitner L, Oberleitner DE, Madden LM, Bollampally P, et al. Pain catastrophizing and pain acceptance are associated with pain severity and interference among methadone‐maintained patients. J Clin Psychol. 2019;75(12):2233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry DT, Savant JD, Beitel M, Cutter CJ, Moore BA, Schottenfeld RS, et al. Pain and associated substance use among opioid dependent individuals seeking office‐based treatment with buprenorphine–naloxone: a needs assessment study. Am J Addict. 2013;22(3):212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein MD, Herman DS, Bailey GL, Straus J, Anderson BJ, Uebelacker LA, et al. Chronic pain and depression among primary care patients treated with buprenorphine. J Gen Intern Med. 2015;30(7):935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voon P, Hayashi K, Milloy MJ, Nguyen P, Wood E, Montaner J, et al. Pain among high-risk patients on methadone maintenance treatment. J Pain. 2015;16(9):887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. Jama. 2003;289(18):2370–8. [DOI] [PubMed] [Google Scholar]
- 12.Potter JS, Dreifuss JA, Marino EN, Provost SE, Dodd DR, Rice LS, et al. The multi-site prescription opioid addiction treatment study: 18-month outcomes. J Subst Abuse Treat. 2015;48(1):62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldeiro RM, Malte CA, Calsyn DA, Baer JS, Nichol P, Kivlahan DR, et al. The association of persistent pain with out‐patient addiction treatment outcomes and service utilization. Addiction. 2008;103(12):1996–2005. [DOI] [PubMed] [Google Scholar]
- 14.Larson MJ, Paasche‐Orlow M, Cheng DM, Lloyd‐Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102(5):752–60. [DOI] [PubMed] [Google Scholar]
- 15.Dhingra L, Masson C, Perlman DC, Seewald RM, Katz J, McKnight C, et al. Epidemiology of pain among outpatients in methadone maintenance treatment programs. Drug Alcohol Depend. 2013;128(1–2):161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox AD, Sohler NL, Starrels JL, Ning Y, Giovanniello A, Cunningham CO. Pain is not associated with worse office-based buprenorphine treatment outcomes. Subst Abus. 2012;33(4):361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsui JI, Lira MC, Cheng DM, Winter MR, Alford DP, Liebschutz JM, et al. Chronic pain, craving, and illicit opioid use among patients receiving opioid agonist therapy. Drug Alcohol Depend. 2016;166:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barry DT, Beitel M, Garnet B, Joshi D, Rosenblum A, Schottenfeld RS. Relations among psychopathology, substance use, and physical pain experiences in methadone-maintained patients. J Clin Psychiatry. 2009;70(9):1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci. 2012;1248:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHugh RK, Fitzmaurice GM, Carroll KM, Griffin ML, Hill KP, Wasan AD, et al. Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug Alcohol Depend. 2014;145:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fareed A, Vayalapalli S, Stout S, Casarella J, Drexler K, Bailey SP. Effect of methadone maintenance treatment on heroin craving, a literature review. J Addict Dis. 2010;30(1):27–38. [DOI] [PubMed] [Google Scholar]
- 22.Awgu E, Magura S, Rosenblum A. Heroin-dependent inmates’ experiences with buprenorphine or methadone maintenance. J Psychoactive Drugs. 2010;42(3):339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsui JI, Anderson BJ, Strong DR, Stein MD. Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: a longitudinal study. Am J Drug Alcohol Abuse. 2014;40(2):163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. [DOI] [PubMed] [Google Scholar]
- 25.Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev. 2013;37(10):2597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marlatt GA, Donovan DM. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors, 2nd ed. Marlatt GA, Donovan DM, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors, 2nd ed. New York, NY, US: The Guilford Press; 2005. xiv, 416–xiv, 416. [Google Scholar]
- 27.Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl). 2011;218(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin J-L, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin J-L, et al. Exacerbated craving in the presence of stress and drug cues in drug-dependent patients. Neuropsychopharmacology. 2018;43(4):859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solberg Nes L, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: a review. Ann Behav Med. 2009;37(2):173–83. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–26. [DOI] [PubMed] [Google Scholar]
- 32.Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin J-L, et al. Before and after: craving, mood, and background stress in the hours surrounding drug use and stressful events in patients with opioid-use disorder. Psychopharmacology (Berl). 2018;235(9):2713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 35.Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43(3):692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Browne WJ, Lahi MG, Parker RMA. A guide to sample size calculations for random effect models via simulation and the MLPowSim software package. Bristol, United Kingdom Univ Bristol. 2009; [Google Scholar]
- 37.Schultzberg M, Muthén B. Number of Subjects and Time Points Needed for Multilevel Time-Series Analysis: A Simulation Study of Dynamic Structural Equation Modeling. Struct Equ Model [Internet]. 2018;25(4):495–515. Available from: 10.1080/10705511.2017.1392862 [DOI] [Google Scholar]
- 38.Martel MO, Finan PH, McHugh RK, Issa M, Edwards RR, Jamison RN, et al. Day-to-day pain symptoms are only weakly associated with opioid craving among patients with chronic pain prescribed opioid therapy. Drug Alcohol Depend. 2016;162:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biddle SJH, Gorely T, Marshall SJ, Cameron N. The prevalence of sedentary behavior and physical activity in leisure time: a study of Scottish adolescents using ecological momentary assessment. Prev Med. 2009;48(2):151–5. [DOI] [PubMed] [Google Scholar]
- 40.Mun CJ, Thummala K, Davis MC, Karoly P, Tennen H, Zautra AJ. Predictors and social consequences of daily pain expectancy among adults with chronic pain. Pain. 2017;158(7):1224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71(4):474–82. [DOI] [PubMed] [Google Scholar]
- 42.Bjekić J, Živanović M, Purić D, Oosterman JM, Filipović SR. Pain and executive functions: a unique relationship between Stroop task and experimentally induced pain. Psychol Res. 2018;82(3):580–9. [DOI] [PubMed] [Google Scholar]
- 43.Koppel L, Andersson D, Posadzy K, Västfjäll D, Tinghög G. The effect of acute pain on risky and intertemporal choice. Exp Econ. 2017;20(4):878–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakubczyk A, Brower KJ, Kopera M, Krasowska A, Michalska A, Łoczewska A, et al. Physical pain and impulsivity in alcohol-dependent patients. Addict Res Theory. 2016;24(6):458–65. [Google Scholar]
- 45.Tapper K Mindfulness and craving: effects and mechanisms. Clin Psychol Rev. 2018;59:101–17. [DOI] [PubMed] [Google Scholar]
- 46.Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, et al. Mindfulness-based therapy: a comprehensive meta-analysis. Clin Psychol Rev. 2013;33(6):763–71. [DOI] [PubMed] [Google Scholar]
- 47.Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, et al. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA psychiatry. 2014;71(5):547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. J Consult Clin Psychol. 2010;78(3):362–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witkiewitz K, Bowen S, Douglas H, Hsu SH. Mindfulness-based relapse prevention for substance craving. Addict Behav. 2013;38(2):1563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garland EL, Hanley AW, Kline A, Cooperman NA. Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessments from a stage 1 randomized controlled trial. Drug Alcohol Depend. 2019;203:61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casey CY, Greenberg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: a model including cognitive, affective, and trauma factors. Pain. 2008;134(1–2):69–79. [DOI] [PubMed] [Google Scholar]
- 52.Moustafa AA, Parkes D, Fitzgerald L, Underhill D, Garami J, Levy-Gigi E, et al. The relationship between childhood trauma, early-life stress, and alcohol and drug use, abuse, and addiction: An integrative review. Curr Psychol. 2018;1–6. [Google Scholar]
- 53.Novick AM, Levandowski ML, Laumann LE, Philip NS, Price LH, Tyrka AR. The effects of early life stress on reward processing. J Psychiatr Res. 2018;101:80–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.French DP, Sutton S. Reactivity of measurement in health psychology: how much of a problem is it? What can be done about it? Br J Health Psychol. 2010;15(3):453–68. [DOI] [PubMed] [Google Scholar]
- 55.Buu A, Yang S, Li R, Zimmerman MA, Cunningham RM, Walton MA. Examining measurement reactivity in daily diary data on substance use: Results from a randomized experiment. Addict Behav. 2020;102:106198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.