Abstract
Enzymes are the complex protein moieties, catalyze the rate of chemical reactions by transforming various substrates to specific products and play an integral part in multiple biochemical cycles. Advancement in enzyme research and its integration with industries have reformed the biotech industries. It provides a superior monetary and ecological exchange to traditional material measures in an efficient and environmentally sustainable manner. The cost-effective production of pure and highly active enzymes is still a challenge for the biocatalyst industries. The use of high purity substrates further raises the cost of a typical biocatalyst. The use of low-cost plant-based biomasses as an enticing and sustainable substrate for enzyme production is the most cost-effective approach to these problems. Given the relevance of biomass as a substrate for enzyme development, this review article focuses on the key source, composition and major enzyme generated using various biomass residues. Furthermore, the difficulties associated with the use of biomass as a substrate and technical developments in this area, are also addressed. The use of waste biomass as a substrate lowers the ultimate cost for the production of biocatalysts while simultaneously reduces the waste burden from the environment.
Keywords: Bioprocess technologies, Challenges, Commercial feasibility, Industrial applications, Low-cost enzymes, Waste plant biomass
Introduction
Biocatalysts are complex biological molecules, able to convert a substrate to a particular product and catalyze the rate of a biochemical reaction. Biologically, these are synthesized by a number of living organisms and play a crucial role in the number of industrial processes (Hasunuma et al. 2013). With the revolution in biotechnology or biotech industries and advancements in technology, these biological catalysts provide an economic and ecological alternative to conventional processes. The traditional processes involve the use of chemical catalysts that are costly, performed under supercritical conditions and causes pollution. Biocatalysts in terms reduce pollution, work under mild conditions, very little by-product formation, high efficacy and are cost-effective. As it is well known that enzymes are synthesized by a variety of sources (microorganisms, animals and plants), but among all, microbial enzymes are of greater interest and use in the present scenario, because the microbes are able to proliferate at a high rate and produce multiple active compounds (Bhatia et al. 2020d). Hence, microbial enzymes have been explored for various industrial and commercial applications to a greater extent especially in biorefineries, pharmaceuticals, textiles, food and beverages, confectionery, paper and pulp bleaching, cosmetics, chemical productions, biofuel production, etc. (Singh et al. 2016; Raveendran et al. 2018; Bhatia et al. 2020b, 2021).
In 2018, the trade enzymes market was $5.5 billion globally and supposed to reach $7.0 billion by the end of 2023 according to the BCC report. This was projected to grow at a 9.1% CAGR (compound annual growth rate) (BCC 2018). The major bottleneck with the industrial application of biocatalysts is their cost. The extensive production of any product requires a large amount of enzyme and simultaneously a large amount of raw material which is quite costly and influences the overall capital (Bhatia et al. 2020d). The utilization of biocatalysts in different industrial practices circuitously affects the budget of the end product and among that 28% of the total working cost is of raw materials (Klein-Marcuschamer et al. 2012; Raveendran et al. 2018). Thus, the use of a low-cost substrate for the production of enzyme is the need of the hour to provide a boom to the enzyme/biotech industry in an economic and eco-friendly way. The utilization of low-cost lignocellulosic waste as a raw substrate for enzyme fabrication might act as a solution to this issue.
Plant biomass comprises the most abundant, globally available, sustainable source of biomass on earth. Besides being economic, another important advantage of using plant biomass as a potential substrate is that it does affect the production of food crops as it does not require extra land for cultivation. Thus, providing an economic, environment-friendly substitute to other substrates used in various industrial applications (Gronenberg et al. 2013; Liu et al. 2019; Al-Battashi et al. 2019; Fernandes et al. 2020). The composition of plant biomass varies depending upon growth conditions, species and type of plant. Cellulose, hemicellulose and lignin are the main constituents of plant biomass and the remaining components are ash residues composed of silicon, calcium, magnesium, aluminium, sodium and potassium, etc. Some resins, fatty acids, alkaloid, phenolic, salts, phytosterols and other compounds present in lignocellulosic biomass have very low quantity are known as extractives (Saini et al. 2015; Kucharska et al. 2018). Cellulose and hemicellulose are highly diverged polymer of six (C6) and five-carbon (C5) sugars while lignin is a polyphenolic polymer present within and between the cell walls of the tracheid, vessels and fibres of xylem tissue (Luo et al. 2018; Wang et al. 2018). Lignocellulosic biomass obtained from plants is an alternative or low-cost substrate for the fabrication of biocatalysts and a variety of valuable chemicals. In this review, we tried to explore various plant-derived biomasses as raw substrate for the production of enzymes to reduce the overall production cost. This review also provides an overview of various strategies used, challenges faced, in using these low-cost substrates along with pretreatment strategies employed to utilize the component of choice. Technological processes and commercial availability and productivity of using plant biomass were also discussed. This review also provides an overview of the pros and cons associated with the use of plant biomass along with their commercial viabilities.
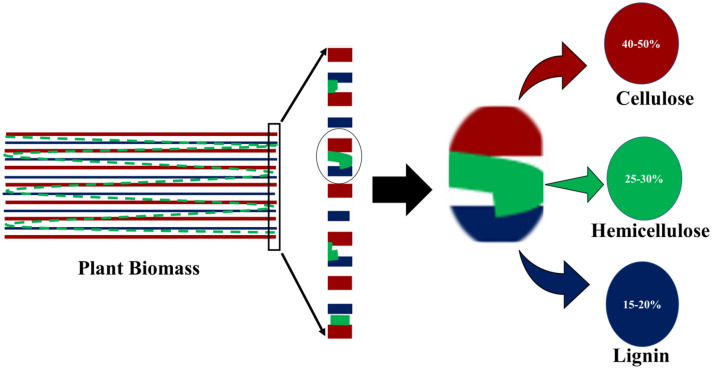
Plant biomass: source and structure
Lignocellulosic biomass is one of the most abundant raw materials available on planet earth that can be used for producing a variety of biocatalysts. It is projected that the global annual production of lignocellulose biomass is around 220 billion tonnes, which show its abundant availability (Bhatt et al. 2018). However, it is still undervalued and underutilized as raw material. Nonetheless, many researchers and industrialists have already started creating viable technologies and processes to convert lignocellulosic residues which are not in use for food or feed purposes into various enzymes. Various residues and biowaste, such as agronomic remain energy harvests, woodland deposits, biodegradable industrial wastes and municipal solid wastes mainly unruffled of starch, extractives, monomers and proteins, etc. as minor constituents (Bhatia et al. 2017). Lignocellulose biomasses are rich in cellulose (40–50%), lignin (10–25%), hemicellulose (20–30%) as major constituents while pectin, starch, simple sugars and extractives (chlorophyll, waxes and non-structural sugars) as minor constituents (Saini et al. 2015; Bhatia et al. 2020c) as shown in Fig. 1. Cellulose and hemicellulose consist of more than half of the entire dry biomass along with some amount of lignin (Dimarogona et al. 2012). These all components are interlinked to each other by van der Wall bonds and hydrogen bonds to form microfibrils and exist as crystalline and amorphous form (Bhatia et al. 2020c). Cellulose consists of 500–15,000 subunits of d-glucopyranose formed by β-1,4-glycosidic linkage, it is mostly crystalline in structure with a somewhat amorphous area (Chen et al. 2017). Hemicellulose is five-carbon sugar of ≤ 200 subunits of d-Xylose, l-Arabinose, d-Mannose, d-Galactose, Glucose, d-glucuronic, 4-O-methyl glucuronic and d-galacturonic acids. It is amorphous in nature (Hasunuma et al. 2013). Lignin is the second most abundant polymer on the earth after cellulose. Monomeric units of lignin are trans-p-coumaryl, trans-coniferyl and trans-sinnapyl connected by trans-p-coumaryl, trans-coniferyl and trans-sinnapyl. It is amorphous in nature (Al-Battashi et al. 2019).
Fig. 1.
Major components of plant biomass
In addition to the above-mentioned constituents, lignocellulosic biomasses also contain pectic compounds, starches, proteins, extractives (terpenes, ash, fats, crude fibres, volatiles) and simple sugars like glucose, fructose, lactose, etc. (Bajpai 2018a; Ravindran et al. 2018). Their composition varies from biomass to biomass-based on their geographical location and environmental conditions.
Sustainable sources of plant biomass
Plant biomass can be utilized as a cheap carbon source to produce various enzymes. Due to the abundant availability of plant biomass, cost-effective management is required to protect the environment. Therefore, integrating it with enzyme production will not help us to reduce the cost of enzymes, but also solves waste management problems while generating revenue. The classification of plant biomass depends on their origin, such as agriculture and horticulture residues, forest residues, biodegradable industry wastes, municipal solid wastes and energy crops (Banerjee et al. 2019; Olatunji et al. 2020; Bhatia et al. 2020d).).
Agricultural and horticultural wastes
Agricultural and horticultural residues were earlier burnt for disposal by farmers which pose an environmental threat. However, these are now seen as an essential element in a green economy and used to generate valuable products of industrial and commercial potential (Ambye-Jensen et al. 2018; Romero et al. 2019). Agricultural and horticultural wastes generally include leaves and stalks of plants, unripe fruits or crops residues left after harvesting (Mohiuddin et al. 2014; Sadh et al. 2018). These wastes are considered as the sustainable alternative to food crops that are purposely grown for the production of these valuable products since they have collected without expanding cropland (Laborel-Préneron et al. 2018; Pavlenko and Searle 2019). These waste materials are rich in cellulose, hemicellulose and lignin along with some minor components, such as starch. However, their composition varies from residue to residue. As agriculture and horticulture sectors are the basis of every country in the world, tonnes of agriculture and horticulture wastes are generated every year globally (five billion metric tons), which provide a better alternative to produce various microbial enzymes (such as α-amylase, amyloglucosidase, cellulase, tannase, xylanase, inulinase, hemicellulases, mannanase, lactase, β-glucanase, invertase, pectinase, protease, transglutaminase, lipases, phytase, laccase and actinobacterial enzymes) at lower cost (Singh et al. 2011; Bharathiraja et al. 2017; Jnawali et al. 2018; Ravindran et al. 2018; Azam and Ahmad 2019; Olatunji et al. 2020).
Forest residue
Forestry residues mainly involve the biomass generated by thinning of plantations, clearing of roads, extracting stem wood and natural attrition. All these operations usually eliminate only 25–50% of waste, remaining residues available as a substrate/carbon source for energy. The interest in forest residue to convert it into valuable products (like biofuels, biopolymers, nanocomposites, enzymes and bioactive molecules) is continuously increasing. Forest waste generally consists of branches, barks, treetops, wood slash, dry leaves, sawdust, trim, etc., which generally produced either naturally in the forest ecosystem or while harvesting forest wood (Bhatia et al. 2018; Nie and Bi 2018). It is the second-largest source of lignocellulose biomass after agriculture residues (Amaniampong et al. 2020). However, the composition of lignin, hemicellulose and cellulose vary from species to species. Softwood hemicellulose contains galactoglucomannan and arabinoglucuronoxylan while hardwood (e.g., willow and poplar) hemicellulose contains glucuronoxylan (Schutyser et al. 2017; Al-Battashi et al. 2019). It is estimated that 2.6 million tonnes of dry forest residues will be available solely in India by 2030 (Pavlenko and Searle 2019). Forest residues act as a major cause of forest fire. Forest fire is one the major problem in forests which cause financial loss, soil corrosion and environment destruction, hence, the use of timberland biomass as a substrate for enzyme fabrication can lessen the delinquent of forest fire (Bhatia et al. 2018). These residues are of varied or low quality and thus require a large number of R&D efforts in order to use as economically viable. Lack of knowledge about forest biomass productivity is one of the major hurdles in using such biomass (Mateos 2019). The major wood products of forest biomass involve firewood, sleepers, lumber, plywood, cellulosic, veneer and piles. Lumber and sleepers possess about 71% of the total wood and produce huge residues (Honorato-Salazar and Sadhukhan 2020).
Plant-based industrial waste
Rapid urbanization and rising product demand are prompting the establishment of new industries. This in terms resulting in the generation of massive amount of waste and waste by-products that, if not recycled or reused, causes immense pressure on the environment. The composition of waste generated from the plant biomass-based industry is depending on the type of industry producing it, such as waste from agro-based industries, paper industries, textile industries, food and fruits industries, forest wood-based industries, distilleries, beverages industries, sugar processing mills, are rich in lignocellulose materials (Ravindran and Jaiswal 2016; Bhatia et al. 2020a, b, c, d; Robak and Balcerek 2018; Ravindran et al. 2018). On the other hand, wastes from dairy industries, fish and meat processing industries lack lignocellulose content, but contains high amounts of proteins, lipids, starch, glucans and diversity of compounds which enhance the growth of microbes can also be used as nitrogen sources for the growth of microorganism and production of enzymes, but more detailed analysis of those wastes are outside the scope of this article (Ravindran and Jaiswal 2016; Liu et al. 2016). The utilization of organic content gained from plant-based industrial waste for the production of enzyme is an outstanding example to validate the vast prospective of waste valorization for the construction of a sustainable society (Bhatia et al. 2020d).
Municipal solid waste
Wastes from domestic, public or commercial organizations collectively called Municipal Solid Waste (MSW) in one or another way contain the residue of plant biomass that can also be utilized for the production of cost-effective enzymes (Dornau et al. 2020). It is estimated that in 2016 world has generated over 2 billion tonnes of MSW and by 2050 world will produce 3.40 billion tonnes of waste every year (Kaza et al. 2018; Dornau et al. 2020). It can be further categorized into two categories, such as organic and inorganic municipal solid waste and organic MSW composition range from 30 to 60% (Dornau et al. 2020). Organic MSW is rich in plant biomass residues and can be used for enzyme production after proper treatment (Gautam et al. 2012). This organic fraction primarily consists of food and garden waste and waste papers and cards that are rich in lignocellulose (Dornau et al. 2020). The diversion of MSW to enzyme production could be very helpful to the environment and also reduce the price of the enzymes which are otherwise very costly. MSW (food waste, craft paper and paper sludge) which are rich in cellulose has shown a promising result at laboratory scale for cellulase production (Gautam et al. 2011).
Enzymes produced using plant biomass as substrate
Advancement in microbiological and biotechnological fields resulted in the production of biocatalysts in laboratories that finally can be used in a number of industries, institutes and day to day products. For example, for clarification of fruit juice cellulases and pectinases are applied, amylases, protease and lipase find their uses in food processing and detergent industries, tannase in tannery wastewater treatment and many more (Ravindran and Jaiswal 2016). Recently, the re-engineering of plant biomass by microbes to produce enzymes has attracted many modern scientists. Several biotechnological processes have been invented and developed to synthesize various value-added stuffs, such as enzymes, biofuels, biopolymers, organic acids, amino acids from plant biomass (Bhatia et al. 2020c). The utilization of plant biomass for the synthesis of enzymes will not only cost-effective, but also an alternative to pollution caused by their disposal in the environment. Carbohydrates polysaccharides i.e., cellulose and hemicellulose in plant biomass act as a low-cost substrate for microbes involved in enzyme production, hence commercially viable. Microbes use these polysaccharides as an energy nutrient necessary for growth and metabolism and in order to do so, microbes secrete extracellular enzymes which hydrolyse the polysaccharides into simple sugars (Abdeshahian et al. 2020).
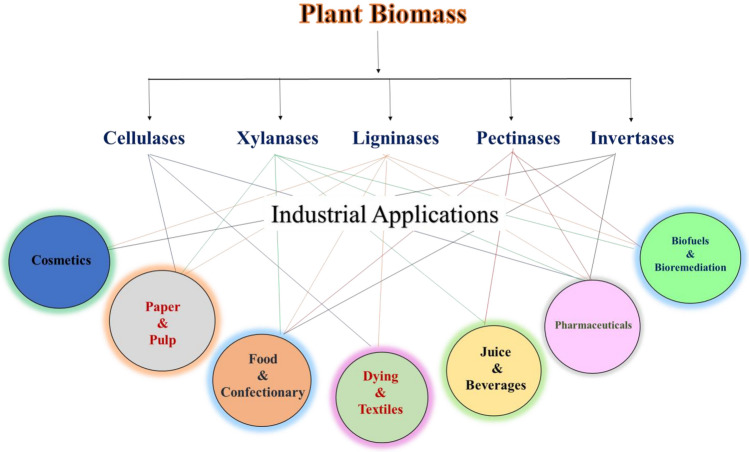
The most common enzymes which can be produced industrially using plant biomass as substrate are lignocellulosic degrading enzymes i.e., cellulase, hemicellulase and ligninases. Ligninases or ligninolytic enzymes can degrade lignin and finds a lot of applications in the textile sector, petroleum, paper and pulp industries and bioremediation (Bharathiraja et al. 2017). On the other hand, cellulase and hemicellulase find application in second-generation biofuel production and in food/fruits processing industries (Ravindran and Jaiswal 2016). Most recently, a variety of enzymes, such as α-amylase, Amyloglucosidase, Tannase, Xylanase, Inulinase, Mannanase, Lactase, β-glucanase, Invertase, Pectinase, Protease, Transglutaminase, Lipases, Phytase and Actinobacterial enzymes can be produced using plant biomass (Ravindran and Jaiswal 2016; Bharathiraja et al. 2017; Ravindran et al. 2018). Among various bacteria, yeast, fungi and actinomycetes, fungi of genera Aspergillus and Trichoderma are widely used for the production of enzymes using plant biomass as substrate (Ravindran et al. 2018). Depending upon the enzyme need to produce, type of feedstock, pretreatment method, microbial strain and fermentation strategy is selected. Table 1 and Fig. 2 summarize the different plant biomasses that have been used as raw material to produce different enzymes. Moreover, plant biomass required some upstream processing based on the type of enzyme one producing before it can be used as raw material for enzyme production (Bhatia et al. 2020d, 2021). For instance, to produce cellulase using plant biomass as substrate, maximum cellulose concentration should be present in plant biomass, hence pretreatment is required which remove lignin and turn crystalline cellulose into amorphous cellulose and ensures cellulose maximum availability. However, the pretreatment process results in the formation of inhibitors; hence in most cases it is avoided.
Table 1.
Summary of the lignocellulosic sources that have been used as raw material for enzyme production
| Enzyme | Feedstock | Bioprocessing condition | Microbial strain | Application | References |
|---|---|---|---|---|---|
|
4-α-d-glucan glucanohydrolase α- amylase endo-1, Random cleavage of α-1, 4 linkages between neighboring glucose subunits |
Rice Bran | pH 9.0, temperature 37 °C and 72 h incubation | Bacillus tequilensis TB5 | Animal nutrition, Aquaculture, Baking, Biofuel, Brewing, Dishwashing and laundry detergents | Paul et al. (2020) |
| Mango Kernel |
Substrate Concentration—5% w/v; temp.—30 °C; pH—4; incubation time—9 days |
Fusarium solan | Panda and Ray (2015) | ||
| Cassava Residues |
SSF; Incubation time—42 h; Temperature—30 °C |
A. niger | dos Santos et al. (2020) | ||
| Date Waste |
SF; pH—6.0; Incubation time—72 h; sugar conc.—15.0 g/l; starch content—5.0 g/l, |
Candida guilliermondii CGL-A10 | Acourene and Ammouche (2012), Chandrasekaran and Bahkali (2013) | ||
| Soybean Husk + Flour Mill Waste (45:55) | Incubation Time—6 days; Without Pre-treatment; temp.—30 °C | A. oryzae NRRL695 | Melnichuk et al. (2020) | ||
| Brewers’ spent grain hydrolysate | SF; Incubation time—30 h | B. subtilis KCC103 | Ravindran and Jaiswal (2016) | ||
| Cassava sago industry’s waste | SSF; Incubation Time—8 days | Rhizopus stolonifer | Panda and Ray (2015) | ||
| Cellulase | Jatropha deoiled cake |
SSF; Temperature—30 °C; inoculum dose—10% |
Paecilomyces variotii | Bleaching, deinking, detergents, refining, starch modification, drainage improvement, decolourization of dyes in effluent, cellulosic and starch-based ethanol, biodiesel, lignocellulose degradation | Pathak et al. (2016) |
| Rice straw | SSF; Temperature—50 °C ; pH—4.8; Growth Period—24 h; 86% initial moisture content | A. terreus | Roohi et al. (2019) | ||
| Apple pomace |
SSF; temperature—30 °C; Incubation time: 48–72 h |
A. niger NRRL-567 | Ravindran and Jaiswal (2016) | ||
| Rice Bran |
SSF; Temp—30 °C; pH—6.0; Incubation time—14 days |
A. flavus | Navaneethapandian et al. (2020) | ||
| Banana fruit stalk | Temperature—35 °C; pH—7.0; Growth Period—3 days |
Purpureocillium Lilacinum |
Roohi et al. (2019) | ||
|
Municipal solid Waste |
Temp.—40 °C; pH—6–7 | A. niger | Gautam et al. (2011) | ||
| Olive pomace + Winery waste | SSF; Temperature—25 °C; Humidity 75% w/w; C/N—15 with urea | A. niger/A. ibericus | Filipe et al. (2020) | ||
|
Municipal solid Waste |
Temp.—45 °C; pH—6.5 | Trichoderma sp. | Gautam et al. (2011) | ||
| Soybean hulls + Wheat bran | Temperature—30 °C; pH—5.0; Growth Period—96 h | A. oryzae | Roohi et al. (2019) | ||
| Banana peel | SSF; temperature—30 °C, Incubation time: 192 h | Trichoderma viride GIM 3.0010 | Ravindran and Jaiswal (2016) | ||
| Coffee Husk | SSF; time—24 h; 62% Moisture Content; (Specialized Consortium) | Pseudoxanthomonas taiwanensis, Sphingobacterium composti) (Cyberlindnera jardinii Barnettozyma californica) | Cerda et al. (2017) | ||
| Banana Waste |
SSF; Temperature—32 °C; Incubation time—15–25 days |
Cellulomonas cartae, P. fluorescens, P. putida and B. megaterium | Dabhi et al. (2014) | ||
|
Coconut fibre Powder |
Temperature—20–50 °C; pH—5.0–9.0; Growth Period—5 days | Cellulomonas | Roohi et al. (2019) | ||
|
Endoglucanase E.C. 3.2.1.4 |
Plantain Peels | SSF; 40 °C, pH—5.0, incubation time—7 days | P. atrovenetum | Biofuel, Paper and Pulp industry, brewing, textiles, detergent industry | Adeleke (2013) |
| Food Waste |
SSF; Inoculum Level—0.5%; Temperature—25 °C; Growth Period—6 days |
A. niger | Tian et al. (2018) | ||
| β-glucanase | Oatmeal orange peel | SSF; temperature—30 °C, pH: 5.5, Incubation time: 4 days |
Rhizomucor miehei CAU432 T. viride MBL |
Brewing, bioethanol | Ravindran and Jaiswal (2016) |
|
β-glucosidase E.C. 3.2.1.2 |
Olive pomace + Winery waste | SSF; Temperature—25 °C; Humidity—75% w/w; C/N—15 with urea | A. niger/A. ibericus | Biofuel Industry; Paper and Pulp industry, brewing, textiles, detergent industry | Filipe et al. (2020) |
| Coffee Industry Waste + Wheat Bran |
SSF; Coffee Industry Waste: wheat bran (1:1 w/w); Temperature—30 °C; pH—5.0; Time—7 2 h |
A. niger F12 | Favaro et al. (2020) | ||
| Pineapple crown leaves + wheat bran | Incubation—8 days; 80% initial moisture content; Temp.—28 °C | A. awamori | Nishida et al. (2018) | ||
| Forage Palm | SSF; Temperature—23 °C; 56% moisture content | P. roqueforti ATCC 10110 | das Neves et al. (2020) | ||
|
Xylanase 1,4-β-xylanxylanohydrolase |
Sorghum straw |
SSF; incubation time—5-day; pH—6.0; temperature—40 °C |
A. tubingensis FDHN1 | Animal fodder, Pulp Bleaching, baking, food, biomedical, Bioethanol industry | Adhyaru et al. (2015) |
| Sugarcane Bagasse | SSF; Incubation time—24 h; temperature-45 °C; supplemented with wheat bran | A. fumigatus | de Oliveira Rodrigues et al. (2017) | ||
| Jatropha deoiled cake | SSF; initial moisture content—70%; incubation temperature—30 °C; inoculum dose—10% | Paecilomyces variotii | Pathak et al. (2016) | ||
| Paddy Straw |
SSF; temperature—50 °C; Moisture Ratio—0.75; Carbon Source—Xylose; N Source—Yeast Extract |
Humicola lanuginosus | Hegde and Ramesh (2016) | ||
| Food Waste | SSF; Moisture Content—77.67%; Inoculum Level—0.5%; Temperature—25 °C; Growth Period—6 days | A. niger | Tian et al. (2018) | ||
| Olive pomace + Winery waste | SSF; Incubation time—7 days; Temperature—25 °C; Humidity—75% w/w; C/N—15 with urea | A. niger/A. ibericus | Filipe et al. (2020) | ||
|
β-Mannanase Degarade Mannans |
Coffee Industry Waste | SSF; pH—5.0; Temp.—30 °C; 5; incubation time—72 h | A. niger F12 | Paper and pulp, textile, pharmaceuticals | Favaro et al. (2020) |
| Transglutaminase | Soy residue (fibrous) |
SSF; Temperature—33 °C, incubation time—48 h |
B. circulans BL32 | Baking, edible film, leather finishing, meat processing, dairy products, cosmetics | Ravindran and Jaiswal (2016) |
|
Protease Hydrolysis the peptide bonds between amino acids |
Coffee Pulp Waste (CPW) | SSF; Incubation time—60 h; pH—8.0; temperature—37 °C | Bacillus sp. BT MASC | Leather, diagnostics, waste management food, pharmaceutical, animal feed | Kandasamy et al. (2016) |
| Rice Bran |
SSF; moisture content—44.44%; temperature—30 °C; Incubation time—72 h |
Rhizopus microsporus NRRL 3671 | Sumantha et al. (2006) | ||
| Corn Cob (CC) | SSF; Incubation time—60 h; pH—8.0; temperature—37 °C | Bacillus sp. BT MASC | Kandasamy et al. (2016) | ||
|
Laccase E.C.1.10.3.2 |
Pearl millet Husks (PM) + Finger Millet (FM) Husk | Incubation time—96; pH—7.0; temperature—40 °C | Bacillus sp. PS | Bleaching, textiles, deinking of paper | Srinivasan et al. (2019) |
| Switchgrass |
SF; Incubation time—54 days; Temperature—30 °C |
Pycnoporus sp. SYBC-L3 | Liu et al. (2013) | ||
| Orange Waste | SSF; Temperature—28 °C; Incubation time—20 days | Pleurotus pulmonarius | Inácio et al. (2015) | ||
|
Phytase Hydrolyse phytic acid |
Orange and citrus peel |
SSF; temperature—50 °C, Incubation time: 72 h |
Klebsiella sp. DB-3FJ711774.1 | Animal fodder with phytase, reduce phosphate concentration of pig and poultry farm effluent | Ravindran and Jaiswal (2016), Ravindran et al. (2018) |
|
Pectinase Catalyse hydrolysis of pectic bond |
Date Fruit Waste |
SSF; Incubation time—72 h; Temperature—37 °C; pH—7.0 |
B. licheniformis KIBGE-IB3 | Processing of starch and wine, juice processing | Aslam et al. (2020) |
| Sapota Peel + Groundnut oil cake | SSF; Temperature—50 °C; pH—4.5; substrate concentration—1.5% | A. oryzae | Panda and Ray (2015) | ||
| Hazelnut Shell | SF; pH—7.0; Incubation time—72 h; Temperature—30 °C | B. subtilis | Uzuner and Cekmecelioglu (2015) | ||
| Orange waste | SSF; Temperature—28 °C; Incubation time—35th day | P. pulmonarius | Inácio et al. (2015) | ||
|
Invertase β‐fructofurano sidase, |
Red carrot jam processing residue | SSF; temperature -30 °C, fermentation time—72 h | S. cerevisiae NRRL Y-12632 | Sucrose hydrolysis; Food additive | Ravindran and Jaiswal (2016) |
|
Inulinase 2,1‐β‐d fructan fructanohydrolase, Acts upon inulin |
Carrot Pomace | SSF; Incubation time—4 days; pH—7.0 | P. oxalicum BGPUP-4 | HFC syrup | Singh et al. (2018) |
| Yacon juice | SF; temperature—30 °C, pH—5, fermentation time—7 days | A. kawachii | Ravindran and Jaiswal (2016) | ||
|
Lipase Triacylglycerol‐acylhydrolases esterification, transesterification, |
Wheat bran + olive oil waste | SSF | A. niger NCIM 1207 | Biopolymers and biodiesel synthesis, enantiopure drugs synthesis and flavouring agent, meat processing, detergents, degreasing, | Sharma et al. (2016) |
| Tannase | Grape peel | SSF; Incubation Period—96 h | P. chrysogenum + Trichoderma viride | Stabilization of malt polyphenols, Food and Pharmaceuticals industry, treatment of tannery effluents; beer and fruit juices clarification | Panda and Ray (2015) |
| Coffee Pulp | SSF; Temperature—30 °C; Incubation time—96 h | P. verrucosum | Bhoite and Murthy (2015) | ||
| Chitinase | Wheat Bran | SSF; Temp—30 °C; Incubation time—72 h | Trichoderma koningiopsis | Biopesticide | Baldoni et al. (2020) |
| Lactase | Fermented ragi | SF; fermentation time—12 h; pH—5.5 | L. acidophilus | Dairy, preparation of lactose-free food products | Ravindran and Jaiswal (2016) |
SF Submerged fermentation, SSF Solid state fermentation, HFC High fructose corn
Fig. 2.
Enzymes produced by utilizing plant biomass as substrate and their industrial applications
Commercial production of biocatalysts
Biocatalysts have a unique place in industrial processes hence their ecofriendly production by utilizing low cost substrates at commercial level is advantageous to fulfill their needs for industries. Various biotransformation strategies have been developed and deployed for the production of biocatalysts at commercial level as discussed below:
Fermentation strategies
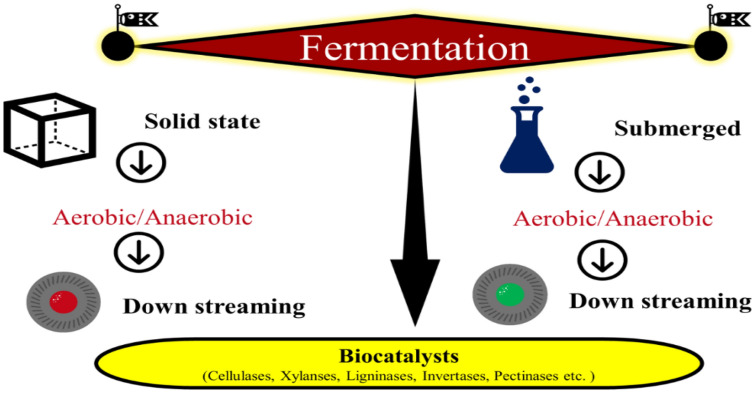
To produce enzymes through microbes, two fermentation processes are commonly used i.e. submerged fermentation (SF) and solid-state fermentation (SSF) as shown in Fig. 3. The submerged fermentation strategy involves a liquid medium (water) that contain nutrients along with lignocellulose substrate for enzyme production. Generally, stirred tank reactors are used which help in maintaining pH, temperature, aeration and agitation. On the other hand, the substrate also acts as energy, carbon and backing material for the growth of microorganisms in solid-state fermentation (Abdeshahian et al. 2020). This type of fermentation encourages fungi growth, but the scale-up of this technology at a large scale still needs a lot of research (Ravindran and Jaiswal 2016). However, on the other hand, submerged fermentation is well established at a larger scale. However, in terms of yield, many studies have proved that SSF is better than SF. In addition, low wastewater generation, SSF fermentation technology has some other advantages like low energy consumption and low nutrition requirements and low cost down streaming. Significant rise in temperature, maintaining moisture and low aeration are some of the issues which are faced during enzyme production through SSF (Srivastava et al. 2020). Recently, sequential fermentation (solid state and submerged) for enzyme production has also gained popularity (Cunha et al. 2012).
Fig. 3.
Fermentation strategies for production of value-added products
SSF find its use in a wide range of industries as a significant process for the synthesis of bio-active compounds and metabolites. The quest for a manageable, sustainable, feasible and biologically cordial process to substitute conventional processes for manufacturing various items has changed the modern area (Lizardi-Jiménez and Hernández-Martínez 2017). Consequently, SSF is pertinent, since it has a few attributes that make it eco-accommodating, for example, lower energy utilization, less wastewater age and the work of rural and agro-industrial squanders as substrates, keeping away from ecological issues brought about by their removal (de Castro and Sato 2015; Hyseni et al. 2018). It involves the use of a solid matrix in the absence or very little presence of water for the cultivation of microbes (mostly aerobic) (Lizardi-Jiménez and Hernández-Martínez 2017).
SF performed under the presence of excess liquid on the plant biomass substrate. The biological entities thus produced and extracted from the fermentation broth. The process is formed under both aerobic and anaerobic conditions (Subramaniyam and Vimala 2012). It is a rapid process and involves excesses and fast utilization of nutrients and thus requires constant supplementation of nutrient (continuous flow fermentation). This process is generally used for the microbes to efficiently grow under moist conditions. The down streaming in SF is easier than that of SSF (Renge et al. 2012; Mishra and Malik 2013). The pros and cons of both the processes are given below in Table 2. Various industries (baking, brewing, detergents, food, pharmaceuticals, biofuels, textiles and leather processing) employ different enzymes (such as amylase, lipase, uricase, laccase, cellulase, hemicellulase, protease, pectinase, catalase etc.) at different stages to produce valuable products (Bhatia et al. 2020d). The use of microbial enzymes in different industries is growing rapidly and with continued advances in R&D activities, they have proven themselves as one of the vital components in various industrial processes. Currently, the food and beverage industry dominate in the terms of the use of industrial enzymes (Bhatia et al. 2021). Hence, become one of the key drivers of the industrial enzyme market. Nowadays, many companies produce these enzymes commercially, not only for industrial purposes but also for scientific and analytical purposes. The commercial and abundant availability of these microbial enzymes has replaced or reduced the use of harmful radioactive elements, heavy metals and catalysts which not only made a threat to the environment but also harmful to humankind (Singh et al. 2016; Bhatia et al. 2020d).
Table 2.
Advantages and disadvantages of SSF and SF
| Fermentation type | Advantages | Disadvantages | References |
|---|---|---|---|
| SSF |
Less Pre-treatment required by substrate Contaminants are restricted due to low moisture content Forced aeration is easier Low wastewater generation Low cost in downstream processing High volumetric productivity |
Low moisture content restrict growth Rise in temperature Difficult in monitoring process parameters |
Ravindran and Jaiswal (2016), Srivastava et al. (2020), Abdeshahian et al. (2020) |
| SF |
Easy to establish and operate Even distribution of nutrient and microbes Easy to monitor process parameters Abundant availability of water for microbes |
Expensive equipment Expensive media Lot of wastewater generated. Need post treatment technology which increase cost Complex downstream processing High power consumption |
Among all industrial enzymes available, proteases account for the largest share in the industrial enzyme market (Ramesh et al. 2020). Various microbial species, such as Bacillus sp. and Aspergillus sp. are extensively studied for the production of protease at a large scale (Ramesh et al. 2020). For instance, Novozymes (Denmark) supplies protease (Product Trade Name: Alcalase) produced from B. licheniformis and have activity ≥ 0.75 Anson units/ml whereas Amano Pharmaceuticals Ltd. produces protease (Product Trade Name: Protease P) from Aspergillus sp. having activity ≥ 0.5 units/g (Razzaq et al. 2019). Various researches have been done to find a cheap substrate for protease production. Several plant biomass (such as sugar cane bagasse, wheat straw, pigeon pea waste, orange peel waste, pineapple waste, rice bran, wheat bran, sugarcane bagasse, raw potato starch, raw sweet potato starch, coffee pulp, copra pasta, grape wastes, among other) have been evaluated under solid-state fermentation conditions which are already have been proven superior to submerged fermentation in terms of economics. Among all, rice bran has been reported to be the best substrate to produce protease from Bacillus sp. with 0.13 IU at 48 h (Sharma et al. 2017).
Amylase is also one of the industrially important enzymes. It was the first industrial enzyme to be produced from a fungal source in 1894 (Ramesh et al. 2020). It finds application in almost all industries, such as the detergent industry, food industry, paper and pulp industry, textile industry, leather industry, biofuel industry, petroleum industry, pharmaceutical industry, cosmetic industry and wastewater treatment plants. In a recent study, Khalid-Bin-Ferdaus et al. (2018) scaled down the production cost of the alpha-amylase in the pilot project using Wheat Bran as substrate and A. niger for the fermentative process. The maximum activity was observed at 28 ℃ and pH 6.2 and the enzyme was extracted by centrifugation (Khalid-Bin-Ferdaus et al. 2018). Almanaa et al. (2020) have also reported that wheat bran with starch as a supplement enhances enzyme yield (threefold) and activity (670 U/g) under solid-state fermentation with B. subtilis D19 (Almanaa et al. 2020).
Lipase, also known as lipolytic enzymes is the third most important group of industrial enzymes. It is mainly used in the pharmaceutical industry. However, the use of lipases at the industrial level is still limited due to high cost, especially when needed in high quantity. Hence, several types of research have been undergoing to reduce the cost of commercial lipase. Many microorganisms, such as bacteria (B. subtilis, B. licheniformis, B. pumilus, Pseudomonas sp., Burkholderia sp. and Staphylococcus sp.), fungi (Rhizopus sp., Aspergillus sp., Penicillium sp., Geotrichum sp., Mucor sp.) and yeast (Candida rugosa, Rhodotorula sp.) are potential producers of lipases (Bharathi and Rajalakshmi 2019). However, lipase from yeast and filamentous fungi is more attractive as they produce more stable lipase. Most recently, recombinant lipase is produced using hosts, such as E. coli and Komagataella phaffii (Contesini et al. 2020). Sethi et al. 2016, has reported production of lipase (most active at pH 6, 50 ℃ and substrate concentration of 1.5%) from mustard oil cake by A. terreus NCFT 4269.10 under solid state fermentation with a yield of 8.44% and the protein had a molecular weight of 46.3 kDa as determined by SDS-PAGE and also suggested further research to establish at industrial level (Sethi et al. 2016). The lipase B (CALB) from C. antarctica, Lipozyme® TL IM from Thermomyces lanuginosus and Novozym® 40,086 from Rhizomucor miehei by Novozymes, Copenhagen, Denmark are some commercially available lipases (Contesini et al. 2020; Bhatia et al. 2021). Nema et al. (2019) performed lipase production from A. niger under solid-state fermentation using a mixture of rice husk, cottonseed cake and red gram husk. Maximum activity of 21.19 U/gds of lipase obtained at temperature 40 ℃, moisture content 75% (v/w), pH 6.0 and 1000 g of substrate concentration using tray fermenter (Nema et al. 2019).
Lignocellulolytic enzymes, such as cellulases (endoglucanase, β-glucosidase, etc.), hemicellulases (xylanases, etc.) and ligninases (laccases, lignin peroxidases, etc.) are some of the emerging groups of industrial enzymes. They play important role in reducing the overall cost of biorefinery (Ríos-Fránquez et al. 2019). Kamsani et al. (2016) have identified Aspergillus sp., Bacillus sp. and Brevibacillus sp. from Bulbitermes sp. termite gut and performed solid-state fermentation using sawdust as substrate. The yield of lignocellulolytic enzymes (particularly endoglucanase, β-glucosidase, xylanase, lignin peroxidase and laccase) were approximate 17–93% higher in co-culture of Aspergillus sp., Bacillus sp. and Brevibacillus sp. compared to a single culture. Several studies also suggest that sawdust can be used as an alternative cheap substrate at the industrial level after some optimization for the production of lignocellulolytic enzymes by using fungal–bacterial co-cultures (Kamsani et al. 2016). Namnuch et al. (2020) reported that A. flavus KUB2 is a potential cellulase and xylanase producer under submerged fermentation by using sugarcane bagasse waste as a substrate (Namnuch et al. 2020).
In addition, to the above-mentioned enzymes, several other enzymes are also employed commercially. For instance, most recently, a study has reported that Aspergillus sp. Gm has potential for commercial production of pectinase at 30 ℃, pH 5.8 and 0.5% substrate (mature ripened orange fruits) concentration via submerged fermentation (KC et al. 2020). Dhital et al. (2014), have also reported A. niger MG1 has potential for commercial pectinase production. Phytase that is used for producing animal feed is produced commercially by Novozyme Company from A. oryzae and Alltech Company from A. niger (Jatuwong et al. 2020).
Challenges and major limitations associated with biomass substrate
Biocatalysts need to be produced in a cost-effective and environmentally sustainable way by utilizing the waste plant biomass. One major advantage of biomass as a suitable feedstock for the production of biocatalysts is its easy availability. However, being available easily of plant waste biomass is not enough as there are a number of other obstacles that need to be address to reduce the cost of biocatalyst for their commercial applications. Limitations being faced by the scientific community to use the waste plant biomass as substrate for the production of biocatalysts are discussed below:
Plant biomass as raw material
Plant biomass consists of polysaccharides (cellulose and hemicellulose) and phenolic compounds (Lignin) collectively called as lignocellulose. Biological degradation of these polysaccharides is mainly prevented by lignin due to its recalcitrant nature (Zeng et al. 2014). Hence, in order to remove lignin from biomass effective pretreatment is needed (Bhatia et al. 2020c). The crystalline nature of cellulose is also an additional obstacle while utilizing plant biomass as raw material. Hence, conversion of this into an amorphous form is necessary for enzymatic degradation, it will not be susceptible to enzymatic degradation (Ravindran and Jaiswal 2016). Cellulose and hemicellulose are the two main components in plant biomass that release fermentable sugars (Cellulose gives glucose; Hemicellulose gives xylose, arabinose, galactose, etc.) after enzymatic hydrolysis and most microbes need glucose as a carbon source (Bhatia et al. 2020a). Hence, the rate of glucose formation and breakdown of cellulose is directly prepositional to cellulose and inversely to hemicellulose or lignin (DeMartini et al. 2013). However, the different enzyme production depends upon the specific substrates present in plant biomass (Ravindran and Jaiswal 2016). For instance, whole-plant biomass utilization for laccase production, starch for amylase production, etc. One more constraint of using plant biomass is that its compositional variability. It is found that the same biomass can be available in different composition due to change in the geographical area, harvesting time and upstream processing (Salihu et al. 2015; Dhyani and Bhaskar 2018; Sadh et al. 2018; Olatunji et al. 2020). Also, the use of cellulose for enzyme production can cut off land cultivation competition, but it still needs in farming and forestry system (Verdade et al. 2015). For instance, some researchers argue that waste residues especially agriculture and horticulture residues are needed in the soil to maintain soil fertility, prevent soil erosion and are important in conservation tillage practices. Using forest residues has also shown some disadvantages like it was reported that increased forest biomass use reduces carbon stocks in Finnish forest (Webb and Coates 2012). Furthermore, deadwood also provides habitat to many forest species. Recent reports have also indicated that plant biomass derived from some dedicated energy crops requires larger land area, more water and resources and have more greenhouse gases emission than food crops which question their sustainability (Verdade et al. 2015). Moreover, even plant biomass are abundantly available at a low cost, but its and transportation and processing are a little bit complex which ultimately raises the cost of the whole production process. It is also reported that the cost of plant biomass vary from region to region and mainly depends upon the supply chain. And hence, careful research and improvements in the current supply chain will reduce the feedstock cost and ultimately product cost (Singhvi and Gokhale 2019).
Pre-treatment of lignocellulose
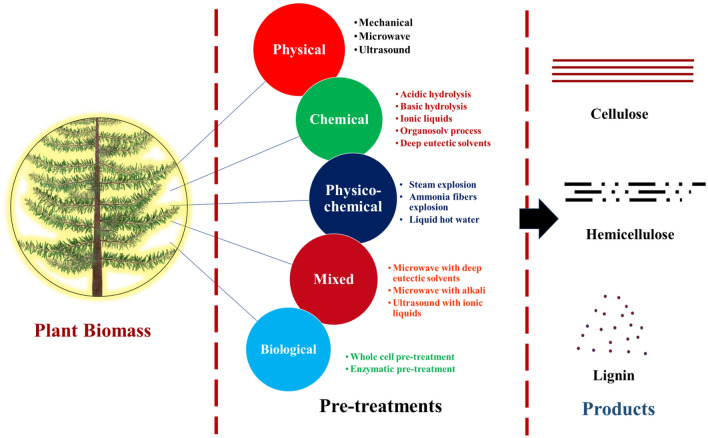
The lignocellulose biomass recalcitrance is majorly influenced by lignin because it is rich in aromatic residues which promote its hydrophobicity and forms a physical barrier around cellulose and hemicellulose (Bhatia et al. 2017; Rathour et al. 2018; Zoghlami and Paës 2019). Lignin directly indirectly bonded to hemicellulose and cellulose which makes the whole structure resistant against enzymatic action, microbial attack and oxidative stress (Andlar et al. 2018). Hence, when utilization of hemicellulose or cellulose is required for enzyme production, delignification step is required so that maximum cellulose or hemicellulose is available for utilization as a carbon source. The pretreatment process mainly categorized into physical, chemical, physicochemical and biological or combinations of two or more types (Bhatia et al. 2020b). However, all these pretreatment methods (Fig. 4) have some benefits and weaknesses as summarizes in Table 3. The main objective of pretreatment is to reduce the size and crystallinity of plant biomass, remove lignin and make polysaccharides (hemicellulose and cellulose) more accessible (Singhvi and Gokhale 2019). However, various pretreatment methods produce many inhibitors (furfural, HMF, levulinic acid and acetic acid) which can have a negative influence on microbial growth (Robak and Balcerek 2018). Hence, the detoxification step is needed before fermentation which ultimately increases the cost of the end product (Artifon et al. 2018). Pretreatment of the plant biomass is still an unresolved problem and hence, the future of using plant biomass as a substrate for enzyme production is expected to lie in the development of cost-effective pretreatment technologies (Singhvi and Gokhale 2019). Several bioengineering types of research have been going on to increase efficiency and reduce production cost. One of the methods is consolidated bioprocessing (CBP) in which pretreatment, saccharification and fermentation are done by the same microorganism or consortium. Hence, the enzyme produces during this process can be recycled (Zhang and Zhang 2013). This will not only generate revenue or reduce the production cost, but inhibitors formation is also minimal since biological pretreatment is more environmentally friendly (Zhang and Zhang 2013; Bušić et al. 2018).
Fig. 4.
Different methods of pretreatment
Table 3.
Comparison of different methods of pretreatment
| Pre-treatment | Mode of action | Sugar production | Inhibitor formation | Investment cost | Additional remarks | References |
|---|---|---|---|---|---|---|
| Mechanical | – | Very low | No or low inhibitors | Low operational cost |
Adsul et al. (2011), Shafiei et al. (2015), Kumar and Sharma (2017), Wang et al. (2018), Kucharska et al. (2018), Al-Battashi et al. (2019), |
|
| Radiations | Removal of lignin and hemicellulose | Less amount | No or low inhibitors | High operational cost | Environmental impact | |
| Steam explosion | Removal of lignin and alteration of lignin structure | Fermentable sugars are produced is less amount | High amount of inhibitors | Low operational cost | Low environmental impact | |
| Alkali | Removal of lignin and hemicellulose | High yield of sugars | No or low inhibitors | Low operational cost | Increases cost of downstream processing | |
| Acid |
Weak acid: Remove hemicellulose and modify lignin structure Strong acid: Hydrolyses both cellulose and hemicellulose |
Large amount of fermentable sugars | High amount of inhibitors | Low operational cost | Suitable for low lignin content. Performed under super critical conditions hence having hazardous, toxic and corrosive effects | |
| Oxidative delignification | Remove lignin and De-crystallization cellulose | Large amount | No or very less inhibitors | Low operational cost | degradation is limited to lignin | |
| Organosolv | Remove lignin and hemicellulose | High yield of fermentable sugars | No or low inhibitors | Cell growth may be inhibited by high quality lignin solvent |
Choice of microorganism
Enzymes of microbial origin are found to be more advantageous over the enzymes of plant, animal, human or mammalian cell origin. Because the life cycle of microbes is shorter, they can be easily manipulated at the genetic level and enzymes produced are more active stable and can be produced in large quantity in comparison to wild counterparts (Anbu et al. 2017). For industry use, enzymes are generally available as enzyme preparations that contain metabolites, additional preservatives and stabilizers along with the desired enzyme ((Bhatia et al. 2020d, 2021). However, enzymes produced by microbes must pass some safety tests. Hence, strain should be non-pathogenic and non-toxic. Generally, commercial enzymes produced by using non-pathogenic microbes (Ravindran and Jaiswal 2016).
Among various bacteria, yeast, fungi and actinomycetes, fungi of genera Aspergillus and Trichoderma are widely employed for industrial production of enzymes using plant biomass as substrate (Ravindran et al. 2018; Abdeshahian et al. 2020). In bacteria some Bacillus sp. has also been employed in the commercial production of enzymes using plant biomass as substrate (Ravindran and Jaiswal 2016). Recently, genetic engineering employed on several microorganisms, such as E. coli K-12, F. venenatum and P. fluorescens etc. which have no history of use in industrial production of native enzymes has been successfully utilized in commercial production of enzymes using plant biomass as substrate (Ravindran and Jaiswal 2016). Thermophilic microbes which are isolated from exotic location have also shown potential to produce industrially important enzymes. There are several advantages of using thermophilic microbes like reduce the risk of contamination, low viscosity, high substrate solubility and high product yield (Coker 2016). Wild-type strains can also be used to produce enzymes that have industrial value. The common method to exploit these microbes is bioprospecting in which first microbes are collected from the environment and culture in the lab and their enzyme production is screened (Ravindran et al. 2018).
Technological progress in plant biomass as a low-cost substrate
Eco-friendly and economic production of a variety of products uses plant biomass as a key feedstock. Although its utilization is a favorable substitute to many existing chemical challenges thus, there is a need of some technological process (pretreatment, hydrolysis and fermentation) in order to make it more suitable. Extensive researches have been undertaken to utilize plant biomass as raw material to synthesize new chemicals, biofuels, PHA and certain other metabolites including biocatalysts (Ge et al. 2018; Bhatia et al. 2020c). Recently, agriculture, forest, marine, the industrial and municipal waste residue has expanded as abundant feedstock worldwide for the production of biocatalysts as shown in Table 1. However, the efficiency and production yield depend on the nature, climate conditions, source and harvesting time of plant biomass (Al-Battashi et al. 2019).
The conversion of plant biomass to sugar is also a complicated and most expensive process. Varieties of pretreatment techniques are generally used for plant biomass hydrolysis (Singhvi and Kim 2020). Plant biomass is converted into commodity products by two main routes either ‘‘acid-based’’ or ‘‘enzyme-based’’. The enzyme-based approach is highly complex, but advantageous over the chemical route mainly because of no toxic chemicals’ generation (Adsul et al. 2011). The cellulosic portion of lignin is C6 sugar, it can be further converted into fermentable sugars i.e. glucose and finally to biofuels (Wang et al. 2018). Besides this, cellulose is also utilized to produce other value-added products, such as microbial polysaccharides, single-cell proteins (SCPs), methane and other fine chemicals (Nangul and Bhatia 2013; Kumar et al. 2018). The hemicellulosic portion of plant biomass forms 20–35% of its total weight, mainly comprising of C5 sugars i.e., xylose, arabinose comprising d-xylose and arabinose. These pentose sugars formed after pretreatment of plant biomass can be used for the production of xylitol (a sugar-free sweetener), SCPs (single-cell proteins), lactic acid, fumaric acid and amino acids (Kumar et al. 2018). The third main constituent of plant biomass is lignin, an aromatic phenolic polymer formed by three monomeric units i.e., coumaryl, coniferyl and sinapyl. A variety of value-added products can be formed from lignin, depending upon its type. 98% of lignin is used as a fuel while only 2% is used for the production of polymeric products (Bhatia et al. 2019). It is used for the synthesis of dyes, jet printing ink, asphalt, vanillin, benzene, toluene, xylene, styrene, bio-based polyethene terephthalate, polyurethane and further production of pharmaceuticals, fragrances and flavouring agents (Pandey and Kim 2011; Bajpai 2018b).
Although a large number of value-added products can be formed from plant biomass by development in research and technological intervention still a break exists between the predicted and actual commercial yield. Therefore, several difficulties i.e., collection, harvesting, handling, pretreatment, depolymerization, needed to be overcome in order to make use of these abundant and cheap feedstocks.
Commercial viability of plant biomass for biocatalyst scenario
Enzymes find usage in various industries across the globe and are preferred over chemical catalysts because of the great degree of substrate specificity. With modernization, the commercial production of enzymes is expected to increase with a 4.7% of annual growth rate from 2016 to 2021 which is $5.0 billion in 2016 to $6.3 billion in 2021 globally (Ravindran and Jaiswal 2016; Ravindran et al. 2018). Since the beginning, enzyme production is limited to only a few companies, such as Novozymes and Danisco. 70% of the total production is dominated by these two companies (de Souza Vandenberghe et al. 2016). Despite such high demands, enzymes are still expensive and thus the cost to process to which these are employed is also high. Plant biomass may serve or is serving as one of the best low-cost substrates for the production of the enzyme. This only just the cost of the whole process, but also help to solve the problem of waste generation which if does not manage sustainably can be a threat to both environment and humankind. However, using plant biomass at commercial levels comes with some challenges as discussed in the previous section. To lower the overall production cost of the enzyme, setting up a lignocellulose biorefinery seems like a more viable option, rather than setting up a company for just one product (Chandel et al. 2018). The concept of biorefinery will not lower the cost of the enzyme produced by completely utilizing lignocellulose for producing various value-added products, but also help to achieve sustainable development goals and make a green circular economy that generates no waste (Bušić et al. 2018). Using plant biomass with or in place of costly feedstock will definitely increase returns (Ravindran et al. 2018). Hence, robust research is needed so that cost-effective and eco-friendly technologies can be developed to valorize lignocellulose. Table 4 summarized some of the enzyme producers and important enzymes being produced commercially.
Table 4.
Commercial hydrolases and their manufacturers
| Enzyme | Manufacturer | Microbe used | References |
|---|---|---|---|
| Termamyl 120L (Amylase) | Novozymes | B. licheniformis |
de Souza Vandenberghe et al. (2016), Ravindran et al. (2018), Bhatia et al. (2021) |
| Cellubrix | Novozymes | T. longibrachiatum | |
| SP526 (Lipase) | Nova Nordisk | A. Antarctica | |
| Pectinex | Schweizerische Ferment A.G | – | |
| Allzym PT | Alltech | A. niger |
Conclusion and future prospective
The enzyme industry is increasing rapidly with prompt industrialization and a major drawback in enzyme production is its cost. Plant biomasses, an economical substrate for the production of a variety of enzymes find their applications in the majority of industries. The development of new and cost-effective techniques using waste biomass as a substrate is a challenging task. A desirable amount of enzymes and other biochemical without any byproduct formation needs integration of systemic as well as synthetic biology. This would aid in the construction of new technologies, new metabolic pathways and fewer side products formation. The potential of these biomasses can be exploited with the use of microbes as it is reflected as the most feasible method to search for desirable enzymes. Despite this, the effectiveness of industrial enzyme production from biomass still needs improved pretreatment strategies. Further, research on the influence of waste biomass and the possible roles of lignocellulosic enzymes in industrial processes is still needed. The available information demonstrates that plant-based biomass is an attractive substrate for the production of value-added products. Although its utilization is a difficult process, but biotechnological intervention and advancement in applied sciences make it easier and useful that will help in solving two critical issues, the never-ending pollution and low-cost products.
Acknowledgements
Authors are highly grateful to the Department of Environment Science and Technology (DEST) Govt. of HP for providing JRF to Ms. Ranju Kumar Rathour and University Grant Commission (UGC) (Grant no. No. F./PDFSS201415SCHIM8434) New Delhi, India for providing financial assistance in the form of PDF to Dr. Ravi Kant Bhatia. All the facilities provided by the Department of Biotechnology HP University Shimla are also duly acknowledged.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Deepak Sakhuja, Hemant Ghai, Ranju Kumari Rathour, Pradeep Kumar, Arvind Kumar Bhatt and Ravi Kant Bhatia have contributed equally.
References
- Abdeshahian P, Kadier A, Rai PK, Silva SS (2020) Lignocellulose as a renewable carbon source for microbial synthesis of different enzymes. In: Lignocellulosic Biorefining Technologies. Wiley, pp 185–202
- Acourene S, Ammouche A. Optimization of ethanol, citric acid, and α-amylase production from date wastes by strains of Saccharomyces cerevisiae, Aspergillus niger, and Candida guilliermondii. J Ind Microbiol Biotechnol. 2012;39:759–766. doi: 10.1007/s10295-011-1070-0. [DOI] [PubMed] [Google Scholar]
- Adeleke AJ. Endoglucanase production by Penicillium atrovenetum using plantain peels as substrate. AU JT. 2013;16:140–146. [Google Scholar]
- Adhyaru DN, Bhatt NS, Modi HA. Optimization of upstream and downstream process parameters for cellulase-poor-thermo-solvent-stable xylanase production and extraction by Aspergillus tubingensis FDHN1. Bioresour Bioprocess. 2015;2:3. doi: 10.1186/s40643-014-0029-1. [DOI] [Google Scholar]
- Adsul MG, Singhvi MS, Gaikaiwari SA, Gokhale DV. Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresour Technol. 2011;102:4304–4312. doi: 10.1016/j.biortech.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Al-Battashi HS, Annamalai N, Sivakumar N, et al. Lignocellulosic biomass (LCB): a potential alternative biorefinery feedstock for polyhydroxyalkanoates production. Rev Environ Sci Biotechnol. 2019;18:183–205. doi: 10.1007/s11157-018-09488-4. [DOI] [Google Scholar]
- Almanaa TN, Vijayaraghavan P, Alharbi NS, et al. Solid state fermentation of amylase production from Bacillus subtilis D19 using agro-residues. J King Saud Univ Sci. 2020;32:1555–1561. doi: 10.1016/j.jksus.2019.12.011. [DOI] [Google Scholar]
- Amaniampong PN, Asiedu NY, Fletcher E, et al. Conversion of lignocellulosic biomass to fuels and value-added chemicals using emerging technologies and state-of-the-art density functional theory simulations approach. In: Daramola MO, Ayeni AO, et al., editors. Valorization of biomass to value-added commodities, green energy and technology. Switzerland: Springer Nature; 2020. pp. 193–220. [Google Scholar]
- Ambye-Jensen M, Balzarotti R, Thomsen ST, et al. Combined ensiling and hydrothermal processing as efficient pretreatment of sugarcane bagasse for 2G bioethanol production. Biotechnol Biofuels. 2018;11:336. doi: 10.1186/s13068-018-1338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbu P, Gopinath SCB, Chaulagain BP, Lakshmipriya T. Microbial enzymes and their applications in industries and medicine 2016. Biomed Res Int. 2017;2017:1–3. doi: 10.1155/2017/2195808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andlar M, Rezić T, Marđetko N, et al. Lignocellulose degradation: an overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng Life Sci. 2018;18:768–778. doi: 10.1002/elsc.201800039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artifon W, Bonatto C, Bordin ER, et al. Bioethanol production from hydrolyzed lignocellulosic after detoxification via adsorption with activated carbon and dried air stripping. Front Bioeng Biotechnol. 2018 doi: 10.3389/fbioe.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam F, Ansari A, Aman A, et al. Production of commercially important enzymes from Bacillus licheniformis KIBGE-IB3 using date fruit wastes as substrate. J Genetic Eng Biotechnol. 2020;18:46. doi: 10.1186/s43141-020-00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam MT, Ahmad A. Date palm waste: an efficient source for production of glucose and lactic acid. In: Naushad M, Lichtfouse E, editors. Sustainable agriculture reviews. Cham: Springer; 2019. pp. 155–178. [Google Scholar]
- Bajpai P (2018a) Wood and fiber fundamentals. In: Biermann’s handbook of pulp and paper. Elsevier, pp 19–74
- Bajpai P (2018b) Value-added products from lignin. In: Biotechnology for pulp and paper processing. Springer Singapore, Singapore, pp 561–571
- Baldoni DB, Antoniolli ZI, Mazutti MA, et al. Chitinase production by Trichoderma koningiopsis UFSMQ40 using solid state fermentation. Braz J Microbiol. 2020;51:1897–1908. doi: 10.1007/s42770-020-00334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Patti AF, Ranganathan V, Arora A. Hemicellulose based biorefinery from pineapple peel waste: Xylan extraction and its conversion into xylooligosaccharides. Food Bioprod Process. 2019;117:38–50. doi: 10.1016/j.fbp.2019.06.012. [DOI] [Google Scholar]
- BCC (2018) Global Markets for Enzymes in Industrial Applications. In: BCC Research. https://www.bccresearch.com/market-research/biotechnology/global-markets-for-enzymes-in-industrial-applications.htm. Accessed 25 Feb 2021
- Bharathi D, Rajalakshmi G. Microbial lipases: an overview of screening, production and purification. Biocatal Agric Biotechnol. 2019;22:101368. doi: 10.1016/j.bcab.2019.101368. [DOI] [Google Scholar]
- Bharathiraja S, Suriya J, Krishnan M, et al (2017) Production of enzymes from agricultural wastes and their potential industrial applications. In: Advances in food and nutrition research. Elsevier, pp 125–148 [DOI] [PubMed]
- Bhatia SK, Kim S-H, Yoon J-J, Yang Y-H. Current status and strategies for second generation biofuel production using microbial systems. Energy Convers Manag. 2017;148:1142–1156. doi: 10.1016/j.enconman.2017.06.073. [DOI] [Google Scholar]
- Bhatia SK, Joo H-S, Yang Y-H. Biowaste-to-bioenergy using biological methods—a mini-review. Energy Convers Manag. 2018;177:640–660. doi: 10.1016/j.enconman.2018.09.090. [DOI] [Google Scholar]
- Bhatia SK, Gurav R, Choi T-R, et al. Bioconversion of barley straw lignin into biodiesel using Rhodococcus sp. YHY01. Bioresour Technol. 2019;289:121704. doi: 10.1016/j.biortech.2019.121704. [DOI] [PubMed] [Google Scholar]
- Bhatia RK, Sakhuja D, Mundhe S, Walia A. Renewable energy products through bioremediation of wastewater. Sustainability. 2020;12:7501. doi: 10.3390/su12187501. [DOI] [Google Scholar]
- Bhatia RK, Ramadoss G, Jain AK, Dhiman RK, Bhatia SK, Bhatt AK. Conversion of waste biomass into gaseous fuel: present status and challenges in India. Bioenergy Res. 2020;13:1046–1068. doi: 10.1007/s12155-020-10137-4. [DOI] [Google Scholar]
- Bhatia SK, Jagtap SS, Bedekar AA, et al. Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges. Bioresour Technol. 2020;300:122724. doi: 10.1016/j.biortech.2019.122724. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Vivek N, Kumar V, Chandel N, Thakur M, Kumar D, Yang YH, Pugazendhi A, Kumar G. Molecular biology interventions for activity improvement and production of industrial enzymes. Bioresour Technol. 2020;24:124596. doi: 10.1016/j.biortech.2020.124596. [DOI] [PubMed] [Google Scholar]
- Bhatia RK, Ullah S, Hoque HZ, Ahmad I, Yang YH, Bhatt AK, Bhatia SK. Psychrophiles: a source of cold-adapted enzymes for energy efficient biotechnological industrial processes. J Environ Chem Eng. 2021;9:104607. doi: 10.1016/j.jece.2020.104607. [DOI] [Google Scholar]
- Bhatt AK, Bhatia RK, Thakur S, et al (2018) Fuel from waste: a review on scientific solution for waste management and environment conservation. Prospects of alternative transportation fuels. Energy, Environment, and Sustainability. Springer Singapore, Singapore, pp 205–233
- Bhoite RN, Murthy PS. Biodegradation of coffee pulp tannin by Penicillium verrucosum for production of tannase, statistical optimization and its application. Food Bioprod Process. 2015;94:727–735. doi: 10.1016/j.fbp.2014.10.007. [DOI] [Google Scholar]
- Bušić A, Marđetko N, Kundas S, et al. Bioethanol production from renewable raw materials and its separation and purification: a review. Food Technol Biotechnol. 2018 doi: 10.17113/ftb.56.03.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda A, Mejías L, Gea T, Sánchez A. Cellulase and xylanase production at pilot scale by solid-state fermentation from coffee husk using specialized consortia: the consistency of the process and the microbial communities involved. Biores Technol. 2017;243:1059–1068. doi: 10.1016/j.biortech.2017.07.076. [DOI] [PubMed] [Google Scholar]
- Chandel AK, Garlapati VK, Singh AK, et al. The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization. Bioresour Technol. 2018;264:370–381. doi: 10.1016/j.biortech.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran M, Bahkali AH. Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology—review. Saudi J Biol Sci. 2013;20:105–120. doi: 10.1016/j.sjbs.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Liu J, Chang X, et al. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol. 2017;160:196–206. doi: 10.1016/j.fuproc.2016.12.007. [DOI] [Google Scholar]
- Coker JA. Extremophiles and biotechnology: current uses and prospects. F1000Research. 2016;5:396. doi: 10.12688/f1000research.7432.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contesini FJ, Davanço MG, Borin GP, et al. Advances in recombinant lipases: production, engineering, immobilization and application in the pharmaceutical industry. Catalysts. 2020;10:1032. doi: 10.3390/catal10091032. [DOI] [Google Scholar]
- Cunha FM, Esperança MN, Zangirolami TC, et al. Sequential solid-state and submerged cultivation of Aspergillus niger on sugarcane bagasse for the production of cellulase. Bioresour Technol. 2012;112:270–274. doi: 10.1016/j.biortech.2012.02.082. [DOI] [PubMed] [Google Scholar]
- da Romero CWS, Berni MD, Figueiredo GKDA, et al. Assessment of agricultural biomass residues to replace fossil fuel and hydroelectric power energy: a spatial approach. Energy Sci Eng. 2019;7:2287–2305. doi: 10.1002/ese3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabhi BK, Vyas RV, Shelat HN. Use of banana waste for the production of cellulolytic enzymes under solid substrate fermentation using bacterial consortium. Int J Curr Microbiol App Sci. 2014;3:337–346. [Google Scholar]
- das Neves CA, de Menezes LHS, Soares GA, et al. Production and biochemical characterization of halotolerant β-glucosidase by Penicillium roqueforti ATCC 10110 grown in forage palm under solid-state fermentation. Biomass Convers Biorefin. 2020 doi: 10.1007/s13399-020-00930-8. [DOI] [Google Scholar]
- de Castro RJS, Sato HH. Enzyme production by solid state fermentation: general aspects and an analysis of the physicochemical characteristics of substrates for agro-industrial wastes valorization. Waste Biomass Valoriz. 2015;6:1085–1093. doi: 10.1007/s12649-015-9396-x. [DOI] [Google Scholar]
- de Oliveira RP, dos Santos BV, Costa L, et al. Xylanase and β-glucosidase production by Aspergillus fumigatus using commercial and lignocellulosic substrates submitted to chemical pre-treatments. Ind Crops Prod. 2017;95:453–459. doi: 10.1016/j.indcrop.2016.10.055. [DOI] [Google Scholar]
- de Souza Vandenberghe LP, de Carvalho JC, Libardi N, et al (2016) Microbial enzyme factories. In: Agro-industrial wastes as feedstock for enzyme production. Elsevier, pp 1–22
- DeMartini JD, Pattathil S, Miller JS, et al. Investigating plant cell wall components that affect biomass recalcitrance in poplar and switchgrass. Energy Environ Sci. 2013;6:898. doi: 10.1039/c3ee23801f. [DOI] [Google Scholar]
- Dhital R, Panta OP, Karki TB. Optimization of cultural conditions for the production of pectinase from selected fungal strain. J Food Sci Technol Nepal. 2014;8:65–70. doi: 10.3126/jfstn.v8i0.11752. [DOI] [Google Scholar]
- Dhyani V, Bhaskar T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy. 2018;129:695–716. doi: 10.1016/j.renene.2017.04.035. [DOI] [Google Scholar]
- Dimarogona M, Topakas E, Christakopoulos P. Cellulose degradation by oxidative enzymes. Comput Struct Biotechnol J. 2012;2:e201209015. doi: 10.5936/csbj.201209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornau A, Robson JF, Thomas GH, McQueen-Mason SJ. Robust microorganisms for biofuel and chemical production from municipal solid waste. Microb Cell Fact. 2020;19:68. doi: 10.1186/s12934-020-01325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos KA, da Costa Ilhéu Fontan R, Santos LS, et al. Partitioning of amylase produced by Aspergillus niger in solid state fermentation using aqueous two-phase systems. Process Biochem. 2020;94:116–125. doi: 10.1016/j.procbio.2020.03.028. [DOI] [Google Scholar]
- Favaro C, Baraldi I, Casciatori F, Farinas C. β-Mannanase production using coffee industry waste for application in soluble coffee processing. Biomolecules. 2020;10:227. doi: 10.3390/biom10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JMC, Fraga I, Sousa RMOF, et al. Pretreatment of grape stalks by fungi: effect on bioactive compounds, fiber composition, saccharification kinetics and monosaccharides ratio. Int J Environ Res Public Health. 2020;17:5900. doi: 10.3390/ijerph17165900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe D, Fernandes H, Castro C, et al. Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Biofuels Bioprod Biorefin. 2020;14:78–91. doi: 10.1002/bbb.2073. [DOI] [Google Scholar]
- Gautam SP, Bundela PS, Pandey AK, et al. Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol Res Int. 2011;2011:1–8. doi: 10.4061/2011/810425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam SP, Bundela PS, Pandey AK, et al. Diversity of cellulolytic microbes and the biodegradation of municipal solid waste by a potential strain. Int J Microbiol. 2012;2012:1–12. doi: 10.1155/2012/325907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Chang C, Zhang L, et al. Chapter five—conversion of lignocellulosic biomass into platform chemicals for biobased polyurethane application. In: Li Y, Ge XBT-A, et al., editors. Advances in bioenergy. Elsevier; 2018. pp. 161–213. [Google Scholar]
- Gronenberg LS, Marcheschi RJ, Liao JC. Next generation biofuel engineering in prokaryotes. Curr Opin Chem Biol. 2013;17:462–471. doi: 10.1016/j.cbpa.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasunuma T, Okazaki F, Okai N, et al. A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresour Technol. 2013;135:513–522. doi: 10.1016/j.biortech.2012.10.047. [DOI] [PubMed] [Google Scholar]
- Hegde A, Ramesh Ch. Isolation and screening of fungi for the production of xylanase using solid-state fermentation from Sirsi Region of Western Ghats of Karnataka, India. Int J Curr Microbiol Appl Sci. 2016;5:547–556. doi: 10.20546/ijcmas.2016.502.062. [DOI] [Google Scholar]
- Honorato-Salazar JA, Sadhukhan J. Annual biomass variation of agriculture crops and forestry residues, and seasonality of crop residues for energy production in Mexico. Food Bioprod Proc. 2020;119:1–19. doi: 10.1016/j.fbp.2019.10.005. [DOI] [Google Scholar]
- Hyseni B, Aytekin AÖ, Nikerel E. Solid state fermentation for enzyme production for food industry. J Microbiol Biotechnol Food Sci. 2018;7:615–622. doi: 10.15414/jmbfs.2018.7.6.615-622. [DOI] [Google Scholar]
- Inácio FD, Ferreira RO, de Araujo CAV, et al. Production of enzymes and biotransformation of orange waste by oyster mushroom, Pleurotus pulmonarius. Adv Microbiol. 2015;5:1–8. doi: 10.4236/aim.2015.51001. [DOI] [Google Scholar]
- Jatuwong K, Suwannarach N, Kumla J, et al. Bioprocess for production, characteristics, and biotechnological applications of fungal phytases. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jnawali P, Kumar V, Tanwar B, et al. Enzymatic production of xylooligosaccharides from brown coconut husk treated with sodium hydroxide. Waste Biomass Valoriz. 2018;9:1757–1766. doi: 10.1007/s12649-017-9963-4. [DOI] [Google Scholar]
- Kamsani N, Salleh MMd, Yahya A, Chong CS. Production of lignocellulolytic enzymes by microorganisms isolated from Bulbitermes sp. Termite Gut in Solid-State Fermentation. Waste Biomass Valoriz. 2016;7:357–371. doi: 10.1007/s12649-015-9453-5. [DOI] [Google Scholar]
- Kandasamy S, Muthusamy G, Balakrishnan S, et al. Optimization of protease production from surface-modified coffee pulp waste and corncobs using Bacillus sp. by SSF. 3 Biotech. 2016;6:167. doi: 10.1007/s13205-016-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaza S, Yao LC, Bhada-Tata P, van Woerden F. What a waste 2.0: a global snapshot of solid waste management to 2050. Washington, DC: World Bank; 2018. [Google Scholar]
- Kc S, Upadhyaya J, Joshi DR, et al. Production, characterization, and industrial application of pectinase enzyme isolated from fungal strains. Fermentation. 2020;6:59. doi: 10.3390/fermentation6020059. [DOI] [Google Scholar]
- Khalid-Bin-Ferdaus KMd, Hossain MdF, Mansur SA, et al. Commercial production of alpha amylase enzyme for potential use in the textile industries in Bangladesh. Int J Biosci (IJB) 2018;13:149–157. doi: 10.12692/ijb/13.4.149-157. [DOI] [Google Scholar]
- Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng. 2012;109:1083–1087. doi: 10.1002/bit.24370. [DOI] [PubMed] [Google Scholar]
- Kucharska K, Rybarczyk P, Hołowacz I, et al. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules. 2018;23:2937. doi: 10.3390/molecules23112937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chandra R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon. 2020;6:e03170. doi: 10.1016/j.heliyon.2020.e03170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AK, Sharma S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess. 2017;4:7. doi: 10.1186/s40643-017-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Binod P, Sindhu R, et al. Bioconversion of pentose sugars to value added chemicals and fuels: recent trends, challenges and possibilities. Bioresou Technol. 2018;269:443–451. doi: 10.1016/j.biortech.2018.08.042. [DOI] [PubMed] [Google Scholar]
- Laborel-Préneron A, Magniont C, Aubert J-E. Characterization of barley straw, hemp shiv and corn cob as resources for bioaggregate based building materials. Waste Biomass Valoriz. 2018;9:1095–1112. doi: 10.1007/s12649-017-9895-z. [DOI] [Google Scholar]
- Liu J, Wang ML, Tonnis B, et al. Fungal pretreatment of switchgrass for improved saccharification and simultaneous enzyme production. Biores Technol. 2013;135:39–45. doi: 10.1016/j.biortech.2012.10.095. [DOI] [PubMed] [Google Scholar]
- Liu J, Dantoft SH, Würtz A, et al. A novel cell factory for efficient production of ethanol from dairy waste. Biotechnol Biofuels. 2016;9:33. doi: 10.1186/s13068-016-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu Y-J, Feng Y, et al. Construction of consolidated bio-saccharification biocatalyst and process optimization for highly efficient lignocellulose solubilization. Biotechnol Biofuels. 2019;12:35. doi: 10.1186/s13068-019-1374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi-Jiménez MA, Hernández-Martínez R. Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech. 2017;7:44. doi: 10.1007/s13205-017-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Cao J, McDonald AG. Cross-linking of technical lignin via esterification and thermally initiated free radical reaction. Ind Crops Prod. 2018;121:169–179. doi: 10.1016/j.indcrop.2018.05.007. [DOI] [Google Scholar]
- Mateos E (2019) Study on the potential of forest biomass residues for bio-energy. In: Multidisciplinary digital publishing institute proceedings. vol 2, pp 1420
- Melnichuk N, Braia MJ, Anselmi PA, et al. Valorization of two agro-industrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Manag. 2020;106:155–161. doi: 10.1016/j.wasman.2020.03.025. [DOI] [PubMed] [Google Scholar]
- Mishra A, Malik A. Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol. 2013;43:1162–1222. doi: 10.1080/10934529.2011.627044. [DOI] [Google Scholar]
- Mohiuddin A, Saha MK, Hossian MS, Ferdoushi A. Usefulness of banana (Musa paradisiaca) wastes in manufacturing of bio-products: a review. Agriculturists. 2014;12:148–158. doi: 10.3329/agric.v12i1.19870. [DOI] [Google Scholar]
- Namnuch N, Thammasittirong A, Thammasittirong SN-R. Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology. 2020 doi: 10.1080/21501203.2020.1806938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangul A, Bhatia RK. Micro-organism: a marvelous source of single cell protein. J Microbiol Biotechnol Food Sci. 2013;3(1):15–18. [Google Scholar]
- Navaneethapandian U, Kumar AG, Liduja K, et al. Biocatalyst: cellulase production in solid state fermentation (ssf) using rice bran as substrate. Biointerface Res Appl Chem. 2020;11:7689–7699. doi: 10.33263/BRIAC111.76897699. [DOI] [Google Scholar]
- Nema A, Patnala SH, Mandari V, et al. Production and optimization of lipase using Aspergillus niger MTCC 872 by solid-state fermentation. Bull Natl Res Centre. 2019;43:82. doi: 10.1186/s42269-019-0125-7. [DOI] [Google Scholar]
- Nie Y, Bi X. Life-cycle assessment of transportation biofuels from hydrothermal liquefaction of forest residues in British Columbia. Biotechnol Biofuels. 2018;11:23. doi: 10.1186/s13068-018-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida VS, de Oliveira RF, Brugnari T, et al. Immobilization of Aspergillus awamori β-glucosidase on commercial gelatin: an inexpensive and efficient process. Int J Biol Macromol. 2018;111:1206–1213. doi: 10.1016/j.ijbiomac.2018.01.146. [DOI] [PubMed] [Google Scholar]
- Olatunji O, Akinlabi S, Madushele N. Application of lignocellulosic biomass (plant biomass) In: Daramola MO, Ayeni AO, editors. Valorization of biomass to value-added commodities. Cham: Springer International Publishing; 2020. pp. 3–19. [Google Scholar]
- Panda SK, Ray RC. Microbial processing for valorization of horticultural wastes. In: Sukla LB, Pradhan N, Panda S, Mishra BK, editors. Environmental microbial biotechnology. Cham: Springer; 2015. pp. 203–221. [Google Scholar]
- Pandey MP, Kim CS. Lignin depolymerization and conversion: a review of thermochemical methods. Chem Eng Technol. 2011;34:29–41. doi: 10.1002/ceat.201000270. [DOI] [Google Scholar]
- Pathak R, Sharma A, Adak A, et al. Role of Jatropha curcas Deoiled cake as substrate for the production of cellulases and xylanase and additive in vermicomposting of kitchen waste. J Pure Appl Microbiol. 2016;10:3163–3172. doi: 10.22207/JPAM.10.4.93. [DOI] [Google Scholar]
- Paul JS, Beliya E, Tiwari S, et al. Production of biocatalyst α-amylase from agro-waste ‘rice bran’ by using Bacillus tequilensis TB5 and standardizing its production process. Biocatal Agric Biotechnol. 2020;26:101648. doi: 10.1016/j.bcab.2020.101648. [DOI] [Google Scholar]
- Pavlenko N, Searle S (2019) The potential for advanced biofuels in India: assessing the availability of feedstocks and deployable technologies. Working Paper (2019–22)
- Ramesh A, Harani Devi P, Chattopadhyay S, Kavitha M. Commercial applications of microbial enzymes. In: Arora NK, Mishra J, Mishra V, editors. Microbial enzymes: roles and applications in industries. Springer Nature, Singapore: microorganisms for sustainability; 2020. pp. 137–184. [Google Scholar]
- Rathour RK, Ahuja V, Bhatia RK, Bhatt AK. Biobutanol: new era of biofuels. Int J Energy Res. 2018;42:4532–4545. doi: 10.1002/er.4180. [DOI] [Google Scholar]
- Raveendran S, Parameswaran B, Ummalyma SB, et al. Applications of microbial enzymes in food industry. Food Technol Biotechnol. 2018;56:16–30. doi: 10.17113/ftb.56.01.18.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Jaiswal A. Microbial enzyme production using lignocellulosic food industry wastes as feedstock: a review. Bioengineering. 2016;3:30. doi: 10.3390/bioengineering3040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Hassan S, Williams G, Jaiswal A. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering. 2018;5:93. doi: 10.3390/bioengineering5040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq A, Shamsi S, Ali A, et al. Microbial proteases applications. Front Bioeng Biotechnol. 2019 doi: 10.3389/fbioe.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renge VC, Khedkar SV, Nandurkar NR. Enzyme synthesis by fermentation method: a review. Sci Rev Chem Commun. 2012;2:585–590. [Google Scholar]
- Ríos-Fránquez FJ, Rojas-Rejón ÓA, Escamilla-Alvarado C. Microbial enzyme applications in bioethanol producing biorefineries: overview. In: Ray RC, Ramachandran S, editors. Bioethanol production from food crops. Academic Press; 2019. pp. 249–266. [Google Scholar]
- Robak K, Balcerek M. Review of second-generation bioethanol production from residual biomass. Food Technol Biotechnol. 2018 doi: 10.17113/ftb.56.02.18.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohi, Bano K, Parveen S, et al (2019) Advancements in bioprocess technology for cellulase production. In: New and future developments in microbial biotechnology and bioengineering. Elsevier, pp 115–126
- Sadh PK, Duhan S, Duhan JS. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess. 2018;5:1. doi: 10.1186/s40643-017-0187-z. [DOI] [Google Scholar]
- Saini JK, Saini R, Tewari L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech. 2015;5:337–353. doi: 10.1007/s13205-014-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu A, Abbas O, Sallau AB, Alam MdZ. Agricultural residues for cellulolytic enzyme production by Aspergillus niger: effects of pretreatment. 3 Biotech. 2015;5:1101–1106. doi: 10.1007/s13205-015-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutyser W, Renders T, van den Bossche G, et al. Nanotechnology in catalysis. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2017. Catalysis in lignocellulosic biorefineries: the case of lignin conversion; pp. 537–584. [Google Scholar]
- Sethi BK, Nanda PK, Sahoo S. Characterization of biotechnologically relevant extracellular lipase produced by Aspergillus terreus NCFT 4269.10. Braz J Microbiol. 2016;47:143–149. doi: 10.1016/j.bjm.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei M, Kumar R, Karimi K. Pretreatment of lignocellulosic biomass. In: Keikhosro K, editor. Lignocellulose-based bioproducts. Biofuel and biorefinery technologies. Cham: Springer; 2015. pp. 85–154. [Google Scholar]
- Sharma R, Oberoi HS, Dhillon GS (2016) Fruit and vegetable processing waste. In: Agro-industrial wastes as feedstock for enzyme production. Elsevier, pp 23–59
- Sharma KM, Kumar R, Panwar S, Kumar A. Microbial alkaline proteases: optimization of production parameters and their properties. J Genetic Eng Biotechnol. 2017;15:115–126. doi: 10.1016/j.jgeb.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Tuteja S, Singh N, Bishnoi NR. Enhanced saccharification of rice straw and hull by microwave–alkali pretreatment and lignocellulolytic enzyme production. Biores Technol. 2011;102:1773–1782. doi: 10.1016/j.biortech.2010.08.113. [DOI] [PubMed] [Google Scholar]
- Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6:174. doi: 10.1007/s13205-016-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RS, Chauhan K, Singh J, et al. Solid-State fermentation of carrot pomace for the production of inulinase by Penicillium oxalicum BGPUP-4. Food Technol Biotechnol. 2018;56:31–39. doi: 10.1713/ftb.56.01.18.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi MS, Gokhale DV. Lignocellulosic biomass: hurdles and challenges in its valorization. Appl Microbiol Biotechnol. 2019;103:9305–9320. doi: 10.1007/s00253-019-10212-7. [DOI] [PubMed] [Google Scholar]
- Singhvi M, Kim BS. Current developments in lignocellulosic biomass conversion into biofuels using nanobiotechology approach. Energies. 2020;13:5300. doi: 10.3390/en13205300. [DOI] [Google Scholar]
- Srinivasan P, Selvankumar T, Kamala-Kannan S, et al. Production and purification of laccase by Bacillus sp. using millet husks and its pesticide degradation application. 3 Biotech. 2019;9:396. doi: 10.1007/s13205-019-1900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N, Mishra PK, Upadhyay SN (2020) Microbial cellulase production. In: Industrial enzymes for biofuels production. Elsevier, pp 19–35
- Subramaniyam R, Vimala R. Solid state and submerged fermentation for the production of bioactive substances: a comparative study. IJSN. 2012;3:480–486. [Google Scholar]
- Sumantha A, Deepa P, Sandhya C, et al. Rice bran as a substrate for proteolytic enzyme production. Braz Arch Biol Technol. 2006;49:843–851. doi: 10.1590/S1516-89132006000600019. [DOI] [Google Scholar]
- Tian M, Wai A, Guha TK, et al. Production of endoglucanase and xylanase using food waste by solid-state fermentation. Waste Biomass Valoriz. 2018;9:2391–2398. doi: 10.1007/s12649-017-0192-7. [DOI] [Google Scholar]
- Uzuner S, Cekmecelioglu D. Enhanced pectinase production by optimizing fermentation conditions of Bacillus subtilis growing on hazelnut shell hydrolyzate. J Mol Catal B Enzym. 2015;113:62–67. doi: 10.1016/j.molcatb.2015.01.003. [DOI] [Google Scholar]
- Verdade LM, Piña CI, Rosalino LM. Biofuels and biodiversity: challenges and opportunities. Environ Dev. 2015;15:64–78. doi: 10.1016/j.envdev.2015.05.003. [DOI] [Google Scholar]
- Wang W, Tan X, Yu Q, et al. Effect of stepwise lignin removal on the enzymatic hydrolysis and cellulase adsorption. Ind Crops Prod. 2018;122:16–22. doi: 10.1016/j.indcrop.2018.05.053. [DOI] [Google Scholar]
- Webb A, Coates D (2012). Biofuels and Biodiversity. Secretariat of the Convention on Biological Diversity. Montreal, Technical Series No. 65, 69 pp
- Zeng Y, Zhao S, Yang S, Ding S-Y. Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr Opin Biotechnol. 2014;27:38–45. doi: 10.1016/j.copbio.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang X-Z, Zhang Y-HP. Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. Hoboken: Wiley; 2013. Cellulases: characteristics, sources, production, and applications; pp. 131–146. [Google Scholar]
- Zoghlami A, Paës G. Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis. Front Chem. 2019 doi: 10.3389/fchem.2019.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]