Abstract
Purpose of Review
Megaprosthesis and Allograft Prosthesis Composite (APC) are the established treatment modalities for massive skeletal defects. There are a handful of studies comparing the use of megaprosthesis and APC in the management of substantial bone loss and it has always been a topic of debate regarding the superiority of one modality over the other. Therefore, we aim to compare the functional outcome and implant survivorship of each modality including complications, revision rates, amputation rate and mortality.
Recent Findings
The Allograft Prosthesis Composite (APC) constitutes a skeletal allograft implanted with a revision type prosthesis in it. The biological environment provided by the allograft allows attachment of the muscles and tendons imparting better stability and function. However, the literature is not kind enough with APC due to associated risk of infection, disease transmission and nonunion at the graft–host junction. The megaprosthesis (MP) on the other hand is a nonbiologic modality with better survivorship but subservient functional outcome. Infection has been a major issue in both the modalities. Advancement in metallurgy using silver coated megaprosthesis also failed to provide strong evidence in preventing infection.
Summary
The functional outcome is better with APC in both the upper and lower limbs. However, the survivorship is better with megaprosthesis, especially in the upper limb when revision rates were compared between the two modalities. Deep infection and mechanical complications were significantly higher in the APC group. There was no significant difference between the two groups in terms of amputation rate, mortality, and local recurrence.
Level of Evidence (CEBM)
2a
Keywords: Megaprosthesis, Allograft Prosthesis Composite, Massive skeletal defects, Bone tumors, Comparative study, Meta-analysis
Introduction
Several causes are attributed to the loss of bone stock, including bone tumor, metastasis, failed total hip arthroplasty, periprosthetic fractures, failed osteosynthesis, and infections [1–3]. The management of these large skeletal defects is a challenge for orthopedic surgeons. The major treatment options employed include osteoarticular allograft, megaprosthesis (MP) and structural Allograft Prosthesis Composite (APC).
With high rates of mechanical complications reported with osteoarticular allografts, the alternative is either megaprosthesis or an APC [4, 5]. The megaprosthesis has an advantage of the relative ease of reconstruction, less time-consuming, and no risk of nonunion, graft resorption, fracture, and disease transmission. However, these implants are unable to heal the soft tissue sleeve around them and are associated with escalated dangers of dislocation and poorer functional outcomes [6, 7]. Alternatively, APC has an advantage of biological fixation of the soft tissue sheath to the allograft bone, thereby reducing the risk of dislocation and hence leads to better functional outcomes. Nevertheless, APCs also carry their own risks of graft resorption, fracture, nonunion, disease transmission, and infection [8, 9].
There are a handful of studies comparing the use of megaprosthesis and APC in the management of substantial bone loss. We present a meta-analysis of the literature comparing the functional outcomes, implant survivorship, complications, revision rates, mortality, and amputation of each treatment modality for managing substantial bone loss.
Methods
We explored multiple databases comprehensively searching for comparative studies using the Preferred Reporting Items for Systematic Reviews and meta-analysis (PRISMA) guidelines [10].
Search Strategy
A systematic search of PubMed, Embase, Scopus, Cochrane and Web of Science was conducted from Jan 1, 1985, to Aug 31, 2020, to select all relevant studies following the PICO criteria (patient/population, intervention, comparison, and outcome) [11]. The Boolean search string used in the advanced search was “Allograft Prosthesis Composite OR allograft prosthesis OR massive skeletal allograft” AND “endoprosthesis OR megaprosthesis OR mega-prosthesis.” Only human studies were included, and additional papers were explored from the references.
Eligibility Criteria
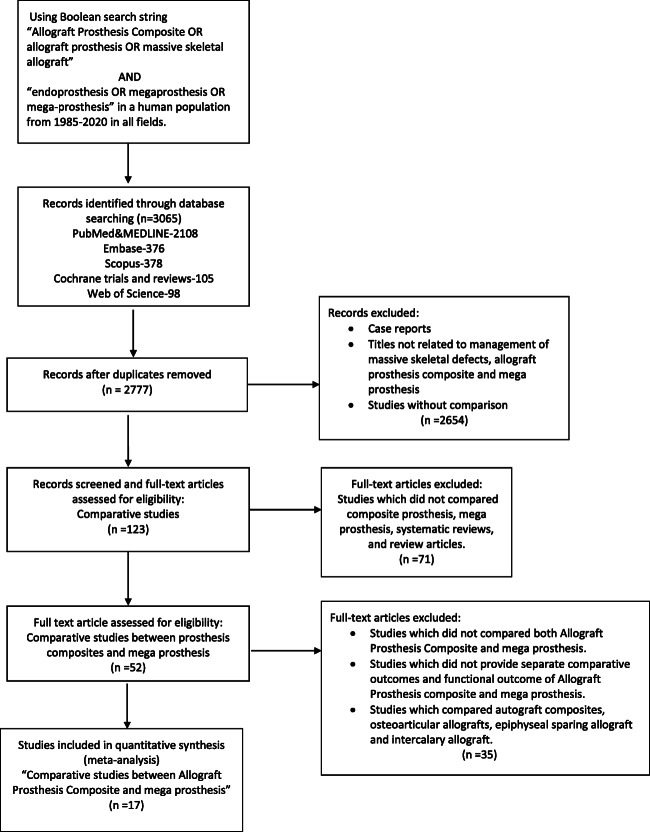
Two independent reviewers (RM&DG) screened all the articles. All the articles that reported the use of a megaprosthesis or APC were included in the first screening stage. Articles published in a language other than English were also included in the study. Studies comparing these two modalities in skeletal defects caused by reasons other than tumors were also included. Studies that used only osteoarticular allograft, autograft composites, epiphyseal sparing allografts, intercalary allografts, and those which did not compare megaprosthesis and APC were excluded. Isolated case reports and previously published systematic reviews were also excluded. Studies that did not evaluate separate comparative and functional outcomes were also excluded. Finally, only studies that reported the comparative outcome between the uses of the mega-prosthesis vs. Allograft Prosthesis Composite was included for further analysis. The search strategy is given in Fig. 1.
Fig. 1.
Flow chart demonstrating search strategy and the included studies according to the PRISMA guidelines
Study Characteristics
All studies included were comparative studies published from 1996–2020. Sixteen of 17 studies were retrospective, and one was prospective. Fourteen of 17 articles were published in English, two were in French, and one was in Chinese. Eight studies compared only APC and megaprosthesis, while remaining nine studies compared other modalities also such as osteoarticular allograft, arthrodesis, standard replacement components, autograft along with these two surgical procedures. All studies except one, dealt with skeletal defects caused by bone tumors, either malignant or benign. One study pertained to bony defects caused by periprosthetic fractures in the elderly population. Six studies were related to defects around the proximal humerus, five were related to defects around the proximal femur, three were related to defects around the knee joint and three were related to defects around the pelvis and periacetabular region. Fifteen of 17 studies used Musculoskeletal Tumor Society score (MSTS) as the functional outcome score. One study related to bony defects due to periprosthetic fractures in elderly used Knee Society Score (KSS) and the other one used International Society of Limb Salvage (ISOLS) score instead of MSTS score. Further detailed attributes of the study are illustrated in Table 1.
Table 1.
General detailed attributes of the included studies
| Name of the study | Type of study | Total number of patients | M/F ratio | Number of malignant and metastatic tumor | Site of the defect | Outcome studied | Disclosure | Level of evidence |
|---|---|---|---|---|---|---|---|---|
| Wang et al [12] | Retrospective | 25 | 10/15 | 20 | Proximal humerus tumor | Functional outcome (MSTS), complications | Not reported | IV |
| Michiel van de sande [13] | Retrospective | 37 | 21/16 | 28 | Proximal humerus tumor | Functional outcome (MSTS), complications and implant survival | Not reported | IV |
| Benedetti [14] | Retrospective | 20 | 17/3 | 14 | Proximal femur tumor | Functional assessment (MSTS) and gait analysis | None | IV |
| Anract [15] (French) | Retrospective | 41 | Not reported | 38 | Proximal femur tumor | Functional outcome (MSTS), complications, implant survival | Not reported | IV |
| Farid [16] | Retrospective | 72 | 45/27 | 69 | Proximal femur tumor | Functional outcome (MSTS), complications, implant survival | None | IV |
| Zehr [17] | Retrospective | 33 | 16/17 | 30 | Proximal femur tumor | Functional outcome (MSTS), complications, implant survival | Not reported | IIB |
| Muller [18] | Retrospective | 42 | 21/21 | 26 | Proximal tibia tumor | Functional outcome (ISOLS), complications, implant survival | None related to the subject of this article. | IV |
| Wunder [19] | Prospective | 75 | Not reported | 75 | Distal femur and proximal tibia tumor | Functional outcome (MSTS), complications, risk of operative revision | Not reported | IV |
| Saidi [20] | Retrospective | 23 | 8/15 | - | Distal femur periprosthetic fracture | Perioperative mobility, complications and return to independence (KSS) | Reported but details not accessible. | IIIB |
| Kassab [21] (French) | Retrospective | 29 | 20/9 | 29 | Proximal humerus tumor | Clinica (MSTS) and radiological results | Not reported | IV |
| Manfrini [22] | Retrospective | 61 | Not reported | 61 | Proximal humerus tumor | Evolution of various limb salvage procedures, radiographic and functional outcome (MSTS) | None | IV |
| Potter [23] | Retrospective | 49 | 29/20 | 43 | Proximal humerus tumor | Functional outcome (MSTS), complications, implant survival | This study was supported by a restricted educational grant from Stryker, and one of the authors is a paid consultant of Stryker | IV |
| Jin Wang [24] (Chinese) | Retrospective | 62 | 29/33 | 62 | Proximal femur tumor | Functional outcome (MSTS) | – | – |
| Hillmann [25] | Retrospective | 110 | 65/45 | 110 | Pelvis tumor | Functional outcome (MSTS) and complications | Not reported | IV |
| Schwameis [26] | Retrospective | 30 | 12/18 | 30 | Pelvis tumor | Functional outcome (MSTS), complications and oncological result | Not reported | IV |
| Nota [27••] | Retrospective | 150 | 74/76 | 150 | Proximal humerus tumor | Functional outcome (MSTS) and complications | None related to the subject of this article | IV |
| Ippolito [28] | Retrospective | 23 | 9/14 | 19 | Periacetabular tumor | Functional outcome (MSTS) and complications | Not reported | IV |
MSTS, musculoskeletal tumour society scoring system; ISOLS, International Society of Limb Salvage; KSS, knee society score
Data Extraction
Information on patient demographics; number of benign, malignant, and metastatic cases; the site of the defect; indications for surgery; average follow-up time; length of the resection; amputation rate; mortality; functional outcome scores; implant survivorship; and complications, including revisions and failures, was extracted from the full texts and supplementary materials, when applicable. Full text of the Chinese language article was not assessed but sufficient amount of data was available in the abstract to be included in the study [24].
Outcome Measures
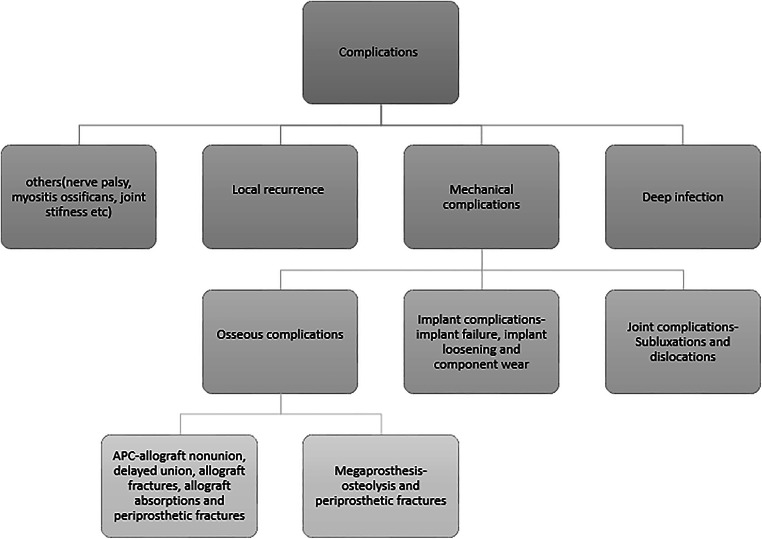
The primary measurements included functional outcome scores and implant survivorship of each treatment modality. The secondary outcome measures included complications, revision rates, amputation rates and mortality. The outcome scores were the reported scores for the appropriate anatomic sites or the overall follow-up scores to assess improvement in function. Complications were defined as any untoward outcome related to the surgical procedure, irrespective of the requirement of revision and resurgery. The revision was defined as any surgery that required the implantation of a new prosthesis or an allograft, or the removal of the either including amputation; resurgery included any surgical intervention other than revision performed for complications in the surgery. Failure in our meta-analysis was defined as any result requiring revision. Complications were subgrouped into deep infection, mechanical complications and local recurrence, and others, which included nerve palsy, joint stiffness, and myositis (Fig. 2). Mortality following the surgery was accounted for if it was directly related to the disease. Amputation was reported as nil if it was not reported or mentioned in a study.
Fig. 2.
Subgroups of complications reported in the studies. APC, Allograft Prosthesis Composite
Quality Assessment
Two independent observers conducted the analysis of the quality of the included studies using Newcastle-Ottawa quality assessment scale (Appendix 1) [29]. According to this scale four studies had five stars, five studies had six stars, five had seven stars and two had eight stars (Table 2). One article was excluded from the quality assessment as the full text of the Chinese article was inaccessible.
Table 2.
Quality assessment of the included studies
| Name of the study | Selection | Comparability | Outcome | Total score |
|---|---|---|---|---|
| Wang et al [12] | *** | ** | 5 | |
| Michiel van de sande [13] | *** | * | *** | 7 |
| Benedetti [14] | **** | * | ** | 7 |
| Anract [15] | ** | * | ** | 5 |
| Farid [16] | **** | *** | 7 | |
| Zehr [17] | **** | * | *** | 8 |
| Muller [18] | *** | * | ** | 6 |
| Wunder [19] | **** | ** | 6 | |
| Saidi [20] | **** | ** | ** | 8 |
| Kassab [21] | *** | * | ** | 6 |
| Manfrini [22] | **** | * | * | 6 |
| Potter [23] | **** | * | ** | 7 |
| Jin Wang [24] | Could not be assessed as full text was not available. | |||
| Hillmann [25] | *** | * | * | 5 |
| Schwameis [26] | *** | ** | 5 | |
| Nota [27••] | **** | ** | 6 | |
| Ippolito [28] | *** | * | *** | 7 |
*Scoring system in a particular category according to Newcastle- Ottawa quality assessment scale. The number of stars indicates its score in each category
Levels of Evidence
Only four studies reported their levels of evidence. To make the assessment of levels of evidence more uniform, and to improve the relevance of levels of evidence, the authors have used the levels of evidence as cataloged by Oxford Centre for Evidence-based Medicine—Levels of Evidence [30, 31]. According to these, 14 studies were of level IV, and only one each of level IIB and IIIB. One article was excluded from this assessment as the full text of the Chinese article was inaccessible.
Statistical Analysis
For continuous variables like MSTS, mean difference and 95% confidence interval were estimated by adopting random-effects model that includes the mean of the variable, standard deviation, and the total number of patients. Dichotomous variables such as complications, revisions, amputation risk ratio, and 95% confidence interval were calculated using the number of events that happened and the number of patients in each group. Risk ratio more than one suggests increased risk in the first group. Heterogeneity refers to the variation of the different outcomes between the studies. Qualitative evaluation of heterogeneity was done using chi-square test and p value. p value less than 0.05 was considered significant. Quantification of heterogeneity was done using I2 test. It represents the percentage of variation across the studies where <25%, 50%, and >50% indicate low, moderate, and high heterogeneity, respectively. The outcomes were pooled using the random effect model wherever heterogeneity was encountered. For studies not reporting standard deviations for MSTS score, they were derived from either ranges or by statistical imputation [32]. We used the RevMan 5 Software (Cochrane Collaboration, London SW1Y 4QX, UK) for statistical analysis.
Results
Seventeen studies with 882 patients were the subjects for meta-analysis. The study characteristics are given in Table 3 and 4. The megaprosthesis group consists of 427 patients while the APC group consisted of 201 patients and the other interventions consists of 254 patients. The differences in the mean age (APC vs. megaprosthesis: 42.3 ± 11 years vs. 39.8 ± 12 years, p=0.540) follow up (APC vs. megaprosthesis: 65.8 ± 28.1 months vs. 72.9 ± 41.2 months, p=0.571), mean length of resection (APC vs. megaprosthesis: 15 ± 2.6 cm vs. 14.1 ± 3.1 cm, p = 0.392) were not statistically significant. The proportion of malignant tumors in APC was 137/164 while in the megaprosthesis group, it was 369/385, and the difference was statistically significant (p<0.001).
Table 3.
Results of the included studies in megaprosthesis group
| Name of the Study | Number of patients | Mean age at surgery (years) | Mean follow up (in months) | Length of resection (in cms) | Implant survival | ||||
|---|---|---|---|---|---|---|---|---|---|
| 3 years | 5 years | 8 years | 10 years | 15 years | |||||
| Wang [12] | 6 | 32 | 48 | 11 | |||||
| Michiel van de sande [13] | 14 | 44 | 120 | 9.6 | 100% | ||||
| Benedetti [14] | 10 | 41 | 118 | 16 | |||||
| Anract [15] (French) | 20 | 43 | 72 | 17.5 | 73% | 49% | |||
| Farid [16] | 52 | 39 | 146 | 14.4 | 81.8 | 85.7% | 85.7% | ||
| Zehr [17] | 17 | 49.1 | 114 | Not reported | 65% | 58% | |||
| Muller [18] | 23 | 37.8 | 62 | 12.6 | 78% | ||||
| Wunder [19] | 64 | 33 | 50.7 | 18.1 | 91% | 85% | |||
| Saidi [20] | 7 | 76 | 6 | Not required | |||||
| Kassab [21] (French) | 15 | 37.5 | 85 | 13 | |||||
| Manfrini [22] | 25 | 10.5 | 136.8 | 15.3 | |||||
| Potter [23] | 16 | 53.6 | 34 | 13.6 | 100% | ||||
| Jin Wang [24] (Chinese) | 39 | 35 | 64 | - | |||||
| Hillmann [25] | 16 | 32 | 45.5 | Not reported | 90% | 80-90% | 80% | ||
| Schwameis [26] | 10 | 15.39 | 49.3 | Not reported | |||||
| Nota [27••] | 84 | 53 | 31.2 | 11 | |||||
| Ippolito [28] | 9 | 42.8 | 80.3 | Not reported | |||||
Table 4.
Results of the included studies in Allograft Prosthesis Composite group
| Name of the Study | Number of patients | Mean age at surgery (years) | Mean follow up (in months) | Length of resection (in cms) | Implant survival | |||
|---|---|---|---|---|---|---|---|---|
| 3 years | 5 years | 10 years | 15 years | |||||
| Wang [12] | 7 | 32 | 48 | 11 | ||||
| Michiel van de sande [13] | 10 | 34 | 120 | 12 | 90 | |||
| Benedetti [14] | 10 | 31 | 60 | 14 | ||||
| Anract [15] (French) | 21 | 40 | 50 | 17.7 | 77.12 | 77.12 | ||
| Farid [16] | 20 | 44 | 76 | 19.8 | 100% | 85.7% | 85.7% | |
| Zehr [17] | 16 | 40.7 | 63 | Not reported | 76 | 76 | ||
| Muller [18] | 19 | 41.8 | 62 | 14.4 | 93.7 | |||
| Wunder [19] | 11 | 42 | 31.1 | 15.6 | 22% | |||
| Saidi [20] | 7 | 79 | 6 | Not required | ||||
| Kassab [21] (French) | 10 | 37.5 | 85 | 13 | ||||
| Manfrini [22] | 3 | 8.6 | 52.8 | 17 | ||||
| Potter [23] | 16 | 56.3 | 82 | 12.5 | 91% | |||
| Jin Wang [24] (Chinese) | 14 | 35 | 64 | - | ||||
| Hillmann [25] | 6 | 32 | 45.5 | Not reported | ||||
| Schwameis [26] | 2 | 14 | 11 | Not reported | ||||
| Nota [27••] | 20 | 53 | 55 | 14 | ||||
| Ippolito [28] | 9 | 148 | 148 | Not reported | 85–95% | 60–70% | ||
Clinical and Functional Outcome Scores
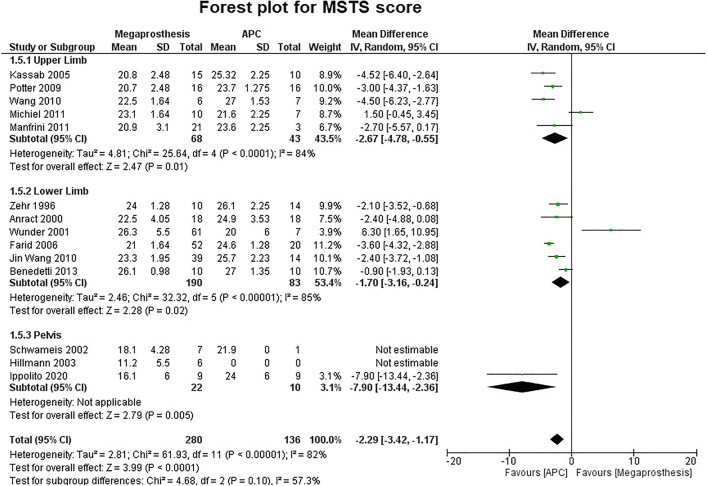
Fifteen studies contributed to MSTS score as the functional outcome score but 14 were used in our meta-analysis. Nota et al. reported similar MSTS score in both groups, but were excluded from analysis due to the unavailability of standard deviation [27••]. Statistical analysis revealed a significant difference between the two groups in favor of APC (MD, −2.29 95% CI, −3.42 −1.17; p=0.0001; I2 = 82%). Five studies involving upper limb six studies involving lower limb and three studies involving the pelvis also revealed a statistically significant difference in favor of APC (upper limb: MD, −2.67; 95% CI, −4.78 −0.55; p=0.01; I2 = 84%; lower limb: MD, −1.70; 95% CI, −3.16 −0.24; p=0.02; I2 = 85%; pelvis: MD, −7.90; 95% CI, −13.44 −2.36; p=0.005) (Fig. 3). One study reported ISOLS score instead of MSTS score and the result were slightly, but not significantly better in the APC group (APC: Excellent, 10/16; good, 5/16; fair, 1/16; Mega prosthesis: Excellent, 6/18; good, 11/18; fair, 1/18) [18]. Another study reported Knee Society Score and no significant difference noticed in 6 weeks (APC: 66, SE 7; MP: 74, SE 5) or 6 months (APC: 75, SE 7; MP: 92, SE 5) Knee Society Scores [20]. Limp was recorded in four studies which were involved in defects around the proximal femur [14–17]. Statistical analysis revealed a significant difference between the two groups in favor of APC (APC, 31/62, 50%; MP, 79/90, 87.7%; p<0.001). Two studies reported on muscle strength. One study found a significant difference in favor of the APC group (p=0.004; only one of 52 patients in the MP group had 5/5 MRC grade while 11 of 20 patients in APC had 5/5 strength), another study although favored APC, but the difference was not significant.
Fig. 3.
Forest plot analysis for musculoskeletal tumor society (MSTS) score. APC, Allograft Prosthesis Composite
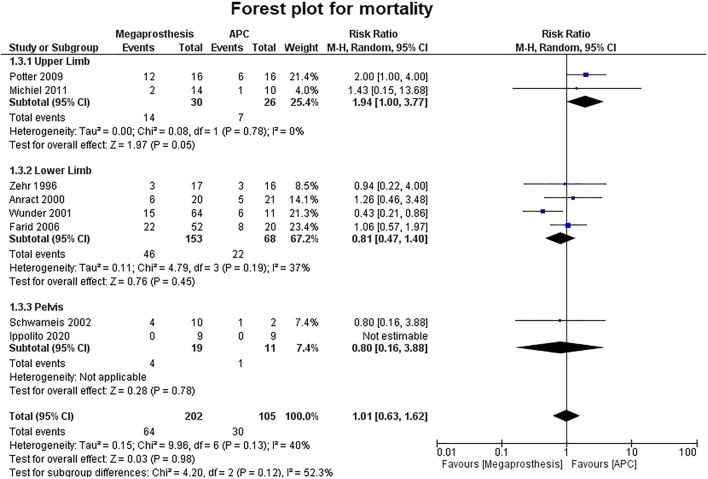
Mortality
Eight studies described mortality in separate groups associated with the disease. There was no statistically significant difference between the two groups (p = 0.98) (Fig. 4).Two studies involved the upper limb, and the difference was significantly better in favor of APC (RR = 1.94; 95% CI, 1.00–3.77; p = 0.05; I2 = 0%) while in other studies involving the lower limb and pelvis, no statistically significant difference was noted between the two groups (lower limb: p = 0.45; pelvis: p = 0.78).
Fig. 4.
Forest plot analysis for mortality. APC, Allograft Prosthesis Composite
Implant Survival and Revision
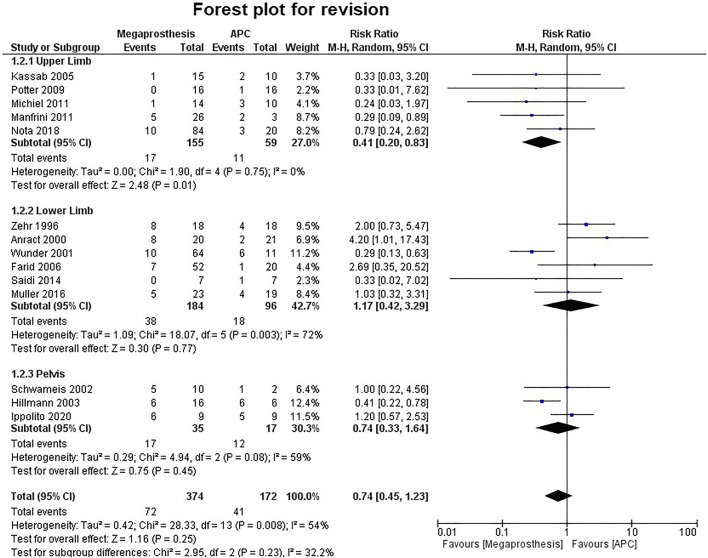
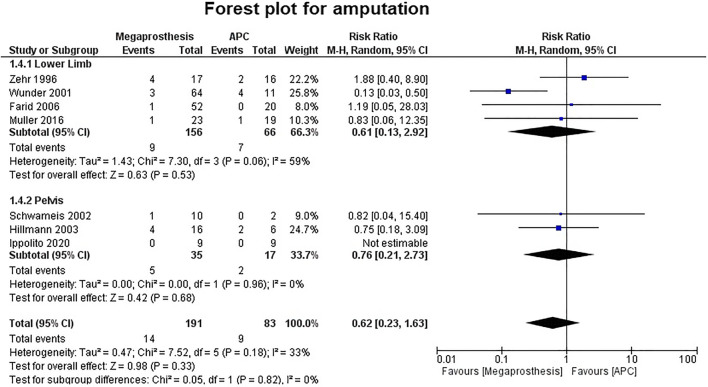
Seven studies contributed to 5-year implant survival, which ranges from 65 to 100% in the megaprosthesis group, while it ranges from 76 to 100% in the APC group. Five studies contributed to 10-year implant survival, which ranges from 58 to 90% in the megaprosthesis group, while it ranges from 76 to 93.7% in the APC group. Fourteen studies analyzed revision rates. The revision was more common in the APC group, but the difference was not statistically significant (RR.0.74; 95% CI, 0.45 −1.23; p=0.25; I2 = 54%) (Fig. 5).Five studies were involved in the analysis of upper limb revision and the difference was statistically significant in favor of megaprosthesis (RR.0.41; 95% CI, 0.20 −0.83; p=0.01; I2 = 0%), while six studies, which involved the lower limb (p=0.77) and three studies, which involve the pelvis (p=0.08) did not reveal any significant difference. Deep infection was the leading cause for revision that was found in both groups (APC, 18/41, 43.9% vs. megaprosthesis, 21/72, 29.1%). Aseptic loosening (17/72, 23.6%) was the next most common reason for revision in the megaprosthesis group, while allograft fracture (9/41, 21.9%) was the next most common reason for revision in the APC group. Amputation was the reported outcome in seven studies and their analyses failed to establish any significant difference between the two groups (p=0.33) (Fig. 6).
Fig. 5.
Forest plot analysis for revision rates. APC, Allograft Prosthesis Composite
Fig. 6.
Forest plot analysis for amputation rate. APC, Allograft Prosthesis Composite
Complications
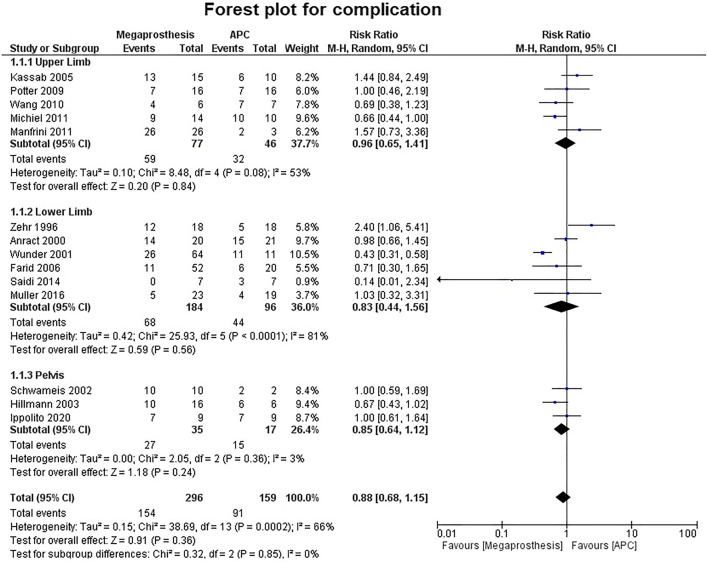
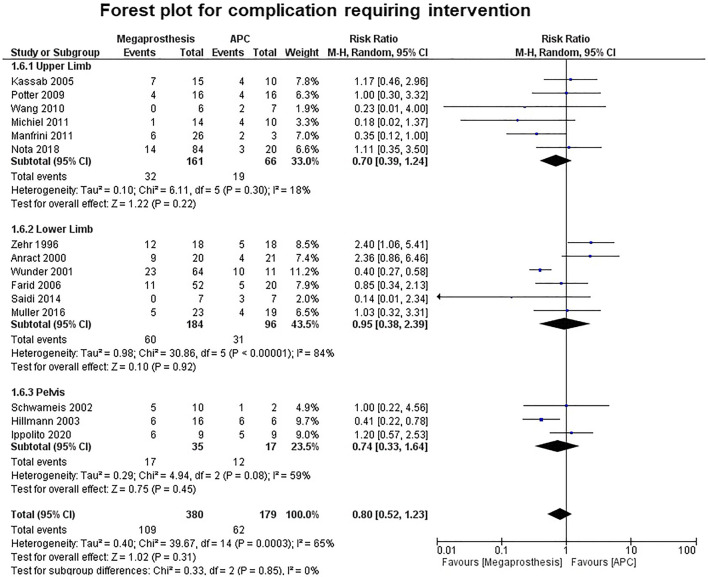
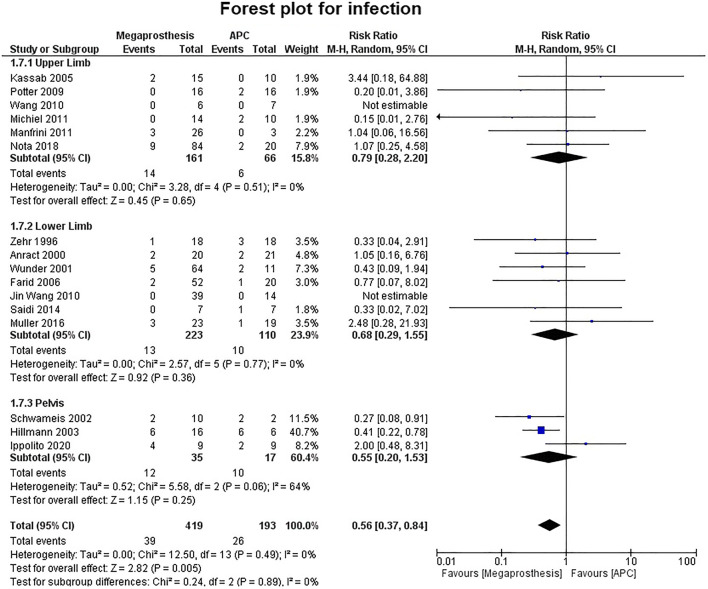
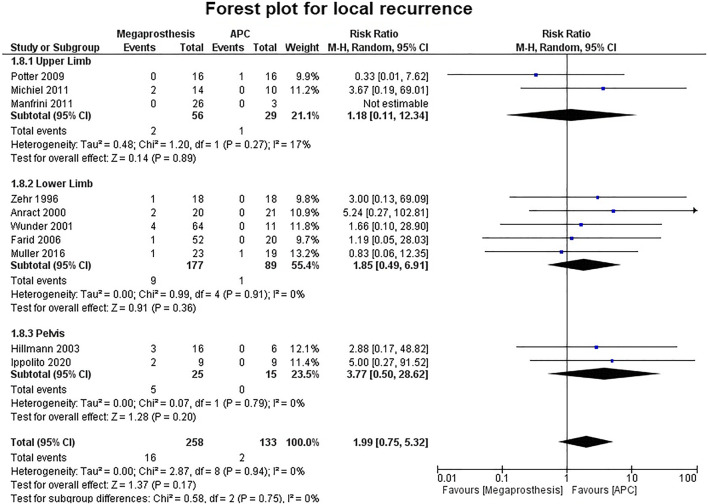
Fourteen studies contributed to the analysis of the number of complications and no significant difference was established between the two groups (p=0.36) (Fig. 7). Five studies were of upper limb, six studies of lower limb, three studies of pelvis and no significant difference was established between the two groups (upper limb: p=0.84; lower limb: p=0.56; pelvis: p=0.24). No significant difference was established between the two groups in the number of complications requiring intervention (p=0.31) (Fig. 8). Among the complications as a group, mechanical complications were the commonest followed by deep infection and local recurrence. Statistical analysis demonstrated significantly increased proportion of mechanical complications in the APC group (Fig. 9a and b) (APC, 102/144, 0.71; megaprosthesis, 163/276, 0.59; p=0.023). Among the mechanical complications, in the megaprosthesis group, joint-related instability complications (105/163, 64.4%) were the commonest complications followed by implant-related (43/163, 26.3%) and osseus complications (15/163, 9.2%), while in the APC group, allograft-related complications (54/102, 52.9%) were the commonest followed by joint-related (38/102, 37.2%) and implant-related complications (10/102, 9.8%). Statistical analysis of these complications demonstrated significantly increased proportion of joint-related (APC, 0.37; megaprosthesis, 0.64; p<0.001) and implant-related complications (APC, 0.09; megaprosthesis, 0.26; p<0.002) in the megaprosthesis group and significantly increased proportion of osseus complications in the APC group (APC, 0.52; megaprosthesis, 0.09; p<0.001). Dislocations followed by implant loosening were the most common mechanical complications in the megaprosthesis group, whereas allograft nonunion and delayed union were the most frequent mechanical complications in the APC group. Sixteen studies contributed to the analysis of the incidence of deep infection and the difference was significantly better in the megaprosthesis group (RR.0.56; 95% CI, 0.37 −0.84; p=0.005; I2 = 0%). Subgroup analysis of upper limb, lower limb and pelvis did not affirm any significant difference between the two groups (upper limb: p=0.65; lower limb: p=0.36; pelvis: p=0.25) (Fig. 10). Local recurrence was reported in 10 studies and no statistically significant difference was established between the two groups (p= 0.17). Subgroup analysis of upper limb, lower limb and pelvis also did not affirm any significant difference between the two groups (upper limb: p=0.89; lower limb: p=0.36; pelvis: p=0.20) (Fig. 11). Individually, deep infection is the most frequent complication in both groups, followed by dislocations and implant loosening in the megaprosthesis group, and allograft nonunion and delayed union in the APC group.
Fig. 7.
Forest plot analysis for complications. APC, Allograft Prosthesis Composite
Fig. 8.
Forest plot analysis for complications requiring intervention. APC, Allograft Prosthesis Composite
Fig. 9.
a Pie chart showing the proportions of different complications in the Allograft Prosthesis Composite Group (APC). b Pie chart showing the proportions of different complications in the megaprosthesis group
Fig. 10.
Forest plot analysis for deep infection. APC, Allograft Prosthesis Composite
Fig. 11.
Forest plot analysis for local recurrence. APC, Allograft Prosthesis Composite
Discussion
There has been a considerable debate concerning the preferable mode of intervention for substantial skeletal defects in the appendicular skeleton. APC and megaprosthesis are the two most commonly accepted methods of choice. However, there has not been enough consensus and conclusive evidence regarding the preference of one over another [33, 34, 35•].
Our meta-analysis demonstrated that MSTS score was significantly better in the APC group for all three sites, i.e., upper limb, lower limb, and pelvis as well. This is in contrast to a systematic review by Teunis et al. , which showed no significant difference between the two groups in the upper limb [33]. A review of the literature on the reconstruction of the proximal humerus had reported MSTS scores ranging from 61 to 87% in the megaprosthesis group and 66.6 to 100% in the APC group [36–42]. Similarly, in our meta-analysis, five studies were involved in the analysis of upper limb scores and it ranged from 72 to 90% in the APC group, while it ranges from 69 to only 77% in the megaprosthesis group. Similar functional results compared to our meta-analysis have been reported in lower limb and pelvis. Jansseen et al. in his systematic review of reconstruction on proximal femur tumors reported MSTS score ranging from 56 to 94% in the megaprosthesis group and 58–93% in the APC group [43•]. Brown et al. in his systematic review of the pelvis reconstruction reported MSTS score ranging from 51 to 64% in the megaprosthesis group and 72% in the allograft group [44]. Wang et al. in his article on the proximal humerus reconstruction reported that range of motion, particularly shoulder abduction was better in the APC group [12]. Potter et al. in his series stated that majority of the patients had abduction more than 90° in the APC group and the difference was statistically significant (12/16 in APC vs. 4/16 in megaprosthesis groups) [23]. Kassab et al. in their study on proximal humerus reconstructions after resection of the tumor reported that range of motion was better in the APC group, but it was the reverse shoulder prosthesis that fared better [21]. They recommended either megaprosthesis or scapulohumeral arthrodesis as options if rotator cuff and deltoid muscle (axillary nerve) were resected during resection. However, APC is an option if resection preserves the rotator cuff and the deltoid muscle, securing the cuff muscles to the allograft bone. Lazerges et al. stated that sometimes sacrifice of rotator cuff is necessary to achieve clear margins during composite reverse shoulder arthroplasty. In that scenario, reverse shoulder arthroplasty provides a logical solution and a reasonable result by providing better mobility, functional recovery, and quality of life [45].
One important component of MSTS score is gait and improved muscular strength means better gait and less limp [16]. Our meta-analysis demonstrates the superiority of APC in terms of better gait and significantly less limp. Anract et al., explained the importance of biological reinsertion of periarticular muscles on allograft as the insertions on a metal body in MP eventually fails [15]. Zehr et al. stated that method for attaching the hip abductor muscles (direct suturing, trochanteric reattachment, or suturing to the surrounding soft tissues) made no difference to the functional outcome of either type of reconstruction [17] .
Our meta-analysis demonstrated that revision rates in upper limb were significantly better in the megaprosthesis group (10.96% in the megaprosthesis group vs. 18.6% in APC) without any heterogeneity. This is in contrast to other individual studies that have reported revision rates in the upper limb. Ruggieri et al. [41] in series of proximal humerus reconstruction with APC demonstrate 14.2% revision rate. Similarly, Abdeen et al. [39] reported revision rate of 8.3% in proximal humerus defects treated with APC. Goulding et al. reported the revision rate of 46.1% on use of compressive osseointegration endoprostheses for reconstruction of the upper limb after tumor resection while Tang et al. reported 16% revision rates in the reconstruction of the elbow with custom made prosthesis [46••, 47].
Deep infection was the most frequent complication and the most common cause of revision in our meta-analysis. The incidence of deep infection in our study was 13.4% in the APC group and 9.3% in the megaprosthesis group. Various studies have reported infection rates ranging from 0% to 24% in APC [8, 41, 48, 49], and 0 to 33% in the megaprosthesis group [47–53]. Groundland et al. in his systematic review concluded that infection was the most common cause of revision in the endoprosthesis group while allograft fracture and deep infection were the most common cause of revision in the APC group [54]. Moreover, Schmidt-Braekling et al. in his review of literature on silver-coated megaprosthesis concluded that there is not enough evidence of its use in preventing infection. Statistically significant more number of infections in the APC group highlights the importance of future research in allograft processing and procurement [55]. Few studies have used irradiated allograft [15, 19, 21]. The irradiation of an allograft can sometimes alter their mechanical properties, but the dose that can cause a risk of fracture remains controversial [19, 56]. A few studies that used irradiated large segment allografts for oncologic reconstructions found fracture rates similar to nonirradiated grafts [57–60].
Limitations
There are certain limitations to this meta-analysis. The most important controlling factor was the number of patients and had a vast difference in the number of patients in both groups in many studies, and the number of malignant patients in both groups [13, 16, 19, 21, 22, 25, 26, 27••]. This heterogeneity may be responsible for potential bias in implant survival and the complication rate. Though these comparative studies demand RCTs, practically it is difficult to have RCTs in these cases. Moreover, most of these studies were retrospective in nature leading to the inherent flaws of the study [12–18, 20–26, 27••, 28].
The second most important controlling factor was the mean follow-up period where complications and implant survival were studied. There were few studies where follow-up was 5 or more years in both groups [13, 14, 16–18, 21, 24, 28]. There was a large difference in the follow-up period in some studies [15–17, 19, 22, 23, 26, 28]. Potter et al., followed up the megaprosthesis group for 34 months while it followed up the APC group for 80 months [23]. Given the fact that 5-year implant survival is 100% in megaprosthesis groups cannot be accredited as an advantage to the megaprosthesis group. Saidi et al. compared APC’s and megaprosthesis in supracondylar periprosthetic fractures around the knee and follow-up period was only 6 months [20]. No complications were reported in the megaprosthesis group. Even though all other confounding factors were taken into consideration, the follow-up period was too little to report most complications. Hillmann et al. in his article on the pelvis reconstruction could not report functional outcome in the APC group as all cases in that group underwent revision [25]. This did not correlate well with the overall revision rates in the meta-analysis, as there was no significant difference between the two groups. This highlights the importance of sample size and the other confounding factors in any study.
Benedetti et al. did a functional assessment by gait analysis and the important confounding factor in their study was the difference in mean age [14]. Although they have used the same surgical technique and postoperative rehabilitation, yet outcome in favor of the APC group could be attributed to the younger age group in the said population. The quality of a cohort can be defined in terms of whether the comparison groups were defined or not, whether the outcome was measured in a blinded, objective way or if it failed to control known confounding factors. Sampling biased toward the group having the favorable outcome is also an indicator of quality of cohort studies [31]. A couple of studies in our meta-analysis give the impression that sampling was biased in favor of the group having the desired outcome [16, 19]. Few studies [13, 15, 16, 19, 22–26, 27••] could not control for confounding factors, while others [12–14, 21, 22, 25, 26, 28] did not mention that the outcome was measured in a blinded way. Finally, some studies failed to clearly define the comparison group separately [12, 13, 18, 21, 22, 24, 26, 27••].
Previously published systematic review of reconstruction of proximal femur tumors by Janssen et al. could not report the outcomes and complications in different groups separately [43•]. With the exception of few studies and complications such as local recurrence, mortality, and amputation rate in our meta-analysis, most of the other studies have reported different complications separately in both the groups [12, 20, 21, 24, 26, 27••]. No meta-analysis has published to date on this topic, and most included studies have used uniform method of surgical technique and allograft procurement and functional scoring system.
Conclusion
APC and megaprosthesis are the time-tested and proven techniques for the reconstruction of skeletal defects with different advantages of each over the other. Our first of a kind meta-analysis on this topic has statistically concluded the superiority of APC over megaprosthesis in overall functional outcome scores and significantly reduced limp and better muscle strength in the lower limb. Overall revision rates did not have a significant difference between the two groups but revision in upper limb significantly favor megaprosthesis. This is particularly important in the younger or pediatric age group where revisions are more likely to occur frequently, but patients adapt better to meet their functional demands. Although, both groups have high rates of complications, significantly more mechanical complications in APC demands further improvement in allograft procurement and processing. Early detection, neoadjuvant chemotherapy, radiotherapy and improved diagnostic and surgical techniques have made amputation and mortality rates almost similar in both groups. With the increase in long-term survival of patients with tumors, functional demands placed on these reconstructions will increase and will need to be addressed. The authors, based on the results of the meta-analysis recommended the use of megaprosthesis, especially in upper limb to reduce the number of revisions following infections and mechanical complications. APCs in high demand young individuals could be an option considering the significantly better functional outcome provided the risk of infection should be explained to the patient. However, further high-quality studies are required to provide a better understanding of the outcomes of megaprosthesis and APC, and future research should be directed toward improving surgical techniques, implants, and allograft processing. The authors recommend multicenter prospective comparative studies between the two treatment modalities and invite the researchers around the globe for future participation.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable
Acronyms
- APC
Allograft Prosthesis Composite
- MP
Megaprosthesis
- RCT
Randomized controlled trial
- MSTS
Musculoskeletal Tumor Society Scoring system
- KSS
Knee Society Score
- ISOLS
International Society of Limb Salvage
- MD
Mean difference
- CI
Confidence interval
- RR
Risk ratio
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors consented for the publication and the order of authorship according to their contribution.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deepak Gautam, Email: cmcdeepak@yahoo.com.
Nitish Arora, Email: narora8756@gmail.com.
Saurabh Gupta, Email: dr.saurabhortho@gmail.com.
Jaiben George, Email: jaibengeorge@gmail.com.
Rajesh Malhotra, Email: rmalhotra62@gmail.com.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Sakellariou VI, Babis GC. Management bone loss of the proximal femur in revision hip arthroplasty: update on reconstructive options. World journal of orthopedics. 2014;5(5):614–622. doi: 10.5312/wjo.v5.i5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panegrossi G, Ceretti M, Papalia M, et al. Bone loss management in total knee revision surgery. Int Orthop. 2014;38(2):419–427. doi: 10.1007/s00264-013-2262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groundland JS, Binitie O. Reconstruction after tumor resection in the growing child. The Orthopedic clinics of North America. 2016;47(1):265–281. doi: 10.1016/j.ocl.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 4.M. P. A. Bus MAJvdS. Taminiau AHM, Dijkstra PDS. Is there still a role for osteoarticular allograft reconstruction in musculoskeletal tumour surgery? A long-term follow-up study of 38 patients and systematic review of the literature. Bone Joint J. 2017;99-B(4):522–530. doi: 10.1302/0301-620X.99B4.BJJ-2016-0443.R2. [DOI] [PubMed] [Google Scholar]
- 5.Toy PC, White JR, Scarborough MT, et al. Distal femoral osteoarticular allografts: long-term survival, but frequent complications. Clin Orthop Relat Res. 2010;468(11):2914–2923. doi: 10.1007/s11999-010-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houdek MT, Wagner ER, Wilke BK, et al. Long term outcomes of cemented endoprosthetic reconstruction for periarticular tumors of the distal femur. Knee. 2016;23(1):167–172. doi: 10.1016/j.knee.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Pala E, Henderson ER, Calabro T, et al. Survival of current production tumor endoprostheses: complications, functional results, and a comparative statistical analysis. J Surg Oncol. 2013;108(6):403–408. doi: 10.1002/jso.23414. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra R, Kiran Kumar GN. V KD, Kumar V. The clinical and radiological evaluation of the use of an allograft-prosthesis composite in the treatment of proximal femoral giant cell tumours. Bone Joint J. 2014;96-B(8):1106–1110. doi: 10.1302/0301-620X.96B8.33611. [DOI] [PubMed] [Google Scholar]
- 9.Mayle RE, Jr, Paprosky WG. Massive bone loss: allograft-prosthetic composites and beyond. The Journal of bone and joint surgery British. 2012;94(11 Suppl A):61–64. doi: 10.1302/0301-620X.94B11.30791. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris JD, Quatman CE, Manring MM, et al. How to write a systematic review. Am J Sports Med. 2014;42(11):2761–2768. doi: 10.1177/0363546513497567. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Guo Z, Li J, et al. Functional outcomes and complications of reconstruction of the proximal humerus after intra-articular tumor resection. Orthop Surg. 2010;2(1):19–26. doi: 10.1111/j.1757-7861.2009.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Sande MA, Dijkstra PD, Taminiau AH. Proximal humerus reconstruction after tumour resection: biological versus endoprosthetic reconstruction. Int Orthop. 2011;35(9):1375–1380. doi: 10.1007/s00264-010-1152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedetti MG, Bonatti E, Malfitano C, Donati D. Comparison of allograft-prosthetic composite reconstruction and modular prosthetic replacement in proximal femur bone tumors: functional assessment by gait analysis in 20 patients. Acta Orthop. 2013;84(2):218–223. doi: 10.3109/17453674.2013.773119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anract P, Coste J, Vastel L, et al. Proximal femoral reconstruction with megaprosthesis versus allograft prosthesis composite. A comparative study of functional results, complications and longevity in 41 cases. Revue de chirurgie orthopedique et reparatrice de l'appareil moteur. 2000;86(3):278–288. [PubMed] [Google Scholar]
- 16.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. doi: 10.1097/01.blo.0000181491.39048.fe. [DOI] [PubMed] [Google Scholar]
- 17.Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res. 1996;322:207–223. [PubMed] [Google Scholar]
- 18.Muller DA, Beltrami G, Scoccianti G, et al. Allograft-prosthetic composite versus megaprosthesis in the proximal tibia—what works best? Injury. 2016;47(Suppl 4):S124–SS30. doi: 10.1016/j.injury.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Wunder JS, Leitch K, Griffin AM, et al. Comparison of two methods of reconstruction for primary malignant tumors at the knee: a sequential cohort study. J Surg Oncol. 2001;77(2):89–99. doi: 10.1002/jso.1076. [DOI] [PubMed] [Google Scholar]
- 20.Saidi K, Ben-Lulu O, Tsuji M, et al. Supracondylar periprosthetic fractures of the knee in the elderly patients: a comparison of treatment using allograft-implant composites, standard revision components, distal femoral replacement prosthesis. J Arthroplast. 2014;29(1):110–114. doi: 10.1016/j.arth.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Kassab M, Dumaine V, Babinet A, et al. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. Revue de chirurgie orthopedique et reparatrice de l'appareil moteur. 2005;91(1):15–23. doi: 10.1016/s0035-1040(05)84271-0. [DOI] [PubMed] [Google Scholar]
- 22.Manfrini M, Tiwari A, Ham J, et al. Evolution of surgical treatment for sarcomas of proximal humerus in children: retrospective review at a single institute over 30 years. J Pediatr Orthop. 2011;31(1):56–64. doi: 10.1097/BPO.0b013e318202c223. [DOI] [PubMed] [Google Scholar]
- 23.Potter BK, Adams SC, Pitcher JD, et al. Proximal humerus reconstructions for tumors. Clin Orthop Relat Res. 2009;467(4):1035–1041. doi: 10.1007/s11999-008-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Yin J, Zou C, et al. Surgical treatment of proximal femoral malignant tumors. Chinese journal of reparative and reconstructive surgery. 2010;24(7):881–884. [PubMed] [Google Scholar]
- 25.Hillmann A, Hoffmann C, Gosheger G, et al. Tumors of the pelvis: complications after reconstruction. Arch Orthop Trauma Surg. 2003;123(7):340–344. doi: 10.1007/s00402-003-0543-7. [DOI] [PubMed] [Google Scholar]
- 26.Schwameis E, Dominkus M, Krepler P, et al. Reconstruction of the pelvis after tumor resection in children and adolescents. Clin Orthop Relat Res. 2002;402:220–235. doi: 10.1097/00003086-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Nota S, Teunis T, Kortlever J, et al. Functional outcomes and complications after oncologic reconstruction of the proximal humerus. The Journal of the American Academy of Orthopaedic Surgeons. 2018;26(11):403–409. doi: 10.5435/JAAOS-D-16-00551. [DOI] [PubMed] [Google Scholar]
- 28.Ippolito J, Thomson J, Beebe K, et al. Outcomes following periacetabular tumor resection: a 25-year institutional experience. J Surg Oncol. 2020. 10.1002/jso.26088 Online ahead of print. [DOI] [PubMed]
- 29.Wells GA, Shea B, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp: Ottawa Hospital Research Institute; 2014.
- 30.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bob Phillips CB, Dave Sackett, Doug Badenoch, et al. Oxford Centre for Evidence-based Medicine – Levels of Evidence. 2009. CEBM, university of oxford2009. Available from: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/.
- 32.Weir CJ, Butcher I, Assi V, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol. 2018;18(1):25. doi: 10.1186/s12874-018-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teunis T, Nota SP, Hornicek FJ, et al. Outcome after reconstruction of the proximal humerus for tumor resection: a systematic review. Clin Orthop Relat Res. 2014;472(7):2245–2253. doi: 10.1007/s11999-014-3474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubina A, Shiu B, Gilotra M, et al. What is the optimal reconstruction option after the resection of proximal humeral tumors? A systematic review. The open orthopaedics journal. 2017;11:203–211. doi: 10.2174/1874325001711010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautam D, Malhotra R. Megaprosthesis versus allograft prosthesis composite for massive skeletal defects. Journal of clinical orthopaedics and trauma. 2018;9(1):63–80. doi: 10.1016/j.jcot.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Connor MI, Sim FH, Chao EY. Limb salvage for neoplasms of the shoulder girdle. Intermediate reconstructive and functional results. J Bone Joint Surg Am. 1996;78(12):1872–1888. doi: 10.2106/00004623-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77(8):1154–1165. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Smolle MA, Andreou D, Tunn PU, Leithner A. Advances in tumour endoprostheses: a systematic review. EFORT open reviews. 2019;4(7):445–459. doi: 10.1302/2058-5241.4.180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdeen A, Hoang BH, Athanasian EA, et al. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406–2415. doi: 10.2106/JBJS.H.00815. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Shen J, Dickinson IC. Functional outcome of arthrodesis with a vascularized fibular graft and a rotational latissimus dorsi flap after proximal humerus sarcoma resection. Ann Surg Oncol. 2011;18(7):1852–1859. doi: 10.1245/s10434-010-1443-z. [DOI] [PubMed] [Google Scholar]
- 41.Ruggieri P, Mavrogenis AF, Guerra G, Mercuri M. Preliminary results after reconstruction of bony defects of the proximal humerus with an allograft-resurfacing composite. The Journal of bone and joint surgery British volume. 2011;93(8):1098–1103. doi: 10.1302/0301-620X.93B8.26011. [DOI] [PubMed] [Google Scholar]
- 42.Black AW, Szabo RM, Titelman RM. Treatment of malignant tumors of the proximal humerus with allograft-prosthesis composite reconstruction. J Shoulder Elb Surg. 2007;16(5):525–533. doi: 10.1016/j.jse.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 43.• Janssen SJ, Langerhuizen DWG, Schwab JH, Bramer JAM. Outcome after reconstruction of proximal femoral tumors: a systematic review. Journal of surgical oncology. 2019; 119(1): 120-9. Lower soft tissue failure for APC can be explained by allowing better reconstruction of soft tissue, and a higher rate of structural failure in the APC group requiring revision is predominantly caused by allograft fracture, allograft resorption, and allograft-host bone nonunion. [DOI] [PubMed]
- 44.Brown TS, Salib CG, Rose PS, et al. Reconstruction of the hip after resection of periacetabular oncological lesions: a systematic review. Bone Joint J. 2018;100-B(1 Supple A):22–30. doi: 10.1302/0301-620X.100B1.BJJ-2017-0548.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazerges C, Dagneaux L, Degeorge B, et al. Composite reverse shoulder arthroplasty can provide good function and quality of life in cases of malignant tumour of the proximal humerus. Int Orthop. 2017;41(12):2619–2625. doi: 10.1007/s00264-017-3538-7. [DOI] [PubMed] [Google Scholar]
- 46.Goulding KA, Schwartz A, Hattrup SJ, et al. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: a viable option in the upper extremity. Clinical orthopaedics and related research. 2017;475(6):1702–1711. doi: 10.1007/s11999-017-5258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang X, Guo W, Yang R, et al. Custom-made prosthesis replacement for reconstruction of elbow after tumor resection. J Shoulder Elb Surg. 2009;18(5):796–803. doi: 10.1016/j.jse.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 48.Biau DJ, Davis A, Vastel L, et al. Function, disability, and health-related quality of life after allograft-prosthesis composite reconstructions of the proximal femur. J Surg Oncol. 2008;97(3):210–215. doi: 10.1002/jso.20936. [DOI] [PubMed] [Google Scholar]
- 49.Donati D, Colangeli M, Colangeli S, et al. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin Orthop Relat Res. 2008;466(2):459–465. doi: 10.1007/s11999-007-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda T, Kakunaga S, Takenaka S, et al. Constrained total hip megaprosthesis for primary periacetabular tumors. Clin Orthop Relat Res. 2013;471(3):741–749. doi: 10.1007/s11999-012-2625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmolders J, Koob S, Schepers P, et al. Silver-coated endoprosthetic replacement of the proximal humerus in case of tumour-is there an increased risk of periprosthetic infection by using a trevira tube? Int Orthop. 2017;41(2):423–428. doi: 10.1007/s00264-016-3329-6. [DOI] [PubMed] [Google Scholar]
- 52.Kinkel S, Lehner B, Kleinhans JA, et al. Medium to long-term results after reconstruction of bone defects at the knee with tumor endoprostheses. J Surg Oncol. 2010;101(2):166–169. doi: 10.1002/jso.21441. [DOI] [PubMed] [Google Scholar]
- 53.Shih ST, Wang JW, Hsu CC. Proximal femoral megaprosthesis for failed total hip arthroplasty. Chang Gung Med J. 2007;30(1):73–80. [PubMed] [Google Scholar]
- 54.Groundland JS, Ambler SB, Houskamp LD, et al. Surgical and functional outcomes after limb-preservation surgery for tumor in pediatric patients: a systematic review. JBJS reviews. 2016;4(2):01874474-201602000-00002. doi: 10.2106/JBJS.RVW.O.00013. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt-Braekling T, Streitbuerger A, Gosheger G, et al. Silver-coated megaprostheses: review of the literature. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2017;27(4):483–489. doi: 10.1007/s00590-017-1933-9. [DOI] [PubMed] [Google Scholar]
- 56.Currey JD, Foreman J, Laketic I, et al. Effects of ionizing radiation on the mechanical properties of human bone. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1997;15(1):111–117. doi: 10.1002/jor.1100150116. [DOI] [PubMed] [Google Scholar]
- 57.Donati D, Capanna R, Campanacci D, et al. The use of massive bone allografts for intercalary reconstruction and arthrodeses after tumor resection. A multicentric European study. La Chirurgia degli organi di movimento. 1993;78(2):81–94. [PubMed] [Google Scholar]
- 58.Hernigou P, Delepine G, Goutallier D, Julieron A. Massive allografts sterilised by irradiation. Clinical results. The Journal of bone and joint surgery British volume. 1993;75(6):904–913. doi: 10.1302/0301-620X.75B6.8245080. [DOI] [PubMed] [Google Scholar]
- 59.Lietman SA, Tomford WW, Gebhardt MC, et al. Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop Relat Res. 2000;375:214–217. doi: 10.1097/00003086-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 60.Loty B, Courpied JP, Tomeno B, et al. Bone allografts sterilised by irradiation. Biological properties, procurement and results of 150 massive allografts. Int Orthop. 1990;14(3):237–242. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.