Abstract
Introduction
There are limited data on the effects of forced medication switching for a nonmedical reason in patients with obstructive airway conditions. This study evaluated disruption in care resulting from a nonmedical medication switch for patients with asthma and/or chronic obstructive pulmonary disease who previously received the inhaled corticosteroid/long-acting β2-agonist budesonide/formoterol.
Methods
This retrospective pharmacy benefit prescription claims analysis evaluated Medicare Part D patients who filled a prescription for budesonide/formoterol as their last inhaled corticosteroid/long-acting β2-agonist in 2016 and were affected by a formulary block of budesonide/formoterol in 2017. Changes to respiratory maintenance therapy, length of gaps in care during which a patient was not in possession of a respiratory controller medication, acute medication use indicative of disease exacerbations, and medication adherence were assessed.
Results
A total of 42,553 patients were included in the analysis. Following the formulary block, 30,016 patients (71%) switched to another controller; 20,628 of these patients (69%) switched to a new inhaled corticosteroid/long-acting β2-agonist, 7081 (23%) stepped down to a monotherapy, and 2307 (8%) switched to a non-inhaled corticosteroid-containing controller. Despite the formulary block, 22,903 patients (54%) attempted to fill budesonide/formoterol as their first postblock controller, and 6624 patients (16%) attempted to return to budesonide/formoterol after switching to another controller. On average, patients experienced a gap in care of approximately 4 months without a controller medication. Also, 9674 (23%) did not fill any controller over the 1-year postblock period. Of those patients who experienced a gap in care, 14,926 (47%) filled a prescription indicative of a possible exacerbation during the gap period (i.e., oral corticosteroids for patients with asthma and oral corticosteroids and/or antibiotics for patients with chronic obstructive pulmonary disease).
Conclusions
The Medicare Part D formulary block was associated with disruption in the management of patients’ respiratory conditions and may have adversely impacted disease control.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41030-021-00147-8.
Keywords: Asthma, Budesonide/formoterol, Chronic obstructive pulmonary disease, Database
Key summary points
| Why carry out this study? |
| Nonmedical medication switches are particularly challenging for patients with obstructive airway conditions, as inhaler switching and multiple device use can lead to errors in inhalation technique, reduced disease control and quality of life, increased use of health care resources, and greater chance of unsuccessful treatment. |
| This study evaluated how a forced nonmedical medication switch affected disease management and control for patients with asthma or chronic obstructive pulmonary disease. |
| What was learned from the study? |
| In this prescription claims analysis of 42,553 Medicare Part D patients affected by a formulary block of the inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) budesonide/formoterol, 30,016 patients (71%) switched to another controller; 20,628 of these patients (69%) switched to an ICS/LABA, 7081 (23%) stepped down to a monotherapy, and 2307 (8%) switched to a non–ICS-containing controller. |
| The average gap in care when patients had no controller medication was 114 days; 47% of patients with a gap in care filled a prescription indicative of an exacerbation. |
| Nonmedical medication switches can significantly disrupt disease management, resulting in substantial morbidity for patients with asthma or chronic obstructive pulmonary disease. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13643246
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are obstructive airway conditions requiring individually tailored management to prevent symptoms and exacerbations [1, 2]. Effectiveness of and patient preference for inhaled maintenance therapies, such as an inhaled corticosteroid in combination with a long-acting β2-agonist (ICS/LABA), can vary depending on medication components and inhaler device attributes [3–5]. Additionally, proper use of inhaler devices is critical for effective management [6–9].
Although patients are often switched to alternate medications within a drug class to reduce insurance costs, forced medication switches for nonmedical reasons have been associated with reduced adherence and poor disease control [10, 11]. Nonmedical medication switches are particularly challenging for patients with asthma and COPD, as specific drug properties and inhaler devices differ among similar agents [12, 13]. Device type switching and multiple device use can lead to errors in inhalation technique, reduced disease control and quality of life, increased use of health care resources, and greater chance of unsuccessful treatment [14, 15].
Budesonide/formoterol (BUD/FORM) is a twice-daily ICS/LABA delivered via pressurized metered-dose inhaler (pMDI) for the treatment of asthma and COPD [16]. In 2016, BUD/FORM pMDI was a Medicare Part D payer’s preferred ICS/LABA maintenance inhaler therapy for obstructive lung diseases. Payer drug formularies detail the medications the payer will cover by therapeutic class, and the formularies are typically established on an annual basis. A payer may exclude medications they have elected not to cover or may provide a supplemental exclusion list that details medications not covered for the given year. In 2017, the Medicare Part D payer implemented a National Drug Code (NDC) formulary block on BUD/FORM, preferring two other ICS/LABA medications. When a medication is not included on a payer’s formulary or is listed on a payer’s exclusion list, it is considered to be blocked because access to coverage for the medication is restricted. The NDC formulary block placed on BUD/FORM by the Medicare Part D payer of interest resulted in a forced nonmedical medication switch.
The present quantitative tracking study investigated the impacts of this forced nonmedical medication switch by using patient claims-level data to identify changes in patient behavior and outcomes in the 1-year period following the formulary block. To this end, the main study objectives included the following: (1) describing respiratory maintenance regimen changes following the block; (2) quantifying patient attempts to either remain on BUD/FORM or return to it after initially switching to another controller therapy; (3) assessing prescription fills for acute medications postblock as an indicator of loss of disease control (rescue inhalers) or exacerbations (oral corticosteroid [OCS] fills for patients with asthma and OCS and/or antibiotic fills for patients with COPD); (4) quantifying gaps in care (i.e., length of time without a controller fill) postblock; and (5) evaluating changes in medication adherence for patients switching from twice-daily pMDI to once- or twice-daily ICS/LABAs delivered via dry powder inhaler (DPI). A secondary objective was to calculate patients’ out-of-pocket (OOP) costs from the preblock to postblock periods.
Methods
Study Design
This study was a retrospective pharmacy benefit prescription claims analysis tracking longitudinal behavior for Medicare Part D patients with a diagnosis of asthma and/or COPD following a BUD/FORM formulary block. The study focused on patients previously treated with BUD/FORM for asthma and/or COPD in 2016 and evaluated disruption in respiratory care for these patients after implementation of the formulary block in 2017. The study window was January 2016 to December 2017. The “preblock” and “postblock” periods refer to the time before and after the formulary block on January 1, 2017, respectively.
Data Source
IQVIA Longitudinal Access and Adjudication Data (LAAD) consist of deidentified longitudinal prescription data and capture approximately 85% of all pharmacy benefit prescriptions filled in the United States, including retail and mail-order prescriptions. Data are collected via direct feeds from pharmacies included in the IQVIA data supplier panel. The medications are identified in the database by the NDC. Encrypted patient identification numbers allow IQVIA to account for patients moving across data suppliers within the sample, enabling insight into patient behavior, cost, and payer access. Additionally, IQVIA LAAD are used to identify patient diagnosis via inclusion of diagnosis codes for a large subset of patients. International Classification of Diseases (ICD)-9/10 codes used to identify patients with asthma and/or COPD are described in Table S1 and Table S2 in the electronic supplementary material.
The data were previously collected and statistically deidentified and are compliant with the deidentification conditions set forth in Sections 164.514 (a)–(b)1ii of the Health Insurance Portability and Accountability Act of 1996 Privacy Rule. The authors only had access to the final database after the data were developed into an anonymized longitudinal patient-level dataset that is widely used across the industry. The authors used the final version of these data; therefore, additional data cleansing specific to this study was not required. The provisions in the Privacy Rule allow for use of health information that neither identifies nor provides a reasonable basis to identify an individual; therefore, approval from an institutional review board was not sought.
Patient Selection
Patients included in the analysis were those with asthma and/or COPD aged ≥ 12 years who had Medicare Part D coverage in 2016 and 2017 and received BUD/FORM as their last ICS/LABA medication in 2016. Patients moving from the Medicare Part D payer of interest to another insurer in 2017 were excluded. Age, sex, diagnosis, and Medicare Part D subsidy status were assessed. Patients were assigned a diagnosis of asthma, COPD, both asthma and COPD, or unknown based on available information. Across analyzed metrics, patients with both asthma and COPD diagnoses behaved like patients with only COPD, so they were grouped with the latter in the analyses. Furthermore, patients with both diagnoses first received a diagnosis for asthma and subsequently a diagnosis for COPD, implying they progressed from asthma to COPD and were technically patients with COPD during the timeframe of analysis. Patients with unknown diagnoses were assigned diagnoses based on their use of known and approved asthma and COPD treatment regimens. Medicare Part D subsidy status (low-income subsidy [LIS], standard eligible, or unknown subsidy status) was identified by assessing OOP costs across all therapeutic areas.
Main Outcome Measures and Analyses
This study evaluated metrics indicative of disruption of care that may have occurred because of the formulary block. Main outcomes of interest included changes in maintenance therapy, gaps in care when a patient was not in possession of a controller, acute medication use indicative of disease exacerbations, and medication adherence.
Changes in maintenance therapy were defined as: (1) filled no controller medication; (2) switched to a new dual ICS/LABA; (3) stepped down to monotherapy (ICS, LABA, long-acting muscarinic antagonists [LAMA], or leukotriene receptor antagonists [LTRA]); (4) stepped up to triple therapy (ICS/LABA and LAMA, ICS/LABA and LTRA, or ICS/LABA and any other respiratory product); (5) switched to a non-ICS/LABA controller (LAMA/LABA, mast cell stabilizer, methylxanthine, or biologic therapy); or alternatively, (6) remained on, or attempted to switch back to, BUD/FORM.
Length of gap in care, defined as the length of time during which a patient was not in possession of any respiratory controller, was quantified using the BUD/FORM days-supply, the formulary block date for BUD/FORM (January 1, 2017), and the switch date to a new controller. Start of the gap in care was the most recent of the following three dates: (1) last date of BUD/FORM days-supply a patient had on hand in 2017; (2) formulary block date for BUD/FORM; or (3) date a patient was blocked from BUD/FORM in 2017. The last date of the gap in care was the fill date of any controller. If patients had no time without possession of a controller, then they had no gap in care. In the database, an “attempt to fill” is any prescription that is sent to a pharmacy by a health care provider or brought to the pharmacy by a patient.
Acute respiratory medication use was measured during the gap in care as an indicator of possible disease exacerbation and included fill of a prescription of OCS for patients with asthma and OCS and/or antibiotics for patients with COPD, dual, and unknown diagnoses. Acute respiratory medication use was also measured in patients with no gap in care over the entire postblock period.
Adherence to ICS/LABA therapy was determined by proportion of days covered (PDC) and persistence metrics. PDC measures the proportion of days a patient had a drug in hand over the duration of a set time period. The numerator is the sum of the total days of therapy for a given patient across all filled prescriptions for that patient during the observed study period, and the denominator is the sum of the total days for the observed study period. Persistence measures the proportion of patients who remain on therapy month-over-month postinitiation, factoring in a 10-day grace period to fill [17]. For both analyses, patients switching to a once-daily or twice-daily DPI postblock were analyzed and compared with each other during the postblock period and compared with BUD/FORM pMDI in the preblock period. These adherence analyses were further limited to patients with ≥ 6 months of data in the preblock and postblock periods. The PDC analysis included patients who were continuously on either a once-daily or twice-daily DPI for 6 months postblock; patients filling prescriptions for both DPI formulations during the postblock period were not included. The persistence analysis included patients who used both DPI formulations postblock in both groups for the respective time periods during which they had fills for the specific therapy.
Acute respiratory medication use in both the preblock and postblock periods was also evaluated for those patients eligible for the PDC analysis. Number and type of acute medication fills were examined in the preblock and postblock periods.
Secondary Outcome Measures
Changes in year-over-year OOP costs were analyzed for patients who switched to another controller in 2017. For consistency, OOP costs for the same group of patients were analyzed in the preblock and postblock periods. OOP costs for these patients were examined across all pharmacy benefit data, as well as all respiratory classes (controller therapy or acute or rescue medications). OOP costs for patients who switched to a different ICS/LABA therapy or used an acute or rescue medication preblock and postblock were also analyzed separately.
Statistical Analysis
Descriptive statistics were used to summarize observed trends and patient behavior within the IQVIA LAAD dataset for all results. For the primary objective, number and percentage of patients are reported for the following analyses: access to BUD/FORM at the pharmacy postblock; respiratory maintenance regimen changes postblock; gap in care (time in which patient has no evidence of possession of any controller); PDC and persistence with a controller; acute medication use (OCS for patients with asthma, OCS and/or antibiotics for patients with COPD); and rescue inhaler use. For the secondary objective, the number and percentage of patients are reported for the following analyses: age, sex, diagnosis, and Medicare Part D subsidy status; BUD/FORM use postblock; changes in OOP costs preblock and postblock; and total prescription volume impact postblock. Data analyses were carried out using Microsoft® SQL Server Management Studio and/or Microsoft® Excel.
Results
A total of 76,389 patients covered by the Medicare Part D payer of interest who filled BUD/FORM as their last ICS/LABA medication in 2016 were identified; 42,553 were selected for inclusion (Figure S1 in the electronic supplementary material). Patients were predominantly female (64%), aged ≥ 65 years (61%), and classified as LIS (65%). Twenty-eight percent had an asthma diagnosis, 42% a COPD diagnosis, 18% a dual diagnosis of asthma and COPD, and 12% an unknown diagnosis. Age, sex, and disease status did not affect the primary or secondary outcomes; thus, the study reports on all eligible patients together (Table 1).
Table 1.
Baseline variables
| Demographic variable | Number of eligible patients (N = 42,553) |
|---|---|
| Sex | |
| Female | 27,334 (64%) |
| Male | 15,206 (36%) |
| Unknown | 13 (0%) |
| Age, years | |
| < 20 | 62 (< 1%) |
| 20–29 | 223 (1%) |
| 30–39 | 1003 (2%) |
| 40–49 | 2654 (6%) |
| 50–59 | 7314 (17%) |
| 60–64 | 5267 (12%) |
| ≥ 65 | 26,030 (61%) |
| Diagnosis | |
| Asthma | 12,009 (28%) |
| COPD | 17,797 (42%) |
| Dual (asthma and COPD) | 7838 (18%) |
| Unknown | 4909 (12%) |
| Medicare Part D subsidy status | |
| LIS, long-term care | 3397 (8%) |
| LIS, dual eligible | 5352 (13%) |
| LIS, non–dual eligible | 19,052 (45%) |
| Standard eligible | 14,752 (35%) |
COPD chronic obstructive pulmonary disease, LIS low-income subsidy
Respiratory Maintenance Regimen Changes Postblock
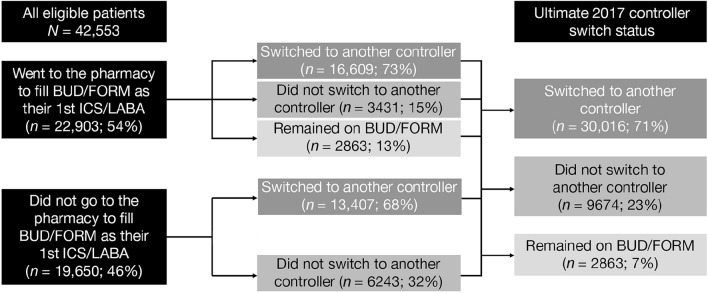
After implementing the formulary block on BUD/FORM, the Medicare Part D payer of interest saw an 88% drop in BUD/FORM prescription volume and a 90% drop in total number of patients taking BUD/FORM from December 2016 to December 2017. Of the 42,553 eligible patients, 54% attempted to fill a BUD/FORM prescription at the pharmacy after implementation of the formulary block (Fig. 1).
Fig. 1.
Eligible patient journey after formulary block.a The complex journey of eligible patients from their first prescription fill to their ultimate 2017 controller status is shown. BUD/FORM budesonide/formoterol, ICS inhaled corticosteroid, LABA long-acting β2-agonist. aNot all percentages sum to 100% as a result of rounding
Ultimately, 7% remained on BUD/FORM, 71% switched to another controller, and 23% did not switch to any controller (Fig. 1). Of note, for patients who dropped off of controller therapy postblock, the average BUD/FORM PDC preblock was 36%.
Some 23% of patients stepped down to monotherapy, 8% switched to a non-ICS/LABA combination of controllers, and 69% switched to a new ICS/LABA. Of the 20,628 patients who switched to another ICS/LABA, 8241 were prescribed a twice-daily DPI, 9822 a once-daily DPI, and 2565 switched to either a twice-daily pMDI ICS/LABA or multiple ICS/LABAs postblock (Table S3 in the electronic supplementary material).
Despite the formulary block, some patients remained on or attempted to return to BUD/FORM. A total of 8887 patients (20%) attempted to fill BUD/FORM in 2017, 2263 remained on BUD/FORM, and 6624 patients switched to another controller but then attempted to switch back to BUD/FORM. Of these patients, 2642 were ultimately approved for BUD/FORM. Two-thirds of patients attempting to switch back to BUD/FORM requested to do so within 60 days of beginning their new controller, 25% attempted to switch back multiple times, and 63% filled an acute medication indicative of a possible disease exacerbation within 30 days of their attempted switch. For comparison, of these patients who attempted to switch back to BUD/FORM during the postblock study period, a similar proportion (67%) had filled an acute care medication for their diagnosis over the entire 1-year 2016 preblock period.
Gap in Care
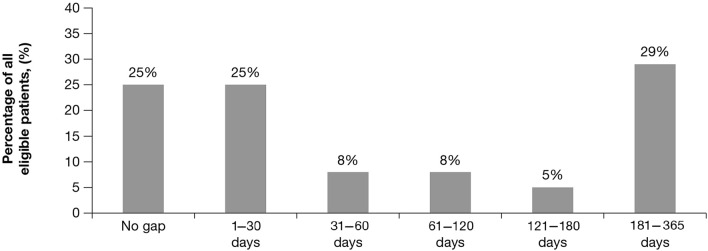
Postblock, the average gap in care (time without possession of any controller) experienced by the total group of 42,553 patients was 114 days. A total of 10,571 patients (25%) experienced no gap in care after implementation of the formulary block, 10,521 (25%) experienced a gap of ≤ 30 days, and 21,461 (50%) experienced a gap of > 30 days. A total of 12,320 (29%) patients had a gap in care of ≥ 6 months (Fig. 2).
Fig. 2.
Percentage of all eligible patients by length of gap in care (N = 42,553). The percentages of patients experiencing gaps in care are shown in 30-day increments
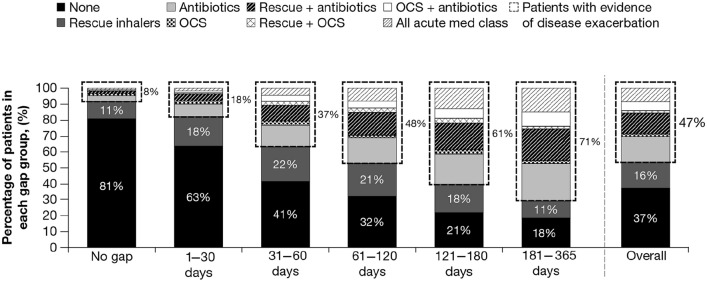
The percentage of patients filling acute medication prescriptions at the pharmacy during their gap in care, potentially showing evidence of disease exacerbation, increased as the length of the gap increased. Only 18% of patients (1915) with a gap in care of 1–30 days filled an acute medication, whereas 71% of patients (8755) with a gap of ≥ 6 months demonstrated evidence of a possible exacerbation. Patients with no gap in care were considered to represent a baseline level of acute medication fills when no gap in controller medications exists. Only 8% of patients with continuous controller possession (828) filled acute medications for the entire postblock period, whereas 47% (14,926) with any gap in care filled acute medication prescriptions during their gap period (Fig. 3).
Fig. 3.
Acute medication use: all eligible patients by length of gap in care (N = 42,553).a med medication, OCS oral corticosteroid. aNot all percentages sum to 100% as a result of rounding. b“Patients with evidence of disease exacerbation” are patients with asthma who filled prescriptions for OCS or patients with COPD who filled prescriptions for OCS and/or antibiotics. These patients were not included in the group of patients who filled only rescue inhalers because filling a rescue inhaler is not direct evidence of an exacerbation. c“All acute med class” includes any of the acute medications shown: antibiotics, rescue inhalers, or OCS. Specific medications included are shown in Table S4 in the electronic supplementary material
Preblock versus Postblock Patient Adherence and Acute Medication Fills
PDC, persistence, and acute medication use indicative of disease exacerbation were analyzed for patients with ≥ 6 months of data in the pre- and postblock periods. For patients who switched from BUD/FORM pMDI to a once-daily or twice-daily DPI, there were no observed differences in the PDC, persistence, or acute medication use between the preblock and postblock periods (or between once- vs. twice-daily therapies). Average PDC for patients switching to a twice-daily DPI was 64% preblock and 59% postblock; corresponding values for patients switching to a once-daily DPI were 63% and 60%, respectively. Similarly, no differences based on ICS/LABA device type or dosing regimen were observed for preblock and postblock persistence (averaging 40% at 6 months) or for fills of acute medications (70% of the study population). The majority (90%) of prescriptions were for a 30-day supply, 5% were for a 60-day supply, and 5% were for a 90-day supply.
Changes in OOP Cost From Preblock to Postblock Period
Changes in OOP costs year-over-year were analyzed for 32,879 patients who switched to another controller because of the formulary block. An average increase of 6% in OOP costs was observed across all therapeutic areas from the preblock to postblock periods. For all respiratory controllers, acute medications, and rescue medications, patients’ average OOP costs increased by 12%. Postblock, patients spent 49% of their total pharmacy OOP spend on respiratory products. For those patients switching to another ICS/LABA, average OOP costs rose 26%, whereas patients using acute or rescue medications experienced an average OOP cost increase of 18% (Table 2).
Table 2.
Average total OOP cost over time
| Respiratory OOP cost time period | Jan–Dec 2016 | Jan–Dec 2017 | Absolute dollar change | Percentage change (%) |
|---|---|---|---|---|
| All therapeutic areas (n = 32,879) | $550.65 | $581.93 | $31.21 | 6 |
| Respiratorya (n = 32,879) | $253.80 | $284.46 | $30.66 | 12 |
| ICS/LABA (n = 23,491) | $101.48 | $127.72 | $26.24 | 26 |
| Acute/rescue medicationsb (n = 25,974) | $53.44 | $62.84 | $9.40 | 18 |
COPD chronic obstructive pulmonary disease, ICS inhaled corticosteroid, LABA long-acting β2-agonist, OCS oral corticosteroid, OOP out-of-pocket
aIncludes all controller, acute, and rescue medications
bIncludes OCS for asthma, OCS and/or antibiotics for COPD, and rescue medication
Discussion
This forced nonmedical switch was associated with a disruption in disease management for patients with asthma or COPD, with 71% switching to another controller and 23% completely dropping off controller medications. Postblock, patients experienced an average gap in care of approximately 4 months without possession of any controller. During this gap period, signs of possible disease exacerbation were observed, with OCS and/or antibiotic medication fills increasing proportionate to the gap length.
The observations of this study are in line with others in the literature and the Global Initiative for Chronic Obstructive Lung Disease that underscore the need for inhaler device choice [18–20]. This study is also consistent with previous findings that show forcing patients to switch controller therapies for nonmedical reasons is associated with patient morbidity [10, 11]. Part of this morbidity may be due to a lack of effective communication by the insurer to patients and health care providers, resulting in delays for patients attempting to fill their medications: as evidenced by their attempts to obtain BUD/FORM postblock, more than 50% of the study patients may have been unaware of the forced nonmedical switch. Because this study utilized prescription claims data, we do not have direct knowledge of whether physicians were clearly informed of the formulary block and the resulting forced switch from BUD/FORM. Although physicians were likely informed by mail, it is not certain they would see or react to this type of correspondence prior to a prescription being written or a patient’s visit to a pharmacy.
Despite the formulary block, 20% of patients and their providers sought to fill BUD/FORM or attempted to switch back after filling another controller. Of patients attempting to switch back to BUD/FORM, 67% had filled an acute care medication over the entire 1-year 2016 preblock period; notably, a comparable proportion (63%) filled an acute medication within 30 days of their attempted switch in the postblock period, and the majority of switches were requested within 60 days of starting the new controller. These findings indicate that a large proportion of patients experienced exacerbations shortly prior to their switch attempt—similar to the proportion of those who experienced exacerbations during the entire year prior to the formulary block—and suggest that exacerbations were potentially proximally related to their attempts to switch back to BUD/FORM, especially given the longer 1-year period for a similar incidence of exacerbations prior to the forced switch. Acute exacerbations of COPD have an independent negative impact on patient prognosis, resulting in a burden on the health care system and an increase in mortality with the frequency of severe exacerbations [21, 22].
Although literature exists showing improved patient adherence for once- over twice-daily dosing for oral medications, this study focusing on inhaled combination ICS/LABA respiratory therapies demonstrated no differences between once- and twice-daily adherence [23–25]. Markers of loss of disease control also did not differ between once- and twice-daily ICS/LABA medications.
This study is similar to others showing that device consistency contributes to better outcomes for patients with obstructive lung disease [10, 11, 26, 27]. A total of 63% of patients who attempted to switch back to BUD/FORM pMDI, a device that is also used for rescue therapy, from their new non-pMDI controller therapy had evidence of a possible exacerbation in the 30 days prior to their request. Correct use of inhaler devices is fundamental to effective asthma and COPD management but represents an important challenge for the patient [18, 28–30]. Inhaler misuse has been associated with older age, lower level of education, and lack of instruction from a health care provider [29]. The fact that more than 50% of patients may not have been aware of the formulary change and went to the pharmacy to obtain a BUD/FORM prescription may also indicate that many were likely never trained on and/or observed for correct inhaler technique with their new controller. Moreover, some health care providers (including pharmacists and nurses) may not be proficient in teaching patients how to use these devices and do not often do so [31].
It is often thought that changes in formulary access represent an attempt to lower prescription drug costs for patients [32]. However, patients in this study saw OOP costs for ICS/LABA therapies increase 26% from the preblock to postblock period. Patients may also have to face costs of disease exacerbations, such as copays for emergency room visits and time off work. Moreover, insurers may also have to bear some costs of the resulting exacerbations [10, 11].
Limitations
Study findings should be interpreted with an understanding of potential limitations related to dataset capture, patient selection, and methodology. The current study is limited to patients who filled prescriptions through a specific Medicare Part D payer, and therefore generalizability of results is limited to patients with Medicare Part D insurance in the United States. The study was designed to control for patients switching to pharmacies outside of IQVIA’s network by making it an exclusion criterion that patients had to show pharmacy fill activity in any therapeutic area in the first half and second half of the preblock period (2016) and postblock period (2017). Additionally, the exact reasons behind a patient’s desire to switch (or not to switch) from one controller to another cannot be ascertained from the data. Furthermore, there is no way to determine if patients actually use a medication that they fill at a pharmacy or if they have obtained therapy outside of a pharmacy fill.
The study corrected for time with access to therapy for adherence and persistence, thus allowing direct comparisons for these metrics in the preblock and postblock periods. As a result of the limitations of the tools used for the data analysis, statistical significance could not be evaluated for the comparisons preblock and postblock. The study was not designed for direct comparisons of acute medication use or gaps in care in the preblock and postblock periods because the data alone cannot account for all of the reasons driving differences in these measures. Because the dataset is limited to pharmacy benefit claims, the study could not capture whether pharmacy claims for medications indicative of acute exacerbations might have resulted in emergency department or unscheduled ambulatory visits or hospitalizations; therefore, it is not known how such events might have contributed to the overall costs of the nonmedical switch. Acute respiratory medication use was not examined by the type of respiratory product to which patients switched.
Conclusions
Nonmedical medication switches can significantly disrupt disease management, resulting in substantial morbidity for patients with asthma or COPD. A large percentage of patients who switched to a different controller for nonmedical reasons filled prescriptions for acute medication in the 30 days prior to requesting to switch back to BUD/FORM. Similarly, for those who experienced a gap in care as a result of switching, the percentage of patients filling acute medication increased with the duration of the care gap. These findings suggest patients may have had more exacerbations after switching and thus the nonmedical switch may have adversely impacted disease control. Patients’ OOP costs also increased after the switch. Because of these impacts on disease control and economic costs to the patient, it is important to ensure that switching a patient’s inhaler includes consideration of the individual patient’s clinical status, medical needs, and ability to use the device correctly.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by AstraZeneca (Wilmington, DE, USA), the manufacturer of budesonide/formoterol. The journal’s Rapid Service Fee was funded by AstraZeneca.
Editorial and Other Assistance
Editorial assistance in the preparation of this article was provided by Shane Walton, PhD, CMPP, of MedErgy (Yardley, PA, USA). Support for this assistance was funded by AstraZeneca (Wilmington, DE, USA). Additional editorial support was provided by Carrie Lancos, ELS, CMPP, of AstraZeneca. The authors thank Jill R. Davis, MS, of AstraZeneca for her contributions to the early development of this study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
These data were presented in part as an oral presentation titled “Disruption in care after a forced formulary switch in inhaled respiratory medications” at the American College of Chest Physicians (CHEST) Annual Meeting; October 6–10, 2018; San Antonio, TX, USA.
Disclosures
Ileen Gilbert is an employee of AstraZeneca. Aanam Aslam Mahmood is an employee of IQVIA, and Katie Devane was an employee of IQVIA at the time this study was conducted and is currently an employee of Hayden Consulting Group. IQVIA was contracted by AstraZeneca to conduct this analysis. Laren Tan is an advisor for AstraZeneca; consultant for Boston Scientific; and speaker for AstraZeneca, Boehringer Ingelheim, Sanofi Genzyme, and Regeneron. A named author is an employee of AstraZeneca; therefore, AstraZeneca was involved in the study design, collection, analysis, and interpretation of data and the development and review of the manuscript. The decision to submit the manuscript for publication was made by the authors.
Compliance with Ethics Guidelines
The data were previously collected and statistically deidentified and are compliant with the deidentification conditions set forth in Sections 164.514 (a)-(b)1ii of the Health Insurance Portability and Accountability Act of 1996 Privacy Rule. The provisions in the Privacy Rule allow for the use of health information that neither identifies nor provides a reasonable basis to identify an individual; therefore, approval from an institutional review board was not sought.
Data Availability
The datasets generated and/or analyzed during the current study are third-party data available from the proprietary IQVIA LAAD and are therefore not publicly available. The database used for this study may be requested from IQVIA for a fee. The authors confirm they did not have any special access privileges that others would not have.
References
- 1.Wang M, Yao Y, Jiang S, Tao F, Tang R, Sun J. How to control asthma with personalized management: where do we stand now? Curr Drug Metab. 2018;19:1157–1167. doi: 10.2174/1389200219666180129111810. [DOI] [PubMed] [Google Scholar]
- 2.Singh D, Miravitlles M, Vogelmeier C. Chronic obstructive pulmonary disease individualized therapy: tailored approach to symptom management. Adv Ther. 2017;34:281–299. doi: 10.1007/s12325-016-0459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjermer L. The importance of continuity in inhaler device choice for asthma and chronic obstructive pulmonary disease. Respiration. 2014;88:346–352. doi: 10.1159/000363771. [DOI] [PubMed] [Google Scholar]
- 4.Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27:22. doi: 10.1038/s41533-017-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DePietro M, Gilbert I, Millette LA, Riebe M. Inhalation device options for the management of chronic obstructive pulmonary disease. Postgrad Med. 2018;130:83–97. doi: 10.1080/00325481.2018.1399042. [DOI] [PubMed] [Google Scholar]
- 6.Braido F, Baiardini I, Sumberesi M, Blasi F, Canonica GW. Obstructive lung diseases and inhaler treatment: results from a national public pragmatic survey. Respir Res. 2013;14:94. doi: 10.1186/1465-9921-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braido F, Lavorini F, Blasi F, Baiardini I, Canonica GW. Switching treatments in COPD: implications for costs and treatment adherence. Int J Chron Obstruct Pulmon Dis. 2015;10:2601–2608. doi: 10.2147/COPD.S79635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4:184–191. doi: 10.4168/aair.2012.4.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhand R, Mahler DA, Carlin BW, et al. Results of a patient survey regarding COPD knowledge, treatment experiences, and practices with inhalation devices. Respir Care. 2018;63:833–839. doi: 10.4187/respcare.05715. [DOI] [PubMed] [Google Scholar]
- 10.Bjornsdottir US, Gizurarson S, Sabale U. Potential negative consequences of non-consented switch of inhaled medications and devices in asthma patients. Int J Clin Pract. 2013;67:904–910. doi: 10.1111/ijcp.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornsdottir US, Sigurethardottir ST, Jonsson JS, et al. Impact of changes to reimbursement of fixed combinations of inhaled corticosteroids and long-acting β(2)-agonists in obstructive lung diseases: a population-based, observational study. Int J Clin Pract. 2014;68:812–819. doi: 10.1111/ijcp.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jena AB, Ho O, Goldman DP, Karaca-Mandic P. The impact of the US Food and Drug Administration chlorofluorocarbon ban on out-of-pocket costs and use of albuterol inhalers among individuals with asthma. JAMA Intern Med. 2015;175:1171–1179. doi: 10.1001/jamainternmed.2015.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle S, Lloyd A, Williams A, et al. What happens to patients who have their asthma device switched without their consent? Prim Care Respir J. 2010;19:131–139. doi: 10.4104/pcrj.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klijn SL, Hiligsmann M, Evers S, Roman-Rodriguez M, van der Molen T, van Boven JFM. Effectiveness and success factors of educational inhaler technique interventions in asthma and COPD patients: a systematic review. NPJ Prim Care Respir Med. 2017;27:24. doi: 10.1038/s41533-017-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miravitlles M, Montero-Caballero J, Richard F, et al. A cross-sectional study to assess inhalation device handling and patient satisfaction in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:407–415. doi: 10.2147/COPD.S91118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clearie KL, Williamson PA, Meldrum K, et al. Pharmacokinetic and pharmacodynamic comparison of hydrofluoroalkane and chlorofluorocarbon formulations of budesonide. Br J Clin Pharmacol. 2011;71:504–513. doi: 10.1111/j.1365-2125.2010.03857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 18.Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28:219–228. doi: 10.1089/jamp.2014.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105:435–441. doi: 10.1016/j.rmed.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019 Report. http://www.goldcopd.org/. Accessed 15 Oct 2020.
- 21.Suissa S, Dell'Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laliberte F, Bookhart BK, Nelson WW, et al. Impact of once-daily versus twice-daily dosing frequency on adherence to chronic medications among patients with venous thromboembolism. Patient. 2013;6:213–224. doi: 10.1007/s40271-013-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goette A, Hammwöhner M. How important it is for therapy adherence to be once a day? Eur Heart J Suppl. 2016;18:I7–I12. doi: 10.1093/eurheartj/suw048. [DOI] [Google Scholar]
- 25.Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–434. doi: 10.2147/PPA.S44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosnic-Anticevich S, Chrystyn H, Costello RW, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis. 2017;12:59–71. doi: 10.2147/COPD.S117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HS, Yoon D, Lee HY, et al. Real-life effectiveness of inhaler device switch from dry powder inhalers to pressurized metred-dose inhalers in patients with asthma treated with ICS/LABA. Respirology. 2019;24:972–979. doi: 10.1111/resp.13559. [DOI] [PubMed] [Google Scholar]
- 28.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 29.Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Vanderman AJ, Moss JM, Bailey JC, Melnyk SD, Brown JN. Inhaler misuse in an older adult population. Consult Pharm. 2015;30:92–100. doi: 10.4140/TCP.n.2015.92. [DOI] [PubMed] [Google Scholar]
- 31.Leung J, Bhutani M, Leigh R, Pelletier D, Good C, Sin DD. Empowering family physicians to impart proper inhaler teaching to patients with chronic obstructive pulmonary disease and asthma. Can Respir J. 2015;22:266–270. doi: 10.1155/2015/731357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Children's Hospital of Philadelphia. When medication switching threatens care of children with asthma. https://policylab.chop.edu/sites/default/files/pdf/publications/When_Medication_Switching_Threatens_Care_Of_Children_With_Asthma.pdf. Accessed 15 Oct 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are third-party data available from the proprietary IQVIA LAAD and are therefore not publicly available. The database used for this study may be requested from IQVIA for a fee. The authors confirm they did not have any special access privileges that others would not have.