Abstract
Introduction
In the IMPACT trial, single-inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) reduced moderate/severe exacerbation rates versus FF/VI or UMEC/VI dual therapy in patients with chronic obstructive pulmonary disease (COPD); however, pneumonia incidence was higher in FF-containing arms. As COPD is a growing problem in Asia, we compared the efficacy and safety of FF/UMEC/VI in Asia versus non-Asia regions.
Methods
IMPACT was a double-blind, 52-week trial in symptomatic COPD patients with ≥ 1 moderate/severe exacerbation in the prior year. This pre-specified analysis evaluated the annual rate of moderate/severe exacerbations, change from baseline in trough forced expiratory volume in 1 s, and St George’s Respiratory Questionnaire total score, mortality, and safety (including pneumonia) in Asia versus non-Asia regions.
Results
The intent-to-treat population comprised 10,355 patients (Asia n = 1644 [16%]). Rate ratios (95% confidence intervals) for moderate/severe exacerbations with FF/UMEC/VI were 0.89 (0.76–1.05) versus FF/VI and 0.86 (0.71–1.04) versus UMEC/VI in Asia, and 0.84 (0.79–0.90) and 0.74 (0.68–0.80) in non-Asia. Efficacy of FF/UMEC/VI on other endpoints was similar in both regions. There was an increased incidence of investigator-reported pneumonia in patients in Asia (FF/UMEC/VI: 13%; FF/VI: 14%; UMEC/VI: 6%) compared with non-Asia (FF/UMEC/VI: 6%; FF/VI: 5%; UMEC/VI: 4%). The increased risk of pneumonia in patients in Asia was most marked in patients with lower body mass index, lower lung function, and taking inhaled corticosteroids. In post hoc analysis of adjudicated on-treatment all-cause mortality, probabilities of death were numerically lower in both regions with FF/UMEC/VI (Asia: 1.16%; non-Asia: 1.35%) and FF/VI (Asia: 1.77%; non-Asia: 1.21%) versus UMEC/VI (Asia: 1.91%; non-Asia: 2.23%).
Conclusions
FF/UMEC/VI provides similar benefits in COPD patients in Asia and non-Asia regions. Clinical benefits of treatment, including reduction in mortality risk, should be weighed against risk of pneumonia, taking account of all known risk factors.
Trial Registration
ClinicalTrials.gov identification, NCT02164513.
Electronic supplementary material
The online version of this article (10.1007/s41030-020-00136-3) contains supplementary material, which is available to authorized users.
Keywords: Asia, Chronic obstructive, Drug therapy, Mortality, Pneumonia, Pulmonary disease
Key Summary Points
| Why carry out this study? |
| Physiological differences between regional populations have been shown to influence the efficacy and safety of COPD therapies. |
| The IMPACT trial demonstrated that single-inhaler triple therapy was more efficacious than dual therapy in patients with COPD. |
| This analysis evaluated differences in efficacy, mortality, and pneumonia risk between Asia and non-Asia regions. |
| What was learned from the study? |
| This analysis demonstrated that FF/UMEC/VI provides similar benefits in COPD patients in Asia and non-Asia regions. |
| Clinical benefits of treatment, including reduction in mortality risk, should be weighed against risk of pneumonia, taking account of all known risk factors. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13089458.
Introduction
The incidence of chronic obstructive pulmonary disease (COPD) is predicted to increase in Asia over the next 30 years [1]. The 2020 Global initiative for chronic Obstructive Lung Disease (GOLD) strategy document recommends that pharmacological therapy for COPD should be used to reduce symptoms and the frequency and severity of exacerbations [2], which contribute to the burden of the disease [3, 4]. Most data assessing therapeutic options have been generated in Western populations, but patients in Asia have different genotypes, social circumstances, and disease characteristics, including a higher prevalence of emphysema [5–10], which may affect the magnitude of benefit and the likelihood of adverse events (AEs) such as pneumonia.
The InforMing the PAthway of COPD Treatment (IMPACT) trial evaluated the relative benefits of single-inhaler triple therapy with the inhaled corticosteroid/long-acting muscarinic antagonist/long-acting β2-agonist (ICS/LAMA/LABA) fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) compared with FF/VI and UMEC/VI in patients with symptomatic COPD and at risk of exacerbations. Overall, the IMPACT study demonstrated that FF/UMEC/VI reduced moderate/severe COPD exacerbations, and improved lung function and health-related quality of life (HRQoL) compared with both dual combination therapies [11]. Sixteen percent of the patients in the IMPACT study were from Asia, allowing an assessment of the efficacy and safety of FF/UMEC/VI in this region. This pre-specified analysis evaluated the efficacy and safety of FF/UMEC/VI in Asia versus non-Asia regions.
Methods
Study Design
Details of the IMPACT trial design (GSK study number CTT116855; ClinicalTrials.gov registration: NCT02164513) have been published previously [11, 12]. It was a phase III, randomized, double-blind, parallel-group, multicenter study. Patients were randomized 2:2:1 to once-daily FF/UMEC/VI 100/62.5/25 µg, FF/VI 100/25 µg, or UMEC/VI 62.5/25 µg, administered via the Ellipta inhaler. The primary objective was to evaluate the effect of FF/UMEC/VI on the annual rate of moderate/severe COPD exacerbations compared with both dual combinations over 52 weeks [11]. Changes in trough forced expiratory volume in 1 s (FEV1) and St George’s Respiratory Questionnaire (SGRQ; validated in different languages) total score, proportion of SGRQ responders (≥ 4 units decrease from baseline in SGRQ total score) at week 52, mortality and safety, including incidence of pneumonia were also examined. In the pre-specified analyses presented here, results for the primary and key secondary efficacy outcomes, and safety outcomes were compared in patients from the Asia and non-Asia regions. The Asia region included China, Japan, South Korea, Philippines, Thailand, Singapore, Vietnam, and Hong Kong. The trial was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and received approval from independent ethics committees and local institutional review boards; all patients provided written informed consent.
Patients
Eligible patients were ≥ 40 years of age and symptomatic (COPD Assessment Test [CAT] score of ≥ 10) with a FEV1 < 50% of predicted and a history of ≥ 1 moderate or severe exacerbation in the previous year, or FEV1 of 50– < 80% of predicted and ≥ 2 moderate or ≥ 1 severe exacerbation in the previous year. Full inclusion/exclusion criteria have been described previously [11, 12].
Assessments and Variables
Demographics and baseline characteristics were assessed at screening. Further details on the identification of chronic mucus hypersecretion (CMH), definitions of exacerbation severity, and other assessments are given in Supplementary Appendix S1. Pneumonia and other AEs were reported as AEs of special interest (AESI) pre-defined as a group of Medical Dictionary for Regulatory Activities (MedDRA) preferred terms. All patients enrolled in the study were required to have a chest X-ray at, or within 3 months prior to, screening and the trial protocol specified that “every effort should be made” to conduct a chest X-ray within 48 h of a moderate or severe exacerbation, or suspected pneumonia. All chest X-rays were reviewed centrally to determine if there were new radiographic findings compatible with pneumonia. As with other serious AE (SAE) reports, pneumonia events were reviewed by an independent committee which adjudicated whether pneumonia was the primary event.
Statistical Methods
The annual rate of moderate/severe exacerbations, change from baseline at week 52 in trough FEV1 and total SGRQ score, and proportion of SGRQ responders at week 52 were analyzed prospectively using regression models with the inclusion of terms for region (Asia/Not Asia) and treatment by region interaction to estimate treatment effects for each region separately. Specific details of the models can be found in Supplementary Appendix S1.
AEs, SAEs, incidences of pneumonia, including pneumonia reported as an AESI, and mortality were summarized descriptively. Two multivariate analyses of the time-to-first pneumonia were performed post hoc. The first examined if Asia was an independent risk factor for pneumonia in each treatment arm after allowing for other known risk factors (age, body mass index [BMI], post-bronchodilator percent predicted FEV1, pneumonia history, sex, smoking status, treatment group) [13]. The second analysis quantified known and potential risk factors for pneumonia (age, BMI, exacerbation history, post-bronchodilator percent predicted FEV1, neutrophils, pneumonia history, sex, smoking status, and ICS treatment) fitting separate models in each region independently. A third post hoc analysis examined the probability of adjudicated on-treatment all-cause mortality in Asia and non-Asia regions. Analysis methods are described in Supplementary Appendix S1.
Results
Patients
The total intent-to-treat (ITT) population comprised 10,355 patients; 1644 (16%) were in Asia (China [5% of ITT], Japan [4%], South Korea [3%], Philippines [1%], Thailand [1%], and Singapore, Vietnam, and Hong Kong [each < 1%]). Patients from Asia were slightly older (67.8 vs. 64.8 years), had a significantly lower BMI (22 vs. 27 kg/m2, p < 0.001), were predominantly male (95 vs. 61%), had a better CAT score (18.3 vs. 20.4) and were less likely to be current smokers (21 vs. 37%) than those in the non-Asia region. More patients from Asia were in the low (< 21 kg/m2) BMI group than in the non-Asia region (39 vs. 13%). No other clinically important differences in baseline characteristics, including lung function and exacerbation history, were seen between the regions (Table 1).
Table 1.
Baseline characteristics
| Asia (N = 1644) |
Non-Asia (N = 8711) |
|
|---|---|---|
| % of overall ITT population | 16 | 84 |
| Age, mean (SD) | 67.8 (7.81) | 64.8 (8.27) |
| Male, n (%) | 1560 (95) | 5310 (61) |
| Height (cm), mean (SD) | 164.7 (6.80) | 168.0 (9.55) |
| Weight (kg), mean (SD) | 60.24 (11.536) | 77.80 (19.338) |
| BMI (kg/m2), mean (SD) | 22.15 (3.650) | 27.47 (6.095) |
| BMI, n (%) | ||
| < 21 kg/m2 | 641 (39) | 1135 (13) |
| 21 to < 25 kg/m2 | 643 (39) | 2107 (24) |
| ≥ 25 kg/m2 | 360 (22) | 5466 (63) |
| CAT score, mean (SD) | 18.3 (5.76) | 20.4 (6.13) |
| Current smoker, n (%) | 340 (21) | 3247 (37) |
| FEV1 (l), mean (SD) | 1.187 (0.4199) | 1.289 (0.4959) |
| Percent predicted FEV1 (%), mean (SD) | 45.6 (15.33) | 45.5 (14.75) |
| FVC (l), mean (SD) | 2.827 (0.7183) | 2.707 (0.8353) |
| FEV1/FVC ratio, mean (SD) | 0.423 (0.1145) | 0.479 (0.1185) |
| Exacerbation history in previous year, n (%) | ||
| 1 moderate | 532 (32) | 3010 (35) |
| ≥ 2 moderate | 717 (44) | 4160 (48) |
| 1 severe | 449 (27) | 1851 (21) |
| ≥ 2 severe | 79 (5) | 292 (3) |
| CMH status n, (%) | ||
| CMH positive | 966 (59) | 5417 (63) |
| CMH negative | 665 (41) | 3202 (37) |
| Influenza or pneumococcal vaccination, n (%) | 251 (15) | 1460 (17) |
| Pneumococcal vaccine | 61 (4) | 734 (8) |
| Blood eosinophils, mean (SD) | 0.257 (0.3151) | 0.216 (0.2144) |
| Blood eosinophils counta | ||
| < 150/µl, n (%) | 726 (44) | 3756 (43) |
| ≥ 150/µl, n (%) | 917 (56) | 4934 (57) |
| Any comorbidity, n (%) | 909 (55) | 6103 (70) |
| Cardiac comorbidity, n (%) | 164 (10) | 1456 (17) |
BMI body mass index, CAT COPD Assessment Test, CMH chronic mucus hypersecretion, COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, ITT intent-to-treat, SD standard deviation
aAsia: N = 1643: non-Asia: N = 8690
Prior to randomization, numerically fewer patients in Asia were receiving ICS + LABA + LAMA (35 vs. 41%) and more were receiving ICS + LABA (36 vs. 31%) and LAMA monotherapy (12 vs. 7%) than in non-Asia regions (Supplementary Table S1). Mean blood eosinophil counts were higher, pneumococcal vaccination rates lower, and fewer patients had cardiac or any comorbidities in patients from Asia than in the non-Asia region in each treatment arm.
Primary Outcome
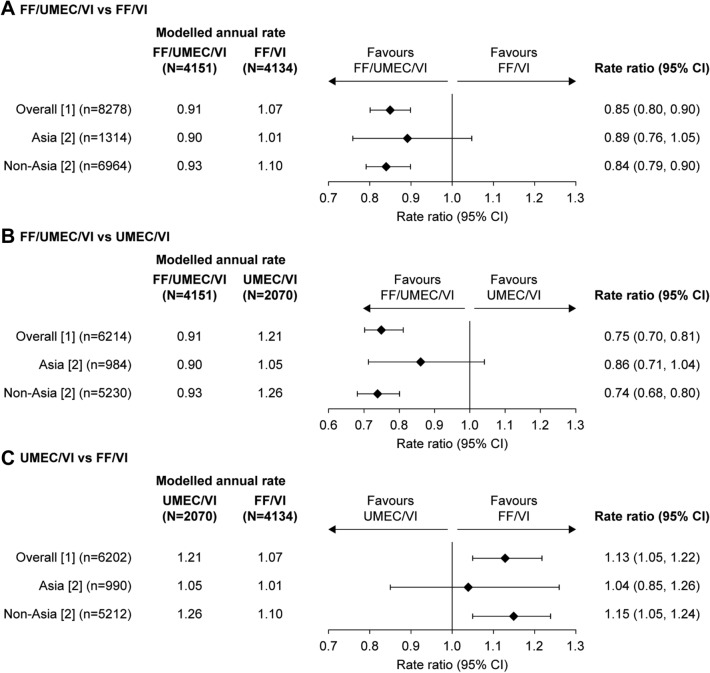
In patients from Asia, the annual rate of on-treatment moderate/severe exacerbations with FF/UMEC/VI was 0.90 (95% confidence interval [CI]: 0.80–1.01) versus 1.01 (95% CI: 0.90–1.13) on FF/VI and 1.05 (95% CI: 0.89–1.23) on UMEC/VI (Fig. 1). The annual rate of moderate/severe exacerbations was similar in patients treated with FF/UMEC/VI in both the Asia and non-Asia regions (0.90 and 0.93, respectively; Fig. 1). The rate of moderate/severe exacerbations in Asia in the UMEC/VI arm was lower than in the non-Asia region (1.05 vs. 1.26), and similar to the rates in the FF/VI arm in Asia and non-Asia (1.01 and 1.10, respectively). Overall, there were no significant interactions between Asia and non-Asia regions and the effect of treatment on the primary outcome (p = 0.360).
Fig. 1.
Rate of on-treatment moderate and severe exacerbations by regional subgroup. a FF/UMEC/VI vs. FF/VI; b FF/UMEC/VI vs. UMEC/VI; c UMEC/VI vs. FF/VI. n is the number of subjects included in the analysis for the two treatment groups of interest. Rate of exacerbations (generalized linear models assuming a negative binomial distribution) included covariates of treatment group, sex, exacerbation history (≤ 1, ≥ 2 moderate/severe), smoking status (screening), post-bronchodilator percent predicted FEV1 (screening) [1] and geographical region [2] and region (Asia/non-Asia) and treatment group by visit by region interactions. CI confidence interval, FEV1 forced expiratory volume in 1 s, FF fluticasone furoate, UMEC umeclidinium, VI vilanterol
Secondary Outcomes
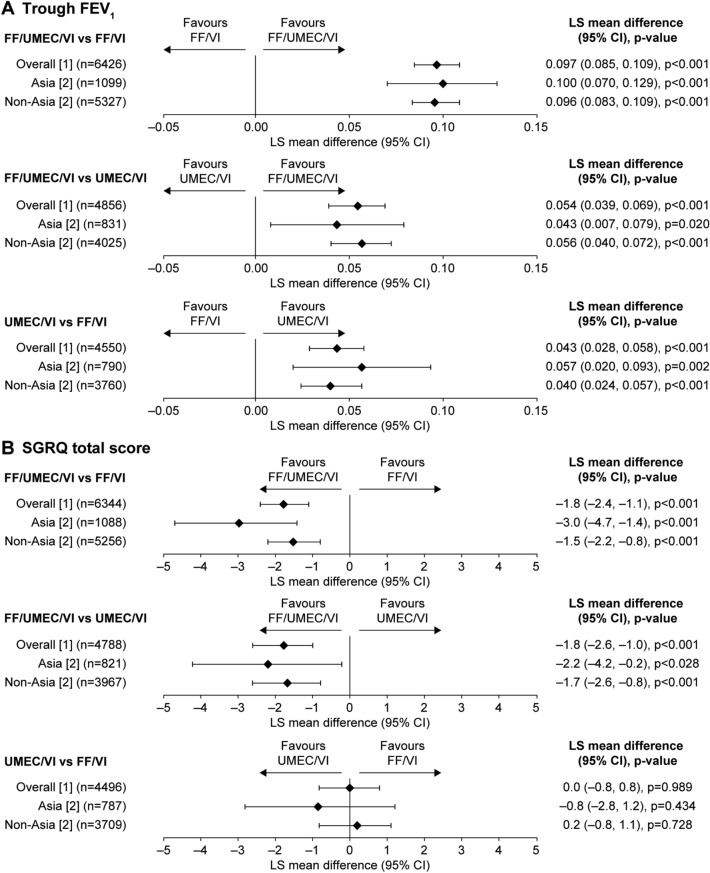
In patients from Asia, the least squares (LS) mean change in trough FEV1 from baseline at week 52 with FF/UMEC/VI (71 ml) was significantly greater than with FF/VI (− 28 ml; difference 100 ml [p < 0.001]) or UMEC/VI dual therapy (28 ml; difference 43 ml [p = 0.02]) and these differences between treatment groups were similar in magnitude to those in patients from the non-Asia region (LS mean change from baseline: FF/UMEC/VI 98 ml, FF/VI 2 ml, UMEC/VI 42 ml; difference: 96 ml for FF/UMEC/VI vs. FF/VI [p < 0.001] and 56 ml for FF/UMEC/VI vs. UMEC/VI [p < 0.001]) (Fig. 2a). The change in trough FEV1 from baseline with UMEC/VI was significantly greater than with FF/VI in patients from Asia (difference 57 ml [p = 0.002]) and was also similar to that in patients from the non-Asia region (difference 40 ml [p < 0.001]) (Fig. 2a).
Fig. 2.
Change from baseline in trough FEV1 (l) and SGRQ total score at week 52. a Change from baseline in trough FEV1 with FF/UMEC/VI vs. FF/VI, FF/UMEC/VI vs. UMEC/VI and UMEC/VI vs. FF/VI; b change from baseline in SGRQ total score at week 52 with FF/UMEC/VI vs. FF/VI, FF/UMEC/VI vs. UMEC/VI and UMEC/VI vs. FF/VI. n is the number of subjects included in the analysis for the two treatment groups of interest. Trough FEV1 and SGRQ total score (repeated measures models) included covariates of treatment group, smoking status (screening), visit, baselines, baseline by visit, treatment group by visit interactions [1] and geographical region [2] and region (Asia/non-Asia), treatment group by region, visit by region and treatment group by visit by region interactions. CI confidence interval, FEV1 forced expiratory volume in 1 s, FF fluticasone furoate, LS least squares, SGRQ St George’s Respiratory Questionnaire, UMEC umeclidinium, VI vilanterol
There was a larger mean improvement from baseline in SGRQ at week 52 with FF/UMEC/VI in Asia than in the non-Asia region (LS mean [95% CI] change from baseline: Asia − 7.1 points [− 8.3 to − 6.0], non-Asia - 5.1 points [− 5.6 to − 4.6]). In both regions, the LS mean improvement in SGRQ from baseline with FF/UMEC/VI was greater than the minimally clinically important difference of 4 points [14] and was significantly greater than that observed with FF/VI and UMEC/VI (Fig. 2b). There were no apparent differences in the proportions of SGRQ responders with FF/UMEC/VI, FF/VI or UMEC/VI between patients in Asia and in the non-Asia region (Supplementary Figure S1). In both cases, there were significantly more responders to FF/UMEC/VI than to either dual therapy.
Overall Safety, Pneumonia, and Mortality
In Asia, the incidences of on-treatment AEs and SAEs were slightly higher than the incidences in the non-Asia region in all treatment groups. The incidences of AEs leading to permanent discontinuation of study treatment or study withdrawal were similar in both regions (Supplementary Table S2).
Investigator-reported pneumonia was more common in Asia than in the non-Asia region in all treatment groups (Table 2). Similar results were seen for pneumonia confirmed by chest radiograph. More pneumonia events were investigated with a chest radiograph or computed tomography scan in Asia than in the non-Asia region, but the proportion of investigations showing infiltrates was not higher, and overall only approximately half of reported pneumonias were confirmed by radiology. Pneumonia reported as an AESI was also more common in Asia than non-Asia in all treatment groups. Pneumonia reported as a serious AESI by investigators was more common in Asia in patients treated with FF/UMEC/VI and FF/VI, but not in those treated with UMEC/VI; however, when these events were adjudicated, rates were higher in Asia but similar in all three treatment groups (Table 2). Pneumonia AESI rates were similar in those with and without CMH in all treatment groups in both regions.
Table 2.
Rates of radiological investigation and pneumonia in patients treated with FF/UMEC/VI, FF/VI and UMEC/VI in Asia and the non-Asia regions
| FF/UMEC/VI | FF/VI | UMEC/VI | ||||
|---|---|---|---|---|---|---|
| Asia N = 654 |
Non-Asia N = 3497 |
Asia N = 660 |
Non-Asia N = 3474 |
Asia N = 330 |
Non-Asia N = 1740 |
|
| Number of patients with investigator-reported pneumonia, n (%) | 88 (13) | 224 (6) | 92 (14) | 190 (5) | 19 (6) | 76 (4) |
| Number of patients with investigator-reported pneumonia supported by infiltrate on CXR/CT, n (%) | 47 (7) | 107 (3) | 59 (9) | 88 (3) | 11 (3) | 29 (2) |
| Number of investigator-reported pneumonia events, n [ratea] | 99 [163.2] | 247 [79.5] | 109 [187.7] | 210 [73.0] | 20 [69.4] | 81 [57.4] |
| Number of investigator-reported pneumonia events for which CXR/CT taken, n/N (%) | 91/99 (92) | 193/247 (78) | 102/109 (94) | 161/210 (77) | 16/20 (80) | 57/81 (70) |
| Proportion of CXR/CT showing an infiltrate, n/N (%) | 51/91 (56) | 115/193 (60) | 65/102 (64) | 91/161 (57) | 11/16 (69) | 29/57 (51) |
| Number of investigator-reported pneumonia events with infiltrate on CXR/CT, n/N (%) | 51/99 (52) | 115/247 (47) | 65/109 (60) | 91/210 (43) | 11/20 (55) | 29/81 (36) |
| Number of patients with pneumonia AESIb, n (%) | 91 (14) | 226 (6) | 98 (15) | 194 (6) | 21 (6) | 76 (4) |
| Number of pneumonia AESIb, n [ratea] | 105 [173.1] | 251 [80.8] | 119 [204.9] | 215 [74.7] | 23 [79.8] | 81 [57.4] |
| Number of patients with serious pneumonia AESI, n (%) | 58 (9) | 142 (4) | 66 (10) | 105 (3) | 10 (3) | 47 (3) |
| Number of serious pneumonia AESI, n [ratea] | 65 [107.1] | 153 [49.2] | 74 [127.4] | 113 [39.3] | 11 [38.2] | 49 [34.7] |
| Number of patients with fatal serious pneumonia AESI, n (%) | 3 (< 1) | 9 (< 1) | 3 (< 1) | 2 (< 1) | 1 (< 1) | 4 (< 1) |
| Number of fatal serious pneumonia AESI, n [ratea] | 4 [6.6] | 9 [2.9] | 4 [6.9] | 2 [0.7] | 1 [3.5] | 4 [2.8] |
| Number of patients with adjudicated SAR pneumonia/RTIc, n (%) | 48 (7) | 128 (4) | 58 (9) | 122 (4) | 26 (8) | 50 (3) |
| Number of adjudicated SAR pneumonia/RTIc, n [ratea] | 60 [98.9] | 147 [47.3] | 64 [110.2] | 135 [46.9] | 32 [111.1] | 55 [39.0] |
| Pneumonia AESI rate according to CMH statusd, n [ratea] | ||||||
| CMH + | 71 [196.7] | 158 [82.4] | 64 [187.0] | 142 [78.3] | 11 [67.3] | 53 [60.9] |
| CMH− | 34 [141.9] | 91 [78.4] | 54 [232.2] | 71 [68.6] | 12 [97.1] | 28 [53.4] |
AESI adverse event of special interest, CMH chronic mucus hypersecretion, CT computerized tomography, CXR chest X-ray, FF fluticasone furoate, RTI respiratory tract infection, SAR serious adverse report, UMEC umeclidinium, VI vilanterol
aRate per 1000 subject-years
bPneumonia AESI included the following terms: pneumonia bacterial, pulmonary tuberculosis, lung infection, pneumonitis, tuberculosis, Aspergillus infection, empyema, pneumonia fungal, pneumonia Haemophilus, pneumonia Klebsiella, pneumonia necrotizing
cWith and without COPD exacerbation, adjudicated as the primary event in the serious adverse report; adjudicated on-treatment adverse events are those that occur between study treatment start date and 1 day after study treatment stop date, inclusive
dBased on SGRQ responses
The rate of deaths due to pneumonia as assessed by investigators and reported as serious AESIs was higher in Asia compared with non-Asia in the ICS-containing treatment groups (FF/UMEC/VI: 6.6 vs. 2.9 per 1000 patient-years, respectively; FF/VI: 6.9 vs. 0.7, respectively), and slightly higher with UMEC/VI (3.5 vs. 2.8) (Table 2). However, the rate of adjudicated pneumonia-related deaths was similar between Asia and non-Asia regions with FF/UMEC/VI (1.6 vs. 1.9 per 1000 patient-years), and higher in Asia versus non-Asia region with FF/VI (3.4 vs. 0.0 per 1000 patient-years) and UMEC/VI (6.8 vs. 1.4 per 1000 patient-years), although the small number of events limits interpretation (Table 3). In the post hoc analysis of adjudicated on-treatment all-cause mortality, the probability of death was lower in the ICS-containing treatment groups compared with the UMEC/VI group in both Asia (FF/UMEC/VI: 1.16% [95% CI: 0.55, 2.41]; FF/VI: 1.77% [0.96, 3.27]; UMEC/VI: 1.91% [0.86, 4.21]) and non-Asia (FF/UMEC/VI: 1.35% [95% CI: 1.00, 1.82]; FF/VI: 1.21% [0.87, 1.69]; UMEC/VI: 2.23% [1.59, 3.12]).
Table 3.
On-treatment adjudicated deaths in the Asia and non-Asia region
| FF/UMEC/VI | FF/VI | UMEC/VI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asia | Non-Asia | Asia | Non-Asia | Asia | Non-Asia | |||||||
| Total duration at risk (patient-years) | 617.0 | 3163.6 | 591.3 | 2932.0 | 293.3 | 1437.6 | ||||||
| Patients, n (%) | Rate [#] | Patients, n (%) | Rate [#] | Patients, n (%) | Rate [#] | Patients, n (%) | Rate [#] | Patients, n (%) | Rate [#] | Patients, n (%) | Rate [#] | |
| Adjudicated on-treatment deathsa | 8 (1) | 13.0 [8] | 42 (1) | 13.3 [42] | 10 (2) | 16.9 [10] | 39 (1) | 13.3 [39] | 6 (2) | 20.5 [6] | 33 (2) | 23.0 [33] |
| Cardiovascular | 3 (< 1) | 4.9 [3] | 13 (< 1) | 4.1 [13] | 4 (< 1) | 6.8 [4] | 17 (< 1) | 5.8 [17] | 2 (< 1) | 6.8 [2] | 13 (< 1) | 9.0 [13] |
| Respiratory | 2 (< 1) | 3.2 [2] | 13 (< 1) | 4.1 [13] | 5 (< 1) | 8.5 [5] | 7 (< 1) | 2.4 [7] | 2 (< 1) | 6.8 [2] | 7 (< 1) | 4.9 [7] |
| Adjudicated pneumonia-related deaths† | 1 (< 1) | 1.6 [1] | 6 (< 1) | 1.9 [6] | 2 (< 1) | 3.4 [2] | 0 | 0 | 2 (< 1) | 6.8 [2] | 2 (< 1) | 1.4 [2] |
| Cancer | 0 | 0 | 4 (< 1) | 1.3 [4] | 0 | 0 | 4 (< 1) | 1.4 [4] | 1 (< 1) | 3.4 [1] | 1 (< 1) | 0.7 [1] |
| Unknown | 2 (< 1) | 3.2 [2] | 9 (< 1) | 2.8 [9] | 1 (< 1) | 1.7 [1] | 7 (< 1) | 2.4 [7] | 1 (< 1) | 3.4 [1] | 10 (< 1) | 7.0 [10] |
| Other | 1 (< 1) | 1.6 [1] | 3 (< 1) | 0.9 [3] | 0 | 0 | 4 (< 1) | 1.4 [4] | 0 | 0 | 2 (< 1) | 1.4 [2] |
COPD chronic obstructive pulmonary disease, FF fluticasone furoate, RTI respiratory tract infection, UMEC umeclidinium, VI vilanterol
aFatal serious adverse reports that are categorized by the adjudicators based on the primary cause of death; †Primary cause of death adjudicated as COPD exacerbation with evidence of pneumonia or pneumonia/RTI without COPD exacerbation. Rate is event rate per 1000 patient-years; #: number of events. On-treatment deaths are those which occur between study treatment start date and 7 days after study treatment stop date, inclusive
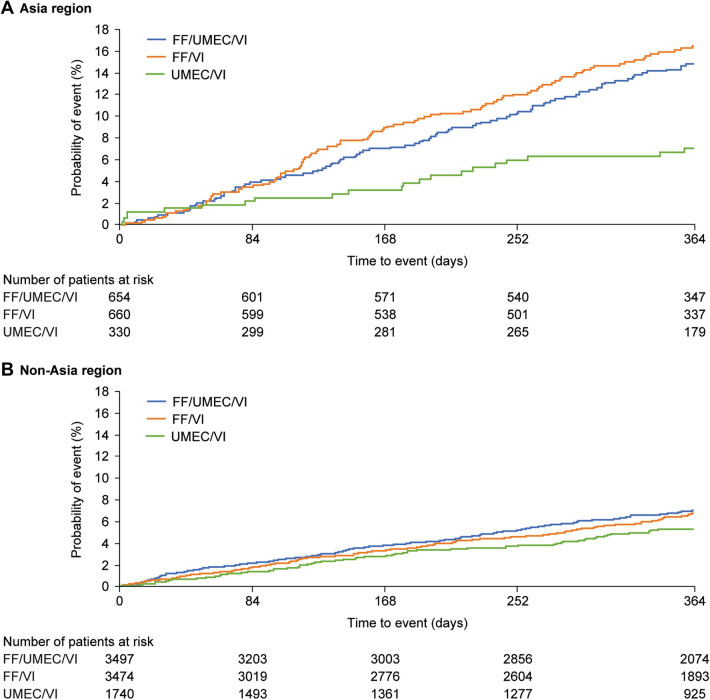
The probability of having pneumonia reported as an AESI was higher in patients in Asia treated with ICS-containing therapies compared with UMEC/VI (Fig. 3). Asia was an independent risk factor for pneumonia AESI in multivariate analysis after allowing for other risk factors in patients treated with FF/UMEC/VI and FF/VI (hazard ratios [HR] [95% CI] for Asia vs. non-Asia respectively: 1.87 [1.44–2.42] and 2.26 [1.75–2.93]), but not for those treated with UMEC/VI (HR 1.06 [95% CI: 0.65–1.73]). Table 4 shows the factors significantly associated with pneumonia AESI in patients in Asia and in the non-Asia region in the second set of multivariate models. Factors that had a statistically significant association were largely the same in both regions, namely increasing age, lower BMI, low FEV1, a history of pneumonia and ICS treatment. Blood neutrophil counts were significantly associated with pneumonia risk in the non-Asia region, but not in Asia, although the magnitude of effect was similar (Table 4). Exacerbation history, sex, and smoking status were not related to pneumonia risk. There appeared to be a greater risk of pneumonia in patients in Asia in relation to low BMI and use of ICS than in the non-Asia region (Table 4).
Fig. 3.
Time-to-first on-treatment pneumonia reported as an AESI in regional subgroups. Kaplan–Meier plot of time-to-first on-treatment event in the pneumonia AESI group in a Asia and b non-Asia regions. AESI adverse event of special interest, FF fluticasone furoate, UMEC umeclidinium, VI vilanterol
Table 4.
Hazard ratios and p values for significant covariates in the multivariate Cox analysis of risk factors for pneumonia in Asia and the non-Asia regions
| Asia | Non-Asia | |||
|---|---|---|---|---|
| HR | p value | HR | p value | |
| Age (per 10 years) | 1.50 (1.24, 1.82) | < 0.001 | 1.40 (1.25, 1.58) | < 0.001 |
| BMI | ||||
|
21 – < 25 vs. < 21 kg/m2 21 – < 25 vs. ≥ 25 kg/m2 < 21 vs. ≥ 25 kg/m2 |
0.70 (0.52, 0.95) 1.24 (0.82, 1.89) 1.77 (1.19, 2.65) |
0.007 |
0.95 (0.71, 1.27) 1.25 (1.01, 1.54) 1.31 (1.01, 1.70) |
0.036 |
| Predicted FEV1 (%) (< 50% vs. ≥ 50%) | 1.78 (1.27, 2.51) | < 0.001 | 1.37 (1.10, 1.69) | 0.004 |
| Neutrophils (109/l) (per 1 × 109/l unit increase) | 1.05 (0.98, 1.12) | NS | 1.05 (1.01, 1.10) | 0.019 |
| History of pneumonia (yes vs. no) | 2.40 (1.80, 3.20) | < 0.001 | 2.19 (1.83, 2.63) | < 0.001 |
| ICS treatment (yes vs. no) | 2.59 (1.65, 4.07) | < 0.001 | 1.32 (1.03, 1.69) | 0.027 |
BMI body mass index, FEV1 forced expiratory volume in 1 s, HR hazard ratio, ICS inhaled corticosteroid
Supporting Analyses on Pneumonia in Patients from Asia from Other Trials
In light of the results of this analysis showing that patients in Asia receiving FF in the IMPACT study had an increased risk of pneumonia compared with non-Asia region, we looked at whether similar results were seen in other studies where relatively large numbers of patients had been recruited in Asia and other regions. In the SUMMIT study [15], 2693 (16.2%) patients were randomized in Asia and 13,897 (83.8%) in other regions. This was an event-driven study in patients with COPD with moderate airflow limitation and a history or at increased risk of cardiovascular disease, primarily examining the effect of FF/VI, FF, VI and placebo on all-cause mortality. In SUMMIT, being a patient in Asia was a significant risk factor for pneumonia in all treatment arms, including placebo and the non-ICS containing arm (HR [95% CI]: FF/VI 2.46 [1.86–3.27], FF 2.21 [1.64–2.98], VI 2.15 [1.51–3.05], placebo 2.92 [2.18–3.91]) (Supplementary Figure S2).
Discussion
In Asia, as in the overall IMPACT population [11], FF/UMEC/VI provided clinical benefits compared with either dual therapies, providing numerical reductions in the rate of moderate/severe COPD exacerbations and significant improvements in lung function and HRQoL. FF/UMEC/VI improved trough FEV1 in patients from Asia by a similar amount to that in the non-Asia region. The magnitude of improvement in SGRQ total score with FF/UMEC/VI in Asia was larger than that seen in the non-Asia region. A larger effect on SGRQ was also seen in patients from Asia in the FLAME study compared with the overall population [6] but no regional effect was seen in UPLIFT [5].
The rates of moderate/severe exacerbations were numerically lower in patients from Asia treated with FF/VI or UMEC/VI than in the non-Asia region. This may reflect greater efficacy of LAMA/LABA and ICS/LABA therapy in this population or it may reflect lower background rates or under-reporting of exacerbations [16–18]. It has been previously reported that Japanese patients with COPD experience fewer exacerbations than Caucasian patients [16], and data from FLAME and UPLIFT studies showed lower rates of exacerbations in patients treated with LAMA/LABA (FLAME) and LAMA (UPLIFT) in the Asian cohort than in the overall population [5, 6]. However, in an analysis of the FULFIL study, the incidence of moderate/severe exacerbations was higher in patients from China in than in other regions [19]. It should be noted that the moderate/severe exacerbation rate ratios for the comparison of triple therapy with both dual therapies were not statistically significant in the Asia region, possibly as a result of the smaller number of patients in each group as reflected in the wider CIs.
Patients enrolled in IMPACT were at a higher risk of pneumonia than those in SUMMIT or the trials of FF/VI versus VI [13] by virtue of the entry criteria: low lung function and more frequent exacerbations are well-recognized risk factors for pneumonia in patients with COPD, and a history of severe exacerbations has been shown to nearly triple the risk [20]. Previous studies have shown that advancing age, lower BMI, lack of vaccination and comorbidities increase the risk of pneumonia. In line with these findings, this analysis shows that in both regions there was a significantly higher risk of pneumonia in patients with lower FEV1, lower BMI, advanced age, and those with a history of pneumonia.
The reported incidences of pneumonia were higher in Asia than in non-Asia regions, across all treatment groups. Pneumonia may occur more commonly in Asia, but differences in diagnostic processes may also partly underly the higher rates of pneumonia found in Asia [21, 22].
ICS-containing treatments have a well-known class effect of increasing the risk of pneumonia [23, 24]. In both regions, there was an increased rate of pneumonia in patients receiving ICS, but in Asia this increase was more pronounced: the rate of pneumonia was more than twofold greater with the two ICS-containing treatment regimens compared with UMEC/VI in Asia. In addition, in the post hoc analyses, patients in Asia receiving ICS were more likely to be reported as having pneumonia than patients in the non-Asia region after adjusting for other risk factors including age, BMI, sex, and smoking status. The incidence of pneumonia reported as a serious AESI was also more than twofold higher with ICS-containing therapies compared with UMEC/VI in Asia. This was in contrast to an exploratory analysis of pneumonia in the SUMMIT study, which shows that being in Asia led to an increase in the risk of pneumonia of a similar magnitude to that seen in IMPACT but that the increase was seen in all the treatment arms, including placebo, and not just those containing ICS. However, when serious adverse event reports in IMPACT were independently adjudicated, the rates of pneumonia were higher in Asia versus non-Asia regions but similarly high across the three treatment arms; there was no trend towards an increased rate in the ICS-containing groups. Furthermore, the results of this analysis suggest that while patients in Asia may experience a greater risk of pneumonia with ICS-containing treatments compared with patients in the non-Asia region, this does not translate into an increased risk of mortality. In fact, the probabilities of adjudicated on-treatment death from any cause were numerically lower in ICS-containing treatment arms compared with UMEC/VI.
Other factors significantly associated with an increased risk of pneumonia in both regions included lower FEV1, lower BMI, advanced age and a history of pneumonia. These findings are in agreement with previous reports that advancing age, lower BMI, lack of vaccination, and comorbidities increase the risk of pneumonia [23]. In the current analysis, lower BMI appeared to be associated with a greater risk of pneumonia in Asia than in non-Asia regions.
The pneumonia risk associated with ICS treatment should be considered in the context of the overall treatment benefits [25]. These considerations have led to questions regarding whether a lower dose of ICS may lead to a better benefit:risk profile of inhaled triple therapy [26]. The recently published ETHOS trial investigated triple therapy with budesonide/glycopyrrolate/formoterol (BUD/GLY/FOR) at a BUD dose of 160 µg or 320 µg [26]. BUD/GLY/FOR at a BUD dose of 160 µg was associated with a similar increase in the incidence in pneumonia compared with GLY/FOR as that seen for BUD/GLY/FOR at a BUD dose of 320 µg (BUD [160]/GLY/FOR vs. GLY/FOR: 1.6-fold increase; BUD [320]/GLY/FOR vs. GLY/FOR: 1.9-fold increase), but with reduced treatment benefits, particularly with regards to severe exacerbations and all-cause mortality [26]. The increased pneumonia incidence observed in ETHOS was similar to that seen in IMPACT with FF/UMEC/VI versus UMEC/VI (1.6-fold), although it is worth noting that both studies used different pneumonia capture and assessments, and that while conducted in similar patient populations, the proportion of patients from Asia was lower in ETHOS (8%) compared with IMPACT (16%). Taken together, the ETHOS results suggest a worse overall benefit:risk profile of the lower ICS dose triple therapy, although this question has not specifically been addressed in Asian patients.
Limitations of this analysis include the fact that the IMPACT study was not powered to detect differences between treatment groups in the sub-populations. In addition, the Asia region was defined by geography and as such different Asian populations were included in this region. While multivariate analyses were conducted to adjust for other factors known to influence the risk of pneumonia, there may be further demographic or clinical characteristics of these different sub-populations that could affect the risk and therefore the results.
With the burden of COPD in Asia set to increase in the coming decades [27, 28], evaluation of the efficacy and safety of therapeutic options, such as triple therapy, in patients with COPD from Asia is of considerable clinical importance to validate the treatment effects seen globally [29, 30]. This analysis has shown that the efficacy of FF/UMEC/VI compared with UMEC/VI and FF/VI in patients with symptomatic COPD and at risk of exacerbations was generally similar in Asia to that seen in the non-Asia population. There was an increased risk of pneumonia in Asia compared with non-Asia and evidence of some factors, including ICS treatment, which may be associated with an increased risk. Nevertheless, there was no evidence of an increased risk of death. Ultimately, in both Asian and non-Asian countries, clinicians must weigh the clear clinical benefits of triple therapy versus dual therapy against possible adverse effects, including the risk of pneumonia, on an individual patient basis, taking account of all identified risk factors and make treatment decisions on this basis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were funded by GSK (study number CTT116855). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship Contributions
David M.G. Halpin, Gerard J. Criner, and Mark T. Dransfield were involved in the acquisition and analysis/interpretation of data. MeiLan K. Han, Benjamin Hartley, Catherine Harvey, C. Elaine Jones, Motokazu Kato, Peter Lange, Sally Lettis, David A. Lomas, Fernando J. Martinez, Neil Martin, Dave Singh, Robert Wise, and Jinping Zheng were involved in the analysis/interpretation of data. David A. Lipson was involved in the conception/design of the study and analysis/interpretation of data.
Editorial Assistance
Editorial support (in the form of writing assistance, assembling figures, collating author comments, grammatical editing, and referencing) was provided by Katie Baker and Philip Chapman, at Fishawack Indicia Ltd, UK, and was funded by GSK.
Disclosures
David M.G. Halpin has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, and Pfizer, and non-financial support from Boehringer Ingelheim and Novartis. Gerard J. Criner has received personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, CSA Medical, Eolo, GSK, HGE Technologies, Novartis, Nuvaira, Olympus, Pulmonx, and Verona. Mark T. Dransfield has received grant support from the Department of Defense, NIH, and the American Lung Association, personal fees from Boehringer Ingelheim, GSK, AstraZeneca, PneumRx/BTG, Genentech, Quark Pharmaceuticals and Mereo, and contracted clinical trial support from Boehringer Ingelheim, GSK, Novartis, AstraZeneca, Yungjin, PneumRx/BTG, Pulmonx and Boston Scientific. MeiLan K. Han has received personal fees from AstraZeneca, Boehringer Ingelheim, GSK, Merck, and Mylan, and research support from Novartis and Sunovion. Benjamin Hartley is a contingent worker with a Contract Research Organisation working on behalf of GSK and holds shares in GSK. Catherine Harvey, C. Elaine Jones, Sally Lettis, Neil Martin, and David A. Lipson are employees of GSK and hold stock/shares in GSK. Motokazu Kato has received lecture honoraria from GSK, AstraZeneca, Nippon Boehringer Ingelheim, and Novartis Pharma. Peter Lange has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi and GSK, and grant support from Boehringer Ingelheim. David A. Lomas has received personal fees from GSK and Grifols, and chaired the GSK Respiratory Therapy Area Board 2012–2015. Fernando J. Martinez has received personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Genentech, GSK, Inova Fairfax Health System, Methodist Hospital, National Society for Continuing Education/Haymarket, Novartis, Pearl Pharmaceuticals, PeerView Communications, Physicians Education Resource, Chiesi, CSL Behring, Sunovion, University of Alabama Birmingham, Physicians Education Resource, Canadian Respiratory Network, CME Outfitters, Teva, Vindico, and Dartmouth, non-financial support from Bioscale/ProterrixBio, Nitto and Zambon, personal fees from MD Magazine, New York University, UpToDate, WebMD/MedScape, Patara/Respivant, Rockpointe, Rare Disease Healthcare Communications, France Foundation and Prime Education, and other support from AstraZeneca, Boehringer Ingelheim, Bioscale/ProterrixBio, Afferent/Merck, Gilead, Patara/Respivant, Stromedix/Biogen, Veracyte, Prometic, Bayer, Bridge Biotherapeutics, Bristol Myers Squibb, Physicians Education Resource, ProMedior/Roche, twoXR, and Gala. Dave Singh has received personal fees from GSK, Cipla, Genentech and Peptinnovate, and personal fees and grant support from AstraZeneca, Boehringer Ingelheim, Chiesi, Glenmark, Menarini, Mundipharma, Novartis, Pfizer, Pulmatrix, Theravance and Verona. Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC). Robert Wise has received personal fees and grant support from AstraZeneca/MedImmune/Pearl, Boehringer Ingelheim and GSK, personal fees from Contrafect, Pulmonx, Roche, Spiration, Sunovion, Merck, Circassia, Pneuma, Verona, Mylan/Theravance, Propeller Health, AbbVie, Kiniksa, Galderma, and Novartis, and grant support from Pearl Therapeutics and Sanofi-Aventis. Jinping Zheng conducted a cohort study supported by GSK and is an AstraZeneca and Boehringer Ingelheim advisory board member. Ellipta is owned by or licensed to the GSK Group of Companies.
Compliance with Ethics Guidelines
The trial was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and received approval from independent ethics committees and local institutional review boards; all patients provided written informed consent.
Data Availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
References
- 1.Teramoto S, Yamamoto H, Yamaguchi Y, Matsuse T, Ouchi Y. Global burden of COPD in Japan and Asia. Lancet. 2003;362:1764–1765. doi: 10.1016/S0140-6736(03)14865-9. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020 Report. 2020. https://www.goldcopd.org/. Accessed 5 Feb 2020.
- 3.Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20:11–23. doi: 10.5588/ijtld.15.0472. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21:14–23. doi: 10.1111/resp.12660. [DOI] [PubMed] [Google Scholar]
- 5.Fukuchi Y, Fernandez L, Kuo HP, et al. Efficacy of tiotropium in COPD patients from Asia: a subgroup analysis from the UPLIFT trial. Respirology. 2011;16:825–835. doi: 10.1111/j.1440-1843.2011.01982.x. [DOI] [PubMed] [Google Scholar]
- 6.Wedzicha JA, Zhong N, Ichinose M, et al. Indacaterol/glycopyrronium versus salmeterol/fluticasone in Asian patients with COPD at a high risk of exacerbations: results from the FLAME study. Int J Chron Obstruct Pulmon Dis. 2017;12:339–349. doi: 10.2147/COPD.S125058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichinose M, Taniguchi H, Takizawa A, et al. The efficacy and safety of combined tiotropium and olodaterol via the Respimat(®) inhaler in patients with COPD: results from the Japanese sub-population of the Tonado(®) studies. Int J Chron Obstruct Pulmon Dis. 2016;11:2017–2027. doi: 10.2147/COPD.S110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto S, Ikeuchi H, Murata S, Kitawaki T, Ikeda K, Banerji D. Efficacy and safety of indacaterol/glycopyrronium in Japanese patients with COPD: a subgroup analysis from the SHINE study. Int J Chron Obstruct Pulmon Dis. 2016;11:2543–2551. doi: 10.2147/COPD.S111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan WC, Ng TP. COPD in Asia: where East meets West. Chest. 2008;133:517–527. doi: 10.1378/chest.07-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatsumi K, Kasahara Y, Kurosu K, et al. Clinical phenotypes of COPD: results of a Japanese epidemiological survey. Respirology. 2004;9:331–336. doi: 10.1111/j.1440-1843.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- 11.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 12.Pascoe SJ, Lipson DA, Locantore N, et al. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. Eur Respir J. 2016;48:320–330. doi: 10.1183/13993003.02165-2015. [DOI] [PubMed] [Google Scholar]
- 13.Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12:27–34. doi: 10.1513/AnnalsATS.201409-413OC. [DOI] [PubMed] [Google Scholar]
- 14.Jones PW. St George's respiratory questionnaire: MCID. COPD. 2005;2:75–79. doi: 10.1081/COPD-200050513. [DOI] [PubMed] [Google Scholar]
- 15.Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: the SUMMIT trial. Respir Med. 2017;131:27–34. doi: 10.1016/j.rmed.2017.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Ishii T, Nishimura M, Akimoto A, James MH, Jones P. Understanding low COPD exacerbation rates in Japan: a review and comparison with other countries. Int J Chron Obstruct Pulmon Dis. 2018;13:3459–3471. doi: 10.2147/COPD.S165187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu W, Collet JP, Shapiro S, et al. Negative impacts of unreported COPD exacerbations on health-related quality of life at 1 year. Eur Respir J. 2010;35:1022–1030. doi: 10.1183/09031936.00079409. [DOI] [PubMed] [Google Scholar]
- 18.Yang T, Cai B, Cao B, et al. REALizing and improving management of stable COPD in China: a multi-center, prospective, observational study to realize the current situation of COPD patients in China (REAL) - rationale, study design, and protocol. BMC Pulm Med. 2020;20:11. doi: 10.1186/s12890-019-1000-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J, Zhong N, Wang C, et al. The efficacy and safety of once-daily fluticasone furoate/umeclidinium/vilanterol versus twice-daily budesonide/formoterol in a subgroup of patients from China with symptomatic COPD at risk of exacerbations (FULFIL trial) COPD. 2018;15:334–340. doi: 10.1080/15412555.2018.1481022. [DOI] [PubMed] [Google Scholar]
- 20.Mullerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106:1124–1133. doi: 10.1016/j.rmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Berrington de Gonzalez A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 22.Kumamaru KK, Machitori A, Koba R, Ijichi S, Nakajima Y, Aoki S. Global and Japanese regional variations in radiologist potential workload for computed tomography and magnetic resonance imaging examinations. Jpn J Radiol. 2018;36:273–281. doi: 10.1007/s11604-018-0724-5. [DOI] [PubMed] [Google Scholar]
- 23.Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34:641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 24.Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD010115. doi: 10.1002/14651858.CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Medicines Agency (EMA). Pharmacovigilance Risk Assessment Committee (PRAC), recommendation 2016. Inhaled corticosteroids (ICS) containing medicinal products indicated in the treatment of chronic obstructive pulmonary disease (COPD). 2016. https://www.ema.europa.eu/medicines/human/referrals/inhaled-corticosteroids-containing-medicinal-products-indicated-treatment-chronic-obstructive. Accessed 30 Sep 2020.
- 26.Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 27.Tan WC, Seale P, Ip M, et al. Trends in COPD mortality and hospitalizations in countries and regions of Asia-Pacific. Respirology. 2009;14:90–97. doi: 10.1111/j.1440-1843.2008.01415.x. [DOI] [PubMed] [Google Scholar]
- 28.Halpin DMG, Celli BR, Criner GJ, et al. It is time for the world to take COPD seriously: a statement from the GOLD board of directors. Eur Respir J. 2019;54:1900914. doi: 10.1183/13993003.00914-2019. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–423. doi: 10.1038/clpt.2008.141. [DOI] [PubMed] [Google Scholar]
- 30.Martin A, Badrick E, Mathur R, Hull S. Effect of ethnicity on the prevalence, severity, and management of COPD in general practice. Br J Gen Pract. 2012;62:e76–81. doi: 10.3399/bjgp12X625120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.