Abstract
Background: Magnetic resonance guided focused ultrasound (MRgFUS) is a relatively novel technique to treat essential tremor (ET). The objective of this review was to analyze the efficacy and the safety profile of MRgFUS for ET.
Methods: A systematic literature review was done. The post procedure changes in the Clinical Rating Scale for Tremor (CRST) score, hand score, disability and quality of life scores were analyzed.
Results: We found 29 studies evaluating 617 patients. DTI based targeting was utilized in six cohorts. A significant difference was observed in the pooled standard mean difference between the pre and postoperative total CRST score (p-value < 0.001 and 0.0002), hand score (p-value 0.03 and 0.02); and the disability at 12 months (p-value 0.01). Head pain and dizziness were the most in procedure complications. The immediate pooled proportion of ataxia was 50%, while it was 20% for sensory complications, which, respectively, declined to 31 and 13% on long term follow up. A significant reduction (p = 0.03) in immediate ataxia related complications was seen with DTI targeting.
Conclusion: MRgFUS for ET seems to be an effective procedure for relieving unilateral tremor. Use of DTI based targeting revealed a significant reduction in post procedure ataxia related complications as compared to traditional targeting techniques. Analysis of other complications further revealed a decreasing trend on follow up.
Keywords: cerebellothalamic tract, diffusion tensor imaging, essential tremor, magnetic resonance guided focused ultrasound, targeting technique comparison, ventral intermediate nucleus
Introduction
Essential tremor (ET) is the most common form of adult movement disorder (1, 2), with an estimated prevalence of 4–6% (3, 4). Although not life threatening, it carries significant morbidity due to functional impairment from loss of hand function (5). Medications such as propranolol and primidone are the first line therapy, but many patients with ET become drug refractory (5). These patients can be good candidates for surgical treatments such as deep brain stimulation, radiofrequency thalamotomy, focused ultrasound thalamotomy, or gamma knife thalamotomy (GKT).
Stereotactic radiofrequency ablation of basal ganglia and thalamic structures, including the VIM nucleus was one of the first surgical interventions to be offered to ET patients (6). The higher risk of side effects eventually led clinicians to consider DBS as a choice for surgical treatment of ET (7–9). However, there are several drawbacks related to its use, such as implant related complications and the requirement of frequent hospital visits for programming (10, 11). GKT was developed as a relatively less invasive thalamotomy method, but suffered from unpredictable lesion size limitation and time taken for the clinical benefit to become apparent (12). MRgFUS integrates ultrasonic waves with magnetic resonance imaging for therapeutic transcranial ablation (13). MRgFUS thalamotomy is an image guided procedure with no incision. It is a precision thalamotomy, in other words. Advantages of MRgFUS include non-invasiveness, real time real-time visualization of the thermal spot, and temperature monitoring while testing for a clinical response during lesion creation. Moreover, there are no hardware-related complications, and the patients do not require repeated hospital visits for programming. New advances are being made to improve the results of MRgFUS. Diffusion tensor imaging, which allows the delineation of the CTT, has been incorporated in recent times in an effort to improve the target of the ultrasonic waves (14).
An earlier review published on the topic included only nine studies (15). Many errors were pointed out in the article, including an insufficient number of studies to draw relevant conclusions (16). Additionally, no long-term data were available at that time. Since then, several centers around the world have embraced this technique. This review summarizes the latest available evidence in literature in terms of efficacy and complications of MRgFUS for ET. Owing to the paucity of Class I evidence and the unlikelihood of prospective studies comparing the various surgical techniques available for treating ET, this meta-analysis strives to provide pooled results of a number of smaller studies on the topic.
Methods
Literature Search
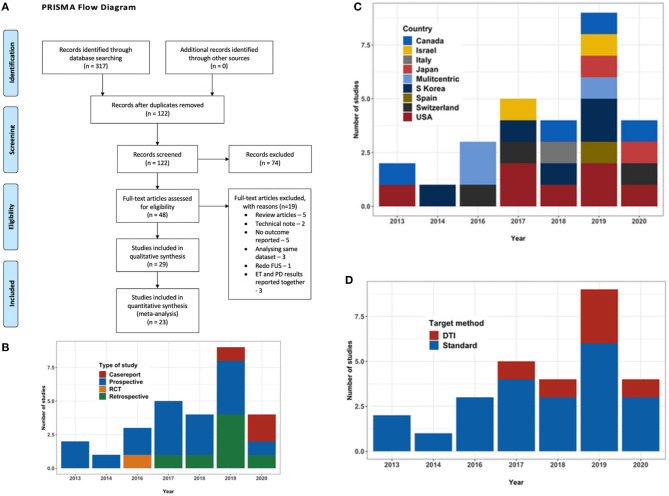
A search for published literature till May 2020 was done on PubMed, Google Scholar, Cochrane library database and Medline using the keywords “MR guided,” “focused ultrasound,” “essential tremor,” “thalamotomy,” “ventral intermediate nucleus,” “cerebellothalamic,” and “diffusion imaging” in various combinations. References of the relevant studies and other review articles on the subject were also studied to supplement the initial search. Only English language articles were considered. Two authors manually and independently reviewed all publications encountered during the search. Disagreements, if any, were resolved with the opinion of a third independent observer. PRISMA guidelines were followed throughout (Figure 1A).
Figure 1.
(A) PRISMA flow diagram outlining the literature search process. (B) Bar chart depicting the type and number of studies with year of publication. (C) Bar chart depicting the country where the study was conducted. (D) Bar chart depicting the trend in targeting technique over the years.
Inclusion and Exclusion Criteria
Studies describing the use of MRgFUS for the treatment of medically refractory ET (unilateral or bilateral) in the adult population were selected for this review. The exclusion criteria were studies that reported outcomes on patients with tremors secondary to any other causes, such as drug-induced tremor, history of preceding trauma within 3 months, psychogenic tremor, or co-morbid Parkinson disease and dystonia were excluded. For studies with mixed diagnoses, we only included outcomes reported for ET patients. To keep the focus primarily on MRgFUS, we excluded cases where a previous procedure such as DBS, radiosurgery or stereotactic ablation was done. To avoid duplication of results, we only included outcomes from a single publication where multiple publications reported outcomes from the same study cohort.
Data Extraction
Data were extracted by two authors independently. Clinical data collected included the maximum reported period of follow up, the total Clinical Rating Scale for Tremor score (maximum score 160) (17, 18), hand score – a subset of CRST Part A and B (maximum score 32), disability as CRST Part C (maximum score 32) and quality of life as determined by the Quality of Life in Essential Tremor Questionnaire score (0–100%). All the data points were collected using a standardized data collection instrument developed in Microsoft Excel (Microsoft Inc., Redmond, WA) template.
The primary outcome variable was the change in CRST score pre and postoperatively at 3 and 12 months. The secondary outcomes were the difference in disability and QOL scores. For the meta-analysis, studies that reported the outcome as a mean value (with standard deviation) of the total CRST score, hand score, CRST Part C score and QUEST score were included. Studies reporting outcome as median or percentage improvement in outcome scores, studies that reported the hand score out of 12/16 were excluded from that part of the analysis. Case reports were excluded from the meta-analysis.
Complications attributed to the procedure were recorded as immediate (occurring during the procedure to within 48 h after the procedure), short-term (from the third day onwards till 3 months), and long-term (persisting/appearing more than 3 months later). The complications were divided into two broad categories - neurologic and minor/treatment related. The neurological complications were further divided into four subcategories – sensory (paresthesia, taste disturbance, dysesthesia, tinnitus), motor (facial or limb weakness), ataxia (dizziness, gait ataxia, dysmetria/hand ataxia) and speech & swallowing. The minor/treatment related complications were categorized as headache and fatigue, sonication, MRI, frame related and others.
Statistical Analysis
Statistical analysis of the pooled data was performed using R software (R Foundation for Statistical Computing, Vienna, Austria) employing the “meta” and “metaphor” packages (19–21). We first performed the analysis using fixed-effect modeling and later, with random-effect methods (after assessing heterogeneity with fixed modeling). Thus, all values reported in the current analysis were from random-effect modeling (was heterogeneity significant for all analyses). The extent of heterogeneity between the studies was quantified using the I2 statistic. Values of I2 < 25%, 25–75%, and >75% were defined as low, moderate and high heterogeneity, respectively (22). The results were expressed as a standardized mean difference with a 95% confidence interval. A negative SMD indicates improvement in the relevant score postoperatively. P-value < 0.05 was considered as statistically significant. Linear regression analysis was performed to detect any significant correlation between parameters.
Risk of Bias Assessment
Studies were assessed for a possible publication bias initially using a funnel plot, which was later quantified using Egger's test. Publication bias was evaluated for reporting of CRST total score at 3 months. Egger's regression test showed that the X-axis intercept occurred at −1.587 with p-value (two-tailed) being 0.04315 (Supplementary Material 1).
Study Quality Assessment
The MINORS criteria were used to assess the methodological quality of non-randomized surgical studies (23) (Table 1).
Table 1.
Methodological index for non-randomized studies (MINORS) scores for included studies*.
| References | Clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to aim of study | Unbiased assessment of the study endpoint | Follow up period appropriate to aim of study | Loss to follow up < 5% | Prospective calculation of study size | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Lipsman et al. (24) | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 12 |
| Elias et al. (25) | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 12 |
| Chang et al. (26) | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 12 |
| Gallay et al. (27) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 11 |
| Zaroor et al. (28) | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 12 |
| Schreglmann et al. (29) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 |
| Kim et al. (30) | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 10 |
| Chazen et al. (31) | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 9 |
| Federau et al. (32) | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 9 |
| Jung et al. (33) | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 12 |
| Iacopino et al. (34) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 11 |
| Krishna et al. (35) | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 13 |
| Boutet et al. (36) | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 10 |
| Park et al. (37) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 13 |
| Hori et al. (38) | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 10 |
| Pineda-Pardo et al. (39) | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 10 |
| Jones et al. (40) | 2 | 2 | 0 | 2 | 2 | 1 | 1 | 0 | 10 |
| Sinai et al. (41) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 11 |
| Chang et al. (42) | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 0 | 11 |
| Miller et al. (43) | 2 | 2 | 0 | 2 | 1 | 1 | 1 | 0 | 8 |
| Krishna et al. (44) | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 10 |
| Gallay et al. (45) | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 12 |
| Fukutome et al. (46) | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 10 |
Score per criterion: 0, not reported; 1, reported but inadequate; 2, reported and adequate. Ideal global score for non-comparative study is 16.
Results
A total of 29 studies (24–52), evaluating a total of 617 patients (156 female) fulfilled the inclusion criteria for the systematic review. Out of these 29 studies, there was only one RCT (47), with the rest being observational studies. There were fourteen prospective and eight retrospective studies and three case reports (Figure 1B). At present, the procedure has been performed in eight countries (Figure 1C). Three studies were reporting long term follow-up (37, 48, 49) of the patients in the RCT. Mean age of the patients ranged from mean 61.7 ± 8.1 to 78 ± 6 years in the studies, except a case report which reported the use of MRgFUS in treating nonagenarians (51). Mean disease duration ranged from 15.4 ± 13.3 to 34.3 ± 22.1 years. The maximum follow-up reported was 5 years by Sinai et al. in two patients (41). The baseline characteristics of the studies are summarized in Table 2.
Table 2.
Study details arranged chronologically by the month and year of publication along with follow up and outcome scores [*For descriptive purposes, the cohort of patients reported by Krishna et al. (35) and Elias et al. (47) were split into two groups, each with its own distinct characteristics].
| References | Study design | Period of recruitment of patients | Place where conducted | No. of patients | Mean age ± SD (range) (in years) | Sex (male, female) | Mean disease duration ± SD (range) (in years) | Target | Localization method | Maximum follow up (range) | Mean total CRST score | CRST part A | Hand score | CRST part C | QUEST | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative (follow up – number of patients) | Preoperative | Postoperative (follow up – number of patients) | Preoperative (maximum score) | Postoperative (follow up – number of patients) | Preoperative | Postoperative (follow up – number of patients) | Preoperative | Postoperative (follow up – number of patients) | |||||||||||
| Lipsman et al. (24) | Prospective, uncontrolled | May 2012– January 2013 | Toronto, Canada | 4 | 70.8 ± 7.8 (58–77) | 4, 0 | 17.8 ± 8.2 (6–25) | VIM | Standard | 3 month | 70.75 ± 17.0 | 35.25 ± 9.5 | NA | NA | 7.25 ± 1.9 (out of 12) | 1.25 ± 0.82 (3 m) | 20.75 ± 3.9 | 10.25 ± 2.8 | NA | NA |
| Elias et al. (25) | Prospective, uncontrolled | February–December 2011 | Virginia, USA | 15 | 66.6 ± 8.0 (53 to 79) | 10, 5 | 32.0 ± 21.3 (4–60) | VIM | Standard | 1 year | 54.9 ± 14.4 | 24.3 ± 14.8 | NA | NA | 20.4 ± 5.2 (out of 32) | 4.3 ± 3.5 (3 m), 5.2 ± 4.8 (1 yr) | 18.2 ± 4.1 | 2.8 ± 3.4 | NA | NA |
| Chang et al. (26) | Prospective, uncontrolled | March–November 2012 | Seoul, Korea | 8 | 66.1 ± 5.3 (61–78) | 7, 1 | 32.1 ± 16.1 (15–57) | VIM | Standard | 6 month | NA | NA | 5.1 ± 1.2 | 1.4 ± 1.4 | NA | NA | 13.5 ± 3.7 | 2.8 ± 2.8 | NA | NA |
| Gallay et al. (27) | Prospective, uncontrolled | NA | Solothurn and Bern, Switzerland | 21 | 69.1 ± 9.2 | 15, 6 | 29.9 ± 15 | CTT | Standard | 1 year | 57.6 ± 13.2 | 25.8 ± 17.6 (1 yr−10) | NA | NA | NA | NA | NA | NA | NA | NA |
| Elias et al. (47) (Treatment group)* | Randomized Control Trial | August 2013–September 2014 | Multicentric-8 | 56 | 70.8 ± 8.7 | 37, 19 | 28.3 ± 16.4 | VIM | Standard | 1 year | 50.1 ± 14.0 | 29.6 ± 13 (3 m); 32.4 ± 14.5 (12 m) | NA | NA | 18.1 ± 4.8 (out of 32) | 9.6 ± 5.1 (3 m), 10.9 ± 4.5 (1 yr) | 16.5 ± 4.6 | 6.2 ± 5.6 (3 m), 6.3 ± 6.2 (1 yr) | 42.6 ± 18.3 | 23.1 ± 16.9 (3 m), 41.4 ± 19.4 (1 yr) |
| Elias et al. (47) (Sham Crossover)* | Randomized Control Trial | August 2013–September 2014 | Multicentric-8 | 21 (19 crossover, 2 retreat) | 71.4 ± 7.3 | 15, 5 | 27.9 ± 14.9 | VIM | Standard | 1 year | 45.43 ± 12.55 | 23.48 ± 10.95 (3 m); 25.00 ± 11.11 (6 m); 18.67 ± 16.02 (1 yr−9) | NA | NA | 16.5 ± 4.21 (out of 32) | 7.43 ± 3.88 (3 m),8.00 ± 3.86 (6 m), 6.71 ± 4.7 (1 yr−9) | NA | NA | NA | NA |
| Chang et al. (48) | 2 year follow up of patients in RCT by Elias et al. (47) | August 2013–September 2014 | Multicentric-8 | 76 (67 followed till 2 years) | 71.0 ± 8.3 (47–89) | 52, 24 | 16.8 ± 12.3 | VIM | Standard | 2 year | NA | NA | NA | NA | 19.8 ± 4.9 (out of 32) | 8.9 ± 4.8 (1 yr−70), 8.8 ± 5.0 (2 yr−67) | 16.4 ± 4.5 | 5.4 ± 5.3 (1 yr −70) 6.5 ± 5.0 (2 yr−67) | NA | NA |
| Halpern et al. (49) | 3 year follow up of patients in RCT by Elias et al. (47) | August 2013–September 2014 | Multicentric-8 | 76 (52 followed till 3 years) | 71.0 ± 8.3 (47–89) | 52, 24 | 16.8 ± 12.3 | VIM | Standard | 3 year | NA | NA | NA | NA | 20.1 ± 4.7 (out of 32) | 9.5 ± 5.4 | 16.4 ± 4.5 | 7.5 ± 6.1 | 43.1 ± 18.3 | 23.8 ± 19.6 |
| Zaroor et al. (28) | Prospective, uncontrolled | November 2013–January 2016 | Haifa, Israel | 18 | 73.1 ± 6.2 (64–87) | 12, 6 | 15.5 ± 9.3 (5–30) | VIM | Standard | 12.5 ± 7.0 (3–24) month | 40.7 ± 11.6 | 9.3 ± 7.1 (1 m); 8.2 ± 5.0 (6 m) | NA | NA | NA | NA | NA | NA | 44.8 ± 12.9 | 13.1 ± 13.2 (1 m); 12.3 ± 7.2 (6 m) |
| Schreglmann et al. (29) | Prospective, uncontrolled | NA | St. Gallen, Switzerland | 6 | 70.7 ± 8.5 (58–82) | 2, 4 | 24.5 ± 22.5 (2–56) | CTT | Standard | 6 month | 43.8 ± 9.8 | 19.8 ± 6.8 | NA | NA | 14.3 ± 4.9 (out of 32) | 2.5 ± 2.6 | NA | NA | NA | 52% improvement |
| Kim et al. (30) | Retrospective | 2012–2014 | Seoul, South Korea | 23 | 64.7 (47–77) | 20, 3 | 20.5 (5–54) | VIM | Standard | 1 year | NA | NA | NA | NA | NA | (>90% improvement was taken as success) 21 patients (91.3%) at 1 m, 18 (78.3%) at 12 m | NA | NA | NA | NA |
| Chazen et al. (31) | Prospective, uncontrolled | NA | New York, USA | 4 | 64.25 ± 11.7 | 3, 1 | NA | CTT | DTI based | NA | NA | NA | NA | NA | 3.75 ± 1.0 (out of 15) | 0.25 ± 0.50 (Immediate post treatment) | NA | NA | NA | NA |
| Federau et al. (32) | Retrospective | August 2013–May 2014 | Stanford, USA | 7 | 78 ± 6 | 5, 2 | NA | VIM | Standard | 1 year | NA | NA | 6.5 ± 1.3 | 2.3 ± 1.1 | 21.5 ± 2.0 (out of 32) | 9.7 ± 5.2 | NA | NA | NA | NA |
| Jung et al. (33) | Prospective, uncontrolled | March 2012–September 2014 | Seoul, South Korea | 20 | 64.1 (47–77) | 17, 3 | 21.2 (5–54) | VIM | Standard | 1 year | 44.75 ± 9.57 | 14.65 ± 9.19 | 12.60 ± 3.80 | 2.75 ± 3.18 | 18.15 ± 3.96 (out of 32) | 5.80 ± 4.53 | 12.80 ± 3.17 | 5.75 ± 4.25 | 64.16 ± 17.75 | 27.38 ± 13.96 |
| Iacopino et al. (34) | Prospective, uncontrolled | January 2015–September 2017 | Palermo, Italy | 13 | 65.22 ± 11.87 | 10, 3 | 22.38 (3–70) | VIM | Standard | 6 month | 40.2 ± 11.8 | 17.3 ± 7.31 (3 m); 17.7 ± 8.80 (11 pts−6 m) | NA | NA | 6.4 ± 2.97 (out of 16) | 2.1 (3 m), 2.2 (6 m−11) | NA | NA | 35.09± 12.25 | 17.09 ± 10.67(3 m), 18.44 ± 13.76 (6 m−11) |
| Krishna et al. (35) | Prospective, uncontrolled | July 2015–September 2016 | Ohio, USA | 10 | 70.8 ± 9.7 | 6, 4 | 34.3 ± 22.1 | VIM | DTI based | 6 month | 59.3 ± 17.3 | 29 ± 16 (3 m), 32 ± 15.9 (6 m−9) | 20.7 ± 8 | 11.6 ± 6.5 (3 m) | 17.4 ± 4.5 (out of 32) | 6.5 ± 3.7 (3 m) | 18.1 ± 5.1 | 4.3 ± 4.4 (3 m) | 81.7 ± 17.7 | 45.3 ± 11.6 (3 m), 45.6 ± 10.8 (6 m−9) |
| Boutet et al. (36) | Retrospective | May 2012–August 2017 | Toronto, Canada | 66 | 72.4 ± 8.4 | 47, 19 | 23.0 ± 14.4 | VIM | NA | 3 month | 59.7 ± 17.4 | 34.8 ± 14.4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Park et al. (37) | Prospective, uncontrolled [4 year follow up of patients reported in RCT by Elias et al. (47)] | October 2013–August 2014 | Seoul, South Korea | 12 | 61.7 ± 8.1 (47–72) | 10, 2 | 17.8 ± 13.03 (7–54) | VIM | Standard | 4 year | NA | NA | NA | NA | 17.4 ± 3.8 (out of 32) | 5.3 ± 3.4 (1 yr), 6.9 ± 5.9 (2 yr), 7.5 ± 5.3 (3 yr), 7.7 ± 4.1 (4 yr) | 12.7 ± 3.0 | 2.9 ± 2.4 (1 yr), 5.1 ± 3.6 (2 yr), 4.4 ± 3.3 (3 yr), 4.7 ± 3.0 (4 yr) | NA | NA |
| Hori et al. (38) | Retrospective | April 2015–October 2017 | Tokyo, Japan | 12 | 76.5 ± 3.8 (67–82) | 9, 3 | Median 15 (10–70) | VIM | Standard | 1 year | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Pineda-Pardo et al. (39) | Prospective, uncontrolled | NA | Madrid, Spain | 24 | 68.0 ± 10.1 | 17, 7 | 18.6 ± 12.8 | VIM + CTT | Atlas + DTI based (to extend the target) | 1 year | 52.9 ± 13.0 | 23.8 ± 8.3 (3 m); 26.4 ± 11.3 (1 yr−19) | 5.6 ± 1.8 | 1.0 ± 0.9 (3 m), 1.5 ± 1.3 (1 yr−19) | NA | NA | 17.3 ± 4.8 | 4.2 ± 4.1 (0–15) (3 m), 5.4 ± 4.9 (0–19) (1 yr−19) | NA | NA |
| Yang et al. (50) | Case Report | NA | Philadelphia, USA | 1 | 74 | 1, 0 | 1 | CTT | DTI based | 3 month | 25 | 5 (3 m) | NA | NA | 10 (out of 32) | 1 (3 m) | 9 | 0 (3 m) | NA | NA |
| Jones et al. (40) | Retrospective | July 2015–July 2018 | Toronto, Canada | 19 low temperature (LT), 30 high temperature (HT) | NA | NA | NA | VIM | Standard | 1 year | NA | NA | NA | NA | 20.5 ± 5.8 (Low Temperature - LT), 20.3 ± 5.0 (Hight Temperature - HT) (out of 32) | Improvement by 53% ± 32 and 51% ± 22% at 3 m, 45% ± 55% and 44% ± 22% (1 yr−9 LT, 27 HT) | NA | NA | NA | NA |
| Sinai et al. (41) | Prospective, uncontrolled | Nov 2013–Nov 2018 | Haifa, Israel | 44 | Median 70.5 (63–87) | 27, 17 | 16.3 ± 10.4 (1–30) | VIM | Standard | Median 12 month | Median 46.0 | Median 12.0 (1 m−44); 18.0 (1 yr−24); 11.0 (2 yr−15); 16.0 (3 yr−10); 14.0 (4 yr−6); 8.0 (5 yr−2) | NA | NA | Median 19 (out of 32) | Median 0.0 (1 m−44); 4.0 (1 yr−24); 4.0 (2 yr−15); 3.5 (3 yr−10); 5.0 (4 yr−6); 3.0 (5 yr−2) | NA | NA | 41.5 | Median 5.5 (1 m−44); 14.0 (0–89) (1 yr−24); 15.0 (2 yr−15); 15.5 (3 yr−10); 14.5 (4 yr−6); 11.0 (5 yr−2) |
| Chang et al. (42) | Prospective, uncontrolled | since 2013 | Seoul, South Korea | 50 | 66.65 ± 9.95 (45–80) | 42, 8 | NA | VIM | Standard | 17.8 ± 19.8 (1–60) month | NA | NA | NA | NA | 12.12 ± 0.51 (out of 32) | 5.88 ± 0.52 | 12.52 ± 0.52 | 3.64 ± 0.47 | NA | NA |
| Miller et al. (43) | Retrospective | July 2014–August 2016 | Baltimore, USA | 4 | NA | NA | NA | VIM + CTT | Atlas + DTI based (to extend the target) | 3 month (1 patient died of unrelated cause after 3 months. For the rest, benefit was sustained till 1 year follow up, no scores mentioned) | 57.5 ± 16.8 | 29.5 ± 6.4 | NA | NA | 6.5 ± 1.0 (out of 16) | 0.75 ± 0.9 | NA | NA | NA | NA |
| Krishna et al. (44) (Pivotal)* | Retrospective | 2013–2015 | Multicentric - 8 | 75 (treatment + sham crossover) | 71.3 ± 8.4 | 51, 24 | 16.8 ± 12.3 | VIM | Standard | 1 year | NA | NA | NA | NA | 19.9 ± 5 (out of 32) | Improvement: 56.3 ± 25.5% (3 m), 52.1 ± 24.9% (1 yr) | NA | Improvement : 68.3 ± 27.6% (3 m), 65.9 ± 30.9% (1 yr) | NA | NA |
| Krishna et al. (44) (Post Pivotal)* | Retrospective | 2015–2016 | Multicentric - 18 | 114 | 71 ± 9.5 | 80, 34 | 15.4 ± 13.3 | VIM | Standard | 1 year | NA | NA | NA | NA | 19.3 ± 5 (out of 32) | Improvement: 63.6 ± 26.1% (3 m), 61.9 ± 24.9% (1 yr) | NA | Improvement: 72.3 ± 25.9% (3 m), 66.1 ± 32.1% (1 yr) | NA | NA |
| Gallay et al. (45) | Prospective, uncontrolled | After 2016 | Solothurn & Bern, Switzerland | 10 | 66 ± 8 years | 8, 2 | 31 ± 14 | CTT (3 patients also had a contralateral centrum medianum thalamotomy) | Standard | 1 year | 48 ± 12 | 16 ± 7 (3 m); 17 ± 8 (1 yr) | 11.8 ± 3.9 | 3.6 ± 1.5 (3 m), 4.3 ± 1.9 (1 yr) | NA | NA | 14.2± 3.4 | 2.6 ± 2.0 (3 m), 3.4 ± 2.6 (1 yr) | NA | NA |
| Paff et al. (51) | Case Report | NA | Toronto, Canada | 1 | 93 | 1, 0 | 40 | VIM | Standard | 1 year | NA | 52% improvement | NA | NA | NA | 64% improvement in hand score | NA | NA | NA | NA |
| Buch et al. (52) | Case Report | NA | Philadephia, USA | 1 | 80 | 1, 0 | NA | VIM + CTT | DTI based | 6 week | NA | NA | NA | NA | 20 (out of 32) | 2 | 21 | 2 | NA | NA |
| Fukutome et al. (46) | Retrospective | May 2016–August 2017 | Nara, Japan | 15 | 62.9 ± 11.3 (41–82) | 11, 4 | 21.5 ± 14.0 (2–47) | VIM | Standard | 1 year | NA | NA | NA | NA | 18.5 ± 5.8 (out of 32) | 4.6 ± 5.7 | NA | NA | NA | NA |
RCT, randomized controlled trial; SD, standard deviation; VIM, ventral intermediate nucleus; CTT, cerebello-thalamic tract; DTI, diffusion tensor imaging; NA, not available; m, month; yr, year.
Targeting Method and Operative Parameters
Majority of the studies followed atlas-based targeting which was further refined by direct targeting based on MRI. DTI based targeting was reported by six studies, two of whom were case reports (Table 2, Figure 1D). Treatment parameters used by various centers have been summarized in Table 3. The skull density ratio (SDR) was more than a mean of 0.45 for all studies, except one which reported a median value of 0.38 (38). The mean number of sonications ranged from 11 ± 3.2 to 22.5 ± 7.6. All studies reported maximum temperature attained as >55°C for the lesioning except Chang et al. (26), who reported 53 ± 3.3°C as the mean temperature attained, and Jones et al. (40), who described a series of 19 patients in whom multiple low-temperature sonications were used to create a lesion. The maximum energy delivered ranged from a mean of 10,320 ± 4,537 to 16,910 ± 8,340 J. The sonication time ranged from a mean of 82.8 ± 30.8 to 105 ± 55 min. A recent case report mentioned 80 min as the sonication time (51). Four centers utilized a 1.5T MRI for the procedure (34, 46, 50, 52) while the rest performed it on a 3T machine.
Table 3.
Treatment parameters [*For descriptive purposes, the cohort of patients reported by Krishna et al. (35) and Elias et al. (47) were split into two groups, each with its own distinct characteristics].
| References | Mean skull density ratio ± SD (range) | No. of sonications ± SD (range) | Maximum energy delivered ± SD (range) (in Joules) | Peak temperature ± SD (range) (in °C) | Mean operative time ± SD (range) (in minutes) | MRI |
|---|---|---|---|---|---|---|
| Lipsman et al. (24) | NA | 22.5 ± 7.6 (12–29) | NA | 59.3 ± 2.9 (56–63) | NA | 3T |
| Elias et al. (25) | NA | 17.9 ± 4.6 (11–26) | 10,320 ± 4,537 (6,500–20,800) | 58.5 ± 2.5 (54–63) | NA | 3T |
| Chang et al. (26) | NA | NA | NA | 53 ± 3.3 (48–61) | 227.5 (169–293) (No vertigo group) to 260.6 (160–354) (Vertigo group) | 3T |
| Gallay et al. (27) | NA | NA | 16,073 ± 6,037 | NA | 285 ± 66 | 3T |
| Elias et al. (47) (Treatment group)* | NA | 18.5 ± 5.2 | 14,497.0 ± 6,695.7 (3,500–34,860) | 55.6 ± 2.3 (50.0–60.7) | NA | 3T |
| Elias et al. (47) (Sham Crossover)* | NA | NA | NA | NA | NA | 3T |
| Chang et al. (48) | NA | 18.5 ± 5.2 | 14,497.0 ± 6,695.7 (3,500–34,860) | 55.6 ± 2.3 (50.0–60.7) | NA | 3T |
| Halpern et al. (49) | NA | 18.5 ± 5.2 | 14,497.0 ± 6,695.7 (3,500–34,860) | 55.6 ± 2.3 (50.0–60.7) | NA | 3T |
| Zaroor et al. (28) | NA | 20.8 ± 6.4 | 12,231.5 ± 3,189.8 | 56.88 ± 2.5 | NA | 3T |
| Schreglmann et al. (29) | NA | 11 ± 3.2 (8–17) | 12,008 ± 4,441 (7,800–19,950) | 62.0 ± 2.5 (58–64) | 271.6 ± 40 (215–305) | 3T |
| Kim et al. (30) | NA | NA | NA | NA | NA | 3T |
| Chazen et al. (31) | NA | NA | NA | NA | NA | 3T |
| Federau et al. (32) | NA | 18.6 ± 5.7 (12–28) | NA | NA | NA | 3T |
| Jung et al. (33) | NA | 16.8 (13–20) | 15,910 ± 5,702.7 | 57.9 | NA | 3T |
| Iacopino et al. (34) | NA | NA | NA | NA | NA | 1.5T |
| Krishna et al. (35) | 0.54 ± 0.1 | 13.9 ± 4.5 | NA | NA | 174.3 ± 41.6 (Sonification time : 82.8 ± 30.8) | 3T |
| Boutet et al. (36) | 0.48 ± 0.1 | NA | NA | 56.6 ± 2.3 | NA | 3T |
| Park et al. (37) | 0.49 ± 0.08 (0.26–0.6) | 17.3 ± 1.6 (15–20) | 15,552.4 ± 6,574.1 (7,150–25,488) | NA | NA | 3T |
| Hori et al. (38) | Median 0.38 (0.27–0.61) | Median 17 (9–26) | Median 23,054 (5,849–38,658) | Median 56 (52–59) | NA | 3T |
| Pineda-Pardo et al. (39) | NA | NA | NA | NA | NA | 3T |
| Yang et al. (50) | NA | 14 | 16,080 | 64 | NA | 1.5T |
| Jones et al. (40) | NA | NA | NA | NA | NA | 3T |
| Sinai et al. (41) | Median 0.47 (0.31–0.67) | Median 19.5 (9–36) | Median 12,077 (6,000–35,500) | NA | NA | 3T |
| Chang et al. (42) | 0.51 ± 0.08 (0.26–0.72) | 15.12 ± 3.88 | NA | 58.76 ± 2.89 | NA | 3T |
| Miller et al. (43) | NA | NA | NA | NA | NA | 3T |
| Krishna et al. (44) (Pivotal)* | 0.55 ± 0.1 (unreported for 17 pts) | 17.4 ± 4.3 | 14,410 ± 7,390 | 55.6 ± 2.8 | 88 ± 40 | 3T |
| Krishna et al. (44) (Post Pivotal)* | 0.5 ± 0.1 (unreported for 4 pts) | 17.1 ± 5.3 | 16,910 ± 8,340 | 56.7 ± 2.5 | 105 ± 55 | 3T |
| Gallay et al. (45) | 0.54 ± 0.06 (0.33–0.62) | NA | 13,720 (5,850–36,000) | NA | NA | 3T |
| Paff et al. (51) | 0.65 | 13 | 18,302 | 59 | 80 | 3T |
| Buch et al. (52) | 0.46 | 16 | 22,559 | 60 | NA | 1.5T |
| Fukutome et al. (46) | 0.45 ± 0.11 (0.30–0.80) | NA | 16,275 ± 8,610 (4,791–33,018) | 57.3 ± 1.9 (54–60) | NA | 1.5T |
Tremor Outcome
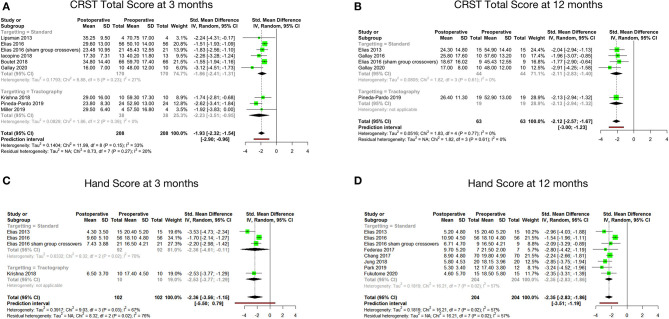
Tremor outcomes, in the form of CRST scores and its subsets, for all studies have been summarized in Table 4. Total CRST scores 3 months after the procedure were reported by nine studies. The pooled standard mean difference between postoperative and preoperative total CRST score at 3 months was −1.93 (95% CI: −2.32 to −1.54, p-value < 0.001). The studies showed moderate heterogeneity with I2 of 33% (Figure 2A).
Table 4.
Summary of outcomes after meta-analysis.
| Outcome variables | Standard mean difference between pre & postoperative score (95% CI) | No. of participants (studies) | p-value | Heterogeneity (I2) |
|---|---|---|---|---|
| Total CRST score (at 3 months) | −1.93 (−2.32 to −1.54) | 208 (9) | <0.001* | Moderate (33%) |
| Total CRST score (at 12 months) | −2.12 (−2.57 to −1.67) | 63 (5) | 0.002* | Low (0%) |
| Hand score (at 3 months) | −2.36 (−3.56 to −1.15) | 102 (4) | 0.03* | Moderate (67%) |
| Hand score (at 12 months) | −2.35 (−2.83 to −1.86) | 204 (8) | 0.02* | Moderate (57%) |
| CRST Part C score (at 3 months) | −2.66 (−3.53 to −1.79) | 104 (5) | 0.08 | Moderate (52%) |
| CRST Part C score (at 12 months) | −2.57 (−3.33 to −1.80) | 202 (7) | 0.01* | Moderate (64%) |
| QUEST score (at 3 months) | −1.49 (−2.93 to −0.04) | 79 (3) | 0.13 | Moderate (51%) |
| QUEST score (at 6 months) | −2.20 (−3.40 to −1.00) | 58 (4) | 0.07 | Moderate (57%) |
CI, confidence interval; NA, not applicable.
Significant. Bold denote significant values.
Figure 2.
Pooled standard mean difference between preoperative and postoperative total CRST score at 3 months (A), total CRST score at 12 months (B), hand score at 3 months (C) and hand score at 12 months (D).
Seven studies reported total CRST scores at 12 months after the procedure. The pooled standard mean difference was −2.07 (95% CI: −2.70 to −1.44). P-value was found to be significant at <0.01. The studies showed high heterogeneity with I2 of 68%. Sensitivity analysis was done, and 2 studies (33, 47) were found to be contributing to heterogeneity. Analysis was redone after removing these 2 studies. Hence, the final analysis for total CRST scores at 12 months after the procedure included five studies. The pooled standard mean difference was −2.12 (95% CI: −2.57 to −1.67). P-value was found to be significant at 0.002. The studies showed low heterogeneity with I2 of 0% (Figure 2B).
Four cohorts reported hand scores (out of a total of 32) at 3 months. The pooled standard mean difference was −2.36 (95% CI: −3.56 to −1.15; p-value - 0.03). The studies showed high heterogeneity with I2 of 67% (Figure 2C). Eight cohorts reported hand scores at 12 months. The pooled standard mean difference was −2.35 (95% CI: −2.83 to −1.86; p-value - 0.02). The studies showed moderate heterogeneity with I2 of 57% (Figure 2D).
The standard mean difference between the preoperative and postoperative total CRST score and hand scores was found to be significant at 3 and 12 months following the procedure. Subgroup analysis of the mean changes in CRST scores according to the targeting technique (standard vs. DTI based) revealed that the difference was not statistically significant between the two groups.
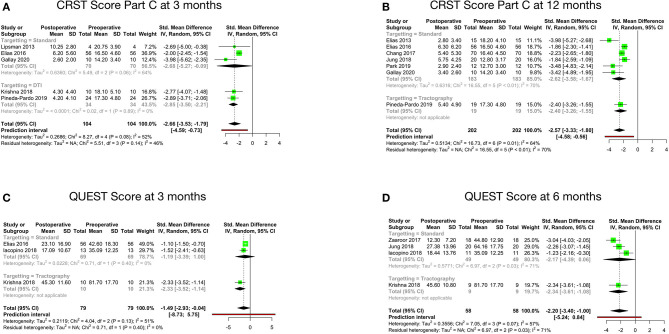
Disability and QOL Outcome
Disability, as per the CRST Part C score at 3 months after MRgFUS, was reported by five studies. The pooled standard mean difference was −2.66 with 95% CI: −3.53 to −1.79 (p-value - 0.08). The studies showed moderate heterogeneity with I2 of 52% (Figure 3A). Disability at 12 months after MRgFUS was reported by eight cohorts. The pooled standard mean difference was −4.54 with 95% CI: −8.95 to −0.12 (p-value < 0.01). The studies showed considerable heterogeneity with I2 of 96%. Sensitivity analysis was done, and 1 study (42) was found to be contributing to heterogeneity. Analysis was redone after removing this study. Hence, the final analysis for disability at 12 months after MRgFUS included seven studies. The pooled standard mean difference was −2.57 with 95% CI: −3.33 to −1.80 (p-value - 0.01). The studies showed moderate heterogeneity with I2 of 64% (Figure 3B).
Figure 3.
Pooled standard mean difference between preoperative and postoperative disability scores at 3 months (A), disability scores at 12 months (B), QUEST scores at 3 months (C) and QUEST scores at 6 months (D).
QOL, as per the QUEST score at 3 months, was reported by three cohorts. The pooled standard mean difference was −1.49 (95% CI: −2.93 to −0.04; p-value - 0.13). The studies showed moderate heterogeneity with I2 of 51% (Figure 3C). Four cohorts reported QOL at 6 months. The pooled standard mean difference was −2.20 (95% CI: −3.40 to −1.00; p-value - 0.07). The studies showed moderate heterogeneity with I2 of 57% (Figure 3D).
The comparison between preoperative and postoperative disability revealed statistically significant difference in the Part C score at 12 months (p-value: 0.01) (Table 4). Further subgroup analysis disclosed no statistically significant difference.
Complications
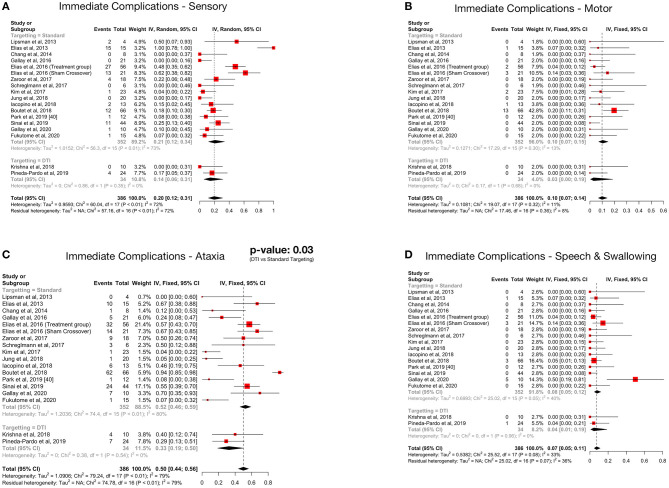
Details regarding the immediate, short term, and long-term complications are provided in the supplementary data (Supplementary Materials 2–4). The total complications were arranged according to the targeting method – standard vs. DTI based (Table 5) The pooled proportion of sensory, motor, ataxia and speech & swallowing related complications was calculated for immediate, early and late (occurring or persisting after 3 months) complications (Table 6, Figure 4, Supplementary Material 5).
Table 5.
Total number of complications (grouped according to the targeting method).
| References | Neurological | Minor/Treatment Related | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensory | Ataxia/gait disturbance | Motor | Speech and swallowing | Headache and fatigue | Sonication related | Frame and MRI related | Other | |||||||||||||||||
| A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | |
| Atlas based targeting | ||||||||||||||||||||||||
| Lipsman et al. (24) | 2 | 1 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA | 1 Deep Vein Thrombosis | 1 Deep Vein Thrombosis | NA |
| Elias et al. (25) | 15 | 4 | 4 | 10 | 5 | 0 | 1 (Grip) | 1 (5 days) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 33 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 |
| Chang et al. (26) | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 3 failed to attain temperature above 50 °C | 0 | 0 |
| Gallay et al. (27) | 0 | 0 | 0 | 5 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elias et al. (47) (Treatment group) | 27 | 17 | 10 | 32 | 17 | 8 | 2 (Grip) | 2 (Grip) | 1 (Grip) | 2 | 2 | 2 | 11 | 3 | 2 | 54 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 |
| Elias et al. (47) (Sham Crossover) | 13 | 8 | 8 | 14 | 7 | 4 | 3 (Grip) | 2 (Grip) | 1 (Grip) | 3 | 2 | 2 | 11 | 2 | 2 | 16 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 |
| Chang et al. (48) | NA | NA | 1 | NA | NA | 12 | NA | NA | 1 | NA | NA | 0 | NA | NA | 0 | NA | NA | 0 | NA | NA | 0 | NA | NA | 0 |
| Halpern et al. (49) | NA | NA | 12 | NA | NA | 10 | NA | NA | 2 | NA | NA | 1 | NA | NA | 1 | NA | NA | 0 | NA | NA | 0 | NA | NA | 1-slow movements |
| Zaroor et al. (28)# | 4 | 4 | 0 | 9 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 39 | 0 | 0 | 8 | 8 | 0 | 0 | 0 | 0 |
| Schreglmann et al. (29) | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kim et al. (30) | 1 | 1 | 0 | 1 | 1 | 0 | 2 (Facial) | 2 (Facial- one resolved in 1 month) | 1 (Facial) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Federau et al. (32) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Jung et al. (33) | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Iacopino et al. (34)# | 2 | 0 | 0 | 6 | 3 | 2 | 1 (Grip) | 1 (Grip - 1 week) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 6 | 0 | 0 | 4 ET patients - aborted treatment due to severe headache; 1 ET patient - failed to attain ablative temperature | 0 | 0 |
| Boutet et al. (36) | 12 | 5 | NA | 62 | 20 | NA | 13 | 6 | NA | 3 | 3 | NA | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | 0 | 0 | NA |
| Park et al. (37) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hori et al. (38) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Jones et al. (40) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Sinai et al. (41) | 11 | 11 | 5 | 24 | 24 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 65 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Chang et al. (42) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Krishna et al. (44) (Pivotal) | NA | NA | 42 | NA | NA | 59 | NA | NA | 4 mild, 2 moderate | NA | NA | 5 | NA | NA | 0 | NA | NA | 0 | NA | NA | 0 | NA | NA | 6 mild, 3 moderate |
| Krishna et al. (44) (Post Pivotal) | NA | NA | 56 | NA | NA | 89 | NA | NA | 16 mild | NA | NA | 17 | NA | NA | 0 | NA | NA | 0 | NA | NA | 0 | NA | NA | 16 mild, 2 moderate |
| Gallay et al. (45) | 1 | 1 | 1 | 7 | 5 | 5 | 0 | 0 | 0 | 5 | 1 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Paff et al. (51) | 0 | 0 | 0 | 1 | 1 | 0 | 1 (c/l lower limb) | 1 (c/l lower limb) (1 m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fukutome et al. (46) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 90 | 54 | 140 | 178 | 104 | 194 | 23 | 15 | 28 | 14 | 8 | 28 | 30 | 9 | 5 | 251 | 0 | 0 | 55 | 10 | 0 | 9 | 1 | 28 |
| DTI based targeting | ||||||||||||||||||||||||
| Chazen et al. (31) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Krishna et al. (35) | 0 | 1 | 0 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pineda-Pardo et al. (39) | 4 | 4 | 4 | 7 | 7 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yang et al. (50) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Miller et al. (43) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Buch et al. (52) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Total | 4 | 5 | 4 | 11 | 10 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Studies reporting zero complications are marked as “0.” Studies in which no complication data was reported for respective time period are marked as “NA.”
A, immediate (during treatment to within 48 h); B, short term (48 h−3 months); C, long term (persisting for more than 3 months). Period in brackets denotes time until when the complication persisted.
Complications not mentioned separately for ET patients.
Table 6.
Summary of complications after meta-analysis.
| Outcome variables | Pooled proportion (95% CI) | No. of participants (studies) | Heterogeneity (I2) | p-value after subgroup analysis (standard vs. DTI based targeting) |
|---|---|---|---|---|
| Immediate | ||||
| Sensory | 20% (12–31%) | 386 (18) | High (72%) | 0.46 |
| Motor | 10% (7–14%) | 386 (18) | Low (11%) | 0.20 |
| Ataxia | 50% (44–56%) | 386 (18) | High (79%) | 0.03* |
| Speech & Swallowing | 7% (5–11%) | 386 (18) | Moderate (33%) | 0.47 |
| Short-term | ||||
| Sensory | 16% (11–23%) | 386 (18) | Moderate (43%) | 0.93 |
| Motor | 6% (4–9%) | 386 (18) | Low (0%) | 0.46 |
| Ataxia | 29% (22–38%) | 386 (18) | Moderate (49%) | 0.95 |
| Speech and swallowing | 4% (3–7%) | 386 (18) | Low (0%) | 0.96 |
| Long-term | ||||
| Sensory | 13% (7–23%) | 368 (16) | High (76%) | 0.88 |
| Motor | 5% (3–7%) | 391 (17) | Low (0%) | 0.86 |
| Ataxia | 31% (24–38%) | 378 (16) | High (87%) | 0.09 |
| Speech and swallowing | 5% (3–8%) | 391 (17) | Low (0%) | 0.77 |
CI, confidence interval.
Significant. Bold denote significant values.
Figure 4.
Forest plots depicting pooled proportions of immediate sensory (A), motor (B), ataxia (C) and speech and swallowing (D) related complications.
Ataxia was the most common postoperative complication. All complications showed a decreasing trend over time. Subgroup analysis revealed significantly less immediate post procedure ataxia related complications in the DTI group, although no significant difference was noted over long term analysis.
Discussion
The first reports of the use of MRgFUS in medically refractory ET were published in 2013 (24, 25). Standard atlas-based targeting was utilized to create a lesion in the contralateral VIM nucleus of the thalamus. The USFDA approved the use of MRgFUS in ET in 2016 after a randomized sham-controlled trial showed favorable results in the MRgFUS group (47). In a short time period, there has been a significant amount of research on the subject. However, most of these studies have small sample size. Our review article summarizes the latest available evidence in literature in terms of efficacy and complications of MRgFUS for ET. Owing to the paucity of studies involving large number of patients, this meta-analysis strives to provide pooled results of a number of smaller studies on the topic. We did an updated systematic review and meta-analysis of the studies describing the outcomes and adverse events following the use of MRgFUS in essential tremor. Though reviews have been published in the past on this topic, but there were significant shortcomings (15, 16). Our review article summarizes the latest available evidence in literature in terms of efficacy and complications of MRgFUS for ET. The primary outcome analyzed was the change in total CRST score and hand score (out of 32) after treatment, while the secondary outcomes measured were the quality of life and the complication rates. We have also tried to find whether any difference in efficacy and complication rate exists according to the area targeted - VIM nucleus of thalamus or CTT in the PSA.
Clinical Efficacy
All studies have reported good postoperative outcomes. We found a significant difference in the pooled SMD between the postoperative and preoperative primary outcome variables, at 3- and 12-months post-procedure. This shows MRgFUS to be an effective surgical modality for the treatment of ET. Additionally, there was a statistically significant improvement in the disability of the patients at 12 months postoperatively, as evaluated by the CRST Part C score. Only one study had a control group; hence between-group comparison was not possible.
For any other surgical technique to replace DBS as the procedure of choice for refractory ET, it has to prove itself as at-par, if not better than DBS. Comparative studies between RFA and DBS have reported better improvement in function and fewer adverse effects with DBS (53). Gamma knife thalamotomy for ET was first described in the 1990s. It's a non-invasive procedure, however, the inability to monitor real-time clinical response, variation in the size of the lesion produced, unpredictable radiation effects, and a delay in clinical response have resulted in GKT being reserved for patients who are otherwise unfit for DBS (12).
Non-invasiveness of the MRgFUS is an advantage of MRgFUS over DBS. Class I evidence in the form of an RCT gave a big impetus to MRgFUS (47). In a retrospective analysis of RFA, DBS, and MRgFUS for ET, outcomes of the procedures between the three groups were not statistically different (30). Another retrospective analysis showed comparable efficacy and QOL between unilateral DBS and MRgFUS (54). A recent study compared a trial on the use of VIM DBS for ET, with the RCT done by Elias et al. (47, 55, 56). They found a greater percentage improvement with DBS, although the patients in the DBS group had worse baseline tremor scores.
Long Term Outcome
Sustained improvement in tremor scores has been demonstrated on long term follow up of patients (37, 41, 48, 49). At 3 years of follow up, the patients enrolled in the RCT had a reduction of 56% in hand score, 63% in disability score and a 50% improvement in the QOL (49). At 4 years of follow up in 12 patients, a 56% reduction in hand score and 63% reduction in the disability scores was seen (37). The maximum available follow up of 5 years in two patients revealed a total CRST score of 8.0 (6–10) and QUEST score of 11.0 (6–16), as compared to a baseline score of 46.0 (16–74) and 41.5 (15–93) respectively (41).
A decline in efficacy over time, in the form of a small increase in the hand tremor and disability scores at 3 years as compared to the scores at 6 months has been noted (49). Four patients out of 76 underwent DBS (49). Sinai et al. observed a return of tremor in 11% of their patients (5/44) (41). Further studies with a greater number of patients are needed to refute this observation. The decline in the efficacy over time may be due to the progressive nature of the disease (57). In such cases, it is feasible to treat the patients again and this is certainly a big strength of MRgFUS.
Treatment Parameters
A meta-analysis of the mentioned treatment parameters could not be done due to the unavailability of adequate data for analysis. Some studies in literature have tried to correlate various treatment parameters with the clinical outcome. SDR was significantly associated with the outcome at 1 and 6 months by Sinai et al. (41), while no such relationship was found in other studies (42, 44, 46). The study which included patients with SDR < 0.4 found no statistically significant difference in the mean SDR of patients who had sustained improvement in symptoms and those who had recurrence of symptoms in this study. Traditionally, a SDR value of <0.40 has been associated with higher energy requirements. But recent clinical series investigating this topic have found no significant difference in the clinical outcome or the complication rate in this subgroup as compared to the patients with SDR value > 0.40 (58, 59).
The higher maximal temperature has been found to significantly influence the percentage change in tremor scores (41, 45). Intraoperative tremor reduction has not been found to correlate to outcome at 3 months, while procedure duration and number of sonications have been shown to be significantly less with the use of DTI (35). Studies have previously found an association between younger age, short disease duration, better baseline tremor scores, fewer number of sonications and a higher maximal temperature during treatment to a better outcome (35, 44, 46). Experience acquired with the technique has also been found to have a positive impact on the outcome positively (44). It has been recommended by some that the procedure should be restricted to a few specialized centers only (41). Barring three patients who underwent bilateral procedures 1 year apart (27), all FUS procedures have been performed unilaterally. ET is a progressive disease, with most patients having bilateral symptoms (57). Thus, more experience with bilateral procedures is required.
Complications
More than 1/3rd of patients developed sonication related complications, amongst which head pain and dizziness were the most common. This seems to be a significant source of discomfort for the patient. Iacopino et al. (34) reported four patients in whom treatment had to be aborted due to severe head pain. None of the sonication or frame related complications persisted beyond 3 months.
Ataxia, which included gait disturbance and hand ataxia, was the most common neurological side effect, followed by sensory deficits. The immediate pooled proportion of ataxia was 50%, while it was 20% for sensory complications, which is considerable. The high complication rate has been postulated to be due to the small size of the VIM nucleus and non-visibility on MRI resulting in the potential overlap of the lesion with the surrounding structures like the ML and CST. The reason why ataxia is the most common acute complication was suggested to be due to the objective nature of assessment (36). Further, it was demonstrated that the area responsible for postoperative ataxia overlapped significantly with the area associated with clinical benefit (36). Fortunately, data suggests that these complications partially resolve with time as evident by the decreased incidence of late complications (Table 4). Furthermore, no additional side effects were observed in each subsequent year of follow up amongst the patients enrolled in the RCT (37, 48, 49). But a major limitation in assessing the long-term complications of the patients undergoing MRgFUS is the high dropout rate (48, 49). Halpern et al. found it to be as high as 31% in 3 years (49). Thus, a true picture of the permanence of the complications is hard to assess.
No hemorrhage, seizure or trajectory related complications have been noted till now with MRgFUS for ET, making it a uniquely safe procedure in this aspect as compared to DBS or RFA (11, 30, 59).
Role of DTI in Target Localization Technique
VIM nucleus of the thalamus, which has traditionally been used as the target for MRgFUS, is not visible on the 3T MRI and surgeons have to target it based on an atlas or an estimate based on their experience. Microelectrode recording is not possible intra-operatively as the procedure is incisionless. A series of low power sonications, delivered before creating the actual lesion, are usually used to observe the resolution of tremor or the appearance of side effects. However, this method of confirming target accuracy has not been scientifically validated. On the other hand, CTT fibers localized to the PSA have been shown to have a high density of clinically relevant fibers for targeting (53, 60, 61). Gallay et al. were the first to target the CTT using atlas-based co-ordinates (27). Boutet et al. identified distinct areas in the thalamus associated with clinical benefit and complications (36).
Advances in DTI have allowed surgeons to visualize the CTT and individualize surgical targeting accordingly. Both the CST and ML can also be localized, thus clearly demarcating the target according to the unique anatomy of each patient (31, 35, 39, 43). The surgical target thus identified has been found to be anterior and lateral to the atlas-based target (62). This should theoretically lead to better postoperative outcomes. Our analysis revealed a significant reduction in ataxia immediately after DTI based targeting. This is noteworthy as post procedure ataxia has been observed to be an important source of patient discomfort. Thus, use of DTI could decrease patient distress and lead to better acceptability of the procedure. A significant benefit was not apparent on analysis of the other complications. This could be due to the smaller number of studies utilizing DTI. Moreover, it must be emphasized that DTI based targeting is not yet standardized. Differences exist in the number of tracts generated for localization. All the centers have generated the ipsilateral CTT tract (one track) for target localization in the VIM region of the thalamus. Many studies have additionally utilized the ipsilateral CST and ML tracts (three tracks) and adjusted the surgical target accordingly to avoid significant motor and sensory side effects. Anatomical considerations of the CTT tract which originates from the contralateral dentate projections and then decussates in the superior cerebellar peduncle to reach the ipsilateral motor cortex via the thalamus, have led some centers to generate the contralateral CTT tract (four track) in addition to the previously defined three tracks, for refining the target localization (62, 63). Thus, there are differences between the various surgical teams performing DTI based MRgFUS and there is no consensus on what are the best practices although there seems to be an increasing trend to the DTI usage (62–65). The utility of DTI in this regard remains to be definitely proven with additional numbers and long-term data.
Limitations
Observational studies formed the majority basis for the analysis as there is only one clinical trial on the subject. These two types of studies are assessed differently in terms of bias and the strength of evidence, and thus recommendations, that they can offer are markedly different. Authors have used various subsets of the CRST scale to report the outcomes, thus precluding standardized comparison. Few studies included patients who underwent bilateral MRgFUS, which is associated with higher rates of complication. However, the data of these patients was not provided separately and could not be excluded from our analysis. The possibility of some overlap between subsets of patients reported from the same center cannot be completely ruled out. DTI based targeting is a novel procedure, and the number of studies utilizing it are quite low in number. A high level of heterogeneity in between studies needs to be kept in mind.
Conclusion
MRgFUS for ET is an effective procedure for relieving unilateral tremor. Use of DTI based targeting revealed a significant reduction in post procedure ataxia related complications as compared to traditional targeting techniques. Analysis of other complications further revealed a decreasing trend on follow up. As of now, it seems to be the procedure of choice for patients unable to tolerate an invasive procedure. For it to replace established surgical options like DBS, further research will be required to prove long term clinical efficacy in both unilateral and bilateral procedures.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MA and KG: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, roles/writing - original draft, and writing - review and editing. RS, RR, and VN: data curation, visualization, and writing - review and editing. MS: project administration, supervision, and writing - review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- MRgFUS
magnetic resonance guided focused ultrasound
- ET
essential tremor
- RFA
radiofrequency ablation
- VIM
ventral intermediate
- DBS
deep brain stimulation
- GKT
gamma knife thalamotomy
- MRI
magnetic resonance imaging
- DTI
diffusion tensor imaging
- CTT
cerebello thalamic tract
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta Analyses
- CRST
clinical rating scale for tremor
- QOL
quality of life
- QUEST
quality of life in essential tremor
- SDR
skull density ratio
- RCT
randomized controlled trial
- PSA
posterior subthalamic area
- CST
corticospinal tract
- ML
medial lemniscus
- SMD
standardized mean difference
- SDC
supplementary digital content.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.654711/full#supplementary-material
References
- 1.Deuschl G, Bain P, Brin M. Consensus statement of the movement disorder society on tremor. Ad Hoc Scientific Committee. Mov Disord. (1998) 13 (Suppl. 3):2–23. 10.1002/mds.870131303 [DOI] [PubMed] [Google Scholar]
- 2.Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. (2018) 33:75–87. 10.1002/mds.27121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. (2010) 25:534–41. 10.1002/mds.22838 [DOI] [PubMed] [Google Scholar]
- 4.Kamble N, Pal PK. Tremor syndromes: a review. Neurol India. (2018) 66 (Suppl. S1):36–47 10.4103/0028-3886.226440 [DOI] [PubMed] [Google Scholar]
- 5.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. (2011) 10:148–61. 10.1016/S1474-4422(10)70322-7 [DOI] [PubMed] [Google Scholar]
- 6.Schreglmann SR, Krauss JK, Chang JW, Martin E, Werner B, Bauer R, et al. Functional lesional neurosurgery for tremor: back to the future? J Neurol Neurosurg Psychiatry. (2018) 89:727–35. 10.1136/jnnp-2017-316301 [DOI] [PubMed] [Google Scholar]
- 7.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. (1991) 337:403–6. 10.1016/0140-6736(91)91175-T [DOI] [PubMed] [Google Scholar]
- 8.Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. (1996) 84:203–14. 10.3171/jns.1996.84.2.0203 [DOI] [PubMed] [Google Scholar]
- 9.Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, Dewey RB, Jr, et al. Evidence-based guideline update: treatment of essential tremor: report of the quality standards subcommittee of the American Academy of Neurology. Neurology. (2011) 77:1752–5. 10.1212/WNL.0b013e318236f0fd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sankhe M, Churi O. Newer advances in lesional surgery for movement disorders. Neurol India. (2018) 66 (Suppl. S1):113–21 10.4103/0028-3886.226463 [DOI] [PubMed] [Google Scholar]
- 11.Wong JK, Hess CW, Almeida L, Middlebrooks EH, Christou EA, Patrick EE, et al. Deep brain stimulation in essential tremor: targets, technology, and a comprehensive review of clinical outcomes. Expert Rev Neurother. (2020) 20:319–31. 10.1080/14737175.2020.1737017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreglmann SR, Krauss JK, Chang JW, Bhatia KP, Kägi G. Functional lesional neurosurgery for tremor: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. (2018) 89:717–26. 10.1136/jnnp-2017-316302 [DOI] [PubMed] [Google Scholar]
- 13.Harary M, Segar DJ, Huang KT, Tafel IJ, Valdes PA, Cosgrove GR. Focused ultrasound in neurosurgery: a historical perspective. Neurosurg Focus. (2018) 44:E2. 10.3171/2017.11.FOCUS17586 [DOI] [PubMed] [Google Scholar]
- 14.Ranjan M, Elias GJB, Boutet A, Zhong J, Chu P, Germann J, et al. Tractography-based targeting of the ventral intermediate nucleus: accuracy and clinical utility in MRgFUS thalamotomy. J Neurosurg. (2019) 1–8. 10.3171/2019.6.JNS19612. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed N, Patra D, Nanda A. A meta-analysis of outcomes and complications of magnetic resonance-guided focused ultrasound in the treatment of essential tremor. Neurosurg Focus. (2018) 44:E4. 10.3171/2017.11.FOCUS17628 [DOI] [PubMed] [Google Scholar]
- 16.Schreglmann SR, Bhatia KP, Hägele-Link S, Werner B, Martin E, Kägi G. Letter to the Editor. Errors in the meta-analysis of outcomes and complications of MRgFUS. Neurosurg Focus. (2018) 45:E15. 10.3171/2018.3.FOCUS1898 [DOI] [PubMed] [Google Scholar]
- 17.Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E. editors. Parkinson's Disease and Movement Disorders. Baltimore, MD: Urban and Schwarzenberg; (1988). p. 225–34. [Google Scholar]
- 18.Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin tremor rating scale in essential tremor. Mov Disord. (2007) 22:833–8. 10.1002/mds.21412 [DOI] [PubMed] [Google Scholar]
- 19.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 20.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag; (2016). [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 24.Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. (2013) 12:462–8. 10.1016/S1474-4422(13)70048-6 [DOI] [PubMed] [Google Scholar]
- 25.Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. (2013) 369:640–8. 10.1056/NEJMoa1300962 [DOI] [PubMed] [Google Scholar]
- 26.Chang WS, Jung HH, Kweon EJ, Zadicario E, Rachmilevitch I, Chang JW. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry. (2015) 86:257–64. 10.1136/jnnp-2014-307642 [DOI] [PubMed] [Google Scholar]
- 27.Gallay MN, Moser D, Rossi F, Pourtehrani P, Magara AE, Kowalski M, et al. Incisionless transcranial MR-guided focused ultrasound in essential tremor: cerebellothalamic tractotomy. J Ther Ultrasound. (2016) 4:5. 10.1186/s40349-016-0049-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson's disease and essential tremor cases. J Neurosurg. (2018) 128:202–10. 10.3171/2016.10.JNS16758 [DOI] [PubMed] [Google Scholar]
- 29.Schreglmann SR, Bauer R, Hägele-Link S, Bhatia KP, Natchev P, Wegener N, et al. Unilateral cerebellothalamic tract ablation in essential tremor by MRI-guided focused ultrasound. Neurology. (2017) 88:1329–33. 10.1212/WNL.0000000000003795 [DOI] [PubMed] [Google Scholar]
- 30.Kim M, Jung NY, Park CK, Chang WS, Jung HH, Chang JW. Comparative evaluation of magnetic resonance-guided focused ultrasound surgery for essential tremor. Stereotact Funct Neurosurg. (2017) 95:279–86. 10.1159/000478866 [DOI] [PubMed] [Google Scholar]
- 31.Chazen JL, Sarva H, Stieg PE, Min RJ, Ballon DJ, Pryor KO, et al. Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. J Neurosurg. (2018) 129:315–23. 10.3171/2017.4.JNS162803 [DOI] [PubMed] [Google Scholar]
- 32.Federau C, Goubran M, Rosenberg J, Henderson J, Halpern CH, Santini V, et al. Transcranial MRI-guided high-intensity focused ultrasound for treatment of essential tremor: a pilot study on the correlation between lesion size, lesion location, thermal dose, and clinical outcome. J Magn Reson Imaging. (2018) 48:58–65. 10.1002/jmri.25878 [DOI] [PubMed] [Google Scholar]
- 33.Jung NY, Park CK, Chang WS, Jung HH, Chang JW. Effects on cognition and quality of life with unilateral magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Neurosurg Focus. (2018) 44:E8. 10.3171/2017.11.FOCUS17625 [DOI] [PubMed] [Google Scholar]
- 34.Iacopino DG, Gagliardo C, Giugno A, Giammalva GR, Napoli A, Maugeri R, et al. Preliminary experience with a transcranial magnetic resonance-guided focused ultrasound surgery system integrated with a 1.5-T MRI unit in a series of patients with essential tremor and Parkinson's disease. Neurosurg Focus. (2018) 44:E7. 10.3171/2017.11.FOCUS17614 [DOI] [PubMed] [Google Scholar]
- 35.Krishna V, Sammartino F, Agrawal P, Changizi BK, Bourekas E, Knopp MV, et al. Prospective tractography-based targeting for improved safety of focused ultrasound thalamotomy. Neurosurgery. (2019) 84:160–8. 10.1093/neuros/nyy020 [DOI] [PubMed] [Google Scholar]
- 36.Boutet A, Ranjan M, Zhong J, Germann J, Xu D, Schwartz ML, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. (2018) 141:3405–14. 10.1093/brain/awy278 [DOI] [PubMed] [Google Scholar]
- 37.Park YS, Jung NY, Na YC, Chang JW. Four-year follow up results of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Mov Disord. (2019) 34:727–34. 10.1002/mds.27637 [DOI] [PubMed] [Google Scholar]
- 38.Hori H, Yamaguchi T, Konishi Y, Taira T, Muragaki Y. Correlation between fractional anisotropy changes in the targeted ventral intermediate nucleus and clinical outcome after transcranial MR-guided focused ultrasound thalamotomy for essential tremor: results of a pilot study. J Neurosurg. (2019) 132:568–73. 10.3171/2018.10.JNS18993 [DOI] [PubMed] [Google Scholar]
- 39.Pineda-Pardo JA, Martínez-Fernández R, Rodríguez-Rojas R, Del-Alamo M, Hernández F, Foffani G, et al. Microstructural changes of the dentato-rubro-thalamic tract after transcranial MR guided focused ultrasound ablation of the posteroventral VIM in essential tremor. Hum Brain Mapp. (2019) 40:2933–42. 10.1002/hbm.24569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RM, Kamps S, Huang Y, Scantlebury N, Lipsman N, Schwartz ML, et al. Accumulated thermal dose in MRI-guided focused ultrasound for essential tremor: repeated sonications with low focal temperatures. J Neurosurg. (2019) 132:1802–9. 10.3171/2019.2.JNS182995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinai A, Nassar M, Eran A, Constantinescu M, Zaaroor M, Sprecher E, et al. Magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: a 5-year single-center experience. J Neurosurg. (2019) 1–8. 10.3171/2019.3.JNS19466. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Chang KW, Park YS, Chang JW. Skull factors affecting outcomes of magnetic resonance-guided focused ultrasound for patients with essential tremor. Yonsei Med J. (2019) 60:768–73. 10.3349/ymj.2019.60.8.768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller TR, Zhuo J, Eisenberg HM, Fishman PS, Melhem ER, Gullapalli R, et al. Targeting of the dentato-rubro-thalamic tract for MR-guided focused ultrasound treatment of essential tremor. Neuroradiol J. (2019) 32:401–7. 10.1177/1971400919870180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishna V, Sammartino F, Cosgrove R, Ghanouni P, Schwartz M, Gwinn R, et al. Predictors of outcomes after focused ultrasound thalamotomy. Neurosurgery. (2020) 87:229–37. 10.1093/neuros/nyz417 [DOI] [PubMed] [Google Scholar]
- 45.Gallay MN, Moser D, Jeanmonod D. MR-guided focused ultrasound cerebellothalamic tractotomy for chronic therapy-resistant essential tremor: anatomical target reappraisal and clinical results. J Neurosurg. (2020) 1–10. 10.3171/2019.12.JNS192219. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Fukutome K, Kuga Y, Ohnishi H, Hirabayashi H, Nakase H. What factors impact the clinical outcome of magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor? J Neurosurg. (2020) 1–6. 10.3171/2020.2.JNS192814. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 47.Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. (2016) 375:730–9. 10.1056/NEJMoa1600159 [DOI] [PubMed] [Google Scholar]
- 48.Chang JW, Park CK, Lipsman N, Schwartz ML, Ghanouni P, Henderson JM, et al. A prospective trial of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: results at the 2-year follow-up. Ann Neurol. (2018) 83:107–14. 10.1002/ana.25126 [DOI] [PubMed] [Google Scholar]
- 49.Halpern CH, Santini V, Lipsman N, Lozano AM, Schwartz ML, Shah BB, et al. Three-year follow-up of prospective trial of focused ultrasound thalamotomy for essential tremor. Neurology. (2019) 93:e2284–93. 10.1212/WNL.0000000000008561 [DOI] [PubMed] [Google Scholar]
- 50.Yang AI, Chaibainou H, Wang S, Hitti FL, McShane BJ, Tilden D, et al. Focused ultrasound thalamotomy for essential tremor in the setting of a ventricular shunt: technical report. Oper Neurosurg. (2019) 17:376–81. 10.1093/ons/opz013 [DOI] [PubMed] [Google Scholar]
- 51.Paff M, Boutet A, Neudorfer C, Elias GJB, Germann J, Loh A, et al. Magnetic resonance-guided focused ultrasound thalamotomy to treat essential tremor in nonagenarians. Stereotact Funct Neurosurg. (2020) 98:182–6. 10.1159/000506817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buch VP, McShane BJ, Beatson N, Yang A, Blanke A, Tilden D, et al. Focused ultrasound thalamotomy with dentato-rubro-thalamic tractography in patients with spinal cord stimulators and cardiac pacemakers. Stereotact Funct Neurosurg. (2020) 98:263–9. 10.1159/000507031 [DOI] [PubMed] [Google Scholar]
- 53.Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. (2000) 342:461–8. 10.1056/NEJM200002173420703 [DOI] [PubMed] [Google Scholar]
- 54.Huss DS, Dallapiazza RF, Shah BB, Harrison MB, Diamond J, Elias WJ. Functional assessment and quality of life in essential tremor with bilateral or unilateral DBS and focused ultrasound thalamotomy. Mov Disord. (2015) 30:1937–43. 10.1002/mds.26455 [DOI] [PubMed] [Google Scholar]
- 55.Harary M, Segar DJ, Hayes MT, Cosgrove GR. Unilateral thalamic deep brain stimulation versus focused ultrasound thalamotomy for essential tremor. World Neurosurg. (2019) 126:e144–52. 10.1016/j.wneu.2019.01.281 [DOI] [PubMed] [Google Scholar]
- 56.Wharen RE, Jr, Okun MS, Guthrie BL, Uitti RJ, Larson P, Foote K, et al. Thalamic DBS with a constant-current device in essential tremor: a controlled clinical trial. Parkinsonism Relat Disord. (2017) 40:18–26. 10.1016/j.parkreldis.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 57.Louis ED, Agnew A, Gillman A, Gerbin M, Viner AS. Estimating annual rate of decline: prospective, longitudinal data on arm tremor severity in two groups of essential tremor cases. J Neurol Neurosurg Psychiatry. (2011) 82:761–5. 10.1136/jnnp.2010.229740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D'Souza M, Chen KS, Rosenberg J, Elias WJ, Eisenberg HM, Gwinn R, et al. Impact of skull density ratio on efficacy and safety of magnetic resonance-guided focused ultrasound treatment of essential tremor. J Neurosurg. (2019) 132:1392–7. 10.3171/2019.2.JNS183517 [DOI] [PubMed] [Google Scholar]
- 59.Boutet A, Gwun D, Gramer R, Ranjan M, Elias GJB, Tilden D, et al. The relevance of skull density ratio in selecting candidates for transcranial MR-guided focused ultrasound. J Neurosurg. (2019) 132:1785–91. 10.3171/2019.2.JNS182571 [DOI] [PubMed] [Google Scholar]
- 60.Gallay MN, Jeanmonod D, Liu J, Morel A. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct. (2008) 212:443–63. 10.1007/s00429-007-0170-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blomstedt P, Sandvik U, Fytagoridis A, Tisch S. The posterior subthalamic area in the treatment of movement disorders: past, present, and future. Neurosurgery. (2009) 64:1029–38. Discussion 1038–1042. 10.1227/01.NEU.0000345643.69486.BC [DOI] [PubMed] [Google Scholar]
- 62.Sammartino F, Krishna V, King NK, Lozano AM, Schwartz ML, Huang Y, et al. Tractography-based ventral intermediate nucleus targeting: novel methodology and intraoperative validation. Mov Disord. (2016) 31:1217–25. 10.1002/mds.26633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamada K, Akazawa K, Yuen S, Goto M, Matsushima S, Takahata A, et al. MR imaging of ventral thalamic nuclei. AJNR Am J Neuroradiol. (2010) 31:732–5. 10.3174/ajnr.A1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soares JM, Marques P, Alves V, Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front Neurosci. (2013) 7:31. 10.3389/fnins.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Donnell LJ, Westin CF. An introduction to diffusion tensor image analysis. Neurosurg Clin N Am. (2011) 22:185–96. 10.1016/j.nec.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.