Abstract
Cooperation and competition are two basic modes of human interaction. Their underlying neural mechanisms, especially from an interpersonal perspective, have not been fully explored. Using the electroencephalograph-based hyperscanning technique, the present study investigated the neural correlates of both cooperation and competition within the same ecological paradigm using a classic motion-sensing tennis game. Both the inter-brain coupling (the inter-brain amplitude correlation and inter-brain phase-locking) and the intra-brain spectral power were analyzed. Only the inter-brain amplitude correlation showed a significant difference between cooperation and competition, with different spatial patterns at theta, alpha and beta frequency bands. Further inspection revealed distinct inter-brain coupling patterns for cooperation and competition; cooperation elicited positive inter-brain amplitude correlation at the delta and theta bands in extensive brain regions, while competition was associated with negative occipital inter-brain amplitude correlation at the alpha and beta bands. These findings add to our knowledge of the neural mechanisms of cooperation and competition and suggest the significance of adopting an inter-brain perspective in exploring the neural underpinnings of social interaction in ecological contexts.
Keywords: cooperation, competition, inter-brain coupling, hyperscanning, electroencephalogram
Introduction
Cooperation and competition are two basic modes of human social interaction (Decety et al., 2004). While cooperation involves the process by which a group of people strive for a common goal, competition entails the process by which each person strives for his or her own goal, even at the expense of others (Deutsch, 1962; Vonk, 1998). Both processes, however, similarly emphasize the monitoring of both one’s own and others’ actions, as well as adopting a specific mental set (Johnson and Johnson, 1989; Decety et al., 2004). Due to their fundamental importance in human daily life, understanding cooperation and competition has been one of the main goals since the birth of modern psychology, and research results have been widely applied in many fields, such as education, business and industry (Piaget, 1950; Vygotsky, 1978; Bandura, 2000; Johnson et al., 2013).
Research on the neural mechanisms of cooperation and competition has been greatly facilitated by the rapid development of hyperscanning methods over the past two decades (Montague et al., 2002; Babiloni and Astolfi, 2014; Mu et al., 2018; Zhang, 2018; Redcay and Schilbach, 2019). Hyperscanning methods address the exploration of neural mechanisms from an inter-brain perspective by simultaneously recording and analyzing brain activities from multiple persons, making it ideal for studying interactive, social processes such as cooperation and competition. Due to the broad conceptual coverage of cooperation and competition, the related laboratory-based studies fall into two main branches with different research focuses. While one branch has explored interactive decision-making using paradigms such as the trust game (e.g. investor–trustee game and the prisoner’s dilemma; Fallani et al., 2010; Hu et al., 2018; Zhang et al., 2019) and the builder game (Liu et al., 2015, 2017; Špiláková et al., 2020) to introduce cooperative or competitive conditions, the other branch has focused on behavioral coordination, in which participants are instructed to (mainly cooperatively) perform specific motor tasks in order to maximize their team’s action performance (Cui et al., 2012; Yun et al., 2012; Cheng et al., 2015; Mu et al., 2016; Hu et al., 2017). By analyzing the coupling of neural activities across multiple brains, findings from these inter-brain studies have highlighted the involvement of the mentalizing system both in interactive decision-making and in behavior coordination, such as the right temporoparietal junction (Dumas et al., 2010; Bilek et al., 2015) and the right inferior frontal gyrus (Saito et al., 2010). Although these findings are largely consistent with psychological theories and previous single-brain studies for cooperation and competition (Decety et al., 2004; Hari and Kujala, 2009; Stanley and Adolphs, 2013; Tsoi et al., 2016), the inter-brain approach is believed to provide a unique added value to promoting the understanding of cooperation and competition (Liu and Pelowski, 2014; Schoot et al., 2016; Mu et al., 2018; Redcay and Schilbach, 2019). Specifically, the inter-brain coupling over the mentalizing regions during cooperation and competition suggests a similarity of the neural activities among interacting partners for a shared representation of the interactive and interpersonal processes.
Meanwhile, emerging research is employing naturalistic paradigms outside the laboratory to further explore the neural mechanisms of cooperation and competition in typical real-world social interaction scenarios. For instance, researchers have conducted hyperscanning studies during music performance (Lindenberger et al., 2009; Sänger et al., 2012; Müller et al., 2013), teamwork of pilots and co-pilots (Astolfi et al., 2011; Toppi et al., 2016), social games (e.g. Jenga and card games; Babiloni et al., 2006; Astolfi et al., 2010; Liu et al., 2016), etc. The rich contextual information in these paradigms is expected to simulate social interactions in daily life that integrate the cognitive processes of both interactive decision-making and behavior coordination. While similar findings to the laboratory-based studies have been reported, these studies with naturalistic paradigms further highlight the functional importance of the mentalizing regions for social interaction in high ecological, real-world settings.
However, most of the abovementioned studies have investigated the neural mechanisms of either cooperation or competition. This was achieved by contrasting the inter-brain neural activity patterns in the cooperation/competition condition with those of a non-cooperation/non-competition condition (i.e. independent tasks; Astolfi et al., 2011; Hu et al., 2017; Miller et al., 2019) or by constructing a permutation condition by calculating inter-brain patterns from randomly shuffled multi-personal data (e.g. Pan et al., 2018; Nastase et al., 2019; Špiláková et al., 2020). Therefore, it could be argued that the between-condition differences were not uniquely devoted to the neural processes of cooperation or competition. Alternatively, the differences could reflect the neural mechanisms related to social interaction in general, as the degrees of social interaction were highly likely to be reduced in the contrasting conditions, leading to possible effects such as the decrease of a general attention level. As both cooperation and competition are similarly demanding in their degrees of social interaction, contrasting the two conditions is expected to identify neural coupling patterns that are more specific to human social functioning, such as self–other monitoring and interpretations (De Cremer and Stouten, 2003; Decety and Sommerville, 2003; Sommerville and Hammond, 2007). Nevertheless, it is challenging to simultaneously introduce comparable conditions to effectively represent cooperation and competition, especially for naturalistic scenarios with high ecological validity. For instance, while the definition of cooperation is clear and straightforward in the music performance context, it is not feasible to concurrently define a meaningful ‘competitive’ condition.
Despite the challenge, there were a few inter-brain studies with both cooperation and competition conditions simultaneously included. Using social games (e.g. Jenga) as well as customized computer games that either focus on interactive decision-making (the trust game and the builder game) or behavior coordination (key press), these studies have reported involvement of distinct brain regions, mainly the frontal and parietal cortex, when contrasting the inter-brain neural coupling patterns between cooperation and competition (Fallani et al., 2010; Cui et al., 2012; Liu et al., 2015, 2016, 2017; Špiláková et al., 2020). Most of these studies, however, have mainly focused on the hemodynamic signature using functional magnetic resonance imaging (fMRI) or functional near-infrared spectroscopy (fNIRS) techniques, with limited explorations on the electrophysiological signature. Nonetheless, electrophysiological techniques such as electroencephalograph (EEG) have been one of the most popular approaches in inter-brain studies, and the EEG could provide rich spectral information about how inter-brain neural coupling is achieved. Although not directly related to cooperation and competition, existing EEG-based inter-brain studies have generally reported increased neural coupling during effective social interaction conditions compared to non-interactive controls. Neural couplings in the theta and alpha bands are commonly reported, although delta, beta and gamma band couplings have also been observed (Babiloni and Astolfi, 2014).
The neural mechanisms of inter-brain neural coupling could be further promoted by EEG techniques by decomposing EEG signals into their amplitude and phase components, which have been demonstrated to play distinct roles in a variety of human cognitive functions (Klimesch, 2012; Engel et al., 2013; Fries, 2015; Zhang et al., 2017a). In single-brain studies, phase response has been related to top-down information exchange between global and local neuronal networks (Sauseng and Klimesch, 2008), whereas amplitude response has been suggested to reflect the excitability of local neural assemblies (David et al., 2005; Klimesch et al., 2007). In contrast, the possible functional significance of amplitude and phase in inter-brain studies remains to be elucidated. While some studies have reported inter-brain phase alignment during social interaction scenarios such as joint guitar playing (Lindenberger et al., 2009; Sänger et al., 2012), coordinated finger movement (Dumas et al., 2010; Yun et al., 2012), joint key press (Mu et al., 2016) and the prisoner’s dilemma (Hu et al., 2018), other studies have found inter-brain amplitude correlations to be related to real-time emotional experiences (Ding et al., 2021), duet piano performances (Zamm et al., 2018) and joint movements (Kawasaki et al., 2018). As inter-brain studies addressed amplitude and phase responses from a completely different perspective as compared to the single-brain studies, it is necessary to reinterpret the functional meanings of these two types of responses before reaching any conclusions.
The present study aimed to investigate the neural mechanisms of both cooperation and competition within the same ecological paradigm. The paradigm was designed based on the classic motion-sensing video game Wii Sports Tennis, which is one of the best-selling video games and is popular among players of varying ages (Collins, 2007; Hartenstein, 2007; Kolan, 2007). It was expected to fully immerse the participants in a state of cooperation or competition, involving not only their behavior coordination but also interactive decision-making. The required movement in the video game was much less intense than that in real tennis games, making it suitable for simultaneous EEG recording. The difference between cooperation and competition was inspected by analyzing both inter-brain amplitude correlation and phase alignment. The contrast of the EEG patterns from the cooperation and the competition conditions in the proposed ecological gaming paradigm is expected to provide more direct evidence for the neural mechanisms underlying cooperation and competition. Following previous studies with both cooperation and competition conditions (Fallani et al., 2010; Cui et al., 2012; Liu et al., 2015, 2016, 2017; Špiláková et al., 2020), between-condition inter-brain coupling difference was expected to be observed within the mentalizing regions but likely to be more localized, with the general social interaction factors controlled. The inter-brain couplings were hypothesized to occur mainly in the theta and alpha bands, as they were most frequently reported in previous EEG studies (Babiloni and Astolfi, 2014). No clear hypothesis regarding the involvement of amplitude and phase responses was formulated due to the limited evidence to date. Any discovery would promote our understanding of the neural mechanisms of cooperation and competition.
Materials and methods
Participants
Sixty healthy adults (30 dyads, all male, 21.8 ± 2.4 years [mean ± S.E.]) participated in the study. The sample size was determined according to previous EEG hyperscanning studies on competition and cooperation (e.g. Fallani et al., 2010; Mu et al., 2016; Balconi and Vanutelli, 2018; Hu et al., 2018) and well exceeds the median sample size in previous hyperscanning studies (33 participants, Reinero et al., 2020). The participants were college students from Tsinghua University with normal or corrected-to-normal vision. All dyads, except one, were not familiar with each other before the experiment. All participants provided written informed consent and were paid for their participation. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the local ethics committee at the Department of Psychology, Tsinghua University.
Paradigm and procedure
The classic motion-sensing video game Wii Sports Tennis (Nintendo, Japan) was used in the present study. The game was run on the Nintendo Wii console and displayed on a 22-inch monitor (DELL, USA), and the sound was played by the monitor’s loudspeaker. Two participants each participated in tennis doubles matches as companions or opponents with the other two players controlled by computer, constituting the cooperation and the competition conditions, respectively. The participants controlled their avatars to play in the virtual tennis field. Specifically, the racket swinging actions of the avatars were controlled by the participants using a motion-sensing controller (Wii Remote Controller). The direction, strength and timing of the swings could be well expressed by the avatars. The movement of the avatars, however, was automatically determined by the computer to follow the tennis ball as well as possible.
Two out of the four avatars were controlled by human participants and the other two were controlled by the computer. The two participants stood side-by-side in front of the monitor and used their right hands to operate the motion-sensing controllers. The distance from the participants to the monitor was approximately 2 m, with a 2 m spacing between the participants. In the cooperation condition, the two participants were assigned to the same team, playing against a team consisting of two computer-controlled avatars. In the competition condition, the two participants were assigned to different teams, playing against each other with their computer-controlled companions. The two conditions are expected to be comparable in terms of their degrees of social interaction, as the participants made their moves under the same rules in similarly competitive ‘tennis game’ atmospheres. In both conditions, the game was played in a two-on-two doubles mode, with the same scoring rules consistent with actual tennis matches. Specifically, a team needs to score four points to win a game. The first three points are displayed as 15 (one point), 30 (two points) and 40 (three points), and the fourth would result in the winning point and the end of that game. If the scores were 40–40, this would be known as deuce. When a game reaches deuce, the player must then win by two clear points. For each match in this study, the experimenter selects from sets of either three or five games. A match is won if a team can win at least two (three) games in a three-game (five-game) set. Unlike real tennis, the match ends after winning the requisite number of games. The experimental paradigm is illustrated in Figure 1.
Fig. 1.

Experimental paradigm. (A) Screenshot from the competitive condition: the screen was divided into left and right parts, each of which presented one participant’s main view. (B) A photo of two participants playing the game while their electroencephalographs were recorded. (C) Illustration of the cooperation and competition conditions.
The experiment was carried out in a normal office space. Upon arrival, two participants first completed four questionnaires to assess their basic dispositional traits related to cooperation and competition, including the Basic Empathy Scale (BES; Jolliffe and Farrington, 2006), the Brief Aggression Questionnaire (BAQ; Webster et al., 2014), the Cooperative/Competitive Strategy Scale (CCSS; Simmons et al., 1988) and the Achievement Motive Scale (AMS; Nygård and Gjesme, 1973). The BES is a 20-item self-reporting measure designed to assess empathy, i.e. a person’s ability to understand and share in the emotional experiences of others. Each item is scored on a five-point ordinal scale (ranging from 1 [strongly disagree] to 5 [strongly agree]). The internal consistency of BES in this study, as measured by Cronbach’s alpha coefficient score, was acceptable (α = 0.72). The 12-item BAQ is a short form of the most commonly used trait aggression measure, the 29-item Buss–Perry Aggression Questionnaire (Buss and Perry, 1992). Participants completed the BAQ by rating their agreement with each statement along a 1 (strongly disagree) to 5 (strongly agree) response scale. The internal consistency of BAQ in this study was acceptable (α = 0.72). The AMS was used to assess achievement motives. It consists of 30 items that indicate perceived affect in achievement situations. Subjects reply by means of a scale ranging from 1 (strongly disagree) to 4 (strongly agree). The internal consistency of AMS in this study was good (α = 0.86). The 19-item CCSS measures the motivation to use competitive or cooperative strategies to achieve success. Each item is followed by five response options ranging from always (5) to never (1). The internal consistencies for the Cooperative Strategy subscale and the Competitive Strategy subscale in this study were α = 0.59 and 0.73, respectively.
Next, the experimenter explained the paradigm and conducted a brief demonstration of the Wii Sports Tennis game. The participants were given a practice session of 20 min (10 min for each participant) to play in tennis doubles mode independently (i.e. each participant was teamed with a computer-controlled companion against two computer-controlled opponents). To reduce interfering muscle activity, participants were instructed to restrict their body movements as much as possible and avoid extreme facial expressions. Additionally, oral communication between the participants was not allowed. The formal experiment started after the practice session. The experiment consisted of two sessions, one for cooperation and one for competition. The order of the two sessions was counter-balanced across the participant dyads. Within each session, the dyad first played multiple three-out-of-five matches. Only when the experimenter considered the remainder of that session insufficient for a complete three-out-of-five match did they switch to a two-out-of-three match. Each session lasted for approximately 10–20 min, depending on the progress of these matches.
During the formal experiment, the participants’ EEGs were recorded by two wireless EEG amplifiers and a web camera was used to record the game screen at 30 frames per second. Before the start of the formal experiment, three consecutive pure-tone beep sounds at 1000 Hz (duration: 1000 ms; inter-beep interval: 1500 ms) were played by a computer. The beep sounds were recorded by the camera and input to the trigger device of the EEG amplifier, serving as the event marker to synchronize the EEG recordings of both participants, as well as the game screen recordings.
EEG recording and data preprocessing
EEG signals of the dyad were recorded simultaneously using two wireless EEG amplifiers (NeuSen.W32, Neuracle, China) at a sampling frequency of 250 Hz. EEG signals were recorded from 32 electrodes arranged according to the international 10–20 system (Fp1/2, Fz, F3/4, F7/8, FC1/2, FC5/6, Cz, C3/4, T3/4, CP1/2, CP5/6, Pz, P3/4, P7/8, PO3/4, PO7/8, Oz, O1/2), with the reference at CPz and a forehead ground at Fpz. Electrode impedances were kept below 10 kOhm for all electrodes throughout the experiment. Due to technical issues during data recording, four dyads’ EEG data were incomplete and rejected. Data from 26 dyads were used for data analysis.
The EEG data were first bandpass filtered to 0.1–40 Hz and then subjected to an artifact rejection procedure using independent component analysis (ICA). Independent components (ICs) with large weights over the frontal or temporal areas, together with a corresponding temporal course showing eye movement or muscle movement activities, were removed. The remaining ICs were then back-projected onto the scalp EEG channels, reconstructing the ICA-cleaned EEG signals. On average, 1–2 ICs were rejected per participant. The EEG data of the cooperation and competition sessions were then extracted. The beginning of a session was defined as the moment at which ‘Start’ first appeared on the screen, and the ending was defined as the moment at which ‘WiiSports’ was displayed when one of the two teams won the last match.
As playing the motion-sensing game was likely to induce more transient artifacts due to instantaneous movements, a further artifact rejection procedure was performed on the ICA-cleaned EEG signals. Specifically, the EEG data were segmented into 1 sec non-overlapping epochs, which were visually inspected for outliers that were different from the majority in mean, standard deviation, minimum or maximum. The time duration of 1 sec was decided to minimize the impact of transient artifacts on data rejection and maximize the proportion of data to be retained. This is especially important in the context of inter-brain analysis, which was only possible when clean data were available at the same moments from both participants. The above artifact rejection procedure was performed using the manual artifact rejection function provided in the FieldTrip Toolbox (Oostenveld et al., 2011). On average, 81.2% epochs (ranging from 78.8% to 83.7%) were retained per dyad. Figure 2 illustrates one typical EEG segment before and after the abovementioned artifact rejection procedure. A complete overview of the data quality is provided in Supplementary Figure S1.
Fig. 2.
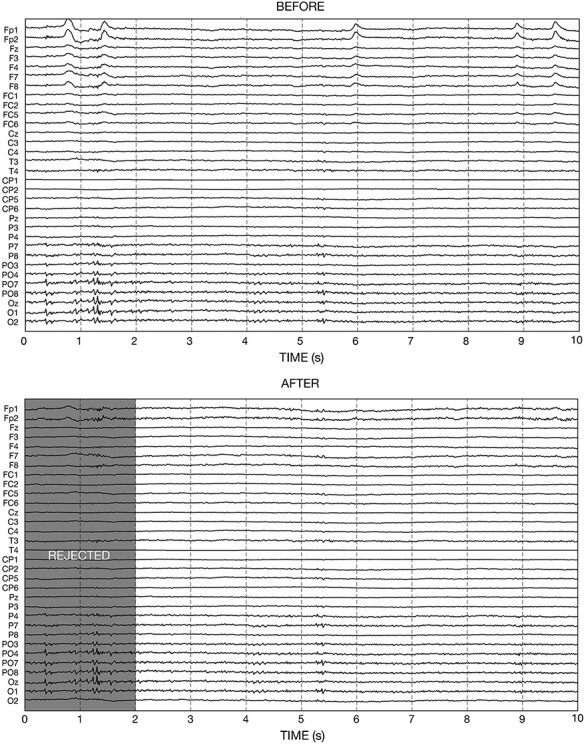
An illustration of one typical electroencephalograph segment before and after the artifact rejection procedure. Vertical dashed lines indicate onset and offset of the epochs. The eye-movement artifacts at the frontal channels and the muscle artifacts at the temporal and occipital channels were largely removed by ICA. The two epochs with still extensive artifacts after ICA were manually identified and rejected.
To explore the possible functional role of different oscillatory frequency bands, the ICA-cleaned data were then bandpass filtered into four frequency bands, namely delta (1–5 Hz), theta (5–8 Hz), alpha (8–14 Hz) and beta (14–30 Hz). The filtered data were then segmented into 1 sec epochs, and the above epoch-based rejection decisions were applied to these filtered epochs.
Behavioral data analysis
The recorded game video was inspected by the experimenter, and the game events were manually coded. The average point length (a point starts at one serve and ends when a point is scored) in different social contexts was calculated for all dyads to provide evidence for the occurrence of valid cooperative/competitive interactions during the experiment.
EEG data analysis
Inter-brain coupling was assessed by computing the inter-brain amplitude correlation and inter-brain phase-locking (phase alignment) between the data epochs filtered at the four frequency bands of all dyads. The filtered signals at these bands could be considered as narrow-band signals, for the well-established similarity of brain activities within each band. Accordingly, a number of studies have applied Hilbert transform on EEG signals at these classical bands and more insights have been gained on the possible role of the oscillatory activities for a variety of cognitive functions (Ng et al., 2012; Pérez et al., 2017; Assaneo and Poeppel, 2018; Goldstein et al., 2018; Ding et al., 2021).
Specifically, these data epochs were subjected to a Hilbert transform
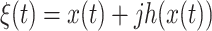 |
(1) |
where t is time, 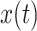 represents EEG data,
represents EEG data, 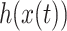 represents its Hilbert transform and j is the imaginary unit. Thus, the instantaneous amplitude
represents its Hilbert transform and j is the imaginary unit. Thus, the instantaneous amplitude 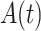 and the instantaneous phase
and the instantaneous phase 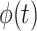 are
are
 |
(2) |
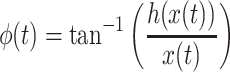 |
(3) |
The above formulae were applied to the EEG data of all channels from all participants, at all the four frequency bands, for all epochs.
Inter-brain coupling was then computed as follows. The inter-brain amplitude correlation (termed as AmpCorr) was obtained by calculating the Pearson’s correlation coefficient between the EEG amplitudes of two channels (j, k) within an epoch:
 |
(4) |
where n is the epoch index. AmpCorr varies between −1 and 1. High AmpCorr values indicate synchronous amplitude fluctuations at the specific frequency band between two channels in a specific epoch. AmpCorr can detect synchrony between channels independent of phase coherence, thus presenting a promising metric for assessing inter-brain correspondence of amplitude dynamics between individuals in social interactions (Zamm et al., 2018).
Inter-brain phase-locking was assessed using the phase-locking value (PLV), which was calculated as the consistency of phases between two channels (j, k) over time:
 |
(5) |
where T is the number of time points within the epoch. PLV varies between 0 and 1; 0 indicates randomly dispersed phases and 1 indicates fully phase-locked oscillations between two channels in a specific epoch. Note that PLV is an amplitude-independent measurement as it assigns a constant unit amplitude for each epoch.
Inter-brain amplitude correlation and inter-brain phase-locking were estimated by examining PLV and AmpCorr for all the matched-channel pairs (i.e. the same channel from the two EEG datasets) from all dyads, at all the four frequency bands, and for all epochs. Fisher’s Z-transformation was performed on the correlation coefficients to generate a normal distribution, after which the inter-brain coupling was averaged across all the epochs within each context.
To examine whether these neural measures could differentiate cooperation from competition, paired t-tests were performed on the within-dyad averaged inter-brain amplitude correlation and phase-locking values in the two conditions across all dyads, separately for all the channels at the four frequency bands. To account for multiple comparisons, a non-parametric cluster-based permutation test was applied (Maris and Oostenveld, 2007; Sassenhagen and Draschkow, 2019). For each frequency band, the statistics results from the t-tests were spatially clustered and compared with a permutation distribution to identify significant channel clusters. Specifically, clusters were first formed if two or more neighboring channels showed significant differences (P < 0.05) in AmpCorr or PLV between the two conditions; cluster-level statistics (cluster-t) were then defined as the sum of the t-values of all channels within a given cluster. Next, a permutated distribution of the cluster-t values was generated by temporally shuffling the data epochs. Specifically, the data epochs within each condition per dyad were randomly matched by shuffling the temporal order of epochs for one participant while keeping that of the other. Inter-brain couplings were computed based on the randomly matched data (separately for the two types of EEG components at the four frequency bands). The shuffling was repeated 5000 times and the same statistical clustering procedure as described above was applied on these shuffled data to obtain a permutation distribution of the cluster-t values. A cluster would be considered as significant only if its cluster-level statistics were significantly larger (positive t-statistics) or smaller (negative t-statistics) than the cluster-level statistics calculated from the permutated data at the 0.05 level. The cluster-based permutation test was performed for the dyad EEG data separately for the four frequency bands and the two types of EEG components (AmpCorr and PLV).
In addition, an intra-brain analysis was performed by calculating the relative spectral power of the four bands (compared to the summed spectral power of all four bands) and comparing the spectral power values in a way similar to that described above. Note that inter-brain phase alignment could not be computed at the intra-brain level for the present dataset.
Since only the inter-brain amplitude correlation showed a significant difference between the two conditions (see Results), further statistical tests were applied to explore whether the inter-brain amplitude correlation in each of the two conditions could significantly differ from a permutated baseline condition. To this end, the inter-brain amplitude correlations in cooperation or competition conditions were tested against zero (one-sample t-test), and the formed clusters were subject to a similar cluster-based permutation procedure as introduced above (i.e. permutated data generated by the temporally shuffled dyad EEG epochs).
Subsequently, to explore the possible behavioral relevance of the inter-brain neural coupling, Pearson correlation coefficients were calculated between the significant inter-brain signatures and the dyad-level dispositional trait. Significant inter-brain signatures were obtained as the mean values of the inter-brain coupling results from all the channels within a significant cluster. The dyads’ mean questionnaire scores were taken to represent the dyad-level dispositional trait (Liu et al., 2017).
In the aforementioned data analysis, the EEG signals from the two interacting players were analyzed in a ‘synchronous’ way by temporally aligning the two EEG signals with a zero lag, as done in many previous studies on cooperation and competition (Lindenberger et al., 2009; Sänger et al., 2012; Müller et al., 2013; Sinha et al., 2016; Balconi and Vanutelli, 2017, 2018; Liu et al., 2017). This ‘synchronous’ strategy was selected as the two players were equally engaged in the game by design, hereby with no clear leader–follower relationships. Nevertheless, the inter-brain phase-locking could capture non-zero-lag relationships if there were consistent phase delays between the two signals. Although the inter-brain amplitude correlation was conducted with zero lag, it was expected to be less sensitive to short temporal delays, as the amplitude response of the oscillatory activities was a slow-varying signal that fluctuated at the pace of the oscillatory cycles. All the EEG processing procedures, as well as the statistical analyses, were conducted using the FieldTrip toolbox (Oostenveld et al., 2011).
Results
Behavioral results
The average point lengths for cooperation and competition were 12.6 s and 10.7 s, respectively, which were comparable to the average point length (8 s) in real-world tennis (Reid and Duffield, 2014). Since less energy is used when playing the Wii version than when actually playing tennis (Graves et al., 2007), the slightly longer point lengths in the study were consistent with the less intense characteristic of the motion-sensing sports game. Although paired t-tests revealed a significant difference in average point length for cooperation and competition contexts (t(25) = 3.15, P < 0.01, Cohen’s d = 0.62), the point lengths were sufficiently long, providing preliminarily support for a good mastery of the gaming tasks by the participants.
Different inter-brain neural signatures between cooperation and competition
The neural signatures for differentiating cooperation and competition conditions are summarized in Figure 3A. The non-parametric cluster-based permutation tests revealed a significant difference between the two conditions. The observed difference was reflected by the inter-brain amplitude correlations over a right-lateralized central-parietal-occipital cluster at the theta band (cluster-t = 29.75, P < 0.001), an occipital cluster at the alpha band (cluster-t = 14.54, P < 0.001) and an occipital cluster at the beta band (cluster-t = 16.75, P < 0.001). All these clusters showed positive cluster-t values, indicating larger inter-brain coupling during the cooperation condition compared to that during the competition condition. The distributions of the cluster-t values from the permutated computation are shown in Figure 3B; the three clusters had higher cluster-t values (red lines) than those from the 5000-times permutation. The inter-brain amplitude correlation values in the two conditions from representative channels in the three clusters are shown in Figure 4B. No statistically significant clusters were obtained for inter-brain phase-locking and intra-brain spectral power.
Fig. 3.

(A) T-maps from comparison of the cooperation and competition conditions for different neural measures. Color represents t-value from the comparison of cooperation and competition conditions (a positive t-value indicates that the neural measure is larger during cooperation than cooperation, and a negative t-value indicates the opposite). Black dots denote channel clusters with significantly different outcomes between the two contexts (all the dots in each topoplot belong to one single cluster). (B) Permutation distributions for the three significant clusters of inter-brain amplitude correlation (AmpCorr) in (A). Red lines indicate the positions of true cluster t-values.
Fig. 4.

Inter-brain amplitude correlation (AmpCorr) results. (A) T-maps of AmpCorr during cooperation, competition and their contrasts. Color in the first two columns represents t-values from the comparison of Coop/Comp AmpCorr and zero. Black dots denote channel clusters with significant AmpCorr in certain context). Color in the third column represents t-values from the comparison of AmpCorr during the cooperation and competition conditions. Black dots denote channel clusters with significantly different AmpCorr between the two contexts (all the dots in each topoplot belong to one single cluster). (B) Individual differences of the AmpCorr values between the two contexts at three representative channels. Each linked pair of points represents data from a single participant. (C) Permutation distributions for the four clusters with significant Coop/Comp AmpCorr in (A). Red lines indicate the positions of the true cluster-t.
Inter-brain amplitude correlations during cooperation and competition
Figure 4A illustrates the significant clusters with inter-brain amplitude correlations in the cooperation and the competition conditions separately. When the dyads cooperated, significantly positive inter-brain amplitude correlations were observed over a right-lateralized fronto-central cluster at the delta band (cluster-t = 31.23, P < 0.05) and a widespread cluster at the theta band (cluster-t = 108.80, P < 0.001). When the dyads competed with each other, significantly negative inter-brain amplitude correlations were observed over the occipital area at both the alpha and the beta bands (cluster-t = −6.44, P = 0.001 and cluster-t = −15.51, P < 0.001, respectively). Figure 4C further demonstrates the permutated distribution for the four clusters.
Correlation between inter-brain coupling and dyad-level dispositional traits
To explore possible behavioral relevance of the inter-brain neural coupling, Pearson correlation analyses were conducted between the significant inter-brain amplitude correlation and the dyad-level dispositional trait. Specifically, the mean AmpCorr (for significant clusters in each condition) or AmpCorr difference between conditions (for clusters with significant conditional difference) at the corresponding frequency band within the seven significant clusters from the above tests (see Figure 4A) were obtained, resulting in seven inter-brain signatures.
The correlation results for all inter-brain signatures and dispositional traits are summarized in Table 1. Only one significant association was found: the participants’ alpha-band AmpCorr difference and empathy level were negatively associated (r = −0.43, uncorrected P < 0.05). Notably, such negative correlation did not survive correction for multiple comparisons. No other association was statistically significant.
Table 1.
Coefficients and P-values (in parentheses) for correlations between all AmpCorr indices and traits
| Empathy | Aggression | Competitive strategy | Cooperative strategy | Achievement motive | |
|---|---|---|---|---|---|
| Coop_Delta | 0.04 (0.84) | 0.05 (0.80) | −0.32 (0.11) | −0.06 (0.76) | −0.01 (0.97) |
| Coop_Theta | 0.20 (0.33) | −0.14 (0.51) | −0.13 (0.52) | 0.13 (0.53) | −0.16 (0.44) |
| Diff_Theta | −0.12 (0.57) | 0.02 (0.92) | −0.11 (0.58) | 0.04 (0.83) | −0.23 (0.26) |
| Comp_Alpha | 0.16 (0.44) | 0.32 (0.12) | 0.04 (0.85) | −0.17 (0.40) | −0.24 (0.26) |
| Diff_Alpha | −0.43 (0.03) | −0.05 (0.79) | 0.07 (0.72) | 0.19 (0.34) | 0.26 (0.21) |
| Comp_Beta | 0.10 (0.63) | 0.05 (0.80) | 0.24 (0.23) | −0.05 (0.82) | −0.10 (0.63) |
| Diff_Beta | −0.08 (0.69) | −0.24 (0.24) | −0.26 (0.19) | 0.28 (0.17) | 0.25 (0.22) |
‘Coop_delta’ represents the mean inter-brain amplitude correlation during cooperation at the delta frequency band from the delta-band cluster with significant amplitude correlation during cooperation and so forth.
Discussion
The present study explored the neural mechanisms of both cooperation and competition within the same ecological paradigm based on a motion-sensing tennis game, using the EEG-based hyperscanning technique. Significant conditional differences in inter-brain neural coupling were found at the theta, alpha and beta bands, in which the cooperation condition manifested increased inter-brain amplitude correlation over the competition condition. Further inspection revealed distinct inter-brain amplitude correlation patterns for cooperation and competition. In the cooperative context, significant positive inter-brain amplitude correlation at the delta and theta bands was found in extensive brain regions; competition, however, was associated with negative occipital inter-brain amplitude correlation at the alpha and beta bands.
Most importantly, cooperation and competition were found to be associated with inter-brain amplitude correlation rather than phase alignment. Our observation is consistent with some of the previous studies supporting the similarity of amplitude fluctuations for effective social interaction (Kawasaki et al., 2018; Zamm et al., 2018). The disagreement with other studies (Dumas et al., 2010; Sänger et al., 2012; Yun et al., 2012; Mu et al., 2016) could be attributed to the difference in the experimental paradigms; the tasks in these studies, such as joint guitar playing and joint key press, might require relatively precise time synchronization between the participants. Although behavior coordination was needed for the present paradigm, the two participants always made different motor actions (even in cooperation), and it was more likely to have higher level of interaction beyond simple motor action similarity. Nevertheless, the ecological setting in the present study resembles a large body of social interactions in daily life, in which precise time synchronization is not always necessary. In these cases, it is plausible to expect inter-brain neural coupling at a coarser time scale as expressed by amplitude correlation, which is not as precisely timed as phase-locking. Moreover, this observation could contribute to extending our understanding of the functional role of the amplitude fluctuation. Specifically, whereas single-brain studies have mostly considered amplitude for bottom-up information processing (Kashiwase et al., 2012; Zhang et al., 2017a), our results from an inter-brain perspective suggest a possible different functional role of amplitude responses for representing social interaction related top-down information: the inter-brain coupling of the amplitude responses between the two players was shown to carry information for differentiating the social interaction modes of cooperation and competition during the game playing.
The difference between cooperation and competition was reflected by inter-brain amplitude correlations at the theta band over the right central and parietal regions. The spatial distribution of the theta-band cluster is consistent with previous fMRI-based and fNIRS-based hyperscanning studies on social interactions (Bilek et al., 2015; Liu et al., 2017), suggesting the possible involvement of a series of sociocognitive processes, such as attention orientation, the sense of agency, self-other discrimination and perspective-taking (Blakemore and Frith, 2003; Decety and Lamm, 2007). Both the spatial distribution and the theta-band finding are also in accordance with a previous EEG-based hyperscanning study (Yun et al., 2012) using a coordinated finger movement paradigm. The theta-band rhythm was suggested to reflect some implicit processes essential for social interactions, as in classical single-brain studies it has been related to prediction (Huang et al., 2015), outcome monitoring (Cavanagh and Frank, 2014) and behavioral adaptation (Cavanagh et al., 2010; Billeke et al., 2013). The inter-brain results extend previous understanding of theta-band rhythm, highlighting the joint effort of the dyad to coordinate their behaviors as a team during cooperation. In addition, the decomposition into amplitude and phase responses in the present study further suggest the importance of amplitude response over these regions at theta band for the regulation of a cooperative social context.
The between-condition difference of inter-brain amplitude correlations at the alpha and beta bands, however, could reflect the similarity in the dyad’s visual information processing related to the Wii tennis game. Compared to competition, the cooperating dyad could have a more similar attention focus and, consequently, interpretation of the visual information (Yu and Zhou, 2006; van Dijk et al., 2008; Kang et al., 2010; Leng and Zhou, 2010; Samaha and Postle, 2015), leading to the enhanced alpha- and beta-band occipital inter-brain amplitude correlation. Interestingly, the separate-condition analysis revealed a significant negative inter-brain amplitude correlation during the competition condition, suggesting that the participants might adopt individualistic strategies, mainly focus on themselves, and reduce interpersonal tuning during competition (Balconi and Vanutelli, 2018). In summary, the different findings of the inter-brain patterns at different bands suggest distinct functional roles of different oscillatory activities from an inter-brain perspective.
We did not find significant intra-brain differences between conditions in the present study, which is consistent with previous studies (Cui et al., 2012; Simony et al., 2016; Balconi et al., 2017b), implying the significance of adopting an inter-brain perspective in the study of cooperation and competition. Nevertheless, as a few other studies have reported intra-brain results related to social interactions (e.g. Tognoli et al., 2007; Balconi et al., 2017a), the current finding might be limited by our analyses. We only focused on the overall intra-brain neural activities, and the various events that happened during the whole interacting process were not fully coded and analyzed. Intra-brain differences between conditions might be revealed if detailed events are taken into consideration (Ding et al., 2018; Pan et al., 2020). Capturing the rich information embedded in such ecological paradigms remains a challenge, which we hope to explore in future studies. Alternatively, the null intra-brain results might be explained by the employment of the two vs two mode of the Wii Sports Tennis game. While the two vs two mode was designed for the introduction of the comparable cooperation and competition conditions, the involvement of the two computer-controlled avatars could lead to additional cooperative and competitive factors for the human players: the two participants were competing against the two computer-controlled avatars in the cooperation condition, and both of them were cooperating with one computer-controlled avatar in the competition condition. Similar neural activation in some of the same areas activated by human partners could be elicited even when the participants interact with such virtual partners (Sebanz et al., 2006; Dumas et al., 2020; Moreau et al., 2020). Hereby, the participants might have comparable senses of cooperation and competition in both conditions (Era et al., 2020), resulting in null results for the between-condition comparison in the current intra-brain analyses.
It should also be noted that the inter-brain coupling analyses in the present study were conducted with matched-channel pairs only. This choice was made mainly based on the balanced nature of the experiment design, in which the roles of the two players in the game were interchangeable in both conditions. The inter-brain coupling between non-corresponding channels in previous studies has often been interpreted as arising from differential social roles in the interactions such as being an imitator or a follower (e.g. Kuhlen et al., 2015). Admittedly, the present study could also benefit from a cross-channel analysis for a more thorough investigation of the inter-brain coupling patterns. The application of a cross-channel analysis for the present data will be considered in future studies, with a clear hypothesis for the possibly unbalanced relationship between the interacting partners as well as a properly designed methodological framework (e.g. for treating the unbalanced coupling results).
In addition, it could be argued that the inter-brain coupling results could be the by-product of interaction in the game. Indeed, the inter-brain coupling patterns in each single condition (i.e. the first two columns in Figure 4A) could be affected by the actual interaction between the two participants, e.g. reflecting the shared sensory information. These single-condition findings were of limited scientific implications and need to be interpreted with caution. Nevertheless, the two conditions were expected to introduce comparable senses of cooperation and competition, and the most notable between-condition difference was the assignment of the human participants. Besides, although a naturalistic paradigm was adopted in this study, some of our results are similar to those reported by traditional laboratory studies, e.g. the theta-band inter-brain coupling in central and parietal regions (Yun et al., 2012) during cooperation. Such similarities might suggest that the current findings reflect the difference between cooperation and competition, rather than merely the by-product of interaction.
Finally, there are some other limitations to be noted. First, the study only included male participants. Considering the possibly different inter-brain patterns of inter-brain coupling among mixed-sex, male and female dyadic interactions (Cheng et al., 2015; Zhang et al., 2017b), future research with female participations is necessary to extend the validity of the present findings. Second, only one game was adopted in the present paradigm, introducing possible confusing effects specific to this game or this type of sport game. More games could be utilized to better explore the diverse forms of cooperation and competition toward more solid conclusions on the inter-brain neural signatures of human social interaction.
Supplementary Material
Contributor Information
Huashuo Liu, Department of Psychology, School of Social Sciences, Tsinghua University, Beijing 100084, China.
Chenying Zhao, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing 100084, China.
Fei Wang, Department of Psychology, School of Social Sciences, Tsinghua University, Beijing 100084, China; Tsinghua Laboratory of Brain and Intelligence, Tsinghua University, Beijing 100084, China.
Dan Zhang, Department of Psychology, School of Social Sciences, Tsinghua University, Beijing 100084, China; Tsinghua Laboratory of Brain and Intelligence, Tsinghua University, Beijing 100084, China.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 61977041 and U1736220); the National Science Foundation of China (NSFC) and the German Research Foundation (DFG) in project Crossmodal Learning (grant number: NSFC 61621136008/DFG TRR-169/C1, B1); the National Key Research and Development Plan (grant number: 2016YFB1001200); the National Social Science Foundation of China (grant number: 17ZDA323); and the Tsinghua University Initiative Scientific Research Program (grant number: 20197010006).
Conflict of interest
The authors declare no conflict of interest.
Supplementary data
Supplementary data are available at SCAN online.
References
- Assaneo, M.F., Poeppel, D. (2018). The coupling between auditory and motor cortices is rate-restricted: evidence for an intrinsic speech-motor rhythm. Science Advances, 4(2), eaao3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi, L., Cincotti, F., Mattia, D., et al. (2010). Simultaneous estimation of cortical activity during social interactions by using EEG hyperscannings. Engineering in Medicine and Biology Society (EMBC), In: 2010 Annual International Conference of the IEEE, Buenos Aires, Argentina, IEEE, 2814–7. [DOI] [PubMed] [Google Scholar]
- Astolfi, L., Toppi, J., Borghini, G., et al. (2011). Study of the functional hyperconnectivity between couples of pilots during flight simulation: An EEG hyperscanning study. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, IEEE, 2338–41. [DOI] [PubMed] [Google Scholar]
- Babiloni, F., Cincotti, F., Mattia, D., et al. (2006). Hypermethods for EEG hyperscanning. In: 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, IEEE, 3666–9. [DOI] [PubMed] [Google Scholar]
- Babiloni, F., Astolfi, L. (2014). Social neuroscience and hyperscanning techniques: past, present and future. Neuroscience and Biobehavioral Reviews, 44, 76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi, M., Crivelli, D., Vanutelli, M.E. (2017a). Why to cooperate is better than to compete: brain and personality components. BMC Neuroscience, 18(1), 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi, M., Pezard, L., Nandrino, J.-L., Vanutelli, M.E. (2017b). Two is better than one: the effects of strategic cooperation on intra- and inter-brain connectivity by fNIRS. PLoS One, 12(11), e0187652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi, M., Vanutelli, M.E. (2017). Brains in competition: improved cognitive performance and inter-brain coupling by hyperscanning paradigm with functional near-infrared spectroscopy. Frontiers in Behavioral Neuroscience, 11, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi, M., Vanutelli, M.E. (2018). Functional EEG connectivity during competition. BMC Neuroscience, 19(1), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura, A. (2000). Exercise of human agency through collective efficacy. Current Directions in Psychological Science, 9(3), 75–8. [Google Scholar]
- Bilek, E., Ruf, M., Schäfer, A., et al. (2015). Information flow between interacting human brains: identification, validation, and relationship to social expertise. Proceedings of the National Academy of Sciences of the United States of America, 112(16), 5207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeke, P., Zamorano, F., Cosmelli, D., Aboitiz, F. (2013). Oscillatory brain activity correlates with risk perception and predicts social decisions. Cerebral Cortex, 23(12), 2872–83. [DOI] [PubMed] [Google Scholar]
- Blakemore, S.-J., Frith, C. (2003). Self-awareness and action. Current Opinion in Neurobiology, 13(2), 219–24. [DOI] [PubMed] [Google Scholar]
- Buss, A.H., Perry, M. (1992). The aggression questionnaire. Journal of Personality and Social Psychology, 63(3), 452–9. [DOI] [PubMed] [Google Scholar]
- Cavanagh, J.F., Frank, M.J., Klein, T.J., Allen, J.J.B. (2010). Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. NeuroImage, 49(4), 3198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh, J.F., Frank, M.J. (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X., Li, X., Hu, Y. (2015). Synchronous brain activity during cooperative exchange depends on gender of partner: a fNIRS-based hyperscanning study. Human Brain Mapping, 36(6), 2039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, J. (2007). Seniors becoming old hands at Wii. Available: https://www.marketplace.org/2007/12/11/seniors-becoming-old-hands-wii/. [June 17, 2020, Date accessed].
- Cui, X., Bryant, D.M., Reiss, A.L. (2012). NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage, 59(3), 2430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, O., Harrison, L., Friston, K.J. (2005). Modelling event-related responses in the brain. NeuroImage, 25(3), 756–70. [DOI] [PubMed] [Google Scholar]
- De Cremer, D., Stouten, J. (2003). When do people find cooperation most justified? The effect of trust and self–other merging in social dilemmas. Social Justice Research, 16(1), 41–52. [Google Scholar]
- Decety, J., Jackson, P.L., Sommerville, J.A., Chaminade, T., Meltzoff, A.N. (2004). The neural bases of cooperation and competition: an fMRI investigation. NeuroImage, 23(2), 744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety, J., Lamm, C. (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The Neuroscientist, 13(6), 580–93. [DOI] [PubMed] [Google Scholar]
- Decety, J., Sommerville, J.A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences, 7(12), 527–33. [DOI] [PubMed] [Google Scholar]
- Deutsch, M. (1962). Cooperation and trust: some theoretical notes. In: Jones, M.R., editor. Nebraska Symposium on Motivation. Lincoln: University of Nebraska Press, 275–319. [Google Scholar]
- Ding, Y., Hu, X., Li, J., et al. (2018). What makes a champion: the behavioral and neural correlates of expertise in multiplayer online battle arena games. International Journal of Human–Computer Interaction, 34(8), 682–94. [Google Scholar]
- Ding, Y., Hu, X., Xia, Z., Liu, Y. J., Zhang, D. (2021). Inter-Brain EEG Feature Extraction and Analysis for Continuous Implicit Emotion Tagging During Video Watching. IEEE Transactions on Affective Computing, 12 (1), 92–102 . [Google Scholar]
- Dumas, G., Nadel, J., Soussignan, R., Martinerie, J., Garnero, L. (2010). Inter-brain synchronization during social interaction. PLoS One, 5(8), e12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas, G., Moreau, Q., Tognoli, E., Kelso, J.A.S. (2020). The human dynamic clamp reveals the fronto-parietal network linking real-time social coordination and cognition. Cerebral Cortex, 30(5), 3271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, A.K., Gerloff, C., Hilgetag, C.C., Nolte, G. (2013). Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron, 80(4), 867–86. [DOI] [PubMed] [Google Scholar]
- Era, V., Aglioti, S.M., Mancusi, C., Candidi, M. (2020). Visuo-motor interference with a virtual partner is equally present in cooperative and competitive interactions. Psychological Research, 84(3), 810–22. [DOI] [PubMed] [Google Scholar]
- Fallani, F.D.V., Nicosia, V., Sinatra, R., et al. (2010). Defecting or not defecting: how to “read” human behavior during cooperative games by EEG measurements. PLoS One, 5(12), e14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries, P. (2015). Rhythms for cognition: communication through coherence. Neuron, 88(1), 220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, P., Weissman-Fogel, I., Dumas, G., Shamay-Tsoory, S.G. (2018). Brain-to-brain coupling during handholding is associated with pain reduction. Proceedings of the National Academy of Sciences of the United States of America, 115(11), E2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves, L., Stratton, G., Ridgers, N.D., Cable, N.T. (2007). Comparison of energy expenditure in adolescents when playing new generation and sedentary computer games: cross sectional study. BMJ, 335(7633), 1282–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari, R., Kujala, M.V. (2009). Brain basis of human social interaction: from concepts to brain imaging. Physiological Reviews, 89(2), 453–79. [DOI] [PubMed] [Google Scholar]
- Hartenstein, M. (2007). Nintendo adds an ‘I’ to Wiimbledon. ABC News. Available: https://abcnews.go.com/amp/WN/story?id=3313922&page=1 [June 17, 2020, Date accessed].
- Hu, Y., Hu, Y., Li, X., Pan, Y., Cheng, X. (2017). Brain-to-brain synchronization across two persons predicts mutual prosociality. Social Cognitive and Affective Neuroscience, 12(12), 1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y., Pan, Y., Shi, X., et al. (2018). Inter-brain synchrony and cooperation context in interactive decision making. Biological Psychology, 133, 54–62. [DOI] [PubMed] [Google Scholar]
- Huang, Y., Chen, L., Luo, H. (2015). Behavioral oscillation in priming: competing perceptual predictions conveyed in alternating theta-band rhythms. The Journal of Neuroscience, 35(6), 2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, D.W., Johnson, R.T., Holubec, E.J. (2013). Cooperation in the Classroom. Edina, MN: Interaction Book Company. [Google Scholar]
- Johnson, D.W., Johnson, R.T. (1989). Cooperation and Competition: Theory and Research. Edina, MN: Interaction Book Company. [Google Scholar]
- Jolliffe, D., Farrington, D.P. (2006). Development and validation of the Basic Empathy Scale. Journal of Adolescence, 29(4), 589–611. [DOI] [PubMed] [Google Scholar]
- Kang, S.K., Hirsh, J.B., Chasteen, A.L. (2010). Your mistakes are mine: self-other overlap predicts neural response to observed errors. Journal of Experimental Social Psychology, 46(1), 229–32. [Google Scholar]
- Kashiwase, Y., Matsumiya, K., Kuriki, I., Shioiri, S. (2012). Time courses of attentional modulation in neural amplification and synchronization measured with steady-state visual-evoked potentials. Journal of Cognitive Neuroscience, 24(8), 1779–93. [DOI] [PubMed] [Google Scholar]
- Kawasaki, M., Kitajo, K., Yamaguchi, Y. (2018). Sensory-motor synchronization in the brain corresponds to behavioral synchronization between individuals. Neuropsychologia, 119, 59–67. [DOI] [PubMed] [Google Scholar]
- Klimesch, W., Sauseng, P., Hanslmayr, S. (2007). EEG alpha oscillations: the inhibition–timing hypothesis. Brain Research Reviews, 53(1), 63–88. [DOI] [PubMed] [Google Scholar]
- Klimesch, W. (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolan, P. (2007). Wii Tennis Tourney in Melbourne. Available: https://www.ign.com/articles/2007/01/12/wii-tennis-tourney-in-melbourne [June 17, 2020, Date accessed].
- Kuhlen, A.K., Allefeld, C., Anders, S., Haynes, J.-D. (2015). Towards a multi-brain perspective on communication in dialogue. In: Willems, R.M., editor. Cognitive Neuroscience of Natural Language Use. Cambridge: Cambridge University Press, 182–200. [Google Scholar]
- Leng, Y., Zhou, X. (2010). Modulation of the brain activity in outcome evaluation by interpersonal relationship: an ERP study. Neuropsychologia, 48(2), 448–55. [DOI] [PubMed] [Google Scholar]
- Lindenberger, U., Li, S.-C., Gruber, W., Müller, V. (2009). Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neuroscience, 10(1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, N., Mok, C., Witt, E.E., et al. (2016). NIRS-based hyperscanning reveals inter-brain neural synchronization during cooperative Jenga game with face-to-face communication. Frontiers in Human Neuroscience, 10, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T., Saito, H., Oi, M. (2015). Role of the right inferior frontal gyrus in turn-based cooperation and competition: a near-infrared spectroscopy study. Brain and Cognition, 99, 17–23. [DOI] [PubMed] [Google Scholar]
- Liu, T., Saito, G., Lin, C., Saito, H. (2017). Inter-brain network underlying turn-based cooperation and competition: a hyperscanning study using near-infrared spectroscopy. Scientific Reports, 7(1), 8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T., Pelowski, M. (2014). Clarifying the interaction types in two-person neuroscience research. Frontiers in Human Neuroscience, 8, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris, E., Oostenveld, R. (2007). Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods, 164(1), 177–90. [DOI] [PubMed] [Google Scholar]
- Miller, J.G., Vrtička, P., Cui, X., et al. (2019). Inter-brain synchrony in mother-child dyads during cooperation: an fNIRS hyperscanning study. Neuropsychologia, 124, 117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague, P.R., Berns, G.S., Cohen, J.D., et al. (2002). Hyperscanning: simultaneous fMRI during linked social interactions. NeuroImage, 16(4), 1159–64. [DOI] [PubMed] [Google Scholar]
- Moreau, Q., Candidi, M., Era, V., Tieri, G., Aglioti, S.M. (2020). Midline frontal and occipito-temporal activity during error monitoring in dyadic motor interactions. Cortex, 127, 131–49. [DOI] [PubMed] [Google Scholar]
- Mu, Y., Guo, C., Han, S. (2016). Oxytocin enhances inter-brain synchrony during social coordination in male adults. Social Cognitive and Affective Neuroscience, 11(12), 1882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, Y., Cerritos, C., Khan, F. (2018). Neural mechanisms underlying interpersonal coordination: a review of hyperscanning research. Social and Personality Psychology Compass, 12(11), e12421. [Google Scholar]
- Müller, V., Sänger, J., Lindenberger, U. (2013). Intra- and inter-brain synchronization during musical improvisation on the guitar. PLoS One, 8(9), e73852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastase, S.A., Gazzola, V., Hasson, U., Keysers, C. (2019). Measuring shared responses across subjects using intersubject correlation. Social Cognitive and Affective Neuroscience, 14(6), 667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, B.S.W., Logothetis, N.K., Kayser, C. (2012). EEG phase patterns reflect the selectivity of neural firing. Cerebral Cortex, 23(2), 389–98. [DOI] [PubMed] [Google Scholar]
- Nygård, R., Gjesme, T. (1973). Assessment of achievement motives: comments and suggestions. Scandinavian Journal of Educational Research, 17(1), 39–46. [Google Scholar]
- Oostenveld, R., Fries, P., Maris, E., Schoffelen, J.-M. (2011). FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Computational intelligence and neuroscience, 2011, 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y., Novembre, G., Song, B., Li, X., Hu, Y. (2018). Interpersonal synchronization of inferior frontal cortices tracks social interactive learning of a song. NeuroImage, 183, 280–90. [DOI] [PubMed] [Google Scholar]
- Pan, Y., Dikker, S., Goldstein, P., et al. (2020). Instructor-learner brain coupling discriminates between instructional approaches and predicts learning. NeuroImage, 211, 116657. [DOI] [PubMed] [Google Scholar]
- Pérez, A., Carreiras, M., Duñabeitia, J.A. (2017). Brain-to-brain entrainment: EEG interbrain synchronization while speaking and listening. Scientific Reports, 7(1), 4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget, J. (1950). The Psychology of Intelligence. New York: Harcourt, Brace & Company. [Google Scholar]
- Redcay, E., Schilbach, L. (2019). Using second-person neuroscience to elucidate the mechanisms of social interaction. Nature Reviews Neuroscience, 20(8), 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, M., Duffield, R. (2014). The development of fatigue during match-play tennis. British Journal of Sports Medicine, 48(Suppl 1), i7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinero, D.A., Dikker, S., Van Bavel, J. J. (2020). Inter-brain synchrony in teams predicts collective performance. Social cognitive and affective neuroscience, 16(1-2), 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, D., Tanabe, H., Izuma, K., et al. (2010). “Stay tuned”: inter-individual neural synchronization during mutual gaze and joint attention. Frontiers in Integrative Neuroscience, 4, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha, J., Postle, B.R. (2015). The speed of alpha-band oscillations predicts the temporal resolution of visual perception. Current Biology, 25(22), 2985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sänger, J., Müller, V., Lindenberger, U. (2012). Intra- and interbrain synchronization and network properties when playing guitar in duets. Frontiers in Human Neuroscience, 6, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenhagen, J., Draschkow, D. (2019). Cluster-based permutation tests of MEG/EEG data do not establish significance of effect latency or location. Psychophysiology, 56(6), e13335. [DOI] [PubMed] [Google Scholar]
- Sauseng, P., Klimesch, W. (2008). What does phase information of oscillatory brain activity tell us about cognitive processes? Neuroscience and Biobehavioral Reviews, 32(5), 1001–13. [DOI] [PubMed] [Google Scholar]
- Schoot, L., Hagoort, P., Segaert, K. (2016). What can we learn from a two-brain approach to verbal interaction? Neuroscience and Biobehavioral Reviews, 68, 454–9. [DOI] [PubMed] [Google Scholar]
- Sebanz, N., Knoblich, G., Prinz, W., Wascher, E. (2006). Twin peaks: an ERP study of action planning and control in coacting individuals. Journal of Cognitive Neuroscience, 18(5), 859–70. [DOI] [PubMed] [Google Scholar]
- Simmons, C.H., Wehner, E.A., Tucker, S.S., King, C.S. (1988). The cooperative/competitive strategy scale: a measure of motivation to use cooperative or competitive strategies for success. The Journal of Social Psychology, 128(2), 199–205. [Google Scholar]
- Simony, E., Honey, C.J., Chen, J., et al. (2016). Dynamic reconfiguration of the default mode network during narrative comprehension. Nature Communications, 7(1), 12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, N., Maszczyk, T., Zhang, W., Tan, J., Dauwels, J. (2016). EEG hyperscanning study of inter-brain synchrony during cooperative and competitive interaction. In: 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Budapest, Hungary, IEEE, 4813–8. [Google Scholar]
- Sommerville, J.A., Hammond, A.J. (2007). Treating another’s actions as one’s own: children’s memory of and learning from joint activity. Developmental Psychology, 43(4), 1003–18. [DOI] [PubMed] [Google Scholar]
- Špiláková, B., Shaw, D.J., Czekóová, K., Mareček, R., Brázdil, M. (2020). Getting into sync: data-driven analyses reveal patterns of neural coupling that distinguish among different social exchanges. Human Brain Mapping, 41(4), 1072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, D.A., Adolphs, R. (2013). Toward a neural basis for social behavior. Neuron, 80(3), 816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoli, E., Lagarde, J., DeGuzman, G.C., Kelso, J.A.S. (2007). The phi complex as a neuromarker of human social coordination. Proceedings of the National Academy of Sciences of the United States of America, 104(19), 8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppi, J., Borghini, G., Petti, M., et al. (2016). Investigating cooperative behavior in ecological settings: an EEG hyperscanning study. PLoS One, 11(4), e0154236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi, L., Dungan, J., Waytz, A., Young, L. (2016). Distinct neural patterns of social cognition for cooperation versus competition. NeuroImage, 137, 86–96. [DOI] [PubMed] [Google Scholar]
- van Dijk, H., Schoffelen, J.-M., Oostenveld, R., Jensen, O. (2008). Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. The Journal of Neuroscience, 28(8), 1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk, R. (1998). Effects of cooperative and competitive outcome dependency on attention and impression preferences. Journal of Experimental Social Psychology, 34(3), 265–88. [Google Scholar]
- Vygotsky, L.S. (1978). Mind in Society. Cambridge, MA: Harvard University Press. [Google Scholar]
- Webster, G.D., DeWall, C.N., Pond, R.S. Jr, et al. (2014). The brief aggression questionnaire: psychometric and behavioral evidence for an efficient measure of trait aggression. Aggressive Behavior, 40(2), 120–39. [DOI] [PubMed] [Google Scholar]
- Yu, R., Zhou, X. (2006). Brain responses to outcomes of one’s own and other’s performance in a gambling task. Neuroreport, 17(16), 1747–51. [DOI] [PubMed] [Google Scholar]
- Yun, K., Watanabe, K., Shimojo, S. (2012). Interpersonal body and neural synchronization as a marker of implicit social interaction. Scientific Reports, 2(1), 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamm, A., Debener, S., Bauer, A.-K.R., et al. (2018). Amplitude envelope correlations measure synchronous cortical oscillations in performing musicians. Annals of the New York Academy of Sciences, 1423(1), 251–63. [DOI] [PubMed] [Google Scholar]
- Zhang, D., Hong, B., Gao, S., Röder, B. (2017a). Exploring the temporal dynamics of sustained and transient spatial attention using steady-state visual evoked potentials. Experimental Brain Research, 235(5), 1575–91. [DOI] [PubMed] [Google Scholar]
- Zhang, D. (2018). Computational EEG analysis for hyperscanning and social neuroscience. In: Im, C.-H., editor. Computational EEG Analysis: Methods and Applications. Singapore: Springer Singapore, 215–28. [Google Scholar]
- Zhang, D., Lin, Y., Jing, Y., Feng, C., Gu, R. (2019). The dynamics of belief updating in human cooperation: findings from inter-brain ERP hyperscanning. NeuroImage, 198, 1–12. [DOI] [PubMed] [Google Scholar]
- Zhang, M., Liu, T., Pelowski, M., Jia, H., Yu, D. (2017b). Social risky decision-making reveals gender differences in the TPJ: a hyperscanning study using functional near-infrared spectroscopy. Brain and Cognition, 119, 54–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


