Abstract
Recently, mesenchymal stem/stromal cells (MSCs) and their widespread biomedical applications have attracted great consideration from the scientific community around the world. However, reports have shown that the main populations of the transplanted MSCs are trapped in the liver, spleen, and lung upon administration, highlighting the importance of the development of cell-free therapies. Concerning rising evidence suggesting that the beneficial effects of MSC therapy are closely linked to MSC-released components, predominantly MSC-derived exosomes, the development of an MSC-based cell-free approach is of paramount importance. The exosomes are nano-sized (30100nm) lipid bilayer membrane vesicles, which are typically released by MSCs and are found in different body fluids. They include various bioactive molecules, such as messenger RNA (mRNA), microRNAs, proteins, and bioactive lipids, thus showing pronounced therapeutic competence for tissues recovery through the maintenance of their endogenous stem cells, the enhancement of regenerative phenotypic traits, inhibition of apoptosis concomitant with immune modulation, and stimulation of the angiogenesis. Conversely, the specific roles of MSC exosomes in the treatment of various tumors remain challenging. The development and clinical application of novel MSC-based cell-free strategies can be supported by better understanding their mechanisms, classifying the subpopulation of exosomes, enhancing the conditions of cell culture and isolation, and increasing the production of exosomes along with engineering exosomes to deliver drugs and therapeutic molecules to the target sites. In the current review, we deliver a brief overview of MSC-derived exosome biogenesis, composition, and isolation methods and discuss recent investigation regarding the therapeutic potential of MSC exosomes in regenerative medicine accompanied by their double-edged sword role in cancer.
Keywords: Mesenchymal stem/stromal cells (MSCs), Exosomes, Regenerative medicine, Cancer, MicroRNAs (miRNAs)
Introduction
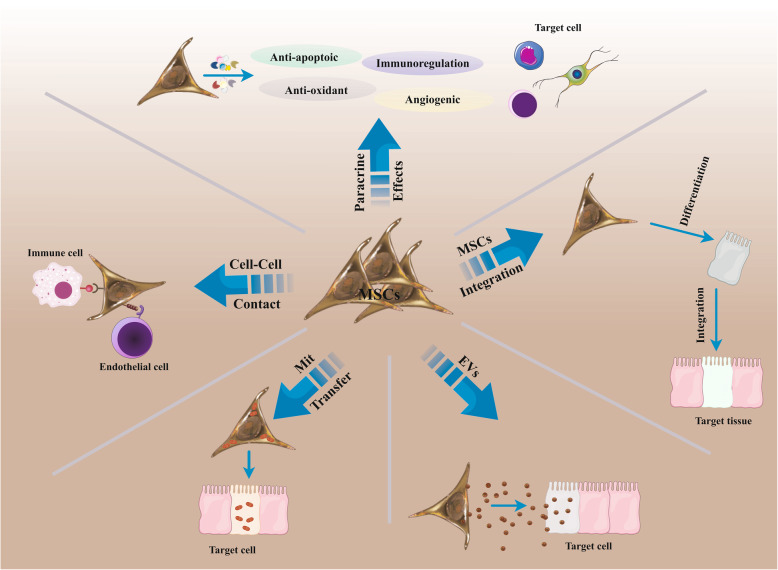
Mesenchymal stem/stromal cells (MSCs) are unique cell populations showing a large potential for unrestricted self-renewal, differentiating into a wide variety of diverse cell lines and forming distinct colonies [1,3]. They are multipotent fibroblast-like stem cells typically found in a variety of human tissue, including bone marrow (BM), adipose tissue, liver, intestine, lung, connective muscle tissue, spleen, skin, placenta, umbilical cords, and other tissues [4,6]. Currently, excellent progress has been established in stem cell technology with promising therapeutic possibilities for the treatment of various diseases [7]. Remarkably, MSCs unique attributes, including differentiation into multiple tissue types, the ability to recruit into damaged sites and tumors, easy isolation process from various tissues, the ability of ex vivo manipulation, and lower ethical concerns [8,10] describe them as an effective therapeutic strategy to induce tissue restoration. Now, considerable efforts have been made to use the MSCs natural interesting capability for homing to inflammation areas, such as those existing in cancer [11, 12]. Meanwhile, another attractive property of MSCs is their lower immunogenicity due to the absence of expressing any co-stimulatory molecules, thus indicating that there is no requirement for the use of immunosuppressive agents for allogeneic transplantation [13, 14]. Based on the literature, they can affect target cells and tissue by cell-cell contact, induction of paracrine effect, the release of exosomes and other secretions, and mitochondria (mito) transfer accompanied by differentiation into a specific adult cell and integration into a target tissue, thus delivering a new platform for cellular and cell-free stem cell therapy (Fig.1) [15, 16]. There is increasing evidence implying that the therapeutic functions and immunoregulatory activities of transplanted MSCs depend extensively on paracrine rather than cellular factors [17, 18]. These studies have shown that most of the transplanted MSCs are commonly trapped in the liver, spleen, and lung, and only less than 1% of MSCs reach and replace at the injured site, emphasizing the prominence of the development of cell-free therapies [19]. The MSCs not only secrete soluble factors (e.g., growth factor, cytokine, chemokine) but also generate and release extracellular vesicles (EVs) which enable eliciting of the therapeutic effects via the exchange of cytoplasmic and genetic material [20, 21]. Moreover, these vesicles resolve safety challenges such as gene mutation and unregulated cell division, and immune rejection commonly is shown during cell-based therapy [15, 22]. In these EVs, exosomes are prominently involved in cell communication and immunomodulatory activities [17]. They are nano-sized (30100nm) lipid bilayer membrane vesicles secreted by MSCs and detected in different body fluids [23,25]. Exosomes may transfer an enormous amount of bioactive molecules to nearby damaged recipient cells, which in turn, cause phenotypic change and then modulate regenerative programs of various organs [26, 27]. These phenotypic changes arise from several mechanisms, ranging from prevention of apoptosis in recipient cells, induction of target cell proliferation, stimulation of immunomodulatory responses, and reduction of oxidative stress in target cells to the enhancement of oxygen supply [28]. Regardless of the role of MSCs in tissue recovery, there is an inconsistency respecting the supportive or suppressive role of MSCs in tumorigenesis or tumor regression [29, 30].
Fig. 1.
The corresponding mechanisms of MSC-based therapy. MSCs can stimulate restoration in the damaged tissue by differentiation into adult cell lineages and by modifying immune reactions. The immunoregulatory capability of MSCs contains paracrine activity, cell-cell contact, and interaction, mitochondrial (mito) transfer, and secretion of extracellular vesicles (EVs). MSCs; mesenchymal stem/stromal cells
Herein, we discuss MSC-derived exosomes biogenesis and composition and also review the recent investigation concerning the therapeutic potential of MSC exosome in regenerative medicine and their dual role in cancer.
Exosome biogenesis
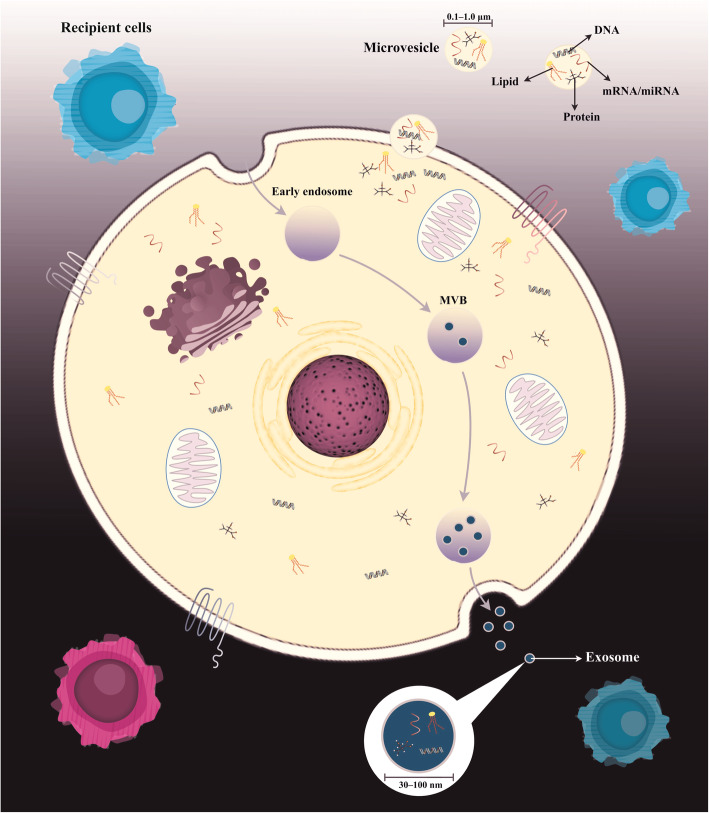
Extracellular vesicles (EVs) are lipid bilayer-delimited particles identified as a novel mediator of intercellular communication [31, 32]. They have been categorized according to their size, content, and mechanism of creation over the last decade [33]. There exist three well-known subtypes of EVs, including microvesicles (MVs), apoptotic bodies, and exosomes. The MVs are 100nm1m in diameter and are released via direct budding of plasma membrane cells. The other subtype of the EVs are exosomes, 30100nm in diameter, which is generated by inward budding of the intracellular endosomal membrane following the formation of multivesicular bodies (MVBs) (Fig.2) [34, 35]. Finally, apoptotic bodies range from 50 to 5000nm in diameter and commonly are derived from apoptotic cells. The exosomes are frequently secreted by a variety of cell types in culture, including epithelial cells, stem cells, immune cells, and tumor cells. Also, they are commonly present in various biological fluids, such as blood, saliva, urine, and breast milk [36]. Exosome biogenesis and secretion is a complex and unique process that is influenced by cellular origins and intracellular homeostasis, as well as the cells microenvironment and external stimuli [37]. Regarding recent studies, Wnt and mTOR pathways, which increase -catenin expression, are introduced as master regulators required both for MSC exosome secretion and also supporting the self-renewal of MSCs [38,40]. Furthermore, various physiological or pathological conditions can influence the mechanism of biomolecule packing into MSC exosomes as well as their biological function. For example, exosomes derived from hypoxia-exposed MSCs contain higher rates of the angiogenic proteins, such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF), and exert more angiogenic activity than normoxia-exposed MSC-derived exosomes [41, 42]. The mechanism of exosome production during exosome biogenesis includes initiation; the plasma membrane is internalized to form an endocytic vesicle termed an early endosome, followed by the early endosome developments into the late endosome. The budding of late endosomal membranes leads to the development of intraluminal vesicles (ILVs) within large MVBs [43]. Most ILVs are secreted into the extracellular space following merging with the plasma membrane, termed exosomes [44, 45]. In detail, exosome biogenesis is regulated by two separate molecular mechanisms, including endosomal sorting complex required for transport (ESCRT) machinery-dependent and ESCRT-independent. Considering literature, each ESCRT machine complex includes several components. Meanwhile, the ESCRT-0 complex recognizes ubiquitinated cytosolic domains of the transmembrane protein and sorts them into the endosomal membrane [46, 47]. ESCRT-I, II complexes bind to the outer part of the endosomal membrane and induce direct intraluminal buds to form MVBs; on the other hand, the ESCRT-III complex is assembled on the outer surface of the endosomal membrane and facilitates the release of new ILVs from the endosomal membrane during the MVB generation process. Recent evidence also indicates that lipids such as sphingosine-1-phosphate, ceramides, sphingolipids, heat-shock proteins, and tetraspanin proteins (e.g., CD81, CD82, and CD9) are involved in the sorting of cellular material in MVBs and the regulation of exosome release in ESCRT-independent pathways. Therefore, both ESCRT-dependent and ESCRT-independent pathways are synergistically critical for exosome biogenesis and function [33, 48,51]. Interestingly, the formation, content, and quantity of secreted exosomes will depend on whether the cells are exposed to various stress factors or external stimuli. Importantly, under various conditions, exosomes derived from the same cells can contain distinct components [32, 52]. The secreted exosomes are guided and taken up by recipient cells residing in the microenvironment or transported to distant sites by biological fluids [53]. Exosomes are taken up by the target cell through direct binding to the plasma membrane, by ligand-receptor interaction, or via phagocytosis [54,56]. Exosomes from different original cell types contain a particular collection of molecules that are required for their biogenesis and functional properties. MSC-derived exosomes, in particular, not only contain the common tetraspanin proteins CD63, CD9, and CD81, which are involved in the development of intraluminal buds, but also include CD29, CD73, CD90, CD44, and CD105, which are MSC-specific and play an influential role in exosome biogenesis [57].
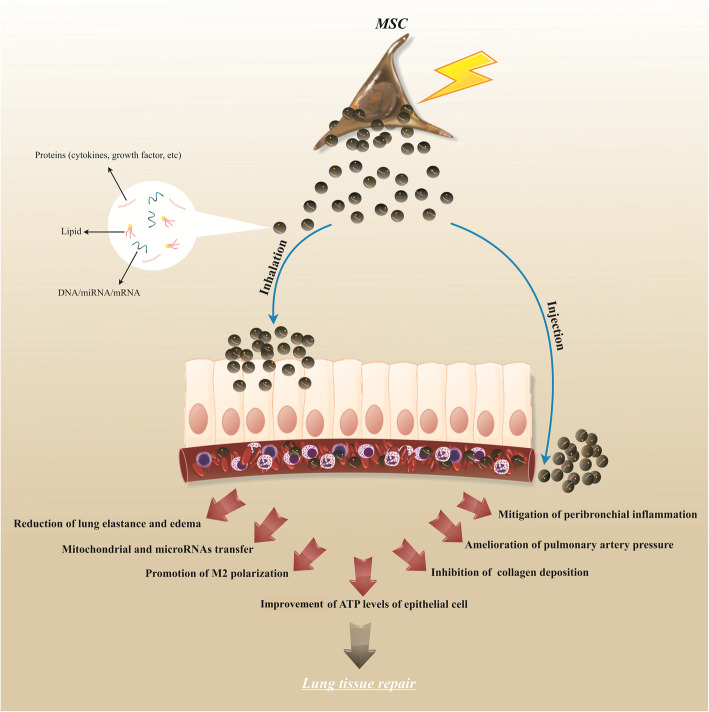
Fig. 2.
The therapeutic capacity of MSC-derived exosomes in lung disorders. The MSC exosomes could elicit desired therapeutic outcomes in transplanted patients upon administration by intravenous (IV) or inhalation route possibly mediated by their anti-inflammatory, immunomodulatory, pro-angiogenic, and also anti-fibrotic properties. MSCs; mesenchymal stem/stromal cells
Components of MSC-derived exosomes
The molecular composition of exosomes depends substantially on the types of donor cells, epigenetic changes, and various physiological and pathological environmental conditions [54, 58]. The exosomes are highly enriched in various components, including lipids, proteins, nucleic acids, and other components. The lipid component of exosomes encompasses cholesterol, sphingomyelin, hexosyl ceramides, phosphatidylserine, and prostaglandins, enabling exosomes key functions and activities such as exosome rigidity, exosomal endocytosis, membrane trafficking, and signaling [59, 60]. MSC exosomes also contain proteins, mRNAs, and microRNAs (miRNAs), which are delivered to recipient cells and can alter the behavior of adjacent cells. Indeed, MSC exosomes contribute to cellular processes such as transcription, proliferation, adhesion, migration, and differentiation [61]. Furthermore, MSC exosomes are involved in the induction of angiogenesis, inhibition of fibrosis, enhancing of the neuronal survival and differentiation, stimulation of extracellular matrix remodeling, abrogation of local inflammation response, and also the regulation of immune cells activities [42]. Proteomic analyses have revealed more than 4000 different proteins within exosomes playing multifaceted functions [62]. Exosomes protein components include tetraspanins (CD9, CD63, CD81, CD82), which are localized on the exosome surface and participate in cellular interactions, exosome biogenesis components (e.g., ESCRT complex, ALG2-interacting protein X (ALIX), thiazide-sensitive Na-Cl cotransporter (TSC101) and syntenin protein, membrane transport) and membrane fusion proteins (e.g., Ras associated with diabetes (Rab) GTPase and annexins), and also heat-shock proteins (HSP70) [63,65]. Exosome protein components can participate in cancer treatment and also play critical functional roles in the repair of human damaged tissue [42, 66]. It has been documented that PDGF-D derived from the umbilical cord (UC)-MSC-EVs could support the recovery process of infarcted heart cells [67]. Furthermore, it has been shown that the connection of the proinflammatory cytokine CCL2 with its receptor expressed on BM-MSC-EVs could reduce inflammation and support improvement of acute renal injury by inhibiting macrophage activation [68]. Other experiments have documented that MSC-EVs derived from mouse BM contain immunomodulatory proteins such as programmed death ligand-1 (PD-L1), galectin-1, and transforming growth factor-beta (TGF-). Besides, BM-MSC-EVs have higher levels of cyclooxygenase 2 (COX-2) and prostaglandin E2 (PGE2) contributed to the negatively regulating pro-inflammatory cytokine effects in the splenocytes [69]. Other predominant parts of the exosomal composition, nucleic acids include mRNA, miRNA, transfer RNA, ribosomal RNA, nucleolar RNA, long non-coding RNA (lncRNA), and DNA fragments. Importantly, RNA conveys genetic information, which in turn, affects protein expression and biological activity in the recipient cells [70,72]. Undoubtedly, one of the most important contents of exosomes is miRNAs due to their competence to elicit multifaceted function in human damaged or cancerous tissue by targeting a myriad of molecules and axis. Numerous training has compared the composition of miRNAs between MSC exosomes and their parental MSCs. Correspondingly, the expression of miR-21 and miR-15 involved in the regulation of cardiac functions was meaningfully lower in MSC-EVs in comparison to the parental MSCs [73]. Also, miRNA array analyses have demonstrated that miRNA profiles for human glioma-associated MSC exosomes and human BM-MSC exosomes are altered from their parental cells. Other studies have indicated that the top four miRNAs that are more obviously enriched in MSC exosomes than MSCs include miR-4485, miR-150-5p, miR-6087, and miR-486-5p, and conversely, top four miRNAs, which largely are found in MSCs, involve miR-34a-5p, miR-34c-5p, miR-15a-5p, and miR-136-3p [74]. Other reports signify that exosomal let-7f, miR-20b, and miR-30e-3p levels are modified in the plasma of patients with small-cell lung cancer [75], and also the existence of the close association between ovarian cancer incidence and progress with miR-21 and miR-141 plasma levels has been robustly evidenced [76].
Exosome isolation methods
Various researches indicate that there are some challenges in the clinical use of exosomes as among various purification processes, there is no standard method for separating exosomes from other micro-particles and also for the separation of various exosome subsets [77, 78]. One of the traditional and most widely used techniques for exosomal isolation is differential centrifugation, which purifies exosomes based on density and size. This method is simple, effective, and economical, but this procedure has a low output and specificity and may also be contaminated with other EVs of a similar dimension. Also, the high speed of centrifugation in this process may damage exosomes. Currently, differential centrifugation has been combined with a sucrose density gradient or sucrose cushions to improve the yield and purity of isolated exosomes [79, 80]. Filtration/ultrafiltration is another option that isolates exosomes based on the pore size of the filter. The filtration method takes less time and troublesome than the differential ultracentrifugation; however, since this approach is based on size, the separation of exosomes from other particles of the same size like apoptotic bodies, or microbubbles is difficult [36, 81]. Size-exclusion chromatography, similar to filtration, isolates exosomes depending on size using columns filled with pore beads. Although the yield of exosome purification is high in this procedure, this technique is time-consuming and is not the epitome for the obtaining of exosomes from large sample volumes [81, 82]. Further, the immunoaffinity strategy has a high purity and yield in exosome purification by binding exosome-associated antigens to antibodies in chromatography columns, magnetic beads, and micro-fluidic systems [83]. On the other hand, though precipitation can also be used for the purification of exosomes, the process is not specific and may co-precipitate other particles and also the chemical treatment may also destroy exosomes thru this process [81]. Moreover, the resuspension of the pellet is complicated and inappropriate for functional use. Overall, although each insolation method has its benefit and downside, its drawback may be dissolved by combining two or more purification procedures and improving both purity and quantity.
Application of MSC-derived exosomes in regenerative medicine
As known, human MSCs have a great competence to sustain tissue homeostasis as their high potential to home into damaged tissue and their effect on a variety of biochemical and cellular processes such as immune regulation, bioenergy, and tissue regeneration [84,86]. These processes are attributed to direct interactions with different types of cells or by paracrine mechanisms so through the production of soluble biological factors like exosomes [87]. Therefore, a large number of studies have focused on the beneficial protective and therapeutic effect of MSCs in the field of regenerative medicine. Exosomes and other EVs secreted by MSCs have an influential role in sustaining and restoring tissue microenvironment homeostasis supported by the high biochemical potential of their bioactive contents, such as proteins and RNAs [3]. Exosomes as nano-sized particles with less immunoreactivity than whole stem cells possess no potential of differentiating to adult cell lineage or tumor formation, attracting particular attention as a therapeutic vehicle for tissue recovery goal in cell-free based therapies (Table1) [117,119]. Moreover, studies show that exosomes produced by MSCs of different origins contain significantly different functional molecules and exhibit heterogeneous characteristics [120,122]. Exosomes from MSCs from bone marrow, adipose tissue, and umbilical cord, for example, showed a major variation in their proteomic profile and potentially therapeutic. Besides, BM-MSC exosomes have pronounced regeneration capacity by induction of the angiogenesis and also AT-MSC exosomes show the most influential secretory activity and immune response regulation compared with MSCs derived from other sources, while UC-MSC exosomes mostly participate in tissue repair, according to Wang et al. reports [123]. The exact underlying mechanism of MSC-derived exosomes in the restoration of damaged tissue is unclear, while a large number of investigations have suggested that exosomes contribute to the regeneration of damaged tissues by several mechanisms, including (a) the maintenance of endogenous stem cells by proliferating, self-regenerating and differentiating [124, 125]; (b) the induction of regenerative phenotypes by promoting cell proliferation, angiogenesis, and regeneration of nerves [126,129]; (c) the protection of cells from apoptosis by various mechanisms and the amelioration of tissue damage [69]; (d) the attenuation of oxidative stress and the adjustment of the immune response through the delivery of immunomodulatory mediators to damaged tissue [41, 130, 131]. Accordingly, EVs derived from MSCs, especially exosomes, can restore tissue damages and uphold their therapeutic efficacy by transferring biologically active molecules and affecting target molecules, finally regulating the gene expression and phenotype of damaged recipient cells [124, 132,134]. Growing proof shows that MSC-derived exosomes have a diagnostic/therapeutic potential in different pathological conditions, covering cardiovascular diseases, liver, kidney, lung injuries, and neurological disorders [130, 135].
Table 1.
Therapeutic potential of MSC exosomes in the context of regenerative medicine
| Condition | MSC source | Isolation method | Mechanism | Main outcome | Ref |
|---|---|---|---|---|---|
| MI | Cardiac MSC | Precipitation at 4C with 5 polyethylene glycol 4000 | Transferring miRs | Promoting cardiac angiogenesis and activating cardiomyocyte proliferation (animal study) | [88] |
| MI | BM-MSC | ExoQuick Exosome Precipitation Solution | Delivering miR-221 and miR-19a | Higher protective effects in cardiomyocytes promoted the growth of cardiomyocytes and prevented cell apoptosis (animal study) | [89, 90] |
| MI | BM-MSC | Ultracentrifugation (110,000g) | Delivering miR-210 that decreases the expression of ephrin-A3 | Promoted angiogenesis and cardiac function (animal study) | [91] |
| Myocardial IRI | HuES9.E1-derived MSC | Chromatography columns | Stimulating anti-apoptotic pathways and also inhibiting pro-apoptotic pathways | Enhanced bioenergetics and pro-survival signaling of cardiac cells (animal study) | [92] |
| MI | BM-MSC | ExoQuick-TC | n.a | Inducing neovascularization and suppressing inflammatory response (animal study) | [93] |
| MI | BM-MSC | Ultracentrifugation (200,000g) | Delivering MiR-125b-5p | Inhibited apoptosis of cardiomyocyte through inhibition of pro-apoptotic genes p53 and BCL2-antagonist/killer 1 (BAK1) (animal study) | [94] |
| Autoimmune hepatitis | BM-MSC | Ultracentrifugation (100,000g) | Delivering exosomal miR-223 | Downregulating various inflammatory genes and proteins and inhibition of hepatocyte death (animal study) | [95] |
| Hepatic oxidant injury | UC-MSC | Ultracentrifugation (100,000g) | Delivering glutathione peroxidase 1 (GPX1) | Suppressed oxidative stress and apoptosis on CCl4-and H2O2-induced hepatic damage (animal study) | [96] |
| LF | HuES9.E1-derived MSC | High-performance liquid chromatography (HPLC) | Increasing the expression of cyclin D1 and PCNA as proliferative proteins and enhancing expression of Bcl-xL as an anti-apoptotic factor | Promoting hepatocyte proliferation and inhibiting hepatocyte apoptosis (animal study) | [97] |
| Liver fibrosis | BM-MSC | Ultracentrifugation (100,000g) | Lowering the plasma level of alanine aminotransferase and modulating expression level of inflammatory cytokines and regulatory T cells | Inducing hepatoprotective and anti-inflammatory effects (animal study) | [98] |
| LF | ESC-MSC | Ultracentrifugation (100,000g) | n.a | Enhanced anti-apoptosis, anti-fibrosis, and regenerative capacity (animal study) | [99] |
| LF | BM-MSC | Ultracentrifugation (100,000g) | Delivering Y-RNA-1 | Modulated inflammatory response and by inducing multiple anti-apoptotic genes (animal study) | [100] |
| AD | WJ-MSC | Ultracentrifugation (100,000g) | Delivering catalase | Decreasing neuronal oxidative stress and synapse damage (animal study) | [101] |
| Brain stroke | BM-MSC | Ultracentrifugation (100,000g) | Transferring miR-133b to neighboring astrocytes and neuron cells | Increased functional regeneration (animal study) | [102] |
| SCI | BM-MSC | n.a | Enhanced neuro-vascularization and weaken neuronal apoptosis, as well as prevent inflammation and inhibit the neurotoxic of A1 astrocytes | Boosted SCI functional recovery (animal study) | [103] |
| SNI | UC-MSCs | Ultracentrifugation | Promoted the generation of axons and Schwann cells, minimized muscular atrophy, and induced anti-inflammatory reactions | Improved functional recovery and peripheral nerve regeneration (animal study) | [104] |
| SNI | Gingiva MSC | ExoQuick Exosome Precipitation Solution (SBI Systems Biosciences) | Expression of phenotype-related genes, c-Jun, Notch1, GFAP, Sox2 and the stimulation of JNK pathways | Regeneration of peripheral nerve (animal study) | [105] |
| MS | AT-MSC | Ultracentrifugation (100,000g) | Reducing the number of Iba-1-positive microglial cells and also the plasma levels of inflammatory cytokines | Modulated microglial activity and improved immunomodulatory activities (animal study) | [88] |
| AKI | UC-MSCs | Ultracentrifugation (100,000g) | Transferring hepatocyte growth factor (HGF) mRNA | Improved dedifferentiation and proliferation of damaged cells (animal study) | [89] |
| Kidney IRI | n.a | n.a | Delivering miR-22 for mTOR/HIF feedback interaction pathway and suppressing (PDCD4)/NFKB pathways | Stimulating kidney protection by inducing anti-inflammatory responses and reducing epithelial cell damage (animal study) | [106] |
| Kidney IRI | WJ-MSC | Ultracentrifugation (100,000g) | Reduced kidney cell apoptosis and boosted anti-inflammatory responses | Downregulated CX3CL1 expression and improved kidney function (animal study) | [107] |
| AKI | UC-MSCs | Ultracentrifugation (100,000g) | Activation of the ERK 1/2 pathway and inhibition of the p38MAPK pathway | Suppressing oxidative stress, inducing cell growth, and reducing kidney cell apoptosis (animal study) | [108] |
| CKD | Urinary MSC | Ultracentrifugation (100,000g) | Delivering effective growth factors | Inhibited cell apoptosis and increased vascular regeneration (animal study) | [109] |
| Kidney artery stenosis | AT-MSC | n.a | Delivering anti-inflammatory cytokine interleukin (IL) 10 | Attenuated kidney inflammation and fibrosis, increased kidney blood supply, and suppressed glomerular filtration (animal study) | [110] |
| Kidney fibrosis | BM-MSC | ExoQuick Exosome Precipitation Solution | Delivering exogenous microRNA let7c | Suppression of kidney inflammation (animal study) | [111] |
| ALI | UCB-MSC | Ultracentrifugation (100,000g) | Delivering VEGF | Restored impaired alveoli function, angiogenic effects, and anti-inflammatory responses and minimized cell apoptosis (animal study) | [112] |
| ALI | AT-MSC | Ultracentrifugation | Delivering miRNA | Attenuating ALI damage and macrophages polarization (animal study) | [113] |
| Lung IRI | BM-MSCs | n.a | Transferring miR-21-5p | Inhibited lung edema and improved the function of wound healing (animal study) | [114] |
| ALI | BM-MSC | Ultracentrifugation | Transferring KGF | Promoted immunomodulatory effects and increased intracellular ATP levels of alveolar epithelial type 2 cell (animal study) | [115] |
| ALI | BM-MSC | Ultracentrifugation (100,000g) | Transferring mitochondrial | Inhibited the production of inflammatory cytokines and enhanced phenotype 2 of alveolar macrophages (animal study) | [116] |
| PAH | BM-MSC | Ultracentrifugation (100,000g) | Alleviated pressure, inhibited right ventricle hypertrophy, and restored pulmonary function | Improved pulmonary artery hypertension (animal study) | [117] |
Note: ALI acute lung injury, MI myocardial infarction, LF liver failure, IRI ischemia-reperfusion injury, PAH pulmonary arterial hypertension, AKI acute kidney injury, CKD chronic kidney disease, SCI spinal cord injury, SNI spared nerve injury, AD Alzheimers diseases, MS multiple sclerosis, BM bone marrow, AT adipose tissue, UC umbilical cord, UCB umbilical cord blood, WJ Whartons jelly, miRs microRNAs, n.a not available
Cardiovascular disease
Cardiovascular disease (CVD) has become a public health concern because it is the main cause of mortality in the world [110, 136]. The ability of endogenous cardiac injury to repair and regenerate is significantly poor due to the insufficient proliferation of existing myocytes concomitant with the weak recruitment and division of resident cardiac progenitor cells [137, 138]. Emerging evidence has shown the cardio-protective role of MSC exosomes in myocardial damage. Ju et al. reported that the delivery of cardiac MSC exosomes after myocardial infarction (MI) enhances cardiac activity by promoting cardiac angiogenesis and activating cardiomyocyte (CMC) proliferation with an unclear mechanism. Cardiac MSC exosomes may enable ischemic myocardium to release chemokines, such as stromal cell-derived factor (SDF) or vascular endothelial growth factor (VEGF), supporting angiogenesis. These exosomes may also upsurge the proliferation of CMC by transferring miRNAs to the ischemic myocardium and by stimulating specific phases of the cell cycle in target cells [88]. Yu et al. have described that exosomes derived from MSC transduced by the growing family of related transcription factor (GATA)-4 genes have enriched various miRNAs, such as miR-221 and miR-19a, which are capable of generating higher protective effects on CMC and also the regeneration of ischemic injury. The miR-221 could improve the survival of myocytes in ischemic injury by reducing the expression of the P53-upregulated apoptosis modulator (PUMA), a member of the B cell lymphoma 2 (Bcl-2) proapoptotic protein families. Also, miR-19a could attenuate the expression of phosphatase and tensin homolog (PTEN), a mediator of Akt and extracellular signal-regulated kinase (ERK) survival signaling pathway, thereby leading to the growth of CMC and preventing cell apoptosis in ischemic myocardium by activating these signaling pathways [89, 90]. Additionally, MSC exosomes contain a high amount of miR-210 that could diminish the expression of ephrin-A3, an angiogenesis suppressor molecule, in endothelial cells to promote angiogenesis and cardiac function in mouse MI models [91]. Another study suggested that MSC exosomes had a direct effect on cardiac cells and enhanced their bioenergetics and survival by reducing oxidative stress, enhancing the production of ATP and NADH, and stimulating anti-apoptotic pathways through Akt and glycogen synthase kinase-3 (GSK3) phosphorylation, and also mitigation of the activation of the proapoptotic pathways mediated by downregulation of c-Jun NH2-terminal kinase (c-JNK). MSC exosomes also could restore cardiac activity and reduce infarct size in the ischemic/reperfuse mouse model of the myocardium [92]. Furthermore, Teng et al. demonstrated that MSC exosomes could recover heart function and repair post-myocardial infarction via inducing neovascularization and suppressing inflammatory response after ischemic injury [93]. In another study, Zhu et al. reported that exposure of BM-MSC to the hypoxia culture medium altered their exosomal content compared to the normoxia culture medium. The miRNA content was shown to be significantly different between normoxia-exposed BM-MSC exosomes (nor-exosomes) and hypoxia-exposed BM-MSC exosomes (hypo-exosomes). Analysis implied that anti-apoptotic miR-125b-5p was one of the most enriched miRNAs in hypo-exosomes with high therapeutic effectiveness in the treatment of MI. Correspondingly, systemic administration of hypo-exosomes inhibited apoptosis of CMC through inhibition of proapoptotic genes p53 and bcl2-antagonist/killer 1 (BAK1) and consequently improved ischemic heart repair in the rodent MI model [94]. Besides, reports have indicated that cardiac MSC-EVs facilitate strong proangiogenic behaviors as well as enhanced cell proliferation, tube forming, and survival of human umbilical vein endothelial cells (HUVECs). These proangiogenic effects of human cardiac MSC-EVs were mainly mediated by positive regulation of the angiopoietin-1 (Ang1) / tyrosine kinase Tie-2 signaling pathway [139]. Other studies have shown that exposure to PDGF enhanced the proangiogenic activity of EVs secreted by AT-MSCs. In EVs derived from PDGF-treated MSCs, the levels of proangiogenic c-kit and stem cell factor (SCF) improved and consequently resulted in induced angiogenesis in vitro and in vivo [140].
Liver injury and fibrosis
Several studies have also revealed that MSC exosomes possess the ability to rescue liver injury and fibrosis through the delivery of various bioactive molecules to hepatocyte cells and other cells in the liver tissue, and thereby change their function and/or phenotype. MSC exosomes have substantial hepatoprotective effects related to exosomal miRNAs, especially miR-223. MiR-223 plays a vital role in the modulation of the immune system as well as the protection of the liver by downregulating various inflammatory genes and proteins, such as cytokines and nucleotide-binding domain and leucine-rich repeat pyrin containing 3 (NLRP3) and also caspase-1. Exosomal miR-223 suppresses NLRP3/caspase-1 signaling pathway and pyroptosis resulting from this signaling pathway, which may minimize liver inflammation and hepatocyte death in the autoimmune hepatitis animal model [95]. Similarly, Yan et al. have already found that glutathione peroxidase 1 (GPX1) containing MSC exosomes could suppress oxidative stress and apoptosis in carbon tetrachloride (CCl4)-and H2O2-induced hepatic damage and finally restore liver function in vivo. The GPX1 is a main antioxidant enzyme that reduces the formation of reactive oxygen species (ROS) through different mechanisms in inflamed liver tissue. Too, MSC-derived exosomes could increase the spread of hepatocytes and decrease their apoptosis through various pathways [96]. Tan et al. reported that MSC exosomes improved hepatic regeneration of CCl4-induced liver damage in a mouse model through promoting hepatocyte proliferation and inhibiting their apoptosis. They found that MSC exosomes stimulate the hepatocyte cell cycle by upregulation of cyclin D1 and proliferating cell nuclear antigen (PCNA) concurrently suppression of the apoptosis of liver cells by increasing the expression of anti-apoptotic Bcl-xL [97]. In parallel, Tamura et al. investigated the inhibitory effect of MSC-derived exosomes on immune-induced liver damage via injection of concanavalin A (con A). They found that MSC-derived exosomes had hepatoprotective and anti-inflammatory competencies through reducing the expression level of proinflammatory cytokines along with promoting the expression of anti-inflammatory cytokines and the frequency of regulatory T cells [98]. To prolong the bioavailability of MSC-derived exosomes in chronic liver disease, Mardpour et al. encapsulated exosomes in polyethylene glycol (PEG) hydrogels to sustain their release in the systemic circulation. They found that hydrogel-mediated delivery caused boosted accumulation of exosomes in fibrotic tissue over prolonged periods, enabling superior anti-apoptosis, anti-fibrosis, and regenerative capacity over free-exosome delivery [99]. Besides, evaluation of the potential of MSC exosomes as a therapeutic approach for tissue restoration and functional recovery by modifying inflammatory response and inducing multiple anti-apoptotic genes in the experimental acute hepatic failure model suggested that MSC-derived exosome intravenous injection resulted in upregulated Y-RNA-1 as a non-coding RNA in damaged tissue, which in turn, supported defensive mechanisms in the microenvironment of the injured liver [100].
Neurological diseases
Concerning the observations, restoring deteriorated neural tissue remains a big problem in regenerative medicine due to a complication of the physiology system and a limited ability to self-regenerate [101, 141]. A wide spectrum of reports has demonstrated that MSC exosomes have promising therapeutic potential in neurological disorders, such as neurodegenerative diseases. Recent researches have shown that MSC exosomes can protect hippocampal neurons by decreasing neuronal oxidative stress and synapse damage in an animal model of Alzheimers disease (AD). Exosomes contain multiple active enzymes, in particular catalases, which can reduce the formation of ROS in hippocampal neurons, and thereby protect them from AD and other neurodegenerative conditions [142]. As well, Xin et al. proposed that MSC exosomes induced the remodeling of neuritis and subsequently facilitated functional regeneration in the rat stroke model via miR-133b transfer to adjacent astrocytes and neuron cells [102]. In another study, intravenous administration of MSC exosomes improved neuro-functional recovery and boosted post-stroke neurogenesis and neurovascular remodeling in the rat model. Liu et al. indicated that BM-MSC exosomes could enhance neurovascular development and attenuate neuronal apoptosis. Moreover, these exosomes inhibited neuroinflammation and also suppressed the neurotoxic and reactive state of A1 astrocytes following traumatic spinal cord injury (SCI) in rats, implying their competence to exploit for SCI therapy [103]. Additionally, exosomes derived from MSCs could provide a significant protective role in peripheral nerve damage. In this regard, exosomes derived from UC-MSCs could aggregate in nerve defect, promote the generation of axons and Schwann cells, minimize muscular atrophy, and exert anti-inflammatory reactions in injured nerve tissues by downregulating proinflammatory cytokines (IL-6 and IL-1) concomitant with upregulating anti-inflammatory cytokines (IL-10). Therefore, UC-MSC exosomes could establish a favorable stromal condition for increased functional recovery and amelioration of sciatic nerve defects in rats [104]. Mechanistically, Mao et al. suggested that gingiva MSC exosomes promote regeneration of peripheral nerve via upregulation of genes participated in Schwann cell dedifferentiation or repair, including c-Jun, notch1, glial fibrillary acidic protein (GFAP), and SRY (sex-determining region Y)-box 2 (Sox2) and the stimulation of c-Jun N-terminal kinase (JNK) pathways in Schwann cells. Hence, these findings provide proof of the concept that gingiva MSC exosomes activate repair phenotype in Schwann cells and promote their proliferation and migration [105]. Besides, AT-MSC exosomes demonstrated inhibitory effects on inflammation-mediated demyelinating disease, in particular, multiple sclerosis (MS). Exosomes also greatly modulate microglial activity by altering microglial cell morphology and reducing the number of Iba-1-positive microglial cells in the brains of the Theilers murine encephalomyelitis virus (TMEV)-infected mice. Regarding observations, administration of exosomes intravenously improved immunomodulatory activities by reducing the plasma levels of inflammatory cytokines, substantially the concentration of Th1 cell-generated IFN- and Th17 cell-produced IL-17A, resulted in ameliorated motor deficits of TMEV-infected mice [88]. As well, systemic administration of prostaglandin E2 receptor 4 (EP4) antagonists induced MSC-EV secretion and increased the production of anti-inflammatory cytokines and other proteins such as IL-2, IL-10, RANTES, vascular endothelial growth factor (VEGF), and brain-derived neurotrophic factor (BDNF) which could alleviate memory and learning deficiencies, improve blood-brain barrier integrity and anti-inflammatory responses, and also elicit functional recovery in a mouse model with hippocampus damage [143].
Kidney disease
Preclinical researches have demonstrated that MSC exosomes have a marked capacity to rescue acute and chronic kidney disease via the secretion of various mRNAs, miRNAs, and immunosuppressive factors into the tissue environment [127, 144, 145]. The molecular mechanisms responsible for kidney regeneration rely on the control of immune responses, the prevention of apoptosis and necrosis of epithelial tubular cells, and the reduction of oxidative stress in the kidney tissue. Ju et al. have shown that MSC exosomes improved dedifferentiation and proliferation of damaged tubular cells by transfer of hepatocyte growth factor (HGF) mRNA and enhanced HGF synthesis, thereby accelerating kidney regeneration and delaying fibrosis [89]. Also, another study verified the role of MSC-generated miRNAs in kidney protection by suggesting the anti-inflammatory ability of miR-22 in the relief of I/R-induced acute kidney injury (AKI). In detail, miR-22 suppresses the programmed cell death 4 (PDCD4)/ nuclear factor-B (NF-B) pathways required for dendritic cell (DC) maturation, which consequently attenuates its potential for the secretion of proinflammatory cytokines. MiR-22 also reduces epithelial cell damage via targeting the mammalian target of rapamycin (mTOR)/ hypoxia-inducible factor (HIF) feedback interaction pathway, enabling AKI recovery [106]. Further, Zou et al. found that the exosomes derived from human Whartons Jelly MSC (WJ-MSC) downregulated chemokine C-X3-C motif ligand 1 (CX3CL1) expression and improved kidney function in rats with kidney ischemia-reperfusion injury (IRI). They found that the exosomes could reduce kidney cell apoptosis and raise the proliferation of these cells concomitant with the enhancement of anti-inflammatory responses by decreasing the macrophage frequencies in wounded sites [107]. Moreover, human UC-MSC exosomes could potently improve cisplatin-induced AKI in laboratory models by suppressing oxidative stress upon decreasing oxidant enzymes and increasing antioxidant enzymes cellular levels and also through mitigation of kidney cell apoptosis by inhibition of the p38 mitogen-activated protein kinase (MAPK) pathway [108]. Besides, intra-kidney delivery of MSC exosomes could restore kidney injury in pigs with metabolic syndrome and kidney artery stenoses possibly mediated by increased kidney blood supply, suppressed glomerular filtration, modified kidney inflammation, and abrogated kidney fibrosis and medullary oxygenation, supporting kidney normal function. It has been supposed that MSC exosomes elicited these protective effects upon IL-10 upregulation as an anti-inflammatory cytokine [110]. Another research indicated that BM-MSC exosomes could deliver exogenous microRNA let7c with strong anti-fibrotic activity via suppression of kidney inflammation in rodent models [111]. Respecting to the glial cell line-derived neurotrophic factor (GDNF) neurotrophic property, exosomes derived from GDNF-overexpressed AT-MSCs were found to stimulate angiogenesis and enhance peritubular capillary loss in tubulointerstitial fibrosis in vivo by activating the SIRT1 signaling pathway and inducing the production of phosphorylated endothelial nitric oxide synthase (p-eNOS) [146]. Moreover, in an experimental model of type I diabetes, intravenous administration of MSC exosomes significantly ameliorated diabetes-induced kidney injury by suppressing apoptosis of podocytes and tubular epithelial cells and inducing tubular vascular survival and regeneration. MSC exosomes contained angiogenesis-related factors such as transforming growth factor- (TGF-), angiogenin, and bone morphogenetic protein-7 (BMP-7) played central roles in these processes [147].
Lung diseases
Rising preclinical evidence shows that MSC-derived exosomes have substantial therapeutic potential in the regeneration and rehabilitation of multiple lung diseases by mediating different molecular pathways and affecting different target cells in the lung tissue environment, such as lung endothelial and epithelial cells and immune cells, leading to the desired therapeutic outcomes following injection by various routes in particular intravenous or inhalation route [148, 149] (Fig.3). Ahn et al. recently found that VEGF plays a crucial function in the protection of exosomes in hyperoxic lung injuries. Correspondingly, exosomes generated by human UC-MSCs have significant protective efficacy by restoring impaired alveoli function and angiogenic effects, lessening cell apoptosis, and suppressing macrophages and proinflammatory reactions in newborn hyperoxic lung injuries in a rat model [112]. Compartmental analysis of the therapeutic capacity of fresh MSC exosomes and aged MSC exosome demonstrated that fresh exosomes had superiority over aged exosomes in terms of the attenuating acute lung injury (ALI) and macrophage polarization because of the existing differences in miRNA content [113]. Besides, Li et al. reported that intratracheal administration of MSC-generated exosomes had a protective effect in the treatment of the murine model with lung IRI by transporting miR-21-5p, as evidenced by inhibited lung edema and improved tissue regeneration by downregulating PTEN and PDCD4. MSC exosomes also shifted alveolar macrophage polarization from M1 to M2 phenotype caused inhibited proinflammatory responses in a miR-21-5p-dependent manner in the murine ischemia-reperfusion injury (IRI) model lung [114]. Interestingly, Monsel and his colleagues found that MSC exosomes could improve ALI induced by E. coli pneumonia by secretion of the keratinocyte growth factor (KGF) and binding to its CD44 receptor on the surface of monocytes and alveolar epithelial cells in the murine model. Administration of MSC exosomes intravenously improved immunomodulatory effects in bacterial pneumonia injuries by improved phagocytosis potential of monocytes and decreased inflammatory cytokine secretion. In particular, exosomes restored alveolar epithelial type 2 cell metabolism by increasing their intracellular ATP levels [115]. In another study, MSC exosomes mediated mitochondrial transfers, which in turn, inhibited the production of inflammatory cytokines and enhance phenotype 2 of alveolar macrophages to mitigate ALI in vivo [116]. Additionally, MSC exosomes could improve pulmonary artery hypertension (PAH) through alleviating mean pulmonary artery pressure (mPAP) and mean right ventricle pressure (mRVP), inhibiting right ventricle hypertrophy, and restoring pulmonary functions in the murine PAH models [117]. Besides, hepatocyte growth factor (HGF) derived from MSC-MVs has been shown to reduce pulmonary microvascular endothelial paracellular and transcellular permeability by facilitating the expression of junction proteins VE-cadherin and occluding under pathological conditions such as acute lung injury [150]. Moreover, MSC-MVs prevented endothelial cell apoptosis, increased IL-10 expression, and reduced IL-6 expression in endothelial cell culture media via an HGF-dependent action in vitro. In another study, the therapeutic capacity of hUC-MSC-EVs versus hUC-MSCs on the treatment of chronic obstructive pulmonary disease (COPD) in a rat model was investigated. Both hUC-MSC-EVs and hUC-MSCs ameliorated peribronchial and perivascular inflammation and thus decreased alveolar septal thickening in the emphysematous lung of COPD by decreasing the synthesis of protein kinase C zeta and NF-B subunits p50 and p65, which control several pathways associated with innate and adaptive immune response [151].
Fig. 3.
Extracellular vesicle (EVs) biogenesis. Exosomes are smaller luminal vesicles (30100nm in diameter) crated from late endosomes that are shaped by inward budding of the limited multivesicular body (MVB) membrane; however, microvesicles (MVs) are small membrane vesicles (0.11m in diameter) secreted from the cell membrane surface. The content, or cargo, of EVs, includes lipids, nucleic acids, and proteins, in particular, proteins related to the plasma membrane, cytosol, and those that participated in lipid metabolism
As well, another study in 30 participants with COPD indicated that administration of MSC exosomes was well tolerated because none of them experienced any unwanted events [152]. Moreover, investigation of the safety and efficacy of exosomes (ExoFlo) derived from allogeneic BM-MSCs in 24 patients with severe 2019 coronavirus disease (COVID-19) revealed that systemic injection of the ExoFlo caused restored patients clinical condition and oxygenation without any serious events during 2weeks follow-up, suggesting MSC exoxomes as a capable therapeutic candidate for severe COVID-19 [153].
Double-edged sword role of MSC-derived exosomes in tumors
The microenvironment around the tumor cells includes extracellular matrix (ECM) and soluble factors along with various tumors stromal cell types, such as MSCs, fibroblasts, immune cells, epithelial cells, and endothelial cells, which have a potent effect on tumor growth and progression modulation [154,156]. The MSCs are a significant constituent of tumor stromal cells which may be recruited at tumor sites with ambiguously defined biological mechanisms and may support the formation of a tumor-associated microenvironment. The crosstalk of MSCs with tumor cells within the tumor stroma affects various cancer features [156] and can exert contradictory functions in various tumors. These investigations have shown that MSCs can either support tumor development and angiogenesis (Fig.4), but can also suppress tumor growth and progression [157, 158]. Recent studies have suggested that MSC-derived exosomes have a potential role in the shaping of the communication between MSCs and tumor cells and also evidenced the regulatory effects of MSCs on the microenvironment of the tumor by distributing soluble factors and genetic biomolecules (Table2) [124, 178].
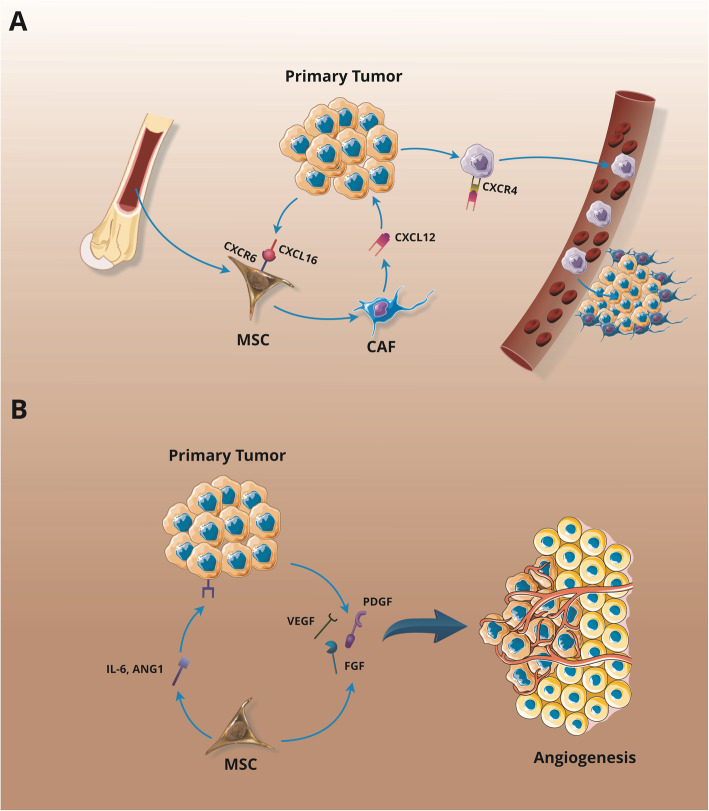
Fig. 4.
MSCs role in tumor metastasis (a) and angiogenesis (b). a The cancer cells release CXCL16 which can elicit the recruitment of MSCs in the tumor site. Upon the bindings of CXCL16 to CXCR6 on MSCs, they are transformed into CAFs producing high levels of CXCL12. CXCL12 stimulates the EMT process on cancer cells that promote CXCR4 expression in cancer cells, leading to their metastasis. b The MSCs commonly generate and release varied angiogenic mediators surrounding angiopoietin-1 (ANG-1) and IL-6 that, in turn, provoke the release of VEGF and other angiogenic factors stimulating tumor angiogenesis. CAF; cancer-associated fibroblast, EMT; epithelial-to-mesenchymal transition, MSC; mesenchymal stem/stromal cell, FGF; fibroblast growth factor, ANG-1; angiopoietin-1, PDGF; platelet-derived growth factor, VEGF; vascular endothelial growth factor
Table 2.
Dual role of MSC exosomes in the context of cancer therapy
| Donor cells | Cell line/condition | Exosome content | Function | Pathway | Ref |
|---|---|---|---|---|---|
| Promoting effect | |||||
| BM-MSCs | MM | miR-15a | Enhanced MM cell proliferation (animal study) | n.a | [159] |
| MSCs | BRCA cells | miR-222/223 | Supported the survival and growth of cancer cells (in vitro study) | Promoted quiescence in a subset of cancer cells and confers drug resistance | [160] |
| AT-MSCs | HUVECs | miR-125a | Promoted angiogenesis in vitro and in vivo | Repressed the expression of the angiogenic inhibitor delta-like 4 (DLL4) | [111] |
| AT-MSCs | Breast cancer MCF7 cells | n.a | Promoted MCF7 migration (in vitro study) | Activating Wnt signaling pathway | [161] |
| BM-MSCs | Osteosarcoma MG63 and gastric SGC7901 cells | n.a | Supported MG63 and SGC7901 cell growth (in vitro study) | Activating Hedgehog signaling pathway | [162] |
| AT-MSCs | HMEC | c-KIT and SCF | Induced angiogenesis (animal study) | Promoted survival, migration, and angiogenesis | [140] |
| MSCs | Gastric cancer | n.a | Stimulated the proliferation and migration of gastric cancer cells ex vivo (animal study) | Activation of the Akt pathway | [163] |
| AT-MSCs | Glioblastoma U87MG cells line | n.a | Induced cell proliferation (in vitro study) | n.a | [164] |
| GC-MSCs | Gastric cancer cells | miR-214, miR-221, and miR-222 | Promoted tumor proliferation and migration (in vitro study) | Downregulating several tumor suppressor target genes | [165] |
| Inhibitory effect | |||||
| MSCs | Breast cancer cells | miR-379 | Inhibition of angiogenesis, tumor amplification, and metastasis (in vitro study) | Regulation of COX-2 mRNA and protein | [166] |
|
UC-MSCs BM-MSCs |
Glioblastoma U87MG cells | n.a | Decreased cell proliferation and induced apoptosis of glioblastoma cells (in vitro study) | n.a | [164] |
| MSCs | Gastric cancer | n.a | Induced drug resistance in gastric cancer cells (animal study) | Triggered the activation of calcium/calmodulin-dependent protein kinases (CaM-Ks) and Raf/MEK/ERK kinase cascade | [167] |
| BM-MSCs | Glioma | miR-146b | Reduced glioma cell invasion, migration, viability, and expression of EGFR (animal study) | Decreased EGFR and inhibited NF-B, as well as its upstream regulator TRAF6 | [168] |
| MSCs | Breast cancer | miR-16 | Suppressed angiogenesis (animal study) | Downregulated VEGF expression in tumor cells in vitro and in vivo | [169] |
| WJ-MSCs | Burkitts lymphoma cells | miR-34a and let-7 family | Arrested cell division of lymphoma cells in the S phase and induced apoptosis by using oxidative stress pathways (in vitro study) | Alteration of antioxidant enzymes | [170] |
| AT-MSCs | Hepatocellular carcinoma (HCC) | miR-122 | Increased the sensitivity of HCC cells to chemotherapeutic agents and induction of apoptosis and cell cycle arrest (animal study) | Reduced expression of cyclin G1 (CCNG1 ) and inhibition of the PI3K/Akt pathway | [171] |
| BM-MSCs | Breast cancer | miR-23b | Induced dormant phenotypes (animal study) | Suppression of MARCKS gene | [172] |
| MSCs | Breast cancer cells | miR-100 | Suppressed angiogenesis (in vitro study) | Modulated the mTOR/HIF-1/VEGF signaling axis | [173] |
| WJ-MSCs | Bladder cancer cells | n.a | Inhibited proliferative viability and induced apoptosis (in vitro study) | Downregulated phosphorylation of Akt and upregulated cleaved Caspase 3 | [174] |
| BM-MSCs | Oral cancer | miR-101-3p | Inhibited proliferation, invasion, migration, and tumor growth (animal study) | Downregulated type X collagen gene | [175] |
| BM-MSCs | Leukemia THP-1 cells | miR-222-3p | Inhibited cell proliferation and promoted cell apoptosis (in vitro study) | Negatively regulating IRF2-INPP4B signaling | [176] |
| AT-MSCs | Ovarian A2780 and SKOV-3 cancer cells | miRNAs | Inhibited the proliferation and growth (in vitro study) | Upregulated pro-apoptotic proteins, as well as downregulated the anti-apoptotic protein | [177] |
Note: BM bone marrow, AT adipose tissue, UC umbilical cord, WJ Whartons jelly, miRs microRNAs, GC-MSCs gastric cancer-derived MSCs, MM multiple myeloma, HMEC human microvascular endothelial cells, HUVECs human umbilical vein endothelial cells, MARCKS myristoylated alanine-rich C-kinase substrate, IRF2-INPP4B interferon-regulatory factor 2 -inositol polyphosphate-4-phosphatase type-II, n.a not available
Tumor growth
Tumor progression and angiogenesis are related to a range of growth factor receptors and the activation of signaling pathways associated with these receptors. Stimulation or phosphorylation of the intracellular domain of these receptors leads to downstream signaling via Akt, PKC, and ERK kinase pathways which promote malignant cell proliferation and migratory phenotypes in tumor cells [179]. MSC exosomes are recognized to deliver their content to adjacent tumor cells and to affect tumor development with tumor-supporting or tumor-inhibiting mechanisms.
Tumor-supporting effects of MSC exosomes
Extensive research has related the transfer of tumor-associated factors present in MSC-derived exosomes to cancer cell proliferation promotion [37]. Vallabhaneni et al. indicated that exosomes secreted from stressed hMSCs stimulated the proliferation of breast cancer cells and metastases via the transmission of significant quantities of tumor-supportive miRNAs, such as miR-21 and miR-34a, and various proteins that are recognized as tumor-promoting factors [180]. Also, Dong et al. found that miR-410 from hUC-MSC-derived exosomes directly suppressed PTEN expression in lung adenocarcinoma cells and supported the growth of lung adenocarcinoma through enhanced proliferation and reduced apoptosis of these cells [181]. Genetic and protein composition has also been found to differ between multiple BM-MSC-derived exosomes and normal BM-MSC-derived exosomes. Lower levels of the tumor suppressor miR-15a and higher levels of adhesion molecules, oncogenic proteins, and cytokines in multiple myeloma BM-MSC-derived exosomes could promote the progression of multiple myeloma disorders, showing that the various origins of MSC-derived exosomes result in unexpected therapeutic outcome [159]. Moreover, Zhu et al. have demonstrated that MSC-derived exosomes increased the expression of VEGF in tumor cells through the activation of ERK1/2 and p38 MAPK pathways resulted in increased tumor growth and angiogenesis in vivo [182]. Other investigations revealed that human BM-MSC exosomes stimulated the Hedgehog (Hh) signaling pathway by influencing the expression of various components of this signaling cascade in osteosarcoma MG63 and gastric cancer SGC7901 cells and promoting tumor progression [162].
Tumor-inhibiting effects of MSC exosomes
Oppositely, the inhibitory effects of exosomes on tumor growth have also been reported. MiR-146b in MSC exosomes can suppress tumor growth in culture. MiR-146b from MSC exosomes binds to epidermal growth factor receptor (EGFR) mRNA, and eventually reduced growth, migration, and invasion of cancer cells in culture [168]. Intra-tumoral injection of MSC-derived exosomes containing miR-146b could also suppress glioma development in a rat primary brain tumor model [168]. Similarly, Takahara et al. discovered that AT-MSC exosomes greatly suppressed human prostate cancer cell proliferation by delivering miR-145 to these cells. Moreover, miR-145 as a tumor suppressor could inhibit the progression of prostate cancer and induce apoptosis in these cells via activating the caspase-3/7 mediated apoptosis pathway and suppressing the anti-apoptotic activity of Bcl-xL [183]. Likely, human WJ-MSC secretome showed anti-proliferative and apoptosis-inducing effects on human bladder cancer T24 cells in vitro and bladder cancer experimental models mediated by restraining phosphorylation of Akt protein kinase and upregulating p53/p21 and cleaved caspase 3 [174]. It seems that the differences in the periods of MSC expansion, the culture medium compounds, and the MSC passage number may act as influential factors that determine the consequence of the employment of exosomes for tumor therapy. Consistent outcomes in the performance and content of exosomes can therefore be obtained by monitoring the preparation conditions of MSCs [164, 179].
Tumor angiogenesis and metastasis
Neovascularization is known to be a critical biochemical step in tumor development and metastases. Both tumor and stromal cells in the tumor microenvironment participate a pivotal role in the regulation of tumor angiogenesis through the secretion of diverse signaling molecules and factors [184, 185]. Studies in different tumors have shown that exosomes produced from different cell types, in particular MSCs, could affect angiogenic response by targeting diverse molecules of angiogenetic signaling pathways. Latest experiments have shown that MSC-derived exosomes release components that act as angiogenic stimuli or angiogenic repressors. Thus, conflicting effects of MSC-derived exosomes on angiogenesis in different tumors may arise due to several elements, for example, the variance in the form of MSCs (i.e., BM-MSCs versus tissue-derived MSCs), the variability of MSC growth conditions, and the different dosage or interval of MSC infusion. Exosomes derived from MSCs have been shown to dramatically increase the expression of VEGF in tumor cells by activation of ERK1/2 and p38 MAPK pathways, sustaining tumor growth, and angiogenesis [182]. Also, AT-MSC-derived exosomes can deliver miR-125a as a proangiogenic factor to endothelial cells and consequently facilitate the development of endothelial tip cells and angiogenesis by suppressing the expression of repressing angiogenic inhibitor delta-like 4 (DLL4) expression [111]. Conversely, exosomal miR-16 has been reported to significantly reduce VEGF expression in breast cancer cells leading to the inhibition of angiogenesis in vitro and in vivo [169]. Additionally, Pakravan et al. revealed that the transfer of miR-100 from human BM-MSC-derived exosomes to breast cancer cells decreased VEGF expression and secretion by modulating the mTOR/HIF-1/VEGF signaling axis [173]. MSC-derived exosomes can also be used as an efficient modulating angiogenetic factor for tumor therapy.
Tumor metastases initiate with the separation of the tumor cells from the primary neoplasm and moving to the distant organ site, which includes a series of distinct multistep biological events. Any of these events are influenced by the biological and molecular alteration of cancer cells and the collaboration of stromal cells in the microenvironment of the tumor [186, 187]. Emerging results have confirmed that MSC-derived exosomes contribute to tumor metastases and the development of a pre-metastatic niche [188]. Studies in MCF-7 breast cancer cells revealed that exosomes secreted by AT-MSCs could successfully upregulate Wnt/-catenin signaling pathways and increase expressions of Wnt target genes such as Axin2 and Dickkopf-1 (Dkk1), thereby facilitating the progression and migration of cancer cells [161]. Similarly, MSC-derived exosomes promote the migration and invasion of HGC-27gastric cancer cells via enhancing the development of epithelial-mesenchymal transition (EMT) and boosting the expression of mesenchymal markers, and reducing the expression of epithelial markers in HGC-27 cells. It was further demonstrated that the expression of OCT4 and Lin28B increased in HGC-27 cells after treatment with MSC exosomes. Besides, these exosomes promoted the development and metastatic potential of HGC-27 cells by activating the Akt signaling pathway [163].
On the other hand, several reports are demonstrating that treatment with MSC-derived exosomes could prevent tumor migration and invasion. Shimbo et al. found that the transfer of miR-143 enriched exosomes into osteosarcoma cells may substantially alter the physiological features of osteosarcoma cells and suppress the migration ability of these cells [189]. Moreover, Ono et al. have demonstrated that the exosomal transfer of miR-23b from the BM-MSCs to breast cancer cells suppressed the target gene, myristoylated alanine-rich C-kinase substrate (MARCKS), which is responsible for promoting cell cycling and motility. Therefore, these findings suggest that exosomes maintained breast cancer cells in dormancy and prevents their recurrence [172]. In conclusion, these observations show that MSC-derived exosomes affect cell-to-cell communication from different pathways which may be essential for the modulation of tumor metastases.
Tumor cell-free therapy by MSC exosomes
Exosomes are recognized as promising natural nanovesicles for clinical therapeutic and diagnostic applications due to their effective biocompatibility and biodistribution properties. Further, engineered/modified exosomes can be used as effective therapeutic options for cancer because of their properties as natural important intercellular contact mediators and their innate ability in packaging and transition of therapeutic agents such as medicines, certain unique mRNAs, miRNAs, regulatory miRNAs, lipids, and proteins [190]. Over the past decades, the use of MSCs and their unlimited capacity in the development of exosomes as a nanocarrier in anticancer therapy have been extensively studied. It has been shown that the induction of MSCs to secrete exosomes loaded with antagomyR-222/223 and systemic administration of these exosomes could sensitize breast cancer cells to carboplatin-based therapy and enhance the cell dormancy of cancer cells to increase host survival in breast cancer [160]. Furthermore, it has been reported that MSC-derived exosomes loaded with anticancer protein, tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), elicited considerable apoptosis in a series of cell lines [191]. Besides, the use of exosomes as a natural drug delivery vehicle has several benefits over artificial delivery systems as exosomes are derived from natural cell supplies with low immunogenicity and reduced toxicity following delivery to the recipient cells. On the other hand, exosomes have a phospholipid bilayer structure that expresses ligands and receptors that can bind with specific ligands and receptors or target cell membranes and internalize their encapsulated contents to target cells. The small size of exosomes increases their ability for long circulation and thus inhibits deterioration or elimination of reticuloendothelial (RES) organs, enhances their extravasation from the blood vessels, and ultimately penetrates deep tissue. The capacity of exosomes in the sustained release of a specific amount of drugs increases their therapeutic potency due to extended circulation of drugs and their accumulation in the recipient cells [192, 193]. Meanwhile, MSC-released exosomes loaded with paclitaxel (PTX) demonstrate the potent anticancer activity by inhibiting the proliferative activity of the CFPAC-1 pancreatic cell line [194]. Also, it has been shown that PTX-loaded MSC-derived exosomes hindered the development of different tumor cell populations in vitro and obstructed the appearance of metastasis in primary tumors in vivo [195]. Despite the promising future of exosomes as a drug vehicle for the treatment of different forms of the tumor, the lack of consistent protocols for the production, isolation, and purification of exosomes leads to contradictory findings that limit their usage. Variable conditions in the MSC culture medium and the harvesting of exosomes, as well as the type of tumor, may affect the pro- or anti-tumor attributes of the MSC exosomes. These discrepancies in the features of MSC exosomes can vary the microenvironment of the tumor and the systemic environment of the host from tumor inhibition to tumor promotion, thereby affecting the outcome of treatment [37, 196]. Therefore, the achievement of favorable clinical effects with limited undesired toxicity requires further exploration in the field of engineering exosomes accompanied by advancement in the production and isolation of exosome techniques.
Conclusions and prospects
Identifying exosomes, in particular MSC exosomes, as a cell-free material that produce inherently beneficial therapeutic effects offers a new paradigm for their application in cancer therapy and regenerative medicine. Exosomes maintain the therapeutic benefit of their origin cells without stimulating the pivotal concerns such as possible tumorigenesis and unwanted gene mutation of MSC therapies. Besides, the therapeutic effects of MSC exosomes may be improved by genetically modified MSC exosomes aiming to express special ligands that direct them toward a favorable target and transfer small molecular drugs directly to the target sites as a drug delivery mechanism. Correspondingly, various clinical trials have been designed and conducted or are ongoing to address their safety, feasibility, and efficacy in the human (Table3); however, various issues remain challenging. First, various techniques for the purification, isolation, and characterization of MSC exosomes used in different laboratory studies can lead to inconsistent results. Further, the MSCs commonly have a limited capacity to produce exosomes on a large scale required to exert beneficial clinical effects. Also, the characterization of MSC exosomes in various subpopulations and precise content characterization of the cargo must be investigated because of the existence of heterogeneity in exosome contents, which in turn, may modify the effect of the intervention on the target tissue [197]. Moreover, to investigate MSC-EV as a therapeutic agent, an appropriate method for evaluating their mechanism of biodistribution and targeting, therapeutic efficiency, and manner and function of MSC-EV in vivo should be used. Appropriate safety profiles, on the other hand, must be developed to assess the optimal therapeutic dose and potential side effects of repeated administration [198]. Further, the progress of an exosome isolation protocol that meets good manufacturing practice (GMP) standards is of paramount importance [197]. In sum, investigation of the therapeutic application of MSC exosomes is still in the early stages and the precise functional mechanism of exosomes remains largely unclear. Therefore, further experimental research is also needed to establish the optimal therapeutic dose, interval, suitable route, and isolation methods along with optimizing the culture conditions of exosomes for reaching favorable therapeutic outcomes with minimal untoward effects in patients suffering from various organ disorders or cancers. Future research must be directed toward the design of a conventional isolation system as well as the development of a new generation of engineered MSCs capable of producing an improved amount of exosomes capable of transferring various efficient safe molecules for the treatment of human disorders.
Table 3.
Evaluation of MSC exosomes therapeutic potential in clinical trial registered in ClinicalTrials.gov (January 2021)
| Condition | Study phase | Participant number | Cell source | Cell type | Delivery route | Location | NCT number |
|---|---|---|---|---|---|---|---|
| ARDS | 1/2 | 169 | n.a | Allogeneic | Inhalation | China | NCT04602104 |
| COVID-19 | 1 | 24 | AT | Allogeneic | Inhalation | China | NCT04276987 |
| MODS | n.a | 60 | n.a | Allogeneic | IV | China | NCT04356300 |
| Pulmonary infection | 1/2 | 60 | AT | Allogeneic | Inhalation | China | NCT04544215 |
| AIS | 1/2 | 5 | n.a | Allogeneic | IP | Iran | NCT03384433 |
| Dry eye | 1/2 | 27 | UC | Allogeneic | Inhalation | China | NCT04213248 |
| Macular holes | 1 | 44 | n.a | Allogeneic | Intravitreal | China | NCT03437759 |
| Preeclampsia | n.a | 200 | UC | Allogeneic | n.a | Egypt | NCT03562715 |
| AD | 1/2 | 9 | AT | Allogeneic | Intranasal | China | NCT04388982 |
Note; ARDS acute respiratory distress syndrome, COVID-19 coronavirus disease 2019, MODS multiple organ dysfunction syndromes, AIS acute ischemic stroke, AD Alzheimer's diseases, AT adipose tissue, UC umbilical cord, IV intravenous, IP intraperitoneal, n.a not available
Acknowledgements
Not applicable.
Abbreviations
- MSCs
Mesenchymal stem/stromal cells
- mRNA
Messenger RNA
- miRNAs
MicroRNAs
- EVs
Extracellular vesicles
- MVBs
Multivesicular bodies
- PDGF
Platelet-derived growth factor
- FGF
Fibroblast growth factor
- EGF
Epidermal growth factor
Authors contributions
All authors contributed to the conception and the main idea of the work. A. H, H. S. R, A. M, J. J. E, A.O.Z, F. M, M. N, M. A. J. K, N. B, and M. S. C drafted the main text, figures, and tables. M. J supervised the work and provided the comments and additional scientific information. M.S.C, A.H, and M.J also reviewed and revised the text. All authors read and approved the final version of the work to be published.
Funding
No funders.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interests.
Footnotes
Publishers Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ali Hassanzadeh, Email: alihassanzadeh1369@yahoo.com.
Heshu Sulaiman Rahman, Email: Heshu.rhman@univsul.edu.iq.
Alexander Markov, Email: alexdoktor@inbox.ru.
Judi Januadi Endjun, Email: judijanuadi@gmail.com.
Angelina Olegovna Zekiy, Email: sfmsmu@mail.ru.
Max Stanley Chartrand, Email: chartrandmax@aol.com.
Nasrin Beheshtkhoo, Email: beheshtkhoo.nano@gmail.com.
Mohammad Amin Jadidi Kouhbanani, Email: aminjadidi1993@gmail.com.
Faroogh Marofi, Email: farooghmarofi@gmail.com.
Marzieh Nikoo, Email: mrz.nikoo@gmail.com.
Mostafa Jarahian, Email: mostafajarahian@gmail.com.
References
- 1.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22:617–624. doi: 10.1634/stemcells.22-4-617. [DOI] [PubMed] [Google Scholar]
- 3.Maumus M, Jorgensen C, Nol D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95:2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Shariati A, Nemati R, Sadeghipour Y, Yaghoubi Y, Baghbani R, Javidi K, Zamani M, Hassanzadeh A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: aA promising frontier. Eur J Cell Biol. 2020;99:151097. doi: 10.1016/j.ejcb.2020.151097. [DOI] [PubMed] [Google Scholar]
- 5.Tavakoli S, Ghaderi Jafarbeigloo HR, Shariati A, Jahangiryan A, Jadidi F, Jadidi Kouhbanani MA, Hassanzadeh A, Zamani M, Javidi K, Naimi A. Mesenchymal stromal cells; a new horizon in regenerative medicine. J Cell Physiol. 2020;235:9185–9210. doi: 10.1002/jcp.29803. [DOI] [PubMed] [Google Scholar]
- 6.Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, Pathak Y, Marofi F, Shamlou S, Hassanzadeh A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021;12:1–30. doi: 10.1186/s13287-021-02265-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Fatima F, Nawaz M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chinese J Cancer. 2015;34:1–13. doi: 10.1186/s40880-015-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Blanc K, Tammik L, Sundberg B, Haynesworth S, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 9.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 10.Marofi F, Hassanzadeh A, Solali S, Vahedi G, Mousavi Ardehaie R, Salarinasab S, et al. Epigenetic mechanisms are behind the regulation of the key genes associated with the osteoblastic differentiation of the mesenchymal stem cells: The role of zoledronic acid on tuning the epigenetic changes. J Cell Physiol. 2019;234:1510822. [DOI] [PubMed]
- 11.Schweizer MT, Wang H, Bivalacqua TJ, Partin AW, Lim SJ, Chapman C, Abdallah R, Levy O, Bhowmick NA, Karp JM. A phase I study to assess the safety and cancer-homing ability of allogeneic bone marrow-derived mesenchymal stem cells in men with localized prostate cancer. Stem Cells Transl Med. 2019;8:441–449. doi: 10.1002/sctm.18-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie C, Yang Z, Suo Y, Chen Q, Wei D, Weng X, Gu Z, Wei X. Systemically infused mesenchymal stem cells show different homing profiles in healthy and tumor mouse models. Stem Cells Transl Med. 2017;6:1120–1131. doi: 10.1002/sctm.16-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasr MB, Vergani A, Avruch J, Liu L, Kefaloyianni E, DAddio F, Tezza S, Corradi D, Bassi R, Valderrama-Vasquez A. Co-transplantation of autologous MSCs delays islet allograft rejection and generates a local immunoprivileged site. Acta Diabetol. 2015;52:917–927. doi: 10.1007/s00592-015-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Blanc K, Ringden O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18:586–591. doi: 10.1016/j.coi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.OConnor ML, Xiang D, Shigdar S, Macdonald J, Li Y, Wang T, Pu C, Wang Z, Qiao L, Duan W. Cancer stem cells: a contentious hypothesis now moving forward. Cancer Lett. 2014;344:180–187. doi: 10.1016/j.canlet.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med. 2019;8:1025. doi: 10.3390/jcm8071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giebel B, Kordelas L, Brger V. Clinical potential of mesenchymal stem/stromal cell-derived extracellular vesicles, Stem cell investigation, 4. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repaircurrent views. Stem cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 20.Gyrgy B, Szab TG, Pszti M, Pl Z, Misjk P, Aradi B, Lszl V, Pllinger E, Pap E, Kittel A. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis A. The origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 22.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 23.Lsser C, Alikhani VS, Ekstrm K, Eldh M, Paredes PT, Bossios A, Sjstrand M, Gabrielsson S, Ltvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:1–8. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nawaz M, Camussi G, Valadi H, Nazarenko I, Ekstrm K, Wang X, Principe S, Shah N, Ashraf NM, Fatima F. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol. 2014;11:688. doi: 10.1038/nrurol.2014.301. [DOI] [PubMed] [Google Scholar]
- 25.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 26.Camussi G, Deregibus M-C, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98. [PMC free article] [PubMed] [Google Scholar]
- 27.Nawaz M, Fatima F, Zanetti BR, de Lima Martins I, Schiavotelo NL, Mendes ND, Silvestre RN, Neder L. Microvesicles in gliomas and medulloblastomas: an overview, J Cancer Ther. 2014;18219.
- 28.Motavaf M, Pakravan K, Babashah S, Malekvandfard F, Masoumi M, Sadeghizadeh M. Therapeutic application of mesenchymal stem cell-derived exosomes: aA promising cell-free therapeutic strategy in regenerative medicine. Cell Mol Biol. 2016;62:74–79. doi: 10.14715/cmb/2016.62.14.13. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Qu Z, Fei ZW, Wu JH, Jiang CP. Role of stem cell-derived exosomes in cancer. Oncol Lett. 2017;13:2855–2866. doi: 10.3892/ol.2017.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., III Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 32.Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomed. 2019;14:2847. doi: 10.2147/IJN.S200036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 34.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et Biophys Acta (BBA)-Gen Subjects. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111:3100. doi: 10.1111/cas.14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DAlimonte I, Lannutti A, Pipino C, Di Tomo P, Pierdomenico L, Cianci E, Antonucci I, Marchisio M, Romano M, Stuppia L. Wnt signaling behaves as a master regulator in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev Rep. 2013;9:642–654. doi: 10.1007/s12015-013-9436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 40.Kim J-A, Choi H-K, Kim T-M, Leem S-H, Oh I-H. Regulation of mesenchymal stromal cells through fine tuning of canonical Wnt signaling. Stem Cell Res. 2015;14:356–368. doi: 10.1016/j.scr.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Ju Z, Ma J, Wang C, Yu J, Qiao Y, Hei F. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation. 2017;40:486–496. doi: 10.1007/s10753-016-0494-0. [DOI] [PubMed] [Google Scholar]
- 42.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:1–11. doi: 10.1186/s13287-019-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brgger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 45.Alenquer M, Amorim MJ. Exosome biogenesis, regulation, and function in viral infection. Viruses. 2015;7:5066–5083. doi: 10.3390/v7092862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, Rong Y, Chuang Y-S, Peng D, Emr SD. Ubiquitin-dependent lysosomal membrane protein sorting and degradation. Mol Cell. 2015;57:467–478. doi: 10.1016/j.molcel.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Piper RC, Luzio JP. Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr Opin Cell Biol. 2007;19:459–465. doi: 10.1016/j.ceb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowal J, Tkach M, Thry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Gorabi AM, Kiaie N, Barreto GE, Read MI, Tafti HA, Sahebkar A. The therapeutic potential of mesenchymal stem cellderived exosomes in treatment of neurodegenerative diseases. Mol Neurobiol. 2019;56:815767. [DOI] [PubMed]
- 52.Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, Egger D. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front Bioeng Biotechnol. 2019;7:292. doi: 10.3389/fbioe.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, Tak S, Lefebvre O, Schwab Y, Goetz JG. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211:27–37. doi: 10.1083/jcb.201504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alcayaga-Miranda F, Varas-Godoy M, Khoury M. Harnessing the angiogenic potential of stem cell-derived exosomes for vascular regeneration. Stem Cells Int. 2016:3409169. [DOI] [PMC free article] [PubMed]
- 55.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 56.Kahroba H, Hejazi MS, Samadi N. Exosomes: from carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell Mol Life Sci. 2019;76:1747–1758. doi: 10.1007/s00018-019-03035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramos TL, Snchez-Abarca LI, Muntin S, Preciado S, Puig N, Lpez-Ruano G, Hernndez-Hernndez , Redondo A, Ortega R, Rodrguez C. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Sign. 2016;14:1–14. doi: 10.1186/s12964-015-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beckler MD, Higginbotham JN, Franklin JL, Ham A-J, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:1–9. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD, Clayton A. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9:1324–1338. doi: 10.1074/mcp.M000063-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frydrychowicz M, Kolecka-Bednarczyk A, Madejczyk M, Yasar S, Dworacki G. Exosomesstructure, biogenesis and biological role in non-small-cell lung cancer. Scand J Immunol. 2015;81:2–10. doi: 10.1111/sji.12247. [DOI] [PubMed] [Google Scholar]
- 65.Thry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 66.Bissig C, Gruenberg J. Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harbor Perspectives Biol. 2013;5:a016816. doi: 10.1101/cshperspect.a016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes derived from AKt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med. 2017;6:51–59. doi: 10.5966/sctm.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen B, Liu J, ZhangF, WangY, Qin Y, Zhou Y, Qiu J, Fan Y. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016;1240301. [DOI] [PMC free article] [PubMed]
- 69.Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, Vykoukal JV, Ruppert KA, Cox CS, Jr, Olson SD. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36:79–90. doi: 10.1002/stem.2730. [DOI] [PubMed] [Google Scholar]
- 70.Konala VBR, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13–24. doi: 10.1016/j.jcyt.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gusachenko O, Zenkova M, Vlassov V. Nucleic acids in exosomes: disease markers and intercellular communication molecules. Biochem (Moscow) 2013;78:1–7. doi: 10.1134/S000629791301001X. [DOI] [PubMed] [Google Scholar]
- 73.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018;9:320. doi: 10.1186/s13287-018-1069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Lanzn MP, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N. Human bone marrow-and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silva J, Garca V, Zaballos , Provencio M, Lombarda L, Almonacid L, Garca JM, Domnguez G, Pea C, Diaz R, Herrera M, Varela A, Bonilla F. Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur Respir J. 2011;37:617–623. doi: 10.1183/09031936.00029610. [DOI] [PubMed] [Google Scholar]
- 76.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 77.Gardiner C, Vizio DD, Sahoo S, Thry C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeo Y, Wee R. Efficiency of exosome production correlates inversely with the developmental maturity of MSC donor. 2013. [Google Scholar]
- 79.Zhang M, Jin K, Gao L, Zhang Z, Li F, Zhou F, Zhang L. Methods and technologies for exosome isolation and characterization. Small Methods. 2018;2:1800021. doi: 10.1002/smtd.201800021. [DOI] [Google Scholar]
- 80.Yamashita T, Takahashi Y, Nishikawa M, Takakura Y. Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood circulation. Eur J Pharmaceutics Biopharm. 2016;98:1–8. doi: 10.1016/j.ejpb.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 81.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bing AN, Van Der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3:23430. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protocols Cell Biol. 2006;30:3.22. 21–23.22. 29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 84.R.C. Lai, R.W.Y. Yeo, S.K. Lim, Mesenchymal stem cell exosomes, in: Seminars in cell & developmental biology, Elsevier, 2015, pp. 82-88. [DOI] [PubMed]
- 85.Wei X, Yang X, Han Z-p, Qu F-f, Shao L, Shi Y-f. Mesenchymal stem cells: a new trend for cell therapy. Acta pharmacol Sinica. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baba MA, Bouchriti Y, Achbani A, Kharbach A, Sine H, Naciri A. Risk of COVID-19 for patients with cancer: a narrative overview. Eur J Med Educ Technol. 2020;13:em2008. doi: 10.30935/ejmets/8257. [DOI] [Google Scholar]
- 87.Salmenkari H, Laitinen A, Forsgrd RA, Holappa M, Linden J, Pasanen L, Korhonen M, Korpela R, Nystedt J. The use of unlicensed bone marrowderived platelet lysateexpanded mesenchymal stromal cells in colitis: a pre-clinical study. Cytotherapy. 2019;21:175–188. doi: 10.1016/j.jcyt.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 88.Laso-Garca F, Ramos-Cejudo J, Carrillo-Salinas FJ, Otero-Ortega L, Feli A, Gmez-de Frutos M, Mecha M, Dez-Tejedor E, Guaza C, Gutirrez-Fernndez M. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. Plos One. 2018;13:e0202590. doi: 10.1371/journal.pone.0202590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ju G-q, Cheng J, Zhong L, Wu S, Zou X-y, Zhang G-y, Gu D, Miao S, Zhu Y-j, Sun J. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. Plos one. 2015;10:e0121534. doi: 10.1371/journal.pone.0121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, Ashraf M, Xu M. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. Plos one. 2013;8:e73304. doi: 10.1371/journal.pone.0073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, Zhou F, Yang X, Yang J, Zeng C. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2017;1863:2085–2092. doi: 10.1016/j.bbadis.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 92.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 93.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 2015;37:2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 94.Zhu L-P, Tian T, Wang J-Y, He J-N, Chen T, Pan M, Xu L, Zhang H-x, Qiu X-T, Li C-C. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L, Lu F-b, Chen D-z, Wu J-l, Xu L-m, Zheng M-h, Li H, Huang Y, Jin X-y, Gong Y-w. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol. 2018;93:38–46. doi: 10.1016/j.molimm.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 96.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25:465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tan CY, Lai RC, Wong W, Dan YY, Lim S-K, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tamura R, Uemoto S, Tabata Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflammation Regen. 2016;36:26. doi: 10.1186/s41232-016-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mardpour S, Ghanian MH, Sadeghi-Abandansari H, Mardpour S, Nazari A, Shekari F, Baharvand H. Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl Mater Interfaces. 2019;11:37421–37433. doi: 10.1021/acsami.9b10126. [DOI] [PubMed] [Google Scholar]
- 100.Haga H, Yan IK, Takahashi K, Matsuda A, Patel T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cells Transl Med. 2017;6:1262–1272. doi: 10.1002/sctm.16-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boni R, Ali A, Shavandi A, Clarkson AN. Current and novel polymeric biomaterials for neural tissue engineering. J Biomed Sci. 2018;25:90. doi: 10.1186/s12929-018-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, Zhou Z, Zhou Z, Xu T, Jiang T. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36:469–484. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- 104.Ma Y, Dong L, Zhou D, Li L, Zhang W, Zhen Y, Wang T, Su J, Chen D, Mao C. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med. 2019;23:2822–2835. doi: 10.1111/jcmm.14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mao Q, Nguyen PD, Shanti RM, Shi S, Shakoori P, Zhang Q, Le AD. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng Part A. 2019;25:887–900. doi: 10.1089/ten.tea.2018.0176. [DOI] [PubMed] [Google Scholar]
- 106.Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y, Hu J, Jia P, Teng J, Ding X. miR-21 protects against ischemia/reperfusion-induced acute kidney injury by preventing epithelial cell apoptosis and inhibiting dendritic cell maturation. Front Physiol. 2018;9:790. doi: 10.3389/fphys.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human Whartons Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:1–13. doi: 10.1186/scrt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang Z-z, Liu Y-m, Niu X, Yin J-y, Hu B, Guo S-c, Fan Y, Wang Y, Wang N-s. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. doi: 10.1186/s13287-016-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eirin A, Zhu X-Y, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cellderived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114–124. doi: 10.1016/j.kint.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182–2189. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- 112.Ahn SY, Park WS, Kim YE, Sung DK, Sung SI, Ahn JY, Chang YS. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50:1–12. doi: 10.1038/s12276-018-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang R, Qin C, Wang J, Hu Y, Zheng G, Qiu G, Ge M, Tao H, Shu Q, Xu J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging. 2019;11:7996. doi: 10.18632/aging.102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.J. wei Li, L. Wei, Z. Han, Z. Chen, Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p, Eur J Pharm. 2019: 852;68-76. [DOI] [PubMed]
- 115.Monsel A, Zhu Y-g, Gennai S, Hao Q, Hu S, Rouby J-J, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic effects of human mesenchymal stem cellderived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, OKane CM, Krasnodembskaya AD. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J-y, An R, Liu Z-j, Wang J-j, Chen S-z, Hong M-m, Liu J-h, Xiao M-y, Chen Y-f. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacol Sinica. 2014;35:1121–1128. doi: 10.1038/aps.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yeo RWY, Lai RC, Tan KH, Lim SK. Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell. Exosomes Microvesicles. 2013;1:7. [Google Scholar]
- 119.Kunter U, Rong S, Boor P, Eitner F, Mller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Ame Soc Nephrol. 2007;18:1754–1764. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- 120.Assuno-Silva RC, Mendes-Pinheiro B, Patrcio P, Behie LA, Teixeira FG, Pinto L, Salgado AJ. Exploiting the impact of the secretome of MSCs isolated from different tissue sources on neuronal differentiation and axonal growth. Biochimie. 2018;155:83–91. doi: 10.1016/j.biochi.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 121.Pires AO, Mendes-Pinheiro B, Teixeira FG, Anjo SI, Ribeiro-Samy S, Gomes ED, Serra SC, Silva NA, Manadas B, Sousa N. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis. Stem Cells Dev. 2016;25:1073–1083. doi: 10.1089/scd.2016.0048. [DOI] [PubMed] [Google Scholar]
- 122.Hoang DH, NguyenTD, Nguyen HP, Nguyen XH, Do PTX, Dang VD, Dam PTM, Bui HTH, Trinh MQ, Vu DM. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum-and xeno-free condition, Front Mol Biosci. 2020;7:119. [DOI] [PMC free article] [PubMed]
- 123.Wang Z-g, He Z-y, Liang S, Yang Q, Cheng P, Chen A-m. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:1–11. doi: 10.1186/s13287-019-1471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak M. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 125.Gradilla A-C, Gonzlez E, Seijo I, Andrs G, Bischoff M, Gonzlez-Mendez L, Snchez V, Callejo A, Ibez C, Guerra M. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun. 2014;5:1–13. doi: 10.1038/ncomms6649. [DOI] [PubMed] [Google Scholar]
- 126.McBride JD, Rodriguez-Menocal L, Guzman W, Candanedo A, Garcia-Contreras M, Badiavas EV. Bone marrow mesenchymal stem cell-derived CD63+ exosomes transport Wnt3a exteriorly and enhance dermal fibroblast proliferation, migration, and angiogenesis in vitro. Stem Cells Dev. 2017;26:1384–1398. doi: 10.1089/scd.2017.0087. [DOI] [PubMed] [Google Scholar]
- 127.Ranghino A, Bruno S, Bussolati B, Moggio A, Dimuccio V, Tapparo M, Biancone L, Gontero P, Frea B, Camussi G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther. 2017;8:1–15. doi: 10.1186/s13287-017-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ho JH, Chen Y-F, Ma W-H, Tseng T-C, Chen M-H, Lee OK. Cell contact accelerates replicative senescence of human mesenchymal stem cells independent of telomere shortening and p53 activation: roles of Ras and oxidative stress. Cell Transplant. 2011;20:1209–1220. doi: 10.3727/0963689109X546562. [DOI] [PubMed] [Google Scholar]
- 129.Xin H, Wang F, Li Y, Lu Q-e, Cheung WL, Zhang Y, Zhang ZG, Chopp M. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant. 2017;26:243–257. doi: 10.3727/096368916X693031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yin L, Liu X, Shi Y, Ocansey DKW, Hu Y, Li X, Zhang C, Xu W, Qian H. Therapeutic advances of stem cell-derived extracellular vesicles in regenerative medicine. Cells. 2020;9:707. doi: 10.3390/cells9030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci. 2000;97:3213–3218. doi: 10.1073/pnas.97.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens. 2010;19:7–12. doi: 10.1097/MNH.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- 133.Eldh M, Ekstrm K, Valadi H, Sjstrand M, Olsson B, Jerns M, Ltvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PloS one. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Valadi H, Ekstrm K, Bossios A, Sjstrand M, Lee JJ, Ltvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 135.Vishnubhatla I, Corteling R, Stevanato L, Hicks C, Sinden J. The development of stem cell-derived exosomes as a cell-free regenerative medicine. J Circ Biomark. 2014;3:3–2. doi: 10.33393/jcb.2014.2043. [DOI] [Google Scholar]
- 136.Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 137.Beltrami AP, Urbanek K, Kajstura J, Yan S-M, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 138.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marbn E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.A.P.J.B.M.I.T.F.J.K.M.N.Y.K. Marcin Wysoczynski1, Hong Li1 & Roberto Bolli1, Pro-angiogenic actions of CMC-derived extracellular vesicles rely on selective packaging of angiopoietin 1 and 2, but not FGF-2 and VEGF, Stem Cell Rev Rep. 2019: 15;530542. [DOI] [PMC free article] [PubMed]
- 140.Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:1–12. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 142.Bodart-Santos V, de Carvalho LR, de Godoy MA, Batista AF, Saraiva LM, Lima LG, Abreu CA, De Felice FG, Galina A, Mendez-Otero R. Extracellular vesicles derived from human Whartons jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid- oligomers. Stem Cell Res Ther. 2019;10:1–13. doi: 10.1186/s13287-019-1432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen SY, Lin MC, Tsai JS, He PL, Luo WT, Herschman H, Li HJ. EP4 antagonist-elicited extracellular vesicles from mesenchymal stem cells rescue cognition/learning deficiencies by restoring brain cellular functions. Stem Cells Transl Med. 2019;8:707–723. doi: 10.1002/sctm.18-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Y, Cui J, Wang H, Hezam K, Zhao X, Huang H, Chen S, Han Z, Han Z-C, Guo Z. Enhanced therapeutic effects of MSC-derived extracellular vesicles with an injectable collagen matrix for experimental acute kidney injury treatment. Stem Cell Res Ther. 2020;11:1–12. doi: 10.1186/s13287-019-1471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nargesi AA, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017;8:1–12. doi: 10.1186/s13287-016-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen L, Wang Y, Li S, Zuo B, Zhang X, Wang F, Sun D. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics. 2020;10:9425. doi: 10.7150/thno.43315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang Z-z, Liu Y-m, Niu X, Yin J-y, Hu B, Guo S-c, Fan Y, Wang Y, Wang N-s. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:1–13. doi: 10.1186/s13287-016-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:1–13. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bogan DR. A snapshot of racial and geographic distribution of lung and bronchus cancer incidence and mortality in Mississippi, 2008-2012. Eur J Environ Public Health. 2017;1:01. doi: 10.20897/ejeph.201701. [DOI] [Google Scholar]
- 150.Wang H, Zheng R, Chen Q, Shao J, Yu J, Hu S. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF) Stem Cell Res Ther. 2017;8:1–10. doi: 10.1186/s13287-016-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ridzuan N, Zakaria N, Widera D, Sheard J, Morimoto M, Kiyokawa H, Isa SAM, Singh GKC, Then K-Y, Ooi G-C. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD) Stem Cell Res Ther. 2021;12:1–21. doi: 10.1186/s13287-020-02088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harrell CR, Miloradovic D, Sadikot R, Fellabaum C, Markovic BS, Miloradovic D, Acovic A, Djonov V, Arsenijevic N, Volarevic V. Molecular and cellular mechanisms responsible for beneficial effects of mesenchymal stem cell-derived product Exo-d-MAPPS in attenuation of chronic airway inflammation. Anal Cell Pathol. 2020;2020:3153891. doi: 10.1155/2020/3153891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Corcoran C, Rani S, OBrien K, ONeill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. Plos one. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 157.Nomoto-Kojima N, Aoki S, Uchihashi K, Matsunobu A, Koike E, Ootani A, Yonemitsu N, Fujimoto K, Toda S. Interaction between adipose tissue stromal cells and gastric cancer cells in vitro. Cell Tissue Res. 2011;344:287–298. doi: 10.1007/s00441-011-1144-3. [DOI] [PubMed] [Google Scholar]
- 158.Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 159.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai Y-T, Reagan M, Azab F, Flores LM, Campigotto F, Weller E. BM mesenchymal stromal cellderived exosomes facilitate multiple myeloma progression. J Clin Investig. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, Isenalumhe LL, Greco SJ, Ayer S, Bryan M. Mesenchymal stem cellderived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 2016;76:5832–5844. doi: 10.1158/0008-5472.CAN-16-1092. [DOI] [PubMed] [Google Scholar]
- 161.Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383:13–20. doi: 10.1007/s11010-013-1746-z. [DOI] [PubMed] [Google Scholar]
- 162.Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y, Li Y, Chen H, Yang L, Zhu H, Li Y. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem. 2017;42:2242–2254. doi: 10.1159/000479998. [DOI] [PubMed] [Google Scholar]
- 163.Gu H, Ji R, Zhang X, Wang M, Zhu W, Qian H, Chen Y, Jiang P, Xu W. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol Med Reports. 2016;14:3452–3458. doi: 10.3892/mmr.2016.5625. [DOI] [PubMed] [Google Scholar]
- 164.Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L, Alessandri G, Pessina A, Perrotta A, Fierabracci A. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther. 2015;15:495–504. doi: 10.1517/14712598.2015.997706. [DOI] [PubMed] [Google Scholar]
- 165.Obrien K, Khan S, Gilligan K, Zafar H, Lalor P, Glynn C, OFlatharta C, Ingoldsby H, Dockery P, De Bhulbh A. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37:2137–2149. doi: 10.1038/s41388-017-0116-9. [DOI] [PubMed] [Google Scholar]
- 166.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, Wang S, Wu X, Yang T, Huang F. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110:1199–1210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, Wang M, Zhu W, Qian H, Xu W. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14:2473–2483. doi: 10.1080/15384101.2015.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee J-K, Park S-R, Jung B-K, Jeon Y-K, Lee Y-S, Kim M-K, Kim Y-G, Jang J-Y, Kim C-W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. Plos one. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin HD, Fong CY, Biswas A, Choolani M, Bongso A. Human Wharton's jelly stem cells, its conditioned medium and cell-free lysate inhibit the growth of human lymphoma cells. Stem Cell Rev Rep. 2014;10:573–586. doi: 10.1007/s12015-014-9514-3. [DOI] [PubMed] [Google Scholar]
- 171.Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:1–11. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R-u, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 173.Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1/VEGF signaling axis in breast cancer cells. Cell Oncol. 2017;40:457–470. doi: 10.1007/s13402-017-0335-7. [DOI] [PubMed] [Google Scholar]
- 174.Wu S, Ju G-Q, Du T, Zhu Y-J, Liu G-H. Microvesicles derived from human umbilical cord Whartons jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. Plos one. 2013;8:e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xie C, Du L-Y, Guo F, Li X, Cheng B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol Cell Biochem. 2019;458:11–26. doi: 10.1007/s11010-019-03526-7. [DOI] [PubMed] [Google Scholar]
- 176.Zhang F, Lu Y, Wang M, Zhu J, Li J, Zhang P, et al. Exosomes derived from human bone marrow mesenchymal stem cells transfer miR-222-3p to suppress acute myeloid leukemia cell proliferation by targeting IRF2/INPP4B. Mol Cell Probes. 2020;51:101513. [DOI] [PubMed]
- 177.Reza AMMT, Choi Y-J, Yasuda H, Kim J-H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 2016;6:1–15. doi: 10.1038/srep38498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Buhmeida A, Dallol A, Merdad A, Al-Maghrabi J, Gari MA, Abu-Elmagd MM, Chaudhary AG, Abuzenadah AM, Nedjadi T, Ermiah E. High fibroblast growth factor 19 (FGF19) expression predicts worse prognosis in invasive ductal carcinoma of breast. Tumor Biol. 2014;35:2817–2824. doi: 10.1007/s13277-013-1374-y. [DOI] [PubMed] [Google Scholar]
- 179.Sharma A. Role of stem cell derived exosomes in tumor biology. Int J Cancer. 2018;142:1086–1092. doi: 10.1002/ijc.31089. [DOI] [PubMed] [Google Scholar]
- 180.Vallabhaneni KC, Penfornis P, Dhule S, Guillonneau F, Adams KV, Mo YY, Xu R, Liu Y, Watabe K, Vemuri MC. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6:4953. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dong L, Pu Y, Zhang L, Qi Q, Xu L, Li W, Wei C, Wang X, Zhou S, Zhu J. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9:1–13. doi: 10.1038/s41419-017-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 183.Takahara K, Ii M, Inamoto T, Nakagawa T, Ibuki N, Yoshikawa Y, Tsujino T, Uchimoto T, Saito K, Takai T. microRNA-145 mediates the inhibitory effect of adipose tissue-derived stromal cells on prostate cancer. Stem Cells Dev. 2016;25:1290–1298. doi: 10.1089/scd.2016.0093. [DOI] [PubMed] [Google Scholar]
- 184.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 185.Kerbel RS. Tumor angiogenesis. New Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 187.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med. 2013;91:431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S, Ochi M. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445:381–387. doi: 10.1016/j.bbrc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 190.Syn NL, Wang L, Chow EK-H, Lim CT, Goh B-C. Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35:665–676. doi: 10.1016/j.tibtech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 191.Yuan Z, Kolluri KK, Gowers KH, Janes SM. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles. 2017;6:1265291. doi: 10.1080/20013078.2017.1265291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fitts CA, Ji N, Li Y, Tan C. Exploiting exosomes in cancer liquid biopsies and drug delivery. Adv Healthcare Mater. 2019;8:1801268. doi: 10.1002/adhm.201801268. [DOI] [PubMed] [Google Scholar]
- 193.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes, Science, 367. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pascucci L, Cocc V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Vigan L, Locatelli A, Sisto F, Doglia SM. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 195.Melzer C, Rehn V, Yang Y, Bhre H, von der Ohe J, Hass R. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers. 2019;11:798. doi: 10.3390/cancers11060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.T. Lener, M. Gimona, L. Aigner, V. Brger, E. Buzas, G. Camussi, N. Chaput, D. Chatterjee, F.A. Court, H.A.d. Portillo, Applying extracellular vesicles based therapeutics in clinical trialsan ISEV position paper. J extracell Vesicles. 2015:4;30087. [DOI] [PMC free article] [PubMed]
- 197.Forsberg MH, Kink JA, Hematti P, Capitini CM. Mesenchymal Stromal Cells and Exosomes: Progress and Challenges. Front Cell Dev Biol. 2020;8:665. doi: 10.3389/fcell.2020.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol. 2020;8:149. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.