Abstract
Background
Intraoperative imaging is frequently made use of in Orthopaedic surgery. Historically, conventional 2-dimensional fluoroscopy has been extensively used for this purpose. However, 2D imaging falls short when it is required to visualise complex anatomical regions such as pelvis, spine, foot and ankle etc. Intraoperative 3D imaging was introduced to counter these limitations, and is increasingly being employed in various sub-specialities of Orthopaedic Surgery.
Objectives
This review aims to outline the clinical and radiological outcomes of surgeries done under the guidance of intraoperative 3D imaging and compare them to those done under conventional 2D fluoroscopy.
Methods
Three electronic databases (PubMed, Embase and Scopus) were searched for relevant studies that directly compared intraoperative 3D imaging with conventional fluoroscopy. Case series on intraoperative 3D imaging were also included for qualitative synthesis. The outcomes evaluated included accuracy of implant placement, mean surgical duration and rate of revision surgery due to faulty implants.
Results
A total of 31 studies from sub-specialities of spine surgery, pelvi-acetabular surgery, foot and ankle surgery and trauma surgery, having data on a total of 658 patients were analysed. The study groups which had access to intraoperative 3D imaging was found to have significantly increased accuracy of implant positioning (Odds Ratio 0.35 [0.20, 0.62], p = 0.0002) without statistically significant difference in mean surgical time (p = 0.57). Analysis of the studies that included clinical follow up showed that the use of intraoperative 3D imaging led to a significant decrease in the need for revision surgeries due to faulty implant placement.
Conclusion
There is sufficient evidence that the application of intraoperative 3D imaging leads to precise implant positioning and improves the radiological outcome. Further research in the form of prospective studies with long term follow up is required to determine whether this superior radiological outcome translates to better clinical results in the long run.
Keywords: Intraoperative computed tomography, Intraoperative CT, iCT, Intraoperative 3D imaging, 3D C-Arm, 3D fluoroscopy, Orthopaedics, Spine surgery
1. Introduction
Intraoperative radiography is a key element of Orthopaedic surgical practice. Classically, this involved the use of two-dimensional imaging using fluoroscopy. This method, however may not be adequate when dealing with complex anatomical regions.
Recent advancements in Computer Tomography (CT) technology have allowed for the use of it intraoperatively, thus opening up new horizons in advanced and accurate surgical techniques. This could be based on either a C-Arm which rotates automatically around the region of interest with the help of a motor, acquiring a large number of images in the process (Siemens Arcadis Orbic/Siremobil, Ziehm 3D Vision), or the recently introduced mobile intraoperative CT scanner. In Orthopaedic surgery, the main objectives of intraoperative CT (iCT) are the confirmation of anatomic reduction and accurate placement of implants, for which it may be used as a part of an image guided navigation system. This technique has found wide applications and is currently used most extensively in spine surgery. It is also being employed in pelvi-acetabular surgery, onco-surgery, foot and ankle surgery, and in the treatment of intra-articular fractures.1, 2, 3, 4 Its application is also likely to expand to other areas in the future. This article aims to describe in detail the current utility, pros and cons of iCT scan in Orthopaedic practice and compare it with conventional fluoroscopy.
2. Materials and methods
2.1. Protocol and registration
The protocol for this systematic review was submitted for pre-registration in medRxiv.org bearing the ID MEDRXIV/2021/252670.5
2.2. Search methodology
This review was conducted conforming to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines (flowchart depicted in Fig. 1). A literature search was conducted on 22/01/2021 using the search string “Intraoperative AND CT OR (computed tomography) AND Orthopaedics” in the following electronic databases: PubMed, Embase and Scopus. The search included articles published from inception till date of search. A total of n = 254 articles were obtained. Duplicates and studies in languages other than English were excluded and n = 133 articles were selected. Furthermore, a manual search was conducted on the bibliography of the included studies for potentially suitable articles.
Fig. 1.
Prisma flowchart.
2.3. Inclusion and exclusion criteria
All articles that directly compared the outcomes of iCT with conventional fluoroscopy were included. Relevant published case series that reported on the application of iCT were also included. Case reports, case series without appropriate data on the outcomes of iCT, Conference abstracts, review articles and editorials were excluded. Articles that were published in languages other than English were also excluded.
2.4. Data collection and analysis
The title and abstract of the articles that were extracted were screened by two authors separately (VK and VB) based on the inclusion and exclusion criteria. In the course of screening, if there was any uncertainty regarding the merit of any article, the full text was obtained and evaluated. Any difference of opinion was clarified by discussion between the authors.
Data extracted from the selected studies, including the author, year of publication, study design, make and model of intraoperative CT scanner used, demographic characteristics, and various outcome parameters were compiled in an excel sheet. A meta-analysis was then performed using Review Manager (RevMan [Computer program] Version 5.4.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
2.5. Risk of bias assessment
Risk of bias in all the included studies were assessed using MINORS tool.6 All 15 non-comparative studies had a score of 10 or above out of a total 16, and all the 16 comparative studies had 16 and above scores out of 24. The summary of risk of bias of individual studies are as shown in Figs. 1 and 2 in supplementary data. The studies having data for mean surgical time and rate of revision surgery were found to be relatively free of publication bias, showing uniform distribution of data on either side of the mean (funnel plot-Figs. 3 and 4, supplementary data). Studies included for calculating accuracy of implant positioning showed some evidence of publication/short study bias evident from skewing of the funnel plot (shown in Fig. 5, supplementary data).
3. Results
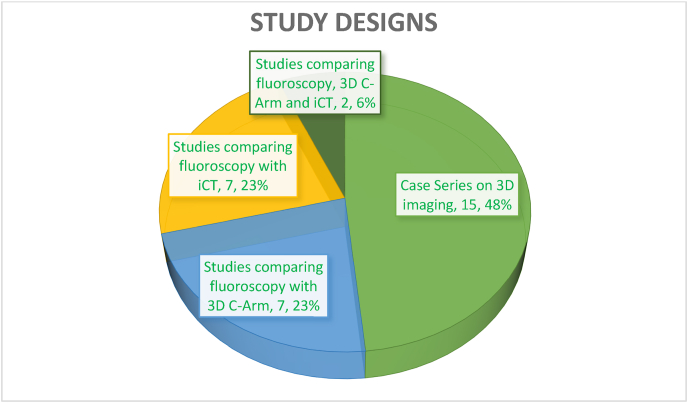
A total of 31 studies were included in the final review (Table 1 in supplementary materials). The various sub-specialities in which 3D imaging was employed included spine surgery (n = 22 studies), Pelviacetabular surgery (n = 2), Foot and ankle surgery (n = 4) and trauma surgery (n = 3). The study designs of included studies are as depicted in Fig. 2.
Fig. 2.
Types of studies included.
Fig. 3 outlines the year of publication of the articles and illustrates an increasing trend of usage of intraoperative 3D imaging in recent years.
Fig. 3.
Year of publication of included studies.
The various 3D imaging devices that were made use of are listed in Table 1.
Table 1.
Make and model of 3D imaging appliances used in the included articles.
| Make and Model | Number of studies |
|---|---|
| O-Arm, Medtronic, USA | 15 |
| Iso-C 3D, Siemens, Germany | 4 |
| Airo, BrainLab, Germany | 3 |
| Somatom, Siemens, Germany | 2 |
| Arcadis Orbic 3D, Siemens, Germany | 1 |
| BodyTom, NeuroLogica- Samsung, USA | 1 |
| CereTom, NeuroLogica- Samsung, USA | 1 |
| CIOS Spin, Siemens, Germany | 1 |
| LightSpeed VCT, GE Healthcare, USA | 1 |
| Spine and Trauma iCT, BrainLab, Germany | 1 |
| Toshiba Medical systems iCT, Toshiba, Japan | 1 |
Studies based on both 3D C-Arm imaging and portable intraoperative CT scanners were initially considered together (henceforth referred to as “iCT”) and were compared with conventional fluoroscopy. Twelve studies (658 patients; 3686 implants) were found to have appropriate data directly comparing the accuracy of conventional fluoroscopy with 3D imaging. The result of meta-analysis of these studies are depicted in the forest plot shown in Fig. 4, which shows that the application of iCT was found to significantly improve the accuracy of implant positioning (Odds Ratio 0.35 [0.20, 0.62], p = 0.0002). On independent comparison, both 3D C-Arms and intraoperative CT were separately found to be superior to conventional fluoroscopy in terms of accuracy of implant placement, but this difference was found to be non-significant in the case of 3D C-Arms (p = 0.08). Intraoperative CT demonstrated the highest level of accuracy (Odds Ratio 0.24 [0.14, 0.42], p < 0.00001) (Forest plots given in Figs. 6 and 7, supplementary data).
Fig. 4.
Forest plot comparing the accuracy of implant positioning between conventional fluoroscopy and iCT.
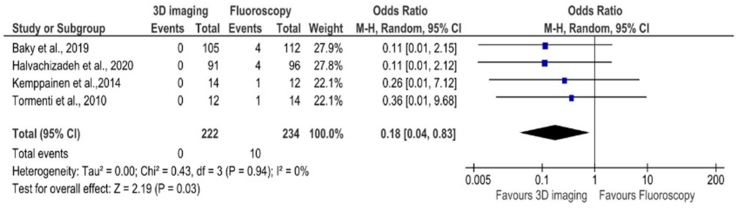
Only 10 out of the 31 studies had followed up the patients to evaluate clinical outcome. On analysing the studies which has appropriate comparative data, the need for revision surgeries due to mal-positioned implants were significantly lower in the groups that had access to iCT (Odds Ratio 0.18 [0.04, 0.83] p = 0.03) (Fig. 5).
Fig. 5.
Forest plot comparing the revision rates of surgeries in which iCT was employed to those done using conventional fluoroscopy.
For studies that made use of an iCT scan after reduction or implant placement, in 13.5% cases, the iCT scan findings led to alteration of surgical procedure or intraoperative revision of the implant.3,7, 8, 9, 10, 11
The mean surgical time was found to be higher for surgeries in which intraoperative 3D imaging was utilised (mean difference 4.19 min) but this was not statistically significant (p = 0.57). The average blood loss could not be compared as an insufficient number of studies had reported on the same.
4. Discussion
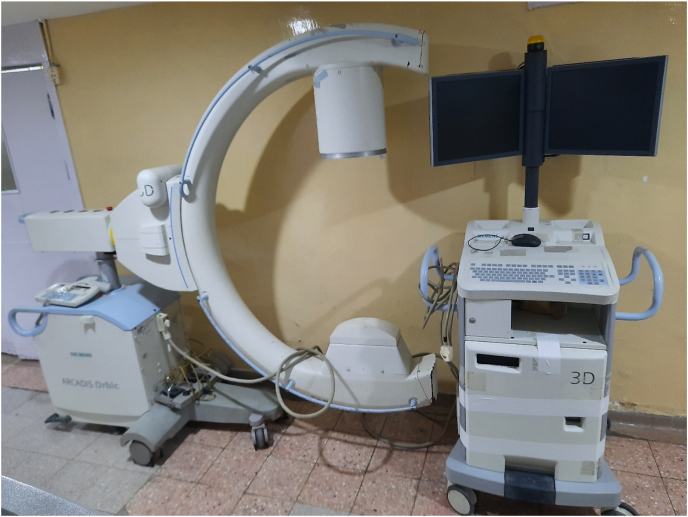
Conventional 2D fluoroscopy allows for a very limited radiological assessment during Orthopaedic surgery, especially while dealing with areas such as pelvis, acetabulum, or spine which have a complex anatomy with extensive soft tissue coverage. In order to enhance intraoperative evaluation of such areas, intraoperative 3D imaging was introduced in 2001.1 This was achieved using a mobile C-Arm, which acquires multiple automated images by 190° motorised movement around the area of interest (Fig. 6). The acquired images are then used to create a 3-dimensional dataset similar to that of a CT, which can be accessed by the operating team.
Fig. 6.
Siemens Arcadis Orbic 3D C-Arm used in our institution.
However, these mobile C-Arms have some limitations. The image quality is usually inferior to conventional CT scanners. Metallic implants cause artifacts that obscure visualisation of relevant structures, and the methods of artifact reduction used in conventional CT scanners are not available for 3D C-Arms. The field of view is often limited to cubic volumes of edge length 12–14 cm, which might be too small to adequately visualise all the relevant structures. Moreover, the best image resolution will be at the centre of rotation, so it becomes very important to adequately position the C-Arm. This ideal position may not always be possible due to various factors such as patient positioning and distinct anatomy of the target region.12
In order to counter these limitations of 3D C-Arms, intraoperative CT scanners were introduced. These allow multiplanar reconstruction of the target area with an increased field of view and better image quality compared to 3D C-Arms. Earlier models were fixed conventional CT scanners that were incorporated into the operating room during construction.13 These had limitations of their own including-need for separate construction, excessive space consumption, small gantry opening and limited intraoperative mobility. More recently, mobile intraoperative CT scanners have been developed with a larger gantry opening that overcame a lot of these limitations (Samsung BodyTom, BrainLab Airo). They offer an increased field of view, better image quality, and less metal artifacts. A cadaveric study has shown that these mobile CT scanners are more precise than 3D fluoroscopy in visualising articular impaction.14
Intraoperative 3D imaging in rapidly gaining popularity in various Orthopaedic surgeries, as evident from the increasing trend of publications on the same in recent years as shown in Fig. 3. The most commonly used device for 3D imaging appears to be the O-Arm, from Medtronic USA which was used in almost half of included studies.
The current systematic review compared intraoperative fluoroscopy with iCT by analysing the available evidence in literature and establishes that the application of iCT leads to significantly better accuracy in terms of implant positioning. Such optimal positioning of implants are especially critical in areas such as spine surgery, where even a minute mal-position of implants could have disastrous consequences. This improved accuracy would more than compensate for the slight increase in mean surgical time as a result of usage of iCT. Theoretically, iCT guided navigation should lead to a decrease in blood loss since a lesser number of attempts would be required to obtain ideal implant positioning. A few studies have reported on the same, but they were insufficient to draw generalised conclusions.15, 16, 17
Spine Surgery- In Orthopaedics, iCT is used most extensively in spine surgery. In the cervical spine, pedicle screw fixation results in excellent stabilisation and has been shown to have a better holding strength.18 However, pedicle screw placement is not routinely done in cervical spine due to the complex pedicle anatomy that is highly variable between individuals, and the proximity of pedicles to the spinal cord and vertebral artery. Instrumentation of the thoracic pedicles are also challenging due to the relatively smaller pedicle size, and more complex anatomy. Accurate targeting of such pedicles using conventional fluoroscopy is very difficult. The significantly higher accuracy accorded by iCT, as shown in this meta-analysis could pave the way for easy instrumentation of these challenging pedicles.7 The use of iCT would also be justified for instrumentation of even the less complicated pedicles, such as those of the lumbar spine as the improved accuracy would result in greater screw purchase and lesser chance of inadvertent injury to adjacent structures.19,20
The extent of neural decompression has been shown to be accurately assessed using iCT in minimally invasive spine surgery reducing the need for additional postoperative imaging. This will allow for accurate prediction of radiological outcome, and also gives intraoperative feedback for the surgeon to decide if more decompression is required.21
In addition to these, iCT has been used in spine surgery for percutaneous repair of pars defect using a cannulated screw, percutaneous discectomy, minimally invasive transforaminal interbody fusion, treatment of spinal deformities and spinal infections.22, 23, 24, 25, 26
Pelvis-acetabulum- Access to iCT is of great benefit for pelvi-acetabular surgeons. Due to the complex anatomy and extensive soft tissue coverage, conventional fluoroscopy offers limited visualisation of the pelvis and acetabulum. Typically, reduction and provisional fixation is done with the help of fluoroscopy, followed by detailed assessment with iCT, and any mal-reduction is corrected then and there. This also precludes the need to have a post-operative CT scan to verify reduction, and hence may ultimately lead to a lower radiation exposure to the patient. Studies have shown that iCT allows for better intraoperative control of reduction and better positioning of implants and screws in acetabular fractures potentially improving biomechanics of the fixation.1,27 There are also reports suggesting that iCT enhances the surgeon's ability to evaluate the stability of hip during stress testing in fractures of the posterior wall of acetabulum.28 Another application of iCT is in the percutaneous placement of iliosacral screws. Studies have shown that the use of iCT results in increased accuracy and lower procedure time of SI joint screws, compared to conventional fluoroscopy.12,29 This technique becomes especially important in patients with difficult anatomy, such as those with obesity and sacral dysmorphism wherein it is crucial to identify safe bony corridors to prevent neurovascular complications.
iCT has been reported to be used to localise a pneumocyst of the iliac bone, which was treated with CT guided injection of bone graft substitute.30
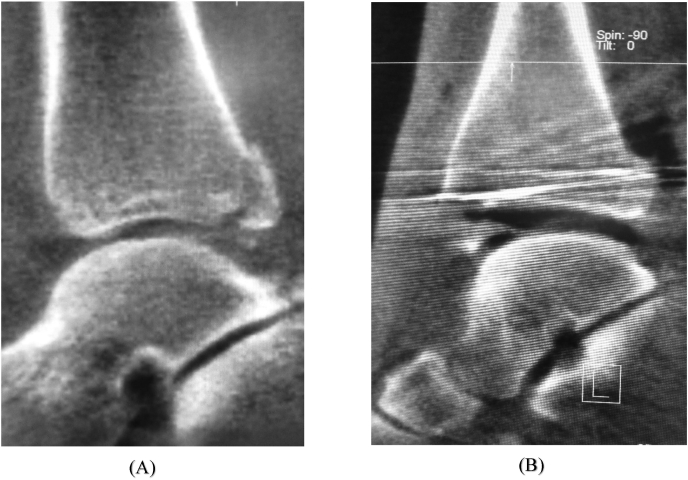
Foot and ankle- In foot and ankle surgery, iCT has been used to assess the adequacy of reduction of the tibio-fibular syndesmosis. Syndesmotic mal-reduction maybe due to several factors, such as inadequate intraoperative radiographic assessment, incorrect reduction techniques, syndesmotic overcompression or excessive rigid fixation, and leads to poor functional results.31 Traditionally, evaluation of the syndesmosis has been done with the help of postoperative CT scans, and if significant mal-reduction was detected, the patient had to be taken up for revision surgery. Making this decision was complicated, as there is no clear consensus as to what constitutes anatomic reduction. This scenario can be avoided if the syndesmosis could be adequately assessed intraoperatively with a CT scan, and necessary modifications can be made at the same sitting (Fig. 7). However some studies have suggested that this method may not be superior to conventional fluoroscopy in the assessment of syndesmotic reduction.10,32
Fig. 7.
Intraoperative CT scan showing a mal-reduced syndesmosis (A), which was detected before conclusion of surgery and was revised. The final CT scan is shown in (B) where the syndesmosis is well reduced.
Richter evaluated the use of iCT for foot and ankle trauma in 62 cases and concluded that iCT provided key information that ordinarily could not be obtained from conventional 2D imaging.3
Hsu et al. used iCT to assess fixation in navicular stress fractures, and concluded that it is a fast and reliable tool to assess guide wire orientation in real-time.33
Kemppainen et al. did a retrospective case control study on the application of iCT in resection of talocalcaneal coalition.11 They found that the quality of resection was greater in the iCT group compared to the control group. They concluded that iCT can change surgical decision making increasing the likelihood of complete resection of the coalition.
Trauma-iCT has been used in the management of complex intra-articular fractures. These fractures require perfect anatomic reconstruction and restoration of the articular surface in order to have a fair clinical outcome. The fracture reduction and provisional fixation are carried out with the help of conventional fluoroscopy. Once satisfactory fixation is confirmed by fluoroscopy, iCT imaging is done prior to definitive fixation/closure (Fig. 8). Atesok et al. used iCT in the treatment of 72 closed intra-articular fractures, and this resulted in 8 fractures (11%) identified as being malreduced, requiring intraoperative revision.4 Halvachizadeh et al. found a decrease in revision rate and improved screw positioning with the use of iCT in their retrospective comparative study on patients with complex distal radius fractures.34 In fractures of the distal radius, it is important to support the subchondral bone with screws of adequate length to prevent postoperative loss of radial length. At the same time, excessive screw length should be avoided as it may lead to articular penetration or penetration of the dorsal cortex leading to irritation and rupture of extensor tendons. Here, the length and direction of screws become very important, and achieving this ideal position using 2D imaging become quite difficult, especially for the less experienced surgeon. iCT guided navigation has been used in volar plating of distal radius fractures to insert screws accurately in the intra-articular dorsal fragments with good clinical and radiological outcomes.35,36
Fig. 8.
(A) Intraoperative CT scan demonstrating a small chip fracture of the posterior malleolus, (B) shows the same fracture after provisional fixation and reassessment with iCT showing a satisfactory reduction. The adequacy of reduction would have been much more difficult to assess using conventional fluoroscopy alone.
Tumor- In Orthopaedic oncology, iCT guided navigation would find application in difficult surgeries, such as intra-epiphseal resection near a joint, bone resection in difficult to access and complex anatomic regions such as the pelvis and spine etc. This technique would also facilitate resection with adequately wide margins, that are immediately confirmed intraoperatively thereby reducing the chances of recurrence.2,37 Osteoid osteomas occurring in inaccessible regions may be percutaneously excised or subjected to radio-frequency ablation under iCT guided navigation.38, 39, 40
Despite the many advantages of iCT, there are certain drawbacks. The high initial costs to purchase and set up a mobile CT scanner is a deterrent to many institutes especially in developing countries. Operating a mobile CT scanner involves an additional learning curve. Increased radiation exposure to the patient and possibly to the staff, and additional time required for image acquisition are other limitations. Moreover, this system requires a fully radiolucent operating table without any metal objects to facilitate 360° image acquisition devoid of artifacts.
A limitation of this systematic review was the unavailability of studies with higher levels of evidence in the included articles due to which observational studies had to be used. Although several studies have demonstrated excellent radiological outcomes with the use of iCT, very few have addressed the crucial question of whether such radiological outcomes translate to good clinical results in the long run. Another limitation was that most of the included articles were based on spine surgery (22 out of 31). Due to the lesser number of studies from other sub-specialities, these could not be independently analysed and compared with each other.
5. Conclusion
iCT is a promising technological development, with continuously expanding applications in several areas of Orthopaedic surgery. There is conclusive evidence that the application of iCT results in better accuracy of implant positioning and reduction. The mean surgical time is also not significantly increased with the use of iCT. However, there is a paucity of prospective studies comparing the final clinical outcome. We suggest further research exploring the use of iCT in various other orthopaedic interventions with clinical follow up.
Declaration of conflict of interest
The authors have no conflict of interests to declare.
Role of funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2021.04.030.
Contributor Information
Vishal Kumar, Email: drkumarvishal@gmail.com.
Vishnu Baburaj, Email: drvbms@gmail.com.
Sandeep Patel, Email: sandeepdrpatelortho@gmail.com, sandeepdrpatelortho@gmail.com.
Siddhartha Sharma, Email: sids82@gmail.com.
Raju Vaishya, Email: raju.vaishya@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Keil H., Beisemann N., Schnetzke M., Vetter S.Y., Grützner P.A., Franke J. First experiences with the Airo mobile intraoperative CT scanner in acetabular surgery-An analysis of 10 cases. Int J Med Robot Comput Assist Surg MRCAS. 2019;15(2) doi: 10.1002/rcs.1986. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara T., Kunisada T., Takeda K. Intraoperative O-arm-navigated resection in musculoskeletal tumors. J Orthop Sci Off J Jpn Orthop Assoc. 2018;23(6):1045–1050. doi: 10.1016/j.jos.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Richter M., Geerling J., Zech S., Goesling T., Krettek C. Intraoperative three-dimensional imaging with a motorized mobile C-arm (SIREMOBIL ISO-C-3D) in foot and ankle trauma care: a preliminary report. J Orthop Trauma. 2005;19(4):259–266. doi: 10.1097/01.bot.0000151822.10254.db. [DOI] [PubMed] [Google Scholar]
- 4.Atesok K., Finkelstein J., Khoury A. The use of intraoperative three-dimensional imaging (ISO-C-3D) in fixation of intraarticular fractures. Injury. 2007;38(10):1163–1169. doi: 10.1016/j.injury.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Kumar V., Baburaj V., Patel S., Sharma S., Vaishya R. Does the use of intraoperative CT scan improve outcomes in Orthopaedic surgery? A protocol for systematic review and meta-analysis. medRxiv. 2021;1 doi: 10.1101/2021.03.01.21252670. Published online January. 2021.03.01.21252670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshii T., Hirai T., Sakai K., Inose H., Kato T., Okawa A. Cervical pedicle screw placement using intraoperative computed tomography imaging with a mobile scanner gantry. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2016;25(6):1690–1697. doi: 10.1007/s00586-016-4508-2. [DOI] [PubMed] [Google Scholar]
- 8.Hecht A.C., Koehler S.M., Laudone J.C., Jenkins A., Qureshi S. Is intraoperative CT of posterior cervical spine instrumentation cost-effective and does it reduce complications? Clin Orthop. 2011;469(4):1035–1041. doi: 10.1007/s11999-010-1603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sembrano J.N., Santos E.R.G., Polly D.W., Jr. New generation intraoperative three-dimensional imaging (O-arm) in 100 spine surgeries: does it change the surgical procedure? J Clin Neurosci. 2014;21(2):225–231. doi: 10.1016/j.jocn.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Summers H.D., Sinclair M.K., Stover M.D. A reliable method for intraoperative evaluation of syndesmotic reduction. J Orthop Trauma. 2013;27(4):196–200. doi: 10.1097/BOT.0b013e3182694766. [DOI] [PubMed] [Google Scholar]
- 11.Kemppainen J., Pennock A.T., Roocroft J.H., Bastrom T.P., Mubarak S.J. The use of a portable CT scanner for the intraoperative assessment of talocalcaneal coalition resections. J Pediatr Orthop. 2014;34(5):559–564. doi: 10.1097/BPO.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 12.Privalov M., Beisemann N., Swartman B. First experiences with intraoperative CT in navigated sacroiliac (SI) instrumentation: an analysis of 25 cases and comparison with conventional intraoperative 2D and 3D imaging. Injury. 2020;19 doi: 10.1016/j.injury.2020.02.093. Published online February. [DOI] [PubMed] [Google Scholar]
- 13.Tonn J.C., Schichor C., Schnell O. Intraoperative computed tomography. In: Pamir M.N., Seifert V., Kiris T., editors. Intraoperative Imaging. Vol 109. Acta Neurochirurgica Supplementum. Springer Vienna; 2011. pp. 163–167. [DOI] [PubMed] [Google Scholar]
- 14.Luxenhofer M., Beisemann N., Schnetzke M. Diagnostic accuracy of intraoperative CT-imaging in complex articular fractures - a cadaveric study. Sci Rep. 2020;10(1):4530. doi: 10.1038/s41598-020-61267-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu M.-H., Huang T.-J., Li Y.-Y., Cheng C.-C., Huang K.-C., Hsu R.W.W. Comparison of the accuracies of transpedicular screw insertion during computed tomography-free, -based, and intraoperative computed tomography spinal surgeries. Formos J Musculoskelet Disord. 2012;3(2):39–42. doi: 10.1016/j.fjmd.2012.03.001. [DOI] [Google Scholar]
- 16.Baky F.J., Milbrandt T., Echternacht S., Stans A.A., Shaughnessy W.J., Larson A.N. Intraoperative computed tomography-guided navigation for pediatric spine patients reduced return to operating room for screw malposition compared with freehand/fluoroscopic techniques. Spine Deform. 2019;7(4):577–581. doi: 10.1016/j.jspd.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohba T., Ebata S., Fujita K., Sato H., Haro H. Percutaneous pedicle screw placements: accuracy and rates of cranial facet joint violation using conventional fluoroscopy compared with intraoperative three-dimensional computed tomography computer navigation. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2016;25(6):1775–1780. doi: 10.1007/s00586-016-4489-1. [DOI] [PubMed] [Google Scholar]
- 18.Abumi K., Shono Y., Taneichi H., Ito M., Kaneda K. Correction of cervical kyphosis using pedicle screw fixation systems. Spine. 1999;24(22):2389. doi: 10.1097/00007632-199911150-00017. [DOI] [PubMed] [Google Scholar]
- 19.Sundaram P.P.M., Oh J.Y.-L., Tan M., Nolan C.P., Yu C.S., Ling J.M. Accuracy of thoracolumbar pedicle screw insertion based on routine use of intraoperative imaging and navigation. Asian Spine J. 2020;22 doi: 10.31616/asj.2020.0068. Published online September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo T.D., Polly D.W.J., Ledonio C.G., Wetjen N.M., Larson A.N. Accuracy of pedicle screw placement in children 10 Years or younger using navigation and intraoperative CT. Clin Spine Surg. 2016;29(3):E135–E138. doi: 10.1097/BSD.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I., Lang G., Navarro-Ramirez R. Can fan-beam interactive computed tomography accurately predict indirect decompression in minimally invasive spine surgery fusion procedures? World Neurosurg. 2017;107:322–333. doi: 10.1016/j.wneu.2017.07.167. [DOI] [PubMed] [Google Scholar]
- 22.Nourbakhsh A., Preuss F., Hadeed M., Shimer A. Percutaneous direct repair of a pars defect using intraoperative computed tomography scan: a modification of the buck technique. Spine. 2017;42(11):E691–E694. doi: 10.1097/BRS.0000000000001929. [DOI] [PubMed] [Google Scholar]
- 23.Oki S., Matsuda Y., Shibata T. Percutaneous discectomy in treatment of herniated lumbar disc using Nucleotome Flex II under computed tomography guidance: a preliminary report. Arch Jpn Chir. 1999;67(2):41–46. [Google Scholar]
- 24.Safaee M., Oh T., Pekmezci M., Clark A.J. 2019. Cone Beam Intraoperative Computed Tomography-Based Image Guidance for Minimally Invasive Transforaminal Interbody Fusion. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien J.R. The use of intraoperative CT and navigation for the treatment of spinal deformity in open and minimally invasive surgery. Spine. 2017;42(Suppl 7):S28–S29. doi: 10.1097/BRS.0000000000002038. [DOI] [PubMed] [Google Scholar]
- 26.Wu M.-H., Dubey N.K., Lee C.-Y. Application of intraoperative CT-guided navigation in simultaneous minimally invasive anterior and posterior surgery for infectious spondylitis. BioMed Res Int. 2017 doi: 10.1155/2017/2302395. 2017((Wu M.-H., maxwutmu@gmail.com; Lee C.-Y., ejaca22@gmail.com; Huang T.-J., tjdhuang@gmail.com) Department of Orthopedic Surgery, Taipei Medical University Hospital, Taipei, Taiwan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebaaly A., Riouallon G., Zaraa M., Jouffroy P. The added value of intraoperative CT scanner and screw navigation in displaced posterior wall acetabular fracture with articular impaction. Orthop Traumatol Surg Res OTSR. 2016;102(7):947–950. doi: 10.1016/j.otsr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham B., Jackson K., Ortega G. Intraoperative CT in the assessment of posterior wall acetabular fracture stability. Orthopedics. 2014;37(4):e328–e331. doi: 10.3928/01477447-20140401-51. [DOI] [PubMed] [Google Scholar]
- 29.Khan J.M., Lara D.L., Marquez-Lara A., Rosas S., Hasty E., Pilson H.T. Intraoperative CT and surgical navigation for iliosacral screws: technique for patients with sacral dysmorphism. J Orthop Trauma. 2018;32 doi: 10.1097/BOT.0000000000001213. Khan J.M.) Division of Orthopaedic Trauma, Department of Orthopaedic Surgery, Wake Forest University School of Medicine, Winston-Salem,):S24-S25. [DOI] [PubMed] [Google Scholar]
- 30.Formby P.M., Kang D.G., Potter B.K., Forsberg J.A. Treatment of symptomatic intraosseous pneumatocyst using intraoperative navigation. Orthopedics. 2015;38(3):e244–e247. doi: 10.3928/01477447-20150305-93. [DOI] [PubMed] [Google Scholar]
- 31.Dowdle S.B., Duchman K.R., Phisitkul P., Amendola A. Intraoperative assessment of syndesmotic injury and how to assess if the syndesmosis is reduced. Tech Orthop. 2017;32(2):86–92. doi: 10.1097/BTO.0000000000000222. [DOI] [Google Scholar]
- 32.Davidovitch R.I., Weil Y., Karia R. Intraoperative syndesmotic reduction: three-dimensional versus standard fluoroscopic imaging. J Bone Jt Surg. 2013;95(20):1838–1843. doi: 10.2106/JBJS.L.00382. [DOI] [PubMed] [Google Scholar]
- 33.Hsu A.R., Lee S. Evaluation of tarsal navicular stress fracture fixation using intraoperative O-arm computed tomography. Foot Ankle Spec. 2014;7(6):515–521. doi: 10.1177/1938640014532130. [DOI] [PubMed] [Google Scholar]
- 34.Halvachizadeh S., Berk T., Pieringer A. Is the additional effort for an intraoperative CT scan justified for distal radius fracture fixations? A comparative clinical feasibility study. J Clin Med. 2020;9(7) doi: 10.3390/jcm9072254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawabata A., Sogabe Y., Morimoto Y., Takamatsu K. Volar locking plate fixation for distal radius fractures by intraoperative computed tomographic–guided navigation. J Hand Surg Glob Online. 2020;2(5):290–296. doi: 10.1016/j.jhsg.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneshiro Y., Hidaka N., Yano K. Intraoperative computed tomography with an integrated navigation system versus freehand technique under fluoroscopy in the treatment of intra-articular distal radius fractures. J Plast Surg Hand Surg. 2019;53(5):255–259. doi: 10.1080/2000656X.2019.1597370. [DOI] [PubMed] [Google Scholar]
- 37.Bosma S.E., Cleven A.H.G., Dijkstra P.D.S. Can navigation improve the ability to achieve tumor-free margins in pelvic and sacral primary bone sarcoma resections? A historically controlled study. Clin Orthop. 2019;477(7):1548–1559. doi: 10.1097/CORR.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkatadass K., Rajasekaran S. Percutaneous excision of difficult osteoid osteomas using intraoperative AIRO CT navigation: a preliminary report. J Pediatr Orthop Part B. 2018;27(5):456–460. doi: 10.1097/BPB.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 39.Flanagin B.A., Lindskog D.M. Intraoperative radiofrequency ablation for osteoid osteoma. Am J Orthop Belle Mead NJ. 2015;44(3):127–130. [PubMed] [Google Scholar]
- 40.Yu F., Niu X.-H., Zhang Q., Zhao H.-T., Xu L.-H., Deng Z.-P. Radiofrequency ablation under 3D intraoperative Iso-C C-arm navigation for the treatment of osteoid osteomas. Br J Radiol. 2015;88(1056):20140535. doi: 10.1259/bjr.20140535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.