Abstract
Frontal fibrosing alopecia (FFA) is an acquired primary lymphocytic cicatricial alopecia characterized by frontotemporal hairline recession, leading to scarring alopecia with a band-like distribution. Prevalence is increasing worldwide, being the most frequent cause of primary scarring alopecia. The natural history of this condition is variable; however, slow progression with spontaneous remission is the most frequent reported outcome. The etiopathogenesis of FFA remains to be elucidated; numerous hypotheses concerning hormonal effects, environmental factors, and genetic predisposition have been proposed. Special interest on genetic basis has emerged since the first familial case was reported. Only a few more familial cases have been published. We report 6 additional cases of female patients with familial FFA (F-FFA) from 3 different families. Sixty-six percent had a family history of autoimmune disease in first-degree relatives; these same patients had a personal history of autoimmune disease. The families described in this cohort study plus the personal and family history of autoimmune disease, as well as the recently described involved genomic loci; reinforced the hypothesis of this disease being genetic. It is important to consider studying this entity since there are scarce data regarding familial cases and this might give us a better insight toward understanding its pathogenesis.
Keywords: Familial frontal fibrosing alopecia, Trichology, Hair loss, Scarring alopecia, Lichen planopilaris
Established Facts
Frontal fibrosing alopecia (FFA) is a scarring alopecia with a distinctive pattern of progressive frontotemporal hairline recession associated with perifollicular hyperkeratosis and erythema.
There is little knowledge on the etiology and pathogenesis of this alopecia. Although an autoimmune reaction and hormonal factors seem to play a role, numerous hypotheses of genetic basis and environmental factors have been proposed.
Novel Insights
The 3 families with familial FFA reported in this cohort study, plus the personal and family history of different autoimmune diseases support the hypothesis of this disease having a genetic etiology.
Introduction
Frontal fibrosing alopecia (FFA) was first described by Kossard in 1994 as a form of primary lymphocytic cicatricial alopecia [1]. FFA is postulated by some authors to be a variant of lichen planopilaris due to their histological similarities [2]. It presents as scarring alopecia with a distinctive pattern of progressive frontotemporal hairline recession associated with perifollicular hyperkeratosis and erythema [3].
There is little knowledge on the etiology and pathogenesis of this alopecia. It was initially believed to have a hormonal origin, as it was first described in postmenopausal women and premenopausal women with a history of hysterectomy. However, subsequent reports revealed cases in younger women and rarely in men [3, 4]. Although an autoimmune reaction and hormonal factors seem to play a role, numerous hypotheses regarding environmental factors and a genetic basis have recently been proposed [2, 5, 6, 7, 8, 9, 10, 11, 12].
We conducted a retrospective cohort study of familial FFA (F-FFA) cases diagnosed at the University Hospital “Dr. José E. González” between 2013 and 2019. Six patients belonging to 3 different families were included. Subjects with F-FFA, diagnosed clinically, dermoscopically, and histopathologically were included. Allergy Patch Testing was conducted in all patients. The North American Standard, Cosmetic, and Photoallergen series were applied. Patient history including gender, age, relationship between familial cases, and relevant medical history was recorded. A review of all cases of F-FFA reported in the literature was done (from 2008 to 2020). The main objective of this study was to report 6 new cases of familial FFA from 3 different families, describing the main demographics and clinical characteristics.
Case Report/Case Presentation
The cases of F-FFA diagnosed in the 5 years of this study comprise 6 females, with a mean age of 60.6 years, from 3 different families. Two of the 3 families were composed of mother and daughter, whereas the third family included 2 sisters. The disease started 34.6 months (range 8–60 months) prior to diagnosis. Four out of 6 patients (66%) had a family history of autoimmune disease in first-degree relatives; these same patients had a personal history of autoimmune disease. The Allergy Patch Testing was positive in 4 patients (66%), and the most common positive allergen was propolis (Table 1).
Table 1.
Clinical and demographic data of 3 families with frontal fibrosing alopecia
| Family | N | Relation | Sex | Age | Menopause | Eyelashes | Eyebrows | Body hair involvement | Facial papules | Depressed frontal veins | Immune-related diseases |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Mother Daughter | 2F | 72 51 |
Surgical No |
Yes No |
Yes Yes |
Pubis Limbs | No Yes |
Yeso No |
RAo LP |
| 2 | 2 | Mother Daughter | 2F | 58 39 |
Yes Surgical |
No No |
Yes No |
Limbs, axillae No |
Yes No |
Yes No |
LP Hypothyroidism |
| 3 | 2 | Sister Sister | 2F | 69 75 |
Yes Yes | Yes Yes |
Yes Yes |
Limbs, axillae Limbs | No Yes |
Yes Yes |
No No |
RA, rheumatoid arthritis; LP, lichen planus pigmentosus.
First Family
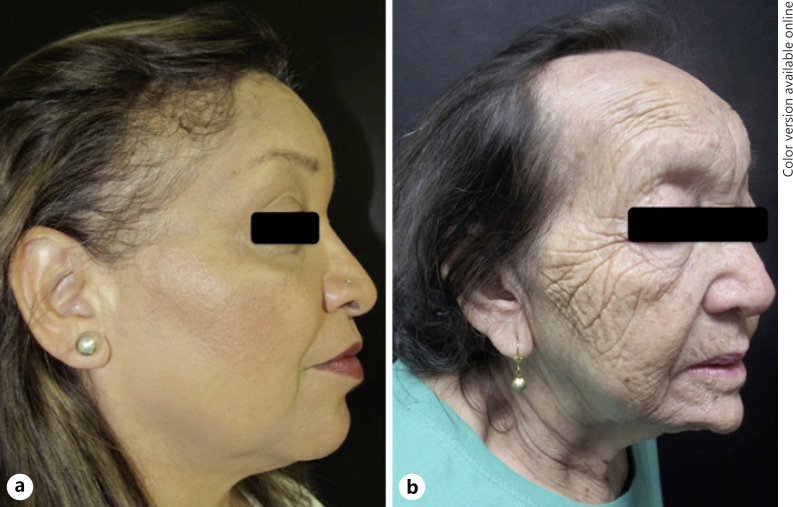
The family included mother and daughter, both of them showed scalp and eyebrows involvement. The mother presented eyelash alopecia and pubic hair loss, while her daughter had hair loss on her arms. The mother's physical examination revealed depressed frontal vein network, while in her daughter we found facial papules (Fig. 1). Both had a history of autoimmune disease; the daughter had lichen planus pigmentosus, and her mother rheumatoid arthritis. The mother had 2 other daughters, one of them with hypothyroidism and another one with vitiligo. Mother and daughter shared cutaneous contact hypersensitivity to propolis.
Fig. 1.
a, b First family, mother and daughter. Both presented scalp and eyebrow alopecia. The daughter's physical examination revealed facial papules and lichen planus pigmentosus in frontal region, chin, and neck (a), while the mother had depressed frontal vein network (b).
Second Family
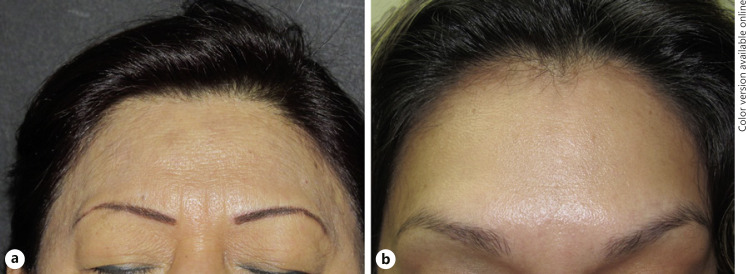
The family included mother and daughter. The mother presented frontotemporal and retro-auricular hairline recession and eyebrow alopecia. During her physical examination, we found facial papules, depressed frontal veins, and alopecic limbs and axillae. Her daughter showed initial signs of frontal receding hairline but no evidence of hair loss in other locations (Fig. 2). The mother presented a personal history of lichen planus pigmentosus, diabetes mellitus, and hypertension, while her daughter had personal history of hypothyroidism. The Allergy Patch Testing of the mother resulted positive to methyldibromo glutaronitrile, 4-phenylenediamine base, methylisothiazolinone, and nickel, but her daughter had no positive patch test results.
Fig. 2.
a, b. Second family, mother and daughter. The mother presented frontotemporal and retro-auricular hairline recession, as well as eyebrow alopecia. a Physical examination revealed facial papules, depressed frontal veins, and alopecic limbs and axillae. b The daughter referred frontal receding hairline, which was barely noticeable clinically, but had dermoscopic and histopathologic characteristics of FFA (b). FFA, frontal fibrosing alopecia.
Third Family
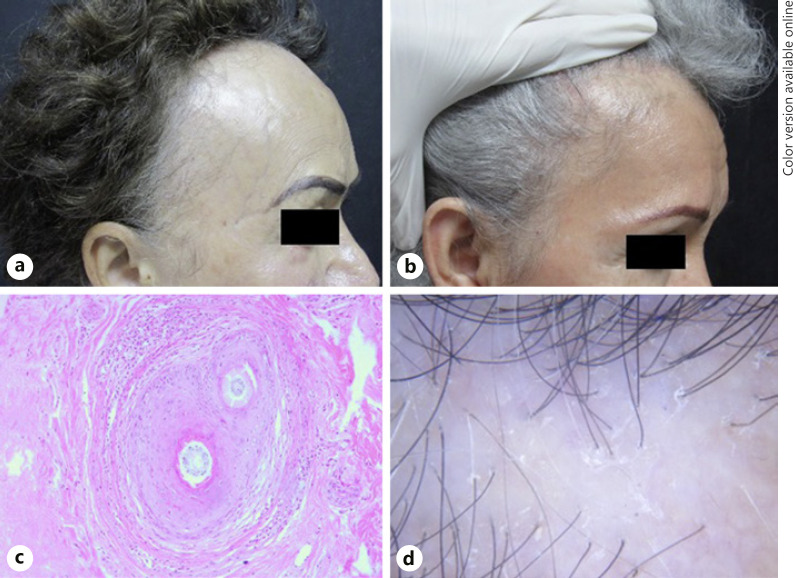
Family included 2 sisters, both with a history of frontotemporal hair loss, eyebrow, eyelash, and limb involvement. The physical examination also revealed hair loss on the axillae in the younger sister and facial papules in the older one. Both had an unremarkable medical history. The Allergy Patch Testing of the younger sister resulted positive to glutaraldehyde 1% and chlorhexidine digluconate 1%, while the test for the elder one resulted negative (Fig. 3).
Fig. 3.
a, b Third family, sisters with FFA. Both present eyebrow and eyelash alopecia and the eldest depressed frontal vein network (a). c Histopathology: perifollicular fibrosis with lymphocytic inflammation (HyE. ×40). d Dermoscopy: perifollicular scale, erythema, and absence of follicular openings.
Discussion/Conclusion
The number of patients with this condition has markedly increased in past years [2, 13]. The reasons for such an increased incidence remain unknown, although awareness of this cicatricial entity might be involved [4, 13].
The etiology of FFA is poorly understood. Hormonal factors are thought to play a role in the pathogenesis of this entity, as it affects predominantly postmenopausal women and due to early onset in women who presented premature menopause or underwent a hysterectomy [3, 13]. Additionally, several autoimmune diseases, such as vitiligo, discoid lupus erythematosus, Sjögren syndrome, and thyroid dysfunction, have been reported to occur concurrently with FFA, suggesting an autoimmune mechanism in the pathogenesis [2, 3, 5, 14].
On the other hand, the fact that FFA develops later in life suggests that environmental factors may play a role in its development [4, 5]. The occurrence of the disease in families could indicate exposure to common environmental triggers [4, 5]. Aldoori et al. [15] reported an association between FFA and the use of facial skin care products containing sunscreen chemicals. It is important to highlight that the authors suggested that prolonged retention within the hair follicle could trigger the immunological response, rather than a specific ingredient of facial products [15]. Nonetheless, the role these products play in the pathogenesis of FFA is unclear and remains controversial.
In view of FFA being increasingly reported in siblings and members of the same family, a genetic basis has aroused keen interest. Tziotzios et al. [10] observed a genome-wide significant association of FFA in 4 genomic loci: 2p22.2, 6p21.1, 8q24.22, and 15q2.1. These findings provide insight into the pathogenesis, as a genetically predisposed immune-inflammatory disease driven by HLA-B+07:02 [10].
The natural history of FFA is not completely known. The initial presentation of a progressive receding frontotemporal hairline leads to a cicatricial alopecia devoid of follicular ostia [2, 3, 4]. Perifollicular erythema and/or scaling may be present in the alopecic areas [3, 7]. Generally, the remaining scalp is unaffected; however, FFA may progress laterally and behind the ears, even toward the occipital area [3, 13]. Partial or total eyebrow alopecia is often observed (50–83%), and it may be preceded or followed by scalp disease. Though it is less common, eyelash involvement may occur (3%). In addition, patients may also develop body hair loss (25%) [2, 3]. Facial papules (forehead, cheeks, and chin), facial erythema, hypo/hyperpigmented macules, and prominence or depression of the facial veins have also been described in FFA-affected patients [2, 4].
To our knowledge, in addition to our 6 patients (3 families), 56 patients within 24 families have been reported in the literature of F-FFA. Of all patients described (including our 6 patients), 56 were women (90.3%) and only 6 were men (9.6%), with a mean age of 58 years (range 21–88). The mean age of onset in our patients was 58 (range 34–72), similar to previous F-FFA reports. Five patients within our 3 families were postmenopausal at the time of diagnosis (83.3%), which is the same percentage obtained by Vañó-Galván et al. [3] in their multicenter study of 355 patients with FFA. Regarding the previous F-FFA published cases, we found no differences in terms of clinical presentation neither by sex nor by menopausal state.
Limb alopecia affected 66.6% of our patients, which is a higher rate compared to the 54% of the published F-FFA cases. Axillary and pubic hairs were affected in 33.3 and 16.6% of our patients, respectively; in contrast to the patients of previous F-FFA reports, who were affected in 21.8 and 15.6%, respectively. Beard involvement was mentioned in 2 of 6 male patients [2, 16], representing 33.3% of the total.
The most common reported feature in F-FFA patients was eyebrow alopecia, found in 89.2% of the previous cases and very similar to the 83.3% observed in our patients. Eyelash alopecia was only mentioned in 9 cases (34.6%), while 50% of our patients had this characteristic. Another important aspect seen in 50% of our cases was the presence of facial papules. This characteristic was only reported in 3 previous studies [7, 12, 16] (Table 2). Furthermore, no difference in regard to the clinical manifestations between familial and nonfamilial FFA subjects was stablished.
Table 2.
Descriptive statistics of F-FFA; includes previous literature and our series
| Families, n | 27 |
| Cases, n | 62 |
| Female, n = 62, n (%) | 56 (90) |
| Relation, n = 62, n (%) | |
| Brother | 4 (6) |
| Sister | 33 (53) |
| Cousin | 2 (3) |
| Daughter | 10 (16) |
| Son | 1 (2) |
| Mother | 10 (16) |
| Father | 1 (2) |
| Niece | 1 (2) |
| Age, years, n = 62 (mean, SD) | 58 (14.3) |
| Menopause women, n = 38, n (%) | 28 (74) |
| Evolution, months, n = 57 (median, IQR) | 24 (12–48) |
| Eyelashes, n = 32, n (%) | 12 (38) |
| Eyebrows, n = 62, n (%) | 55 (89) |
| Limbs, n = 56, n (%) | 31 (55) |
| Pubic area, n = 38, n (%) | 6 (16) |
| Axillae, n = 38, n (%) | 9 (24) |
| Beard, n = 4, n (%) | 2 (50) |
| Facial papules, n = 24, n (%) | 10 (42) |
| Depressed frontal vein network, n = 6, n (%) | 4 (67) |
N, number of data available; SD, standard deviation; IQR, interquartile range; F-FFA, familial FFA.
The associated diseases in our series of cases were systemic arterial hypertension and diabetes mellitus in 1 patient and rheumatoid arthritis and cervical cancer in another. There was 1 case of thyroid disease and lichen planus pigmentosus in 2 others. Notably, 66% percent had a family history of autoimmune disease in first-degree relatives; these were the same patients who had a personal history of an autoimmune disease.
The 3 families with F-FFA reported in this cohort study, plus the personal and family history of different autoimmune diseases support the hypothesis of this disease having a genetic etiology. Additionally, exposure to environmental triggers may play a role in its development. It is important to consider studying this entity since there is scarce data regarding familial cases and this might give us a better insight toward understanding its pathogenesis.
The main limitation in our study was the absence of genome-wide study, to confirm whether a genetic component was involved in our included families. Additional research with a larger number of subjects should be done to determine the etiology and pathogenesis of this complex disease in order to offer prompt diagnosis and definite treatment.
Statement of Ethics
The authors have no ethical conflicts to disclose. The patients provided written informed consent to publish photos and details of the case.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors declare they received no funding in support of this work.
Author Contributions
Study concept and design: S.S. Ocampo. Acquisition of the data: S.S. Ocampo, T.L. Orizaga, and V. Olvera. Analysis and interpretation of the data: S.S. Ocampo, T.L. Orizaga, and V. Olvera. Drafting of the manuscript: S.S. Ocampo and V. Olvera. Critical revision of the manuscript and important intellectual input: all authors. Study supervision: M.E. Herz, S. Chávez, and J. Ocampo. All authors declare that they have sufficiently participated in the submitted work to deserve authorship, and all have had access to the clinical material and have revised the manuscript before submission. The corresponding author (Dr. J. Ocampo) endorses the scientific responsibility of the work reported herein.
Acknowledgement
We have to thank Dr. Sergio Lozano Rodriguez who always helps us with proofreading and traduction.
References
- 1.Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch Dermatol. 1994 Jun;130((6)):770–4. [PubMed] [Google Scholar]
- 2.Porriño-Bustamante ML, López-Nevot MÁ, Aneiros-Fernández J, García-Lora E, Fernández-Pugnaire MA, Arias-Santiago S. Familial frontal fibrosing alopecia: a cross-sectional study of 20 cases from nine families. Australas J Dermatol. 2018 May;60((2)):e113–8. doi: 10.1111/ajd.12951. [DOI] [PubMed] [Google Scholar]
- 3.Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, Arias-Santiago S, Rodrigues-Barata AR, Garnacho-Saucedo G, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014 Apr;70((4)):670–8. doi: 10.1016/j.jaad.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Iorizzo M, Tosti A. Frontal fibrosing alopecia: an update on pathogenesis, diagnosis, and treatment. Am J Clin Dermatol. 2019 Jun;20((3)):379–90. doi: 10.1007/s40257-019-00424-y. [DOI] [PubMed] [Google Scholar]
- 5.Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013 Jan;168((1)):220–2. doi: 10.1111/j.1365-2133.2012.11101.x. [DOI] [PubMed] [Google Scholar]
- 6.Chan DV, Kartono F, Ziegler R. Absence of HLA-DR1 positivity in 2 familial cases of frontal fibrosing alopecia. J Am Acad Dermatol. 2014 Nov;71((5)):e208–10. doi: 10.1016/j.jaad.2014.05.064. [DOI] [PubMed] [Google Scholar]
- 7.Navarro-Belmonte MR, Navarro-López V, Ramírez-Boscà A, Martínez-Andrés MA, Molina-Gil C, González-Nebreda M, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015 Mar;14((1)):64–9. doi: 10.1111/jocd.12125. [DOI] [PubMed] [Google Scholar]
- 8.Otero Rivas MM, Antolín SC, Sánchez Sambucety P, Samaniego González E, García Ruíz de Morales JM, Rodríguez Prieto MÁ. Frontal fibrosing alopecia and lichen planopilaris in HLA-identical mother and daughter. Indian J Dermatol Venereol Leprol. 2015 Mar–Apr;81((2)):162. doi: 10.4103/0378-6323.152284. [DOI] [PubMed] [Google Scholar]
- 9.Porriño-Bustamante ML, López-Nevot MÁ, Aneiros-Fernández J. Study of Human Leukocyte Antigen (HLA) in 13 cases of familial frontal fibrosing alopecia: CYP21A2 gene p.V281L mutation from congenital adrenal hyperplasia linked to HLA class I haplotype HLA-A+33:01; B+14:02; C+08:02 as a genetic marker. Australas J Dermatol. 2019 Aug;60((3)):e195–200. doi: 10.1111/ajd.12985. [DOI] [PubMed] [Google Scholar]
- 10.Tziotzios C, Petridis C, Dand N, Ainali C, Saklatvala JR, Pullabhatla V, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B+07:02. Nat Commun. 2019 Mar;10((1)):1150. doi: 10.1038/s41467-019-09117-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junqueira Ribeiro Pereira AF, Vincenzi C, Tosti A. Frontal fibrosing alopecia in two sisters. Br J Dermatol. 2010 May;162((5)):1154–5. doi: 10.1111/j.1365-2133.2010.09664.x. [DOI] [PubMed] [Google Scholar]
- 12.Rocha VB, Pires MC, Contin LA. Familial fibrosing frontal alopecia in six sisters. An Bras Dermatol. 2020 Jan–Feb;95((1)):125–8. doi: 10.1016/j.abd.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.To D, Beecker J. Frontal fibrosing alopecia: Update and review of challenges and successes. J Cutan Med Surg. 2018 Mar–Apr;22((2)):182–9. doi: 10.1177/1203475417736279. [DOI] [PubMed] [Google Scholar]
- 14.Miteva M, Aber C, Torres F, Tosti A. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011 Aug;165((2)):445–7. doi: 10.1111/j.1365-2133.2011.10382.x. [DOI] [PubMed] [Google Scholar]
- 15.Aldoori N, Dobson K, Holden CR, McDonagh AJ, Harries M, Messenger AG. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016 Oct;175((4)):762–7. doi: 10.1111/bjd.14535. [DOI] [PubMed] [Google Scholar]
- 16.Porriño-Bustamante ML, García-Lora E, Buendía-Eisman A, Arias-Santiago S. Familial frontal fibrosing alopecia in two male families. Int J Dermatol. 2019 Sep;58((9)):e178–80. doi: 10.1111/ijd.14499. [DOI] [PubMed] [Google Scholar]