Abstract
In idiopathic membranous nephropathy, the complement membrane attack complex, more commonly referred to as complement 5b-9 (C5b-9), induces glomerular epithelial cell injury and proteinuria. C5b-9 can also activate numerous mechanisms that restrict or facilitate injury. Recent studies suggest that autophagy and the canonical Wnt signaling pathway serve an important role in repairing podocyte injury. However, the effect of C5b-9 on these pathways and the relationship between them remains unclear. The aim of the present study was to show the effect of C5b-9 on the Wnt/β-catenin signaling pathway and autophagy in podocytes in vitro. Levels of relevant indicators were detected by immunofluorescence staining and capillary western immunoassay. C5b-9 serum significantly activated the Wnt/β-catenin signaling pathway and promoted autophagy. Treatment with Dickkopf-related protein 1 (DKK1), a Wnt/β-catenin pathway blocker, protected podocytes from injury and significantly inhibited autophagy. The results indicated that inhibition of the Wnt/β-catenin pathway physiologically activated autophagy. The results indicated that C5b-9 resulted in a decrease in Akt in podocytes. However, the podocytes preincubated with DKK1 and then attacked by C5b-9 showed an increase in Akt levels. This may explain the observation that blocking the Wnt/β-catenin signaling pathway attenuated C5b-9 podocyte damage, while inhibiting autophagy. The results of the present study also suggest that regulation of these two pathways may serve as a novel method for the treatment of idiopathic membranous nephropathy.
Keywords: autophagy, Dickkopf-related protein 1, Wnt/β-catenin signaling pathway, complement 5b-9, membranous nephropathy, podocyte
Introduction
Relatively little is known regarding the relationship between autophagy and the Wnt/β-catenin signaling pathway in membranous nephropathy, in which both serve an important role in the repair of injured podocytes (1-3). Idiopathic membranous nephropathy is characterized by the diffuse deposition of immune complexes under the glomerular basement membrane of epithelial cells and diffuse thickening of the basement membrane, which is a disease mediated by in situ immune complexes (4). Phospholipase A2 receptor (PLA2R) and thrombospondin type-1 domain-containing 7A, expressed in podocytes, have been identified as the main pathogenic antigens in this condition (5), and the source of PLA2R antibodies may be circulating neutrophils (6). Podocytes are epithelial cells of the glomerular viscera, located outside the glomerular basement membrane, and autoantibodies bind to antigens on the surface of podocytes, forming an in situ immune complex that results in disease (2,5). In situ immune complexes differ from circulating immune complexes in that they do not come into contact with circulating inflammatory mediators and generally do not trigger inflammatory reactions (7). Therefore, they are more likely to trigger podocyte lesion formation by activating complement, leading to proteinuria and renal tissue damage (8). The membrane attack complex, as the final product of complement activation, attacks glomerular podocytes primarily through stimulating membrane phospholipids via phospholipase A2 to generate arachidonic acid and through cyclo-oxygenase to stimulate endoplasmic reticulum stress (9). In addition, oxidative stress, cytoskeletal protein migration and other factors are involved in the occurrence and development of podocyte injury (8,10).
Autophagy is an important evolutionarily conserved mechanism which allows eukaryotic cells to maintain homeostasis and recycle intracellular components (11). During autophagy, cells form a double-layered membrane structure in the cytoplasm to form autophagosomes around damaged or aging organelles and biomacromolecules, and bind with lysosomes to form an autolysosome, in which the material is degraded and the contents reused for synthesis of cellular components (11). Mature podocytes, which are terminally-differentiated cells, have a high basal level of autophagy (12). The formation of autophagosomes to remove excess or damaged proteins and organelles is a vital injury response mechanism on which podocytes rely for survival (13). When mature podocytes are injured by the membrane attack complex complement 5b-9 (C5b-9), multiple injury signaling pathways are activated in the cells, leading to podocyte lesions, and a series of defense mechanisms are activated to limit the damage and promote repair. Lv et al successfully established an in vitro model of C5b-9 complex injury in podocytes (14). This revealed that C5b-9 can cause abnormal podocyte morphology and autophagy activation (14). Wang et al (15) found that the expression of ER stress-related proteins GRP78 and GRP94 in podocytes was abnormally distributed, and the expression of autophagy-related protein LC3 was significantly increased in a rat model of passive Heymann nephritis. In these autophagy related studies, 3-methyladenine (3-MA) is a commonly used inhibitor and rapamycin is a commonly used activator (16,17).
The Wnt/β-catenin signaling pathway serves a crucial role in the adhesion, differentiation and survival of podocytes (18). Activation of the Wnt/β-catenin signaling pathway can lead to podocyte damage, both in vitro and in vivo (2). For example, in diabetic kidney disease, doxorubicin nephropathy and other glomerular diseases, which can lead to podocyte injury, the expression of Wnt in glomerular epithelial cells is significantly increased and the downstream β-catenin activated, resulting in activation of the Wnt/β-catenin signaling pathway. However, specific inhibition of this pathway, such as through the knockout of β-catenin or the use of pathway inhibitors, can alleviate podocyte damage, suggesting a critical role for this pathway in kidney protection (18-20). To the best of our knowledge, the relationship between autophagy and the Wnt/β-catenin signaling pathway has not been elucidated in the repair of podocytes. In our previous study, it was shown that treatment with curcumin protected podocyte cells against leptin-induced damage and that its protective effects were mediated by inhibition of the Wnt/β-catenin signaling pathway in vitro (21). Dickkopf-related protein 1 (DKK1), a specific inhibitor of the Wnt/β-catenin signaling pathways, decreased LC3II significantly (3,22). These results suggest a close relationship between the Wnt/β-catenin signaling pathway and autophagic inhibition in podocytes. However, this observation cannot fully explain the correlation between the Wnt/β-catenin signal pathway and autophagy. Thus, C5b-9 was used to further study the relationship between the canonical Wnt/β-catenin signal pathway and autophagy.
In the present study, podocytes were incubated with C5b-9 serum and treated with DKK1 and changes in autophagy were observed. These results may help us to more fully elucidate the potential mechanism of the influence of Wnt/β-catenin signaling pathway on the autophagy-lysosome pathway. The results of the present study highlight the potential existence of a critical relationship between the canonical Wnt/β-catenin signaling pathway and autophagy.
Materials and methods
Cell culture and treatments
A conditioned immortal mouse podocyte cell line was kindly provided by Professor Maria Pia Rastaldi (S.C. Hospital of the University of Milan, Milan, Italy). Cells were cultured in RPMI-1640 medium (cat. no. 11875093; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (cat. no. 10100147; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin-streptomycin (cat. no. SV30010; Hyclone; Cytiva) and 10 U/ml of mouse recombinant interferon-γ (cat. no. 39127S; Cell Signaling Technology, Inc.) with 5% CO2, at 33˚C to induce proliferation. The podocytes were then cultured with 5% CO2 at 37˚C without interferon-γ when they reached 60-70% confluence. The cells changed from spindle-liked to star-liked after 7-10 days, indicating that they had fully differentiated, At this point, they were used in subsequent experiments.
The complement membrane attack complex, C5b-9, was established using normal human serum as a complement source and treated with zymosan (cat. no. Z4250; Sigma-Aldrich; Merck KGaA) as described by Liu et al (23). The normal human serum used in this study was provided by a 23-year-old male in November 2018. The serum was provided by healthy volunteers that provided written informed consent. The study received ethical approval from the Medical Ethics Committee of Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University (Beijing, China). To evaluate the effect of C5b-9, mouse podocyte cells (MPC) were treated with different concentrations of zymosan activated serum (ZAS) for 1, 2, 4, 8, 12 or 24 h (C5b-9 group), and heat inactivated serum was used as the control (blank group). The volume of zymosan required to increase lactate dehydrogenase (LDH) release by <10% was used as a sublethal dose to induce podocyte injury. The cells were also pretreated with 3-MA (cat. on. 3977; R&D Systems, Inc.) rapamycin (cat. no. 1292; R&D Systems, Inc.) or DKK1 (cat. no. 5897-DK; R&D Systems, Inc.) before treatment with ZAS. The group preincubated with 3-MA before using ZAS was called the 3-MA group, the group preincubated with rapamycin was called the rapamycin group, and the group preincubated with DKK1 was called the DKK1 group.
C5b-9 ELISA
Following the manufacturer's protocol, a C5b-9 ELISA kit (cat. no. A020; Quidel Corporation, Inc) was used to determine the levels of C5b-9 in the serum of samples incubated with zymosan. The optical density was measured at 450 nm using a plate reader.
LDH assay
The optimum concentration and incubation time for C5b-9 was determined using an LDH release test, which utilized a non-radioactive cytotoxicity assay (cat. no. G1780; Promega Corporation). Mature and differentiated MPCs suspended in RPMI1640 medium supplemented with 10% inactivated FBS, were plated in 96 well cell plates (7x103 cells/well). Podocytes were measured following exposure to zymosan for different periods of time at 490 nm using a plate reader, according to the manufacturer's protocol. The formula used to calculate LDH release was: LDH release rate (%) = (experimental-target spontaneous)/(target maximum-target spontaneous) x100. Experimental group refers to the OD value of cell pore after relevant intervention stimulation. Target spontaneous group refers to the OD value of normal cell pores without intervention or stimulation. Target maximum group refers to the OD value of the cell pore after adding the lysate provided by the kit.
Immunofluorescence staining of cultured podocytes
The podocyte samples were fixed with 4% paraformaldehyde for 20 min at room temperature, rinsed with PBS (cat. no. KGB5001; Nanjing KeyGen Biotech Co., Ltd.) and then permeabilized with 0.5% Triton X-100 (Applygen Technologies, Inc.) in PBS for 30 min at room temperature. Non-specific binding was blocked with 5% BSA (cat. no. SW3015; Beijing Solarbio Science & Technology Co., Ltd.) for 1 h at room temperature. The podocytes were incubated with primary antibodies overnight at 4˚C. The following day, the podocytes were washed with PBS, labeled with secondary antibodies using 2 drops/ml for 30 min at room temperature and incubated with Alexa Fluor 488 Phalloidin (cat. no. A12379 Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at room temperature. Finally, podocytes were washed with PBS and sealed using antifade mounting reagent containing DAPI (cat. no. S2110; Beijing Solarbio Science & Technology Co.).
The primary antibodies used were: Anti-C5b-9 rabbit polyclonal antibody (pAb) (1:1,000; cat. no. ab55811; Abcam), anti-podocin rabbit monoclonal antibody (mAb) (1:500; cat. no. ab181143; Abcam), anti-beclin-1 rabbit mAb (1:50; cat. no. ab217179; Abcam), anti-LC3A/B rabbit antibody (1:200; cat. no. 4108; Cell Signaling Technology, Inc.), anti-SQSTM1 mouse mAb (1:100; cat. no. ab109012; Abcam), anti-β-catenin rabbit mAb (1:100; cat. no. 8480; Cell Signaling Technology, Inc.), anti-GSK-3β rabbit mAb (1:50; cat. no. 12456; Cell Signaling Technology, Inc.) and anti-Akt rabbit mAb (1:400; cat. no. 4691; Cell Signaling Technology, Inc. ). The secondary antibodies used were goat anti-rabbit IgG (H+L) Alexa Fluor 594 (cat. no. R37117; Invitrogen; Thermo Fisher Scientific, Inc.) and goat anti-mouse IgG (H+L) Alexa Fluor 594 (cat. no. R37121; Invitrogen; Thermo Fisher Scientific, Inc.).
Capillary western immunoassay (WES)
According to the manufacturer's protocols, the 12-230 kDa separation module (ProteinSimple; Bio-Techne Corporation) and anti-mouse detection module (ProteinSimple; Bio-Techne Corporation) were used for analysis using a WES system (ProteinSimple; Bio-Techne Corporation) as previously described (24). Proteins were extracted from cells using RIPA lysate and protease inhibitor, The concentration of the extracted protein was determined using a BCA kit (cat. no. PC0200, Beijing Solarbio Science & Technology Co.). Extracted cell proteins were diluted with 5x master mix and 0.1x sample buffer provided with the kit. Antibody diluent II provided in the kit was then used to dilute the primary antibody. Diluted protein, antibody diluent II, diluted primary antibody, secondary HRP conjugate, luminol-conjugate mix and wash buffer were added to each row of the plate provided in the kit. Finally, a WES dedicated instrument (ProteinSimple; Bio-Techne Corporation) and Compass software 4.0.0 (ProteinSimple; Bio-Techne Corporation) was used to process the plate. After 2.5 h, the corresponding band of each protein sample was obtained. The concentration of the band was analyzed to determine the corresponding protein content using Compass software.
Anti-nephrin rabbit mAb (1:50; cat. no. ab216341; Abcam), anti-podocin rabbit mAb (1:50; cat. no. ab181143; Abcam), anti-podocalyxin-like 1 mouse antibody (1:10; cat. no. sc-23903; Santa Cruz Biotechnology, Inc.), anti-podoplanin mouse antibody (1:10; cat. no. sc-166906; Santa Cruz Biotechnology, Inc.), anti-beclin-1 rabbit mAb (1:50; cat. no. ab217179; Abcam), anti-LC3A/B rabbit pAb (1:50; cat. no. ab62721; Abcam), anti-SQSTM1 mouse mAb (1:50; cat. no. ab109012; Abcam), anti-β-catenin rabbit mAb (1:50; cat. no. 8480; Cell Signaling Technology, Inc.), anti-phosphorylated (p)-β-catenin rabbit mAb (S675; 1:50; cat. no. 4176; Cell Signaling Technology, Inc.), anti-p-β-catenin rabbit mAb (S552; 1:50; cat. no. 5651; Cell Signaling Technology, Inc.), anti-GSK-3β rabbit mAb (1:50; cat. no. 12456; Cell Signaling Technology, Inc.), anti-p-GSK-3β rabbit mAb (S9; 1:50; cat. no. 5558; Cell Signaling Technology, Inc.), anti-p-GSK-3β rabbit pAb (Y216; 1:50; ab75745; Abcam), anti-Akt rabbit mAb (1:50; cat. no. 4691; Cell Signaling Technology, Inc.) and anti-GAPDH rabbit mAb (1:50; cat. no. 5174; Cell Signaling Technology, Inc.) were used as primary antibodies to detect the expression of their respective protein targets in the different cell groups.
Statistical analysis
SPSS version 20.0 (IBM, Corp.) was used for statistical analysis. Data are expressed as the mean ± standard deviation, or as counts or percentages as relevant. Comparisons between two-groups were performed using an independent-sample t-test unless otherwise indicated. Comparisons between multiple-groups were performed using an ANOVA followed by a least significant difference or Student-Newman-Keuls tests for ≤3 groups or Tukey's test for >3 groups to verify the data. P<0.05 was considered to indicate a statistically significant difference.
Results
C5b-9 serum-mediates injury of podocytes
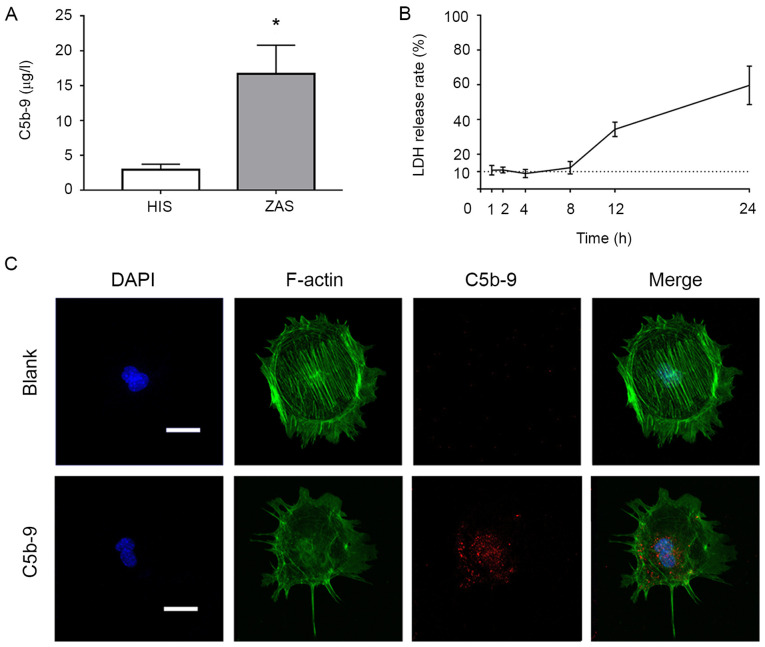
Firstly, to mimic the formation of the membrane attack complex in vitro, using human blood as the complement source, the content of C5b-9 in the extracted serum was measured using ELISA (Fig. 1A). Glomerular epithelial cells were incubated with C5b-9 serum for differing periods of time, and their respective LDH release rates were calculated (Fig. 1B). A duration of 2 h was used as the model of hypo-lysis.
Figure 1.
Effects of C5b-9 and different concentrations of Rapamycin, 3-MA and DKK1 on podocytes. (A) The content of C5b-9 in human serum after incubation with Zymosan was determined by ELISA. (B) LDH release rates at different time points were determined to reflect the cytotoxicity of C5b-9. (C) C5b-9 damaged podocytes. Immunofluorescence staining of the cytoskeleton (green) and C5b-9 (red) in glomerular epithelial cells indicated that cell size in the C5b-9 group was smaller than that of the blank group, that fluorescence intensity was weaker and the cytoskeleton was more disordered. Scale bar, 10 µm. *P<0.05 (Student's t-test). C5b-9, complement 5b-9; 3-MA, 3-methyladenine; DKK1, Dickkopf-related protein 1; LDH, lactate dehydrogenase; F-actin, filamentous actin, HIS, human inactivated serum; ZAS, zymosan activated serum.
Immunofluorescence staining was performed on the cytoskeleton of podocytes in each group with using phalloidin, and it was demonstrated that the cells in the C5b-9 group exhibited weaker fluorescence intensity and a more disordered cytoskeleton in comparison with the blank group (Fig. 1C).
3-MA, rapamycin and DKK1 protect against C5b-9-mediated injury of podocytes
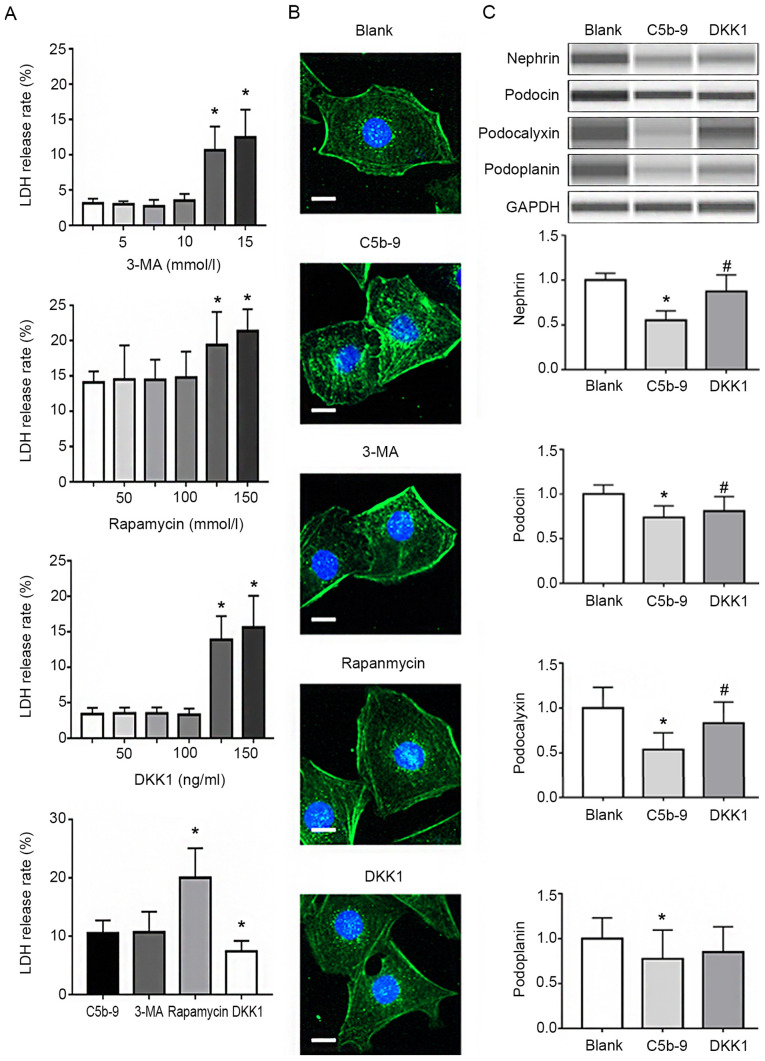
In order to better understand the protective effects of the autophagy and Wnt/β-catenin pathways on podocytes, 3-MA, rapamycin and DKK1 were used to preculture podocytes that were subsequently damaged using C5b-9. Firstly, LDH release rates of podocytes incubated with different concentrations of 3-MA, rapamycin and DKK1 were calculated, and then the maximum concentration possible with minimal cell damage to cells was selected. As shown in Fig. 2A, the concentration of 3-MA used for subsequent experiments was 10 mmol/l, 100 nmol/l for rapamycin and 100 ng/ml for DKK1. Subsequently, the above concentrations were used to pretreat the podocytes prior to C5b-9 injury, and the LDH release rates were measured. DKK1 reduced the release rate of LDH in comparison to that with C5b-9 alone, whereas 3-MA and rapamycin did not. This suggested that 3-MA and rapamycin pretreatment did not protect podocytes, whereas DKK1 pretreatment did (Fig. 2A). Immunofluorescence staining of podocin was used to detect changes in the treated cells. The results of injury analysis were consistent with LDH release rates (Fig. 2B). Finally, WES was used to determine the effects of DKK1 on podocin, nephrin, podoplanin and podocalyxin in podocytes. The results indicated that DKK1 protected podocytes from damage caused by C5b-9 (Fig. 2C).
Figure 2.
C5b-9 can damage podocytes and DKK1 can repair the damage. (A) In order to select the optimal concentration of DKK1, normal podocytes were incubated with different concentrations of Rapamycin, 3-MA and DKK1 and their LDH release rates calculated. The maximum concentration with the least damage was selected. *P<0.05 (Student's t-test). (B) Immunofluorescence staining for podocin in the blank, C5b-9, rapamycin, 3-MA and DKK1 groups. Scale bar, 1 µm. (C) WES was used to determine the levels of nephrin, podocin, podocalyxin and podoplanin of glomerular epithelial cells in the blank, C5b-9 and DKK1 groups. *P<0.05 vs. blank group; #P<0.05 vs. C5b-9 group. C5b-9, complement 5b-9; 3-MA, 3-methyladenine; DKK1, Dickkopf-related protein 1; LDH, lactate dehydrogenase.
Effects of C5b-9 serum on autophagy
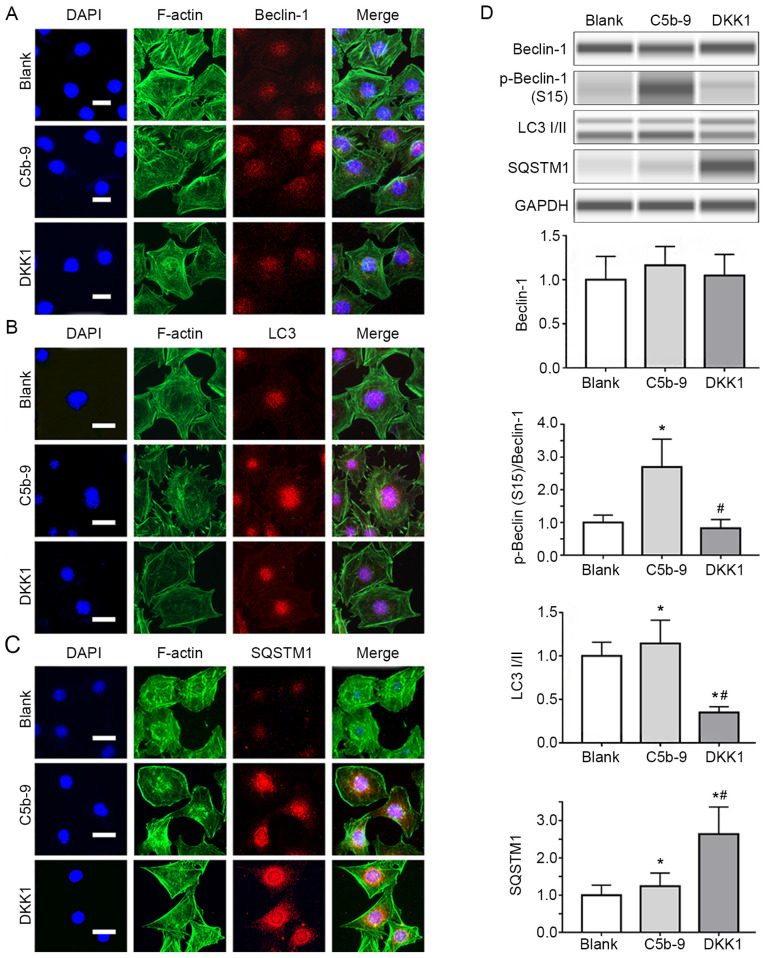
The levels of beclin-1, LC3 and SQSTM1 expression in podocytes of the blank group, C5b-9 group and DKK1 group were detected using immunofluorescence.
The arrangement of the cytoskeleton in the C5b-9 group was more disordered compared with the blank group, and the cytoskeleton of the DKK1 group did not exhibit the disorganization of the C5b-9 group. The fluorescence intensity of beclin-1 showed no significant difference among the three groups (Fig. 3A), and LC3 in the C5b-9 group was stronger compared with the blank group and DKK1 group (Fig. 3B), and the fluorescence intensity of SQSTM1 was stronger compared with the blank group, whereas in the DKK1 group, the fluorescence intensity was slightly stronger compared with the other two groups (Fig. 3C). These results suggested that C5b-9 activated autophagy.
Figure 3.
DKK1 successfully inhibited autophagy activated by C5b-9. (A) Immunofluorescence staining for Beclin-1 in blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (B) Immunofluorescence staining for LC3 in blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (C) Immunofluorescence staining for SQSTM1 in blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (D) Beclin-1, phospho-beclin-1 (S15), LC3I/II and SQSTM1 proteins were determined by WES in blank, C5b-9 and DKK1 groups. *P<0.05 vs. blank group; #P<0.05 vs. C5b-9 group. C5b-9, complement 5b-9; DKK1, Dickkopf-related protein 1; LDH, lactate dehydrogenase; F-actin, filamentous actin; .SQSTM1, sequestosome 1; LC3, microtubule-associated protein light chain 3.
Beclin-1, p-beclin-1 (S15), LC3I/II and SQSTM1 expression levels were detected using WES to reflect the changes in autophagy in podocytes in each group. Beclin-1 expression slightly increased in the C5b-9 group compared with the blank group, and in the DKK1 group, beclin-1 expression was slightly lower than that of the C5b-9 group, and similar to that of the blank group, though the difference was not statistically significant. LC3II and p-beclin-1 (S15) levels increased in the C5b-9 group compared with the blank group and were lower in the DKK1 group compared with the C5b-9 group and similar to that in the blank group. SQSTM1 levels in the C5b-9 group were slightly higher than that in the blank group, while in the DKK1 group its expression was significantly higher compared with the C5b-9 group. This suggests that C5b-9 activates autophagy in glomerular epithelial cells and that this can be inhibited by DKK1 (Fig. 3D).
Effect of C5b-9 serum on the Wnt/β-catenin signaling pathway
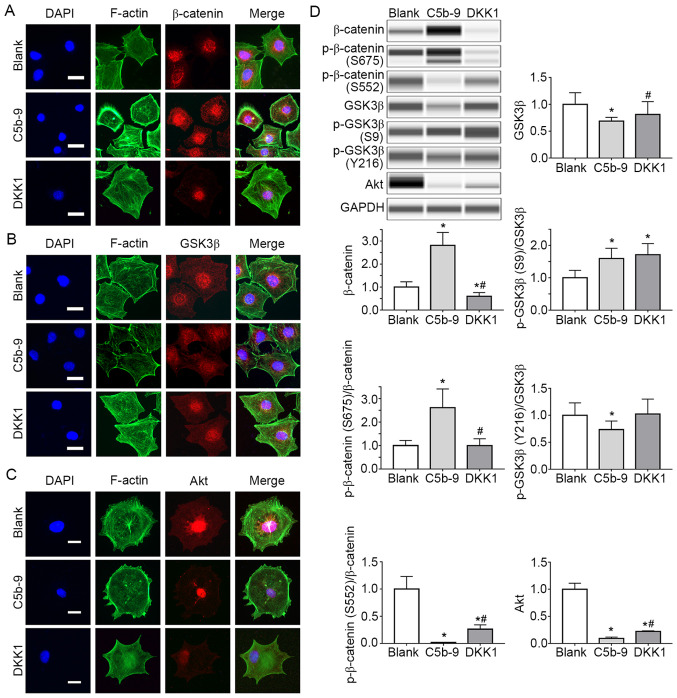
The levels of β-catenin, GSK-3β and Akt in podocytes of blank group, C5b-9 group and DKK1 group were assessed based on immunofluorescence staining. The fluorescence intensity of β-catenin in the C5b-9 group was stronger than that of the blank group and DKK1 group (Fig. 4A), and the fluorescence intensity of the GSK-3β was weaker than that of the blank group and DKK1 group (Fig. 4B). This suggests that C5b-9 activates the Wnt/β-catenin signaling pathway.
Figure 4.
DKK1 successfully inhibited the Wnt/β-catenin signal pathway activated by C5b-9 and simultaneously increased the Akt. (A) Immunofluorescence staining for β-catenin in blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (B) Immunofluorescence staining for GSK-3β in blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (C) Immunofluorescence staining for Akt in blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (D) β-catenin, phosphorylayed-β-catenin (S675, S552), GSK-3β, phosphorylated-GSK-3β (S9, Y216) and Akt proteins were determined by WES in blank, C5b-9 and DKK1 groups. *P<0.05 vs. blank group; #P<0.05 vs. C5b-9 group. C5b-9, complement 5b-9; DKK1, Dickkopf-related protein 1; F-actin, filamentous actin; GSK3, glycogen synthase kinase 3.
At the same time, the changes in the levels of canonical Wnt pathway members [GSK-3β, p-GSK-3β (S9), β-catenin and p-β-catenin (S675, S552)] were detected. GSK-3β expression in the C5b-9 group was significantly lower compared with the blank group, whereas in the DKK1 group, GSK3-β expression was significantly higher than the C5b-9 group and similar to that in the blank group. β-catenin, p-β-catenin (S675) and p-GSK-3β (S9) levels in the C5b-9 group were significantly higher than those in the blank group. However, the β-catenin levels in the DKK1 group were significantly lower than the other two groups. p-β-catenin (675) levels were lower in the C5b-9 group and similar to that of the blank group. p-GSK-3β (S9) levels were significantly higher in the DKK1 group in comparison with the other two groups. These results suggest that the membrane attack complex activates the Wnt/β-catenin pathway in glomerular epithelial cells. However, the p-β-catenin (S552) level in the C5b-9 group was significantly lower compared with the blank group. In addition, p-β-catenin levels in the DKK1 group were lower than in the blank group, but higher than that in the C5b-9 group (Fig. 4D).
In order to explain this phenomenon, immunofluorescence staining and WES were used to detect Akt levels in each group. The results indicated that the fluorescence intensity was weaker in the C5b-9 group, and slightly stronger in the DKK1 group I comparison with the C5b-9 group (Fig. 4A-D). WES showed that Akt expression in the C5b-9 group was significantly lower compared with the blank group, whereas Akt levels in the DKK1 group were higher than those in the C5b-9 group, but still lower than those in the blank group (Fig. 4D).
Discussion
In the present study, three important observations were made: Firstly, the results suggested that C5b-9 activated autophagy and the Wnt/β-catenin signaling pathway. Secondly, it was indicated that DKK1-mediated inhibition of the Wnt/β-catenin signaling pathway reduced C5b-9-induced damage to glomerular epithelial cells. Thirdly, it appeared that autophagy was inhibited and DKK1 protected podocytes.
The purpose of the present study was to observe changes to autophagy and the Wnt/ catenin signaling pathway in podocytes after C5b-9 injury. Therefore, podocytes were pretreated with 3-MA, rapamycin and DKK1 before C5b-9 treatment, and the condition of podocytes was observed by immunofluorescence. The Wnt/β-catenin signaling pathway is associated with autophagy and it has been reported that activation of GSK-3β can inhibit mammalian target of rapamycin complex 1 (mTORC1) to activate autophagy by promoting the binding of tuberous sclerosis 1/2(25). It has also been reported that β-catenin enucleation inhibits autophagy by inhibiting SQSTM1 transcription (22). Thus, inhibition of the Wnt/β-catenin signaling pathway can activate autophagy (26,27). However, the majority of these previous studies were carried out in tumor cells. To the best of our knowledge, the present study is the first to evaluate the effects of C5b-9 on podocyte Wnt/β-catenin signaling pathway and autophagy, mimicking the process in the human body. The aim of the present study was to determine whether damage of glomerular epithelial cells could be reduced, and thus highlight a potential treatment strategy for idiopathic membranous nephropathy.
C5b-9, via the lectin complement pathway, is the primary cause of podocyte injury in idiopathic membranous nephropathy (28). Studies on the pathogenesis of idiopathic membranous nephropathy have revealed the molecular mechanism underlying complement activation (29). IgG4 is a major subtype of IgG deposited in the glomeruli of idiopathic membranous nephropathy (30,31). IgG4 does not activate the classical complement pathway (32). Therefore, there may be other complement activation mechanisms. Preliminary evidence suggests that specific IgG4 anti-PLA2R antibody activates the alternative complement pathway or the mannose-lectin pathway (33,34). The results of the present study are consistent with those commonly seen in patients with idiopathic membranous nephropathy, where C4 is deposited on the glomeruli in the absence of Complement 1q. Eventually, C5b-9 dissolves the glomerular epithelial cells, leading to the formation of proteinuria (10,35). In addition, zymosan, a polysaccharide prepared from the cell walls of saccharomyces cerevisiae, activates the alternative complement pathway (36). Therefore, this complement pathway was stimulated in the present study, and incubated in normal serum with zymosan to reduce the injury to podocytes as much as possible.
Results of the DKK1 pretreatment of podocytes showed that expression of beclin-1 and LC3II were decreased, whereas SQSTM1 expression was significantly increased. DKK1 activates GSK-3β by inhibiting the Wnt/β-catenin pathway, which was hypothesized to indirectly inhibit mTORC1 to activate beclin-1 and thus activate autophagy. However, completely opposing results were obtained in the present experiment. In addition, when the Wnt/β-catenin signaling pathway was activated by complement, GSK-3β (S9) phosphorylation levels were increased, whereas β-catenin (S552) phosphorylation was significantly reduced, and thus, this phenomenon may be explained simply by Akt protein abnormalities (37). As Akt phosphorylates both GSK-3β and β-catenin, GSK3β is inhibited and β-catenin is activated, thereby activating the Wnt/β-catenin pathway (38). Thus, it was hypothesized that this anomaly was related to Akt, and this was demonstrated in subsequent experiments. Thus, the canonical Wnt signaling pathway and other related signal proteins may have variations in the cell line used. However, it is also possible that complement attack mediated damage activated or inhibited the targets of multiple signaling pathways, thus resulting in the aforementioned phenomenon.
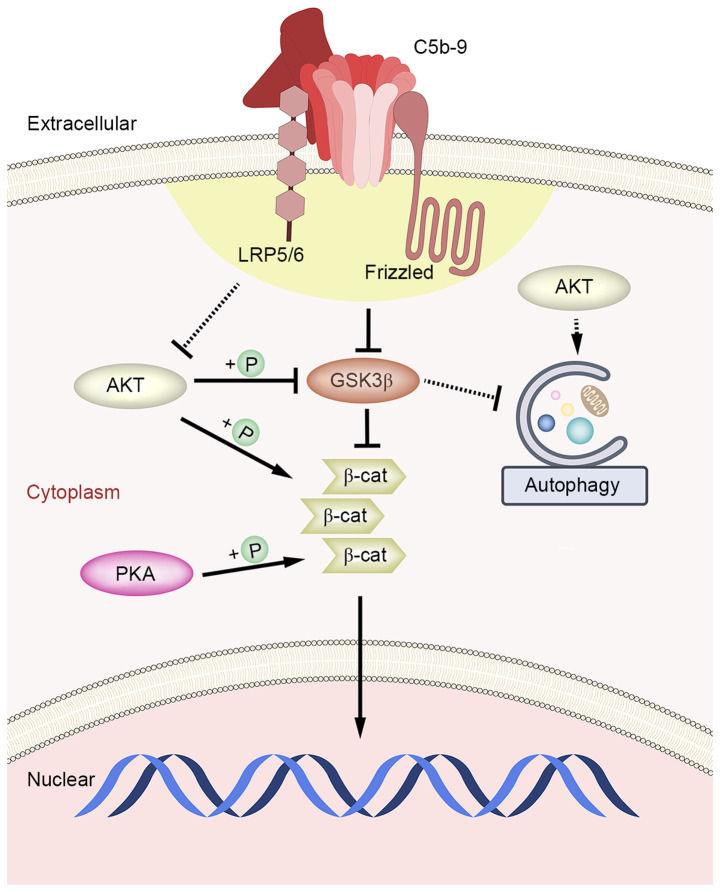
The results of the present study indicated that Akt protein was damaged by C5b-9, and that DKK1 inhibited the canonical Wnt pathway, which can repair cell damage and restore Akt, which in-turn phosphorylates β-catenin (S552) and reduces the levels of p-GSK-3β (S9). Thus, autophagy may be inhibited due to the activation of Akt (39). This suggests that C5b-9, while activating the Wnt/β-catenin signaling pathway, acts directly on low density lipoprotein receptor-related protein 5/6 (LRP5/6), which is the target of DKK1(40), and other proteins together to cause damage to Akt. DKK1 inhibits LRP5/6 and restores Akt function to some extent, but not completely (Fig. 5). In addition, variations in signaling pathways in various cells and in complement, which are common pathogenic factors in the human body that are not commonly assessed in cellular experimental methods, require further study.
Figure 5.
Schematic diagram of the mechanism of the effect of C5b-9 on Wnt/β-catenin signaling pathway and autophagy in glomerular epithelial cells. After the activation of Wnt/β-catenin signaling pathway by C5b-9, GSK3 was weakened, thus might playing an indirect inhibitory effect on autophagy. However, C5b-9 also damaged Akt while activating the Canonical Wnt pathway in glomerular epithelial cells, leading to the activation of autophagy ultimately. C5b-9 group. C5b-9, complement 5b-9; GSK3, glycogen synthase kinase 3; AKT, protein kinase B; PKA, protein kinase A; +P, phosphorylation; β-cat, β-catenin.
In conclusion, inhibition of the Wnt/β-catenin signaling pathway may protect podocytes from damage caused by complement within a certain period of time, and one of the mechanisms of this is through inhibition of autophagy. However, the specific role of signaling proteins, such as GSK-3β and β-catenin remains unclear and requires further study. The results of the present study also suggest that complement aggravates the damage to glomerular epithelial cells in idiopathic membranous nephropathy through over-activation of the Wnt/β-catenin signaling pathway, and pathway inhibitors such as DKK1 may serve as a novel approach to treat this disease.
Acknowledgements
The authors are grateful for associate researcher Yan Lin and Lei Zhang and assistant researcher Lu Zhang as staff of the Institute of Traditional Chinese Medicine of Beijing Hospital of Traditional Chinese Medicine for their guidance on the experimental operation.
Funding Statement
Funding: This work was supported by grants from the National Natural Science Foundation of China (grant nos. 81673907 and 81973793 to LB), Natural Science Foundation of Beijing Municipality (grant no. 7182070 to LB) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of special funding support (grant no. XLMX201833 to LB).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BL, HD and WeiL were responsible for conception of the study. BL, ZD, FL, YG, ZZha and FM were responsible for the cell experiments and the detection of related indicators. HD, XX and ZZhu were responsible for data analysis and interpretation. ZD and FL participated in the drafting of the manuscript. BL, ZF and WenL were responsible for conception of the study and responsible for critical revision of important content, and undertook part of the data analysis and interpretation work. BL and HD are responsible for approving the final version to be published and agree to be responsible for all aspects of the work and to ensure that issues relating to the accuracy or completeness of any part of the work are properly investigated and resolved. The authenticity of the original data in this paper has been confirmed by BL and HD and they are responsible for this. All authors read and approved this manuscript.
Ethics approval and consent to participate
Ethical approval was obtained from the Medical Ethics Committee of Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University. Blood donors provided their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhou L, Liu Y. Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015;11:535–545. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai H, Liu Q, Liu B. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res. 2017;2017(2615286) doi: 10.1155/2017/2615286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai H, Liu F, Qiu X, Liu W, Dong Z, Jia Y, Feng Z, Liu Z, Zhao Q, Gao Y, et al. Alleviation by Mahuang Fuzi and Shenzhuo decoction in high glucose-induced podocyte injury by inhibiting the activation of Wnt/β-catenin signaling pathway, resulting in activation of podocyte autophagy. Evid Based Complement Alternat Med. 2020;2020(7809427) doi: 10.1155/2020/7809427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronco P, Debiec H. Pathophysiological advances in membranous nephropathy: Time for a shift in patient's care. Lancet. 2015;385:1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Gao C, Dai H, Zheng Y, Dong Z, Gao Y, Liu F, Zhang Z, Liu Z, Liu W, et al. Immunological pathogenesis of membranous nephropathy: Focus on PLA2R1 and its role. Front Immunol. 2019;10(1809) doi: 10.3389/fimmu.2019.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Gao C, Liu Z, Dai H, Feng Z, Dong Z, Zheng Y, Gao Y, Tian X, Liu B. Idiopathic membranous nephropathy: Glomerular pathological pattern caused by extrarenal immunity activity. Front Immunol. 2020;11(1846) doi: 10.3389/fimmu.2020.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nangaku M, Shankland SJ, Couser WG. Cellular response to injury in membranous nephropathy. J Am Soc Nephrol. 2005;16:1195–1204. doi: 10.1681/ASN.2004121098. [DOI] [PubMed] [Google Scholar]
- 9.Cybulsky AV, Takano T, Papillon J, McTavish AJ. Complement-induced phospholipase A2 activation in experimental membranous nephropathy. Kidney Int. 2000;57:1052–1062. doi: 10.1046/j.1523-1755.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- 10.Takano T, Elimam H, Cybulsky AV. Complement-mediated cellular injury. Semin Nephrol. 2013;33:586–601. doi: 10.1016/j.semnephrol.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asanuma K, Tanida I, Shirato I, Ueno T, Takahara H, Nishitani T, Kominami E, Tomino Y, Arnold SJ, Lindenmeyer MT, et al. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J. 2003;17:1165–1167. doi: 10.1096/fj.02-0580fje. [DOI] [PubMed] [Google Scholar]
- 14.Lv Q, Yang F, Chen K, Zhang Y. Autophagy protects podocytes from sublytic complement induced injury. Exp Cell Res. 2016;341:132–138. doi: 10.1016/j.yexcr.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Hong Q, Lv Y, Feng Z, Zhang X, Wu L, Cui S, Hou K, Su H, Huang Z, et al. Autophagy can repair endoplasmic reticulum stress damage of the passive Heymann nephritis model as revealed by proteomics analysis. J Proteomics. 2012;75:3866–3876. doi: 10.1016/j.jprot.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Fan T, Chen L, Huang Z, Wang W, Zhang B, Xu Y, Mao Z, Hu H, Geng Q. Autophagy activation by rapamycin before hypoxia-reoxygenation reduces endoplasmic reticulum stress in alveolar epithelial cells. Cell Physiol Biochem. 2017;41:79–90. doi: 10.1159/000455953. [DOI] [PubMed] [Google Scholar]
- 17.Unno R, Kawabata T, Taguchi K, Sugino T, Hamamoto S, Ando R, Okada A, Kohri K, Yoshimori T, Yasui T. Deregulated MTOR (mechanistic target of rapamycin kinase) is responsible for autophagy defects exacerbating kidney stone development. Autophagy. 2020;16:709–723. doi: 10.1080/15548627.2019.1635382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB, Susztak K. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem. 2011;286:26003–26015. doi: 10.1074/jbc.M111.223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu BL, Chen YP, Cheng H, Wang YY, Rui HL, Yang M, Dong HR, Han DN, Dong J. The protective effects of curcumin on -obesity-related glomerulopathy are associated with inhibition of Wnt/β-catenin signaling activation in podocytes. Evid Based Complement Alternat Med. 2015;2015(827472) doi: 10.1155/2015/827472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petherick KJ, Williams AC, Lane JD, Ordóñez-Morán P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik K, Paraskeva C, et al. Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J. 2013;32:1903–1916. doi: 10.1038/emboj.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu WJ, Li ZH, Chen XC, Zhao XL, Zhong Z, Yang C, Wu HL, An N, Li WY, Liu HF. Blockage of the lysosome-dependent autophagic pathway contributes to complement membrane attack complex-induced podocyte injury in idiopathic membranous nephropathy. Sci Rep. 2017;7(8643) doi: 10.1038/s41598-017-07889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beekman C, Janson AA, Baghat A, van Deutekom JC, Datson NA. Use of capillary Western immunoassay (Wes) for quantification of dystrophin levels in skeletal muscle of healthy controls and individuals with Becker and Duchenne muscular dystrophy. PLoS One. 2018;13(e0195850) doi: 10.1371/journal.pone.0195850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao J, Tyburczy ME, Moss J, Darling TN, Widlund HR, Kwiatkowski DJ. Tuberous sclerosis complex inactivation disrupts melanogenesis via mTORC1 activation. J Clin Invest. 2017;127:349–364. doi: 10.1172/JCI84262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Liu T, Yu M, Li K, Li W. The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells. J Exp Clin Cancer Res. 2018;37(7) doi: 10.1186/s13046-018-0678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li RN, Liu B, Li XM, Hou LS, Mu XL, Wang H, Linghu H. DACT1 overexpression in type I ovarian cancer inhibits malignant expansion and cis-platinum resistance by modulating canonical Wnt signalling and autophagy. Sci Rep. 2017;7(9285) doi: 10.1038/s41598-017-08249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo W, Olaru F, Miner JH, Beck LH Jr, van der Vlag J, Thurman JM, Borza DB. Alternative pathway is essential for glomerular complement activation and proteinuria in a mouse model of membranous nephropathy. Front Immunol. 2018;9(1433) doi: 10.3389/fimmu.2018.01433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronco P, Debiec H. Pathogenesis of membranous nephropathy: Recent advances and future challenges. Nat Rev Nephrol. 2012;8:203–213. doi: 10.1038/nrneph.2012.35. [DOI] [PubMed] [Google Scholar]
- 30.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pellé T, Gaspari F, Suardi F, Gagliardini E, Orisio S, Benigni A, et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borza DB. Alternative pathway dysregulation and the conundrum of complement activation by IgG4 immune complexes in membranous Nephropathy. Front Immunol. 2016;7(157) doi: 10.3389/fimmu.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glassock RJ. Pathogenesis of membranous nephropathy: A new paradigm in evolution. Contrib Nephrol. 2013;181:131–142. doi: 10.1159/000348472. [DOI] [PubMed] [Google Scholar]
- 34.Ma H, Sandor DG, Beck LH Jr. The role of complement in membranous nephropathy. Semin Nephrol. 2013;33:531–542. doi: 10.1016/j.semnephrol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couser WG, Nangaku M. Cellular and molecular biology of membranous nephropathy. J Nephrol. 2006;19:699–705. [PubMed] [Google Scholar]
- 36.Paréj K, Kocsis A, Enyingi C, Dani R, Oroszlán G, Beinrohr L, Dobó J, Závodszky P, Pál G, Gál P. Cutting edge: A new player in the alternative complement pathway, MASP-1 is essential for LPS-induced, but not for zymosan-induced, alternative pathway activation. J Immunol. 2018;200:2247–2252. doi: 10.4049/jimmunol.1701421. [DOI] [PubMed] [Google Scholar]
- 37.Sastre-Perona A, Riesco-Eizaguirre G, Zaballos MA, Santisteban P. β-catenin signaling is required for RAS-driven thyroid cancer through PI3K activation. Oncotarget. 2016;7:49435–49449. doi: 10.18632/oncotarget.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soutto M, Peng D, Katsha A, Chen Z, Piazuelo MB, Washington MK, Belkhiri A, Correa P, El-Rifai W. Activation of β-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut. 2015;64:1028–1039. doi: 10.1136/gutjnl-2014-307191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiza C, Nascimento EB, Ouwens DM. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am J Physiol Endocrinol Metab. 2012;302:E1453–E1460. doi: 10.1152/ajpendo.00660.2011. [DOI] [PubMed] [Google Scholar]
- 40.Katoh M, Katoh M. Molecular genetics and targeted therapy of WNT-related human diseases (Review) Int J Mol Med. 2017;40:587–606. doi: 10.3892/ijmm.2017.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.