Abstract
Psoriasis is a chronic inflammatory skin disease whose etiology has not yet been determined. MicroRNAs (miRs) regulate the early stages of psoriasis and are targets for therapeutic intervention. The present study aimed to investigate the functional role of miR-489-3p in psoriasis. The present study first assessed the expression levels of miR-489-3p and Toll-like receptor (TLR)4 mRNA using reverse transcription-quantitative PCR, and also detected the protein expression levels of TLR4 and NF-κB via western blot analysis. TargetScan and miRDB target gene prediction tools were used to confirm the regulation of Toll-like receptor (TLR)4 by miR-489-3p. Moreover, a Cell Counting Kit (CCK)-8 assay was conducted to evaluate cell viability, while cell cycle and colony formation assays were performed to evaluate cell proliferation. Human keratinocytes (HaCaT) were co-transfected with TLR4-small interfering RNA and miR-489-3p-inhibitor plasmids, and analysis of cell proliferation and inflammatory cytokine secretion was performed using CCK-8 assay and ELISA. It was found that miR-489-3p expression was downregulated in patients with psoriasis. Bioinformatics analysis identified that TLR4 was a direct target of miR-489-3p. This was confirmed via luciferase reporter assays in HaCaT cells. The overexpression of miR-489-3p inhibited the TLR4/NF-κB signaling pathway and reduced cell proliferation. TLR4 silencing alleviated the effects of miR-489-3p, and enhanced cell proliferation and inflammatory cytokine secretion. Taken together, these data suggested that miR-489-3p may be a key effector of psoriasis, which promotes inflammatory responses by direct targeting of TLR4. miR-489-3p therefore represents a promising prognostic biomarker and therapeutic target for psoriasis treatment.
Keywords: psoriasis, miR-489-3p, hyperproliferation, inflammation, TLR4/NF-κB signaling, KCs
Introduction
The typical clinical manifestation of psoriasis include widely distributed scaly erythema or plaques that can affect the skin, joints and fingernails (1). Skin, as the first barrier against infection and injury, contains a large number of immune cells from the epidermis and dermis, including keratinocytes (KCs), Langerhans cells, dermal dendritic cells, macrophages and T lymphocyte (2). Psoriasis is characterized by the excessive proliferation and aberrant differentiation of KCs, but is fully reversible with appropriate therapy (3). Previous studies have reported that psoriasis can be complicated with diabetes, vascular disease, arthritis, Crohn's disease, lymphoma and depression (4,5). The World Health Organization reported in 2016 that the global prevalence of psoriasis ranged from 0.09-11.43%, and it can occur at any age (6). Psoriasis has therefore emerged as a public health concern.
MicroRNAs (miRNAs/miRs) are a class of small, endogenous, single-stranded, non-coding RNAs that serve a key role in proliferation, apoptosis, differentiation, invasion and metabolism (7). miRNAs bind to the 3' untranslated regions (UTR) of specific target mRNAs via complementary base pairing, leading to mRNA degradation and the inhibition of translation (8). miRNAs can directly or indirectly interact with key genes of the inflammatory pathway to regulate inflammatory responses, and serve an important role in classic inflammatory pathways (9). In recent years, the specific roles of miRNAs in psoriasis have become a hot topic of research. For example, Chang et al (10) revealed that miR-126 served an important role in promoting the proliferation and migration of KCs during skin wound healing, but the mechanism of action of miR-489-3p was not reported. The present study performed bioinformatics predictions that identified the ability of miR-489-3p to directly bind to the 3'UTR of TLR4 and downregulate its expression. The activation of TLR4 triggers its downstream effector NF-κB, which translocates to the nucleus (11) and enhances the expression of IL-1β, IL-6, TNF-α and other pro-inflammatory cytokines (12,13). Therefore, preventing inflammatory responses in psoriasis could alleviate disease progression.
KCs can sense injury-related molecular model molecules and/or pathogenesis-related molecular model molecules and activate inflammatory bodies, which induces the inflammatory response in psoriasis (14). The activation of NF-κB in psoriatic KCs, leading to the production of a variety of immune-related proteins, and lead to further inflammatory response (15). In the present study, human KCs (HaCaT cell line) were used for studying psoriasis in vitro, and to evaluate the molecular mechanism of miR-489-3p in inhibiting the inflammatory response of psoriasis.
Materials and methods
Research subjects and sample collection
The specimens were collected from 15 patients with psoriasis between March 2018 and March 2019 in The Third People's Hospital of Hangzhou, including 8 men and 7 women, with an average age of 33.5 years (age range, 24-68 years). The course of disease ranged from 14 months to 12 years (mean, 6.5 years). All patients with psoriasis were diagnosed clinically and pathologically, and all plaques were of progressive plaque type. The Psoriasis Area and Severity Index (PASI) score ranged from 10-20(16). The inclusion criteria were as follows: Patients with psoriasis had typical erythematous; scaling plaques confirmed by histopathology; systemic therapy including immunosuppressants, biological agents, glucocorticoids and retinoic acids were not used within the first 3 months, and external drug therapy and phototherapy ceased for 1 month prior to the study; no accompanying autoimmune disease, neoplastic disease or inflammatory disease. The exclusion criteria were as follows: Patients with chronic plaque psoriasis in the involuting and stable stage; pregnancy, lactation and menstruation in female patients. The healthy controls showed no history of psoriasis or other skin defects, and no autoimmune or systemic disease, as well as were comparable to the sex and age of the patients.
All participants provided written informed consent for their participation. The study protocol was designed and implemented in accordance with the principles of the Helsinki Declaration and was approved by The Third People's Hospital of Hangzhou Ethics Review Committee.
Cell culture
Human KCs (HaCaT cell line) were purchased from Guangzhou Jennio Biological Technology (Guangzhou, China). Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific Inc.) containing 1% non-essential amino acids and 10% FBS (Thermo Fisher Scientific, Inc.) at 37˚C with 5% CO2. Medium was replaced every 2 days and cells were passaged when 70-80% confluent.
Cell transfections
Cells were digested with trypsin and then seeded in 6-well cell culture plates at 5x105/well. When the cells reached 60% confluence, they were transfected with 100 nM miR-489-3p mimic, 100 nM miR-489-3p mimic negative control using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's procedure for 24 h at 37˚C with 5% CO2. miR-489-3p mimic, miR-489-3p mimic negative control (mimic-con), miR-489-3p inhibitor and miR-489-3p inhibitor negative control (inhibitor-con) were purchased from Shanghai GenePharma Co., Ltd. (miR-489-3p mimics-con, 5'-UUCUCCGAACGUGUCACGUTT-3'; miR-489-3p mimic 5'-GUGACAUCACAUAUACGGCAGC-3'; miR-489-3p inhibitor-con, 5'-CAGUACUUUUGUGUAGUACAA-3'; and miR-489-3p inhibitor 5'-GCUGCCGUAUAUGUGAUGUCAC-3'). Transfection efficiency was verified 24 h later via reverse transcription-quantitative (RT-q)PCR.
TLR4 silencing
Cells (1x106) were transfected with 1.0 µg TLR4 small interfering (si) RNA5'-CCGGCCGCTGGTGTATCTTTGAATACTCGAGTATTCAAAGATACA CCAGCGGTTTTTG-3') or scrambled siRNA (si-con, 5'-CCGGCTCCGGGTGTATCGTTTAATACTCGAGTCTAT AGAAATACACCAGGGCTTTTTG-3') as a negative control (cat. no. sc-40260; Santa Cruz Biotechnology, Inc.) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's procedure for 24 h at 37˚C with 5% CO2.
Post-transfection, cells were cultured in complete media for 48 h. TLR4-siRNAs were purchased from Shanghai GenePharma Co., Ltd. Transfection efficiency was verified 48 h later via RT-qPCR.
miRNA target prediction and dual luciferase reporter assays
TargetScan v.7.2 (http://www.targetscan.org/mamm_31/) and miRDB (http://mirdb.org/) miRNA target prediction database were used to identify the target genes of has-miR-489-3p. TLR4 3'UTR wild-type (WT) and TLR4 3'UTR mutant (MUT) plasmids were cloned into pGL3 luciferase reporter vectors (Promega Corporation). HaCaT cells were seeded into 6-well plates at a density of 5xl05/well. Cells were co-transfected with TLR4 luciferase reporter constructs (1 µg), miR-489-3p mimics (100 nM) or miR-489-3p mimic-con using Lipofectamine 2000 at 37˚C with 5% CO2 for 24 h. Luciferase activity was assessed using a Dual-Luciferase Reporter Assay system (Promega Corporation) after 24 h post-transfection. Relative luciferase activities were calculated by firefly luciferase activities/Renilla luciferase activities.
Cell proliferation assays
Cell proliferation was determined using Cell Counting Kit-8 (CCK-8) assays (MedChemExpress) according to the manufacturer's protocol Cells were seeded at the density of 4x104 cells/well into 96-well plates and incubated for 0-3 days, and then CCK-8 (10 µl) were added at different time points (0, 24, 48 and 72 h) at 37˚C and further cultured for 2 h. The optical densities were measured at 450 nm using an automatic microplate reader (INFINITE M200; Tecan Group, Ltd.).
Colony formation assays
Cells were seeded in 12-well plates (~300 cells/well) 24 h post-transfection. Cells were cultured at 37˚C with 5% CO2 for 14 days, and the medium was replaced every 3 days. Cells were stained with crystal violet (2%) at 37˚C for 30 min and observed and photographed under a light microscope (Olympus Soft Imaging Solutions GmbH) and colonies ≥50 cells were counted with Image J software v.1.52 (National Institutes of Health). Experiments were performed on a minimum of three independent occasions.
Cell cycle assays
Cell cycle analysis was performed via flow cytometry. Cells (2x106 cells/ml) in 6-well plates were trypsinized and fixed in pre-chilled 75% ethanol at 4˚C overnight. Cells were harvested by centrifugation at 800 x g at 4˚C for 10 min. The supernatants were discarded and then resuspended in PBS with 50 µg/ml propidium iodide (PI), 100 µg/ml RNase A and 0.2% Triton X-100 and incubated in the dark for 30 min at room temperature. PI, RNase A and Triton X-100 were all purchased from Sigma-Aldrich; Merck KGaA. Cells were captured on a flow cytometer (FACSCalibur; BD Biosciences), and cell cycle data was analyzed using CellQuest software v.2.0 (BD Biosciences) and ModFit LT v.2.0 (Verity Software House, Inc.).
RT-qPCR
Total RNA was extracted using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.), and RNA concentrations and purity were measured via UV spectrophotometry (Nanodrop Technologies). A total of 1 µg total RNA was reverse transcribed using PrimeScript RT reagent kit (Takara Bio, Inc.). The conditions for RT were as follows: 37˚C for 10 min, followed by 85˚C for 5 sec and followed by holding at 4˚C. SYBR Premix Ex Taq II (Tli RNaseH Plus) (Takara Bio, Inc.) was used to detect miR-489-3p and TLR mRNA expression levels using the ABI PRISM 7000 instrument (Applied Biosystems; Thermo Fisher Scientific Inc.). The housekeeping gene GAPDH was used as the control of TLR4 and the small nuclear RNA U6 was used as the control of miR-489-3p. The thermocycling conditions used were as follows: Denaturation at 95˚C for 30 sec, followed by 40 cycles at 95˚C for 5 sec and 60˚C for 30 sec. The following primers were used: miR-489-3p, forward, 5'-GCGCGGTGACATCACATATAC-3' and reverse, 5'-AGTGCAGGGTCCGAGGTATT-3'; U6 forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3'and reverse, 5'-CGCTTCACGAATTTGCGTGTCAT-3'; TLR4 forward, 5'-TTTGGACAGTTTCCCACATTGA-3' and reverse, 5'-AAGCATTCCCACCTTTGTTGG-3'; and GAPDH forward, 5'-ACAACTTTGGTATCGTGGAAGG-3' and reverse, 5'-GCCATCACGCCACAGTTTC-3'. Melting curves were used to monitor non-specific amplifications. Relative Ct values were quantitated using the 2-∆ΔCq method (17) were ΔΔCq=(Cq target gene-Cq control gene) experimental group-(Cq target gene-Cq control gene) control group.
Western blot analysis
Cell proteins were extracted in RIPA buffer (Sigma-Aldrich; Merck KGaA). Nuclear proteins were extracted using CelLytic™ NuCLEAR™ reagent (Sigma-Aldrich; Merck KGaA). Proteins were quantified using a BCA assay, and equal concentrations (50 µg/lane) were resolved on 12.5% polyacrylamide gels at 130 V. Proteins were transferred to PVDF membranes and blocked in 2.5% skimmed milk at 37˚C for 1 h. Membranes were probed with primary antibodies overnight at 4˚C, including anti-rabbiti-TLR4 (1:1,000; cat no. 13867; Abcam), anti-rabbit-NF-κB p65 (1:1,000; cat no. 3034; Cell Signaling Technologies, Inc.), phospho-NF-κB p65 (1:1,000; cat no. 3033; Cell Signaling Technologies, Inc.), anti-rabbit-Lamin B (1:2,000; cat no. 194109; Abcam) and anti-rabbit-β-actin (1:2,000; cat no. 115777; Abcam). Membranes were washed in TBS containing 0.1% Tween 20 and labeled with Goat Anti-Rabbit IgG H&L (HRP) antibodies (1:3,000; cat no. 7090; Abcam) for 1 h at room temperature. Membranes were then washed and proteins were visualized using the ECL detection system (Amersham; Cytiva) on a ChemiDoc Bio-Rad system (Bio-Rad Laboratories, Inc.). Band intensities were semi-quantified using ImageJ software v.1.52v (National Institutes of Health).
ELISA
HaCaT cells (1x106) were seeded into 6-well plates to 80-85% confluence. The cell culture supernatant were collected by centrifugation at 2,000 x g for 10 min at 4˚C in order to remove debris. ELISA assays were performed according to the manufacturer's instructions to detect the secretion of TNF-α (cat no. ab181421; Abcam), IFN-γ (cat. no. ab46025; Abcam), IL-22 (cat. no. ab216170; Abcam), IL-4 (cat no. ab215089; Abcam) and IL-1β (cat no. ab214025; Abcam). Gradient dilution of the standard was used to draw a standard curve. The OD value was evaluated at 450 nm using an automatic microplate reader (INFINITE M200; Tecan Group, Ltd.). Three replicates were performed.
Statistical analysis
Data were analyzed using SPSS 20.0 (IBM Corp.). Unpaired t-tests were used to compare between two groups, and a one-way ANOVA was used to compare multiple groups, followed by Dunnett's post hoc analysis. Pearson's correlation coefficient analysis was used to determine the correlation between two variables. All experiments were repeated three times. P<0.05 was considered to indicate a statistically significant difference. Data are presented as the mean ± SD.
Results
miR-489-3p targets the TLR4/NF-κB axis in psoriasis
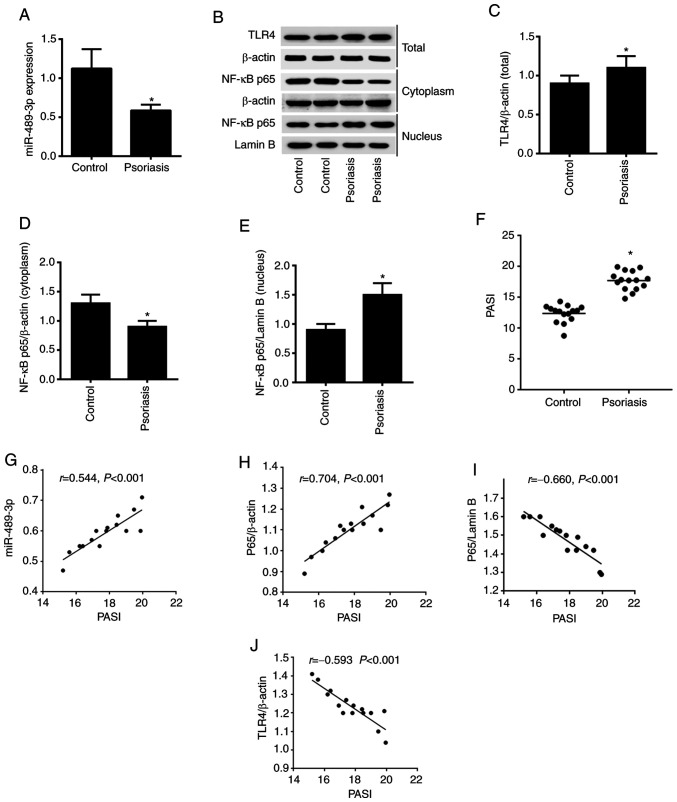
The present study first assessed the expression level of miR-489-3p via RT-qPCR analysis. Downregulation of miR-489-3p expression was observed in psoriasis vs. healthy tissue (Fig. 1A). In addition, upregulation of TLR4 and nuclear NF-κB expression levels, downregulation of cytoplasmic NF-κB expression levels were detected using western blot analysis (Fig. 1B-E). A comparison of the PASI between the patient and health control samples revealed that the PASI was significantly higher in patients with psoriasis (P<0.05; Fig. 1F). The correlation between PASI and miR-489-3p, NF-κB p65 and TLR4 expression levels was determined using Pearson's correlation coefficient analysis. The results demonstrated that PASI was positively correlated with miR-489-3p expression and cytoplasmic NF-κB p65 expression. Moreover, PASI was negatively correlated with NF-κB p65/Lamin B and TLR4 expression (P<0.001; Fig. 1G-J). These results suggested that miR-489-3p expression may promote enhanced inflammation in psoriasis by suppressing NF-κB/TLR4 expression.
Figure 1.
Expression levels of miR-489-3p and TLR4/NF-κB signaling in psoriasis. (A) Expression level of miR-489-3p in skin specimens from patients with psoriasis was detected via reverse transcription-quantitative PCR. (B) Western blot analysis of the protein expression levels of (C) TLR4, (D) cytoplasmic NF-κB p65 and (E) nuclear NF-κB p65 in skin specimens from patients with psoriasis. (F) PASI in patients with psoriasis and healthy controls. Correlations between PASI and (G) miR-489-3p, (H) cytoplasmic NF-κB p65, (I) nuclear NF-κB p65 and (J) TLR4. *P<0.05 vs. Control. TLR4, Toll-like receptor 4; PASI, Psoriasis Area and Severity Index; miR, microRNA.
miR-489-3p inhibits TLR4/NF-κB signaling
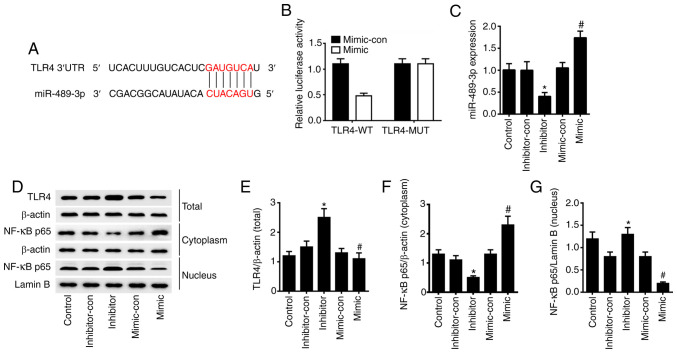
To confirm the regulation of TLR4 by miR-489-3p, TargetScan and miRDBA tools were used to predict potential target genes. From this analysis, it was found that miR-489-3p could bind to the 3'UTR of TLR4 (Fig. 2A). Dual luciferase assays in HaCaT cells transfected with TLR4-WT luciferase reporter identified a significant loss of TLR4 activity in cells co-expressing miR-489-3p mimic (P<0.05). However, no such effect was observed for the TLR4-MUT reporter (P>0.05) in miR-489-3p overexpressing cells (Fig. 2B). RT-qPCR analysis was conducted to verify the transfection efficiency, and the results demonstrated that the expression levels of miR-489-3p were significantly upregulated in HaCaT cells transfected with miR-489-3p mimic, compared with cells transfected with miR-489-3p mimic-con. Furthermore, the expression levels of miR-489-3p were significantly downregulated in HaCaT cells transfected with miR-489-3p inhibitor, compared with cells transfected with miR-489-3p inhibitor-con (P<0.05; Fig. 2C). Western blot analysis confirmed that the overexpression of miR-489-3p inhibited TLR4 and nuclear NF-κB p65 expression and upregulated cytoplasmic NF-κB p65; when miR-489-3p expression was inhibited, the TLR4 and nuclear NF-κB p65 expression were upregulated and the cytoplasmic NF-κB p65 was downregulated (P<0.05; Fig. 2D-G). The results suggested that miR-489-3p upregulation inhibits the inflammatory response of skin cells via TLR4/NF-κB signaling.
Figure 2.
miR-489-3p inhibits TLR4/NF-κB signaling. (A) TLR4 was the target gene of miR-489-3p, as predicted via bioinformatics analysis. (B) HaCaT cells were transfected with luciferase reporter plasmids containing WT or MUT TLR4 3'UTR, together with miR-489-3p mimic or miR-489-3p mimic-con. (C) miR-489-3p expression in HaCaT cells transfected with different plasmids, as detected via reverse transcription-quantitative PCR. (D) Western blot analysis of the protein expression levels of (E) TLR4, (F) cytoplasmic NF-κB p65 and (G) nuclear NF-κB p65 in HaCaT cells transfected with different plasmids. *P<0.05 vs. inhibitor-con; #P<0.05 vs. mimic-con. WT, wild-type; MUT, mutant; UTR, untranslated region; con, negative control; miR, microRNA; TLR4, Toll-like receptor 4.
miR-489-3p inhibits KC hyperproliferation and inflammation
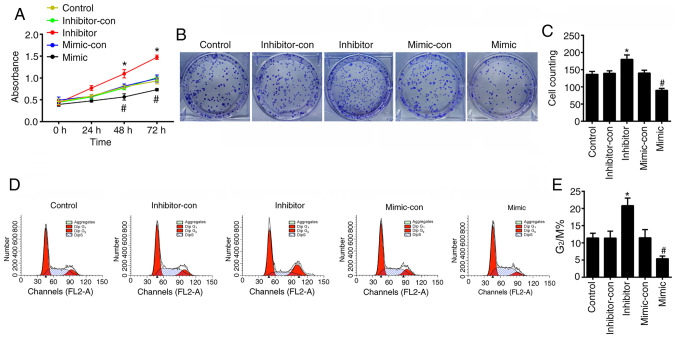
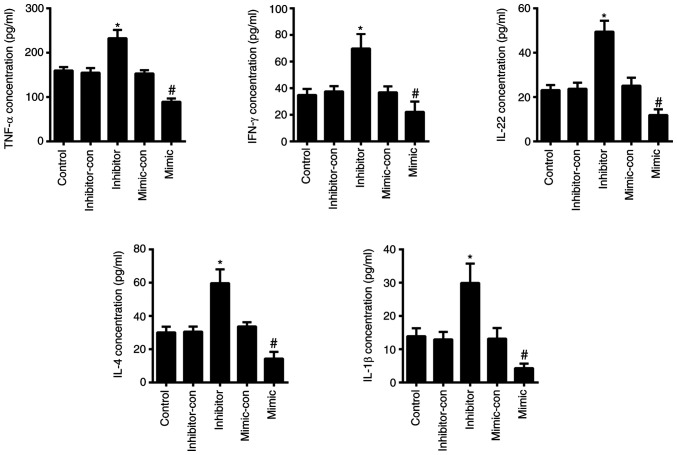
To verify the effects of miR-489-3p on the proliferation of KCs in vitro, the viability of HaCaT cells was assessed using CCK-8, cell cycle and colony formation assays to determine cell proliferation. The CCK-8 assay revealed that the viability of HaCaT cells transfected with miR-489-3p mimic was significantly decreased (P<0.05), whilst cells transfected with miR-489-3p inhibitors showed higher levels of viability in a time-dependent manner (P<0.05; Fig. 3A). Colony formation was impaired in HaCaT cells transfected with miR-489-3p mimic (P<0.05), whilst the colony forming ability of HaCaT cells transfected with miR-489-3p inhibitor was increased (P<0.05; Fig. 3B and C). Flow cytometry assays identified that miR-489-3p overexpression inhibited G2/M cell cycle progression (P<0.05; Fig. 3D and E), which was enhanced by the transfection of miR-489-3p inhibitor (P<0.05; Fig. 3D and E). The ELISA results demonstrated that the levels of TNF-α, IFN-γ, IL-22, IL-4 and IL-1β were significantly increased in cells transfected with miR-489-3p inhibitor (P<0.05; Fig. 4), but were declined in cells transfected with miR-489-3p mimic (P<0.05; Fig. 4). These data indicated that miR-489-3p inhibited the proliferation of KCs and the secretion of inflammatory cytokines.
Figure 3.
miR-489-3p inhibits the hyperproliferation of keratinocytes. (A) A Cell Counting Kit-8 assay was used to analyze cell proliferation. (B) Colony forming ability was analyzed using a colony formation assay, (C) and the results were quantified. (D) Flow cytometry was used to assess the cell cycle and (E) the results of G2/M were quantified. *P<0.05 vs. inhibitor-con; #P<0.05 vs. mimic-con. con, negative control; miR, microRNA; TLR4, Toll-like receptor 4.
Figure 4.
MicroRNA-489-3p inhibits inflammation. ELISA was used assess the secretion of various inflammatory cytokines. *P<0.05 vs. inhibitor-con; #P<0.05 vs. mimic-con. con, negative control.
miR-489-3p targets TLR4 to inhibit KC hyperproliferation and inflammation
To confirm that miR-489-3p inhibits the TLR-mediated inflammatory response, HaCaT cells were co-transfected with TLR4-siRNA and miR-489-3p inhibitor plasmids, and cell proliferation and inflammatory cytokine secretion were assessed. A RT-qPCR assay was conducted to verify the transfection efficiency, and the results indicated that the expression levels of TLR4 were significantly downregulated in HaCaT cells transfected with TLR4 siRNA compared with those transfected with TLR4 si-con (P<0.05; Fig. 5A). Moreover, the expression levels of TLR4 were significantly upregulated in HaCaT cells transfected with miR-489-3p inhibitor + TLR4 si-con compared with transfected with TLR4 si-con + inhibitor-con (P<0.05; Fig. 5B), while the expression levels of TLR4 were significantly downregulated in HaCaT cells transfected with miR-489-3p inhibitor + TLR4 si-RNA compared with cells transfected with TLR4 si-con + miR-489-3p inhibitor (P<0.05; Fig. 5B).
Figure 5.
TLR4 silencing prevents microRNA-489-3p inhibitor-induced hyperproliferation in KCs. (A) Transfection efficiency of si-TLR4. (B) Expression level of TLR4 in HaCaT cells transfected with different plasmids was detected via reverse transcription-quantitative PCR. (C) Cell Counting Kit-8 assays were used analyze cell proliferation. (D) Colony forming ability was analyzed using a colony formation assay, (E) and the results were quantified. (F) Flow cytometry was used to assess the cell cycle and (G) the results of G2/M were quantified. *P<0.05 vs. inhibitor-con + si-con; #P<0.05 vs. inhibitor + si-con. con, negative control; TLR4, Toll-like receptor 4; si, small interfering RNA.
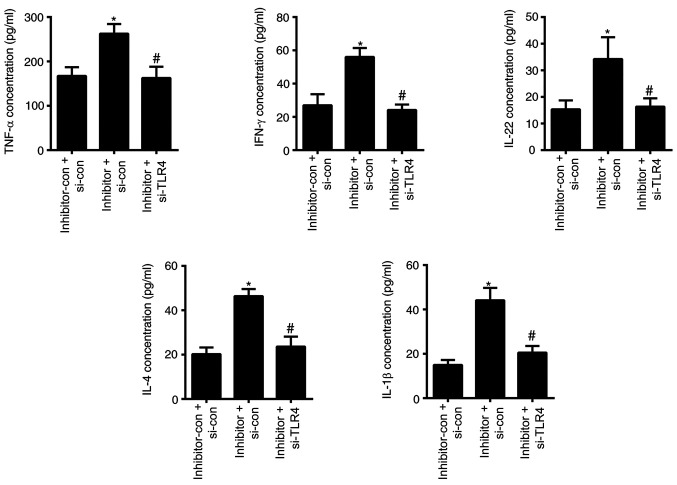
The ability of cells to survival and form colonies were significantly upregulated in the cells transfected with miR-489-3p-inhibitor + si-TLR4-con compared with the cells transfected with miR-489-3p-inhibitor-con + i-TLR4-con and significantly downregulated in the cells transfected with inhibitor + si-TLR4 compared with the cells transfected with miR-489-3p-inhibitor + si-TLR4-con (P<0.05; Fig. 5C-E). Consistent with these findings, G2/M progression was also significantly increased in the miR-489-3p-inhibitor+ si-TLR4-con group compared with the miR-489-3p-inhibitor-con + si-TLR4-con group; and decreased in the miR-489-3p-inhibitor + si-TLR4 group compared with the miR-489-3p-inhibitor + si-TLR4-con group (P<0.05; Fig. 5F and G). The secretion levels of the inflammatory cytokines TNF-α, IFN-γ, IL-22, IL-4 and IL-1β in cells transfected with miR-489-3p-inhibitor + si-TLR4-con were significantly higher compared with those transfected with inhibitor-con + TLR4 si-con and the secretion levels were significantly lower in the cells transfected with miR-489-3p-inhibitor + si-TLR4 compared with the cells transfected with miR-489-3p-inhibitor + si-TLR4-con (P<0.05; Fig. 6). These results suggested that miR-489-3p regulated KC proliferation by inhibiting TLR4 and the inflammatory response.
Figure 6.
TLR4 silencing inhibits microRNA-489-3p inhibitor-induced inflammation in KCs. ELISA was used to assess the secretion of various inflammatory cytokines. *P<0.05 vs. inhibitor-con + si-con; #P<0.05 vs. inhibitor + si-con. con, negative control; TLR4, Toll-like receptor 4; si, small interfering RNA.
Discussion
The pathogenesis of psoriasis is both complex and poorly characterized, involving multiple factors such as immunity, inflammation, cell proliferation and apoptosis, as well as a common immune-mediated pathway to cause abnormal proliferation, differentiation, apoptosis and local inflammation of KCs (3). Various cells (including dendritic cells, T lymphocytes, vascular endothelial cells and KCs), cytokines, chemokines and intracellular signal transduction are involved in this pathological process (18,19). Abnormal KC hyperproliferation, accompanied by hyperkeratosis, disfunction and inflammatory cell infiltration, are the main pathological features of psoriasis (20), and KCs serve an important role in the development of psoriasis. KCs form the epidermis of the skin, and maintain its mechanical barrier function by promoting wound healing. KCs, which have undergone a complete differentiation cycle, overlap and arrange with adjacent cells in the stratum corneum, forming a strong skin barrier (21). As key players in innate immune defenses, KCs express a variety of pattern recognition receptors, including TLRs 1-6 and 9, and retinoic acid-inducible gene I-like receptors (22), that form the initial responses to internal and external environmental stimuli. KCs undergo terminal differentiation via the spinous layer and granular layer of the epidermis, and begin to secrete keratin (K) 1 and K10 from the spinous layer, resulting in a loss of the nucleus in the stratum corneum (23).
The excessive proliferation of KCs is an important histopathological cause of psoriasis (19). During the terminal differentiation process, the nucleus is retained and the differentiation cycle is incomplete, which is accompanied by lipid secretion and reduced horny transparent particles, thus disrupting the normal skin barrier (24). In recent years, the regulation of KCs via psoriatic-associated miRNAs has been an area of intense research interest. Tsuru et al (25) reported that miR-424 was poorly expressed in psoriasis and influenced cell proliferation by regulating the expression levels of mitogen-activated protein kinase 1 and cyclin E1 in KCs. Moreover, Xu et al (26) revealed that miR-125b was lowly expressed in patients with psoriasis and that its overexpression inhibited KC proliferation and differentiation. Chang et al (10) also observed that miR-126 served an important role in the proliferation and migration of KCs during wound healing. However, to the best of our knowledge, the relationship between miR-489-3p and psoriasis has not been previously reported, and studies on miR-489-3p have focused on its role in cancer development. The overexpression of miR-489-3p may can delay the development of various diseases. For example, the overexpression of miR-489-3p in bladder cancer cells can inhibit tumor cell proliferation and invasion (27), whilst in osteosarcoma, miR-489-3p inhibits tumor cell metastasis via the paired box 3/MET pathway (28). Furthermore, Jiang et al (29) reported that miR-489-3p inhibited neuronal growth via its regulation of PI3K/AKT signaling during spinal cord injury. The present study demonstrated that miR-489-3p expression was downregulated in psoriasis, and that its overexpression in human immortalized KCs in vitro inhibited cell proliferation and colony forming ability. This indicated that the overexpression of miR-489-3p in psoriasis inhibited the proliferation of KCs.
Bioinformatics predictions identified that TLR4 was a target of miR-489-3p, the negative regulation of which was confirmed using a luciferase assay. To further corroborate the present findings, the expression levels of TLR4 and NF-κB were detected in KCs transfected with miR-489-3p-mimic or miR-489-3p-inhibitor plasmids. To confirm these findings, TLR expression was silenced using siRNA technology. The results demonstrated that miR-489-3p negatively regulated TLR4 and NF-κB expression in KCs.
TLRs serve a crucial role in protecting the host from a variety of exogenous and endogenous pathogens (30). Moreover, TLRs are key mediators of innate and adaptive immunity, and are activated upon stimulation by pro-inflammatory factors to activate the inflammatory response (31). NF-κB is a multi-directional nuclear transcription factor that is ubiquitous in the cytoplasm (32,33). In response to TLR activation, NF-κB translocates to the nucleus and induces the expression of an array of genes that mediate innate and acquired immune regulation, cell adhesion, inflammatory response and anti-apoptotic stimuli (34,35). NF-κB activates TNF-α, IFN-γ, IL-22, IL-4, IL-1β and other cytokines, all of which serve key roles in the inflammatory response (36). Thus, inflammatory cytokines can be targeted therapeutically for the treatment of psoriasis (37). The relationship between TNF-α and miRNAs in psoriasis is also well-characterized. For instance, Pivarcsi et al (37) reported that miR-146a and miR-125b regulated the secretion of TNF-α. After treatment with TNF-α targeting compounds, miR-128a was highly expressed, while miR-142-3p and miR-181a were downregulated (37). The present study demonstrated that miR-489-3p inhibited the secretion of TNF-α, IFN-γ and IL-22 in KCs. It has been shown that TLR4/NF-κB negatively regulated the inflammatory responses of miRNAs. For example, Loubaki et al (38) reported that miR-146a regulated immune responses via TLR4 signaling in sepsis, while Liu et al (39) revealed that miR-129-5p targeted high mobility group box 1 to inhibit autoimmune encephalomyelitis-related epilepsy via the TLR4/NF-κB axis.
Psoriasis is caused by the involvement of the skin in an autoinflammatory reaction caused by the abnormal interaction between epidermal KCs and immune cells (40). The present study simply used HaCaT cells as the cellular model, and this was a single in vitro study that did not consider the complex immune mechanism of psoriasis. In future studies, animal models will be established to further examine the role of miR-489-3p in the treatment of psoriasis.
In summary, the present study demonstrated that miR-489-3p negatively regulated the expression of TLR4 at the post-transcriptional level and inhibited the proliferation of KCs and prevented the secretion of inflammatory cytokines via inhibition of the TLR4/NF-κB pathway in psoriasis. These results highlight miR-489-3p as a promising therapeutic target for psoriasis.
Acknowledgements
Not applicable.
Funding Statement
Funding: This research was financially supported by a grant from Zhejiang Traditional Chinese Medicine Administration (grant no. 2020ZA090).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YY designed the study and performed the experiments. PW collected data and interpreted the results. FZ contributed to the preparation and revision of the manuscript for important intellectual content. YY and FZ evaluated the authenticity of the original data. All authors agreed to be accountable for the content of the work, and read and approved the final manuscript.
Ethics approval and consent to participate
The study complied with ethics committee regulations of The Third People's Hospital of Hangzhou and was performed with informed consent of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Langley RG, Krueger GG, Griffiths CE. Psoriasis: Epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64 (Suppl 2):ii18–ii25. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang K, Shen ZA. Research advances on immunological properties and gene regulation of skin keratinocytes. Zhonghua Shao Shang Za Zhi. 2021;37:89–92. doi: 10.3760/cma.j.cn501120-20200106-00007. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 3.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: A prospective study of US female nurses. Arch Dermatol. 2009;145:379–382. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis-part I: Clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 6.Rizwan M, Khan A. Association of psoriasis and serum uric acid levels: A case control study. Pakistan Armed Forces Med J. 2019;69:408–412. [Google Scholar]
- 7.Pradyuth S, Rapalli VK, Gorantla S, Waghule T, Dubey SK, Singhvi G. Insightful exploring of microRNAs in psoriasis and its targeted topical delivery. Dermatol Ther. 2020;33(e14221) doi: 10.1111/dth.14221. [DOI] [PubMed] [Google Scholar]
- 8.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 9.Rebane A, Akdis CA. MicroRNAs: Essential players in the regulation of inflammation. J Allergy Clin Immunol. 2013;132:15–26. doi: 10.1016/j.jaci.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Liang J, Xia X, Chen X. miRNA-126 enhances viability, colony formation, and migration of keratinocytes HaCaT cells by regulating PI3 K/AKT signaling pathway. Cell Biol Int. 2019;43:182–191. doi: 10.1002/cbin.11088. [DOI] [PubMed] [Google Scholar]
- 11.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 12.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 13.Wei W, Dejie L, Xiaojing S, Tiancheng W, Yongguo C, Zhengtao Y, Naisheng Z. Magnolol inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. Inflammation. 2015;38:16–26. doi: 10.1007/s10753-014-0003-2. [DOI] [PubMed] [Google Scholar]
- 14.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Xiao C, Dang E, Cao J, Zhu Z, Fu M, Yao X, Liu Y, Jin B, Wang G, Li W. CD100-Plexin-B2 promotes the inflammation in psoriasis by activating NF-κB and the inflammasome in keratinocytes. J Invest Dermatol. 2018;138:375–383. doi: 10.1016/j.jid.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Wu AG, Conway J, Barazani L, Roy B, Cline A, Pereira F. Is clear always clear? Comparison of psoriasis area and severity index (PASI) and the Physician's global assessment (PGA) in psoriasis clearance. Dermatol Ther (Heidelb) 2020;10:1155–1163. doi: 10.1007/s13555-020-00435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol. 2012;9:302–309. doi: 10.1038/cmi.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 20.Han G, Williams CA, Salter K, Garl PJ, Li AG, Wang XJ. A role for TGFbeta signaling in the pathogenesis of psoriasis. J Invest Dermatol. 2010;130:371–377. doi: 10.1038/jid.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tattersall D, Scott CA, Gray C, Zicha D, Kelsell DP. EKV mutant connexin 31 associated cell death is mediated by ER stress. Hum Mol Genet. 2009;18:4734–4745. doi: 10.1093/hmg/ddp436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalali BN, Köllisch G, Mages J, Müller T, Bauer S, Wagner H, Ring J, Lang R, Mempel M, Ollert M. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J Immunol. 2008;181:2694–2704. doi: 10.4049/jimmunol.181.4.2694. [DOI] [PubMed] [Google Scholar]
- 23.Aldehlawi H, Usman S, Lalli A, Ahmad F, Williams G, Teh MT, Waseem A. Serum lipids, retinoic acid and phenol red differentially regulate expression of keratins K1, K10 and K2 in cultured keratinocytes. Sci Rep. 2020;10(4829) doi: 10.1038/s41598-020-61640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni X, Lai Y. Keratinocyte: A trigger or an executor of psoriasis? J Leukoc Biol. 2020;108:485–491. doi: 10.1002/JLB.5MR0120-439R. [DOI] [PubMed] [Google Scholar]
- 25.Tsuru Y, Jinnin M, Ichihara A, Fujisawa A, Moriya C, Sakai K, Fukushima S, Ihn H. miR-424 levels in hair shaft are increased in psoriatic patients. J Dermatol. 2014;41:382–385. doi: 10.1111/1346-8138.12460. [DOI] [PubMed] [Google Scholar]
- 26.Xu N, Brodin P, Wei T, Meisgen F, Eidsmo L, Nagy N, Kemeny L, Ståhle M, Sonkoly E, Pivarcsi A. MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J Invest Dermatol. 2011;131:1521–1529. doi: 10.1038/jid.2011.55. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Qu W, Jiang Y, Sun Y, Cheng Y, Zou T, Du S. miR-489 suppresses proliferation and invasion of human bladder cancer cells. Oncol Res. 2016;24:391–398. doi: 10.3727/096504016X14666990347518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Yang G, Qian Y. Loss of MicroRNA-489-3p promotes osteosarcoma metastasis by activating PAX3-MET pathway. Mol Carcinog. 2017;56:1312–1321. doi: 10.1002/mc.22593. [DOI] [PubMed] [Google Scholar]
- 29.Jiang R, Zhang C, Gu R, Wu H. MicroRNA-489-3p inhibits neurite growth by regulating PI3K/AKT pathway in spinal cord injury. Pharmazie. 2017;72:272–278. doi: 10.1691/ph.2017.6972. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee S, Thompson WE, Chowdhury I. Emerging roles of microRNAs in the regulation of Toll-like receptor (TLR)-signaling. Front Biosci (Landmark Ed) 2021;26:771–796. doi: 10.2741/4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 32.Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-kappaB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 33.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human Toll signaling pathway: Divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayden MS, Ghosh S . NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Ann Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 36.Sun W, Gao Y, Yu X, Yuan Y, Yi J, Zhang Z, Cheng Y, Li Y, Peng X, Cha X. ‘Psoriasis 1’ reduces psoriasis-like skin inflammation by inhibiting the VDR-mediated nuclear NF-κB and STAT signaling pathways. Mol Med Rep. 2018;18:2733–2743. doi: 10.3892/mmr.2018.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pivarcsi A, Meisgen F, Xu N, Ståhle M, Sonkoly E. Changes in the level of serum microRNAs in patients with psoriasis after antitumour necrosis factor-α therapy. Br J Dermatol. 2013;169:563–570. doi: 10.1111/bjd.12381. [DOI] [PubMed] [Google Scholar]
- 38.Loubaki L, Chabot D, Paré I, Drouin M, Bazin R. MiR-146a potentially promotes IVIg-mediated inhibition of TLR4 signaling in LPS-activated human monocytes. Immunol Lett. 2017;185:64–73. doi: 10.1016/j.imlet.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Liu AH, Wu YT, Wang YP. MicroRNA-129-5p inhibits the development of autoimmune encephalomyelitis-related epilepsy by targeting HMGB1 through the TLR4/NF-κB signaling pathway. Brain Res Bull. 2017;132:139–149. doi: 10.1016/j.brainresbull.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Nicolas JF. Psoriasis: How the epithelium influences the immune response: Keratinocytes, dendritic cells and T lymphocytes. Bull Acad Natl Med. 2014;198:17–30. (In French) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.