Abstract
This hypothesis-testing study evaluated the relationship between mercury (Hg)-based dental amalgams and arthritis diagnoses among adults in the United States (US). A total of 86 305 425 weighted-persons with ⩾1 dental amalgam filling surface (DAFS) (exposed group) and 32 201 088 weighted-persons with ⩾1 other dental filling surface (ODFS) (no DAFS, unexposed group) were examined in the 2015 to 2016 National Health and Nutritional Examination Survey (NHANES). All persons were 20 to 80 years-old with known demographic characteristics and arthritis status. Survey logistic regression and survey frequency modeling in SAS were employed with and without adjustment of covariates. The arthritis rate was significantly increased in the exposed group compared to the unexposed group in the unadjusted (7.68-fold) and adjusted (4.89-fold) models. Arthritis (per 10 000 weighted-person-years) was 6.0-fold significantly increased in the exposed group (6.2) compared to the unexposed group (1.06). A significant bimodal dose-dependent relationship between DAFS and arthritis rate was observed. The arthritis rate increased with increasing DAFS (peak among persons with 4-7 DAFS) and, subsequently, decreased among those with >6 DAFS. A significant decrease in arthritis rate among persons with >13 DAFS as compared to those persons with 4 to 7 DAFS was observed. A significant association between DAFS and arthritis risk and a dose-dependent DAFS associated immune-stimulation/immune-suppression with arthritis risk were observed. An estimated additional $96 835 814 US dollars (USD) are spent on annual medical costs and $184 797 680 USD are lost in annual wages from reported new onset arthritis attributably associated with DAFS (annual total cost = $281 633 494 USD).
Keywords: Arthritis, cross-sectional, dental amalgam, joint disorder, mercury
Introduction
As described by the United States (US) Centers for Disease Control and Prevention (CDC), arthritis is a leading cause of disability, and causes pain, aching, stiffness, and swelling of the joints with accompanying physical and mental adverse effects.1 The initial pathogenic causes of most cases of arthritis remain unknown. Investigators suggested that underlying genetic2 or epigenetic3,4 susceptibility to autoimmunity were important in the etiology of arthritis, but investigators also suggested that exposure to environmental toxicants, such as mercury (Hg), could play a part in the pathogenesis of arthritis.5
Dental amalgam fillings are a mixture of mercury (Hg), silver, tin, copper, as well as several other metals.6 Dental amalgam fillings have been traditionally used for filling cavities in posterior teeth for more than 150 years and are perceived by some dentists to be a strong, well-retained, and cost-effective material.7 Dental amalgam fillings were also observed to have significant antibacterial properties against the most common bacterium known to induce dental caries.8
On a weight basis, dental amalgam fillings are about 50% Hg. Of particular concern, Hg0 vapor at body temperature is continuously released from dental amalgam fillings. Hg0 vapor is easily absorbed into the human body through mucus membranes and the lungs where it is rapidly oxidized to other forms.6
In light of the importance of the potential role for Hg exposures in the etiology of arthritis, the purpose of the present hypothesis-testing study was to evaluate the relationship between increasing exposure to dental amalgam filling surfaces and the risk of arthritis in adults by undertaking a cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) database.
Methods
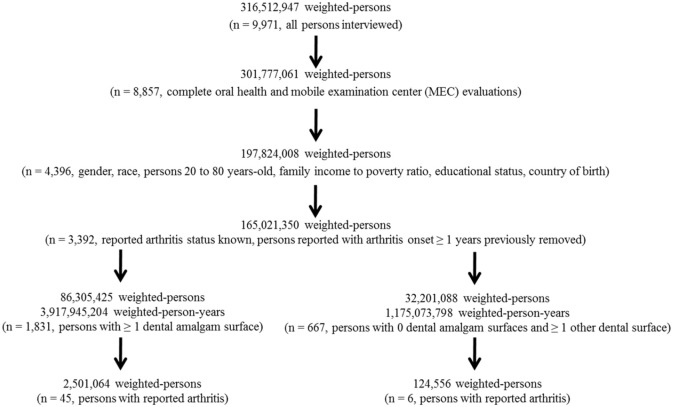
The SAS system for Windows, version 9.4 (Cary, NC, USA) was used to examine the NHANES data. This study integrated the 2015-2016 NHANES data from demographic survey questions, oral health examinations, and arthritis diagnoses. The 2015-2016 NHANES data collection methods were approved by the NCHS Research Ethics Review Board (ERB) (Protocol#2011-17). Each study subject provided informed consent to participate in the NHANES program. The health information collected in the NHANES program is kept in strictest confidence, and is only used for stated purposes. Figure 1 presents a schematic flowchart of the data examined in the present study.
Figure 1.
A schematic flowchart of the data examined in the present study.
Study participants
An overall population of 165 021 350 weighted-persons 20 to 80 years-old with non-missing values for the demographic survey questions, oral health examinations, and medical condition survey questions relating to arthritis diagnoses were examined. The number of weighted-persons was derived by applying the full sample two-year mobile examination weight variable (WTMEC2YR) to each person examined in this study. The WTMEC2YR variable was created by the NHANES program. The WTMEC2YR is a measure of the number of persons in the general population that a sampled individual represents and is needed to obtain unbiased estimates of population parameters when sample participants are chosen with unequal probabilities. The demographic variables for the population were identified from within the NHANES demographic dataset. The variables examined were as follows: gender, age in years at examination, race (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanic, or other), educational level (less than 9th grade, 9-11th grade, high school graduate/general equivalence degree, some college or associates degree, or college graduate or above), country of birth, and socioeconomic status (poverty income ratio (PIR) – a ratio of family income to poverty threshold). The PIR variable was created by the NHANES program.
Exposures
The dental amalgam filling surface exposure variable was determined by examining the NHANES oral health—dentition dataset. For each person, the oral health examination was conducted by dental examiners, who were dentists licensed in at least 1 US state. A health technician assisted in entering all examiner observations directly into a computerized data collection system. All oral health assessments took place in a designated room at the mobile examination center (MEC) that included a portable dental chair, light, and compressed air.
For all survey participants aged 1 and older, the oral health examination began with a tooth count assessment. Next, their teeth were assessed for coronal caries, including untreated dental decay and teeth treated or extracted due to caries. The 2015 to 2016 coronal caries assessment was similar to the protocols used in 1999 to 2004 and 2011 to 2014, with the following exception: filled surfaces were assessed by restoration type (amalgam or other). The cumulative numbers of dental amalgam filling or other dental filling surfaces were computed for each person examined in this study. The study groups examined were 86 305 425 weighted-persons with ⩾1 dental amalgam filling surface and ⩾0 other dental filling surfaces (exposed group) and 32 201 088 weighted-persons with 0 dental amalgam filling surfaces and ⩾1 other dental filling surface (unexposed group). All persons without any dental filled surfaces (dental amalgam or other) were not examined.
Outcomes
The medical conditions NHANES dataset was examined to determine the incidence rate of the reported outcome of arthritis examined in this study. Among the persons examined, arthritis was determined based upon each person reporting whether a doctor ever said they had arthritis and the age at which they were told they had arthritis (the age of onset of arthritis). Only persons reported with onset of arthritis at the same age as when they were examined were included in this study, persons with onset of arthritis previously were not examined.
Statistical analyses
In all statistical analyses, the statistical package in SAS was utilized, and a two-sided P-value < .05 was considered statistically significant. The number of persons examined in this study was sufficient to allow for adequate statistical power for the analyses undertaken. The null hypothesis was that there would be no difference in the incidence rate of reported arthritis regardless of dental amalgam filling status.
Survey logistic regression modeling was utilized to evaluate the incidence rate of reported arthritis among those persons in the dental amalgam filling surface group as compared to the other filling surface group. The covariates of race, gender, socioeconomic status, country of birth, age, and education level were considered in adjusted models. In addition, survey frequency modeling was employed to evaluate the incidence rate of reported arthritis in the dental amalgam filling surface group as compared to the other filling surface group. In order to adjust for the differences in age between the 2 groups examined, the total number of weighted-person-years (age of person at the time of the NHANES survey multiplied by WTMEC2YR).
Additional analyses were undertaken to evaluate the potential dose-dependent relationship between the incidence rate of reported arthritis and the number of dental amalgam filling surfaces using survey logistic modeling. The covariates of race, gender, socioeconomic status, country of birth, age, and education level were considered in adjusted models. Further, analyses were undertaken to determine, what, if any, threshold existed between the incidence rate of reported arthritis and the number of dental amalgam filling surfaces.
Results
Table 1 displays the demographic characteristics of the population of persons examined in the NHANES database. The mean age in the dental amalgam filling surface group (45.4 years-old) was older than the mean age in the other dental filling surface group (36.5 years-old) and the male/female ratio was greater in the dental amalgam filling surface group (1.08) as compared to the other dental filling surface group (0.73). When comparing the dental amalgam filling surface group to the other dental filling surface group, the racial distribution revealed more non-Hispanic white persons (66.2% vs 62.0%) and fewer non-Hispanic Asian persons (5.0% vs 8.2%) and the country of birth distribution revealed more born in the 50 US states or Washington, DC (83.8% vs 77.9%). The education level distribution revealed that more there were more high school graduates in the dental amalgam filling surface group (20.8%) as compared to the other dental filling surface group (15.5%) and fewer persons were at least college graduates in the dental amalgam filling surface group (35.5%) as compared to the other dental filling surface group (41.2%). The socioeconomic status measurements were similar in the dental amalgam filling surface and other dental filling surface groups.
Table 1.
Demographic characteristics of the dental amalgam filling groupa and the other dental filling exposed groupb examined in the NHANES database.
| Parameter examined | Dental amalgam filling surface group | Other dental filling surface group |
|---|---|---|
| Age | ||
| Mean age ± std (age range: 20-80) | 45.4 ± 13.9 | 36.5 ± 14.4 |
| Gender (%) | ||
| Male | 44 924 792 (52.0%) | 13 484 780 (42.2%) |
| Female | 41 380 632 (48.0%) | 18 618 067 (57.8%) |
| Race (%) | ||
| Non-Hispanic White | 57 155 034 (66.2%) | 19 972 404 (62.0%) |
| Non-Hispanic Black | 8 489 281 (9.8%) | 3 632 713 (11.3%) |
| Non-Hispanic Asian | 4 292 172 (5.0%) | 2 632 455 (8.2%) |
| Hispanic | 13 511 065 (15.7%) | 5 093 402 (15.8%) |
| Otherc | 2 857 873 (3.3%) | 870 113 (2.7%) |
| Country of birth (%) | ||
| Born in 50 US states or Washington, DC | 72 290 720 (83.8%) | 25 072 390 (77.9%) |
| Others | 14 014 704 (16.2%) | 7 128 697 (22.1%) |
| Education level (%) | ||
| Less than 9th grade | 3 415 212 (3.9%) | 1 283 265 (4.0%) |
| 9 to 11th grade (includes 12 grade with no diploma) | 5 820 408 (6.7%) | 1 794 028 (5.6%) |
| High school graduate/GED or equivalent | 17 915 360 (20.8%) | 5 013 176 (15.5%) |
| Some college or AA degree | 28 551 319 (33.1%) | 10 853 470 (33.7%) |
| College graduate or above | 30 603 126 (35.5%) | 13 257 149 (41.2%) |
| Socioeconomic status (score range: 0-5) | ||
| Mean PIR score ± std | 3.2 ± 1.6 | 3.1 ± 1.7 |
| Exposure status | ||
| Mean number of dental amalgam filling surfaces ± std | 7.2 ± 6.3 | 0 ± 0 |
| Mean number of other dental filling surfaces ± std | 4.3 ± 5.5 | 6.6 ± 5.3 |
| Period of examination | ||
| Total weighted-person-years | 4 053 143 632 | 1 175 073 798 |
| Reported to be diagnosed with arthritis | ||
| Frequency per 10,000 weighted-person-years (n) | 6.2 (45) | 1.06 (6) |
Abbreviations: AA, associate degree; GED, general education development; PIR, poverty income ratio; std, standard deviation of the mean.
Those weighted-persons with ⩾1 amalgam filling surfaces.
Those weighted-persons with ⩾1 other dental filling surfaces and 0 dental amalgam filling surfaces.
Includes persons of mixed race.
Table 2 shows the results of survey logistic regression models examining the incidence rate of reported arthritis in the dental amalgam filling surface group in comparison to the other dental filling surface group. The risk of reported arthritis was significantly increased by 7.68-fold in the unadjusted and 4.89-fold in the adjusted models when comparing the dental amalgam filling surface group to the other dental filling surface group. In the adjusted model, the covariates of country of birth and age were also significantly related to the risk of reported arthritis.
Table 2.
Survey logistic regression modelsa examining the incidence rate of reported arthritis in the dental amalgam filling surfaces group as compared to the other dental filling surfaces group in the NHANES database.
| Outcome | Variables | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|---|
| Arthritis b | Dental amalgam filling surfaces group versus other dental filling surfaces group | 7.68 | 2.53 to 23.2 | .0003 |
| Dental amalgam filling surfaces group versus other dental filling surfaces group | 4.89 | 1.55 to 15.5 | .0069 | |
| Race | 0.89 | 0.74 to 1.07 | .20 | |
| Gender | 0.87 | 0.33 to 2.26 | .77 | |
| Socioeconomic status | 1.01 | 0.82 to 1.25 | .90 | |
| Country of birth | 0.47 | 0.27 to 0.82 | .0086 | |
| Age | 1.06 | 1.04 to 1.08 | <.0001 | |
| Education level | 0.99 | 0.75 to 1.3 | .95 |
Bold-Italicized results are statistically significant.
The survey logistic model employed used stratum, cluster, and weight variables. The incidence rate of reported arthritis in the year of the NHANES survey was evaluated among those weighted-persons with ⩾1 amalgam filling surfaces (dental amalgam filling surfaces group) in comparison to those weighted-persons with ⩾1 other dental filling surfaces and 0 dental amalgam filling surfaces (other dental filling surfaces group).
The incidence rate of reported arthritis was derived from NHANES survey data among those persons with reported arthritis in the year of the NHANES survey collection. Those persons with reported arthritis at any time prior to the year of the NHANES survey collection were eliminated from the analyses.
Table 3 reveals the results of survey frequency modeling evaluating the incidence rate of reported arthritis in the dental amalgam filling surface group as compared to the other dental filling surface group. The rate ratio = 6.0 was significantly increased with an attributable rate difference per 10 000 weighted-person-years = 5.3.
Table 3.
A summary of survey frequency modelinga to evaluate the incidence rate of reported arthritis in the dental amalgam filling surfaces group as compared to the other dental filling surfaces group in the NHANES database.
| Exposure group examined | Total weighted persons reported to be diagnosed with arthritisb | Total weighted person-years | Outcome measurements |
|---|---|---|---|
| Dental amalgam filling surfaces group | 2 501 064 | 3 917 945 204 | |
| Other dental filling surfaces group | 124 556 | 1 175 073 798 | |
| Rate ratio (95% CI) | 6.0 (2-21) | ||
| P value | <.001 | ||
| Attributable rate difference per 10,000 (95% CI) | 5.3 (2-9) |
Bold-Italicized results are statistically significant.
Abbreviation: CI, confidence interval.
The survey logistic model employed used stratum, cluster, and weight variables. The incidence rate of reported arthritis in the year of the NHANES survey was evaluated among those weighted-persons with ⩾1 amalgam filling surfaces (dental amalgam filling surfaces group) in comparison to those weighted-persons with ⩾1 other dental filling surfaces and 0 dental amalgam filling surfaces (other dental filling surfaces group).
The incidence rate of reported arthritis was derived from NHANES survey data among those persons with reported arthritis in the year of the NHANES survey collection. Those persons with reported arthritis at any time prior to the year of the NHANES survey collection were eliminated from the analyses.
Table 4 shows the results of survey logistic models examining the incidence rate of reported arthritis in relation to the total number of dental amalgam filling surfaces for each person examined in the NHANES database. Overall, it was observed that there was a significant bimodal dose-dependent relationship between increasing total numbers of dental amalgam filling surfaces and the incidence rate of reported arthritis. There was no significant dose-dependent relationship between increasing total numbers of other dental filling surfaces and the incidence rate of reported arthritis.
Table 4.
Survey logistic regression modelsa examining the incidence rate of reported arthritis in relationship to the total number of dental filling surfaces for each person examined in the NHANES database.
| Outcome | Variables | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|---|
| Arthritis b | Total number of dental amalgam filling surfaces | 1.033 | 0.99 to 1.07 | .10 |
| Total number of dental amalgam filling surfaces | 1.017 | 0.97 to 1.06 | .48 | |
| Race | 0.88 | 0.73 to 1.06 | .19 | |
| Gender | 0.86 | 0.34 to 2.18 | .74 | |
| Socioeconomic status | 1.01 | 0.82 to 1.25 | .93 | |
| Country of birth | 0.44 | 0.25 to 0.78 | .0048 | |
| Age | 1.06 | 1.05 to 1.06 | <.0001 | |
| Education level | 0.98 | 0.74 to 1.29 | .88 | |
| Total number of dental amalgam filling surfaces | 1.01 | 0.97 to 1.06 | .62 | |
| Total number of other dental filling surfaces | 0.95 | 0.87 to 1.04 | .28 | |
| Race | 0.87 | 0.72 to 1.05 | .16 | |
| Gender | 0.88 | 0.35 to 2.22 | .78 | |
| Socioeconomic status | 1.03 | 0.83 to 1.27 | .80 | |
| Country of birth | 0.45 | 0.26 to 0.78 | .0043 | |
| Age | 1.06 | 1.05 to 1.08 | <.0001 | |
| Education level | 1.00 | 0.77 to 1.29 | .98 |
Bold-Italicized results are statistically significant.
The survey logistic model employed used stratum, cluster, and weight variables. The incidence rate of reported arthritis in the year of the NHANES survey was evaluated in relation to the total number of dental filling surfaces for each person. The population examined included those persons in the dental amalgam filling surfaces group plus the other dental filling surfaces group.
The incidence rate of reported arthritis was derived from NHANES survey data among those persons with reported arthritis in the year of the NHANES survey collection. Those persons with reported arthritis at any time prior to the year of the NHANES survey collection were eliminated from the analyses.
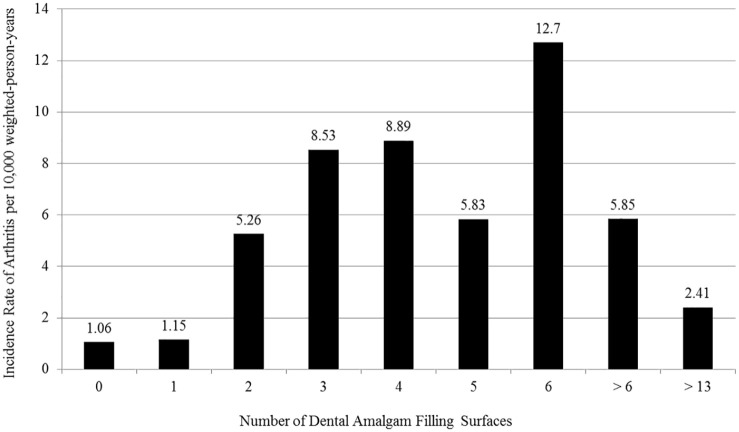
Table 5 and Figure 2 further examine the relationship between the incidence rate of reported arthritis (per 10 000 weighted-person-years) and the total number of dental amalgam filling surfaces. The incidence rate of reported arthritis was similar among those persons examined with no dental amalgam filling surfaces (1.06) or a single dental amalgam filling surface (1.15). The rate of reported arthritis increased from 5.26 among those with 2 dental amalgam filling surfaces to 12.7 among those with 6 dental amalgam filling surfaces. A decrease was observed to 5.85 among those with more than 6 dental amalgam filling surfaces, and an even further decreased was observed to 2.41 among those with more than 13 dental amalgam filling surfaces.
Table 5.
A summary of survey frequency modeling to evaluate the incidence rate of reported arthritis as compared to the total number of dental amalgam filling surfaces per person in the NHANES database.
| Number of dental amalgam filling surfaces (percentile) | Total weighted persons with reported arthritisa | Total weighted-person-years | Incidence rate of persons with reported arthritis (per 10,000 weighted-person-years) |
|---|---|---|---|
| 0 (0-31) | 124 556 | 1 175 073 798 | 1.06 |
| 1 (32-40) | 49 120 | 428 117 858 | 1.15 |
| 2 (41-48) | 224 010 | 426 220 026 | 5.26 |
| 3 (49-56) | 353 700 | 414 411 402 | 8.53 |
| 4 (57-61) | 376 321 | 423 108 009 | 8.89 |
| 5 (62-66) | 139 568 | 239 487 089 | 5.83 |
| 6 (67-71) | 362 098 | 284 794 937 | 12.7 |
| > 6 (72-100) | 996 247 | 1 701 805 883 | 5.85 |
| > 13 (91-100) | 127 091 | 528 207 158 | 2.41 |
There was a significant increase in the incidence rate of reported arthritis (rate ratio = 8.6, P < .01) among those persons with 4 to 7 dental amalgam filling surfaces (9.27 per 10,000 weighted-person-years) as compared to those persons with 0 to 2 dental amalgam filling surfaces (1.08 per 10,000 weighted-person-years). There was a significant increase in the incidence rate of reported arthritis (rate ratio = 3.85, P < .05) among those persons with 4 to 7 dental amalgam filling surfaced (9.27 per 10,000 weighted-person-years) as compared to those persons with >13 dental amalgam filling surfaces (2.41 per 10,000 weighted-person-years).
The incidence rate of reported arthritis was derived from NHANES survey data among those persons with reported arthritis in the year of the NHANES survey collection. Those persons with reported arthritis at any time prior to the year of the NHANES survey collection were eliminated from the analyses.
Figure 2.
An assessment of the relationship between the number of dental amalgam filling surfaces and the incidence rate of arthritisa per 10,000 weighted-person-years.
aThe incidence rate of reported arthritis was derived from NHANES survey data among those persons with reported arthritis in the year of the NHANES survey collection. Those persons with reported arthritis at any time prior to the year of the NHANES survey collection were eliminated from the analyses.
Overall, there was a significant increase in the incidence rate of reported arthritis (rate ratio = 8.6, P < .01) among those persons with 4 to 7 dental amalgam filling surfaces (9.27 per 10 000 weighted-person-years) as compared to those persons with 0 to 2 dental amalgam filling surfaces (1.08 per 10 000 weighted-person-years). There was a significant increase in the incidence rate of reported arthritis (rate ratio = 3.85, P < .05) among those persons with 4 to 7 dental amalgam filling surfaced (9.84 per 10 000 weighted-person-years) as compared to those persons with >13 dental amalgam filling surfaces (2.41 per 10 000 weighted-person-years).
Discussion
The present hypothesis-testing study is the first epidemiological study to evaluate the relationship between exposure to dental amalgam filling surfaces and reported arthritis in American adults. The results revealed that the incidence rate of reported arthritis was significantly increased among those persons in the dental amalgam filling surface group in comparison to those persons in the other dental filling surfaces group. The association between dental amalgam filling surfaces and the increased risk of reported arthritis was observed to remain significant in statistical modeling even when considering multiple covariates. It was further observed that there was a significant bimodal dose-dependent relationship between the number of dental amalgam filling surfaces and the incidence rate of reported arthritis. The incidence rate of reported arthritis increased among persons with increasing dental amalgam filling surfaces (the peak was among observed among those with 4 to 7 dental amalgam filling surfaces) and, subsequently, decreased among those with more than 6 dental amalgam filling surfaces.
As described by the CDC, 23% of all adults, or more than 54 million people, have arthritis in the US. It was estimated that 60% of US adults with arthritis are of working age (18 to 64 years), and it is a leading cause of work disability. Arthritis can limit the type of work people are able to do or keep them from working at all. In fact, 8 million working-age adults report that their ability to work is limited because of their arthritis.9
The results from the present study help to provide important considerations regarding the societal impacts of the association between dental amalgam filling surfaces and the increased risk of arthritis. Survey frequency modeling revealed that the attributable rate difference per 10,000 weighted-person-years for reported arthritis was 5.3 (95% confidence interval = 2-9) among those in the dental amalgam filling surfaces group as compared to those in the other dental filling surfaces group. A total 45 742 weighted-persons (86 305 425 weighted-persons in the dental amalgam filling surfaces group × 0.00053 weighted-person-years) were reported to be newly diagnosed with arthritis attributably associated with their dental amalgam filling surface exposure.
Unfortunately, arthritis is known to have significant economic, personal, and societal impacts. In 2013, it was estimated that the total national arthritis-attributable medical care costs and earnings losses among adults with arthritis were $303.5 billion US dollars.10 Among adults with arthritis compared to those adults without arthritis, it was estimated that an additional $2117 in annual medical costs per adult was spent and $4040 was lost in annual wages.10 It can be estimated from the results observed in the present study that an additional $96 835 814 US dollars are spent on annual medical costs and $184 797 680 US dollars are lost in annual wages from reported new onset arthritis attributably associated with dental amalgam filling surface exposures (annual total cost = $281 633 494 US dollars).
In considering the database examined in the current study, NHANES is a major program of the National Center for Health Statistics (NCHS), which is a part of the US CDC. NHANES is designed to assess the health and nutritional status of adults and children in the US by utilizing survey methods that combine interviews, physical examinations, and laboratory tests. The NHANES program began in the early 1960s, and has been conducted as a series of surveys focusing on different population groups or health topics. In 1999, the survey became a continuous program with components that are adaptable to a variety of health and nutritional measurements in order to meet emerging needs. The NHANES examines a nationally representative sample of about 5000 Americans each year, and these persons are located in counties across the US, 15 of which are visited each year. It is the aim of NHANES to help determine the prevalence of major diseases and risk factors for diseases, and then, from such data, help to develop sound public health policy. Since, detailed information was collected regarding the materials used in dental fillings in the 2015 to 2016 NHANES for the first time, this data was employed in the present study to evaluate the potential relationship between dental filling material and the outcome of arthritis.
In comparing the results observed in this study with previous studies, a number of previous studies have found significant associations between dental amalgam filling exposure and the outcome of arthritis. For example, a previous study evaluated the impact of dental amalgam removal on clinical symptoms among 86 persons examined on average 10 months post-dental amalgam filling removal.11 Of the 86 persons examined, it was described that 20 persons were reported to have arthritis and that among this group, 70% reported significant improvements in their arthritic symptoms and 65% reported less joint pain.
Another study was undertaken to evaluate persons initially complaining of symptoms from dental amalgam fillings who underwent dental amalgam filling removal in comparison to general population controls from a family practice and a dental practice over a 7 year period.12 These investigators observed at baseline musculoskeletal complaints were significant increased among those complaining of adverse effects from dental amalgam fillings in comparison to general population controls. Subsequently, these investigators observed at 2 year and 7 year follow-up examinations that there were no differences in musculoskeletal complaints as compared to general population controls. These investigators concluded that while there was a significant drop in musculoskeletal complaints among those with dental amalgams who underwent dental amalgam removal within the first 2 year period, they predicted that the health effects of removing dental amalgam fillings would only occur after several years due to the assumed long-term negative effects of the fillings. In support of their hypothesis, they described the half-life of Hg in plasma after dental amalgam removal was estimated to be 3 to 4 months, and, since, it was assumed that the decrease in the plasma levels of Hg following dental amalgam removal would follow an exponential curve, the plasma level of Hg would return to the level found in persons with no history of dental amalgams in about 3 years. Therefore, these investigators described that they expected to see a continual reduction of symptoms from 2 to 7 years. However, they did not observe this phenomenon. They concluded that dental amalgams fillings could not be the main cause of the health complaints observed among persons with dental amalgams that were subsequently removed.
In comparing the aforementioned studies to the current study, there are a number of important limitations. First, all of the aforementioned studies examined persons undergoing dental amalgam filling removal. As a result, there may be selection biases present in those persons wanting to undergo dental amalgam filling removal. Namely, the persons examined by the investigators maybe more or less healthy than the general population and they may have preconceived perceptions regarding the risk/benefits of various dental filling materials. The present study examined a representative sample of the US population that participated in NHANES independent of their health status or their dental filling status. Second, the aforementioned studies examined persons with various health complaints. It is not clear, what, if any, relationship existed between the health complaints examined and dental filling status (ie, the health complaints may have started prior to the placement of dental amalgam fillings). The present study examined persons with newly diagnosed arthritis in the same year as their dental filling status was determined. Further, a comparison was made between groups with dental amalgam filling surfaces and other dental filling surfaces. The groups assessed in this study were examined at the same time and under the same circumstances with consideration of multiple other covariates. Thus, the present study was able to isolate differences in material utilized in filling teeth and its relationship with newly diagnosed arthritis.
The results observed in this study are biologically plausible. Investigators recently examined Hg distribution following mice exposed to Hg vapor to determine whether it localize in areas known to be impacted in arthritis.13 These investigators reported that Hg was found to localize in the synovial cells and articular chondrocytes. These cell types are known to be affected in persons suffering from arthritis. In addition, Hg was retained in connective tissue fibroblasts. The investigators concluded that their findings provide mechanistic support for a connection between Hg exposure and arthritis.
Investigators examined activation of the immune system and systematic immune-complex deposits in genetically Hg-susceptible Brown Norway rats as compared to Hg-resistant Lewis rats following placement of dental amalgam fillings as compared with control animals receiving placement of composite resin fillings.14 These investigators observed that dental amalgam fillings, in genetically Hg-susceptible rats, as compared to composite resin fillings significantly increased activation of the immune system and increased systemic immune complex deposits.
Investigators observed the impact of dental amalgam fillings on the immune system in a case-series of patients.15 These investigators reported that removal of all dental amalgam fillings and replacement with other dental filling materials significantly reduced blood pro-inflammatory biomarkers.
Other investigators described a case-report of a 48 year-old man presenting in the emergency room with a 3 day history of joint pain involving the shoulders, elbows, wrists, hands, knees, and feet.16 The man was exposed 11 days previously to mercury vapors from a small amount of mercury boiled in a pot in the kitchen. After an extensive evaluation, the investigators reported that the man’s arthritis was the likely consequence of mercury exposure, especially since previous investigators have described that among the symptoms of mercury poisoning, include: morning stiffness, arthralgia, and/or arthritis. The investigators concluded that mercury intoxication should be considered in arthritis etiology.
In yet another study, investigators examined the presence of hypersensitivity to dental and environmental metals in patients with clinical disorders.17 Of particular relevance to the findings observed in the present study, the investigators reported on a 51 year-old woman with a history of thyroiditis and rheumatoid arthritis. The patient was a surgical assistant in a hospital and her ailments were significantly interfering with her ability to work. She was a heavy smoker, had positive titers for anti-Epstein Barr virus and anti-Cytomegalovirus antibodies, and dental examination revealed the presence of dental amalgam fillings. The patient’s lymphocytes were observed to significantly react with Hg (and several Hg-compounds), gold, and nickel. The patient considered that her ill health was the consequence of her dental amalgam fillings, and she underwent removal and replacement of her dental amalgam fillings. At a follow-up examination 6 months after her dental amalgam fillings were removed, the patient’s lymphocyte reactivity to mercury was significantly decreased while the response to nickel was not affected. The patient clinically reported that within 2 months of dental amalgam replacement her arthritic pain disappeared and her level of fatigue significantly decreased. She was able to return to work, and her health continued to improve 18 months later.
The findings of the aforementioned studies, coupled with the bimodal dose-dependent relationship between dental amalgam filling surfaces and the risk of arthritis observed in this study, suggest an immunological component, in addition to a toxicological basis, for the relationship observed between dental amalgam filling surfaces and arthritis in this study. It was previously hypothesized that since metal-induced sensitization may be caused by chronic low-dose exposure, conventional toxicological approaches comparing concentrations of metals in body tissues may not provide answers regarding the metal-pathology connection.18 Namely, instead, it was suggested that the biological mechanism may involve an immune-mediated allergic-type reaction to metals, especially among immunologically susceptible persons with the consequence that minute concentrations of an allergen can induce systemic reactions in sensitized individuals.17,18 Finally, consistent with the results observed in this study, it was hypothesized that differences in dose and susceptibility to dental amalgam filling surfaces may induce immunosuppression.19
Study limitations
As with any observational cross-sectional study, it was not possible to follow the participants examined on a longitudinal basis to evaluate the relationship between exposures and outcomes overtime, and thus, assess a potential direct causal relationship between exposures and outcomes. Despite this limitation, dental amalgam filling surface exposures and reported arthritis diagnostic status were collected on an independent and contemporaneous basis. Further, persons were examined for reported new arthritis diagnoses in the same year as their dental filling status was assessed. As a result, time lapses between measurements of reported exposure and outcomes were minimized. In addition, recall biases associated with measurements of reported exposures and outcomes were minimized because they were collected independent of 1 another, and independent of the study design employed in this study to examine the data. Furthermore, in support of the associations observed in this study, the results, as described previously, are biologically plausible. It is recommended to more complete evaluate the relationship between dental amalgam filling exposures and the risk of arthritis that future studies examine the relationship in prospective longitudinal observational cohorts and/or in prospective randomized placebo-controlled human trials.
The NHANES program uses the questionnaire method of data collection and participants may recall information wrongly or report information inaccurately. However, the demographic, socioeconomic, and health-related questions are detailed and consistent and asked during an interview with highly trained study personnel. In addition, when comparing the reported arthritis observed in this study, the observations made are consistent with previous CDC studies.20 Finally, for the data examined, it is presumed that limitations or errors in the questionnaire method of data collection employed by NHANES would have applied equally to all participants examined. Conceivably, such errors in the data would have most likely reduced the statistical power of this study to be able to reveal significant relationships. Despite these potential limitations, significant associations were observed between dental amalgam filling surfaces and the outcome of arthritis. It is recommended that future studies employ other tests and measurements of arthritis to more fully evaluate dental amalgam surfaces associated effects on arthritis.
A further potential limitation of this study was that persons with no dental filling surfaces were excluded. These persons were excluded because there may be social and medical attributes that may be associated with avoidance of dental filling surface placement. Hence, by requiring that all persons examined in this study have at least 1 dental filling, the potential for confounding associated with avoidance of dental care was minimized. Similarly, by requiring that every person have at least 1 dental filling, the potential for presence/absence of dental filling surfaces as mediating the relationship between dental amalgam filling surfaces and arthritis was minimized because all persons examined had dental fillings.
It is possible the results observed could have been the result of chance and that would have to be considered as a potential limitation of the study; however, that would be unlikely since only a small number of statistical tests were conducted and the direction and magnitude of the results were biologically plausible. In addition, the data were analyzed using several types of statistical models with consideration of covariates, and all yielded statistically significant results.
Another potential limitation of this study is persons diagnosed with arthritis may visit dental professionals differentially than the general population. Specifically, a population based study of the 1995 National Health Survey revealed that persons diagnosed with arthritis were significantly less likely to see a dental professional than the general population.21 The present study attempted to address this phenomenon by analyzing reported cases of newly diagnosed arthritis within the same year as the NHANES survey, when data was collected regarding the dental filling status of persons. As a result, it is assumed that more long-term trends in differential dental care among persons diagnosed with arthritis versus the general population would have been minimized.
Finally, while the present study observed a significant association between dental amalgam filling surfaces with reported arthritis diagnoses, the present study did not examine the potential impact of other environmental and genetic susceptibility risk factors for arthritis.22,23 For example, it was observed in a study of identical twins that a history of cigarette smoking significantly increased the risk of diagnosed arthritis.24 Consistent with this previous observation, as revealed in Table 6, when utilizing the same statistical models employed in the present study to evaluate the relationship between dental amalgam filling surfaces and the risk of a reported arthritis diagnosis, it was observed that increasing serum cotinine, a known primary metabolite of nicotine that can be used as a markers for active smoking and secondhand smoke exposure,25 was a significant risk factor for a reported arthritis diagnosis. Interestingly, the significant relationship between dental amalgam filling surfaces and the risk of reported arthritis remained significant. It would be worthwhile in future studies to further examine how various different environmental exposures and genetic susceptibility factors may work independently or synergistically to induce arthritis.
Table 6.
Survey logistic regression modelsa examining the incidence rate of reported arthritis in the dental amalgam filling surfaces group as compared to the other dental filling surfaces group in the NHANES database when considering tobacco utilization.
| Outcomes | Variables | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|---|
| Arthritis b | Dental amalgam filling surfaces group versus other dental filling surfaces group | 4.22 | 1.23 to 14.5 | .022 |
| Race | 0.87 | 0.70 to 1.08 | .19 | |
| Gender | 1.01 | 0.42 to 2.44 | .99 | |
| Socioeconomic status | 1.03 | 0.82 to 1.28 | .83 | |
| Country of birth | 0.54 | 0.31 to 0.95 | .031 | |
| Age | 1.06 | 1.04 to 1.08 | <.0001 | |
| Education Level | 1.10 | 0.78 to 1.56 | .59 | |
| Serum cotinine (ng/mL) | 1.003 | 1.002 to 1.004 | <.0001 |
Bold-Italicized results are statistically significant.
The survey logistic model employed used stratum, cluster, and weight variables. The incidence rate of reported arthritis in the year of the NHANES survey was evaluated among those weighted-persons with ⩾1 amalgam filling surfaces (dental amalgam filling surfaces group) in comparison to those weighted-persons with ⩾1 other dental filling surfaces and 0 dental amalgam filling surfaces (other dental filling surfaces group).
The incidence rate of reported arthritis was derived from NHANES survey data among those persons with reported arthritis in the year of the NHANES survey collection. Those persons with reported arthritis at any time prior to the year of the NHANES survey collection were eliminated from the analyses.
Conclusion
This cross-sectional study provides the first epidemiological evidence linking increasing dental amalgam filling surfaces with reported arthritis among adult Americans. It was observed that the association remained significant when considering multiple other covariates and different statistical models. Overall, it was estimated that 45 742 weighted-persons were reported to be newly diagnosed with arthritis attributably associated with their dental amalgam filling surface exposure from this study. The annual total cost from the combined medical costs and lost annual wages to the US was estimated to be $281 633 494 US dollars. It was also revealed that there was a bimodal dose-dependent relationship between dental amalgam filling surface exposure and the risk of reported arthritis. The incidence rate of reported arthritis and dental amalgam filling surfaces increased to a peak and, subsequently, decreased among those with increasing numbers of dental amalgam filling surfaces. It is recommended that future longitudinal cohort studies be conducted for consistency with the observations made in the present study and to further determine the association between dental amalgam filling surfaces and arthritis in other populations. It is also recommended that dentists advise their patients that dental amalgam fillings may be associated with an increased risk of arthritis.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by a grant from the International Academy of Oral Medicine and Toxicology (IAOMT) to the Institute of Chronic Illnesses, Inc.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Mark Geier and Mr. David Geier are directors of the non-profit Institute of Chronic Illnesses, Inc and CoMeD, Inc. The Institute of Chronic Illnesses, Inc and CoMeD, Inc have no financial interests relating to dental filling materials or the outcome of arthritis. Dr. Mark Geier and Mr. David Geier own shares in EmeraMed, Ltd a company developing compounds utilized to treat mercury intoxication.
Authors’ Contributions: Dr. Mark Geier and Mr. David Geier contributed to the conception, analysis, and drafting of the manuscript.
References
- 1. Guglielmo D, Murphy LB, Boring MA, et al. State-specific joint paint and physical inactivity among adults with arthritis–United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCurdy D. Genetic susceptibility to the connective tissue diseases. Curr Opin Rheumatol. 1999;11:399-407. [DOI] [PubMed] [Google Scholar]
- 3. Fietta P, Delsante G. Epigenetics in autoimmune connective tissue diseases. Acta Biomed. 2014;85:91-107. [PubMed] [Google Scholar]
- 4. Klein K, Gay S. Epigenetics in rheumatoid arthritis. Curr Opin Rheumatol. 2015;27:76-82. [DOI] [PubMed] [Google Scholar]
- 5. Stejskal V, Reynolds T, Bjorklund G. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J Trace Elem Med Biol. 2015;31:230-236. [DOI] [PubMed] [Google Scholar]
- 6. Homme KG, Kern JK, Geier DA, King PG, Sykes LK, Geier MR. New science challenges old notion that mercury dental amalgam is safe. Biometals. 2014;27:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rasines Alcaraz MG, Veitz-Keenan A, Sahrmann P, Schmidlin PR, Davis D, Iheozor-Ejiofor Z. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. Cochrane Database Syst Rev 2014;3:CD0055620. [DOI] [PubMed] [Google Scholar]
- 8. Kozmos M, Virant P, Rojko F, et al. Bacterial adhesion of Streptococcus mutans to dental surfaces. Molecules. 2021;26:1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Arthritis in America. Accessed May 7, 2020. https://www.cdc.gov/vitalsigns/arthritis/index.html
- 10. Murphy LB, Cisternas MG, Pasta DJ, Helmick CG, Yelin EH. Medical expenditures and earnings losses among US adults with arthritis in 2013. Arthritis Care Res (Hoboken). 2018;70:869-876. [DOI] [PubMed] [Google Scholar]
- 11. Siblerud RL. Health effects after dental amalgam removal. J Orthomol Med. 1990;5:95-106. [Google Scholar]
- 12. Nerdrum P, Malt UF, Hoglend P, et al. A 7-year prospective quasi-experimental study of the effects of removing dental amalgam in 76 self-referred patients compared with 146 controls. J Psychosom Res. 2004;57:103-111. [DOI] [PubMed] [Google Scholar]
- 13. Pamphlett R, Kum Jew S. Mercury is taken up selectively by cells involved in joint, bone and connective tissue disorders. Mfron Med (Luasanne). 2019;6:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hultman P, Lindh U, Horsted-Bindslev P. Activation of the immune system and systemic immune-complex deposits in Brown Norway rats with dental amalgams. J Dent Res. 1998;77:1415-1425. [DOI] [PubMed] [Google Scholar]
- 15. Bjorkman L, Brokstad KA, Moen K, Jonsson R, Minor changes in serum levels of cytokines after removal of amalgam restorations. Toxicol Lett. 2012;211:120-125. [DOI] [PubMed] [Google Scholar]
- 16. Karatas GK, Tosun AK, Karacehennem, Sepici V. Mercury poisoning: an unusual case of polyarthritis. Clin Rheumatol. 2002;21:73-75. [DOI] [PubMed] [Google Scholar]
- 17. Sterzl I, Prochazkova J, Hrda P, et al. Mercury and nickel allergy: risk factors in fatigue and autoimmunity. Neuro Endorcinol Lett. 1999;20:221-228. [PubMed] [Google Scholar]
- 18. Stejskal J, Stejskal VD. The role of metals in autoimmunity and the link to neuroendocrinology. Neuro Endocrinol Lett. 1999;20:351-364. [PubMed] [Google Scholar]
- 19. Bjorklund G, Dadar M, Aaseth J. Delayed-type hypersensitivity to metals in connective tissue diseases and fibromyalgia. Environ Res. 2018;161:573-579. [DOI] [PubMed] [Google Scholar]
- 20. Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2013–2015. Morb Mortal Wkly Rep. 2017;66:246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pokrajac-Zirojevic V, Slack-Smith LM, Booth D. Arthritis and use of dental services: a population based study. Aust Dent J. 2002;47:208-213. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010:26:355-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamamoto SS, Yacyshyn E, Jhangri G, et al. Household air pollution and arthritis in low- and middle-income countries: cross-sectional evidence from the World Health Organization’s study on global ageing and health. PLoS One. 2019;12:e0226738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum. 1996;29:732-735. [DOI] [PubMed] [Google Scholar]
- 25. Vartiainen E, Seppala T, Lillsunde P, Puska P. Validation of self reported smoking by serum cotinine measurement in a community based study. J Epidemiol Community Health. 2002;56:167-170. [DOI] [PMC free article] [PubMed] [Google Scholar]