Abstract
Viruses are non-living organisms that annually cause many problems for human societies. The spread of some of the most dangerous viruses causing acute pneumonia, including novel Corona virus has led to the largest death toll in the world. With a long incubation period, Corona virus causes many problems for the immune system. Studies have shown that antioxidant enzymes play an important role in reducing infection and boosting the immune system. The immune system of people with chronic infections is often weak. Specific immunity is one of the most important responses to the virus. The present study therefore investigates association of Coronavirus pathogenicity with the level of antioxidants and immune system.
Keywords: Antioxidant enzymes, coronavirus, COVID-19, immune system
Introduction
Corona viruses are a group of long-standing, prevalent and diverse viruses that cause various diseases in human and animal. These viruses are the second leading cause of colds after Rhinovirus.[1,2] They cause diseases in the respiratory, gastrointestinal, and central nervous system. The prominent spike proteins on the coating of these viruses gave them a crown-like appearance, and since the Latin word corona means crown, they were called Corona virus.[3] Prior to 2019, there were only six CoVs capable of infecting human and causing respiratory diseases, including HCoV-229E, HCoV-OC43, HCoV-NL63, HKU1, SARS-CoV, MERS-CoV. In 2019, the WHO introduced a novel Corona virus, 2019-nCoV.[4]
Most viruses infecting the human body are controlled by the immune system.[5] The human immune system is one of the most sophisticated biological systems. It is a complex network of cells and chemicals, and its job is to protect human against foreign organisms and substances. Almost all viral infections induce various types of inflammatory cells, especially macrophages and in some infections, neutrophils.[6] On the other hand, viral infections often cause the production of Radicals and Reactive Oxygen Species (ROS) in cells. ROSs may harm host cells and contribute to pathogenesis.[7]
The production of free ROSs in the lung may contribute to damage the lung tissues through the virus activity.[8] Antioxidants, as substances that delay or inhibit substrate oxidation, play an important role in the body's immune system against ROS. Antioxidants are found in many foods, including fruits and vegetables.[9] The present study therefore investigates association of Corona virus pathogenicity with the level of antioxidants and immune system.
Corona viruses, its size and structure
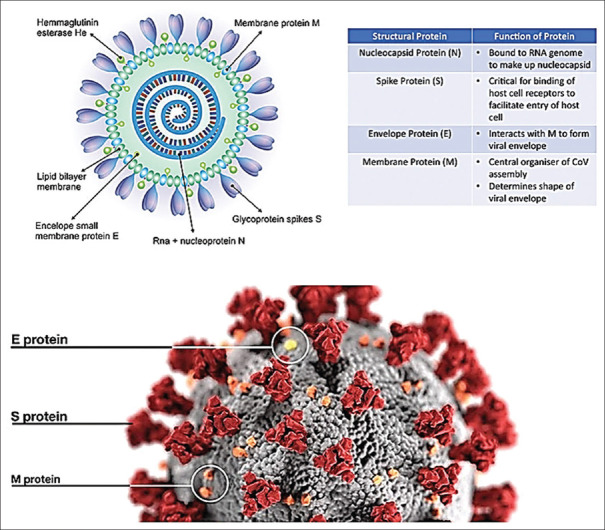
Corona viruses (CoVs) belong to the subfamily Orthocoronavirinae in the family Coronaviridae and the order Nidovirales. These subfamilies include α, coronavirus; β, corona virus; γ, coronavirus, and delta, coronaviru.[10] Coronaviruses have a coating, a helical capsid, and their genome is a positive single-stranded RNA that has the largest genome size among viruses with an approximate size of 26-32 kb.[3,11] These viruses are 80 to 90 nanometers.[2] The coronaviral genome encodes four major structural proteins: the spike (S) protein, nucleocapsid (N) protein, membrane (M) protein, and the envelope (E) protein, all of which are required to produce a structurally complete viral particle.[4] Figure 1 shows the structure, function of the structural proteins and electron microscopic images of the virus. Coronavirus replication begins by binding its spike protein (S) onto the surface molecules of the host cell. Binding of the receptor (S) is important to initiate virus entry into host cells. The virus enters its genetic material after binding to the receptor. In addition, some CoVs may enter the cell with the help of proteases.[11]
Figure 1.
The structure and electron microscopic
The immune system functions
The human immune system is a complex network of specific cells, tissues and organs, with the task of protecting the organism against diseases caused by specific pathogens such as viruses, bacteria, and other parasites.[6] In fact, it is equipped with a mechanism that allows living organisms to differentiate between insider and outsider cells.[12] The first line of defense against pathogens includes physical barriers in the skin and mucous membranes. If pathogens break this barrier of protection, the innate immune system is activated to diagnose and fight them. Thus, its task is to provide powerful nonspecific defenses preventing or limiting infection by most pathogenic microorganisms.[6] For successful performance, the acquired immune system produces a large number of cells, mostly in the bone marrow after childhood. In a routine blood test, there found five different types of white blood cells to defend the body, including.[1] neutrophils: primary responders with the task of phagocytosis and local killing;[2] lymphocytes: provide adaptive immunity and are divided into T and B cells;[3] monocytes: primary responders working on phagocytosis and antigen production. The adult type is called macrophages;[4] basophils; and[5] eosinophils: defend against parasites.[13]
However, T lymphocytes play the most important role in viral infection. In fact, the viruses are specifically identified in a highly complex cellular response by lymphocytes. Specificity in the acquired immune system is that viral antigens are exposed to T lymphocytes by antigen-processing cells. During this process, by entry of the virus into body, the immune system is activated, and by examining the antigenic levels of the pathogen, confirms the entry of the external pathogen. Antigen processor cells, including endothelial, dendritic, macrophages, and B lymphocytes, transcribe and transmit large numbers of viral antigens T lymphocytes through binding to and digesting them. T lymphocytes replicate these antigens and play an important role in the destruction of the target virus by providing surface-associated receptors compatible with the virus.[14,15,16,17,18]
Immune response to coronavirus
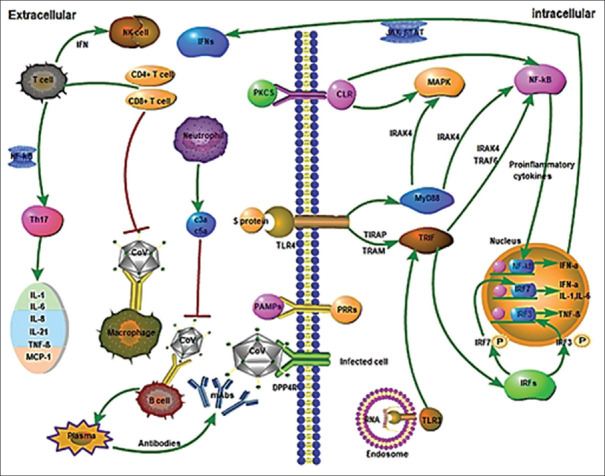
The initiation of an immune response to invading microorganisms requires that the host detects that organism and its components, including non-viral RNA, cellular stress, metabolic changes, and cellular damage resulting from infection.[19] These virus-related molecules, such as DNA and genomic RNA or double-stranded RNA are detected by Pattern Recognition Receptors (PRRs) in the virus-infected cell.[20] Known PRRs mainly include Toll-like receptors (TLRs), RIG-like receptors (RLRs), NOD-like receptors (NLRs), C Lectin-like receptors (CLmin), and free-floating receptors in the cytoplasm such as cGAS, IFI16, STING, and DAI.[3] After detecting viral compounds, PRRs initiate an appropriate and effective antiviral response, which involves the production of a variety of cytokines and adaptive and inflammatory immune responses. Interferons I (IFN_β and IFN-α molecules) are key cytokines produced after virus infection that induce the initiation of immune response and adaptation thereafter.[20] IFNs, as the main antiviral molecules in the host, restrict the virus and play an important role in enhancing phagocytosis of macrophages, antigens, and restricting NK cells of infected target cells and T/B cells. Thus, inhibition of IFNs production had a direct effect on virus survival in the host.[3] Figure 2 illustrates the immune response to the coronavirus. In the Section A, Figure 2, CoV infects macrophages and then macrophages deliver the CoV antigen to T lymphocytes. This process leads to activation and detection of T cells and production of cytokines associated with different T cell subsets (e.g. Th17), followed by extensive release of cytokines to enhance the immune response. Continued production of these intermediates due to virus stability has a negative effect on NK and CD8 T cell activation. However, CD8 T cells provide very effective intermediates for clearing CoV. Part B. Binding of CoV to DPP4R on the host cell via protein S leads to the emergence of genomic RNA in the cytoplasm. The immune response to dsRNA is partially produced by CoV proliferation. TLR-3 is affected by dsRNA and cascades of signaling pathways are activated (IRFs and NF-κB activation) following the production of type I IFNs and pre-inflammatory cytokines. To protect uninfected cells, the production of type I IFNs is crucial to increase the release of antiviral proteins. Occasionally, CoV proteins may interfere with TLR-3 signaling and bind to coronavirus dsRNA during replication and prevent TLR-3 activation and immune response. It is likely that TLR-4 detects S protein and results in activation of pre-inflammatory cytokines through MyD88-dependent signaling pathway. The cell-virus interaction results in a high production of immune mediator. The secretion of large amount of chemokines and cytokines (IL-1, IL-6, IL-8, IL-21, TNF-β and MCP-1) in response to CoV infection increases in infected cells. These chemokines and cytokines, in turn, attract lymphocytes and leukocytes to the site of infection.[3] In people with a strong immune system, lymphocytes, especially T lymphocytes, act more rapidly and specifically kill the pathogen. On the other hand, in people with a weak immune system, COVID-19 causes acute inflammation triggered by the development of pneumonia and increased cytokines. Regarding SARS, which is in the family of Coronaviruses and is genetically similar to the novel coronavirus, the acute stage is associated with a severe decrease in the number of T cells in the blood.[21,22,23,24] indicating the major role of lymphocytes in specific immunity regarding the Coronavirus diseases.
Figure 2.
Innate immune responses and adaptive immune responses to Coronaviruses (CoV) during infection. *Red lines show inhibitory effects and green lines show activation effects (3)
Antioxidants
To balance the oxidation state, plants and animals have complex systems of antioxidants, which help regulate cellular responses.[25] Antioxidant is any substance that significantly inhibits or delays the oxidation of that substance if it is present at low concentrations compared to an oxidizer.[26] Oxidation is a chemical reaction that can produce free radicals (Reactive Oxygen Species), thereby leading to chain reactions and subsequently damaging the cells of organisms.[25] The physiological role of antioxidants is to complete these chain reactions and to prevent the damage of free radicals to the cellular components caused by the chemical reactions.[25,26] ROS is a molecular group produced by cellular metabolism through the activation of mitochondrial oxidases or other cellular components.[25] These molecules have an unpaired electron in their atomic orbit.[26] Low levels of ROS are required for intracellular signaling, though the higher level causes irreversible damage to lipids, proteins, and DNA.[27] Types of antioxidants include catalase, glutathione peroxidase, glutathione reductase, superoxide dismutase, alpha-tocopherol, ascorbate, and glutathione.[26] In infected cells, viral products cause an oxidative environment;[27] and oxidative stress, directly caused by the virus or by the host's immune response, is an important pathological mechanism in the body.[28] Viral infections often produce oxygen radicals in cells. Although ROS can act as a key element in antiviral defense, they can be harmful to host cells and contribute to pathogenesis.[7] Regular consumption of vegetables and fruits containing antioxidants significantly reduces the risk of chronic diseases. Citrus such as orange and lemon contain high levels of natural antioxidants including vitamin C. Also, Blueberry, strawberry, grape, plum, red bean, spinach, cabbage, broccoli, and alfalfa sprout have high levels of antioxidants.[9]
Antioxidants and viruses
Viral infections are associated with oxidative stress, which plays an important role in their pathogenesis. Oxidative processes cause virus replication in infected cells, decrease cell proliferation, and induce cell apoptosis. In patients with neuroviral infections, there is intensification of lipid oxidation processes and severe suppression of the body's antioxidant defense system by free radicals.[29] The role of oxidants in inactivating viruses was discovered in the early 1970s, but the metabolic role of oxidants in viral infections was later elucidated.[30] Antioxidants are used by various mechanisms to prevent or treat various diseases associated with oxidative stress and in many cases have therapeutic effects. Since natural antioxidants such as vitamins C and E (ascorbic acid and α-tocopherol), curcumin, and various polyphenols considered as antiviral agents because they reduce the level of ROS in infected cells and the expression of pre-apoptotic signaling molecules as well as regulate the cellular levels of stress proteins, including c-Jun N-terminal kinase (JNK), mitogen-activated protein phospho-p38 kinase (MAPK), extracellular signal-regulating kinases (ERK-1/2), and NF-kB transcription factor.[29]
Solutions
Antioxidants
Antioxidants are considered to be the first line of defense against free radical damages, which are abundantly found in fruits, vegetables, and teas.[9] The most important aspect of treating viral diseases is to suppress virus replication followed by cell survival. Researchers are looking for antiviral drugs among antioxidants.[29] Studies have shown that the use of antioxidants is very effective in reducing inflammation and has had a beneficial effect on the recovery of chronic lung disease as adjunctive therapy. According to studies on the treatment and control of COVID-19 in China, Chinese medicine, including the use of antioxidants as adjunctive therapy, has a beneficial effect.[30,31,32,33] Since regular consumption of antioxidant vegetables and fruits reduces the risk of chronic diseases, fruits such as blueberry, strawberries, grape, plum, red bean, spinach, cabbage, broccoli, alfalfa sprout, and drinks containing antioxidant are highly recommended. Certainly not all antioxidants provide a 100% response so further investigations should be considered in the case of Coronavirus.
Immune system
Although there have been many studies on the immune system, two were discussed here. Among the factors strengthening the immune system is physical exercise. Physical exercise, by reducing the stress of the cell and regulating cytokines, improve the immune system.[34,35,36,37] Another important issue is stress. Previous studies has clearly demonstrated that psychological stress clinically affects immune functions, including inflammatory processes, wound healing, and responses to infectious agents. Prolonged stress increases the immune system by increasing steroid hormones. In addition, it increases inflammation and consequently oxidative stress in the cells. People with diabetes are exposed to oxidative stress because of the blood sugar, which is an important factor in strengthening the immune system and fighting viruses. Also, viral infection itself is said to create inflammatory and oxidative stress in cells. Studies have shown that everyone who suffers from a particular disease, even the common cold, is exposed to cellular oxidative stress.[38,39,40,41,42,43,44,45,46] As a result of balanced physical exercise, stress reduction, and antioxidant intake will be the contributing factor.
Vaccination
Another solution is to produce an effective vaccine. Since Coronavirus S protein is capable of stimulating neutral antibodies, responding to CD4 and CD8 receptors, and N protein can induce T cell response in humans, both of these proteins are potentially useful candidates capable of producing strong humoral and cellular (innate and acquired) immunity against SARS-CoV. Importantly, previous studies have shown that some vaccines encoding N proteins induces eosinophilic responses.[43] Therefore, these vaccines should be carefully controlled. There is currently no information available on epitopes known in patients with Coronavirus, though investigating epitopes can help develop the vaccine.
Conclusion
Studies have shown that viral diseases, such as corona, activate the innate and acquired immune system to fight the virus, though the role of acquired immunity is especially important for lymphocytes. Also, the more the T lymphocytes enter the fight against the virus, the better the immune system withstands the virus. In addition, viral diseases increase the production of free radicals, and since antioxidants play an important role in eliminating free radicals, reducing viral infection, and boosting the immune system, diets containing these antioxidant can be very useful. Antioxidants help the immune system to function better in its regeneration by reducing oxidative stress and thereby reducing inflammation. It is recommended that a study on patients with Coronavirus be conducted to evaluate antioxidant and lymphocyte levels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Li F. Receptor recognition mechanisms of coronaviruses: A decade of structural studies. J Virol. 2015;89:1954–64. doi: 10.1128/JVI.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ui M, Liu X, Guo D, Zhang Z, Yin CC, Chen Y, et al. Electron microscopy studies of the coronavirus ribonucleoprotein complex. Protein Cell. 2017;8:219–24. doi: 10.1007/s13238-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–32. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seah I, Su X, Lingam G. Revisiting the Dangers of the Coronavirus in the Ophthalmology Practice. Nature Publishing Group; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: What decides the outcome? Nat Rev Immunol. 2010;10:514–26. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao S. Bio-Inspired Computational Algorithms and Their Applications. BoD–Books on Demand. 2012 [Google Scholar]
- 7.Kuzmenko YV, Smirnova OA, Ivanov AV, Starodubova ES, Karpov VL. Nonstructural protein 1 of tick-borne encephalitis virus induces oxidative stress and activates antioxidant defense by the Nrf2/ARE pathway. Intervirology. 2016;59:111–7. doi: 10.1159/000452160. [DOI] [PubMed] [Google Scholar]
- 8.Jacoby DB, Choi AM. Influenza virus induces expression of antioxidant genes in human epithelial cells. Free Radic Biol Med. 1994;16:821–4. doi: 10.1016/0891-5849(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 9.Yadav A, Kumari R, Yadav A, Mishra JP, Srivatva S, Prabha S. Antioxidants and its functions in human body. A review. Res Environ Life Sci. 2016;9:1328–31. [Google Scholar]
- 10.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479–90. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillaiyar T, Meenakshisundaram S, Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov Today. 2020;25:668–88. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McComb S, Thiriot A, Krishnan L, Stark F. Introduction to the Immune System, in Immunoproteomics. Springer. 2013:1–20. doi: 10.1007/978-1-62703-589-7_1. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson LB. The immune system. Essays Biochem. 2016;60:275–301. doi: 10.1042/EBC20160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yewdell JW, Bennink JR. Mechanisms of viral interference with MHC class I antigen processing and presentation. Ann Rev Cell Dev Biol. 1999;15:579–606. doi: 10.1146/annurev.cellbio.15.1.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozano-Ojalvo D, Dunkin D, Mondoulet L, Agudo J, Merad M, Sampson HA, et al. PDL2+ CD11b+ dermal dendritic cells capture topical antigen through hair follicles to prime LAP+ Tregs. Nat Commun. 2018;9:1–12. doi: 10.1038/s41467-018-07716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka Y, Nagashima H, Bando K, Lu L, Ozaki A, Morita Y, et al. Oral CD103 - CD11b+ classical dendritic cells present sublingual antigen and induce Foxp3+ regulatory T cells in draining lymph nodes. Mucosal Immunol. 2017;10:79–90. doi: 10.1038/mi.2016.46. [DOI] [PubMed] [Google Scholar]
- 17.Murray N, McMichael A. Antigen presentation in virus infection. Curr Opin Immunol. 1992;4:401–7. doi: 10.1016/s0952-7915(06)80030-0. [DOI] [PubMed] [Google Scholar]
- 18.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+dendritic cells. Nat Immunol. 2009;10:488–9. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 19.Braciale TJ, Hahn YS. Immunity to viruses. Immunological Rev. 2013;255:5–12. doi: 10.1111/imr.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 21.Park Y-J, Walls AC, Wang Z, Sauer MM, Li W, Tortorici MA, et al. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat Struct Mol Biol. 2019;26:1151–7. doi: 10.1038/s41594-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Frontiers Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–28. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salehi B, Martorell M, Arbiser JL, Sureda A, Martins N, Maurya PK, et al. Antioxidants: Positive or negative actors? Biomolecules. 2018;8:124. doi: 10.3390/biom8040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young I, Woodside J. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–86. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Nagy N, Masucci MG. The Epstein–Barr virus nuclear antigen-1 upregulates the cellular antioxidant defense to enable B-cell growth transformation and immortalization. Oncogene. 2020;39:603–16. doi: 10.1038/s41388-019-1003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–75. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 29.Fedoreyev SA, Krylova NV, Mishchenko NP, Vasileva EA, Pislyagin EA, Iunikhina OV, et al. Antiviral and antioxidant properties of echinochrome A. Mar Drugs, 2018;16:509. doi: 10.3390/md16120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterhans E. Oxidants and antioxidants in viral diseases: Disease mechanisms and metabolic regulation. J Nutr. 1997;127:962S–5S. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- 31.Tenero L, Piazza M, Zanoni L, Bodini A, Peroni D, Piacentini GL. Antioxidant supplementation and exhaled nitric oxide in children with asthma. Allergy Asthma Proc. 2016;37:e8–13. doi: 10.2500/aap.2016.37.3920. [DOI] [PubMed] [Google Scholar]
- 32.Nobakht M, Gh BF, Oskouie AA, Aliannejad R, Rezaei-Tavirani M, Tavallaie S. et al.Pro-oxidant–antioxidant balance in Iranian veterans with sulfur mustard toxicity and different levels of pulmonary disorders. Drug Chem Toxicol. 2016;39:362–6. doi: 10.3109/01480545.2015.1122033. [DOI] [PubMed] [Google Scholar]
- 33.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirowsky JE, Dailey LA, Devlin RB. Differential expression of pro-inflammatory and oxidative stress mediators induced by nitrogen dioxide and ozone in primary human bronchial epithelial cells. Inhal Toxicol. 2016;28:374–82. doi: 10.1080/08958378.2016.1185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8:201–17. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colbey C, Cox AJ, Pyne DB, Zhang P, Cripps AW, West NP. Upper respiratory symptoms, gut health and mucosal immunity in athletes. Sports Med. 2018;48:65–77. doi: 10.1007/s40279-017-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson RJ, Bigley AB, Agha N, Hanley PJ, Bollard CM. Mobilizing immune cells with exercise for cancer immunotherapy. Exerc Sport Sci Rev. 2017;45:163–72. doi: 10.1249/JES.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–46. doi: 10.1016/j.critrevonc.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress—a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547–60. doi: 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- 40.LeRoy AS, Murdock KW, Jaremka LM, Loya A, Fagundes CP. Loneliness predicts self-reported cold symptoms after a viral challenge. Health Psychol. 2017;36:512–20. doi: 10.1037/hea0000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y-Z, Wang Y-X, Jiang C-L. Inflammation: The common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y-R, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85:12201–15. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soltany S, Roham M, Hejrati A, Heidari Beigvand H, Saberinia A. Coronavirus Outbreak and Important Points regarding its Prevention and Biological Cognition? International Journal of Psychosocial Rehabilitation. 2020;24:8402–12. DOI: 10.37200/IJPR/V24I8/PR280846. [Google Scholar]
- 45.Bokaiean R, Momeni M, Sabrjoo P, Dahmardehei M, Roham M, Rahber H. Comparing active Leptospermum honey dressing with conventional dressing in skin graft donor sites. Iranian Journal of Dermatology. 2018;21:1–6. [Google Scholar]
- 46.Roham M, Abbaszadeh A, Zendehdel A, Momeni M, Mirzae N, Gholami M. Prognostic factors of sepsis rapid progression in patients admitted to Intensive Care Unit. Annals of Tropical Medicine and Public Health. 2017;10:1770. [Google Scholar]