Abstract
Introduction:
The characteristics of somatic symptoms seen at the first hospital visit in patients with psychogenic backgrounds remain poorly elucidated till date.
Methodology:
A total of 277 patients who visited the Department of General Medicine at a single university hospital with somatic symptoms were prospectively enrolled in this study. The eventual definite diagnoses were classified into the following three groups: non-psychogenic disease (n = 128), psychogenic symptoms (n = 131), and mental illness (n = 18). Subsequently, the chief complaints and other background information of the patient obtained at the first visit were compared among the three groups.
Results:
More than half of the patient with non-psychogenic diseases (60.2%) presented with a single complaint at their first hospital visit; contrarily, less than half of the patients with psychogenic symptoms (23.7%) or mental illnesses (22.2%) presented with a single complaint at the first visit. Approximately, <10% of the patients with non-psychogenic diseases had four or more multisystemic presentations at the first visit. The results of the receiver operating characteristic curve analysis revealed a fair discriminatory ability of the number of complaints to identify patients with psychogenic diseases or psychiatric backgrounds. Almost half of the non-psychogenic patients with four or more multisystemic presentations were eventually diagnosed with autoimmune-related disorders, such as Sjögren's syndrome or Behçet's disease. In conclusion, the general notion that patients with psychogenic somatic symptoms are likely to present with more complaints than patients with non-psychogenic diseases is correct. However, not a few patients who present with multiple indefinite complaints would certainly have organic diseases such as autoimmune-related disorders or neuromuscular diseases. A careful diagnostic process is required in such patients before attributing their symptoms to psychogenic or psychiatric factors.
Keywords: Chief complaint, mental illness, psychogenic, somatic symptom disorder, somatoform disorder
Introduction
Somatic symptom disorder (SSD), previously known as somatoform disorder, is a disease entity with persistent distressing somatic/physical symptoms that cannot be fully explained by general medical conditions or other mental illnesses.[1,2] Currently, the diagnosis of SSD cannot be obtained by specific diagnostic examinations, and great emphasis is placed on the presence of certain psycho-behavioral features based on perceptual abnormalities (i.e., disproportionality, persistency, and excessiveness of anxiety about the symptoms).[2,3,4] Diagnosis is possible only after the comprehensive exclusion of other conceivable organic medical conditions or mental illnesses.[5,6] The correct diagnosis of SSD is important not only to treat patients with anxiolytics but also to avoid overlooking non-psychotic diseases that can otherwise be treated with proper therapeutic interventions. However, till date, the characteristics of somatic symptoms in patients with psychogenic backgrounds and those with non-psychogenic diseases have not been studied sufficiently. Identifying and systematizing such psychosomatic relationships is important not only for the practice of primary care physicians but also to develop artificial intelligence-based diagnostic aids in the future. The application of artificial intelligence in clinical settings to correctly diagnose diseases based on the complaints and laboratory findings has been attempted worldwide. Clarifying the clinical characteristics of patients with psychogenic somatic symptoms is essential to build a reliable diagnostic tool that will not overlook non-psychogenic medical conditions that require disease-specific treatments.[7,8,9]
In the present study, we first prospectively enrolled undiagnosed patients who were later decided to be with psychogenic or non-psychogenic (i.e., organic or functional) background; thereafter, we tried to clarify the clinical characteristics of patients with psychogenic somatic symptoms by comparing them with the characteristics of patients with non-psychogenic diseases.
Material and Methods
Study design and subjects
All patients who visited the Department of General Medicine at a single university hospital between April 2018 and March 2020 were initially included in this prospective observational study. The inclusion criteria were as follows:
Definite diagnoses were not obtained before consultation at our hospital
The complaints persisted for more than 1 month
Blood test results, including blood cell count, were available
All participating doctors in the department agreed that necessary diagnostic examinations, such as thyroid hormone test, antinuclear antibody, anti-SSA/SSB antibodies, rheumatoid factor, antineutrophil cytoplasmic antibodies, Holter electrocardiography, spirometry, polysomnography, endoscopy, computed tomography, and magnetic resonance imaging, were sufficient and appropriately performed to evaluate all the complaints to establish a correct diagnosis in each patient.
During the enrollment period, seven patients were diagnosed with myalgic encephalomyelitis/chronic fatigue syndrome or fibromyalgia. These patients were excluded from the study as it could not be concluded whether they had psychogenic or non-psychogenic conditions as of March 2020.[10,11,12,13] Besides, three middle-aged female patients who were eventually diagnosed with the postmenopausal syndrome were also excluded from this study because of the difficulty of objectively diagnosing the disorder solely based on laboratory and physiological diagnostic examinations.[14] Consequently, the remaining 277 patients with persistent physical symptoms were considered eligible for the study.
Categories of eventual diagnosis
The enrolled patients who visited our department with unknown diagnoses were eventually categorized into the non-psychogenic or psychogenic groups based on subsequently established definite diagnoses [Figure 1]. Within the psychogenic group, the patients were further subdivided into the “psychogenic symptoms” group and the “mental illness/psychiatric” group based on the psychiatrists' diagnosis. All patients in the mental illness group were either already diagnosed with mental illnesses (i.e., depression, schizophrenia, personality disorders) by psychiatrists before visiting our department or diagnosed later with mental illnesses by psychiatrists after consultation in our department. A list of the definite diagnoses that were categorized into the psychogenic group and a list of mental diseases that were categorized into the mental illness group are presented in Table 1. All patients with persistent physical symptoms were followed for more than 6 months to ascertain whether they fulfill the present diagnostic criteria for SSD.[3] Two female patients were later diagnosed with functional dyspepsia and they were included in the “non-psychogenic” group according to the latest Rome IV diagnostic criteria.[15] One of them presented with a single complaint, and another with two complaints. There was no patient diagnosed with irritable bowel syndrome.
Figure 1.
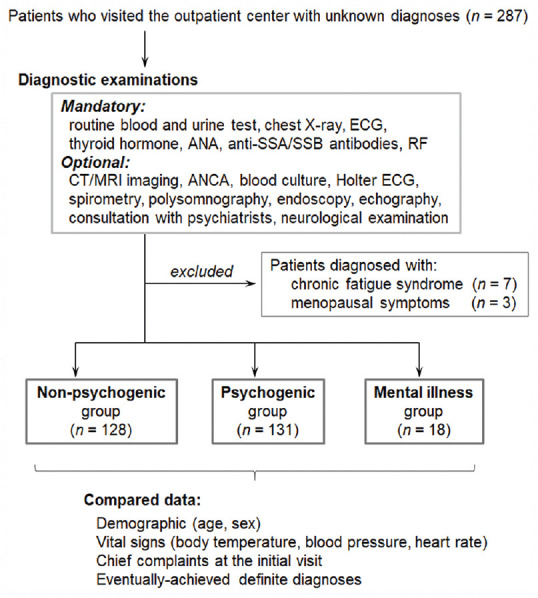
The three patient groups established by the presence of psychogenic or psychiatric background Abbreviations: ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibodies; ECG, echocardiography; RF, rheumatoid factor
Table 1.
List of diagnoses in the psychogenic group and the mental illness group
| List of diagnoses in the psychogenic group |
| Somatic symptom disorder (SSD) |
| Psychosomatic disorder |
| Somatoform disorder |
| Somatoform dissociative symptoms |
| Conversion disorder |
| Hypochondriasis |
| List of diagnoses in the mental illness group |
| Depression |
| Bipolar disorder |
| Schizophrenia |
| Personality disorder |
Studied variables
Demographic data (such as age and sex), numbers and types of the self-reported somatic complaints at the first visit, routine blood tests, routine urine tests, presence of serum antinuclear antibodies; tests for anti-SSA/SSB antibodies, rheumatoid factors, and thyroid hormone levels (T3, T4, thyroid-stimulating hormone) were evaluated in all the patients. The same type of physical symptom in different body parts belonging to a single body system (e.g., dysesthesia in both upper limbs) was counted as one somatic complaint. The Zung self-rating depression scale (SDS) score was also evaluated in all patients at their initial hospital visits. Other diagnostic examinations were performed as needed, based on patients' complaints. All complaints reported by the enrolled patients were checked and evaluated using proper diagnostic examinations. Furthermore, the prevalence of several major and popular complaints (i.e., limb numbness, pharyngolaryngeal dysesthesia, dizziness, tinnitus, slight fever, headache, and fatigue) were separately counted among the enrolled patients. Each patient was assessed by doctors with more than 5 years of experience in the fields of cardiology, pulmonology, neurology, nephrology, or general surgery. If the assessment of any patient revealed the need for consultation with other specialized departments, consultations with the appropriate departments were arranged for correct diagnosis and appropriate treatment.
Statistical analyses and software
Comparisons between quantitative variables with presumed normal distribution were performed with the Student's t-test, and quantitative variables with non-normal distribution were compared using the Mann–Whitney U test. Comparison of quantitative variables between three or more nonpaired groups with presumed normal distribution was performed using analysis of variance or the Kruskal–Wallis test, followed by the post-hoc tests according to the distributional pattern of the evaluated variables. While comparison of qualitative variables using the Chi-square test or Fisher's exact test was performed according to the size of each cell. Binary logistic regression analysis was performed to assess the influence of the abovementioned seven major complaints and to evaluate their predictive values for the eventual diagnoses of psychogenic or psychiatric conditions; these assessments were performed after combining the “psychogenic symptoms” and “mental illness/psychiatric” groups into a single group. Odds ratios (ORs) and 95% confidence intervals (CIs) for each explanatory variable were calculated using logistic regression analysis. Receiver operating characteristic (ROC) curves were generated to estimate and compare the predictive values of the number of complaints, total SDS score, and patient's age to detect associations between psychogenic or psychiatric backgrounds and the manifested somatic symptoms. The area under the curve (AUC), with the measured values from 0–1, was calculated for each ROC curve to compare the discriminatory ability of each variable to diagnose patients with and without psychogenic/psychiatric conditions. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 22 (IBM Corp., USA) or MATLAB R2015a (MathWorks, USA).
Ethics approval
The present study was approved by the Institutional Review Board (IRB) of Tohoku University Graduate School of Medicine (IRB approval number: THK-2019-1-977/Date of approval: 26th March, 2020). This clinical research was conducted following the Helsinki Declaration, as revised in 2013.
Results
Patient background
Among the 277 consecutive patients who were eligible for the following analyses in this study, 110 (39.7%) were males and 167 (60.3%) were females. The mean age at their first visit to our department was 52.8 ± 19.6 years. Among the enrolled patients, 123 (44.4%) were referred from other hospitals with medical referral letters, and 154 (55.6%) directly visited our hospital without medical referral letters.
After comprehensive diagnostic examinations and assessment by experts from appropriate specialties, the patients were eventually categorized into the following three groups: “non-psychogenic symptoms” group (n = 128), “psychogenic symptoms” group (n = 131), and “mental illness/psychiatric” group (n = 18). Detailed background data, vital signs, and chief complaints at the first visit are summarized in the first half of Table 2. The proportion of female patients was slightly higher in the psychogenic group than in the other groups but without any statistical significance. The vital signs evaluated at the first visit were not significantly different among the three groups. The SDS score was the lowest in the non-psychogenic group and the highest in the mental illness group.
Table 2.
Clinical findings in each of the three groups
| Non-psychogenic disease (n=128) | Psychogenic symptoms (n=131) | Mental illness (n=18) | P | |
|---|---|---|---|---|
| Male : Female | 57 : 71 | 47 : 84 | 6 : 12 | 0.31 |
| Age [years] | 52.7±20.3 | 54.5±18.6 | 41.7±18.9 | 0.0333 |
| Vital signs at the first visit | ||||
| BT [°C] | 36.6±0.50 | 36.5±0.48 | 36.6±0.43 | 0.57 |
| sBP [mmHg] | 127.3±22.2 | 127.9±21.5 | 116.6±18.2 | 0.11 |
| dBP [mmHg] | 73.7±14.4 | 74.4±17.6 | 70.1±14.2 | 0.57 |
| HR [bpm] | 83.6±17.2 | 81.5±15.9 | 80.7±15.3 | 0.64 |
| SDS scores | 43.3±10.2 | 47.0±9.4 | 56.3±11.1 | <0.0001 |
| Complaints at the first visit to our department | ||||
| Number of complaints* | 1 [1-2] | 3 [2-4] | 3 [2-5] | <0.0001 |
| Single complaint | 77 (60.2%) | 31 (23.7%) | 4 (22.2%) | <0.0001 |
| 2-3 complaints | 39 (30.5%) | 67 (51.1%) | 6 (33.3%) | 0.0026 |
| 4-6 complaints | 11 (8.6%) | 27 (20.6%) | 6 (33.3%) | 0.0034 |
| ≥ 7 complaints | 1 (0.8%) | 6 (4.6%) | 2 (11.1%) | 0.0341 |
| Prevalence in each of the 7 major complaints | ||||
| Limb numbness | 23 (18.0%) | 33 (25.2%) | 2 (11.1%) | 0.21 |
| Pharyngolaryngeal dysesthesia | 6 (4.7%) | 14 (10.7%) | 0 (0.0%) | 0.0830 |
| Dizziness | 5 (3.9%) | 25 (19.1%) | 5 (27.8%) | 0.0002 |
| Tinnitus | 1 (0.8%) | 6 (4.6%) | 0 (0.0%) | 0.12 |
| Slight fever | 19 (14.8%) | 8 (6.1%) | 3 (16.7%) | 0.0551 |
| Headache | 9 (7.0%) | 22 (16.8%) | 3 (16.7%) | 0.0479 |
| Fatigue | 10 (7.8%) | 21 (16.0%) | 8 (44.4%) | 0.0001 |
BT: body temperature, dBP: diastolic blood pressure, HR: heart rate, sBP: systolic blood pressure, bpm: beats per minute, SDS: Zung self-rating depression scale. Data are presented as mean±standard deviation or as number (percentage). *Median and 25-75 percentile range
Details of complaints at the first hospital visit
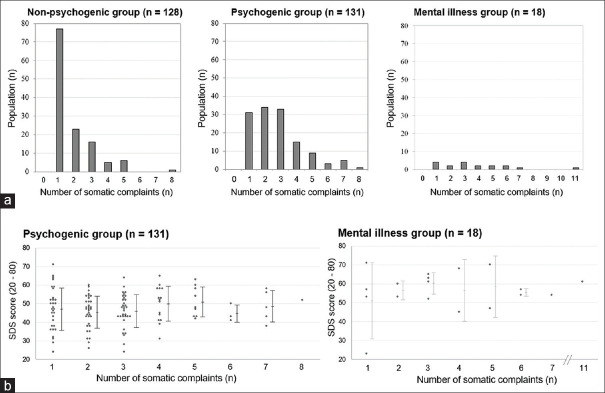
Details of the somatic complaints at the first visit, before the definite diagnoses, are summarized in the second half of Table 2. The number of complaints at the first visit was the highest in the mental illness group and the lowest in the non-psychogenic group. About 77 of the 128 patients with non-psychogenic diseases (60.2%) presented with a single complaint at the first hospital visit, whereas only 31 of the 131 patients with psychogenic symptoms (23.7%) and four of the 18 patients with mental illnesses (22.2%) presented with a single complaint [Figure 2a]. In the total cohort of 277 patients, there was a slight positive correlation between the number of complaints and SDS scores, with a Pearson's R-value of 0.20 (P = 0.0008; the test of no correlation). However, when evaluated separately in each of the three disease groups, no group showed a significant correlation between the number of somatic complaints and SDS scores [Figure 2b].
Figure 2.
Relationship between the number of somatic complaints, psychogenic factor, and SDS score (a) Histograms of the number of somatic complaints at the first hospital visit in each of the three groups. (b) Grouped scatter plots of the SDS score by the number of somatic complaints in each disease group. Abbreviation: SDS, Zung self-rating depression score
Dizziness and fatigue were more prevalent in patients with psychogenic or psychiatric backgrounds than in those with non-psychogenic diseases. The prevalence of headache was slightly higher, whereas that of chronic fever was lower in patients with the psychogenic background; however, the difference was not statistically significant. On binary logistic regression analysis, dizziness (OR: 5.08, 95% CI: 1.85–13.91, P = 0.0016) and fatigue (OR: 3.04, 95% CI: 1.34–6.89, P = 0.0077) showed significant associations with psychogenic or psychiatric backgrounds. Conversely, slight fever (OR: 0.33, 95% CI: 0.13–0.82, P = 0.0172) showed a significant association with non-psychogenic conditions. Other major complaints (i.e., limb numbness, pharyngolaryngeal dysesthesia, tinnitus, headache) were associated with neither the psychogenic/psychiatric group nor the non-psychogenic group.
ROC curve analysis
Next, we performed a ROC curve analysis to compare the discriminatory ability of the number of complaints, total SDS score, and age to diagnose patients with and without psychogenic/psychiatric background [Figure 3]. The measured AUC for the number of complaints was 0.708, which was then compared with that of the SDS total score (AUC, 0.646; P = 0.15). The AUC for the patient's age was 0.502, which was significantly lower than the abovementioned two AUCs (P < 0.0001 for both). These results suggest that both the number of complaints and SDS scores have reasonable discriminating ability to predict psychogenic/psychiatric background, whereas age has no such discriminatory ability.[16]
Figure 3.
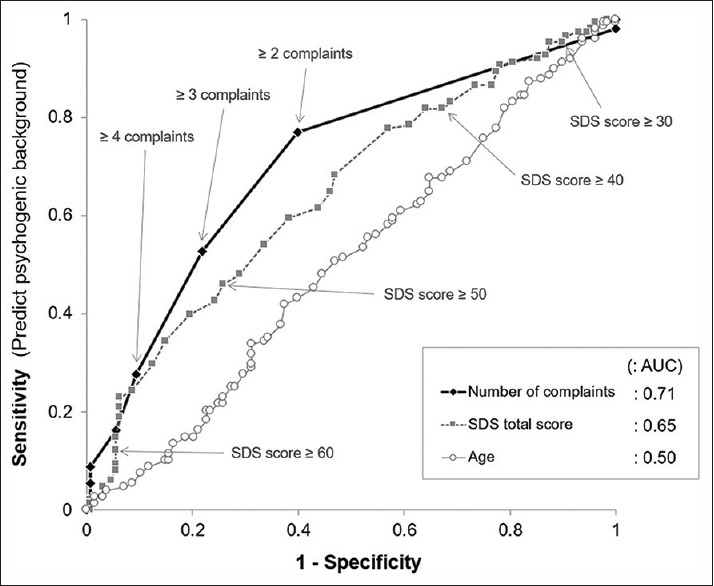
The receiver operating characteristic curves to compare the discriminatory ability to doubt the presence of psychogenic background The measured area under the curve for the number of complaints was fair with 0.71, which was slightly higher than that for the SDS total score. Abbreviations: AUC, the area under the curve; SDS, Zung self-rating depression score
Non-psychogenic diseases with ≥ 4 multisystemic complaints
While more than half of the patients with non-psychogenic diseases presented with a single complaint at the first visit, 12 out of the 128 patients with non-psychogenic diseases (9.4%) presented with ≥ 4 multisystemic complaints at their first visit. A list of the self-reported complaints at the first visit and the eventual definite diagnoses of these 12 patients are shown in Table 3. One of the patients with Behçet's disease had a history of encephalitis about 10 years before, but whether this preceding episode is associated with Behçet's disease or not is unknown. Patients with neuromyelitis optica spectrum disorders (NMOSD) with serum anti-aquaporin-4 antibodies usually present physical symptoms based on optic neuritis or myelitis at their clinical onset[17,18] ; however, some of the patients may present intractable hiccup with anorexia by medullary lesions or allergy-like cutaneous symptoms with skin-color changes by myelitis as observed in the patient in this study.[19,20] If NMOSD with serum anti-aquaporin-4 antibodies is regarded as an autoimmune-related disorder, then seven patients (58.3%) of the 12 patients with ≥ 4 multisystemic complaints had autoimmune-related conditions.
Table 3.
List of non-psychogenic patients with ≥4 complaints at the first visit
| Patient number | Definite diagnosis | Complaints at the first visit |
|---|---|---|
| 1 | Behçet’s disease | Fever, headache, lymphadenopathy, eye pain, genital ulcer, joint pain, muscle pain, axillary pain |
| 2 | Sjögren’s syndrome | Dry mouth, dry eyes, eye pain, fatigue, dysphagia |
| 3 | ALS | Neck weakness, stiff shoulder, fatigability, dysphagia, facial paresthesia |
| 4 | Myotonic dystrophy | Sweating in the hands, grip weakness, stiff back, facial deformity, restlessness |
| 5 | SMA syndrome | Weight loss, dyspnea, dysphagia, dizziness, constipation |
| 6 | Graves’ disease | Swollen eyelid, swollen neck, palpitation, cough, runny nose |
| 7 | RS3PE syndrome | Dysesthesia in the jaw, stiff shoulder, numbness in the limbs, polyarthralgia, swollen hands |
| 8 | Behçet’s disease | Abdominal discomfort, fatigue, dizziness, slight fever |
| 9 | Sjögren’s syndrome | Slight fever, tinnitus, ear fullness, numbness in the limbs |
| 10 | NMOSD | Numbness in the arms and the thighs, headache, dysesthesia in the body trunk, breathlessness, redness in the hands |
| 11 | Sjögren’s syndrome | Palpitation, anorexia, weight loss, pain in upper and the lower limb girdles |
| 12 | Chronic urticaria | Itchy skin rashes, nausea, headache, fatigue |
ALS: amyotrophic lateral sclerosis, NMOSD: neuromyelitis optica spectrum disorders, RS3PE: remitting seronegative symmetrical synovitis with pitting edema, SMA: superior mesenteric artery
Discussion
As suggested by the term “unidentified complaints,” it has been empirically believed that patients with psychogenic conditions are more likely to visit hospitals with more non-systematized, multisystem complaints than patients with non-psychogenic diseases. In the present study, we statistically confirmed that patients with psychogenic or psychiatric backgrounds are likely to present with more complaints than patients with non-psychogenic diseases. This finding was true even after excluding patients with mental illnesses, such as depression, schizophrenia, and personality disorders, from the psychogenic group. Notably, although most of the patients with non-psychogenic diseases visited the hospital with a single or only a few complaints at their first visit, some patients with non-psychogenic diseases presented with ≥ 4 complaints, especially those with autoimmune-related disorders (e.g., Behçet's disease, Sjögren's syndrome) or neuromuscular diseases.
It has been suggested that autoimmune-related disorders, endocrine disorders, systemic infections, and neuromuscular disorders are likely to present a wide spectrum of symptoms, often mimicking the manifestations of somatoform disorders.[21,22,23,24,25] Most of these symptoms usually require an early and correct diagnosis for selecting appropriate, effective therapies.[24,26] If these conditions are not diagnosed correctly, they can impair the quality of life of the affected individuals. Among these non-psychogenic conditions with multiple complaints, Behçet's disease requires additional caution because it is difficult to diagnose and the diagnosis is often delayed, even by skilled physicians. One of the reasons for delayed diagnosis is the lack of disease-specific serum biomarkers (e.g., autoantibodies) for Behçet's disease. Accordingly, this disease must be diagnosed based on the clinical history and careful inspection of the systemic skin lesions.[27,28] The fact that two of the 277 enrolled patients in the present study were eventually diagnosed with Behçet's disease indicates that it is not rare among patients who present with miscellaneous symptoms but without definite diagnoses. The prevalence of Behçet's disease is higher in patients with Asian ethnicities; hence, extra caution is needed if a patient has Asian ancestry.[29,30] If a patient visits the hospital with miscellaneous symptoms, including nonspecific skin lesions, and comprehensive diagnostic laboratory tests fail to achieve a definite diagnosis, clinicians should always consider the possibility of Behçet's disease and should not hesitate to consult rheumatologists and dermatologists, if required.
The present study had some limitations. First, the number of patients in the mental illness group was relatively small. Further research is, therefore, needed to elucidate the clinical differences between psychogenic patients with and without mental illnesses. Moreover, this study was performed at a single university hospital and patients who come to university hospitals probably have different clinical backgrounds than those who visit clinics or community hospitals. However, this limitation may not be critical because the same conclusions were reached when we analyzed the data among 123 of the 277 enrolled patients who were not referred from other hospitals but visited our hospital directly without medical referral letters. Lastly, psychological tests and personality assessments were not performed in the present study. Such additional assessments may offer further insights into the relationship between somatic complaints and psychogenic backgrounds in these patients.
Conclusions
Patients with psychogenic or psychiatric somatic symptoms are likely to present with a greater number of complaints at the first hospital visit than those with non-psychogenic diseases. Dizziness and chronic fatigue increase the likelihood of psychogenic conditions. However, some of the non-psychogenic diseases, especially some autoimmune-related disorders, can also present multisystemic symptoms (≥ 4 complaints), as seen in the current study. Hence, comprehensive laboratory testing with careful physical inspection and history taking is essential before affirming the diagnosis of psychogenic or psychiatric somatic conditions.
Declarations
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of Tohoku University School of Medicine (IRB approval number: THK-2019-1-977). Written informed consent was waived by the IRB, and the process of informed consent was secured in an opt-out manner.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49–54. [PubMed] [Google Scholar]
- 2.Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20:23–31. doi: 10.31887/DCNS.2018.20.1/phenningsen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 4.Toussaint A, Hüsing P, Kohlmann S, Löwe B. Detecting DSM-5 somatic symptom disorder: Criterion validity of the Patient Health Questionnaire-15 (PHQ-15) and the Somatic Symptom Scale-8 (SSS-8) in combination with the Somatic Symptom Disorder-B Criteria Scale (SSD-12) Psychol Med. 2020;50:324–33. doi: 10.1017/S003329171900014X. [DOI] [PubMed] [Google Scholar]
- 5.Dunphy L, Penna M, El-Kafsi J. Somatic symptom disorder: A diagnostic dilemma. BMJ Case Rep. 2019;12:e231550. doi: 10.1136/bcr-2019-231550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okur Güney ZE, Sattel H, Witthöft M, Henningsen P. Emotion regulation in patients with somatic symptom and related disorders: A systematic review. PLoS One. 2019;14:e0217277. doi: 10.1371/journal.pone.0217277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishii T, Akaishi T, Fujimori K, Abe M, Ohara M, Shoji M, et al. Application of large electronic medical database for detecting undiagnosed patients in the general population. Tohoku J Exp Med. 2019;249:113–9. doi: 10.1620/tjem.249.113. [DOI] [PubMed] [Google Scholar]
- 8.Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6:94–8. doi: 10.7861/futurehosp.6-2-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amisha, Malik P, Pathania M, Rathaur VK. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328–31. doi: 10.4103/jfmpc.jfmpc_440_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes Rivera M, Mastronardi C, Silva-Aldana CT, Arcos-Burgos M, Lidbury BA. Myalgic encephalomyelitis/chronic fatigue syndrome: A comprehensive review. Diagnostics (Basel) 2019;9:91. doi: 10.3390/diagnostics9030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bested AC, Marshall LM. Review of myalgic encephalomyelitis/chronic fatigue syndrome: An evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30:223–49. doi: 10.1515/reveh-2015-0026. [DOI] [PubMed] [Google Scholar]
- 12.Eich W, Bär KJ, Bernateck M, Burgmer M, Dexl C, Petzke F, et al. [Definition, classification, clinical diagnosis and prognosis of fibromyalgia syndrome: Updated guidelines 2017 and overview of systematic review articles] Schmerz. 2017;31:231–8. doi: 10.1007/s00482-017-0200-7. [DOI] [PubMed] [Google Scholar]
- 13.Häuser W, Fitzcharles MA. Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci. 2018;20:53–62. doi: 10.31887/DCNS.2018.20.1/whauser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meeta, Digumarti L, Agarwal N, Vaze N, Shah R, Malik S. Clinical practice guidelines on menopause: An executive summary and recommendations. J Midlife Health. 2013;4:77–106. doi: 10.4103/0976-7800.115290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016:S0016–5085. doi: 10.1053/j.gastro.2016.02.032. (16) 00223-7. doi: 10.1053/j.gastro. 2016.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–6. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akaishi T, Nakashima I, Takahashi T, Abe M, Ishii T, Aoki M. Neuromyelitis optica spectrum disorders with unevenly clustered attack occurrence. Neurol Neuroimmunol Neuroinflamm. 2020;7:e640. doi: 10.1212/NXI.0000000000000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology. 2005;65:1479–82. doi: 10.1212/01.wnl.0000183151.19351.82. [DOI] [PubMed] [Google Scholar]
- 20.Akaishi T, Takahashi T, Fujihara K, Misu T, Abe M, Ishii T, et al. Risk factors of attacks in neuromyelitis optica spectrum disorders? J Neuroimmunol. 2020;343:577236. doi: 10.1016/j.jneuroim.2020.577236. doi: 10.1016/j.jneuroim. 2020.577236. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt CW. Questions persist: Environmental factors in autoimmune disease. Environ Health Perspect. 2011;119:A249–53. doi: 10.1289/ehp.119-a248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollard KM, Hultman P, Kono DH. Toxicology of autoimmune diseases. Chem Res Toxicol. 2010;23:455–66. doi: 10.1021/tx9003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamboj A, Lause M, Kumar P. Ophthalmic manifestations of endocrine disorders-endocrinology and the eye. Transl Pediatr. 2017;6:286–99. doi: 10.21037/tp.2017.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spuler S, Stroux A, Kuschel F, Kuhlmey A, Kendel F. Delay in diagnosis of muscle disorders depends on the subspecialty of the initially consulted physician. BMC Health Serv Res. 2011;11:91. doi: 10.1186/1472-6963-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjögren's syndrome in patients with dry eye. Clin Ophthalmol. 2016;10:43–53. doi: 10.2147/OPTH.S80043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitali C, Palombi G, Cataleta P. Treating Sjögren's syndrome: Insights for the clinician. Ther Adv Musculoskelet Dis. 2010;2:155–66. doi: 10.1177/1759720X10363246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeidan MJ, Saadoun D, Garrido M, Klatzmann D, Six A, Cacoub P. Behçet's disease physiopathology: A contemporary review. Auto Immun Highlights. 2016;7:4. doi: 10.1007/s13317-016-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hisamatsu T, Naganuma M, Matsuoka K, Kanai T. Diagnosis and management of intestinal Behçet's disease. Clin J Gastroenterol. 2014;7:205–12. doi: 10.1007/s12328-014-0488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilian NC, Sawalha AH. Behçet's disease in the United States: A single center descriptive and comparative study. Eur J Rheumatol. 2017;4:239–44. doi: 10.5152/eurjrheum.2017.17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennikov A, Alekberova Z, Goloeva R, Kitaichi N, Denisov L, Namba K, et al. Single center study on ethnic and clinical features of Behcet's disease in Moscow, Russia. Clin Rheumatol. 2015;34:321–7. doi: 10.1007/s10067-013-2442-9. [DOI] [PubMed] [Google Scholar]