Abstract
Background:
Antimicrobial resistance (AMR) is a global concern requiring immediate attention. Among many proven measures of decreasing AMR, practice of antimicrobial stewardship is the lowest hanging which can be adapted with negligible financial implications.
Methods:
This is a case record based extended cross-sectional type of observational operation research study conducted at an institute of national importance established by Government of India. Point prevalence of antibiotic usage among the patients admitted in the hospital, on four different days in four different quarters of a year was done to study the impact of antimicrobial stewardship program (AMSP).
Results:
A cumulative 711 patients were exposed on antibiotics among 1396 study participants. There was a significant decrease in antibiotic consumption across the 1st and 4th quarter. The average antibiotic usage was 50.9% (61.75, 60%, 48.4%, and 39% respectively in the 1st to 4th quarter). Among the total number of patients, intravenous antibiotic usage was 47.9% (60.71%, 58.4%, 44.9%, and 34.2% respectively in 1st to 4th quarter). Among the newly admitted patients, the consumption of antibiotic usage decreased from 45.9% to 25.7%. Among the intravenous antibiotics, the top 10 consumed antibiotics were 3rd generation cephalosporin (39.8%), aminoglycoside (14.8%), amoxicillin/amoxy-clav (12.5%), piperacillin-tazobactum (8.5%), carbapenams (6.6%), cefuroxime (6.4%), quinolones (4.3%), vancomycin/linezolid (4.1%), colistin (0.8%), and others (0.8%).
Conclusion:
Government run hospitals can run low budget antimicrobial stewardship program with sustainable impact on antibiotic consumption. For a successful AMSP, it requires change in attitude, commitment, and administrative support rather than a huge financial support.
Keywords: Antibiotic consumption, antibiotic usage, antimicrobial resistance, antimicrobial stewardship, point prevalence of antibiotic
Introduction
Antimicrobial resistance (AMR) is a major public health challenge. In the first decade of this century (2000-2010), there has been an increase in antibiotic consumption by 35%. Most of this increment has occurred for high-end antibiotics like carbepenems (45%) and polymixins (13%) as described by Van Boeckel et al.[1] India happens to be the largest consumer of antibiotics in 2010 and contributing to 23% of the increment of retail antibiotic sales among the BRICS countries as reported by Laxminarayan and Chaudhury (2016).[2] The increasing percentage of resistance to last resort antibiotics like carbapenems and colistins in India is alarming (Gandra et al. 2016).[3] Hence, there is an urgent need of adopting measures to decrease drug resistance and ensuring that the available antibiotics remain useful for patient care saving millions of lives globally. In 2015, World Health Organization (WHO) developed a global action plan on antimicrobial resistance (GAP AMR) wherein the member states are to produce national strategic plans for AMR.[4] India as a responsible signatory to GAP-AMR rolled on the National Action Plan on AMR (NAP AMR) on 19th April 2017.[5]
Antimicrobial stewardship program (AMSP) is one among the globally accepted strategies for decreasing AMR without hampering the patient safety. Practiced properly, AMSP have been found to reduce unnecessary usage of antibiotics and hence reduce AMR. AMSP have resulted in a reduction of 22%–36% antibiotic usage with a significant cost savings in many countries of Europe and America.[6,7,8,9] India have not fared well in AMSP. As per a survey conducted by Indian Council of Medical Research (ICMR) among 20 tertiary health care institutes (HCI) about implementation and practice of AMSP components, it was found that AMSP written documents were available only in 40% of HCI and only 30% HCIs had AMSP implementation strategies in place. Better performance was observed by private HCI as compared to Government run HCI.[10] Identifying the growing AMR and gross deficiency in practice of AMSP, ICMR stepped up to establish country wide antimicrobial resistance surveillance and research network (AMRSN). All India Institute of Medical Sciences, Bhopal (AIIMS Bhopal) is one of the regional centers among the ICMR established AMRSN network of hospitals.[11]
The objectives of the AMRSN network are to conduct sensitization workshops for the residents and faculties of the hospital, formation of hospital infection control committee (HICC), formation of AMSP committee (AMSPC), and development of hospital antibiogram, prescription audit, and formulary restriction. The outcome variables are point prevalence study (PPS) of high priority intravenous (IV) drugs and decrease in multi-drug resistant organisms (MDRO). The present study was undertaken to identify the immediate impact of practice of AMSP at a HCI of national importance run by Government of India.
The present study reemphasizes the importance of AMSP among primary care physicians to combat AMR as a mandate of NAP AMR.
Methods
Design
This is a case record based extended cross-sectional type of observational operation research study.
Setting
The study was carried out at AIIMS Bhopal, an institute of national importance established by Government of India after due approval by Institute Human Ethics Committee (IHEC). AIIMS Bhopal is a regional center for ICMR initiated antimicrobial resistance surveillance and research network (AMRSN) of India. The Institute adopted an antimicrobial stewardship program (AMSP) with support of ICMR as a project mode. The primary objective of the project was to decrease the usage of empirical antibiotic usage and promote culture sensitivity assisted usage of antibiotic.
Participants
As it was a case record based operation research, individual patient consent was not required. The data was collected as anonymous and unique patient identifies were not generated.
Inclusion criteria: all patients admitted in the hospital during the predefined period of 24 h.
Exclusion criteria: none.
Study procedures
The study was undertaken with approval of IHEC. A prefixed date was identified in each quarter of the year. Data was collected for 24 h from records of all patients present in the hospital from morning 8 AM to next day morning 8 AM. All patients moved into operation theatres, investigation centers, or any other service centers were also taken into consideration. The denominator was calculated by all the patients who remained in the hospital even momentarily (within the time period of 24 h) were taken into consideration.
Data collection was entrusted upon the on-duty nursing officers (NO), supervised by the senior nursing officer (SNO) of each ward. The project research associate collated all the data next day and circulated to all the faculties for correction of any ambiguity of data. Data accuracy was checked at multiple levels by NO and SNO, research associate, the faculty in charge of the area, and medical records department (MRD). After thorough review of the data, it was finally presented to the institute administrators and the funding agency.
The current survey focused on the usage of intravenous antibiotics used among patients admitted in the hospital. Point prevalence study (PPS) was conducted on 27th November 2018 in the 1st quarter of the study period. Subsequent PPS was conducted on 16th March 2019, 27th July 2019 and on 30th December 2019.
Sample size: A formal sample size was not considered as the study was for a definite period and all cases who were eligible for the study were taken into account.
Statistical analysis
Compiled and collated data was entered in spreadsheet. The data was summarized as frequencies and percentages for categorical variables. Percentage was calculated to one decimal value. A confidence interval 95% was taken into account and Chi-square P value of < 0.05 was taken as significant. Microsoft excel and Open epi software was utilized for statistical analysis.
Results
A cumulative number of 1396 patients were surveyed at 4 different days of conduction of the study. A total number of 711 patients (711/1396 = 50.93%) were on some form of antibiotic oral or intravenous (IV). Though the average antibiotic usage was 50.9%, quarter wise proportion of antibiotic usage was 61.75%, 60%, 48.4%, and 39%, respectively, in 1st to 4th quarter [Table 1]. This decrease in total antibiotic usage across 1st and 4th quarter was significant as evident by Chi-square P < 0.0000001. A total of 975 units of antibiotics were used in 711 different patients amounting to average consumption of 0.69 (975/1396) units among the total 1396 number of patients.
Table 1.
Summary statement of total antibiotic usage
| Point prevalence study quarter | Total number of Patients surveyed | Number of patients on (oral + IV) antibiotics | Number of patients on IV antibiotics | Number of patients on oral antibiotics | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 1 | 280 | 173 | (173/280) 61.79% | 170 | (170/280) 60.71% | 3 | (3/280) 1.76% |
| 2 | 308 | 185 | (185/308) 60.06% | 180 | (180/308) 58.44% | 5 | (5/308) 2.78% |
| 3 | 396 | 192 | (192/396) 48.48% | 178 | (178/396) 44.95% | 14 | (14/396) 7.87% |
| 4 | 412 | 161 | (161/412) 39.08% | 141 | (141/412) 34.22% | 20 | (20/412) 14.18% |
| Total | 1396 | 711 | (711/1396) 50.93% | 669 | (669/1396) 47.92% | 42 | (42/1396) 6.28% |
Among the total number of 1396 patients, intravenous antibiotic usage was 47.9% (60.71%, 58.4%, 44.9%, and 34.2%, respectively, in 1st to 4th quarter as in Figure 1). This decrease in total IV antibiotic usage across 1st and 4th quarter was significant as evident by Chi-square P < 0.0000001. Among the total antibiotic consumption, intravenous (IV) antibiotic consumption proportion was 95.7% (933/975). The proportion wise usage of IV antibiotic usage is provided in Table 2.
Figure 1.
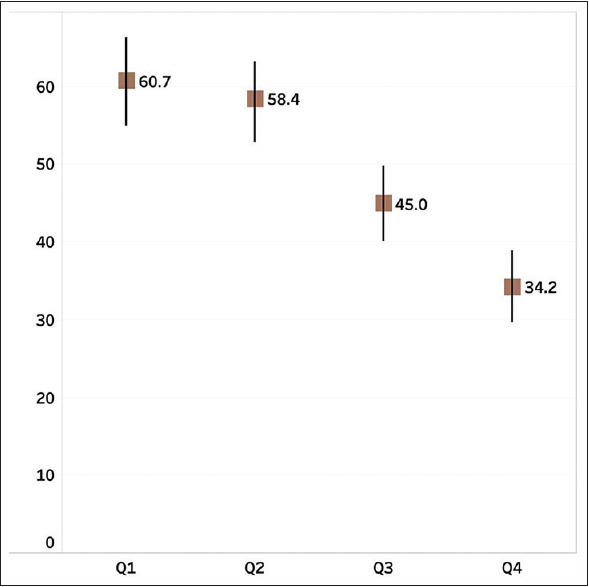
Comparative usage of IV antibiotics in different quarters. X axis: Q1 (quarter 1), Q2 (quarter 2), Q3 (quarter 3), Q4 (quarter 4) Y axis: proportion in percentage with 95% confidence interval
Table 2.
Proportion of different intravenous (IV) antibiotics
| Antibiotic | Frequency | Proportion (among IV antibiotic) | Proportion (among total patients) |
|---|---|---|---|
| 3rd gen Cephalosporin (Ceftriaxone, cefotaxime, cefoperazone-sulbactam) | 372 | 372/933=39.8% | 372/1396=26.6% |
| Aminoglycosides | 139 | 133/933=14.8% | 139/1396=9.9% |
| Amoxy/amoxy-clav | 117 | 117/933=12.5% | 117/1396=8.3% |
| Pipercillin-tazobactum | 80 | 80/933=8.5% | 80/1396=5.7% |
| Penams (meropenam, imipenam) | 62 | 62/933=6.6% | 62/1396=4.4% |
| Cefuroxime | 60 | 60/933=6.4% | 60/1396=4.2% |
| Quinolones (levofloxacin, ofloxacin, ciprofloxacin) | 41 | 41/933=4.3% | 41/1396=2.9% |
| Vancomycin/linezolid | 39 | 39/933=4.1% | 39/1396=2.7% |
| Colistin | 8 | 8/933=0.8% | 8/1396=0.5% |
Out of the total number of 1396 patients surveyed, 195 (14%) patients were new cases admitted during the cumulative study period and 1201 (86%) were prior hospitalized patients. Among these 669 IV antibiotic exposed patients during the study period, 77% (515/669) were already continuing antibiotic (prior hospitalized cases), 16% (107/669) of patients were started on antibiotic on the study period (prior hospitalized and new cases), and 7% (47/669) were switched over from oral to IV antibiotic during the study period (prior hospitalized cases). Among the 195 patients who were admitted during the study period, 37.4% (73/195) were initiated on IV antibiotics. The proportion of IV antibiotic usage among the newly admitted patients across the 4 quarters was 45.9%, 43.7%, 40.9%, and 25.7% as in Table 3.
Table 3.
Spectrum of usage of Intravenous (IV) antibiotics
| Character | 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Total number of patients surveyed | 280 | 280/280=100% | 308 | 308/308=100% | 396 | 396/396=100% | 412 | 412/312=100% | 1396 | 1396/1396=100% |
| New admission | 37 | 37/280=13.2% | 48 | 48/308=15.6% | 44 | 44/396=11.1% | 66 | 66/412=16% | 195 | 195/1396=14% |
| Prior admitted | 243 | 243/280=86.8% | 260 | 260/308=84.4% | 352 | 352/396=88.9% | 346 | 346/412=84% | 1201 | 1201/1396=86% |
| Total number of patients on IV antibiotic | 170 | 170/280=60.7% | 180 | 180/308=58.4% | 178 | 178/396=44.9% | 141 | 141/412=34.2% | 669 | 669/1396=47.9% |
| New admission | 17 | 17/170=10% | 21 | 21/180=11.7% | 18 | 18/178=10.1% | 17 | 17/141=12.1% | 73 | 73/669=10.9% |
| Prior admitted | 153 | 153/170=90% | 159 | 159/180=88.3% | 160 | 160/178=89.9% | 124 | 124/141=87.9% | 596 | 596/669=89.1% |
| Number of patients already on antibiotic treatment | 138 | 138/170=81.1% | 140 | 140/180=77.8% | 130 | 130/178=73% | 107 | 107/141=75.9% | 515 | 515/669=77% |
| Number of patients started on IV antibiotic | 22 | 22/170=12.9% | 30 | 30/180=16.7% | 28 | 28/178=15.7% | 27 | 27/141=19.1% | 107 | 107/669=16% |
| New admission | 17 | 17/22=77.2% | 21 | 21/30=70% | 18 | 18/28=64.3% | 17 | 17/27=63% | 73 | 73/107=68.2% |
| Prior admitted | 5 | 5/22=22.8% | 9 | 9/30=30% | 10 | 10/28=35.7% | 10 | 10/27=37% | 34 | 34/107=31.8% |
| Number of patients with change of antibiotic | 10 | 10/170=5.8% | 10 | 10/180=5.6% | 20 | 20/178=11.2% | 7 | 7/141=5% | 47 | 47/669=7% |
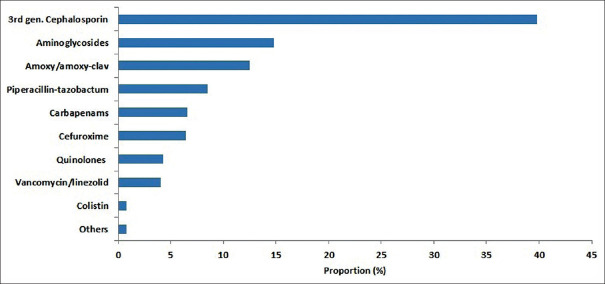
Among the total consumption of IV antibiotics, the top 10 highest consumed IV antibiotics in our study were 3rd generation cephalosporin (372/933 = 39.8%), aminoglycosides (14.8%), amoxicillin/amoxicillin-clav (12.5%), piperacillin-tazobactum (8.5%), carbapenams (6.6%), cefuroxime (6.4%), quinolones (4.3%), vancomycin/linezolid (4.1%), colistin (0.8%), and others (0.8%) as shown in Table 2 and Figure 2.
Figure 2.
Proportion of usage of intravenous (IV) antibiotics. 3rd gen cephalosporin (ceftriaxone, cefotaxime, cefoperazone-sulbactam); carbapenams (meropenam, imipenam); quinolones (levofloxacin, ofloxacin, ciprofloxacin)
The details of quarter wise data of antibiotic consumption are given as follows:
1st quarter
A total number of 280 cases were included in the study duration. Out of the total 280 study participants, 173 cases were on some antibiotic (oral and IV). Among these 173 cases, 170 cases (170/280 = 60.7%, 95% CI of proportion was 54.9% to 66.3%) were on IV antibiotic during the study. Among the total 170 cases of patients on IV antibiotic during the study period, 81.1% of cases (138/170) were already on IV antibiotic, 12.9% cases (22/170) were initiated IV antibiotic, and 5.8% cases (10/170) were switched from oral to IV antibiotic. Out of the total 280 study participants, 13.2% (37/280) were admitted during the study period. Among these 37 newly admitted patients during the study period, 45.9% cases (17/37) were put on IV antibiotics.
2nd quarter
A total number of 308 cases were included in the study duration. Out of the total 308 study participants, 185 cases were on some antibiotic (oral and IV). Among these 185 cases, 180 cases (180/308 = 58.4%, 95% CI of proportion was 52.8% to 63.8%) were on IV antibiotic during the study. Among the total 180 cases of patients on IV antibiotic during the study period, 77.8% of cases (140/180) were already on IV antibiotic, 16.7% cases (30/180) were initiated IV antibiotic, and 5.6% cases (10/180) were switched from oral to IV antibiotic. Out of the total 308 study participants, 15.6% (48/308) were admitted during the study period. Among the 48 newly admitted patients during the study period, 43.7% cases (21/48) were put on IV antibiotics.
3rd quarter
A total number of 396 cases were included in the study duration. Out of the total 396 study participants, 192 patients were on some antibiotic (oral and IV). Among these 192 cases, 178 cases (178/396 = 44.9%, 95% CI of proportion was 40.1% to 48.8%) were on IV antibiotic during the study. Among the total 178 cases of patients on IV antibiotic during the study period, 89.9% of cases (160/178) were already on IV antibiotic, 15.7% cases (28/178) were initiated IV antibiotic, and 11.2% cases (20/178) were switched from oral to IV antibiotic. Out of the total 396 study participants, 11.1% (44/396) were admitted during the study period. Among the 44 newly admitted patients during the study period, 40.9% cases (18/44) were put on IV antibiotics.
4th quarter
A total number of 412 cases were included in the study duration. Out of the total 412 study participants, 161 cases were put on some antibiotic (oral and IV). Among these 161 cases, 141 cases (141/412 = 34.2%, 95% CI of proportion was 29.7% to 38.9%) were on IV antibiotic during the study. Among the total 141 cases of patients on IV antibiotic during the study period, 75.9% of cases (107/141) were already on IV antibiotic, 19.1% cases (27/141) were initiated IV antibiotic, and 5% cases (7/141) were switched from oral to IV antibiotic. Out of the total 412 study participants, 16% (66/412) were admitted during the study period. Among the 66 newly admitted patients during the study period, 25.8% cases (17/66) were put on IV antibiotics.
Discussion
This is a unique study in view of extended point prevalence (PP) study of antibiotics spread over a year. There is very few extended point prevalence study in the global literature. The average parameters of the 4 quarters of our study are compared with other studies of relevance published within last 5 years.
Point prevalence (PP) of antibiotic usage has been reported from 28% in Scotland to highest of 80.1% in Nigeria [Table 4].[12,13,14,15,16,17,18,19] The average PP of antibiotic usage of our study is 50.9% (711/1396) which is almost of similar to Saudi Arabia (49.1%).[19] Recent studies from China, Egypt, and Pakistan (56%, 59%, and 77.6%, respectively) have shown higher PP of antibiotic usage than that of our study.[14,15,16] However, studies from Scotland, Switzerland, and Ireland document PP of antibiotic usage much less than us (28%, 33%, and 34.4%, respectively).[12,13,18] Though the overall PP of antibiotic usage of our study is 50.9%, the actual quarter wise values are 61.7%, 60%, 48.4%, and 39%, respectively, in 1st to 4th quarter of the study period [Table 1]. With time the proportions are approaching the levels similar to the best in the world in overall consumption of antibiotics.
Table 4.
Point prevalence of antibiotic usage in different countries
| Country | Point Prevalence of antibiotics among hospitalized patient (%) | Point Prevalence of IV antibiotic among hospitalized patient (%) |
|---|---|---|
| Pakistan[16] | 77.6 | 70.6 |
| China[15] | 56 | 54.5 |
| Saudi Arabia[19] | 49.1 | 40.6 |
| Nigeria[17] | 80.1 | 44.8 |
| Switzerland[12] | 33 | 23.6 |
| Ireland[18] | 34.4 | 20.6 |
| Scotland[13] | 28 | 9.9 |
| Egypt[14] | 59 | Not available |
| India (present study) | 50.9 | 47.9 |
Antimicrobial resistance (AMR) is worst affected by unrestricted usage of intravenous (IV) antibiotics. Thus, we looked in details about the PP of IV antibiotic usage like no other studies reported before. The overall proportion of IV antibiotic usage in our study was 47.9% (669/1396). Our study finding was similar to that of study from Saudi Arabia (40.6%). (19) Our neighboring countries, China and Pakistan reported PP of IV antibiotic usage as 54.5% and 70.6%.[15,16] However, there is much scope of improvement in our institute as Switzerland, Northern Ireland, and Scotland have reported PP of IV antibiotic usage as 23.6%, 20.6%, and 9.9%, respectively, as mentioned in Table 4.[12,13,18] Though the overall PP of IV antibiotic usage of our study is 47.92%, the actual quarter wise values are 60.7%, 58.4%, 44.9%, and 34.2%, respectively, in 1st to 4th quarter of the study period [Table 1]. Though we are approaching to the levels similar to the best in the world in overall consumption of antibiotics, we are far from them in consumption of IV antibiotics which is a matter of concern.
Third generation cephalosporins are the most frequently IV antibiotic used in most studies around the globe. Their proportion varies from 40% in China to 28% in Scotland.[13,15] The proportion of usage of amoxicillin and amoxicillin-clav is higher in European countries (20.3% in Scotland) than that of our study.[13] The usage of aminoglycosides (14.8%) in our institute is similar to that of Scotland (11.5% of gentamicin) but higher than that Egypt (6%).[13,14] The usage of piperacillin-tazobactum is similar to Scotland (8.1%) but higher than that of other Asian countries (China-5%, Pakistan-3.3%).[15,16] Our use of carbapenam (6.6%) was higher than that of Egypt (2%) and Switzerland (3%).[12,14] The usage of IV antibiotics (vancomycin and linezolid) for severe gram positive infections was 4.1% which was of similar proportion to Switzerland and Egypt (<5%) and much less than Scotland (18.6%).[12,13,14] Our proportion of quinolone (4.3%) was similar to Switzerland and Egypt (about 5%) but much less than that of other Asian countries like China and Pakistan.[12,14,15,16]
There is scanty published literature of extended PP usage of antibiotic except from Italy and Northern Ireland.[18,20] The proportion of usage of antibiotic (both oral and IV) in 1st quarter was 61.7% (95% CI = 55.9–67.3). Our study demonstrates a significant shift of antibiotic usage of 61.79% (oral 1.7% and IV 98.2%) initially to 39.08% (oral 12.4% and IV 87.5%) at completion of the study over one year as in Table 1. The study from Northern Ireland by Al-Taani et al. (2018) reported 3 PP antibiotic usages spanning over 5 years.[18] The initial PP of antibiotic usage was 44.1% (oral 37.7% and IV 62.3%) as compared to the 3rd PP of antibiotic usage was 53.6% (oral 31.3% and IV 68.6%). The study could not establish decrease in antibiotic usage over a period of 5 years, but the study documented decrease consumption of oral antibiotics from 37.7% to 31.4%. The study also demonstrates a rise in usage of IV antibiotics from 62.3% to 68.6%. Another study conducted over a decade in Italy by Tersigni et al. (2019) did not reveal any significant change in antibiotic usage (43% to 40%).[20] This is contrary to the expectation that AMSP causes a significant decrease in antibiotic usage.
It is imperative to believe that AMSP is a powerful instrument to decrease the AMR at country level. However, as evidenced by the studies from Italy and Northern Ireland it seems that it is difficult to achieve a sustained decrease in antibiotic usage over a prolonged period after a baseline decline of unnecessary and nonjudicious antibiotic prescription. The possible causes of this discrepancy might be because of floating population of health care worker which requires continuous teaching and training to adhere to AMSP, hospitalization of more and more sick patients over a period of time, pressure for prescribing antibiotics by internet savvy patients, and the fear of suing the health care system in case of poor outcome. However, in our study which spanned over a year, we observed a significant reduction in antibiotic usage although over a shorter time frame of one year than both the earlier studies. The sustainability of this initial positive on consumption of antibiotic would be an exciting challenge to be relished over a decade long time.
Conclusion
Antimicrobial resistance (AMR) is a threat to mankind and needs immediate attention. Antimicrobial stewardship practices are one of the lowest hanging targets in the perseverance of decreasing the AMR. As evidenced in this study, with practice of AMSP, the total consumption of oral and intravenous antibiotic consumption decreased from 61% to 39% over one year. The total number of patients on IV antibiotics decreased from 60% to 47% and use of down the ladder oral antibiotics increased from 1% to 14% at the end of the study.
India as a nation is lagging far behind the American and European countries in AMSP. Government hospitals of India are in real bad shape than the private sector hospitals. However, the time is apt for the government run hospitals to take a lead. This study proves that government run hospitals can run low budget AMSP with sustainable impact on antibiotic consumption. For a successful AMSP, it requires change in attitude, commitment, and administrative support rather than a huge financial support. Needless to say that with AMSP there will be improvement in AMR, reduction in cost of therapy, and positive impact on the environment.
Financial support and sponsorship
Indian Council of Medical Research (ICMR) funded the project and provided the technical assistance.
Conflicts of interest
Dr. Sagar Khadanga received funds for the project from Indian Council of Medical Research.
Acknowledgements
We acknowledge Dr. Abhijit Pakhare, Associate Professor, Dept. of CFM, AIIMS Bhopal for statistical analysis
References
- 1.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14:742–50. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 2.Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: Drivers and opportunities for action? PLoS Med [Internet] 2016;13:e1001974. doi: 10.1371/journal.pmed.1001974. doi: 10.1371/journal.pmed. 1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandra S, Mojica N, Klein EY, Ashok A, Nerurkar V, Kumari M, et al. Trends in antibiotic resistance among major bacterial pathogens isolated from blood cultures tested at a large private laboratory network in India, 2008–2014. Int J Infect Dis. 2016;50:75–82. doi: 10.1016/j.ijid.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global action plan on antimicrobial resistance. 2015 doi: 10.7196/samj.9644. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A. National Action Plan on Antimicrobial Resistance. 2017. pp. 1–57. https://ncdc.gov.in/WriteReadData/l892s/File645.pdf .
- 6.Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis Off Publ Infect Dis Soc Am. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 7.Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, Ramsay CR, Wiffen PJ, Wilcox M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database of Systematic Reviews. 2013;(4) doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Huttner B, Harbarth S, Nathwani D ESCMID Study Group for Antibiotic Policies (ESGAP) Success stories of implementation of antimicrobial stewardship: A narrative review. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2014;20:954–62. doi: 10.1111/1469-0691.12803. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DL. The role of antimicrobial management programs in optimizing antibiotic prescribing within hospitals. Clin Infect Dis. 2006;42(Suppl 2):S90–5. doi: 10.1086/499407. [DOI] [PubMed] [Google Scholar]
- 10.Walia K, Ohri VC, Mathai D Antimicrobial Stewardship Programme of ICMR. Antimicrobial stewardship programme (AMSP) practices in India. Indian J Med Res. 2015;142:130–8. doi: 10.4103/0971-5916.164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AMRSN Network [Internet] cited 2020 Jul 13. Available from: http://iamrsn.icmr.org.in/index.php/amrsn/amrsn-network .
- 12.Zingg W, Metsini A, Gardiol C, Balmelli C, Behnke M, Troillet N, et al. Antimicrobial use in acute care hospitals: National point prevalence survey on healthcare-associated infections and antimicrobial use, Switzerland, 2017? Euro Surveill. 2019;24:1900015. doi: 10.2807/1560-7917.ES.2019.24.33.1900015. doi: 10.2807/1560-7917.ES.2019.24.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seaton RA, Nathwani D, Burton P, McLaughlin C, MacKenzie AR, Dundas S, et al. Point prevalence survey of antibiotic use in Scottish hospitals utilising the Glasgow Antimicrobial Audit Tool (GAAT) Int J Antimicrob Agents. 2007;29:693–9. doi: 10.1016/j.ijantimicag.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Talaat M, Saied T, Kandeel A, El-Ata GAA, El-Kholy A, Hafez S, et al. A point prevalence survey of antibiotic use in 18 hospitals in Egypt. Antibiotics. 2014;3:450–60. doi: 10.3390/antibiotics3030450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie D, Xiang L, Li R, Hu Q, Luo Q, Xiong W. A multicenter point-prevalence survey of antibiotic use in 13 Chinese hospitals. J Infect Public Health. 2015;8:55–61. doi: 10.1016/j.jiph.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Saleem Z, Hassali MA, Versporten A, Godman B, Hashmi FK, Goossens H, et al. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: Findings and implications. Expert Rev Anti Infect Ther. 2019;17:285–93. doi: 10.1080/14787210.2019.1581063. [DOI] [PubMed] [Google Scholar]
- 17.Abubakar U. Antibiotic use among hospitalized patients in northern Nigeria: A multicenter point-prevalence survey. BMC Infect Dis. 2020;20:86. doi: 10.1186/s12879-020-4815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Taani GM, Scott M, Farren D, Gilmore F, Mccullagh B, Hibberd C, et al. Longitudinal point prevalence survey of antibacterial use in Northern Ireland using the European Surveillance of Antimicrobial Consumption (ESAC) PPS and Global-PPS tool. Epidemiol Infect. 2018;146:985–90. doi: 10.1017/S095026881800095X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasser M, Aljabri AK, Alsaadi FN, Rizk LM, Alahmadi RY, Aljuhani SR, et al. A prospective antibiotic point prevalence survey in two primary referral hospitals during and after pilgrims stay in Madinah, Saudi Arabia. Trop J Pharm Res. 2020;19:391–9. [Google Scholar]
- 20.Tersigni C, Montagnani C, D'Argenio P, Duse M, Esposito S, Hsia Y, et al. Antibiotic prescriptions in Italian hospitalised children after serial point prevalence surveys (or pointless prevalence surveys): Has anything actually changed over the years? Ital J Pediatr. 2019;45:127. doi: 10.1186/s13052-019-0722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]