Abstract
Context:
Postoperative nausea (PON) and postoperative vomiting (POV) are the most undesirable morbidity after anaesthesia. There is paucity of data on PONV from the Indian subcontinent.
Aims:
We aim to study the prevalence of PON and POV, associated risk factors and the effect of following standardized risk stratification and prophylaxis protocols in the day care patient population.
Settings and Design:
This was a prospective cohort study at a tertiary care teaching institute.
Methods and Material:
Data from 500 patients undergoing day care surgery over a period of 12 months were analysed. We used the Apfel scoring system for evaluation of risk of post-operative nausea and vomiting (PONV) for each participant. A standard PONV prophylaxis protocol was used intra-operatively.
Statistical analysis used:
Data analysis was done using the Mann-Whitney U test, the Chi-square and Fisher's exact test.
Results:
The period prevalence of post-operative nausea (PON) and post-operative vomiting (POV) was 2.04% and 2.45%, respectively, in this study. The prevalence of PONV in each risk category was lower than that predicted by the Apfel score due to utilization of a standard anti-emetic prophylactic protocol. We found younger age, previous history of nausea, previous history of vomiting, urological surgeries and alcohol consumption as significant risk factors for postoperative nausea. Longer duration of surgery, previous history of nausea, alcohol consumption and higher BMI were the significant risk factors for postoperative vomiting.
Conclusions:
Adherence to preoperative risk stratification and a standard anti-emetic prophylactic protocol can significantly reduce the prevalence of postoperative nausea and vomiting.
Keywords: Ambulatory surgery, ponv prevention
Introduction
Post-operative nausea and vomiting (PONV) is any nausea, retching, or vomiting which occurs during the first 24–48 h after surgery in patients.[1] Systematic reviews have shown that the incidences of post-operative nausea (PON) and post-operative vomiting (POV) after outpatient surgery are about 17% (range 0-55%) and 8% (range 0-16%) respectively.[2] Vomiting and nausea after surgery pose numerous problems to patients and hospital staff; including prolonged hospital stay, use of more resources, increased economic burden, decreased patient satisfaction, unplanned hospital admission etc., About 49% patients have found nausea and vomiting to be an undesirable complications after anaesthesia.[3] It has been estimated that about $253270- $519617 has been spent per year by US health care system because of PONV.[4]
As no studies have focused on the prevalence of PONV in the day care population in India, we conducted this prospective study to assess the period prevalence of Post-Operative Nausea and Post-Operative Vomiting among adults undergoing day care surgeries under general anaesthesia till the discharge time from the post-anaesthesia care unit. We also assessed the effect of risk stratification using the Apfel score and standard anti emetic prophylaxis for each participant on PONV. In addition, we studied the possible risk factors for Post-Operative Nausea (PON) and Post-Operative Vomiting (POV) separately in this patient population. With increasing day care surgeries, evidence-based knowledge of PONV will aid primary care physicians in the counselling and reassurance of the surgical patients regarding PONV in the preoperative and management of patients in postoperative periods.
Subjects and Methods
This prospective, cohort study was conducted over twelve months (1/1/16 to 31/12/16) to assess the period prevalence and risk factors of postoperative nausea (PON) and postoperative vomiting (POV) in adults undergoing day care surgery under general anaesthesia at a tertiary care hospital in South India. We also evaluated to what extent would the addition of a PONV risk scoring system and standardized anti emetic prophylaxis would reduce the occurrence of PON and POV. The study was approved by our Institutional Review Board and ethics committee and written informed consent was taken from all the participants (Ref No: Blue IRB Minute No: 9790 dated 3/12/2015). The exclusion criteria included: patients' refusal for participation, age <18 or >60 years, ASA physical status >=3, patients who were on chemotherapy, patients on palliative therapy with chronic opioid intake, pregnant and lactating women, patients who was on anti-emetic treatment within 24 hours prior to surgery, patients who had previous history of hepatic, renal or cardiopulmonary abnormality, and significant gastrointestinal disorders (for example peptic ulcer disease or gastro oesophageal reflux disease). A detailed history and physical examination were conducted for all the patients. The Apfel risk score [Table 1] was used for prediction of the risk of post-operative nausea and vomiting for each patient.
Table 1.
The risk factors for PONV according to the simplified Apfel score and the interpretation of the scores
| Characteristics | Apfel score |
|---|---|
| Female sex | 1 |
| Previous history of PONV | 1 |
| Non smoker | 1 |
| Post-operative opioid usage | 1 |
| Total | 4 |
*Apfel Simplified Score for Postoperative Nausea and Vomiting
A minimum of six hours of fasting for solids and two hours for clear fluids was ensured before surgery. On the day of surgery, the anaesthetist in day care operation room provided general anaesthesia to these selected patients using endotracheal tube or supraglottic airway device. The patient's heart rate, blood pressure, oxygen saturation, ECG, end tidal CO2 waveforms were recorded throughout the surgery. The anaesthetists followed the standard prophylaxis schedule for preventing PONV as described by Gan et al.[5] [Figure 1].
Figure 1.
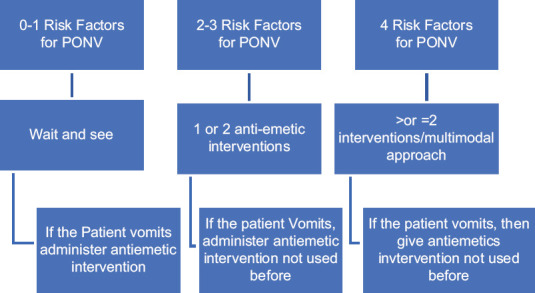
Standard prophylaxis schedule for PONV by Gan et al.
The use of anaesthetic agents and anti-emetic medications was recorded. The patients were transferred to the PACU after the surgery. The patients were monitored for any events of nausea and vomiting every 30 minutes in the PACU. In patients who had PON or POV, the number of episodes of PON and POV and the time to occurrence of the episodes was recorded. The severity of nausea was recorded using the validated Numerical Rating Scale (NRS). Severity of vomiting was measured by the number of vomiting events occurring at least one minute apart during a specific time interval.[1] Severity of retching was measured by the number of retching events occurring at least one minute apart during a specific time interval.[1] The need for single or multiple rescue anti emetics, number of doses required and the timing of administration of the rescue anti-emetic were also recorded. The duration of observation in the PACU was also noted. If the patient was shifted to the ward, the reason for the same was recorded. The data was collected by the primary investigator and co-investigators and were analysed using Epidata (Version 2.0.7.53) and Stata (Version 13.1).
The sample size was calculated using the variable 'gender' (which was the most significant risk factor) from a similar study done by Apfel et al., with an odds ratio (OR) of 2.19.[1] A total sample of 300 (150 males and females) was needed to test the association of gender on incidence of 30% assuming an OR of 2.19 with 80% power at 5% level of significance, adjusted for 20% of missing data. Estimated sample size, n = 118 ~ 120 (each group). Adjusted for 20% missing data, n = 150 (each group). The period prevalence of PON and POV was calculated using the following formula:
Period prevalence = (Persons having PON or POV during a given time period/Study population during the same time period) × 100.
The Mann-Whitney U test, the Chi-square and Fisher's exact test were used in the univariate analyses for comparison of continuous variables and proportions, respectively. Data from 500 patients (out of the 553 patients who were recruited) were analysed and 53 patients had to be excluded from the analysis due to protocol violation in the administration of the antiemetic prophylaxis.
Results
Tables 2 and 3 depicts the demographic characteristics of our patient population. Figure 2 depicts the distribution of patient population according to the Apfel scores. Majority of patients had two of the four risk factors from the Apfel risk score. Table 4 depicts the expected and actual numbers and percentage of patients who had post-operative nausea and post-operative vomiting in relation to their Apfel risk score. Forty-one patients who had an Apfel score of one did not receive anti emetic prophylaxis intra operatively and rescue anti emetic was needed only for two of these 41 patients in the PACU due to vomiting and nausea. Table 5 represents the risk factors for post-operative nausea and post-operative vomiting which were statistically significant in our patient population.
Table 2.
The demographic characteristics of our patient population
| Demography | Frequency | Percentage | |
|---|---|---|---|
| Gender | Male | 346 | 69.2 |
| Female | 154 | 30.8 | |
| Age | 18-28 | 96 | 19.1 |
| 29-38 | 152 | 30.4 | |
| 39-48 | 139 | 27.8 | |
| 49-60 | 113 | 22.6 | |
| Dept. of origin | Open General Surgery | 256 | 51 |
| Urology | 35 | 7 | |
| Orthopaedics | 120 | 24 | |
| Breast surgery | 25 | 5 | |
| ENT | 62 | 13 | |
| Thyroid surgery | 1 | <0.1 | |
| Others | 1 | <0.1 | |
| Comorbid Illness | No comorbid Illness | 419 | 81.2 |
| Diabetes | 47 | 4.7 | |
| Hypertensive | 29 | 4 | |
| Asthma/COPD | 8 | 0.01 | |
| Hypothyroid | 8 | 0.01 | |
| Hyperthyroid | 3 | <0.01 | |
| Seizure disorder | 2 | <0.01 |
Table 3.
Preoperative and intraoperative data of patients
| Demography | Frequency | Percentage |
|---|---|---|
| Previous history of nausea | 21 | 4.2 |
| Previous history of vomiting | 15 | 3 |
| History of smoking | 47 | 9.4 |
| History of alcohol consumption | 48 | 9.6 |
| History of vertigo/tinnitus | 10 | 2 |
| History of motion sickness | 27 | 5.4 |
| History of tobacco consumption | 52 | 10.4 |
| ASA grading | ||
| Grade 1 | 419 | 83.8 |
| Grade 2 | 81 | 16.2 |
| Intraoperative Inhalation agent used | ||
| Isoflurane | 484 | 96.8 |
| Sevoflurane | 14 | 2.8 |
| Nitrous oxide | 17 | 3.4 |
| Mean duration of stay in PACU | 6.99±2.62 h | |
| No of patients who received prophylactic antiemetic | ||
| Ondansetron 4 mg | 447 | 89.4 |
| Dexamethasone 4 mg | 227 | 45.4 |
| Metoclopramide 10 mg | 19 | 3.8 |
Figure 2.
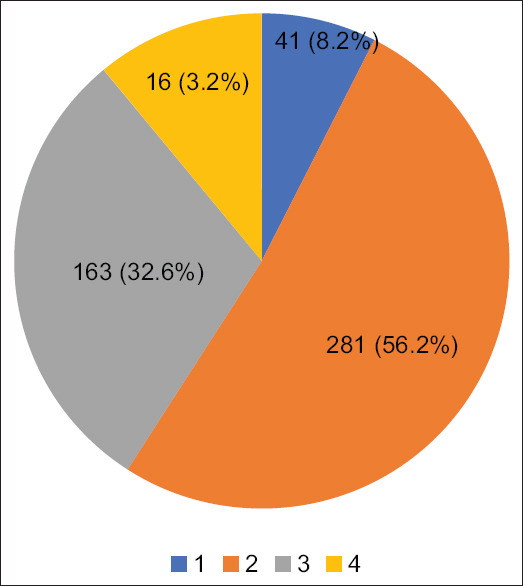
Distribution of patients according to the simplified Apfel risk score for PONV
Table 4.
Expected and actual number and percentage of patients with PON and POV
| Apfel score | Number of Patients | Number predicted to have PONV (%) | Actual number of patients with POn (%) | Actual number of patients with POV (%) |
|---|---|---|---|---|
| 1 | 41 | 9 (21%) | 2 (4.8%) | 2 (4.86%) |
| 2 | 281 | 110 (39%) | 3 (1.07%) | 5 (1.78%) |
| 3 | 163 | 99 (61%) | 4 (2.45%) | 3 (1.84%) |
| 4 | 16 | 12 (78%) | 1 (6.25%) | 2 (12.5%) |
Table 5.
Statistically significant risk factors in our study
| Risk factors for Postoperative Nausea (PON) which were statistically significant in our study | Risk factors for postoperative Vomiting (POV) which were statistically significant in our study |
|---|---|
| Younger age (P=0.0225) | Prolonged duration of surgery (P=0.0522) |
| Previous history of nausea (P=0.019) | Higher BMI (P=0.05) |
| Previous history of vomiting (P=0.003) | Previous history of postoperative vomiting (P=0.001) |
| History of alcohol consumption (P=0.037) | History of alcohol consumption (P=0.028) |
| Urological surgeries (P=0.008) |
We found that the period prevalence of postoperative nausea (PON) was 2.04% (CI 1.1-3.6) and the period prevalence of postoperative vomiting (POV) was 2.45% (CI 1.4-4.1). Our data suggested that young age, previous history of nausea, previous history of vomiting, urological surgeries and alcohol consumption were the significant risk factors for postoperative nausea (PON). Long duration of surgery, previous history of nausea, alcohol consumption and higher BMI were the significant risk factors for post-operative vomiting (POV) on univariate analysis.
Discussion
Postoperative nausea and vomiting have an overall incidence of about 30% and upto 80% in the high risk patients.[1] The prevalence of nausea is about 20% in the post-anaesthesia care unit (PACU), and over 50% after 24 hours; the respective numbers for vomiting are 5% and 25%.[4] Parra-Sanches et al. have found that the incidence of PONV in ambulatory surgery as 37% during hospitalization, 42% on the first postoperative morning and 49% by the third postoperative morning.[5] Another multicentre prospective cohort study reported the incidences of nausea and vomiting in the PACU as 19.9% and 3.9%, respectively.[1]
In our study population the period prevalence of postoperative nausea was 2.05% and the period prevalence of postoperative vomiting was 2.45%. The lower prevalence of nausea and vomiting after surgery in our study population can be attributed to adherence to the pre-operative risk stratification and use of targeted prophylactic anti emetic prophylaxis. Previous studies have also used the algorithm by Gan et al.[5] for preventing postoperative nausea and vomiting with good results.[2]
Reviewers have validated the value of using a risk assessment score rather than using single risk factors to aid decision regarding prophylactic anti emetics.[5] Preoperative risk stratification; tailored, standard anti emetic prophylaxis protocol and avoidance of nitrous oxide resulted in extremely low prevalence of PONV as compared to the predicted prevalence in each risk category of the Apfel score in our study population. Adherence to a standard PONV prophylaxis protocol can limit the undue use of anti-emetic prophylaxis as 41 of our patients with a single risk factor did not receive any prophylactic medication intra operatively. This strategy may therefore have economic implications for the patients and the care providers. Previous studies have shown that strict compliance to risk stratified, multimodal prophylactic therapy resulted in reduced incidence of postoperative nausea and vomiting in the western population.[6,7,8] A 2020 literature review by Peter Kranke et al.[9] has shown that risk stratified approach of antiemetic prophylaxis is the ideal way of preventing PONV. The fourth consensus guidelines released this year have recommended usage of multimodal antiemetic therapy for more than one risk factors, as we had done in this study.[10] The Apfel scoring system is the most accurate scoring system in preventing postoperative nausea and vomiting according to study done by Gunavan et al.[11]
Only 17 of our patients had received nitrous oxide during surgery. Systematic reviews have revealed that non-inclusion of nitrous oxide decreased the incidence of postoperative vomiting, although there was no documented evidence in reduction of postoperative nausea.[12,13,14] Since only 3.4% of our population received nitrous oxide as one of the inhalational anaesthetic agents, this could also explain the lower occurrence of post-operative nausea and postoperative vomiting in our population compared to other studies. In our patient population, previous history of postoperative nausea (p = 0.019) and previous history of postoperative vomiting (p = 0.005) were independent predictors of postoperative nausea. Only previous history of nausea seems to be the predictor for postoperative vomiting in our patient population (p = 0.001). Though there were reviews which have pointed that previous history of PONV is a significant risk factor in predicting postoperative nausea and vomiting,[15,16] they have not analysed postoperative nausea and postoperative vomiting as separate risk factors in predicting postoperative nausea and postoperative vomiting.
Our data suggests that patients who consume alcohol are more prone to develop postoperative nausea and vomiting (p-value = 0.028). Alcohol consumption is correlated with increased risk of vomiting among pregnant women.[17] Numerous trials have shown increased association of alcohol consumption with vertigo.[18,19] One possible explanation is because of altered sensitivity of the vestibular apparatus among alcoholics which induces nausea and vomiting.[20] Some research papers report that vertigo is associated with PONV[21] indicating a possible association between postoperative nausea and vomiting and alcohol consumption. Our results corroborate the findings of Cohen et al.[7] and Lerman et al.[6] who found that higher BMI increased the chance of a person experiencing postoperative vomiting. However, a review by Apfel et al. reported BMI as not a significant risk factor for postoperative nausea and postoperative vomiting.[23] Although a systematic analysis has reported little statistical correlation between patient age and nausea and vomiting after surgery[24] ; our results are similar to others who have reported higher incidences of nausea among younger patients.[25] In contrast to most other studies which have shown gender to be the most significant of all the risk factors for PONV[1,2,20,21,26] ; our data failed to show this association.
Our study results were similar to those of Stadler et al.,[14] who reported that urological surgeries are a risk factor for postoperative nausea[30] . In contrast, other investigators have reported that type of surgery is not a significant risk factor for postoperative nausea and postoperative vomiting.[22,27,28,29]
Regarding the duration of surgery, we found that patients whose surgical duration were more than one hour had higher prevalence of postoperative vomiting (p = 0.029). Previous studies by Koivuranta et al.[13] and Lerman et al.[6] have reported similar results. In his risk score, Koivuranta has included duration of surgery as one of the predictors for postoperative nausea and postoperative vomiting[31] (estimated logistic coefficients 0.68 with standard error of 0.15).
All our patients received opioids in the operative period in the form of fentanyl or morphine. We did not find a statistically significant association between intraoperative and postoperative use of opioids [fentanyl (p = 0.804, P = 0.785) and morphine (p = 0.584, P = 0.854) and PON and POV. Studies by Sneyd et al.[32] and Stadler et al.[27] have reported no significant association between PONV and opioid usage. In contrast, numerous studies and systematic reviews have shown a strong association between PONV and opioid use.[1,2] (OR 1.93; 1.53 to 2.43).
The association between smoking and postoperative nausea (p = 0.948) and postoperative vomiting (p = 0.898) in our study was not statistically significant. We also compared the effects of tobacco chewing (as this is more relevant to the Indian population) on postoperative nausea (p = 0.967) and postoperative vomiting (p = 0.812) but found no statistical association between them either. Although meta-analyses report that non-smokers have high risk for postoperative nausea and postoperative vomiting[11,13,28] we did not find a significant relationship between PONV and non-smoking status.
Limitations of this study
We calculated the sample size for this study assuming an incidence rate of 30% for PONV but our period prevalence of postoperative nausea was 2.04% and for postoperative vomiting was 2.45%. Hence the study sample size was underpowered to establish any causal association. This should be appreciated while interpreting the causative associations between the various risk factors and PON and POV in our study population. Our study population did not include the female patients undergoing gynaecological day-care surgical procedures as these are performed in operation rooms in another building. This may have affected our prevalence rates as female gender is known to be a strong predictor for PONV. This factor may also affect the generalizability of our results.
Although we tried to avoid attrition bias by following a standard protocol for anti-emetic prophylaxis; we had an attrition of about 9.5% population in our study due to violation of the anti-emetic protocol. There may have been recall bias from patients for variables like history of migraine, history of motion sickness, history of smoking, history of alcohol intake, history of tinnitus/vertigo/light headedness, previous history of post-operative nausea, previous history of vomiting and history of tobacco consumption other than smoking. Also, we did not follow up the patients post discharge from the post-anaesthesia care unit so we may have missed patients who developed nausea and vomiting after discharge from the hospital.
In conclusion, following a simplified risk stratification scoring system pre-operatively and providing adequate prophylaxis to patients resulted in the reduction of the incidence of PON and POV to 2.01% and 2.45%. Further studies are needed to identify the problems associated with PONV and the economic impact of a standardized prophylactic protocol for PONV in the day care setting.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Key Messages
Apfel score will predict the risk of PONV enabling primary physician to advice patients regarding preventive strategies. Risk stratification score adherence and standardised antiemetic prophylaxis administration are effective in reducing the incidence of postoperative nausea from 17% to 2.04% and postoperative vomiting from 8% to 2.4% in ambulatory surgery.
Financial support and sponsorship
Internal Fluid Research.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank the Institutional Review Board, Christian Medical College, Vellore for approving this study. We thank our ambulatory surgery nursing staff for monitoring the patients in our study for post-operative nausea and post-operative vomiting.
References
- 1.Apfel CC, Philip BK, Cakmakkaya OS, Shilling A, Shi Y-Y, Leslie JB, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology. 2012;117:475–86. doi: 10.1097/ALN.0b013e318267ef31. [DOI] [PubMed] [Google Scholar]
- 2.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–51. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberhart LHJ, Morin AM, Wulf H, Geldner G. Patient preferences for immediate postoperative recovery. Br J Anaesth. 2002;89:760–1. [PubMed] [Google Scholar]
- 4.Hirsch J. Impact of postoperative nausea and vomiting in the surgical setting. Anaesthesia. 1994;49(Suppl):30–3. doi: 10.1111/j.1365-2044.1994.tb03580.x. [DOI] [PubMed] [Google Scholar]
- 5.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting? Anesthesiol Res Pract. 2011;2011:748031. doi: 10.1155/2011/748031. doi: 10.1155/2011/748031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–28. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 8.Pierre S, Corno G, Benais H, Apfel CC. A risk score-dependent antiemetic approach effectively reduces postoperative nausea and vomiting--A continuous quality improvement initiative. Can J Anaesth. 2004;51:320–5. doi: 10.1007/BF03018235. [DOI] [PubMed] [Google Scholar]
- 9.Kranke P, Meybohm P, Diemunsch P, Eberhart LHJ. Riskadapted strategy or universal multimodal approach for PONV prophylaxis? Best Pract Res Clin Anaesthesiol [Internet] 2020. May 21, cited 2020 Oct 29. Available from: http://www.sciencedirect.com/science/article/pii/S1521689620300367 . [DOI] [PubMed]
- 10.Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131:411–48. doi: 10.1213/ANE.0000000000004833. [DOI] [PubMed] [Google Scholar]
- 11.Gunawan MY, Utariani A, Maulydia M, Veterini AS. Sensitivity and specificity comparison between APFEL, KOIVURANTA, and SINCLAIR score as PONV predictor in post general anesthesia patient. Qanun Medika. 2020;4:69–76. [Google Scholar]
- 12.Divatia JV, Vaidya JS, Badwe RA, Hawaldar RW. Omission of nitrous oxide during anesthesia reduces the incidence of postoperative nausea and vomiting. A meta-analysis. Anesthesiology. 1996;85:1055–62. doi: 10.1097/00000542-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Tramèr M, Moore A, McQuay H. Omitting nitrous oxide in general anaesthesia: Meta-analysis of intraoperative awareness and postoperative emesis in randomized controlled trials. Br J Anaesth. 1996;76:186–93. doi: 10.1093/bja/76.2.186. [DOI] [PubMed] [Google Scholar]
- 14.Lerman J. Surgical and patient factors involved in postoperative nausea and vomiting. Br J Anaesth. 1992;69(7 Suppl 1):24S–32S. doi: 10.1093/bja/69.supplement_1.24s. [DOI] [PubMed] [Google Scholar]
- 15.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology. 1999;91:109–18. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Yoo Y-C, Bai S-J, Lee K-Y, Shin S, Choi EK, Lee JW. Total intravenous anesthesia with propofol reduces postoperative nausea and vomiting in patients undergoing robot-assisted laparoscopic radical prostatectomy: A prospective randomized trial. Yonsei Med J. 2012;53:1197–202. doi: 10.3349/ymj.2012.53.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weigel MM, Weigel RM. The association of reproductive history, demographic factors, and alcohol and tobacco consumption with the risk of developing nausea and vomiting in early pregnancy. Am J Epidemiol. 1988;127:562–70. doi: 10.1093/oxfordjournals.aje.a114831. [DOI] [PubMed] [Google Scholar]
- 18.Sunami K, Tochino R, Tokuhara Y, Yamamoto H, Tomita S, Koshimo N, et al. Effects of cigarettes and alcohol consumption in benign paroxysmal positioning vertigo. Acta Otolaryngol. 2006;126:834–8. doi: 10.1080/00016480500527474. [DOI] [PubMed] [Google Scholar]
- 19.Summala H, Mikkola T. Fatal accidents among car and truck drivers: Effects of fatigue, age, and alcohol consumption. Hum Factors. 1994;36:315–26. doi: 10.1177/001872089403600211. [DOI] [PubMed] [Google Scholar]
- 20.Kuritzky A, Ziegler DK, Hassanein R. Vertigo, motion sickness and migraine. Headache. 1981;21:227–31. doi: 10.1111/j.1526-4610.1981.hed2105227.x. [DOI] [PubMed] [Google Scholar]
- 21.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–84. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: Assessing risk factors for nausea and vomiting. Anesth Analg. 1994;78:7–16. doi: 10.1213/00000539-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Kranke P, Apefel CC, Papenfuss T, Rauch S, Löbmann U, Rübsam B, et al. An increased body mass index is no risk factor for postoperative nausea and vomiting. A systematic review and results of original data. Acta Anaesthesiol Scand. 2001;45:160–6. [PubMed] [Google Scholar]
- 24.Kupietzky A, Tal E, Shapira J, Ram D. Fasting state and episodes of vomiting in children receiving nitrous oxide for dental treatment. Pediatr Dent. 2008;30:414–9. [PubMed] [Google Scholar]
- 25.Silva AC, O’Ryan F, Poor DB. Postoperative nausea and vomiting (PONV) after orthognathic surgery: A retrospective study and literature review. J Oral Maxillofac Surg. 2006;64:1385–97. doi: 10.1016/j.joms.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Apfel CC, Roewer N. [Postoperative nausea and vomiting] Anaesthesist. 2004;53:377. doi: 10.1007/s00101-004-0662-8. [DOI] [PubMed] [Google Scholar]
- 27.Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology. 2003;98:46–52. doi: 10.1097/00000542-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Junger A, Klasen J, Benson M, Sciuk G, Hartmann B, Sticher J, et al. Factors determining length of stay of surgical day-case patients. Eur J Anaesthesiol. 2001;18:314–21. doi: 10.1046/j.0265-0215.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- 29.Chung F, Mezei G. Adverse outcomes in ambulatory anesthesia. Can J Anaesth. 1999;46(5 Pt 2):R18–34. doi: 10.1007/BF03013179. [DOI] [PubMed] [Google Scholar]
- 30.Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443–9. doi: 10.1111/j.1365-2044.1997.117-az0113.x. [DOI] [PubMed] [Google Scholar]
- 31.Eberhart LHJ, Högel J, Seeling W, Staack AM, Geldner G, Georgieff M. Evaluation of three risk scores to predict postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2000;44:480–8. doi: 10.1034/j.1399-6576.2000.440422.x. [DOI] [PubMed] [Google Scholar]
- 32.Sneyd JR, Carr A, Byrom WD, Bilski AJ. A meta-analysis of nausea and vomiting following maintenance of anaesthesia with propofol or inhalational agents. Eur J Anaesthesiol. 1998;15:433–45. doi: 10.1046/j.1365-2346.1998.00319.x. [DOI] [PubMed] [Google Scholar]


