Abstract
Introduction:
Patients with sleep-related breathing disorders (SRBD) have various structural and functional abnormalities of the upper airway during sleep which may get reflected on their pulmonary function tests. The aim of the study was to find the correlation between the spirometric indices and snoring, grades of apnea–hypoapnea index (AHI), and STOPBANG. There is scarcity of literature showing correlation of STOP BANG with spirometric variables.
Material and Methods:
Patient with SRBD fulfilling the inclusion and exclusion criteria were enrolled. The pretest probability sleep score STOPBANG and polysomnography (PSG) were calculated for all the patients. Spirometric indices like forced expiratory volume in one sec (FEV1), forced vital capacity (FVC), postbronchodilator ratio FEVI/FVC (PBDR), and peak expiratory flow rate (PEFR) were studied. Their association with snoring, different grades of obstructive sleep apnea (OSA), and STOPBANG were evaluated using statistical analysis.
Results:
A total of 70 patients were enrolled. Abnormalities of spirometric indices were found to be common in patients with SRBD but their association with snoring, grades of OSA, and STOPBANG were not statistically significant. There is no statistically significant correlation between body mass index (BMI) and grades of AHI.
Conclusion:
This study found no statistically significant correlation between spirometric parameters and STOPBANG and degree of AHI. Primary care physicians should be aware that obstructive lung disease does coexist with the sleep disordered breathing but as per this study, their statistically significant association needs further validation.
Keywords: AHI, BM, OSAS, significant, sleep, spirometry, SRBD, STOPBANG
Introduction
The term sleep-related breathing disorders (SRBD) includes a group of pathophysiologic conditions that characteristically have an abnormal respiratory pattern during sleep ranging from simple snoring to obstructive sleep apnea (OSA). In the 1960s, the only upper airway closure syndrome described was OSA, in addition to the sleep disorder of snoring.[1] Two distinct syndromes have been described now: (a) upper airway resistance syndrome (UARS) and (b) obstructive sleep apnea syndrome (OSAS). Each of these syndromes has a distinct definition, yet both have in common the symptom of excessive daytime sleepiness resulting from repeated upper airway obstruction. These disorders belong to a continuum of a disease process that is based on the severity of the airway obstruction. Partial airway obstruction initially presents as snoring, which may then progress to upper airway resistance syndrome (UARS) and finally to OSAS. Yet, the individual patient may not pass steadily through this continuum; that is, a patient may snore for years and then go straight on to having OSAS, depending on the severity of the upper airway obstruction.[1]
Obstructive sleep apnea syndrome (OSAS), the commonest form of SRBD, is also known as the obstructive sleep apnea–hypopnea syndrome (OSAHS). It involves cessation or a significant decrease in airflow in the presence of breathing effort. The prevalence of OSA is estimated to be 10–20% with about 4% and 2% of middle-aged men and women, respectively, suffering from symptomatic OSA.[2]
Obesity is considered a major risk factor for the development and progression of OSA. The prevalence of OSA in obese or severely obese patients is nearly twice that of normal-weight adults.[3]
Obese patients have reduced functional residual capacity which leads to significant intrathoracic depression at the beginning of inspiration caused by the contraction of the diaphragm and the result is pharyngeal collapse in these patients.[4] A 10% gain in bodyweight could predict a 32% increase in apnea–hypopnea index (AHI).[4]
Spirometric abnormalities have often been noticed among patients suffering from OSAHS.[5]
OSA has been shown to have an effect on the intrathoracic airways. The anatomical aspects, lower airway inflammation and oxidative stress may also have an impact on pulmonary function.[6]
Lung volumes also affect the collapsibility of upper airways which is one of the important pathological factors in OSA patients. Many studies that examined the effect of lung volume on collapsibility have shown that by increasing the functional residual capacity (FRC) in OSA is accompanied by improvement in the pharyngeal collapsibility and decrease in pharyngeal resistance due to increase in pharyngeal size.[7] These studies appear to suggest the involvement of lung volume, in terms of FRC or end expiratory lung volume (EELV), in the pathogenesis of OSA.[7]
The presence of OSA may further increase airway inflammation and possibly lead to further decline of pulmonary functions in patients with respiratory disease.[8]
A number of studies have investigated the association between OSA and Obstructive lung disease (OLD).[9] Studies have shown that up to 20% patients have severe OLD with co-existent OSA.[10]
Various pretest probability scores have been developed to screen patients for a polysomnography (PSG) and assessing risk for OSAS during their baseline work-up. The STOP-BANG questionnaire is a reliable and easy-to-use screening tool which aids in the classification of patients for OSA risk based on their scores.[11]
Spirometric parameters may exert an influence on the diagnostic efficacy of STOP-BANG. This may be explained by the fact that spirometric parameters are associated with body mass index (BMI), neck circumference, and gender.
Various studies have been conducted to find the correlation between pretest probability scores and AHI but there has been a scarcity of literature about the correlation of STOP-BANG and spirometric parameters.
Hence, the present study was undertaken in patients with sleep disordered breathing to study the correlation of various spirometric parameters with AHI, BMI, STOP-BANG and the correlation of BMI with AHI.
Material and Methods
This prospective observational study was conducted at the Department of Internal and Pulmonary medicine of an institute from December 2018 to December 2019. All patients of SRBD who presented with the symptoms of daytime sleepiness, snoring witnessed apnea, shortness of breath, daytime fatigue, and morning headache were enrolled. The detailed history and clinical examinations were noted and the following inclusion/exclusion criteria were applied.
Ethical clearance was not deemed necessary in view of this study being purely observational.
Inclusion criteria
Patients with symptoms of sleep disordered breathing
Adult >18 years of age.
Exclusion criteria
Age <18.
Pregnancy and/or lactation.
Acute respiratory infections.
Acute exacerbation of COPD/bronchial asthma,
Acute myocardial infarction and acute stroke.
Any other lung pathology/malignancy
All patients were subjected to baseline spirometry in pulmonary function test (PFT) laboratory as per the American Thoracic Society criteria. Spirometry was done with computerized MedGraphics Spirometer. After spirometry with postbronchodilator, reversibility were categorized into bronchial asthma and COPD.
Asthma was diagnosed on the basis of Global Initiative for Asthma guidelines (GINA). Diagnosis of COPD was based on the Global Initiative for Obstructive Lung Disease guidelines.
BMI of all the patients were recorded and graded according to the WHO classification of overweight obesity.
Pre-test probability sleep score and STOP-BANG were calculated for all the patients. PSG was done in all patients with SRBD who fulfill the inclusion and exclusion criteria. PSG involved multichannel monitoring, including electroencephalography, electrooculography, submentalis, anterior tibialis electromyography, electrocardiography, airflow (nasal–oral thermistors), respiratory effort (pneumobelts), body position, and oxygen saturation (SaO2—pulse oximetry).
The AHI grading was done according to the American Academy of Sleep Medicine guidelines (AASMA). AHI is the standard metric used to quantitate the severity of OSA.
Data and statistical analysis
Analyses were done using Statistical Package for the Social Sciences (SPSS v 21.0). Descriptive statistics including frequencies, percentages, mean, and standard deviation were calculated for the parameters taken into account. The correlation of spirometric parameters with STOP-BANG and AHI was assessed by using bivariate Pearson's correlation coefficient and represented by scatter plotss. The significance of correlation was determined with a P value < 0.05.
Results
A total of 70 SDB patients were included in the study. Of these, 37 (52.9%) were male and 33 were female (47.1%) patients. The mean age of the study group was 51.42 years, with a standard deviation of 10.2 yrs. The mean AHI recorded is 41.9 and mean BMI measured is 31.47. The mean for the pretest probability score STOP-BANG recorded is 4.9. The mean FEV1 and FVC are 74.63 and 74.54, respectively [Table 1a, 1b].
Table 1a.
Represents the gender distribution
| Frequency | Percent | |
|---|---|---|
| Valid | ||
| Male | 37 | 52.9 |
| Female | 33 | 47.1 |
| Total | 70 | 100.0 |
Table 1b.
Mean values and standard deviation of the parameters under study
| n | Minimum | Maximum | Mean | Std. deviation | |
|---|---|---|---|---|---|
| Age | 70 | 25 | 80 | 51.42 | 10.299 |
| Stop bang | 70 | 2 | 8 | 4.91 | 1.380 |
| AHI | 70 | 2.7 | 125.0 | 41.940 | 31.6789 |
| BMI | 70 | 20.1 | 43.3 | 31.472 | 5.2004 |
| FEV1 | 70 | 30 | 112 | 74.63 | 18.957 |
| FVC | 70 | 30 | 114 | 74.54 | 19.365 |
| PBDR | 70 | 63.0 | 107.0 | 79.705 | 7.5766 |
| PEFR | 70 | 345 | 658 | 493.89 | 95.539 |
| Valid n (listwise) | 70 |
The majority of patients have normal spirometric parameters (35; 50%) followed by obstructive pattern (28; 40%) and restrictive pattern (7; 10%) patients [Table 1c].
Table 1c.
Spirometric abnormalities
| Spirometric findings | No of patients | Percentages |
|---|---|---|
| Normal | 35 | 50% |
| Obstructive pattern | 28 | 40% |
| Restrictive pattern | 7 | 10% |
The majority of patients with severe OSA (34.28%) belonged to the obese class 1 category; however, no statistically significant correlation was observed between AHI and BMI [Table 2 and Figure 1].
Table 2.
Comparison between AHI and BMI Category
| Categories of SDB | BMI | ||||
|---|---|---|---|---|---|
| Normal wt (18.5-24.9) | Pre-obese (25-29.9) | Class 1-obese (30-34.9) | Class 2-obese (35-39.9) | Class 3-obese (>40) | |
| Normal AHI | |||||
| Primary | 1 (33.33%) | 0 (0%) | 1 (33.33%) | 1 (33.33%) | 0 |
| Snorers (<5) | |||||
| Mild OSA | 1 (8.33%) | 2 (16.66%) | 4 (33.33%) | 4 (33.33%) | 1 (8.33%) |
| Moderate OSA | 2 (10.00%) | 9 (45.00%) | 5 (25.00%) | 2 (10.00%) | 2 (10.00%) |
| Severe OSA | 3 (8.50%) | 10 (28.57%) | 12 (34.28%) | 9 (25.71%) | 1 (2.85%) |
| 70 | 7 | 21 | 22 | 16 | 4 |
Figure 1.
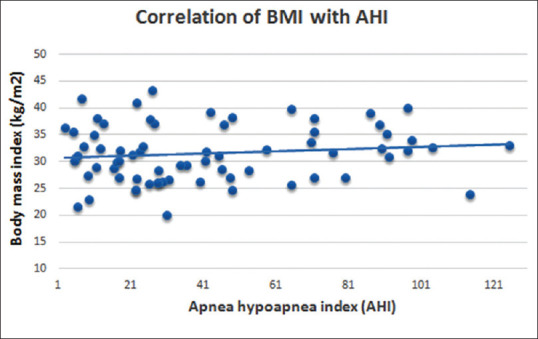
Scatter plot show no Correlation of BMI with AHI (by bivariate Pearson's correlation coefficient)
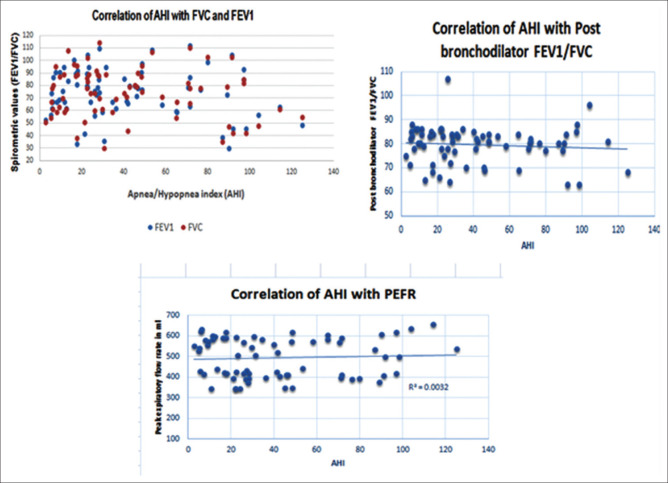
There was no significant correlation between AHI and spirometric abnormalities after applying Pearson's correlation test between AHI grades and spirometric parameters. The P value for FVC was 0.222(>0.05), FEV1 was 0.226 (>0.05), FEV1/FVC was 0.457 (>0.05), and PEFR was 0.641 (>0.05) [Figure 2].
Figure 2.
Scatter plot show no Correlation of spirometry variables with AHI (by bivariate Pearson's correlation coefficient
No significant correlation was found between STOP-BANG and spirometric parameters after applying Pearson's correlation test between pretest probability score STOP-BANG risk catagories for OSA and spirometric parameters. The P value for FVC was 0.166 (>0.05), FEV1 was 0.339 (>0.05), PBR FEV1/FVC was 0.525 (>0.05), and PEFR was 0.213 (>0.05).
Discussion
The abnormalities in spirometric indices have been reported to be present and related to OSAS in view of the presence of various structural and functional abnormalities of the upper airway during sleep in such patients[12,13,14] . Studies have shown that as the depth of sleep increases, there is a reduction in minute ventilation with an increase in upper airway resistance.[10]
In our study, no correlation was found between the severity of AHI and spirometric parameters. This is similar to many studies like those done by Gajanan et al.,[15] Narsimhan et al.[16] and Sharma et al.[17] which showed no correlation between forced expiratory volume (FEV1) and AHI. We found spirometric abnormalities in these patients but such abnormalities have no statistically significant correlation with different groups of SDB. Similarly, Swapnil Manaji Thorve et al.[18] also found that abnormalities in spirometric parametres were common in OSAS patients but their association with grades of OSA were not statistically significant.
Sei Won Kim et al.[6] studied the association between PFT results during daytime and OSA with regard to the severity of OSA and presence of obesity and found that the degree of pulmonary function change was not significantly associated with AHI.
Similar to our study, in many studies, FEV1 was considered to be an unsuitable tool for assessing the functional impact of OSAS as these data did not show a significant difference between participants with and without OSAS during their studies.[4]
In contrast to what we observed, Zerah-Lancer F et al. in their study found that FEV1/FVC decreases significantly as AHI increases.[19] Similarly, Kreiger et al. observed that the severity of airway obstruction, as evidenced by FEV1/FVC ratio, was directly proportional to the severity of OSA.[20]
Arkin et al.[7] in their study found that FRC and Expiratory reserve volume (ERV) were significantly reduced, whereas FEV1/FVC% was slightly raised in the OSA patient and independently associated with the severity of OSA. Orr et al.[21] in their study found a strong association between the severity of OSA and pulmonary function parameters like increased loop gain in those with airflow obstruction (lower FEV1 and higher RV and RV/TLC ratio). Rouatbi et al.[4] found in her study that obesity when associated with OSAS increases the severity of pulmonary function abnormalities.
Obesity is seen as a major risk factor for the development and progression of OSA. Patients with mild OSA who gain 10% of their baseline weights are at a six fold-increased risk of progression of OSA, and similar weight loss can result in more than 20% improvement in OSA severity.[22]
The association between obesity and OSA has long been appreciated. All most all have found a significant association between OSA and measures of excess body weight. Our study showed that a majority of patients with severe OSA (34.28%) belonged to the obese class 1 category; however, no statistically significant correlation was observed between degree of AHI and BMI. This is in accordance with the study done by Narsihaman et al.,[16] Venkateswaran et al.[22] and Sei Won Kim et al.[6] who showed that BMI is not correlated with the degree of AHI. However, on the contrary, studies done by Peppard PE et al.[23] and others demonstrated a positive correlation between AHI and BMI.
STOP-BANG (SBQ) which is an easy-to-use pretest probability score for screening the SDB patients indicated that those who have a higher score in the SBQ would have a greater probability of moderate and severe OSA. However, there is a scarcity of literature that focused on the correlation of SBQ with spirometric parameters. Our study has examined this issue and found that there is no statistically significant correlation between SBQ and spirometric parameters.
In contrast to what we observed, Wu et al.[24] investigated how pulmonary function interferes with scoring questionnaires like STOP-BANG, Berlin Questionnaire (BQ), and modified Berlin Questionnaire (MBQ) and found that STOP-BANG along with two other questionaires was more accurate in subjects with lower FEV1%pred or FVC% pred value. Pulmonary function might exert an influence on the diagnosis efficacy of the three questionnaires through BMI and neck circumference.
A limitation of this study is the small sample size. Therefore, studies with larger sample size are further needed to find the correlation.
Conclusion
This study did not find a statistically significant correlation neither between spirometric parameters and STOP-BANG nor between spirometric parameters and the degree of AHI. Although a majority of severe OSA patients in our study belonged to obese category class1, this observation was not statistically significant. It is apparent from our study that a general approach for OSA diagnosis does not necessarily require the inclusion of conventional pulmonary function test but treatments of OSA per se may be influenced by poor lung function and the presence of COPD in some individuals. Both OSA and OLD may coexist, although a statistically significant coexistence was not seen in our study; however, if they do, then the mortality increases. In the light of these, a primary care physician should not underestimate the importance of doing PFTs during the workup of OSA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge the contribution of Dr Javed Bhat, Assistant Professor, Commerce, Department of Higher Education J&K who performed the statistical analyses of this paper.
References
- 1.Walker RP. Snoring and obstructive sleep apnea. In: Bailey BJ, Johnson JT, Newlands SD, editors. Head & Neck Surgery – Otolaryngology. 4th ed. 46. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 2.Ramirez JM, Garcia AJ, Anderson TM, Koschnitzky JE, Peng YJ, Kumar GK, et al. Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol. 2013;189:344–53. doi: 10.1016/j.resp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somer VK. Interactions between obesity and obstructive sleep apnea. Chest. 2010;137:711–9. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouatbi S, Ghannouchi I, Kammoun R, Saad HB. The ventilatory and diffusion dysfunctions in obese patients with and without obstructive sleep apnea-hypopnea syndrome? Hindawi J Obes. 2020;2020:8075482. doi: 10.1155/2020/8075482. doi: 10.1155/2020/8075482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suri JC, Sen MK. Pulmonary functions in obstructive sleep apnea hypopnea syndrome in a cohort of patients attending the sleep center of a tertiary care hospital. Indian J Sleep Med. 2007;2:21–7. [Google Scholar]
- 6.Kim SW, Kang HH, Ban WH, Lee SH. Pulmonary function and effects of body position in patients with obstructive sleep apnea. Chronobiol Med. 2019;1:157–62. [Google Scholar]
- 7.Abdeyrim A, Zhang Y, Li N, Zhao M, Wang Y, Yao X, et al. Impact of obstructive sleep apnea on lung volumes and mechanical properties of the respiratory system in overweight and obese individuals. BMC Pulm Med. 2015;15:76. doi: 10.1186/s12890-015-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien A, Whitman K. Lack of benefit of continuous positive airway pressure on lung function in patients with overlap syndrome. Lung. 2005;183:389–404. doi: 10.1007/s00408-005-2551-6. [DOI] [PubMed] [Google Scholar]
- 9.Bramen SS. Growing old with asthma: What are the changes and challenges? Expert Rev Respir Med. 2010;2:239–48. doi: 10.1586/ers.10.12. [DOI] [PubMed] [Google Scholar]
- 10.Shah AD, Patel NV, Ajay SF, Pandya RA, Aakkara AG, Shah DN, et al. study of spirometry finding in snorers. J Evid Based Med Health. 2016;3:2482–6. [Google Scholar]
- 11.Utpat K, Bansal S, Desai U, Joshi J. Clinical profile of obstructive sleep apnea syndrome in a tertiary care hospital in Western India. Indian J Sleep Med. 2019;14:1–6. [Google Scholar]
- 12.Haponik EF, Bleecker ER, Allen RP, Smith PL, Kaplan J. Abnormal inspiratory flow-volume curves in patients with sleep-disordered breathing. Am Rev Respir Dis. 1981;124:571–4. doi: 10.1164/arrd.1981.124.5.571. [DOI] [PubMed] [Google Scholar]
- 13.Levent E, Sariman N. Analysis of obstructive sleep apnea patients with “sawtooth sign” on the flow-volume curve. Sleep Breath. 2011;15:357–65. doi: 10.1007/s11325-010-0393-9. [DOI] [PubMed] [Google Scholar]
- 14.Kosmas EN RT, Toukmatzi S, Michaelides S, Polychronopoulos V. Significance of the inspiratory flow-volume loop in the diagnosis of obstructive sleep apnea syndrome. Chest. 1998;114:371S. [Google Scholar]
- 15.Halkanche GV, Mhaisekar DG. Study of correlation between obstructive sleep apnea and obstructive airway disease. MedPulse Int Med J. 2017;4:217–21. [Google Scholar]
- 16.Narasimhan M, Sharma R, Shanmuganathan A, Vallabhaneni V, Rajalingam R, Ganga N, et al. Correlation of severity of Apnea Hypopnoea index with forced expiratory volume (FEV1) in overlap syndrome. J Evid Based Med Health. 2017;4:2349–2562. [Google Scholar]
- 17.Sharma B, Feinsilver S, Owens RL. Obstructive airway disease and obstructive sleep apnea: Effect of pulmonary function. Lung. 2011;189:37–41. doi: 10.1007/s00408-010-9270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorve SM, Gupta V, Mandilwar S, Modi N, Prabhudesai P. Spirometry and flow volume loops in obstructive sleep apnea patients. Int J Contemp Med Res. 2019;6:L5–10. [Google Scholar]
- 19.Zerah-Lancer F, Lofaso F, Coste A, Ricolfi F, Goldenberg F, Harf A. Pulmonary functions in obese snorers with or without sleep apnea syndrome. Am J Respir Crit Care Med. 1997;156:522–7. doi: 10.1164/ajrccm.156.2.9609015. [DOI] [PubMed] [Google Scholar]
- 20.Krieger AC, Patel N, Green D, Modersitzki F, Belitskaya-Levy I, Lorenzo A, et al. Respiratory disturbance during sleep in COPD patients without daytime hypoxemia. Int J Chron Obstruct Pulmon Dis. 2007;2:609–15. [PMC free article] [PubMed] [Google Scholar]
- 21.Orr JE, Schmick CN, Edwards BA, DeYoung PN, Brena R, Sun XS, et al. Pathogenesis of obstructive sleep apnea in individuals with the COPD+OSA Overlap syndrome versus OSA alone. Physiol Rep. 2020;8:e14371. doi: 10.14814/phy2.14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkateswaran S, Tee A. Overlap syndrome between chronic obstructive pulmonary disease and obstructive sleep apnoea in a Southeast Asian teaching hospital. Singapore Med J. 2014;55:488–92. doi: 10.11622/smedj.2014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep disordered breathing. JAMA. 2000;284:301521. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Xie L, Li W, Xiang G, Hu W, Jiang H, et al. Pulmonary function influences the performance of berlin questionnaire, modified berlin questionnaire, and STOP-bang score for screening obstructive sleep apnea in subjects with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:1207–16. doi: 10.2147/COPD.S248139. [DOI] [PMC free article] [PubMed] [Google Scholar]