Abstract
Sepsis is a syndrome characterized by organ dysfunction and an abnormal immune response to infection. A growing body of research has shown the importance of long non-coding RNAs (lncRNAs) in tumorigenesis, virus replication, inflammatory injury and other pathological processes. The aim of the present study was to explore the role and potential mechanism of the lncRNA growth arrest-specific 5 (GAS5) in the lipopolysaccharide (LPS)-induced inflammation and apoptosis of THP-1 cells. An in vitro sepsis model was established by treating THP-1 cells with LPS. Apoptosis was detected by flow cytometry. The expression levels of IL-6, IL-1β and TNF-α were detected using reverse transcription-quantitative PCR (RT-qPCR) and ELISA, and those of GAS5, microRNA (miR)-23a-3p and Toll-like receptor 4 (TLR4) were detected by RT-qPCR. The changes in the biological activity of THP-1 cells induced by the silencing of GAS5 and overexpression of miR-23a-3p and TLR4 were investigated. The relationships among GAS5, miR-23a-3p and TLR4 were analyzed using luciferase reporter assays. The results revealed that LPS increased the expression of GAS5 in THP-1 cells, and GAS5 knockdown effectively inhibited inflammation and cell apoptosis in the LPS-induced sepsis model. In addition, the results of the luciferase reporter assays indicated that both GAS5 and TLR4 directly target miR-23a-3p. The expression of miR-23a-3p was downregulated whereas that of TLR4 was upregulated in the septic cells. Further experiments showed that the overexpression of TLR4 attenuated the suppressive effects of miR-23a-3p overexpression and GAS5 knockdown on LPS-induced inflammation and apoptosis. In conclusion, the present study indicates that GAS5 strengthens LPS-induced inflammation and apoptosis via the miR-23a-3p/TLR4 pathway.
Keywords: sepsis, GAS5, miR-23a-3p, Toll-like receptor 4, apoptosis
Introduction
Sepsis is a systemic inflammatory disease that occurs after severe trauma, burns, infection and major surgery, and is associated with multiple organ failure and mortality (1,2). The excessive inflammation, immunosuppression and tissue damage caused by sepsis can increase susceptibility to secondary infection and greatly aggravate the state of illness of a patient, and may ultimately lead to death (3). Therefore, the early diagnosis of sepsis and septic shock is essential for reducing the mortality rate. Although sepsis has become the leading cause of death in critically ill patients worldwide, the underlying molecular mechanism of sepsis remains poorly understood. Therefore, it is of crucial importance to explore the pathogenesis of the inflammatory response induced by sepsis.
The genome-wide analysis of gene expression in critically ill patients has shown that more >80% of basic genetic factors undergo changes, suggesting that the molecular basis of sepsis may be associated with regulation at the genetic level (4). Long non-coding RNAs (lncRNAs) are non-protein-coding RNAs that are >200 nucleotides in length (5). The mechanisms of action of lncRNAs appear diverse, as they can regulate gene expression at multiple levels and play important roles in tumor formation, virus replication, inflammatory injury and other pathological processes. lncRNAs have also gradually been considered as biomarkers for various diseases, including sepsis (6,7). Studies have reported on several lncRNAs that participate in cellular immune regulation, the release of inflammatory factors and the inflammatory response of sepsis. For example, Chen et al (8) demonstrated that lncRNA NEAT1 is significantly upregulated in patients with sepsis-induced acute kidney injury, and that NEAT1 exacerbates lipopolysaccharide (LPS)-induced cell injury by directly targeting microRNA (miRNA/miR)-204 and activating the NF-κB pathway. Jia et al (9) reported that lncRNA CCL2 upregulates the expression of inflammatory factors in macrophages from septic mice. Growth arrest-specific 5 (GAS5) is a lncRNA that is closely associated with the regulation of malignant tumors. The expression of GAS5 is downregulated in renal cell carcinoma, and its downregulation has been suggested to serve an oncogenic role (10). Also, GAS5 expression is negatively correlated with the malignancy of cervical cancer, and its overexpression has been shown to reduce cell viability and increase apoptosis in cervical cancer cells (11). Furthermore, GAS5 has been reported to be involved in cell proliferation, invasion, metastasis, apoptosis, epithelial-mesenchymal transition and drug resistance through various molecular mechanisms (12). However, studies concerning the functional role and mechanisms of GAS5 in sepsis are lacking.
miRNAs are naturally occurring, non-coding, single-stranded RNA molecules that are 21–25 nucleotides in length, and are able to bind to mRNA and reduce protein translation (13). miRNAs are widely involved in numerous biological processes, including the growth and development of the organism, substance metabolism, hematopoietic cell differentiation, tumor formation and immune regulation, and are expected to become new targets for the treatment of various diseases. A previous study detected a significant reduction in the expression of miR-23a-3p in patients with acute kidney injury caused by sepsis (14). In another study using myeloid leukemia cells, the downregulation of miR-23a-3p inhibited cell proliferation, promoted apoptosis and influenced the cell cycle (15). A number of studies have shown that lncRNA can cause a series of changes in biological functions through mutual regulation with miRNA (16–18). Bioinformatic analysis predicts that miR-23a-3p is a potential target gene of GAS5; however, the interaction between GAS5 and miR-23a-3p in sepsis has not yet been studied.
Accordingly, in the present study, the functional mechanism of GAS5 was explored to evaluate the effects of GAS5 on LPS-induced inflammation and cell apoptosis and provide a theoretical basis for molecular-targeted therapy and the prognostic diagnosis of sepsis.
Materials and methods
Cell culture and treatment
The THP-1 human monocytic leukemia cell line was purchased from the American Type Culture Collection. The cells were cultured in RPMI-1640 medium (HyClone; Cytiva) containing 10% fetal bovine serum (FBS; HyClone; Cytiva) and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 5% CO2 at 37°C. After subculture, cells in the logarithmic phase were taken for the following experiments.
To establish the cell sepsis model, cells were treated with 1 µg/ml LPS for 24 h at 37°C to simulate the sepsis environment. Reverse transcription-quantitative PCR (RT-qPCR) was used to detect the expression levels of IL-6, TNF-α and IL-1β to verify that the sepsis model was constructed successfully.
Cell transfection
Short hairpin RNAs (shRNAs) targeting GAS5 (sh-GAS5-1 and sh-GAS5-2), shRNA control (sh-NC), miR-23a-3p overexpression vector (miR-23a-3p mimic; cat. no. B01001) and miRNA negative control (miR-NC; cat. no. B01001) were obtained from Shanghai GenePharma Co., Ltd. The Toll-like receptor 4 (TLR4) overexpression vector pcDNA-TLR4 and empty control vector pcDNA-NC were constructed by Guangzhou RiboBio Co., Ltd. In strict accordance with the manufacturer's instructions, following LPS treatment, 1 µg plasmids were transfected into THP-1 cells using Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Following incubation for 6 h, the medium was replaced with DMEM with 10% FBS (HyClone; Cytiva). After 48 h of incubation, the cells were collectedand the transfection efficiency was analyzed by RT-qPCR.
Bioinformatics
StarBase v3.0 (http://starbase.sysu.edu.cn/) was used to predict the target miRNA of GAS5 and the downstream target genes of miR-23a-3p.
RT-qPCR analysis
The assay was performed in accordance with the instructions of the respective kits. Total RNAs were isolated from cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Then, the RNAs were reverse transcribed into cDNA using the PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd.). qPCR was then performed using Light Cycler 480 SYBR Green I Master mix (Roche Applied Science). The reaction was carried out under the following conditions: Initial denaturation at 95°C for 1 min 40 sec, followed by 42 cycles of denaturation at 94°C for 30 sec, annealing at 55°C for 1 min and elongation at 72°C for 60 sec, followed by a final extension at 72°C for 5 min. Relative gene expression was analyzed using the 2−ΔΔCq method (19). U6 was used as an endogenous control for miR-23a-3p and GAPDH was used as a control for other genes. The primer sequences used are as follows: IL-6 forward, 5′-GCAAGGGTCTGGTTTCAGCC-3′ and reverse, 5′-TGAGGTAAGCCTACACTTTCCAA-3′; TNF-a forward, 5′-CCCTCTCTCCCCTGGAAAGG-3′ and reverse, 5′-GCCACTGAATAGGGCGAT-3′; IL-1b forward, 5′-ATTGCTCAAGTGTCTGAAGCAG-3′ and reverse, 5′-AGAGAGCACACCAGTCCAA-3′; GAS5 forward 5′-CTTCTGGGCTCAAGTGATCCT-3′ and reverse, 5′-TTGTGCCATGAGACTCCATCAG-3′; TLR4 forward, 5′-GGAGACTTGGCCCTAAACCA-3′ and reverse, 5′-GACATGGAAACACACCCAGG-3′; miR-23a-3p forward 5′-GCGATCACATTGCCAGGG-3′ and reverse, 5′-CAGTGCGTGTCGTGGAGT-3′; GAPDH forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
ELISA
Experiments were performed according to the protocol outlined in the IL-6 (cat. no. 130-094-065; Miltenyi Biotec), TNF-α (cat. no. 130-101-688; Miltenyi Biotec) and IL-1β ELISA kits (cat. no. 130-094-053; Miltenyi Biotec). Cells were incubated at 37°C for 30 min. According to the manufacturer's protocol: A total of 100 µl supernatant was added onto the IL-6/TNF-α/IL-1β antibody-coated plate, and incubated at 25°C for 2 h. After adding the biotin-conjugated detecting IL-6/TNF-α/IL-1β antibody and incubating at 25°C for 2 h, streptavidin-HRP was added and 3,3′-5,5′tetramethylbenzidin was used for development, which was incubated for 20 min at room temperature and protected from light. The optical density value was measured at 450 nm using a Multiskan spectrum spectrophotometer (Thermo Fisher Scientific, Inc.). Experiments were performed in triplicate.
Cell apoptosis analysis
Transfected cells were seeded into 24-well plates at a density of 1×105 cells/well and routinely incubated for 48 h. After this, the cells were collected, resuspended with 100 µl 1X binding buffer and 5 µl Annexin V-FITC and 5 µl PI staining solution (Beyotime Institute of Biotechnology) were added. The suspension was incubated for 10 min at room temperature in the dark. Finally, 400 µl of 1X binding buffer was added. The samples were analyzed by a BD FACSCalibur flow cytometry (BD Biosciences) and FlowJo software (version 7.6.1; Tree Star, Inc.) within 1 h. Each measurement was repeated three times and the mean value was taken.
Luciferase reporter assay
The binding in the GAS5/miR-23a-3p/TLR4 cascade was detected using a Luciferase Reporter Assay System kit (Promega Corporation). miR-23a-3p mimic or miR-NC together with the wild-type (WT) or mutant (MUT) GAS5-3′untranslated region (UTR) or TLR4-3′UTR were co-transfected into 293T cells (Beyotime Institute of Biotechnology) using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). Luciferase activity was detected 48 h after transfection using the dual-luciferase reporter system (Promega Corporation). Firefly luciferase activity was normalized to Renilla luciferase activity.
Western blot analysis
Total intracellular protein was extracted from the cells by lysis with RIPA buffer (Beyotime Institute of Biotechnology) and the protein concentration was detected using a BCA kit (Beyotime Institute of Biotechnology). Proteins (30 µg/lane) were separated by 10% SDS-PAGE and transferred to a PVDF membrane (EMD Millipore). Membranes were then blocked with 5% milk for 2 h at room temperature and incubated with anti-TLR4 (1:1,000; cat. no. 14358; Cell Signaling Technology Inc.), anti-Bcl-2 (cat. no. 4223; 1:1,000; Cell Signaling Technology, Inc.), anti-Bax (cat. no. 14796; 1:1,000; Cell Signaling Technology, Inc.), anti-cleaved caspase-3 (cat. no. 9661; 1:1,000; Cell Signaling Technology, Inc.), anti-caspase-3 (cat. no. 9662; 1:1,000; Cell Signaling Technology, Inc.), anti-cleaved poly (ADP ribose) polymerase (PARP; cat. no. 5625; 1:1,000; Cell Signaling Technology, Inc.), anti-PARP antibodies (cat. no. 9532; 1:1,000; Cell Signaling Technology, Inc.) and anti-GAPDH (1:1,000; cat. no. 5174; Cell Signaling Technology Inc.) primary antibodies overnight at 4°C. Membranes were washed with PBS twice and then incubated with HRP-conjugated second antibody (cat. no. bs-0295G; 1:2,000; BIOSS) at room temperature for 2 h. Enhanced chemiluminescence (Chemilucent Plus western blot enhancing kit; cat. no. 2650; EMD Millipore) was used for film exposure. The gray value of each band was analyzed using ImageJ software v1.8.0 (National Institutes of Health). GADPH served as an internal reference.
Statistical analysis
Data were analyzed using SPSS version 17.0 statistical software (SPSS, Inc.). Measurement data are expressed as the mean ± standard deviation. An unpaired Student's t-test or one-way ANOVA followed by Tukey's post hoc test was employed to examine the difference between two or multiple groups, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
GAS5 is upregulated in LPS-stimulated THP-1 cells
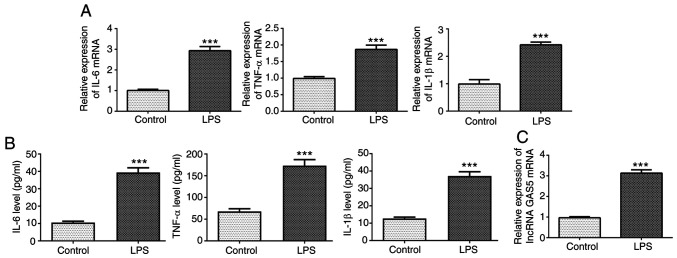
To test the effects of GAS5 on the inflammation and apoptosis induced by sepsis, an in vitro cell model of sepsis was established using LPS-induced THP-1 cells. The RT-qPCR and ELISA results showed that the expression levels of IL-6, TNF-α and IL-1β in THP-1 cells stimulated with LPS were significantly increased compared with those in the untreated control group (Fig. 1A and B). In addition, the expression of GAS5 was significantly increased in the THP-1 cells stimulated with LPS compared with the untreated control (Fig. 1C).
Figure 1.
GAS5 and inflammatory factors are upregulated in LPS-stimulated THP-1 cells. The expression levels of inflammatory factors IL-6, TNF-α and IL-1β were measured by (A) RT-qPCR and (B) ELISA in LPS-induced THP-1 cells. (C) GAS5 expression was detected by RT-qPCR in LPS-induced THP-1 cells. ***P<0.001 vs. untreated control. GAS5, growth arrest-specific 5; RT-qPCR, reverse transcription-quantitative PCR; LPS, lipopolysaccharide.
GAS5 knockdown alleviates LPS-induced inflammation and apoptosis in THP-1 cells
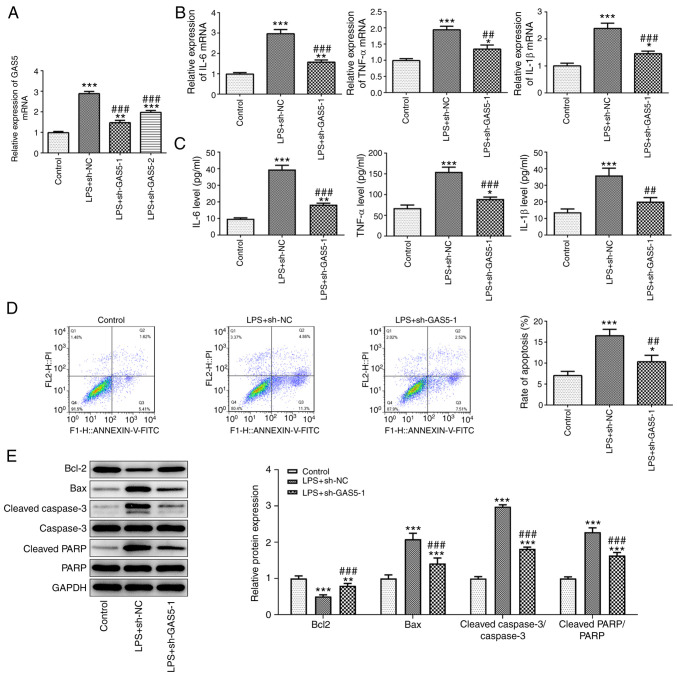
Next, two shRNAs targeting GAS5 were transfected into THP-1 cells to knockdown the expression of GAS5. As shown in Fig. 2A, the expression of GAS5 in the cells transfected with sh-GAS5-1 was the lowest, which indicated that sh-GAS5-1 had the better knockdown efficiency; thus sh-GAS5-1 was selected for use in the following experiments. The effect of GAS5 knockdown on the biological functions of THP-1 cells treated with LPS was then detected. As shown in Fig. 2B and C, GAS5 knockdown significantly reduced the release of the inflammatory cytokines IL-6, TNF-α and IL-1β from THP-1 cells stimulated with LPS. The results of a flow cytometry assay showed that the increase in cell apoptosis induced by LPS treatment was attenuated when GAS5 was knocked down (Fig. 2D). The western blotting results confirmed the increase in apoptosis in the sh-GAS5-1-transfected cells (Fig. 2E). Compared with the LPS plus sh-NC group, Bcl-2 was downregulated and Bax, caspase3 and PARP were upregulated in the LPS plus sh-GAS5-1 group.
Figure 2.
GAS5 knockdown alleviates LPS-induced inflammation and apoptosis in THP-1 cells. (A) The knockdown efficiency of GAS5 expression was detected by RT-qPCR. The effect of GAS5 knockdown on the expression of inflammatory factors IL-6, TNF-α and IL-1β was analyzed by (B) RT-qPCR and (C) ELISA in LPS-induced THP-1 cells. The effect of GAS5 knockdown on apoptosis was determined by (D) flow cytometry and (E) the western blotting of apoptosis-associated proteins in LPS-induced THP-1 cells. *P<0.05, **P<0.01 and ***P<0.001 vs. control; ##P<0.01 and ###P<0.001 vs. LPS+sh-NC. GAS5, growth arrest-specific 5; LPS, lipopolysaccharide; RT-qPCR, reverse transcription-quantitative PCR; sh-, short hairpin; NC, negative control; PARP, poly (ADP ribose) polymerase.
miR-23a-3p is a direct target gene of GAS5
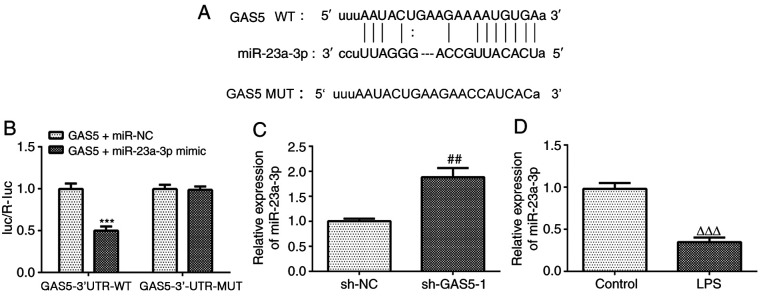
miR-23a-3p was predicted as the target miRNA of GAS5 using the StarBase database. The predicted binding sequence is shown in Fig. 3A. The results of the luciferase reporter assay demonstrated that the miR-23a-3p mimic significantly reduced the luciferase activity of the reporter vector containing GAS5-3′UTR-WT, but had no effect on the luciferase activity of the reporter vector containing GAS5-3′UTR-MUT (Fig. 3B). In addition, GAS5 knockdown significantly increased the expression of miR-23a-3p (Fig. 3C). Furthermore, the expression of miR-23a-3p was downregulated in THP-1 cells treated with LPS (Fig. 3D). These results indicate that GAS5 can specifically regulate the expression of miR-23a-3p and that there is a negative association between GAS5 and miR-23a-3p.
Figure 3.
miR-23a-3p is a direct target gene of GAS5. (A) The StarBase database predicted a targeting relationship between GAS5 and miR-23a-3p. (B) The relationship between GAS5 and miR-23a-3p was analyzed using a luciferase reporter assay. (C) The effect of GAS5 knockdown on the expression of miR-23a-3p was determined by RT-qPCR in LPS-induced THP-1 cells. (D) The effect of LPS on the expression of miR-23a-3p in THP-1 cells was detected by RT-qPCR. ***P<0.001 vs. GAS5+miR-NC; ##P<0.01 vs. sh-NC; ΔΔΔP<0.001 vs. untreated control. miR, microRNA; GAS5, growth arrest-specific 5; RT-qPCR, reverse transcription-quantitative PCR; LPS, lipopolysaccharide; NC, negative control; WT, wild type; MUT, mutant; luc/R-luc, firefly luciferase/Renilla luciferase.
TLR4 is a direct target gene of miR-23a-3p
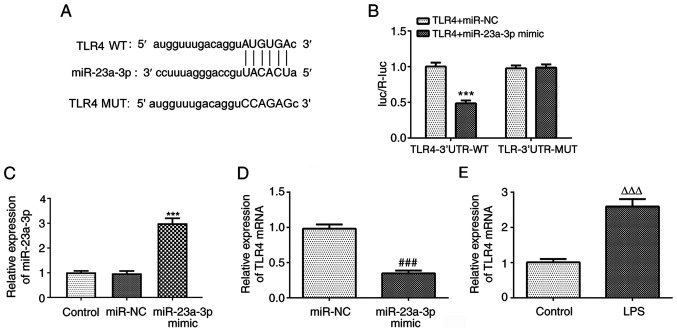
To investigate the downstream target genes of miR-23a-3p, the StarBase database was used, which predicted that TLR4 is a potential target gene of miR-23a-3p. The predicted binding sequence is shown in Fig. 4A. The results of the luciferase reporter assay showed that the miR-23a-3p mimic significantly reduced the luciferase activity of the reporter vector containing TLR4-3′UTR-WT, but had no effect on the luciferase activity of the reporter vector containing TLR4-3′UTR-MUT (Fig. 4B). The expression of miR-23a-3p was successfully increased following transfection with miR-23a-3p mimic (Fig. 4C). Furthermore, miR-23a-3p overexpression significantly reduced the expression of TLR4 (Fig. 4D). In addition, the expression of TLR4 was upregulated in THP-1 cells treated with LPS (Fig. 4E). These results indicate that miR-23a-3p can specifically regulate the expression of TLR4 and that there is a negative association between miR-23a-3p and TLR4.
Figure 4.
TLR4 is a direct target gene of miR-23a-3p. (A) The StarBase database predicted a targeting relationship between TLR4 and miR-23a-3p. (B) The relationship between TLR4 and miR-23a-3p was analyzed using a luciferase reporter assay. ***P<0.001 vs. TLR4+miR-NC. (C) The transfection efficiency of miR-23a-3p mimic was detected by RT-qPCR. ***P<0.001 vs. miR-NC. (D) The effect of miR-23a-3p overexpression on the expression of TLR4 was determined by RT-qPCR in LPS-induced THP-1 cells. (E) The effect of LPS on the expression of TLR4 in THP-1 cells was detected by RT-qPCR. ###P<0.01 vs. miR-NC; ΔΔΔP<0.001 vs. control. TLR4, Toll-like receptor 4; miR, microRNA; RT-qPCR, reverse transcription-quantitative PCR; LPS, lipopolysaccharide; NC, negative control; WT, wild type; MUT, mutant; luc,/R-luc, firefly luciferase/Renilla luciferase.
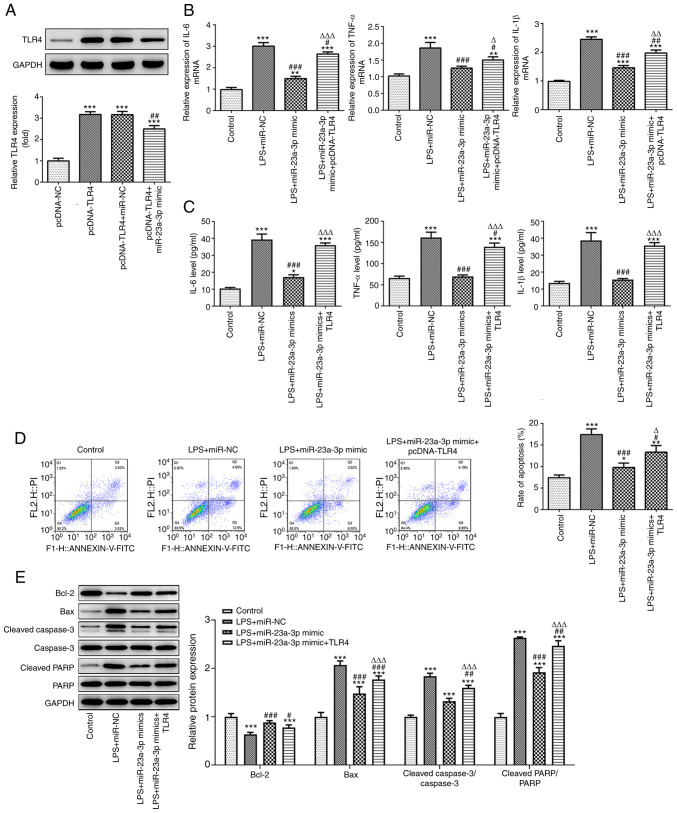
miR-23a-3p affects LPS-induced inflammation and apoptosis by targeting TLR4
The effects of miR-23a-3p and TLR4 on the biological functions of THP-1 cells treated with LPS were studied. Firstly, a TLR4 overexpression plasmid was successfully transfected into THP-1 cells, and the TLR4 overexpression efficiency was verified by western blotting. The results also showed that miR-23a-3p overexpression significantly attenuated the increase in expression of TLR4 induced by the TLR4 plasmid (Fig. 5A). Furthermore, RT-qPCR and ELISA results revealed that the overexpression of miR-23a-3p significantly attenuated the LPS-induced expression of the inflammatory cytokines IL-6, TNF-α and IL-1β in THP-1 cells, and the overexpression of TLR4 reduced the inhibitory effect of miR-23a-3p overexpression on the release of inflammatory factors (Fig. 5B and C). In addition, miR-23a-3p overexpression significantly suppressed the LPS-induced apoptosis of THP-1 cells, and the overexpression of TLR4 alleviated the inhibitory effect of miR-23a-3p overexpression on apoptosis (Fig. 5D and E).
Figure 5.
miR-23a-3p affects LPS-induced inflammatory and apoptosis by targeting TLR4. (A) The efficiency of the TLR4 expression plasmid and the effect of miR-23a-3p overexpression on TLR4 expression were detected by western blotting. ***P<0.001 vs. pcDNA-NC; ##P<0.01 vs. pcDNA-TLR4+miR-NC. The inhibitory effect of miR-23a-3p overexpression on the inflammatory factors IL-6, TNF-α and IL-1β was mediated via the targeting of TLR4, as revealed by (B) RT-qPCR and (C) ELISA in LPS-induced THP-1 cells. The inhibitory effect of miR-23a-3p overexpression on apoptosis was mediated via the targeting of TLR4, as demonstrated by (D) flow cytometry and (E) the western blotting of apoptosis-associated proteins in LPS-induced THP-1 cells. *P<0.05, **P<0.01 and ***P<0.001 vs. control; #P<0.05, ##P<0.01 and #P<0.05 vs. LPS+miR-NC; ΔP<0.05, ΔΔP<0.01 and ΔΔΔP<0.001 vs. LPS+miR-23a-3p mimic. miR, microRNA; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; RT-qPCR, reverse transcription-quantitative PCR; NC, negative control; PARP, poly (ADP ribose) polymerase.
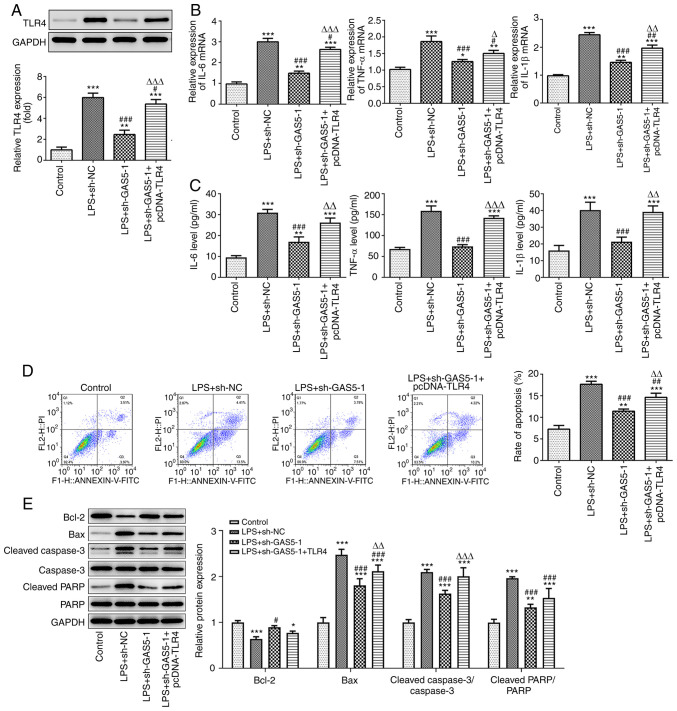
GAS5 affects LPS-induced inflammation and apoptosis by regulating the miR-23a-3p/TLR4 pathway
To investigate the effect of GAS5 and TLR4 on the biological function of THP-1 cells treated with LPS, cells transfected with sh-GAS5-1 and TLR4 overexpression plasmid were used. Western blotting analysis showed that GAS5 knockdown significantly suppressed the expression of TLR4 in LPS-induced THP-1 cells (Fig. 6A). In addition, GAS5 knockdown significantly inhibited the expression of the inflammatory cytokines IL-6, TNF-α and IL-1β in LPS-treated THP-1 cells, and the overexpression of TLR4 attenuated the inhibitory effect of GAS5 knockdown on the release of inflammatory factors (Fig. 6B and C). Furthermore, flow cytometry and western blotting results demonstrated that TLR4 overexpression reduced the inhibitory effect of GAS5 knockdown on apoptosis (Fig. 6D and E). These results demonstrate that the overexpression of TLR4 can reverse the inhibitory effects of GAS5 knockdown and miR-23a-3p overexpression on the inflammatory response and apoptosis of THP-1 cells. In particular, they suggest that GAS5 may affect LPS-induced inflammation and apoptosis by regulating the miR-23a-3p/TLR4 pathway.
Figure 6.
GAS5 affects LPS-induced inflammation and apoptosis by regulating the miR-23a-3p/TLR4 pathway. (A) The effect of GAS5 knockdown on TLR4 expression was detected by western blotting. The inhibitory effect of GAS5 knockdown on the inflammatory factors IL-6, TNF-α and IL-1β was mediated via the regulation of TLR4, as revealed by (B) RT-qPCR and (C) ELISA in LPS-induced THP-1 cells. The inhibitory effect of GAS5 knockdown on apoptosis was mediated via the regulation of TLR4, as demonstrated by (D) flow cytometry and (E) the western blotting of apoptosis-associated proteins in LPS-induced THP-1 cells. *P<0.05, **P<0.01 and ***P<0.001 vs. control; #P<0.05, ##P<0.01 and ###P<0.001 vs. LPS+sh-NC; ΔP<0.05, ΔΔP<0.01 and ΔΔΔP<0.001 vs. LPS + sh-GAS5-1. GAS5, growth arrest-specific 5; LPS, lipopolysaccharide; miR, microRNA; TLR4, Toll-like receptor 4; RT-qPCR, reverse transcription-quantitative PCR; NC, negative control; PARP, poly (ADP ribose) polymerase.
Discussion
An LPS-induced THP-1 cell sepsis model was used in the present study to investigate the expression and mechanism of lncRNA GAS5. The results revealed that the expression of GAS5 was significantly increased in THP-1 cells induced by LPS and was accompanied by the downregulation of miR-23a-3p and upregulation of TLR4, as well as increases in the release of pro-inflammatory factors and the number of apoptotic cells. Further experiments showed that both GAS5 and TLR4 target miR-23a-3p. However, the overexpression of TLR4 was demonstrated to attenuate the protective effects of GAS5 knockdown and miR-23a-3p overexpression on inflammatory injury. These results suggest that the damaging effect of GAS5 on LPS-induced THP-1 cells is mediated via the negative regulation of miR-23a-3p, which subsequently increases the expression of TLR4.
The lncRNA GAS5 was first discovered by Schneider et al (20) in NIH3T3 mouse fibroblasts, who found that serum-starved cells showed growth arrest accompanied by an increase in the expression of GAS5. Further research has shown that GAS5 is aberrantly expressed in a variety of tumors and acts as a tumor suppressor (21,22). However, GAS5 is seldom studied in fields outside oncology. Notably, a previous study showed that the knockdown GAS5 reduced the inflammatory injury of cardiomyocytes induced by high glucose (23). This anti-inflammatory property suggests that an investigation into the potential inhibitory effects of GAS5 knockdown on the inflammatory injury in sepsis is merited. In the present study, the role of GAS5 in sepsis was evaluated for the first time, to the best of our knowledge. The results showed that the expression levels of GAS5 and inflammatory factors were significantly increased in THP-1 cells following treatment with LPS, and that GAS5 knockdown suppressed the inflammatory reaction and apoptosis in the LPS-induced THP-1 cells. This suggests that GAS5 may be involved in the occurrence and development of the inflammatory response caused by sepsis.
TLR4 is expressed on the surfaces of numerous kinds of cells, including neutrophils and macrophages, and participates in the regulation of various physiological functions of cells. Previous studies have shown that the upregulation of TLR4 promotes an inflammatory response and inhibits cell growth (24,25). In another study, miR-23a-3p was reported to inhibit monocyte function and phagocytosis by targeting TLR4/TNF-α/TGF-β1/IL-10 signaling in patients with active tuberculosis with a high bacterial load via interferon regulatory factor 1/transcription factor SP1 (26). In addition, the downregulation of miR-21-5p in patients with obstructive sleep apnea has been shown to regulate intermittent hypoxia and reoxygenation-induced apoptosis and cytotoxicity by targeting pro-inflammatory TNF-α/TLR4 signaling (27). In the present study, TLR4 was identified as a gene that is directly targeted by miR-23a-3p, and a clear negative association between miR-23a-3p and TLR4 expression was observed. The overexpression of miR-23a-3p significantly reduced the expression of the inflammatory factors IL-6, TNF-α and IL-1β, as well as apoptosis, and the overexpression TLR4 was able to reverse this change. This suggests that miR-23a-3p and TLR4 act antagonistically to regulate the inflammatory response as follows: miR-23a-3p inhibits the inflammatory response induced by sepsis, while TLR4 promotes the inflammatory response.
A number of studies have shown that lncRNA and miRNA can regulate each other and participate in the occurrence and development of a variety of diseases by forming a complex molecular regulatory network (28,29). The results of the present study demonstrate that GAS5 targets miR-23a-3p and negatively regulate its expression, and that miR-23a-3p and TLR4 antagonistically regulate the inflammatory response induced by sepsis. Experiments in which the GAS5 knockdown plasmid and TLR4 overexpression plasmid were cotransfected into THP-1 cells demonstrated that TLR4 overexpression attenuated the reductions in inflammatory cytokine levels and apoptosis caused by GAS5 knockdown. Together, these results indicate that GAS5 promotes sepsis-induced inflammation and apoptosis by negatively regulating miR-23a-3p and thereby increasing the expression of TLR4.
In summary, the present study demonstrated that GAS5 is upregulated in LPS-treated THP-1 cells and appears to promote the inflammation and apoptosis of LPS-induced THP-1 cells by modulating the miR-23a-3p/TLR4 axis. Moreover, the GAS5/miR-23a-3p/TLR4 axis may become a new potential therapeutic target in the treatment of sepsis. However, there are some limitations to the present study. Firstly, only in vitro experiments were conducted and no in vivo experiments were performed for validation. In future research, animal models and clinical samples may be used to confirm the findings of the present study. Secondly, the mechanisms underlying the roles of lncRNA GAS5 and miR-23a-3p in the progression of sepsis have not been fully investigated. Therefore, these issues require further in-depth study in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
DH designed the study and was mainly involved in the bioinformatics and data analysis. ZG mainly conducted most of the experiments. Both authors read and approved the final manuscript. ZG and DH confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wu H, Liu J, Li W, Liu G, Li Z. LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of LPS-induced sepsis mice by activating NF-κB pathway. Biochem Biophys Res Commun. 2016;471:240–246. doi: 10.1016/j.bbrc.2016.01.117. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cawcutt KA, Peters SG. Severe sepsis and septic shock: Clinical overview and update on management. Mayo Clin Proc. 2014;89:1572–1578. doi: 10.1016/j.mayocp.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription. 2014;5:e944014. doi: 10.4161/21541272.2014.944014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Qiu J, Chen B, Lin Y, Chen Y, Xie G, Qiu J, Tong H, Jiang D. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-κB pathway. Int Immunopharmacol. 2018;59:252–260. doi: 10.1016/j.intimp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Jia Y, Li Z, Cai W, Xiao D, Han S, Han F, Bai X, Wang K, Liu Y, Li X, et al. SIRT1 regulates inflammation response of macrophages in sepsis mediated by long noncoding RNA. Biochim Biophys Acta Mol Basis Dis. 2018;1864:784–792. doi: 10.1016/j.bbadis.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–1082. doi: 10.7314/APJCP.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 11.Yao T, Lu R, Zhang J, Fang X, Fan L, Huang C, Lin R, Lin Z. Growth arrest-specific 5 attenuates cisplatin-induced apoptosis in cervical cancer by regulating STAT3 signaling via miR-21. J Cell Physiol. 2019;234:9605–9615. doi: 10.1002/jcp.27647. [DOI] [PubMed] [Google Scholar]
- 12.Ghaforui-Fard S, Taheri M. Growth arrest specific transcript 5 in tumorigenesis process: An update on the expression pattern and genomic variants. Biomed Pharmacother. 2019;112:108723. doi: 10.1016/j.biopha.2019.108723. [DOI] [PubMed] [Google Scholar]
- 13.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge QM, Huang CM, Zhu XY, Bian F, Pan SM. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS One. 2017;12:e0173292. doi: 10.1371/journal.pone.0173292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, Wang S, Zhao Y, Du F, Wang W, Lv P, Qi L. Long noncoding RNA NEAT1 modulates cell proliferation and apoptosis by regulating miR-23a-3p/SMC1A in acute myeloid leukemia. J Cell Physiol. 2019;234:6161–6172. doi: 10.1002/jcp.27393. [DOI] [PubMed] [Google Scholar]
- 16.Su Z, Zhi X, Zhang Q, Yang L, Xu H, Xu Z. LncRNA H19 functions as a competing endogenous RNA to regulate AQP3 expression by sponging miR-874 in the intestinal barrier. FEBS Lett. 2016;590:1354–1364. doi: 10.1002/1873-3468.12171. [DOI] [PubMed] [Google Scholar]
- 17.Wang JY, Yang Y, Ma Y, Wang F, Xue A, Zhu J, Yang H, Chen Q, Chen M, Ye L, et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother. 2020;121:109627. doi: 10.1016/j.biopha.2019.109627. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Yujiao W, Fang W, Linhui Y, Ziqi G, Zhichen W, Zirui W, Shengwang W. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res. 2020;53:40. doi: 10.1186/s40659-020-00309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/S0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 21.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 2013;1832:1613–1623. doi: 10.1016/j.bbadis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Liu B, Li C. Knockdown of long noncoding RNA GAS5 protects human cardiomyocyte-like AC16 cells against high glucose-induced inflammation by inhibiting miR-21-5p-mediated TLR4/NF-κB signaling. Naunyn Schmiedebergs Arch Pharmacol. 2019;393:1541–1547. doi: 10.1007/s00210-019-01795-z. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Guo X, Yang J, Ding JW, Li S, Yang R, Fan ZX, Yang CJ. RP105 protects against apoptosis in ischemia/reperfusion-induced myocardial damage in rats by suppressing TLR4-mediated signaling pathways. Cell Physiol Biochem. 2015;36:2137–2148. doi: 10.1159/000430180. [DOI] [PubMed] [Google Scholar]
- 25.Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71:1989–1998. doi: 10.1158/0008-5472.CAN-10-2833. [DOI] [PubMed] [Google Scholar]
- 26.Chen YC, Lee CP, Hsiao CC, Hsu PY, Wang TY, Wu CC, Chao TY, Leung SY, Chang YP, Lin MC. MicroRNA-23a-3p down-regulation in active pulmonary tuberculosis patients with high bacterial burden inhibits mononuclear cell function and phagocytosis through TLR4/TNF-α/TGF-β1/IL-10 signaling via targeting IRF1/SP1. Int J Mol Sci. 2020;21:8587. doi: 10.3390/ijms21228587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YC, Hsu PY, Su MC, Chin CH, Liou CW, Wang TY, Lin YY, Lee CP, Lin MC, Hsiao CC. miR-21-5p under-expression in patients with obstructive sleep apnea modulates intermittent hypoxia with Re-oxygenation-induced-cell apoptosis and cytotoxicity by targeting pro-inflammatory TNF-α-TLR4 signaling. Int J Mol Sci. 2020;21:999. doi: 10.3390/ijms21030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: When ce-bling rivalries go awry. Cancer Discov. 2013;3:1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, Kong R, Xia R, Lu KH, Li JH, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.