Abstract
Toxin–antitoxin (TA) modules are small operons in bacteria and archaea that encode a metabolic inhibitor (toxin) and a matching regulatory protein (antitoxin). While their biochemical activities are often well defined, their biological functions remain unclear. In Type II TA modules, the most common class, both toxin and antitoxin are proteins, and the antitoxin inhibits the biochemical activity of the toxin via complex formation with the toxin. The different TA modules vary significantly regarding structure and biochemical activity. Both regulation of protein activity by the antitoxin and regulation of transcription can be highly complex and sometimes show striking parallels between otherwise unrelated TA modules. Interplay between the multiple levels of regulation in the broader context of the cell as a whole is most likely required for optimum fine‐tuning of these systems. Thus, TA modules can go through great lengths to prevent activation and to reverse accidental activation, in agreement with recent in vivo data. These complex mechanisms seem at odds with the lack of a clear biological function.
Keywords: bacterial stress response, conditional cooperativity, rejuvenation, toxin–antitoxin, transcription regulation
1. INTRODUCTION
Toxin–antitoxin (TA) modules are small genetic elements that are abundant on the chromosomes as well as on the mobile genetic elements of bacteria and archaea. TA modules have been classified into seven main types based on the nature and mode of action of the antitoxin. 1 , 2 Among these, Type II TA modules, where toxin and antitoxin are both proteins and where the antitoxin counteracts the toxin via direct interaction, are the most widespread and will be the focus of this review. Most often, the antitoxin gene precedes the toxin gene. The antitoxin typically contains a toxin neutralizing domain preceded by a DNA binding domain that regulates transcription of the entire operon. This we will refer to as the canonical organization. However, other genetic organizations involving three genes or where the order of the genes are swapped also exist. 3
TA systems were originally discovered as elements that stabilize low copy number plasmids via a mechanism of post‐segregational killing, 4 but it has been argued that TA modules may equally stabilize non‐essential parts of chromosomes such as cryptic prophages. 5 Another suggested function involves stress response where they slow down or halt cell growth via Lon‐mediated activation of toxins, potentially leading to persister cells. This mechanism was nevertheless recently disproven. 6 Still, a direct link was identified between alarmone signaling and the recently discovered ToxSAS‐AntiToxSAS family of TA modules. 7 A further often proposed function is protection against bacteriophages. This is supposed to involve altruistic killing of the host cell, 8 a mechanism that is nonetheless debated. 9 Finally, TA modules might be essentially selfish entities whose first function is self‐preservation. 10 The other biological effects could then just be side effects of this selfish behavior, explaining the perceived inconsistencies of their functions in the literature.
Regardless of their potential function, the biochemical activities of toxins are diverse and always interfere with basic cellular functions such as transcription, translation or cell wall synthesis, granting them a bactericidal or bacteriostatic character. The corresponding antitoxins are at least as diverse than the toxins and their folds and mechanism of action can differ a lot. Even between members of the same TA family, different DNA binding domains and transcription regulation mechanisms can be found (e.g., the DNA binding domains of VapB antitoxins can have an AbrB fold, HTH, Phd/YefM fold, or even RHH fold). In the last years, the function and regulation of TA modules has been a point of focus for many research groups. The regulation involves all possible levels including transcription, translation, protein activity, and degradation. Here, we will focus on transcription regulation and activation/deactivation of TA modules.
2. REGULATION OF TOXIN ACTIVITY BY THE ANTITOXIN
2.1. Direct inhibition by the antitoxin
A hallmark feature of all Type II TA modules is that the antitoxin neutralizes the toxin via direct interaction. The affinity between toxin and antitoxin is typically in the nM to pM range, but cases of even fM affinity are known. 11 Most typical is a toxin‐neutralizing domain that is intrinsically disordered (IDP) and folds upon binding the toxin (Figure 1a,b). This IDP domain then wraps around the toxin, covering a large surface including at least part of the site for interaction with its target, a situation found for ccdAB, 12 mazEF, 13 , 14 , 15 phd/doc, 16 vbhAT, 17 parDE, 18 ataTR, 19 , 20 , 21 vapBC, 22 relBE, 23 yoeB/yefM, 24 dinJ/yafQ, 25 and certain higBA modules. 26 While this is an efficient mechanism of inhibition, antitoxin binding often also induces conformational changes in the toxin that induce an inactive state. Examples are the expulsion of an α‐helix in Escherichia coli RelE that disrupts the active site, 23 or the sliding of a β‐strand in Vibrio cholerae HigB2 that has a similar effect. 26 Also in the MazF family, antitoxin binding results in the reorientation of a catalytic loop. The latter change is even conserved in the structurally very similar but functionally divergent CcdB family. 15
FIGURE 1.
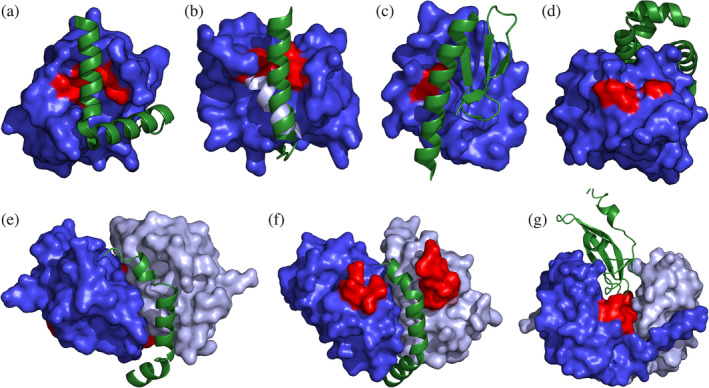
Direct inhibition of toxic activity by antitoxin binding. Toxins are shown in blue surface representation with the active site colored red. For dimeric toxins, two shades of blue are used. Antitoxins are shown as green cartoon representations. (a) E. coli yoeB/yefM (PDB entry 2A6Q). The toxin‐neutralizing intrinsically disordered (IDP)‐domain wraps around the toxin, thereby blocking its active site. No conformational change in the toxin is induced. (b) E. coli relBE (PDB entry 4FXE). Binding by the IDP region of the antitoxin induces a conformational change in the toxin: the C‐terminal α‐helix of RelE (light blue) becomes unfolded, which cripples the active site. (c) E. coli hicAB (PDB entry 6HPB). The antitoxin HicB is a fully folded protein that upon binding HicA covers its sole known catalytic residue, His23 (red). (d) P. vulgaris higB (PDB entry 4MCX). A. The fully folded antitoxin HigA is an allosteric inhibitor of toxin HigB. It binds on the opposite side of the catalytic site (red) and likely inhibits HigB by preventing it to dock on the ribosome. (e) F‐plasmid ccdAB (PDB entry 3HPW). Toxin CcdB forms a dimer that is inhibited by a single copy of the IDP region of CcdA. The latter wraps around the waste of the CcdB dimer close to the dimer interface. This interaction induces a 12° relative rotation of the CcdB monomers, which prevent CcdB to dock onto the GyrA14 fragment of GyrA. The GyrA14 binding region on CcdB (red) is located on the opposite side of the CcdB dimer relative to the binding groove for CcdA. (f) B. subtilis mazEF (PDB entry 4ME7). MazF and CcdB likely share a common ancestor and the MazF dimer strongly resembles the CcdB dimer. A single copy of the IDP region of the MazE antitoxin from Bacillus subtilis binds in a way similar to the binding of CcdA to CcdB. It prevents a crucial MazF loop (ref) to adopt a conformation that is competent for substrate binding and catalysis. (g) H. influenzae vapXD (PDB entry 6ZN8). VapD forms a V‐shaped dimer with its catalytic site located in a deep cleft near the dimer interface. The antitoxin VapX inserts into this cleft, covering the active site and breaking the symmetry of the complex
While the antitoxins of all of the earlier studied TA modules indeed bind their toxin via folding upon binding, more recently it has become clear that there are also several antitoxin families that are fully folded globular proteins. Notable examples are mqsRA, hicAB, hipAB, and two distinct classes of higBA modules. These typically interact with the toxin by covering the active site (Figure 1c). 27 , 28 , 29 , 30 , 31 In the case of Proteus vulgaris higBA and the closely related Pseudomonas putida graTA, the folded antitoxin does not cover the active site, but still inhibits its ribonuclease activity via an allosteric mechanism (Figure 1d). 32 , 33 Also HigB from E. coli and Shigella flexneri are inhibited in a similar way, although the corresponding antitoxin structurally diverges from those in the P. vulgaris and P. putida systems. 34 , 35 , 36
2.2. Stoichiometry of toxin neutralization
For monomeric toxins such as RelE, HigB, HicA, ParE, Doc, or VbhT, each toxin is fully inhibited when it binds one antitoxin monomer equivalent. For CcdB and MazF on the other hand, which form stable dimers, only one antitoxin equivalent needs to interact with a dimer to achieve full inhibition (Figure 1e,f). 12 , 14 , 15 In the case of CcdB, CcdA prevents gyrase binding via allosteric action combined with steric hindrance. In the case of MazF, the IDP domain of MazE prevents substrate binding simultaneously in both catalytic sites of the MazF dimer.
VapD toxins form a conserved dimer with their catalytic site located in a deep cleft near the dimer interface. 37 , 38 Haemophilus influenzae VapD is inhibited by VapX via the formation of a pseudo‐symmetric VapD2‐VapX heterotrimer (Figure 1g). 38 In this arrangement, the active sites of both VapD monomers interact with the single VapX. The ataRT and related kacAT modules equally require the formation of a dimer for activity. 39 , 40 Here the antitoxin AtaR prevents dimer formation of AtaT and a 1:1 binding stoichiometry is achieved. 20 , 41
Some TA pairs form higher order structures. This has been described multiple times for VapBC complexes, which are frequently hetero‐octamers (for a review see Bendtsen and Brodersen, 2017). 42 Higher order association of toxin–antitoxin complexes outside the context of transcription regulation has since then been reported for hicAB, vapXD, and parDE. 28 , 30 , 31 , 38 , 43 In the case of hicAB, these higher order complexes differ between different species with heterotetramers, heterohexamers as well as hetero‐octamers being observed. 28 , 30 , 31 It is tempting to speculate that such higher order structures carry some functionality, for example, further harnessing the activity of the toxin, as was recently suggested. 28 Nevertheless, in the case of the E. coli PaaA2‐ParE2 pair, disruption of the higher order assembly does not affect antitoxin activity in vivo. 43
2.3. Chemical modification of the toxin
For some toxins, there is a further layer of post‐translational control: they can be controlled via auto‐inhibition or auto‐activation. This was first discovered for E. coli HipA that is inactivated via autophosphorylation. 44 In contrast, Pseudomonas fluorescens Fic‐1 and Neisseria meningitidis NmFic require auto‐AMPylation as an activation step. AMPylation of Fic‐domain containing toxins prevents binding of an α‐helix in their active site. 45 , 46 Thus, self‐modification will result in either a reduced time window for activity or in lag time before the toxin becomes active.
A second example of inactivation via post‐translational modification is the poly‐adenylation found in the mntA‐hepT family. 47 , 48 In this case, binding of antitoxin MntA is insufficient to fully inhibit HepT toxicity and the antitoxin MntA additionally functions as an adenylyltransferase. The toxic activity of the HepT RNase is neutralized by MntA via modification of a tyrosine residue close to the toxin's active site.
2.4. Rejuvenation mechanisms
Restoration of normal growth often just requires production of fresh antitoxin and assumes that a basal amount of protein synthesis still continues in cells where a TA toxin is activated. 49 In many cases, the freshly produced antitoxin just needs to inhibit the corresponding toxin. In other cases, the “restart” mechanism is more complex. The ccdAB module presents such a case (Figure 2a). When Gyrase is poisoned by CcdB, the end product is a ternary Gyrase‐DNA‐CcdB complex that needs to be resolved before DNA and RNA synthesis and chromosome segregation can occur. The antitoxin CcdA needs to actively remove CcdB from the ternary CcdB‐Gyrase‐DNA complex in a process called rejuvenation, and which is dependent on the C‐terminal end of the IDP domain of CcdA. 12
FIGURE 2.
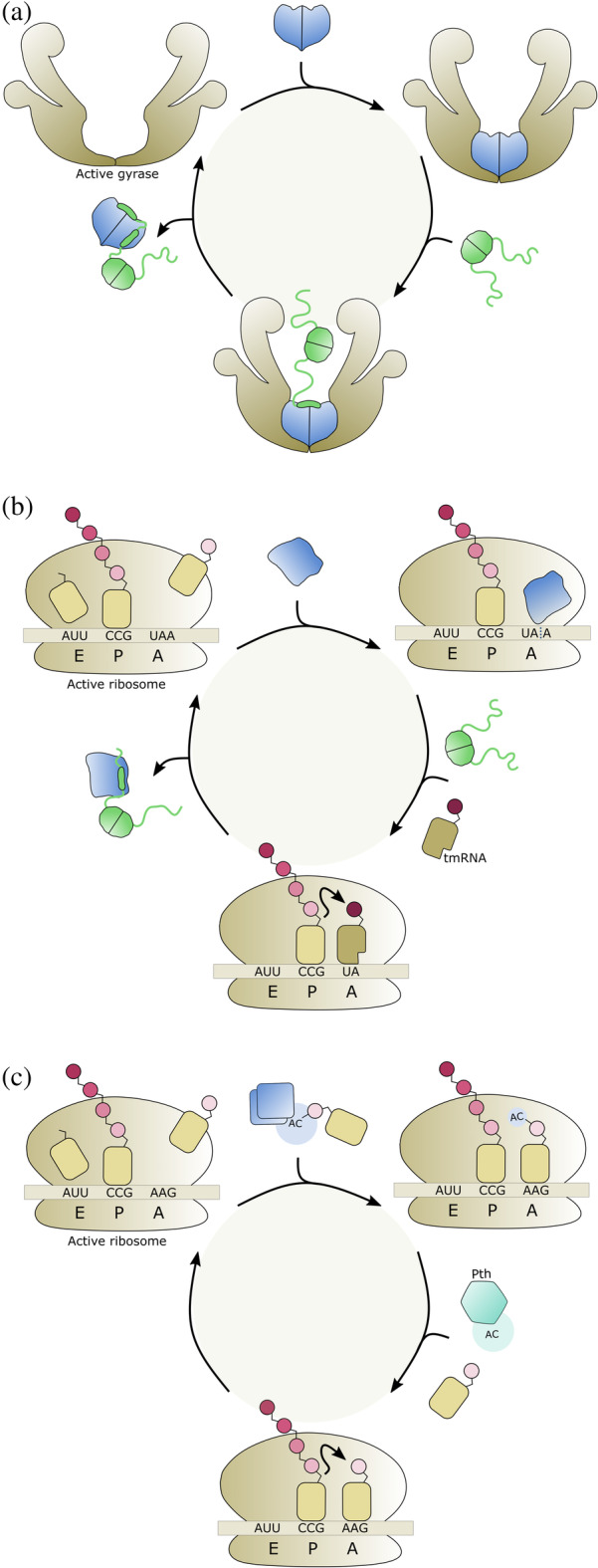
Rejuvenation mechanisms of TA modules. Toxins are colored blue and antitoxins are colored in green. Thinner lines represent intrinsically disordered (IDP) domains and thicker shapes represent folded regions. (a) ccdAB. Binding by CcdB (blue) poisons gyrase. Gyrase is liberated by binding of the antitoxin CcdA (green), the IDP domain of which folds upon binding to CcdB. (b) relBE. RelE (blue) cleaves mRNA at the A‐site of the ribosome. Binding by the antitoxin RelB (green) removes the toxin from the ribosome and renders it inactive, but the resulting ribosome remains stalled due to the bound damaged mRNA. Protein synthesis can be resumed by the action of tmRNA. (c) tacAT. Toxin TacT (blue) acetylates amino acids on charged tRNAs. This prevents these amino acids to be incorporated in the growing polypeptide chain thus stalling the ribosome. The tRNAs are recycled via deacetylation by Pth (cyan)
Sometimes, additional components need to be recruited. For example, RelE toxins stall ribosomes by cleaving mRNA in a codon‐dependent manner while it is being translated. Neutralization of RelE by RelB antitoxin will prevent further ribosomes to be affected but will not allow already stalled ribosomes to be rejuvenated. Therefore, tmRNA is required for rapid recovery of translation and resuscitation of RelE‐inhibited cells (Figure 2b). 50
A second TA module that requires an additional component to restart growth is Salmonella enterica tacAT (Figure 2c). TacT inhibits translation by acetylation of the primary amine group on charged tRNA molecules. 39 A reversal of toxic activity is instated by peptidyl‐tRNA hydrolase (Pth), which recycles the acetylated aa‐tRNAs. In this unusual Type II TA module, the neutralization activity of the antitoxin TacA itself is also modulated by the toxin via acetylation on a specific lysine residue. In its non‐acetylated from, TacA inhibits TacT. But TacT is nevertheless capable of also acetylating TacA next to its aa‐tRNA substrate, the first toxin shown to do so. TacA acetylation enhances TacT activity in vitro as well as in vivo and is reversed by the activity of NAD+‐dependent CobB sirtuin deacetylase. 51
3. REGULATION OF TRANSCRIPTION
3.1. Lack of autoregulation and regulation via external transcription factors
Many antitoxins repress transcription of the TA operon via their DNA‐binding domain. Nevertheless, a number of TA modules exist where the antitoxin does not contain a DNA binding domain but solely consists of a toxin‐neutralizing segment and for which no mechanism for transcription regulation is obvious (Figure 3a). Such highly basic TA modules include relBE homologues from the archaea Methanococcus jannaschii (MjRelBE) and Pyrococcus horikoshii (PhRelBE). 52 , 53 Here, in absence of other factors encoded outside the TA operon that may influence expression, regulation occurs only at the level of translation and degradation.
FIGURE 3.
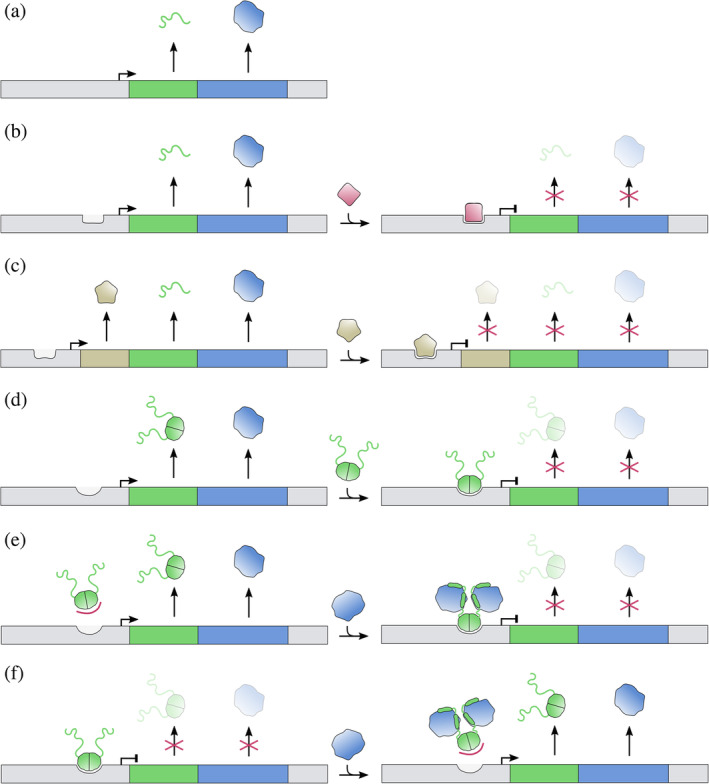
Overview of the simpler mechanisms of transcription regulation of TA modules. Toxins are colored blue and antitoxins green. Thin lines are used to indicate intrinsically disordered (IDP) regions and solid shapes represent folded domains. Activation of transcription is indicated by an arrow and repression by a vertical line at the promoter/operator site. (a) A TA module without (auto)regulation. The antitoxin is IDP and lacks a DNA binding domain. Toxin and antitoxin are translated at constant rates, with the gene order ensuring excess production of antitoxin. Such a situation is encountered for relBE modules in some Archaea. (b) The antitoxin again lacks a DNA binding domain and transcription regulation is performed by an external repressor (red). (c) In three‐component TA modules, the antitoxin lacks a DNA binding domain as well, but transcription regulation is performed by a repressing regulator (beige) of which the gene is located directly upstream of the antitoxin gene. (d) The antitoxin gene possesses a DNA binding domain and acts as a transcriptional repressor of the TA operon without aid of te toxin. (e) The antitoxin has a DNA binding domain but requires bound toxin to repress transcription. (f) The antitoxin acts as a repressor of transcription. Binding by the toxin prevents operator binding, thus alleviating repression and inducing transcription
For some such TA systems, an external regulator is recruited (Figure 3b). For example, the antitoxin SaMazE from the Staphylococcus aureus SamazEF module only consists of a toxin‐neutralizing IDP domain. The SamazEF module can both be transcribed as part of the sigB operon but also as a smaller transcript encoding only SaMazE and SaMazF. 54 Both are negatively regulated by SigB and positively regulated by SarA, making it the only known TA module entirely under control of transcription factors that respond to stress.
Other TA modules are so‐called three‐component systems where in addition to a toxin‐neutralizing antitoxin without DNA binding function, the operon contains a third gene that encodes a transcription regulator (Figure 3c). The best‐known examples hereof are the Streptococcus pyogenes plasmid‐encoded ω‐ε‐ζ zeta module 55 and three‐component versions of parDE modules found on the chromosome of E. coli O157:H7. 56 Only the case of ω‐ε‐ζ has transcription regulation been studied in some detail. The ω protein was shown to act as a simple repressor of the operon and its activity is not influenced by ε or ζ. 57 The situation for the PaaR2 regulator is not studied in detail but may be more complex as the paaA2 and parE2 genes are inserted within the immunity region of a cryptic λ‐like prophage.
3.2. Antitoxin as the sole repressor
In the majority of cases, the antitoxin contains a separate DNA‐binding domain next to its toxin neutralizing domain. The antitoxin then is involved in autoregulation of transcription of the operon. In a few cases the antitoxin is the only factor that influences transcription of the operon (Figure 3d). One such example is the E. coli higBA module, where operator binding by HigA does not seem to be influenced by the presence of HigB, as both HigA and the HigBA complex bind the operator with a similar nanomolar affinity. 58 This may be somewhat surprising given that HigB binding induces a large conformational change to HigA.
A more intricate situation is observed for the E. coli dinJ‐yafQ module. 25 Equally to E. coli HigBA, antitoxin DinJ represses the operon and this activity is not affected by YafQ. However, the module is also controlled externally by LexA, a transcription repressor that is activated through the SOS response system.
3.3. Toxin is required as a co‐repressor
In some cases, the affinity of the antitoxin alone is insufficient to form a stable complex with the operator, and the toxin acts as a co‐repressor (Figure 3e). This is a.o. observed for the vapBC modules. For both Neisseria gonorrhoeae fitAB and Salmonella enterica vapBC it was found that the toxin increases the affinity of the antitoxin for its operator 59 , 60 as is also observed in the first step of conditional cooperativity (see below). Some other otherwise unrelated TA modules may be regulated in the same way, for example, prlF‐yhaV. 61
3.4. Toxin diminishes DNA binding activity of antitoxin
Most antitoxins exhibit a combination of toxin neutralization and DNA repression activity, which typically results in the toxin influencing the DNA binding potential of the antitoxin. In its simplest case, the toxin reduces the affinity of the antitoxin for its operator (Figure 3f). This situation is typically observed for a number of TA modules where the antitoxin is a fully folded protein. Well‐studied examples are E. coli mqsRA, P. putida graTA, and E. coli hicAB. 31 , 33 , 62
The MqsA antitoxin contains two folded domains: a HTH‐XRE domain for operator binding and a Zn2+‐stabilized domain that neutralizes MqsR. 27 As the binding sites partially overlap, binding of DNA and MqsR to MqsA is mutually exclusive, resulting in de‐repression of the operon when MqsR titrates out MqsA. 62
Transcriptional repression of the graTA operon occurs via cooperative binding of two GraA antitoxin dimers at opposite sides of the operator DNA. 33 Binding of GraT to GraA abolishes DNA binding. Most likely, a short 22 amino acid N‐terminal IDP stretch of GraT acts as an entropic barrier that impedes the formation of a GraTA‐DNA complex. The toxin from the closely related higBA module from P. vulgaris plasmid Rts1 does not contain such an N‐terminal IDP region 32 and the HigBA complex binds only two‐fold weaker to the operator than HigA, 63 indicating that even closely related TA modules can differ significantly in their regulation.
Also for the Burkholderia pseudomallei hicAB module such a mechanism seems to be in place. 31 HicA‐HicB complexes with 1:2 stoichiometries show strong operator binding that is not significantly different from what is observed for the antitoxin on its own. However, at a 1:1 ratio, this affinity drops 200‐fold. For Streptococcus pneumoniae hicAB as well, the complex with 1:2 stoichiometry binds with similar affinity as the antitoxin on its own. 29 Unfortunately, no data are available here for a complex of 1:1 stoichiometry.
3.5. Conditional cooperativity
For antitoxins that neutralize their corresponding toxin via folding upon binding of an IDP region, the autoregulation tends to be more complex. The antitoxin is often not an effective repressor because of its low affinity for the DNA. This weak affinity can be owing to an inefficient DNA binding motif of the antitoxin such as in F‐plasmid CcdA, 64 or due to an entropic exclusion effect of its IDP C‐terminus as seen in Phd from bacteriophage P1. 65 In presence of the toxin, effective repression is observed but is dependent on the molar ratio between toxin and antitoxin. Typically, at a toxin: antitoxin ratio around 1:1, DNA binding is most tight. At larger ratios de‐repression is observed. This mechanism has been termed “conditional cooperativity.” 66 In each case, the switch is caused by the formation of a toxin–antitoxin complex of different stoichiometry than the one that binds to the operator. This complex fails to bind at adjacent operator sites due to steric exclusion.
Conditional cooperativity involves two or more evenly spaced operator sites. The antitoxin dimers are bridged by toxins, leading to an increased affinity due to avidity. This is the case for F‐plasmid ccdAB (Figure 4a) and bacteriophage P1 phd/doc (Figure 4b). 12 , 16 , 64 The toxin dimer or monomer has two antitoxin binding sites that differ several orders of magnitude in affinity. Excess toxin then allows derepression by switching from the low to the high affinity interaction. In this situation, an additional toxin is added that disrupts the alternating antitoxin–toxin–antitoxin chain. This prevents binding of the resulting toxin–antitoxin complex to adjacent operator sites. 16 , 64 In the case of phd/doc, an additional mechanism of entropic exclusion prevents high affinity binding of isolated Phd in absence of Doc. 65 In the case of ccdAB, the affinity of the antitoxin for its operator is inherently very weak in absence of toxin. 64
FIGURE 4.
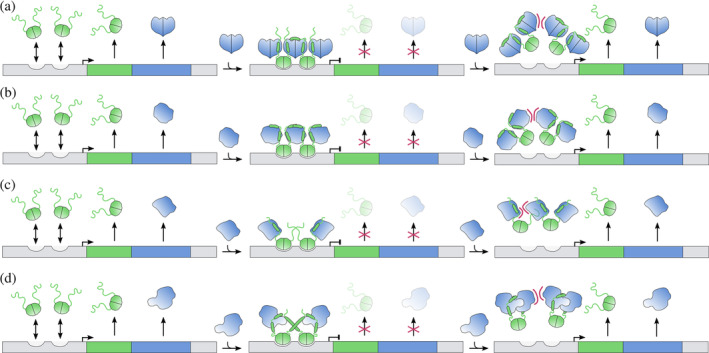
Conditional cooperativity‐based transcription regulation of TA modules. Color coding and symbols are as in Figure 3. (a) ccdAB. The affinity of the antitoxin for its operator is low and does not lead to repression. At T:A ratios around 1, binding is increased because of an avidity effect and transcription is repressed. At higher ratios, a low to high affinity switch occurs which relieves repression due to steric hindrance. (b) phd/doc. Entropic exclusion prevents simultaneous binding of two Phd dimers to the operator. The presence of a bridging Doc toxin increases allows simultaneous binding of two Phd dimers and transcription is repressed. Titration of additional toxin results in TA complexes of different stoichiometry. This introduces steric hindrance and abolishes DNA binding thus activating transcription. (c) relBE. Conditional cooperativity is not mediated by a low to high affinity switch. Two RelB dimers bind cooperatively to adjacent sites and this complex is further stabilized by two RelE monomers that flank the RelB dimer of dimers. Titration of additional RelE results in steric hindrance that abolishes DNA binding and activates transcription. (d) ataRT. AtaR has a weak affinity for its operator DNA. The binding between the AtaT toxin and the AtaR antitoxin positions the DNA‐binding domain of AtaR in a favorable orientation that binds the operator with high affinity. Activation of transcription is achieved by higher toxin to antitoxin ratios, resulting in a TA complex with lower affinity for the operator DNA
Alternatively, antitoxins bind cooperatively on adjacent sites, with the toxin blocking the sides of the operator complex (Figure 4c). The bound toxin may even increase the affinity of the toxin for its operator somewhat by stabilizing its folded, DNA‐binding competent state. Such a situation is observed for E. coli RelBE that binds to its operator as a 2:1 complex. 67 Titration of additional RelE toxin breaks the contacts between the adjacent RelB dimers and sterically prevents two RelB dimers to bind simultaneously to the operator. More recently, the E. coli yoeB/yefm module was shown to follow exactly the same mechanism. 68 YoeB is a RNase toxin that belongs to the RelE superfamily, but the corresponding antitoxin YefM has a structure similar to Phd.
Related but somewhat more complex behavior is seen for the recently discovered GNAT‐toxin–antitoxin modules such as E. coli ataRT and Klebsiella pneumoniae kacAT (Figure 4d). Like for E. coli relBE, the repressing TA complex consists of two contacting antitoxin dimers flanked on each side by a toxin monomer. 19 , 20 , 21 The AtaR‐AtaT complex is kinetically stable and binds the operator with high affinity, resulting in repression of the ataRT operon. This requires that the repressing AtaR‐AtaT complex is formed in vivo before AtaT has a chance to form homodimers, which is its active state. Further titration of the repressing AtaR‐AtaT complex with AtaT leads to dissociation from the operator and the formation of different complex that harbours a catalytically competent AtaT dimer that nevertheless remains inhibited by AtaR. 19
3.6. Double promoter mechanisms for TA modules with inverse gene order
In its most typical incarnation, TA modules encode the antitoxin upstream of the toxin. As a consequence of translation of the corresponding bicistronic transcript, an excess of antitoxin is produced, which is essential to control the activity of the toxin. For TA modules with an inverse gene order, where one would expect that more toxin than antitoxin is produced, this poses a problem as how the cell can cope with this.
For E. coli hicAB, a mechanism is discovered that allows production of excess of antitoxin. E. coli hicAB is transcribed from two promotors (Figure 5). 69 The upstream promoter allows for expression of both toxin and antitoxin genes. It contains a CRP‐S motif and is activated by Sxy. The downstream promoter on the other hand produces an mRNA that only allows for expression of the antitoxin HicB. This promotor is repressed by HicB, and this repression is relieved upon excess of HicA. This mechanism allows for the specific production of antitoxin when the toxin to antitoxin ratio becomes too high and thus prevents activation of the system under normal growth conditions.
FIGURE 5.
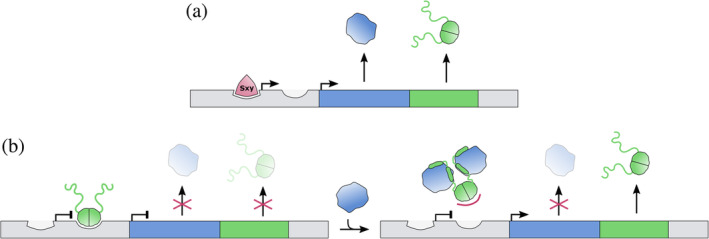
Transcription regulation of the E. coli hicAB module. The inverse genetic organization of this module requires a dual mode of regulation via two promoter/operator sites. (a) An upstream promoter is activated by Sxy (red) and induces transcription of both toxin and antitoxin. (b) A secondary promoter is recognized by the antitoxin HicB, which acts as a repressor of the hicAB operon. Binding of the toxin HicA to HicB abolishes DNA binding and activates transcription from this secondary promoter. The resulting transcript solely allows the production of HicB antitoxin
More recently, an identical regulation mechanism was proposed for H. influenzae toxTA based on RNA‐seq experiments, 70 indicating that this or similar mechanisms might be common in TA modules with inverse gene order. Also for mqsRA, two additional promoters were identified that are located in the toxin sequence and drive the constitutive expression of the antitoxin MqsA. 71 Again, this allows for excess production of the MqsA antitoxin compared to the MqsR toxin. Taken together, these data suggest a common mechanism of regulation for TA modules with an inverse gene regulation.
4. PROTEOLYTIC DEGRADATION OF ANTITOXINS
Already 25 years ago, it was established that Lon‐dependent degradation of F‐plasmid CcdA accounts for the shorter lifetime of CcdA relative to CcdB, a key given to explain post‐segregational killing. 72 This, together with the identification of antitoxins from various other TA families as Lon (and sometimes ClpXP) substrates, led to the widespread notion that during episodes of stress TA toxins are activated by Lon‐dependent proteolysis of antitoxins.
Recently, this notion has been challenged. 6 It was shown that stress‐induced transcription of TA operons indeed follows from antitoxin degradation, showing the relevance of the mechanisms for transcription regulation discussed above. But at the same time, it does not lead to activation of the toxins. These findings are supported by earlier observations that the interaction between toxin and antitoxin is often very tight, making it difficult for a protease such as Lon to differentially degrade the toxin‐bound segment of the antitoxin. This is best illustrated in vivo as well as in vitro for the S. pneumoniae pezAT module where the TA complex is unusually stable. 11
AUTHOR CONTRIBUTIONS
Pieter De Bruyn: Conceptualization; visualization; writing‐original draft; writing‐review & editing. Yana Girardin: Conceptualization; visualization; writing‐original draft; writing‐review & editing. Remy Loris: Conceptualization; funding acquisition; project administration; supervision; visualization; writing‐original draft; writing‐review & editing.
ACKNOWLEDGMENTS
Remy Loris received financial support from FWO‐Vlaanderen (grants No. G.0226.17N and G.0033.20N) and the Onderzoeksraad of the Vrije Universiteit Brussel (grant No SPR13). Yana Girardin was supported via a personal PhD grant from FWO‐Vlaanderen (FWOTM961).
De Bruyn P, Girardin Y, Loris R. Prokaryote toxin–antitoxin modules: Complex regulation of an unclear function. Protein Science. 2021;30:1103–1113. 10.1002/pro.4071
Funding information Fonds Wetenschappelijk Onderzoek, Grant/Award Numbers: 1164620N, G.0033.20N, G.0226.17N, FWOTM961; Vrije Universiteit Brussel, Grant/Award Number: SPR13
REFERENCES
- 1. Page R, Peti W. Toxin‐antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol. 2016;12:208–214. [DOI] [PubMed] [Google Scholar]
- 2. Marimon O, Teixeira JMC, Cordeiro TN, et al. An oxygen‐sensitive toxin‐antitoxin system. Nat Commun. 2016;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loris R, Garcia‐Pino A. Disorder‐ and dynamics‐based regulatory mechanisms in toxin‐antitoxin modules. Chem Rev. 2014;114:6933–6947. [DOI] [PubMed] [Google Scholar]
- 4. Gerdes K, Rasmussen PB, Molin S. Unique type of plasmid maintenance function: Postsegregational killing of plasmid‐free cells. Proc Natl Acad Sci USA. 1986;83:3116–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yao J, Guo Y, Wang P, et al. Type II toxin/antitoxin system ParESO/CopASO stabilizes prophage CP4So in Shewanella oneidensis . Environ Microbiol. 2018;20:1224–1239. [DOI] [PubMed] [Google Scholar]
- 6. LeRoux M, Culviner PH, Liu YJ, Littlehale ML, Laub MT. Stress can induce transcription of toxin‐antitoxin systems without activating toxin. Mol Cell. 2020;79:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jimmy S, Kumar Saha C, Kurata T, et al. A widespread toxin‐antitoxin system exploiting growth control via alarmone signaling. Proc Natl Acad Sci USA. 2020;117:10500–10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hazan R, Engelberg‐Kulka H. Escherichia coli mazEF‐mediated cell death as a defense mechanism that inhibits the spread of phage P1. Mol Genet Genomics. 2004;272:227–234. [DOI] [PubMed] [Google Scholar]
- 9. Song S, Wood TK. Post‐segregational killing and phage inhibition are not mediated by cell death through toxin/antitoxin systems. Front Microbiol. 2018;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magnuson RD. Hypothetical functions of toxin‐antitoxin systems. J Bacteriol. 2007;189:6089–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mutschler H, Reinstein J, Meinhart A. Assembly dynamics and stability of the pneumococcal epsilon zeta antitoxin toxin (PezAT) system from Streptococcus pneumoniae . J Biol Chem. 2010;285:21797–21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Jonge N, Garcia‐Pino A, Buts L, et al. Rejuvenation of CcdB‐poisoned gyrase by an intrinsically disordered protein domain. Mol Cell. 2009;35:154–163. [DOI] [PubMed] [Google Scholar]
- 13. Kamada K, Hanaoka F, Burley SK. Crystal structure of the MazE/MazF complex: molecular bases of antidote‐toxin recognition. Mol Cell. 2003;11:875–884. [DOI] [PubMed] [Google Scholar]
- 14. Simanshu DK, Yamaguchi Y, Park JH, Inouye M, Patel DJ. Structural basis of mRNA recognition and cleavage by toxin MazF and its regulation by antitoxin MazE in Bacillus subtilis . Mol Cell. 2013;52:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zorzini V, Mernik A, Lah J, et al. Substrate recognition and activity regulation of the Escherichia coli mRNA endonuclease MazF. J Biol Chem. 2016;291:10950–10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia‐Pino A, Balasubramanian S, Wyns L, et al. Allostery and intrinsic disorder mediate transcription regulation by conditional cooperativity. Cell. 2010;142:101–111. [DOI] [PubMed] [Google Scholar]
- 17. Engel P, Goepfert A, Stanger FV, et al. Adenylylation control by intra‐or intermolecular active‐site obstruction in Fic proteins. Nature. 2012;482:107–110. [DOI] [PubMed] [Google Scholar]
- 18. Dalton KM, Crosson S. A conserved mode of protein recognition and binding in a ParD‐ParE toxin‐antitoxin complex. Biochemistry. 2010;49:2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jurėnas D, Van Melderen L, Garcia‐Pino A. Mechanism of regulation and neutralization of the AtaR–AtaT toxin–antitoxin system. Nat Chem Biol. 2019;15:285–294. [DOI] [PubMed] [Google Scholar]
- 20. Yashiro Y, Yamashita S, Tomita K. Crystal structure of the enterohemorrhagic Escherichia coli AtaT‐AtaR toxin‐antitoxin complex. Structure. 2019;27:476–484. [DOI] [PubMed] [Google Scholar]
- 21. Qian H, Yu H, Li P, et al. Toxin‐antitoxin operon kacAT of Klebsiella pneumoniae is regulated by conditional cooperativity via a W‐shaped KacA‐KacT complex. Nucleic Acids Res. 2019;47:7690–7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattison K, Wilbur JS, So M, Brennan RG. Structure of FitAB from Neisseria gonorrhoeae bound to DNA reveals a tetramer of toxin‐antitoxin heterodimers containing pin domains and ribbon‐helix‐helix motifs. J Biol Chem. 2006;281:37942–37951. [DOI] [PubMed] [Google Scholar]
- 23. Li G‐Y, Zhang Y, Inouye M, Ikura M. Inhibitory mechanism of Escherichia coli RelE‐RelB toxin‐antitoxin module involves a helix displacement near an mRNA interferase active site. J Biol Chem. 2009;284:14628–14636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamada K, Hanaoka F. Conformational change in the catalytic site of the ribonuclease YoeB toxin by YefM antitoxin. Mol Cell. 2005;19:497–509. [DOI] [PubMed] [Google Scholar]
- 25. Ruangprasert A, Maehigashi T, Miles SJ, Giridharan N, Liu JX, Dunham CM. Mechanisms of toxin inhibition and transcriptional repression by Escherichia coli DinJ‐YafQ. J Biol Chem. 2014;289:20559–20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hadži S, Garcia‐Pino A, Haesaerts S, et al. Ribosome‐dependent Vibrio cholerae mRNAse HigB2 is regulated by a ß‐strand sliding mechanism. Nucleic Acids Res. 2017;45:4972–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown BL, Grigoriu S, Kim Y, et al. Three dimensional structure of the MqsR:MqsA complex: A novel TA pair comprised of a toxin homologous to RelE and an antitoxin with unique properties. PLoS Pathog. 2009;5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manav MC, Turnbull KJ, Jurėnas D, Garcia‐Pino A, Gerdes K, Brodersen DE. The E. coli HicB antitoxin contains a structurally stable helix‐turn‐helix DNA binding domain. Structure. 2019;27:1675–1685. [DOI] [PubMed] [Google Scholar]
- 29. Kim DH, Kang SM, Park SJ, Jin C, Yoon HJ, Lee BJ. Functional insights into the Streptococcus pneumoniae HicBA toxin‐antitoxin system based on a structural study. Nucleic Acids Res. 2018;46:6371–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bibi‐Triki S, de la Sierra‐Gallay IL, Lazar N, et al. Functional and structural analysis of HicA3‐HicB3, a novel toxin‐antitoxin system of Yersinia pestis . J Bacteriol. 2014;196:3712–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winter AJ, Williams C, Isupov MN, et al. The molecular basis of protein toxin HicA‐dependent binding of the protein antitoxin HicB to DNA. J Biol Chem. 2018;293:19429–19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schureck MA, Maehigashi T, Miles SJ, et al. Structure of the Proteus vulgaris HigB‐(HigA)2‐HigB toxin‐antitoxin complex. J Biol Chem. 2014;289:1060–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talavera A, Tamman H, Ainelo A, et al. A dual role in regulation and toxicity for the disordered N‐terminus of the toxin GraT. Nat Commun. 2019;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu BS, Liu M, Zhou K, et al. Conformational changes of antitoxin HigA from Escherichia coli str. K‐12 upon binding of its cognate toxin HigB reveal a new regulation mechanism in toxin‐antitoxin systems. Biochem Biophys Res Commun. 2019;514:37–43. [DOI] [PubMed] [Google Scholar]
- 35. Yang J, Zhou K, Liu P, et al. Structural insight into the E. coli HigBA complex. Biochem Biophys Res Commun. 2016;478:1521–1527. [DOI] [PubMed] [Google Scholar]
- 36. Yoon W‐S, Seok S‐H, Won H‐S, Cho T, Lee SJ, Seo M‐D. Structural changes of antitoxin HigA from Shigella flexneri by binding of its cognate antitoxin HigB. Int J Biol Macromol. 2019;130:99–108. [DOI] [PubMed] [Google Scholar]
- 37. Kwon A‐R, Kim J‐H, Park SJ, et al. Structural and biochemical characterization of HP0315 from Helicobacter pylori as a VapD protein with an endoribonuclease activity. Nucleic Acids Res. 2012;40:4216–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bertelsen MB, Senissar M, Nielsen MH, et al. Structural basis for toxin inhibition in the VapXD toxin‐antitoxin system. Structure. 2021;29:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheverton AM, Gollan B, Przydacz M, et al. A Salmonella toxin promotes persister formation through acetylation of tRNA. Mol Cell. 2016;63:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qian H, Yao Q, Tai C, Deng Z, Gan J, Ou H‐Y. Identification and characterization of acetyltransferase‐type toxin‐antitoxin locus in Klebsiella pneumoniae . Mol Microbiol. 2018;108:336–349. [DOI] [PubMed] [Google Scholar]
- 41. Jurėnas D, Chatterjee S, Konijnenberg A, et al. AtaT blocks translation initiation by N‐acetylation of the initiator tRNAfMet . Nat Chem Biol. 2017;13:640–648. [DOI] [PubMed] [Google Scholar]
- 42. Bendtsen KL, Brodersen DE. Higher‐order structure in bacterial VapBC toxin‐antitoxin complexes. In: Harris J, Marles‐Wright J, editors. Macromolecular protein complexes subcellular biochemistry. Cham: Springer, 2017; p. 381–412. [DOI] [PubMed] [Google Scholar]
- 43. Sterckx YGJ, Jové T, Shkumatov AV, et al. A unique hetero‐hexadecameric architecture displayed by the Escherichia coli O157 PaaA2‐ParE2 antitoxin‐toxin complex. J Mol Biol. 2016;428:1589–1603. [DOI] [PubMed] [Google Scholar]
- 44. Correia FF, D'Onofrio A, Rejtar T, et al. Kinase activity of overexpressed HipA is required for growth arrest and multidrug tolerance in Escherichia coli . J Bacteriol. 2006;188:8360–8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lu C, Nakayasu ES, Zhang L‐Q, Luo Z‐Q. Identification of Fic‐1 as an enzyme that inhibits bacterial DNA replication by AMPylating GyrB, promoting filament formation. Sci Signal. 2016;9:1–11. [DOI] [PubMed] [Google Scholar]
- 46. Stanger FV, Burmann BM, Harms A, et al. Intrinsic regulation of FIC‐domain AMP‐transferases by oligomerization and automodification. Proc Natl Acad Sci USA. 2016;113:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao J, Zhen X, Tang K, et al. Novel polyadenylylation‐dependent neutralization mechanism of the HEPN/MNT toxin/antitoxin system. Nucleic Acids Res. 2020;48:11054–11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Songailiene I, Juozapaitis J, Tamulaitiene G, et al. HEPN‐MNT toxin‐antitoxin system: The HEPN ribonuclease is neutralized by oligoAMPylation. Mol Cell. 2020;80:1–16. [DOI] [PubMed] [Google Scholar]
- 49. Pedersen K, Christensen SK, Gerdes K. Rapid induction and reversal of a bacteriostatic condition by controlled expression of toxins and antitoxins. Mol Microbiol. 2002;45:501–510. [DOI] [PubMed] [Google Scholar]
- 50. Christensen SK, Gerdes K. RelE toxins from bacteria and Archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol Microbiol. 2003;48:1389–1400. [DOI] [PubMed] [Google Scholar]
- 51. VanDrisse CM, Parks AR, Escalante‐Semerena JC. A toxin involved in Salmonella persistence regulates its activity by acetylating its cognate antitoxin, a modification reversed by CobB sirtuin deacetylase. mBio. 2017;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Francuski D, Saenger W. Crystal structure of the antitoxin‐toxin protein complex RelB‐RelE from Methanococcus jannaschii . J Mol Biol. 2009;393:898–908. [DOI] [PubMed] [Google Scholar]
- 53. Takagi H, Kakuta Y, Okada T, Yao M, Tanaka I, Kimura M. Crystal structure of archaeal toxin‐antitoxin RelE‐RelB complex with implications for toxin activity and antitoxin effects. Nat Struct Mol Biol. 2005;12:327–331. [DOI] [PubMed] [Google Scholar]
- 54. Donegan NP, Cheung AL. Regulation of the mazEF toxin‐antitoxin module in Staphylococcus aureus and its impact on sigB expression. J Bacteriol. 2009;191:2795–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de la Hoz AB, Ayora S, Sitkiewicz I, et al. Plasmid copy‐number control and better‐than‐random segregation genes of pSM19035 share a common regulator. Proc Natl Acad Sci USA. 2000;97:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hallez R, Geeraerts D, Sterckx YGJ, Mine N, Loris R, Van Melderen L. New toxins homologous to ParE belonging to three‐component toxin‐antitoxin systems in Escherichia coli O157:H7. Mol Microbiol. 2010;76:719–732. [DOI] [PubMed] [Google Scholar]
- 57. Weihofen WA, Cicek A, Pratto F, Alonso JC, Saenger W. Structures of ω repressors bound to direct and inverted DNA repeats explain modulation of transcription. Nucleic Acids Res. 2006;34:1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jadhav PV, Sinha VK, Chugh S, et al. 2.09 Å resolution structure of E. coli HigBA toxin‐antitoxin complex reveals an ordered DNA‐binding domain and intrinsic dynamics in antitoxin. Biochem J. 2020;477:4001–4019. [DOI] [PubMed] [Google Scholar]
- 59. Wilbur JS, Chivers PT, Mattison K, Potter L, Brennan RG, So M. Neisseria gonorrhoeae FitA interacts with FitB to bind DNA through its ribbon‐helix‐helix motif. Biochemistry. 2005;44:12515–12524. [DOI] [PubMed] [Google Scholar]
- 60. Winther KS, Gerdes K. Regulation of enteric vapBC transcription: Induction by VapC toxin dimer‐breaking. Nucleic Acids Res. 2012;40:4347–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schmidt O, Schuenemann VJ, Hand NJ, et al. prlF and yhaV encode a new toxin‐antitoxin system in Escherichia coli . J Mol Biol. 2007;372:894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brown BL, Lord DM, Grigorius S, Peti W, Page R. The Escherichia coli toxin MqsR destabilizes the transcriptional repression complex formed between the antitoxin MqsA and the mqsRA operon promoter. J Biol Chem. 2013;288:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schureck MA, Meisner J, Hoffer ED, et al. Structural basis of transcriptional regulation by the HigA antitoxin. Mol Microbiol. 2019;111:1449–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vandervelde A, Drobnak I, Hadži S, et al. Molecular mechanism governing ratio‐dependent transcription regulation in the ccdAB operon. Nucleic Acids Res. 2017;45:2937–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garcia‐Pino A, De Gieter S, Talavera A, de Greve H, Efremov RG, Loris R. An intrinsically disordered entropic switch determines allostery in Phd‐doc regulation. Nat Chem Biol. 2016;12:490–496. [DOI] [PubMed] [Google Scholar]
- 66. Overgaard M, Borch J, Jørgensen MG, Gerdes K. Messenger RNA interferase RelE controls relBE transcription by conditional cooperativity. Mol Microbiol. 2008;69:841–857. [DOI] [PubMed] [Google Scholar]
- 67. Bøggild A, Sofos N, Andersen KR, et al. The crystal structure of the intact E. coli RelBE toxin‐antitoxin complex provides the structural basis for conditional cooperativity. Structure. 2012;20:1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xue L, Yue J, Ke J, et al. Distinct oligomeric structures of the YoeB‐YefM complex provide insights into the conditional cooperativity of type II toxin‐antitoxin system. Nucleic Acids Res. 2020;48:10527–10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Turnbull KJ, Gerdes K. HicA toxin of Escherichia coli derepresses hicAB transcription to selectively produce HicB antitoxin. Mol Microbiol. 2017;104:781–792. [DOI] [PubMed] [Google Scholar]
- 70. Black HF, Mastromatteo S, Sinha S, et al. A competence‐regulated toxin‐antitoxin system in Haemophilus influenzae . PLoS One. 2020;15:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fraikin N, Rousseau CJ, Goeders N, Van Melderen L. Reassessing the role of the yype II mqsRA toxin‐antitoxin system in stress response and biofilm formation: mqsA is transcriptionally uncoupled from mqsR . mBio. 2019;10:e02678–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Van Melderen L, Bernard P, Couturier N. Lon‐dependent proteolysis of CcdA is the key control for activation of CcdB in plasmid‐free segregant bacteria. Mol Microbiol. 1994;11:1151–1157. [DOI] [PubMed] [Google Scholar]


