Abstract
Background
Oxidative stress plays critical pathophysiological roles in vascular remodeling-related cardiovascular diseases, including hypertension, atherosclerosis, and restenosis. Previous studies demonstrate that SENP3, a redox-sensitive SUMO2/3-specific protease, is strongly implicated in cancer development and progression. However, the role of SENP3 in vascular remodeling remains unknown.
Methods
We generated three mouse models of vascular remodeling due to low shear stress, hypertension, and atherosclerosis. The expression of SENP3 was determined by western blotting and/or immunofluorescence staining in cultured vascular smooth muscle cells (VSMCs), animal models, and human samples. The biological function of SENP3 in proliferation and migration of VSMC and vascular remodeling was further investigated in vitro and in vivo models.
Findings
SENP3 was highly expressed in VSMCs of remodeled arteries, accompanied by elevated reactive oxygen species (ROS) levels. In cultured VSMCs, SENP3 protein levels were enhanced by oxidized low-density lipoprotein and Angiotensin II in a ROS-dependent manner. SENP3 overexpression significantly promoted and sh-RNA-mediated knockdown markedly inhibited VSMCs proliferation and migration. Immunofluorescence staining showed that SENP3 expression was correlated with intimal area in remodeled arteries. Furthermore, we demonstrated that SENP3 interacted with β-catenin and inhibited its proteasome-dependent degradation via de-SUMOylation of β-catenin. Most importantly, SENP3+/− mice exhibited alleviated vascular remodeling.
Interpretation
Our results highlight the important function of SENP3 as a redox sensor and mediator in vascular remodeling.
Keywords: Oxidative stress, Vascular remodeling, Senp3, Sumoylation, β-catenin
Research in context.
Evidence before this study
Vascular remodeling is a complicated pathophysiological process implicated in many cardiovascular diseases. Mounting evidence strongly implicates oxidative stress as the most common cell stress and common reason for vascular remodeling-related cardiovascular diseases, including hypertension, atherosclerosis, and restenosis. Previous studies demonstrate that SENP3, a redox-sensitive SUMO2/3-specific protease, is strongly implicated in cancer development and progression. However, the role of SENP3 in vascular remodeling remains unknown.
Added value of this study
This study highlights the importance of SENP3, a redox-sensitive protease, in the development of vascular remodeling. SENP3 acts not only as a sensor of oxidative stress, but also as a key mediator in oxidative stress-induced pathological process, via regulation of SUMO/de-SUMOylation balance of numbers of protein substrates and the function of these proteins. Our findings provide a common point of view about how vascular cells sense oxidative stress and mediate the according modulation of intracellular signal transduction, which ultimately leading to the development and progression of vascular remodeling.
Implications of all the available evidence
SENP3 plays an important role in the pathological process of vascular remodeling. Therefore, SENP3 might represent a novel therapeutic target for vascular remodeling-related cardiovascular diseases.
Alt-text: Unlabelled box
1. Introduction
Vascular remodeling is a complicated pathophysiological process implicated in many cardiovascular diseases. Mounting evidences strongly suggest that oxidative stress caused by increased reactive oxygen species (ROS) plays a critical role in vascular remodeling-related cardiovascular diseases [1], [2], [3]. Various risk factors for the development of atherosclerosis and vascular remodeling (e.g. hyperlipidemia, hyperglycemia, hypertension and disturbed shear stress) mediate elevated ROS levels and result in oxidative stress in the vasculature [4]. Therefore, it raises the possibility that oxidative stress might be the common reason for vascular remodeling. However, the mechanism underlying how vascular smooth muscle cells (VSMCs) sense oxidative stress, and which molecules mediate excessive proliferation and migration and ultimately lead to vascular remodeling remains elusive.
The biologic process of oxidative stress-induced vascular remodeling includes the following components: sensors to sense oxidative stress, effectors or mediators to mediate changes in the biologic function of VSMCs, and the resultant structural and functional changes in the vessel wall. Of note, it has been well demonstrated that under oxidative stress, redox-sensitive proteins can sense and be modulated by redox changes, and subsequently mediate reprogramming of multiple signaling processes, leading to the development of specific pathologic processes. Therefore, investigating the role of redox-sensitive proteins in vascular remodeling might help understand the pathogenesis of vascular remodeling.
Posttranslational modifications (PTMs), such as phosphorylation, acetylation, ubiquitination and SUMOylation, are important regulators of cell signaling, due to the transient and often reversible nature of these modifications. Among these, SUMOylation, which conjugates Small Ubiquitin-like Modifier (SUMO) to target proteins and regulates the level, subcellular localization, and transcriptional activity of the modified proteins [5,6], is a very important molecular modification pathway during oxidative stress. It has been demonstrated that this unique modification is a fine sensor for reactive oxygen species [7,8]. The SUMO proteases (SENPs) de-SUMOylate modified proteins and thus are critical for maintaining the SUMOylation level of substrates required for normal physiology. Our previous studies demonstrate that SENP3, a redox-sensitive protein in SENPs family, accumulates under oxidative stress [9]. SENP3 plays important roles in cancer development and progression, which was mediated by its de-SUMOylation of various protein substrates [10], [11], [12], [13]. Regulation of SUMO/deSUMOylation balance by SENP3 may also represent an important regulatory mechanism under oxidative stress during vascular remodeling.
Wnt/β-catenin signaling pathway has been strongly implicated in vascular remodeling [14], [15], [16], [17], [18]. Many physiological stimuli, including oxidized low-density lipoprotein (oxLDL), Angiotensin II (Ang II) and growth factors, activate β-catenin signaling in VSMCs [16], [17], [18]. Activation of this signaling induces the transcription of multiple target genes (e.g., cyclin D1, c-myc, matrix metalloproteinases [MMPs]), which promote VSMC proliferation and migration. PTMs, mainly phosphorylation, play critical roles in the regulation of β-catenin signaling [19]. Whether β-catenin is modified and regulated by other PTM remains unknown.
In this present study, we assessed the expression and function of SENP3 in vascular remodeling; we also examined the SUMO modification of β-catenin. We demonstrated that SENP3, a known redox-sensitive SUMO protease, accumulates under oxidative stress during vascular remodeling. SENP3 promotes the proliferation and migration of VSMCs and mediates vascular remodeling. Mechanistically, SENP3 interacts with β-catenin and promotes its protein stability. These findings suggest that SENP3 is a sensor of oxidative stress in VSMCs, and contributive to vascular remodeling.
2. Materials and methods
2.1. Human tissue specimens
Human artery samples were obtained from patients with atherosclerosis undergoing lower limb amputation. Their use was approved by the Medical Ethics Committee of the Shanghai Jiaotong University School of Medicine, and all subjects gave written informed consent. Femoral and popliteal arteries and their major branches were cut transversely at ~2-cm intervals and immediately frozen in liquid nitrogen until use. Specimens were OCT-embedded, sectioned into 8-μm-thick slices, and mounted on the microscope slides.
2.2. Animal experiments
All animal experiments were performed under the guidelines on animal care of Shanghai Jiaotong University School of Medicine (Permit no. 2014–018). SENP3+/− mice were generated from BayGenomics (San Francisco, CA). ApoE−/− mice (#002052) in a C57BL/6 background were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). ApoE−/− mice and C57BL/6 mice were bred at Shanghai Biomodel Organism Science & Technology Development Co., Ltd.
SENP3+/− and wild-type (WT) mice (male, 6–8 weeks of age) were used in the experiments. Partial ligation of left common carotid artery (LCCA) induced low shear stress in the LCCA and was performed as described previously [20]. In brief, mice were anesthetized using intraperitoneal injection of pentobarbital sodium (50 mg/kg) and body temperature was maintained at 37 °C by using heating pads. After blunt dissection to expose the distal branches of the LCCA, blood flow was reduced by ligation of all branches of the LCCA except for the left thyroid artery. After validation that blood flow was present, the incision was closed with a suture. Sham ligation was performed with suture placement without ligation.
Male C57BL/6 mice (12–16 weeks of age) were used in the mouse model of Ang II-induced vascular remodeling. Briefly, mice were anesthetized with sodium pentobarbital, followed by subcutaneously implantation of Alzet osmotic minipump (Model 2002; ALZA Scientific Products, USA) containing saline alone or Ang II (Sigma, A9525) dissolved in saline. Mice were then continuously infused with saline or Ang II (2.1 mg/kg/d) for 2 weeks.
Female ApoE−/− mice (8 weeks of age) underwent combined partial ligation of the LCCA and left renal artery as previously described [21]. Mice were sacrificed 4 weeks after surgery.
2.3. Tissue collection and processing
Mice were perfused with ice cold isotonic saline, after which left carotid arteries or aortas were isolated. Tissue samples were fixed with 4% paraformaldehyde overnight and embedded in optimal cutting temperature (OCT; Sakura Finetechnical). These OCT-embedded sections (5 μm thick) were cut for use every 200 μm over a 2-mm length of carotid arteries from the distal bifurcation of carotid artery specimens, or of thoracic aortas from the proximal thoracic aortas specimens.
2.4. Histology
The OCT- or paraffin-embedded carotid artery or thoracic aorta sections were stained with hematoxylin and eosin (H&E) and picrosirius red as described previously [18,20]. All images were captured by Olympus digital camera and analyzed using ImagePro Plus software.
2.5. Immunofluorescence
The OCT-embedded sections were washed with PBS, fixed in 4% paraformaldehyde for 20 min, and permeabilized with 0.2% Triton X-100 for 8 min. The sections were then blocked with 5% fetal bovine serum (FBS) for 30 min and incubated with primary antibodies against SENP3 (1:100; Cell Signaling Technology [CST]; #5591), PCNA (1:100; Abgent; #AJ1594a), and α-actin (1:300; Abcam; ab21027) overnight, followed by further staining with secondary antibodies labeled with red fluorescence (1:300; donkey-anti-goat, 555 nm; Invitrogen; #A21432) and green fluorescence (1:300; donkey-anti-rabbit, 488 nm; Invitrogen; #A21206) for 60 min. After staining nuclei with 4, 6-diamino- 2-phenylindole (DAPI; Beyotime), the fluorescence signal was acquired by confocal microscopy (Zeiss LSM 710).
2.6. Detection of ROS
2′, 7′-Dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma) was used to measure the ROS levels in the cultured VSMCs, as previously described [22]. Dihydroethidium (DHE, Sigma) staining was used for the in situ detection of ROS levels in frozen tissue sections. Briefly, unfixed frozen cross-sections were incubated with DHE (5 μmol/l) at 37°C for 30 min in a humidified chamber protected from light, followed by a 5-min wash in PBS. Images were obtained by confocal microscopy (Zeiss LSM 710).
2.7. Cell culture
Primary VSMCs were isolated from the thoracic aortic arteries of Sprague-Dawley rats (6–8 weeks) using mechanical dissociation as described previously [23]. VSMCs were identified via smooth muscle (SM)-α-actin staining. VSMCs at passages 3 to 8 were used in all experiments. VSMCs were cultured in DMEM with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin. Twenty-four hours prior to drug treatment, VSMCs were transferred to serum-free medium for the duration of the experiment. VSMCs were treated with various reagents including oxLDL (Yiyuan Biotechnologies), Ang II (Sigma) and H2O2 (Sigma) as described in the figure legends.
HEK293T and HEK293FT cells were maintained in DMEM with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin in an atmosphere containing 5% CO2.
2.8. Plasmid constructs and transfection
Several tagged (GFP-, RGS-His (RH)- and Flag-tagged) constructs for wild-type SENP3 were generated using standard techniques by cloning the full-length cDNA of SENP3 into the pEGFP-C1 or pcDNA3 vectors as described previously [10], [11], [12], [13]. The RH- and GFP-tagged SENP3 mutant constructs (RH-SENP3/C532A and GFP-SENP3/C532A) were made by site-directed mutagenesis based on the SENP3 wild-type constructs (WT) using a QuikChange mutagenesis kit (Stratagene, La Jolla, CA). RH-SUMO-3 and HA-Ub were used in our previous work [24]. HA-β-catenin and Flag-β-catenin were kindly provided by Prof. Xiao-kun Zhang (Xiaomen University, Xiamen, China). The constructs were transiently transfected into cells using Lipofectamine 2000 (Invitrogen) or X-tremeGENE HP DNA (Roche) Transfection Reagents following the manufacturer's instructions.
2.9. Lentiviral constructs and lentivirus packaging
The SENP3 lentiviral expression construct was made by subcloning the conventional pcDNA3-SENP3 into the pLVX-IRES-ZsGreen1 Vector (Clontech Laboratories, Inc., Mountain View, CA). SENP3 shRNA lentiviral construct was generated by inserting a shRNA oligonucleotide into pLVX-IRES-ZsGreen1 vector. Lentiviral particles are packaged as previously described [25]. Briefly, lentivirus vectors were cotransfected with packaging vectors into HEK293FT cells using Lipofectamine 2000. Virus particles were harvested 48 h after transfection. Virus pellets were rinsed once and then resuspended in PBS.
2.10. Western blotting
Whole cell lysates were prepared and quantitated by BCA assay. Equal amounts of protein per lane were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted using antibodies against SENP3 (1:1000; CST; #5591), PCNA (1:1000; Abgent; #AJ1594a), P27 (1:1000; CST; #2552), green fluorescent protein (GFP) (1:1000; Abcam; #ab290), HA(1:5000; Sigma; #H9658), Flag (1:5000; Sigma; #F1804), RGS (1:1000; Qiagen; #34,650), β-catenin (1:1000; CST; #8480), β-actin (1:5000; Abcam; #ab6276) and horseradish peroxidase-conjugated secondary antibodies. Protein bands were detected using enhanced chemiluminescence (Millipore)
2.11. Flag immunoprecipitation assay
For Flag-immunoprecipitation assay, transfected cells in 10cm-dish were lysed in cold lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris–HCl [pH 7.4], 1 mM EDTA, 1 mM EGTA [pH 8.0], 0.2 mM sodium ortho-vanadate, 1 mM PMSF, 0.5% protease inhibitor cocktail, 0.5% IGEPAL CA-630) on ice for 30 min. Following sonication, the lysates were centrifuged for 30 min at 12,000 rpm. Anti-Flag M2 Affinity Gel (Sigma Aldrich, Cat# A2220) was added to the cell lysates and incubated at 4 °C overnight. The resin was washed with lysis buffer 5 times. After the last washing, the proteins were eluted in elution buffer and subjected to western blotting.
2.12. Ni-NTA pull-down assay
Ni-nitrilotriacetic acid resin (NTA) pull-down assay was performed as previously described [10]. Briefly, the cells transfected with RH-tagged plasmids were lysed in a lysis buffer according to the manufacturer's protocols. Ni2+-NTA-agarose resin (Invitrogen, Cat# R901–15) was then added to the cell lysates and incubated with gentle agitation at 4 °C overnight. The resin was successively washed at room temperature with four different washing buffers. After the last washing, RH-tagged proteins were eluted in elution buffer and subjected to western blotting.
2.13. Cell counts
For cell counts, primary rat VSMCs were plated into a 6-well plate and were infected with GFP-SENP3 lentivirus (GFP-SENP3) and GFP control lentivirus (GFP), or sh-SENP3 lentivirus (sh-SENP3) and sh-control lentivirus (sh-NC). 48 h after infection, the cells were trypsinized and counted on a hemocytometer with an Olympus inverted microscope (Olympus, Tokyo, Japan).
2.14. EdU incorporation assay
EdU incorporation assays were performed using BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 488 in vitro Imaging Kits (Beyotime). Briefly, VSMCs in the logarithmic growth were plated at 5 × 104 cells per well into 12-well plates. After 24 h of the indicated treatment, each well was incubated with 1 ml of 10 μM EdU medium for 2 h. The cells were fixed with 4% paraformaldehyde for 30 min. After washing with PBS in 3 times, the cells were washed with PBS containing 0.5% TritonX-100 for 10 min. Then, click additive solution and click reaction solution were prepared according to the manufacturer's instructions, then added into each well and incubated in the dark for 30 min at room temperature. The staining solution was discarded, and the cells were washed with PBS for 10 min. For the nuclear staining, 1 × Hoechest 33,342 was added for 30 min incubation at room temperature. After washing with PBS, the positive cells were observed by fluorescence microscopy.
2.15. Cell migration assay (In vitro scratch-wound assay and transwell assay)
For the scratch-wound assay, primary rat VSMCs were seeded into 6-well plates and were infected with sh-SENP3 lentivirus (sh-SENP3) and sh-control lentivirus (sh-NC). 48 h after infection, the cells were serum-starved overnight, scraped by sterilized 10 μL pipette tips, washed with PBS to remove the cell debris, and stimulated with Ang II (500 mM) for an additional 12 h. Photomicrographs were taken using an Olympus inverted microscope. The numbers of cells that migrated into the wound area were quantified using Diskus software (Hilgers, Königswinter, Germany).
The transwell assay was performed using a 24-well plate containing transwell inserts (Corning Inc., Corning, NY, USA). Starved VSMCs were seeded into the upper chamber and serum-free medium containing Ang II was added to the lower chamber; the plates were incubated for 6 h. The cells on the upper or lower surfaces of the transwell inserts were then fixed with ice cold methanol for 10 min and stained with crystal violet at room temperature for 30 min. Cells on the upper surface of the filter were scraped. The cells that migrated into the lower section were counted in five random fields (20 × magnification) using an Olympus inverted microscope.
2.16. Statistical analysis
Values were expressed as mean ± standard error of the mean (SEM). The Student's t-test was used for comparison of two groups and the one-way ANOVA was used for multiple comparisons. A p-value<0.05 was considered to indicate a statistically significant result.
3. Results
SENP3 is highly expressed in VSMCs following vascular remodeling due to low shear stress, hypertension and atherosclerosis
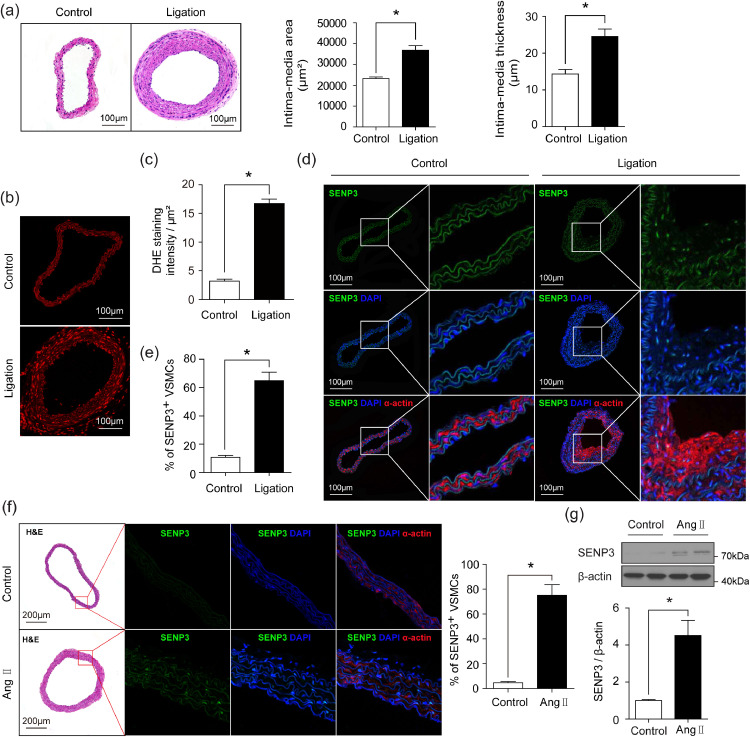
To investigate the role of SENP3 in vascular remodeling, we generated the mouse model of low shear stress-induced vascular remodeling by partial ligation of left common carotid artery (LCCA) and examined the expression of SENP3 in remodeled arteries. As depicted in Fig. 1(a), partial carotid ligation resulted in significant vascular remodeling as assessed by H&E staining and quantified by intima-media area and intima-media thickness. ROS levels were significantly increased in ligated carotid arteries (Fig. 1(b) and (c)), consistent with previous studies [20,26]. Meanwhile, SENP3 expression was dramatically enhanced in the neointimal layer of ligated carotid arteries compared to control arteries (Fig. 1(d) and (e)). Moreover, dual immunofluorescence staining revealed that SENP3 was localized predominantly in neointimal VSMCs (Fig. 1(d)).
Fig. 1.
SENP3 is highly expressed in VSMCs following low shear stress-induced and Ang II-induced vascular remodeling. (a) Representative images of H&E staining and quantification of intima-media area and intima-media thickness in sham control and ligation of left common carotid artery (ligation) groups (*p < 0.05, n = 8). (b and c) Representative images of DHE staining and quantification of DHE staining intensity in control and ligation groups (*p < 0.05, n = 6). (d and e) Representative images of dual immunofluorescence staining of SENP3 and smooth muscle (SM)-α-actin and quantification of percentage of SENP3-positive VSMCs in control and ligation groups (*p < 0.05, n = 6). (f) Representative images of H&E staining, dual immunofluorescence staining of SENP3 and SM-α-actin, and quantification of percentage of SENP3-positive VSMCs in thoracic aortas from Ang II-infused mice compared with control mice (*p < 0.05, n = 6). (g) Western blot analysis of SENP3 protein expression in thoracic aortas from Ang II-infused mice compared with control mice. Values are mean ± SEM.
To further demonstrate the role of SENP3 in vascular remodeling, we generated Ang II-induced vascular remodeling model. SENP3 staining was markedly increased in VSMCs of remodeled thoracic aortas (Fig. 1(f)). The western blotting results consistently showed an over 4-fold upregulation of SENP3 protein level in the thoracic aorta sections of the Ang II-infused mice compared with the control mice (Fig. 1(g)).
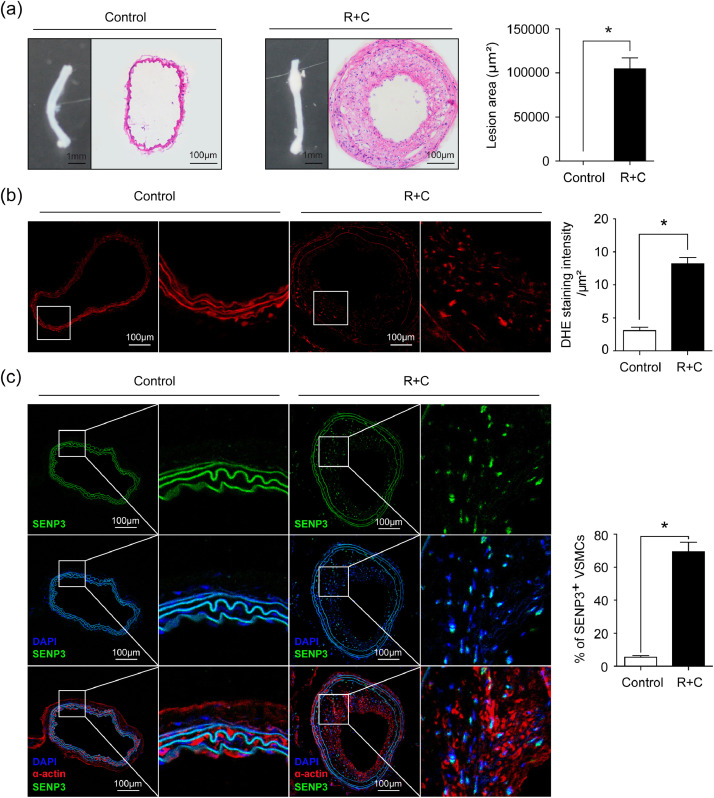
In addition, by combining partial ligation of the left renal artery and LCCA (R + C) in ApoE-/- mice [21], we generated another mouse model of vascular remodeling due to atherosclerosis. As depicted in Fig. 2(a), R + C in ApoE-/- mice significantly induced atherosclerosis in carotid arteries. ROS levels were significantly increased in atherosclerotic lesions (Fig. 2(b)). Moreover, SENP3 was highly expressed in intimal VSMCs (Fig. 2(c)). Taken together, all these results suggest a potential role of redox-sensitive SENP3 in the development of vascular remodeling under various pathological conditions.
Fig. 2.
SENP3 is highly expressed in VSMCs following vascular remodeling due to atherosclerosis. (a) Representative images of gross appearance and H&E staining, and quantification of lesion area in sham control and combined partial ligation of the left renal artery and left common carotid artery (R + C) groups (*p < 0.05, n = 8). (b) Representative images of DHE staining and quantification of DHE staining intensity in sham control and R + C groups (*p < 0.05, n = 6). (c) Representative images of dual immunofluorescence staining of SENP3 and SM-α-actin and quantification of percentage of SENP3-positive cells (*p < 0.05, n = 6). Values are mean ± SEM.
SENP3 expression is upregulated by oxLDL and Ang II in a ROS-dependent manner in primary rat VSMC
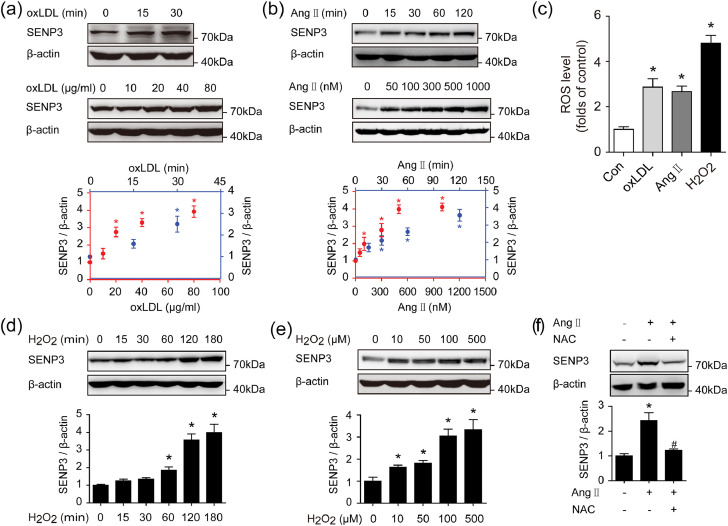
Because SENP3 was mainly up-regulated in VSMCs in remodeled arteries, we next examined the effect of various stimuli relevant to vascular remodeling on SENP3 expression in primary rat VSMCs. Immunoblotting demonstrated that both oxLDL and Ang II markedly increased SENP3 protein expression in a time- and dose-dependent manner (Fig. 3(a) and (b)).
Fig. 3.
SENP3 expression is upregulated by oxLDL and Ang II in a ROS-dependent manner in primary rat VSMC. (a) Primary rat VSMCs were treated with oxLDL (40 μg/ml) for the indicated time points and increasing concentrations of oxLDL for 30 min, respectively. Protein expression of SENP3 was examined by western blotting (n = 3, *p < 0.05). (b) Primary rat VSMCs were stimulated with Ang II (500 nM) for the indicated time and increasing concentrations of Ang II for 1 h, respectively. Protein expression of SENP3 was examined by western blotting (n = 3, *p < 0.05). (c) Primary rat VSMCs were treated with oxLDL (40 μg/ml), Ang II (500 nM) and H2O2 (100 μM) for 30 min. ROS level was detected by flow cytometry after DCFH-DA staining (n = 3, *p < 0.05 vs Con). (d and e) Primary rat VSMCs were stimulated with H2O2 (100 μM) for the indicated time (d) and increasing concentrations of H2O2 for 1 h (e), respectively. (f) Primary rat VSMCs were pretreated with or without 5 mM NAC for 6 h before Ang II stimulation for another 1 h. Protein expression of SENP3 was examined by western blotting (n = 3, *p < 0.05 vs Ang II/NAC(-), #p < 0.05 vs Ang II(+)). Values are mean ± SEM.
It has been demonstrated that SENP3 is a redox-sensitive proteins in carcinogenesis [9]. However, whether and how SENP3 participates in oxidative stress-induced vascular remodeling is unknown. As shown in Fig. 3(c), oxLDL, Ang II and the direct inducer of oxidative stress H2O2 increased intracellular ROS levels in vitro in primary VSMCs. Furthermore, SENP3 protein level was enhanced by H2O2, also in a time-dependently and dose-dependently manner (Fig. 3(d) and (e)), however, no alternations in SENP3 mRNA levels were detected following H2O2 treatment (Fig. S1(a)). Using a proteasome inhibitor MG132, we showed that SENP3 protein level was increased when treated with MG132 alone and remained stable when further exposed to H2O2 (Fig. S1(b)). In addition, the upregulation of SENP3 was blocked by anti-oxidant N-acetyl cysteine (NAC) (Fig. 3(f)). All these results suggest that the upregulation of SENP3 in VSMCs is through ROS-dependent proteasome pathway.
SENP3 promotes proliferation and migration of VSMCs
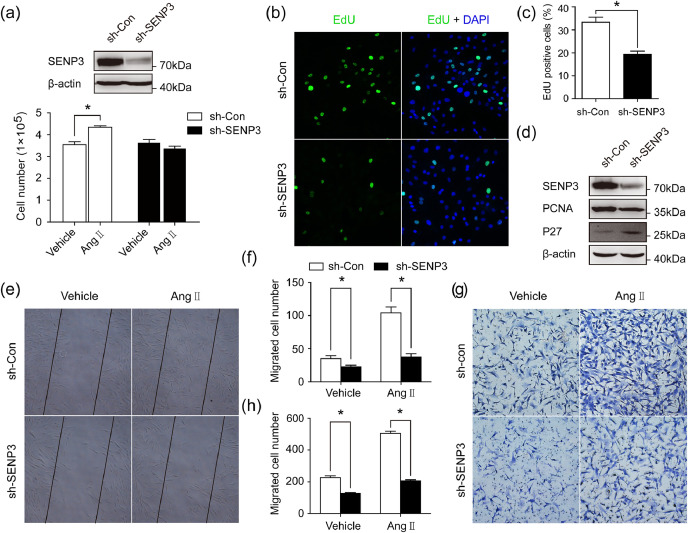
To establish the functional significance of SENP3, we examined the effect of SENP3 knockdown and overexpression on VSMC proliferation and migration. Infection of VSMCs with sh-SENP3 lentivirus significantly reduced endogenous SENP3 expression and abolished Ang II-induced VSMCs proliferation, as determined by cell counting (Fig. 4(a)). Under normal growth conditions, VSMCs infected with sh-SENP3 lentivirus (sh-SENP3 VSMCs) exhibited markedly reduced cell proliferation compared to VSMCs infected with control lentivirus (sh-Con VSMCs) (Fig. 4(b) and (c)). Consistently, protein level of the proliferative marker (PCNA) was down-regulated, whereas protein level of P27, an inhibitor of cyclin-dependent kinase, was up-regulated in sh-SENP3 VSMCs (Fig. 4(d)). Accordingly, when VSMCs were infected with SENP3 lentivirus to overexpress SENP3, the cell proliferation was significantly increased under normal growth condition (Fig. S2(a) and (b)).
Fig. 4.
SENP3 deletion decreases VSMC proliferation and migration in primary VSMCs in vitro. (a) VSMCs which were infected with control lentivirus and sh-SENP3 lentivirus were serum-starved overnight and stimulated with Ang II (500 nM) for 48 h. The expression of endogenous SENP3 was examined by western blotting and cell numbers were quantified using a hemacytometer. (b and c) Representative images of the EdU incorporation assay and quantification of percentage of EdU-positive cells in sh-SENP3 lentivirus-infected cells compared with control lentivirus-infected cells under normal growth condition with 10% FBS DMEM. (d) Protein expression of Cyclin D1, PCNA and P27 were examined by western blotting. (e and f) Monolayer confluent cells were serum-starved and scraped in the presence of Ang II (500 nM) to stimulate VSMC migration toward the wound area. Representative images of the in vitro scratch-wound assay and quantification of migrated cells (number/field) are presented. (g and h) Representative images of transwell migration assay and quantification of migrated cells (number/field). Values are mean ± SEM.
Next, we used in vitro scratch-wound and transwell migration assays to investigate whether SENP3 knockdown would affect VSMC migration. The in vitro scratch-wound assay revealed that cell migration induced by Ang II was significantly decreased in sh-SENP3 VSMCs (Fig. 4(e) and (f)). Additionally, the transwell migration assay also demonstrated that Ang II-stimulated cell migration decreased by approximately 2.5-fold in sh-SENP3 VSMCs, compared with sh-Con VSMCs (Fig. 4(g) and (h)).
Most importantly, in the mouse model of vascular remodeling due to atherosclerosis, we found that the expressions of PCNA and SENP3 were positively correlated with intimal area (r2=0.45, p < 0.05; r2=0.48, p < 0.05, respectively; Fig. S3). These results indicate that SENP3 promotes VSMC proliferation and migration during vascular remodeling.
SENP3 promotes β-catenin stability, whereas SUMOylation facilitates proteasome-dependent degradation of β-catenin.
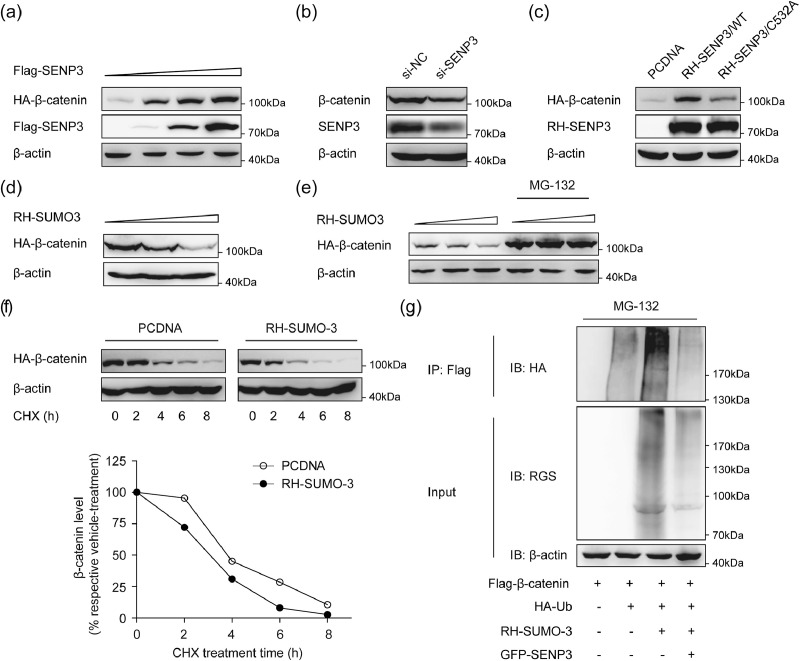
Growing evidences have indicated that β-catenin signaling is involved in the regulation of the proliferation and migration of VSMCs and vascular remodeling [14], [15], [16], [17], [18]. To define the possible mechanism by which SENP3 exerts its effect on VSMCs proliferation and migration, we examined the effect of SENP3 on Wnt/β-catenin signaling. The protein level of β-catenin was dose-dependently increased by SENP3 overexpression (Fig. 5(a)) and decreased by SENP3 knockdown (Fig. 5(b)). As cysteine 532 of SENP3 is responsible for its enzymatic activity [27], we overexpressed an enzymally inactive mutant SENP3 (SENP3-C532A) and found that the protein level of β-catenin was increased by SENP3, not SENP3-C532A (Fig. 5(c)). This result reveals that SENP3 increases the protein level of β-catenin through its enzymatic activity.
Fig. 5.
SENP3 promotes β-catenin stability, while SUMOylation promotes proteasome-dependent degradation of β-catenin. (a) 293T cells were transfected with HA-β-catenin and increasing amounts of Flag-SENP3 for 48 h. The levels of HA-β-catenin in whole cell lysates were determined by western blotting with anti-HA, anti-Flag and anti-β-actin antibodies. (b) 293T cells were transfected with control siRNA (si-NC) and SENP3 siRNA (si-SENP3) for 48 h. Lysates were prepared and analyzed by western blotting. (c) 293T cells were transfected with HA-β-catenin and equal amounts of PCDNA or RH-SENP3 or RH-SENP3/C532A for 48 h. Lysates were prepared and analyzed by western blotting. (d) 293T cells were transfected with HA-β-catenin and increasing amounts of RH-SUMO-3 for 48 h. Lysates were prepared and analyzed by western blotting. (e) 293T cells were transfected with HA-β-catenin and increasing amounts of RH-SUMO-3 for 48 h, in the presence or absence of MG132 (10 μM) for the last 10 h. Lysates were prepared and analyzed by western blotting. (f) Evaluation of β-catenin protein half-life. 293T cells were transfected with HA-β-catenin and PCDNA or RH-SUMO-3 for 36 h and were subsequently exposed to the protein synthesis inhibitor cycloheximide (CHX) for the indicated time. Lysates were prepared and analyzed by western blotting. The relative level of β-catenin was evaluated by densitometry and normalized to β-actin. (g) 293T cells were transfected with Flag-β-catenin, HA-ubiquitin (HA-Ub), RH-SUMO-3 and GFP-SENP3 and treated with MG132 (10 μM) for the last 10 h. The ubiquitination of Flag-β-catenin was determined by immunoprecipitation (IP) assay using Flag-beads and western blotting using antibody against HA.
As SENP3 acts as a SUMO2/3-specific protease, we then examined the effect of SUMOylation (SUMO-3) on β-catenin expression. The protein level of β-catenin was decreased by SUMO-3, which was completely blocked by proteasome inhibitor MG-132 (Fig. 5(d) and (e)). To further demonstrate this point of view, 293 T cells were then transfected with SUMO-3, and cycloheximide (CHX) was used to suppress protein biosynthesis. The half-life of exogenous β-catenin was markedly shortened in cells overexpressing SUMO-3 (Fig. 5(f)). In addition, the ubiquitination of β-catenin was increased when SUMO-3 was overexpressed, which was abolished when SENP3 were simultaneously overexpressed (Fig. 5(g)). Taken together, these results suggest that SENP3 promotes β-catenin stability, while SUMOylation promotes proteasome-dependent degradation of β-catenin.
SENP3 interacts with and de-SUMOylates β-catenin.
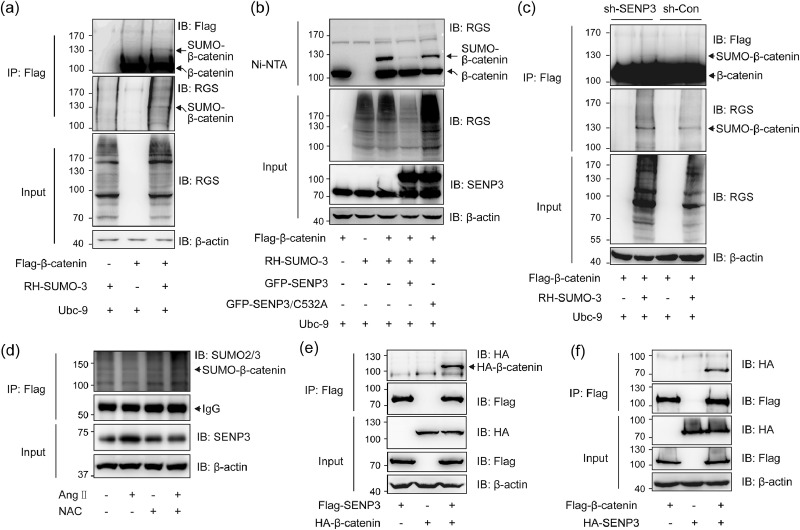
As SENP3 acts as a SUMO2/3-specific protease, and SENP3 promotes β-catenin stability while SUMOylation decreases β-catenin stability, we speculated that β-catenin can be SUMOylated and SENP3 can de-SUMOylate β-catenin. As shown in Fig. 6(a), β-catenin SUMOylation was detected by immunoprecipitation in 293 T cells co-expressed with Flag-β-catenin and RH-SUMO-3. Using Ni-NTA pull-down assays, SUMOylation of β-catenin was further demonstrated. The result showed that SENP3 deconjugated SUMO-3 from β-catenin, while SENP3-C532A mutant that lost de-SUMOylating activity did not change the SUMOylation of β-catenin (Fig. 6(b)). In stable cell line of SENP3 knockdown, we found that SUMOylation of β-catenin was pronounced compared to that in control cells (Fig. 6(c)). Notably, SUMOylation of β-catenin was significantly decreased after Ang II treatment in primary VSMCs, and this effect was reversed after NAC treatment (Fig. 6(d)). The interaction between β-catenin and SENP3 was further demonstrated by co-IP assay in exogenous setting (Fig. 6(e) and (f)). These results suggest that SENP3 de-SUMOylates β-catenin via a direct interaction.
Fig. 6.
SENP3 interacts with and de-SUMOylates β-catenin. (a) 293T cells were transfected with Flag-β-catenin, RH-SUMO-3 and Ubc-9 for 48 h. The SUMOylation of Flag-β-catenin was determined by IP assay using Flag-beads and western blotting using anti-RGS, anti-Flag, and anti-β-actin antibodies. (b) 293T cells were transfected with Flag-β-catenin, RH-SUMO-3, Ubc-9 and GFP-SENP3 or GFP-SENP3/C532A for 48 h. The SUMOylation of Flag-β-catenin was determined by pull-down assay using Ni-NTA agarose beads. (c) 293T cells stably expressing control shRNA (sh-Con) and SENP3 shRNA (sh-SENP3) were transfected with Flag-β-catenin, RH-SUMO-3 and Ubc-9 for 48 h. The SUMOylation of Flag-β-catenin was determined by IP assay using Flag-beads. (d) Primary VSMCs which were transfected with Flag-β-catenin for 24 h were pretreated with or without 5 mM NAC for 6 h before vehicle or Ang II stimulation for another 1 h. The SUMOylation of Flag-β-catenin was determined by IP assay using Flag-beads. (e and f) 293T cells were transfected with Flag-SENP3 or HA-β-catenin either alone or both together (e). 293T cells were transfected with HA-SENP3 or Flag-β-catenin either alone or both together (f). Exogenous interaction between SENP3 and β-catenin was determined by co-immunoprecipitation (co-IP) using Flag-beads.
SENP3+/− mice exhibited reduced vascular remodeling induced by low shear stress
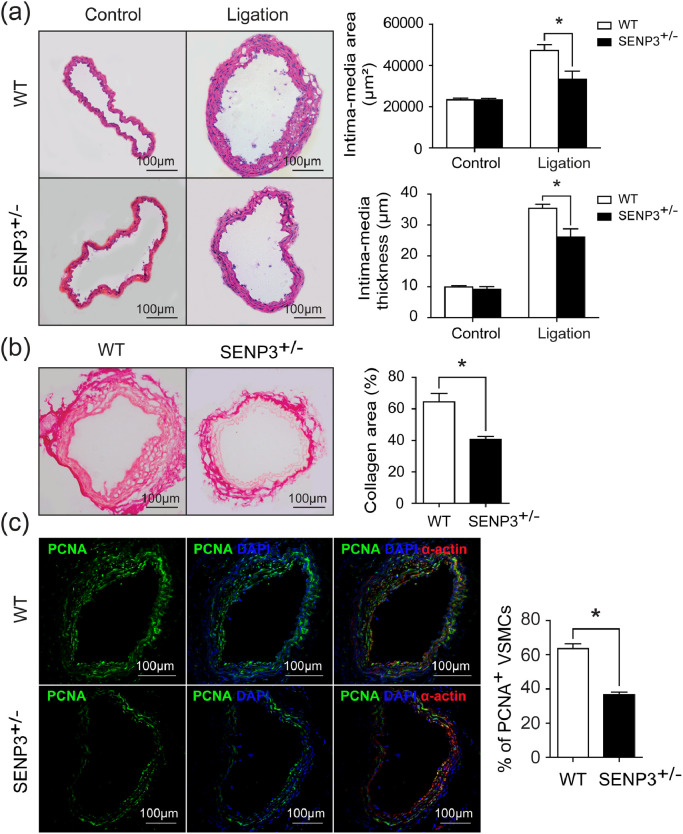
Because SENP3−/− mutation causes embryonic lethality [28], SENP3+/− mice were generated. To further define the in vivo role of SENP3 in vascular remodeling, SENP3+/− mice and wild-type (WT) littermates were subjected to partial ligation of left common carotid artery (LCCA). Body weights were measured before and after the surgery in WT and SENP3+/- mice. There were no differences in body weight between two groups (Fig. S4(a) and (b)). However, four weeks after surgery, SENP3+/− mice exhibited markedly reduced vascular remodeling compared with WT mice (Fig. 7(a)). The quantitative morphometric analysis revealed marked decreases in the intima-media area and intima-media thickness of the carotid arteries from SENP3+/- mice compared with WT mice (Fig. 7(a)). The picrosirius red staining results indicated decreased collagen deposition in carotid arteries from SENP3+/− mice (Fig. 7(b)). Furthermore, dual immunofluorescence staining of PCNA and SM-α-actin was performed in ligated carotid arteries. The percentage of PCNA-positive VSMCs was significantly decreased in ligated carotid arteries from SENP3+/− mice compared with WT mice (Fig. 7(c)). Consistently, the percentages of MMP-8- and MMP-13-positive area in the intima area were significantly decreased in ligated carotid arteries from SENP3+/− mice (Fig. S5(a)-(d)). These results indicated that SENP3 promotes vascular remodeling in vivo.
Fig. 7.
SENP3+/− mice exhibited reduced vascular remodeling induced by low shear stress. (a) Representative images of H&E staining and quantification of intima-media area and intima-media thickness in wild-type (WT) and SENP3+/− mice (*p < 0.05, n = 7–8). (b) Representative images of picrosirius red staining and quantification of collagen area in WT and SENP3+/− mice (*p < 0.05, n = 6). (c) Representative images of dual immunofluorescence staining of proliferating cell nuclear antigen (PCNA) and SM-α-actin and quantification of percentage of PCNA-positive VSMCs in WT and SENP3+/− mice (*p < 0.05, n = 6). Values are mean ± SEM.
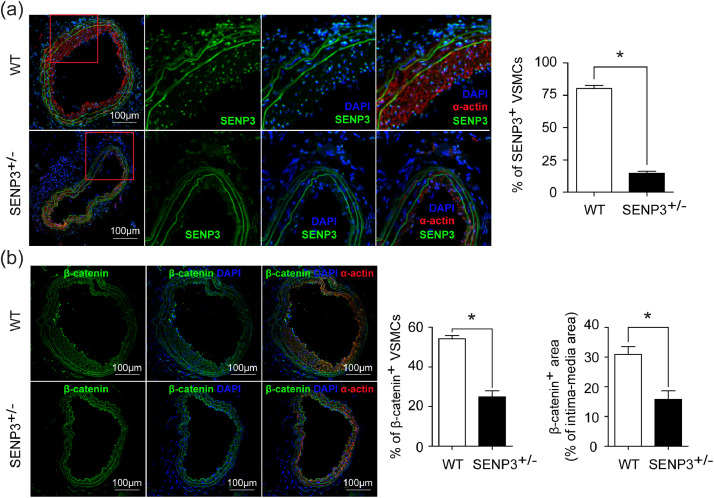
Furthermore, dual immunofluorescence staining of SENP3 and SM-α-actin confirmed that the expression of SENP3 was decreased in VSMCs from SENP3+/− mice compared with WT mice (Fig. 8(a)). Most importantly, the in vivo expression of β-catenin in VSMCs was markedly decreased in the remodeled arteries from SENP3+/− mice compared with WT mice (Fig. 8(b)). This result is consistent with our in vitro data showing that SENP3 promotes the protein stability of β-catenin.
Fig. 8.
SENP3 and β-catenin immunofluorescence staining in remodeled carotid arteries from WT and SENP3+/− mice. (a) Representative images of dual immunofluorescence staining of SENP3 and SM-α-actin and quantification of percentage of SENP3-positive VSMCs in WT and SENP3+/− mice (*p < 0.05, n = 6). (b) Representative images of dual immunofluorescence staining of β-catenin and SM-α-actin and quantification of percentage of β-catenin-positive VSMCs and percentage of β-catenin-positive area in ligated carotid arteries from WT and SENP3+/− mice (*p < 0.05, n = 6). Values are mean ± SEM.
SENP3 expression is positively correlated with β-catenin expression in human neointimal hyperplasia
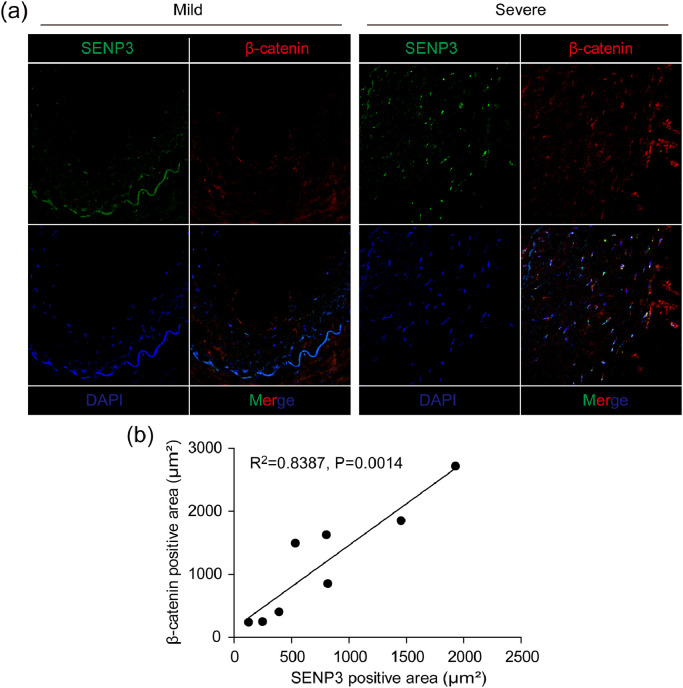
To determine whether the role of SENP3 observed in murine vascular remodeling models translates to human vascular diseases, we investigated the protein levels of SENP3 and β-catenin simultaneously in human specimens of lower extremity artery with neointimal hyperplasia. We found that the protein levels of both SENP3 and β-catenin were relatively low in diseased arteries with mild neointimal hyperplasia, but dramatically increased in lesions with severe neointimal hyperplasia (Fig. 9(a)). Furthermore, we found that the expression of β-catenin was positively correlated with the expression of SENP3 (Fig. 9(b)).
Fig. 9.
SENP3 expression is positively correlated with β-catenin expression in human neointimal hyperplasia. (a) Representative images of dual immunofluorescence staining of SENP3 and β-catenin in human specimens of lower extremity artery with neointimal hyperplasia. (b) Correlation between SENP3 positive area and β-catenin positive area.
4. Discussion
Accumulating evidence strongly implicates oxidative stress as the most common cell stress and common reason for vascular remodeling. However, the precise molecular mechanism underlying this oxidative stress-induced vascular remodeling has not been fully elucidated. The present study has revealed the important role of redox-sensitive SENP3 in mediating the development of vascular remodeling. SENP3 protein levels were rapidly and markedly increased in a ROS-dependent manner after exposure to various stimuli relevant to vascular remodeling, mainly oxLDL, Ang II and low shear stress both in vivo and in vitro. SENP3 interacts with β-catenin and inhibits its proteasome-dependent degradation through de-SUMOylation, which ultimately promotes the proliferation and migration of VSMCs and contributes to vascular remodeling.
SUMO specific proteases (SENPs) are cysteine proteases which play important roles in maintaining the balance between SUMO/de-SUMOylated proteins required for normal cellular physiology. There are six isoforms of SENPs identified in humans (SENP1–3 and 5–7) [29]. Many previous studies have highlighted the pivotal role of SENPs in the development of various diseases, especially cancer [[10], [11], [12], [30], [31], [32]]. However, there are few studies concerning the role of SENPs and SUMOylation in vascular diseases. Recently, Qiu ei al reported that SENP1 regulates the SUMOylation of GATA2 that leads to endothelial dysfunction in graft atherosclerosis [33]. To our knowledge our study is the first study that demonstrates the important role of SENP3, a redox-sensitive protease, in the development of vascular remodeling. We generated three mouse models of vascular remodeling due to low shear stress, hypertension, and atherosclerosis. In all these models, we found SENP3 to be markedly increased in VSMCs accompanied by elevated ROS levels in remodeled arteries. These findings demonstrate that SENP3 and SENP3-mediated reprogramming of cell signaling processes might be a common mechanism of oxidative stress-induced vascular remodeling under various pathological conditions.
Our previous studies have demonstrated that SENP3 plays critical roles in cancer development and progression [10], [11], [12]. SENP3 enhances the transcriptional activity of HIF-1α by SUMOylating P300 and promotes tumor angiogenesis [10]. SENP3 de-SUMOylates PML and Stat3 to increase tumor cell proliferation [11,13]. Additionally, SENP3 promotes epithelial-mesenchymal transition (EMT) in gastric cancer cells via de-SUMOyltion and enhancing the transitional activity of FOXC2 [12]. In the present study, we demonstrated that SENP3 facilitates VSMC proliferation and migration and this effect is mediated, at least in part, via de-SUMOylation of β-catenin and regulation of its protein stability. And certainly, there might be other protein substrates of SENP3, especially those regulating the proliferation, migration and phenotypic switching of VSMCs. Most importantly, our in vivo experiments revealed that SENP3 expression was correlated with intima area in remodeled arteries. Besides, SENP3+/− mice exhibited reduced vascular remodeling. Therefore, SENP3 acts not only as a sensor of oxidative stress, but also as a key mediator or effector in oxidative stress-induced pathological process via regulation of SUMO/de-SUMOylation balance of numbers of protein substrates and subsequent the function of these proteins.
The Wnt/β-catenin pathway is an important signaling pathway that regulates vascular development and homeostasis. We have previously published that β-catenin signaling pathway was activated under Ang II stimulation in vitro and in vivo [18]. And this signaling pathway is negatively regulated by orphan nuclear receptor Nur77 [18]. In the present study, we found that β-catenin is modified by SUMOylation which can be reversibly de-conjugated by SENP3. Moreover, SENP3 promotes β-catenin stability, whereas SUMOylation facilitates proteasome-dependent β-catenin degradation. Recent studies showed that SUMOylation can serve as a targeting signal in the ubiquitin-proteasome system (UPS) [34]. SUMO-targeted Ubiquitin Ligases (STUbLs) are recruited to SUMOylated target proteins or those containing SUMO-like domains to catalyze their ubiquitination and degradation [24,35,36]. Our results indicated that β-catenin is targeted to proteasome for degradation in a SUMOylation-dependent manner. However, as degradation of β-catenin was well demonstrated to be regulated by phosphorylation, whether the SUMO/de-SUMOylation status of β-catenin affects β-catenin degradation directly or indirectly via affecting its phosphorylation status is unknown.
In summary, we provided the first evidence that SENP3 is a redox-sensitive protease that accumulates during oxidative stress-induced vascular remodeling, which promotes VSMC proliferation and migration via regulation of β-catenin protein stability. Further work is needed to fully understand the exact role of SENP3 and SENP3-mediated SUMO/de-SUMOylation status of specific protein substrates in vascular remodeling during hypertension, atherosclerosis, vascular injury, restenosis, and aneurysm formation.
Author contributions
Z.C., Z.W., R.Y., M.C., Y.L., Y.W., and P.N. conducted experiments, data analysis, and interpretation. Z.C. wrote the manuscript. L.S., J.Y., and B.H. conceived and designed the experiments, analyzed the data, and revised the manuscript. All authors approved the final version of the manuscript.
CRediT authorship contribution statement
Zhaohua Cai: Formal analysis. Zi Wang: Formal analysis. Ruosen Yuan: Formal analysis. Mingli Cui: . Yimin Lao: . Ying Wang: . Peng Nie: . Linghong Shen: Formal analysis. Jing Yi: Formal analysis. Ben He: Formal analysis.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by grants numbers 81330006, 81830010, 91539106, 81770428 and 31230037 from the National Natural Science Foundation of China; grand number 2017YFC 0909301 from the National key R&D program; grand number 18411950400 from the Shanghai Municipal Natural Science Foundation, grant number 2013CB910900 from the National Ministry of Science and Technology of China. We thank the members of the Department of Laboratory Animal Science, Shanghai Jiaotong University School of Medicine for their technical support. The funders had no role in the study design, data collection, data analyses, interpretation, or writing of the manuscript.
Data availability statement
All the data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding authors upon reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103386.
Contributor Information
Jing Yi, Email: yijing@shsmu.edu.cn.
Ben He, Email: drheben@126.com.
Appendix. Supplementary materials
References
- 1.Yung L.M., Leung F.P., Yao X., Chen Z.Y., Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targ. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 2.Souza H.P., Souza L.C., Anastacio V.M. Vascular oxidant stress early after balloon injury: evidence for increased NAD(P)H oxidoreductase activity. Free Radic Biol Med. 2000;28:1232–1242. doi: 10.1016/s0891-5849(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 3.Szocs K., Lassegue B., Sorescu D. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 4.Higashi Y., Noma K., Yoshizumi M., Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 5.Geiss-Friedlander R., Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 6.Kerscher O. SUMO junction-what's your function? New insights through SUMO-interacting motifs. EMBO Rep. 2007;8:550–555. doi: 10.1038/sj.embor.7400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bawa-Khalfe T., Yeh E.T. SUMO losing balance: SUMO proteases disrupt SUMO homeostasis to facilitate cancer development and progression. Gen Cancer. 2010;1:748–752. doi: 10.1177/1947601910382555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feligioni M., Nistico R. SUMO: a (oxidative) stressed protein. Neuromol Med. 2013;15:707–719. doi: 10.1007/s12017-013-8266-6. [DOI] [PubMed] [Google Scholar]
- 9.Yan S., Sun X., Xiang B. Redox regulation of the stability of the SUMO protease SENP3 via interactions with CHIP and Hsp90. EMBO J. 2010;29:3773–3786. doi: 10.1038/emboj.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., Han Y., Wang Y. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009;28:2748–2762. doi: 10.1038/emboj.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y., Huang C., Sun X. SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated with accelerated cell proliferation under mild oxidative stress. J Biol Chem. 2010;285:12906–12915. doi: 10.1074/jbc.M109.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren Y.H., Liu K.J., Wang M. De-SUMOylation of FOXC2 by SENP3 promotes the epithelial-mesenchymal transition in gastric cancer cells. Oncotarget. 2014;5:7093–7104. doi: 10.18632/oncotarget.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z., Wang M., Li J. SUMOylation and SENP3 regulate STAT3 activation in head and neck cancer. Oncogene. 2016;35:5826–5838. doi: 10.1038/onc.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Xiao Y., Mou Y., Zhao Y., Blankesteijn W.M., Hall J.L. A role for the beta-catenin/T-cell factor signaling cascade in vascular remodeling. Circ Res. 2002;90:340–347. doi: 10.1161/hh0302.104466. [DOI] [PubMed] [Google Scholar]
- 15.Quasnichka H., Slater S.C., Beeching C.A., Boehm M., Sala-Newby G.B., George S.J. Regulation of smooth muscle cell proliferation by beta-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ Res. 2006;99:1329–1337. doi: 10.1161/01.RES.0000253533.65446.33. [DOI] [PubMed] [Google Scholar]
- 16.Tsaousi A W.H., Lyon C.A., Taylor V., Swain A., Johnson J.L., George S.J. Wnt4/beta-catenin signaling induces vsmc proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–436. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 17.Bedel A., Negre-Salvayre A., Heeneman S. E-cadherin/beta-catenin/T-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ Res. 2008;103:694–701. doi: 10.1161/CIRCRESAHA.107.166405. [DOI] [PubMed] [Google Scholar]
- 18.Cui M., Cai Z., Chu S. Orphan nuclear receptor nur77 inhibits angiotensin ii-induced vascular remodeling via downregulation of beta-catenin. Hypertension. 2016;67:153–162. doi: 10.1161/HYPERTENSIONAHA.115.06114. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi A., Kishida S., Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y., Cai Z., Cui M. The orphan nuclear receptor nur77 inhibits low shear stress-induced carotid artery remodeling in mice. Int J Mol Med. 2015;36:1547–1555. doi: 10.3892/ijmm.2015.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin S.X., Shen L.H., Nie P. Endogenous renovascular hypertension combined with low shear stress induces plaque rupture in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2372–2379. doi: 10.1161/ATVBAHA.111.236158. [DOI] [PubMed] [Google Scholar]
- 22.Yang J., Li H., Chen Y.Y. Anthraquinones sensitize tumor cells to arsenic cytotoxicity in vitro and in vivo via reactive oxygen species-mediated dual regulation of apoptosis. Free Radic Biol Med. 2004;37:2027–2041. doi: 10.1016/j.freeradbiomed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Jovinge S., HultgardhNilsson A., Regnstrom J., Nilsson J. Tumor necrosis factor-alpha activates smooth muscle cell migration in culture and is expressed in the balloon-injured rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:490–497. doi: 10.1161/01.atv.17.3.490. [DOI] [PubMed] [Google Scholar]
- 24.Wang M., Sang J., Ren Y. SENP3 regulates the global protein turnover and the Sp1 level via antagonizing SUMO2/3-targeted ubiquitination and degradation. Protein Cell. 2016;7:63–77. doi: 10.1007/s13238-015-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisgrove S.R., Lee Y.R., Liu B., Peters N.T., Kropf D.L. The microtubule plus-end binding protein EB1 functions in root responses to touch and gravity signals in arabidopsis. Plant Cell. 2008;20:396–410. doi: 10.1105/tpc.107.056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang P.C.Y., Qin L.F., Zielonka J. MyD88-dependent, superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med. 2008;205:3159–3171. doi: 10.1084/jem.20081298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Yang J., Yang K. The biphasic redox sensing of SENP3 accounts for the HIF-1 transcriptional activity shift by oxidative stress. Acta Pharmacol Sin. 2012;33:953–963. doi: 10.1038/aps.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lao Y., Yang K., Wang Z. DeSUMOylation of MKK7 kinase by the SUMO2/3 protease SENP3 potentiates lipopolysaccharide-induced inflammatory signaling in macrophages. J Biol Chem. 2018;293:3965–3980. doi: 10.1074/jbc.M117.816769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A., Zhang K.Y. Advances in the development of SUMO specific protease (SENP) inhibitors. Comput Struct Biotechnol J. 2015;13:204–211. doi: 10.1016/j.csbj.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bawa-Khalfe T., Lu L.S., Zuo Y., Huang C., Dere R., Lin F.M. Differential expression of SUMO-specific protease 7 variants regulates epithelial-mesenchymal transition. Proc Natl Acad Sci USA. 2012;109:17466–17471. doi: 10.1073/pnas.1209378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu J., Fan Y., Liu X. SENP1 protects against myocardial ischaemia/reperfusion injury via a HIF1alpha-dependent pathway. Cardiovasc Res. 2014;104:83–92. doi: 10.1093/cvr/cvu177. [DOI] [PubMed] [Google Scholar]
- 32.Cashman R., Cohen H., Ben-Hamo R., Zilberberg A., Efroni S. SENP5 mediates breast cancer invasion via a TGFbetaRI SUMOylation cascade. Oncotarget. 2014;5:1071–1082. doi: 10.18632/oncotarget.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu C., Wang Y., Zhao H. The critical role of SENP1-mediated GATA2 deSUMOylation in promoting endothelial activation in graft arteriosclerosis. Nat Commun. 2017;8:15426. doi: 10.1038/ncomms15426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schimmel J., Larsen K.M., Matic I. The ubiquitin-proteasome system is a key component of the SUMO-2/3 cycle. Mol Cell Proteom. 2008;7:2107–2122. doi: 10.1074/mcp.M800025-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Sriramachandran A.M., Dohmen R.J. SUMO-targeted ubiquitin ligases. Biochim Biophys Acta. 2014;1843:75–85. doi: 10.1016/j.bbamcr.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Geoffroy M.C., Hay R.T. An additional role for SUMO in ubiquitin-mediated proteolysis. Nat Rev Mol Cell Biol. 2009;10:564–568. doi: 10.1038/nrm2707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding authors upon reasonable request.