Abstract
Background:
The main symptoms of fibromyalgia comprise diffuse pain, disability, depressive symptoms, catastrophizing, sleep disruption and fatigue, associated with dysfunction of the descending pain-modulating system (DPMS).
Objectives:
We aimed to identify patterns of main symptoms of fibromyalgia and neuroplasticity biomarkers (i.e. brain-derived neurotrophic factor (BDNF) and S100B protein) in non-responders to the conditioned pain modulation task (CPM-task) induced by immersion of hand in cold water (0–1°C). Furthermore, we evaluated if these patterns predict responsiveness to CPM-task.
Methods:
This cross-sectional study included 117 women with fibromyalgia ((n = 60) non-responders and (n = 57) responders), with age ranging from 30 to 65 years old. We analysed changes in numerical pain scale (NPS-10) during the CPM-task using a standardized protocol.
Results:
A hierarchical multivariate logistic regression analysis was used to construct a propensity score-adjusted index to identify non-responders compared to responders to CPM-task. The following variables were retained in the models: analgesic use four or more times per week, heat pain threshold (HPT), poor sleep quality, pain catastrophizing, serum levels of BDNF, number of psychiatric diagnoses and the impact of symptoms of fibromyalgia on quality of life. Receiver operator characteristics (ROC) analysis showed non-responders can be discriminated from responders by a composite index of more frequent symptoms of fibromyalgia and neuroplasticity markers (area under the curve (AUC) = 0.83, sensitivity = 100% and specificity = 98%).
Conclusion:
Patterns of fibromyalgia symptoms and neuroplasticity markers may be helpful to predict responsiveness to the CPM-task which might help personalize treatment and thereby contribute to the care of patients with fibromyalgia.
Keywords: Catastrophizing, brain-derived neurotrophic factor, S100B, chronic pain, conditioned pain modulation-task
Introduction
Fibromyalgia is characterized by chronic pain with symptoms disproportionate to the evidence of tissue injury or anatomical damage. Although its pathophysiology is not fully elucidated, the most accepted mechanism to evoking pain hypersensitivity in fibromyalgia is the central sensitization syndrome (CSS).1 It encompasses the impaired functioning of neurons and circuits in nociceptive pathways, with an increase in neuronal excitability and synaptic efficacy and reduced inhibition.2,3 Preclinical studies showed that astrocytic and microglial activation triggers the increase in brain-derived neurotrophic factor (BDNF). It alters the transmembrane anion gradient by downregulating K+-Cl− cotransporter 2 (KCC2), which increases in intracellular Cl−, which leads to changes in GABA- and glycine-evoked responses from inhibitory to excitatory.4–6
This hyperexcitability pattern is a primary underpin mechanism of the CSS. It comprises a cluster of symptoms, such as psychological distress, sleep disturbances, fatigue, pain, allodynia, hyperalgesia and expansion of the receptive field.7,8 To identify symptoms related to CSS, a screening tool has been used, which is denominated by Central Sensitization Inventory (CSI). A higher score on the CSI for chronic pain was positively associated with the inefficiency of the descending pain-modulating system (DPMS).9 The disinhibition on DPMS indicates a defective of DPMS according to a spectrum of responders and non-responders to conditioned pain modulating (CPM)-task in different chronic pain conditions.
The S100B protein can be actively released from glial cells, namely, oligodendrocytes and astroglia in the human brain.10,11 The glial marker protein S100B is elevated during major depressive episodes and decreased following successful treatment.12 While the neuronal activity regulates the transcription of the BDNF gene, the transport of BDNF mRNA protein into dendrites and the secretion of BDNF.13 According to an earlier study, we found that serum levels of S100B were lowest in fibromyalgia than chronic pain conditions with significant structural damage (e.g. osteoarthritis and endometriosis). In contrast, serum levels of BDNF were higher in chronic pain with scarce tissue damage (e.g. fibromyalgia, chronic tensional headache and myofascial pain).14 In addition, the dysfunction in the DPMS was more significant in chronic pain with a scarce tissue injury (e.g. fibromyalgia) compared to osteoarthritis.14
The DPMS can be assessed by the CPM paradigm. During CPM-task, the nociceptive heterotopic stimuli activate the descending inhibitory control (DNIC), and it produces a phenomenon where ‘pain-inhibits pain’.15,16 The neurobiological systems involved in the CPM-task includes serotoninergic, opioidergic and noradrenergic systems,17–19 which are also engaged in the psychological characteristics of chronic pain (i.e. anxiety,20–24 depression21,23 and pain catastrophizing).22 This interplay among neurobiological pathways underpinning to DPMS with the psychological characteristics may explain at least part of the interpersonal variability in pain perception.25 Although a meta-analysis tried to expand the repertoire of psychological factors in CPM response, the authors recognized as major limitations of their analysis, varied approaches on the CPM paradigm, the mix of pain conditions with different pathophysiology and non-standardized protocols for diagnosis criteria.26 Thus, it is pertinent to construct a comprehensive and biologically plausible framework to capture the symptoms and clinical signs associated with the disruption of the endogenous pain-inhibitory system.27
Considering the above-mentioned facts, it is reasonable to assess the CPM paradigm according to a spectrum of responders and non-responders, in integrating perspective, to explore the severity of symptoms frequently found in the fibromyalgia (i.e. pain, disability, depressive symptoms, pain catastrophizing, sleep disruption, fatigue, etc.), psychological measures and serum neuroplasticity biomarkers (BDNF and S100B protein). Our study has two specific aims: (1) to construct a framework that integrates different patterns of symptoms intensity across fibromyalgia and serum markers of neuroplasticity to test the hypothesis that these sets of variables could predict the dysfunction of DPMS and (2) to evaluate the ability of each marker of neuroplasticity (BDNF and S100-B-protein) and a composite index that integrates these measures (symptoms of fibromyalgia, HPT, pain catastrophizing, analgesic use, psychotropic medications, psychiatric disorders, BDNF and S100-B-protein) to differentiate non-responders to CPM-task, compared to responders. The paradigm of CPM was tested by the change in numerical pain scale (NPS scores in the range of 0–10), during the quantitative sensory testing (QST) concurrently with the heterotopic nociceptive stimulus (CPM-task) induced by immersion of hand in cold water (0–1°C) for 60 seconds.
Material and methods
Procedure and participants
The methods and results sections are reported according to the Strengthening The Reporting of OBservational Studies in Epidemiology (STROBE) guidelines. All subjects provided oral and written informed consent before participating in this cross-sectional study. The study followed the guidelines and regulations for clinical research and was approved by the Research Ethics Committee at our institution.
Recruitment, inclusion and exclusion criteria
All patients were recruited by directly contacting them from the institutional chronic pain clinic, by referrals from other clinic units and from a community that is situated in an urban circumscribed geographic area that constitutes the catchment area of the Basic Health Unit linked to the (name of Institution), which serves a population of approximately 8000 living nearby. Recruitment was undertaken in the time from August 2016 to December 2017. Patients were contacted by phone and answered a screening questionnaire. If they met the inclusion criteria, they were invited for medical evaluation, history collection and detailed description of their symptoms. The diagnosis was established by a board-certified pain specialist to (name of country) Board with more than 10 years of experience in patients’ pain care. We included only women, because fibromyalgia is more prevalent in females.28 Women aged between 30 and 65 years who were able to read and write and had a confirmed diagnosis of fibromyalgia according to the criteria of the American College of Rheumatology (2010–2016) were included. Pain score needed to be equal to or greater than six on NPS 0–10 on most days for the last 3 months. We excluded pregnant women and patients with a history of alcohol or drug abuse in the last 6 months, neurological diseases, history of decompensated systemic diseases or any chronic inflammatory disease (e.g. lupus, rheumatoid arthritis and Reiter’s syndrome). Furthermore, we excluded patients with uncompensated hypothyroidism or history of cancer.
Instruments and assessments
Dependent and independent variables of main interest
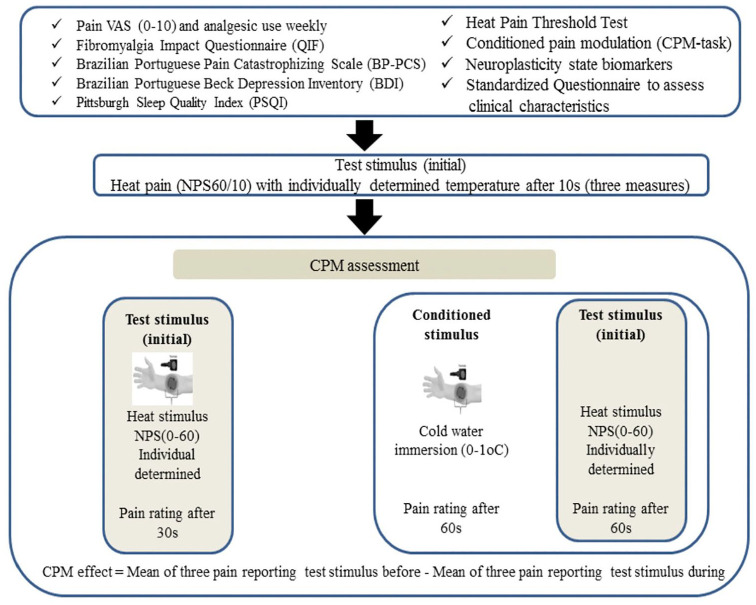
The dependent variable (outcome) was the ‘score on NPS (0–10)’ during the CPM classified as non-responders and responders to CPM-task. The timeline of assessments is presented in Figure 1. The main interest factors in this study were the following: Brazilian Portuguese pain catastrophizing scale (BP-PCS), fibromyalgia impact questionnaire (FIQ), visual analogue pain scale (VAS), heat pain threshold (HPT), beck depression inventory II (BDI-II), Pittsburgh sleep quality index (PSQI), BDNF and S100-B calcium protein.
Figure 1.
Timeline assessments.
Psychophysical measures
HPT test: This measure uses the method of limits with a computer Peltier-based contact thermode (30 × 30 mm2)29,30 attached to the skin on the ventral aspect of the mid-forearm. A computerized version of thermotest (developed by our group) was used to determine the HPT on the volar side of the non-dominant forearm. When heat is set at 32°C, the thermostat is heated at a rate of 1.0°C/second to a maximum of 52°C, when the temperature begins to drop. The HPT is the minimum temperature at which the stimuli become painful. It was obtained by the mean of three assessments performed with an inter-stimuli interval of 40 seconds.29
CPM-task: The sequence of procedures to determine the DPMS was the following: First, we used the same computerized version of thermotest described above on HPT measures to define the average of three temperatures by the QST for patients’ report score 6/10 (NPS, 0–10). The position of the thermode on the non-dominant forearm was slightly altered between trials (although it remained on the left ventral forearm). Second, after 5 minutes, subjects were requested to immerse their dominant hand up to the wrist into the water at a temperature of 0 up to 1°C for 1 minute, and the QST was introduced after 30 seconds. Thus, the pain score on NPS (0–10) in the area of the thermotest was assessed. Third, the CPM was calculated by the difference between the pain score on NPS (0–10) during the cold-water immersion (QST + CPM) at the temperature they set 6/10 on NPS (0–10) during the initial time (T0). Non-responders showed a difference in the count on NPS (HPT1 − HPT0) equal to zero or higher, and for responders, these values would be lower than zero.31
Pain measures, sleep quality, pain catastrophizing, depressive symptoms, demographic data and medical comorbidities
All instruments have been validated for the (name of country) population. Two evaluators with specific training were responsible for all assessments.
FIQ32 was used to evaluate the impact of symptoms on quality of life. The FIQ consists of 10 domains and items comprising questions to assess the patient’s ability to perform routine activities of daily living, fatigue, morning, stiffness, mood, anxiety and depression. Higher scores indicate worst conditions, and the maximum score is 100.32,33
VAS ranged from no pain (0 mm) to worst pain possible (100 mm),34 according to participants’ pain score in most days in the last 3 months.
Analgesic use was defined by an average of analgesic used per week during the previous month. For data analysis, analgesic use was included as a dichotomous variable (the use of analgesics at least four or fewer days per week or the use for more than 4 days per week). This approach was chosen because patients with chronic pain rescue analgesic use change each week, depending on their level of pain.
BP-PCS33 was used to evaluate the pain catastrophizing. The BP-PCS is a self-administered questionnaire that consists of 13 items. It is divided into three dimensions of catastrophizing in response to pain: rumination, magnification and helplessness.35 The BP-PCS total score ranges from 0 to 52 points.
BDI was used to evaluate depressive symptoms.36
PSQI was used to measure the quality and patterns of sleep over the last month.37
Standardized questionnaire: a standardized query was used to assess demographic data and medical comorbidities. Patients were requested to provide information about their age, sex, level of education, marital status and lifestyle habits. They also provided information about their health status, including clinical and psychiatric diagnoses, the latter assessed by the Mini-international Neuropsychiatric Interview (MINI).38
Neuroplasticity state biomarkers (BDNF and S100B)
To assess the neuroplasticity state biomarkers, we evaluated using serum levels of BDNF and S100B collected in plastic tubes and centrifuged for 10 minutes at 4500 r/min at 4°C in a −80°C freezer for further BDNF assays. All samples were assayed in duplicate to avoid intra-assay variation. BDNF serum level was analysed by enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies specific for BDNF (R&D Systems, MN, USA, catalogue number DY248, BDNF with the lowest detection limit = 11.7 pg/mL). The plates were incubated with capture antibody overnight (room temperature). After incubation, the plates were washed. The samples and standards were added in the plate and incubated by 2 hours and washed again. The detection antibody was added and incubated for 2 hours. After another washing, Streptavidin–horseradish peroxidase (HRP) conjugate was added and after 20 minutes of incubation, the last washing step was done. Thus, the substrate solution was added, and after 20 minutes, the reaction was stopped by the addition of acidic solution. Optical density was measured using an ELISA reader at a wavelength of 450 nm (GloMax®-Multi Microplate Reader; Promega, WI, USA) for the assay measurements.
Human S100B ELISA and enzyme-linked immunosorbent assay kit (EZHS100B-33K; Millipore, MI, USA, the lowest detection limit is 2.7 pg/mL) were used to determine serum levels. The sample was incubated by 2 hours after it was washed and the monoclonal anti-human S100B antibody was added. After another washing, Streptavidin–HRP conjugate was added and after 30 minutes of incubation, the last washing step was done. Thus, the substrate solution was added, and after 20 minutes, the reaction was stopped by the addition of acidic solution. All the S100 measurements were performed at 30 °C. Optical density was measured using an ELISA reader at a wavelength of 450 nm (GloMax®-Multi Microplate Reader; Promega, WI, USA) for the assay measurements. For all experiments, the absorbance is proportional to the concentration of S100B, according to the standard curve used.
Efforts to address potential sources of bias
To reduce assessment bias, two researchers with vast clinical expertise to treat outpatients in pain clinic were responsible for making the diagnostics according to pre-specified criteria. Two evaluators with specific training were responsible for all assessments and to apply the standardized protocol to assess the QST and the CPM-task.
Statistical analysis
An estimated 102 subjects would be needed to provide 80% power for a receiver operating characteristic (ROC) study, assuming the area under the curve (AUC) is 0.7 with a two-sided α of 0.05.39 Finally, considering the likely attrition rate and other unexpected factors, we increased the sample by 15%, and the required sample size was 117 patients.
To summarize the main socio-demographic features of the sample, we used a descriptive statistic. t-tests for independent samples or Wilcoxon–Mann–Whitney, chi-square and Fisher’s exact tests were performed to compare variables between responders and non-responders’ group. To control potential confounding variables on the relationship between changes in NPS (0-10) during CPM test, classified as responder and non-responders, we performed a forward stepwise hierarchical binary logistic regression model (Table 3).2,40 A p value of less than 0.10 was required for a factor to be included in a hierarchical regression model. Considering that several variables can affect the DPMS, we constructed a propensity score-adjusted index.22 This multivariate analysis considered the hierarchical relationships between the proposed factors. The first hierarchical model included demographic data that could directly or indirectly determine all the variables analysed in the additional hierarchical levels. The odds ratio (OR) values resulting from the multivariate analysis were not derived from the full model with all variables, but from the equation corresponding to the level at which the factor of interest was first entered. This avoids the possibility that mediating variables will reduce some of the explanatory power of more distant determinants.
Table 3.
ROC analysis to detect the failure of DPMS according to the serum levels of BDNF and S100B protein (n = 117).
| AUC | 95% CI | Cutoffs | Sensitivity | Specificity |
|---|---|---|---|---|
| Serum brain-derived neurotrophic factor (BDNF) (ng/mL) | ||||
| 0.67 | (0.57–0.77) | 7.6900 | 1.000 | 1.000 |
| 10.2100 | 1.000 | 0.982 | ||
| 11.9250 | 1.000 | 0.965 | ||
| 12.4600 | 1.000 | 0.947 | ||
| 13.9700 | 0.983 | 0.947 | ||
| 16.4850 | 0.983 | 0.930 | ||
| 18.3500 | 0.983 | 0.912 | ||
| 19.2550 | 0.983 | 0.895 | ||
| 19.9050 | 0.983 | 0.877 | ||
| 20.1950 | 0.983 | 0.860 | ||
| Serum S100-B protein (ng/mL) | ||||
| 0.58 | (0.45–0.66) | 3.9600 | 1.000 | 1.000 |
| 5.4950 | 1.000 | 0.965 | ||
| 6.1650 | 0.983 | 0.947 | ||
| 6.3450 | 0.983 | 0.930 | ||
| 6.5750 | 0.933 | 0.930 | ||
| 6.9450 | 0.917 | 0.912 | ||
| 7.2900 | 0.900 | 0.895 | ||
| 7.5650 | 0.883 | 0.895 | ||
AUC: area under the curve; CI: confidence interval; ROC: receiver operator characteristics.
The nonparametric ROC analysis was performed to differentiate the properties of propensity score index in each step according to the spectrum of non-responders and responders to CPM-task. The AUCs with exact binomial 95% confidence intervals (CI) are presented. The cutoff values with the highest Youden index, with 90% sensitivity and with 100% specificity is presented each one of four indexes and all they showed a ROC AUC higher than 0.68. Statistical significance was set to a p value of 0.05, two-tailed. Data were analysed using SPSS software version 22.0 (SPSS, Chicago, IL).
Results
Patient characteristics
We screened 164 potential participants with a diagnosis of fibromyalgia, and we included 117 in the study. Thirty-seven not fulfilling the diagnostic criteria for fibromyalgia or presented pain level on most days for the last 3 months was defined in the protocol (NPS 6/10) and 10 had another clinical diagnosis defined as exclusion criteria (rheumatoid arthritis, lupus, non-compensate hypothyroidism, etc.). All enrolled subjects participated in a complete assessment of the study and were included in all analyses (Table 1). All patients presented a detectable level of BDNF and S100B according to their detection limits.
Table 1.
Demographic and clinical characteristics of the study sample. Values are given as the mean (SD) or frequency (n = 117).
| Non-responder (n = 60) | Responder (n = 57) | p value | |
|---|---|---|---|
| Age (years) | 48.43 (9.29) | 50.54 (7.84) | 0.19 |
| Body index | 28.38 (4.70) | 27.83 (5.02) | 0.54 |
| Education (years) | 10.28 (4.20) | 10.60 (4.12) | 0.68 |
| Smoking (Yes/No) | 12/48 | 9/48 | 0.36 |
| Clinical comorbidity (Yes/No) | 25/35 | 28/29 | 0.27 |
| Hypertension (Yes) | 27 | 23 | |
| Diabetes (Yes) | 7 | 9 | |
| Hypothyroidism (Yes) | 0 | 1 | |
| Asthma (Yes) | 1 | 0 | |
| Other (Yes) | 2 | 0 | |
| Number of days analgesic used per week in the last 3 months (<4 times/>4 times)** | 11/49 | 20/37 | 0.03 |
| Acetaminophen/Dipirone (Yes) | 29 | 16 | |
| Dorflex (Yes) | 21 | 16 | |
| NSAID (Yes) | 7 | 5 | |
| Opioid (Yes) | 2 | 0 | |
| Psychiatric disorder according to MINI (Yes/No)* | 23/37 | 20/41 | 0.43 |
| Major depressive episode with dysthymia | 25 | 21 | |
| Manic-depressive disorder | 7 | 6 | |
| Post-traumatic stress disorder | 3 | 3 | |
| Generalized anxiety disorder | 3 | 3 | |
| Active use of central nervous system active medication (Yes/No)** | 22/38 | 16/41 | 0.21 |
| Antidepressant (n/%) | 14 | 15 | |
| Anticonvulsant (n/%) | 2 | 2 | |
| Benzodiazepine (n/%) | 2 | 5 |
NSAID: nonsteroidal anti-inflammatory drugs; MINI: mini-international Neuropsychiatric Interview.
Some patients were using more than one type of drug; *Some patients were more than one psychiatric disorder
Univariate analysis
Relationships between outcome and spectrum of responder and non-responders to CPM test
Pain catastrophizing, disability and quality of life are related to fibromyalgia, pain scores on the VAS, HPT, depressive symptoms, sleep quality and serum levels of BDNF and S100-B calcium protein according to the spectrum of responders and non-responders to CPM-task (Table 2).
Table 2.
Relationship of the outcomes and the main interest factor according to the spectrum of responders and non-responders to CPM-task. Data are presented as mean and standard error (n = 117).
| Non-responders (n = 60) | Responders (n = 57) | p value | |
|---|---|---|---|
| Visual analogue scalea | 7.49 (1.68) | 6.81 (1.58) | 0.03 |
| Beck depression inventory II (BDI-II)a | 25.43 (11.49) | 20.09 (8.87) | 0.00 |
| Brazilian Portuguese pain catastrophizing scalea | 35.73 (10.55) | 26.95 (12.09) | 0.00 |
| Pittsburgh sleep quality index (PSQI)a | 19.45 (7.01) | 15.75 (8.20) | 0.01 |
| Brain-derived neurotrophic factor (BDNF) (ng/mL)a | 50.58 (29.34) | 40.61 (21.14) | 0.03 |
| S100-B calcium protein (pg/mL)a | 22.18 (11.04) | 17.06 (8.22) | 0.02 |
| Fibromyalgia impact questionnaire (FIQ)a | 69.24 (13.06) | 58.75 (13.66) | 0.00 |
| Change in numerical pain scale during CPM-taskb | 1.20 (1.02) | –1.90 (1.38) | 0.00 |
CPM: conditioned pain modulation.
Compared by t-test for independent sample.
Compared by the Mann–Whitney test.
Putative markers of neuroplasticity BDNF and S100-B protein as a tool to detect the failure of DPMS
To explore how the putative markers of neuroplasticity BDNF and S100-B protein are related to CPM dysfunction, their discriminate properties to screen non-responders to CPM-task using their serum levels are presented in Table 3. The respective cutoff points each one of them would offer were at least 90% or higher sensitivity, and they reached 80% or higher in the specificity as shown in Table 3.
The ability of each of these putative markers of neuroplasticity to discriminate non-responders to CPM-task from responders is presented in Figure 2. By setting the AUC, the ability for BDNF (cutoff point >20.19) to distinguish non-responders to CPM-task was 67%, providing 90% sensitivity and 85% specificity. The ability for S100B protein (cutoff point >7.56) to identify correctly non-responders to the CPM-task was 58%, providing 88% sensitivity and 89% specificity.
Figure 2.
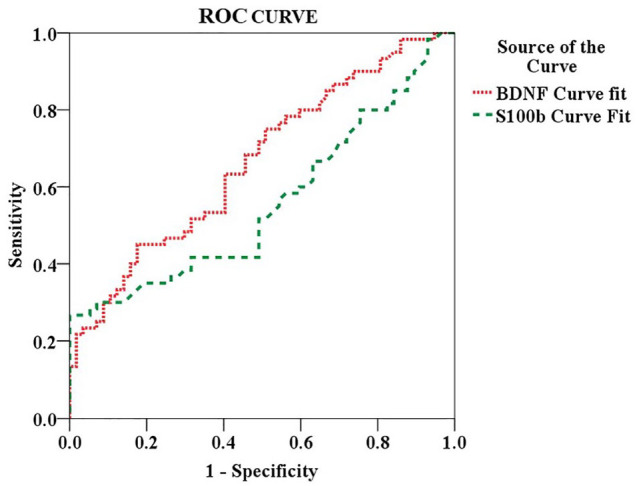
Nonparametric receiver operating characteristics (ROC) analyses. The area under the curve (AUC) with exact binomial 95% confidence intervals (CIs) according to each cutoff point of serum BDNF and S100-B protein is presented in Table 3.
Multiple conditional logistic regression analysis to construct the propensity score-adjusted index to identify factors according to the spectrum of responders and non-responders to CPM-task
The variables retained in the multiple conditional logistic regression analysis to construct the propensity score-adjusted index to quantifying those likely to be non-responders to CPM-task. Potential factors related to a greater propensity to the spectrum of responders and non-responders to CPM-task are summarized in Table 4. The OR values resulting from the multivariate analysis were not derived from the full model with all variables, but from the equation corresponding to the level at which the risk factor of interest was first entered.
Table 4.
Hierarchical multiple conditional logistic regression analysis to assess predictors for responsiveness to CPM-task failure in fibromyalgia (n = 117).
| Beta | SEM | Wald | p | Odds ratio | CI 95% | |
|---|---|---|---|---|---|---|
| Analgesic used (<4 times/⩾4 times per week) | 1.466 | 0.576 | 6.480 | 0.011 | 4.33a | (1.40–13.38) |
| Heat pain threshold (HPT) | 0.168 | 0.069 | 5.959 | 0.015 | 1.18a | (1.03–1.36) |
| Pittsburgh sleep quality index (PSQI, cutoff-Q25 ⩾ 17) | 0.978 | 0.496 | 3.889 | 0.049 | 2.66b | (1.06–7.03) |
| Brain-derived neurotrophic factor (BDNF) ⩾ 58.11(ng/mL) | 1.314 | 0.549 | 5.732 | 0.017 | 3.72b | (1.27–10.91) |
| Brazilian Portuguese pain catastrophizing scale ⩾33 | 1.300 | 0.477 | 7.432 | 0.006 | 3.67b | (1.44–9.34) |
| Number of psychiatric diagnosis | ||||||
| None | 5.827 | 0.054 | ||||
| One diagnosis | 1.537 | 0.753 | 4.163 | 0.041 | 4.65c | (1.06–20.36) |
| Two or more diagnoses | 1.804 | 0.752 | 5.747 | 0.017 | 6.07c | (1.39–26.54) |
| Fibromyalgia impact questionnaire (FIQ) ⩾ 78 | 1.927 | 0.775 | 6.189 | 0.013 | 6.86d | (1.50–31.34) |
FIQ: fibromyalgia impact questionnaire
Hierarchical multiple regression analysis. Cutoff point at quartile (cutoff-Q).
Model 1: First block – age, score on VAS (cutoff ⩾ 7), analgesic use (four or more than 4 days per week), heat pain threshold (HPT), psychotropic medication (antidepressant and anticonvulsants).
Model 2: Second block – model I along with catastrophizing pain (cutoff-Q25 = 33), S100B (cutoff-Q75 = 23.43), BDNF (cutoff-Q75 = 58.11) and PSQI (cutoff-Q25 ⩾ 17).
Model 3: Third block – model II along with depressive symptoms by BDI-II, number of psychiatric diagnoses according to the MINI (major depressive episode with dysthymia, mania-depressive disorder and generalized anxiety disorder).
Model 4: Fourth block – model III along with FIQ (cutoff-Q75 = ⩾78) to assess the impact of symptoms on quality of life.
On the first level of the model, the variables such as analgesic use (four or more than four time per weeks) and the HPT presented a higher risk for nor-responders to CPM-task with an OR = 4.33 and OR = 1.18, respectively. Lowering each Celsius degree to detect the HPT increased the risk for no responders to CPM-task by 18%. On the second level, a higher propensity for non-responders to CPM-task was associated with poor sleep quality (OR = 2.66), higher pain catastrophizing (OR = 3.67) and higher levels of serum BDNF (OR = 3.72). On the third level, compared to the absence of psychiatric with one and two or more psychiatric diagnoses showed a higher risk for non-responders to CPM-task (OR 4.65 and 6.07, respectively). On the fourth level, a higher impact of symptoms of fibromyalgia on quality of life had a strong association with non-responders to CPM-task (OR = 6.86).
The composite index of symptoms associated with fibromyalgia and putative neuroplasticity markers as a tool to detect the failure of DPMS
To screen non-responders to CPM-task using the scores of each one of these indexes according to each step, variables retained in each level of hierarchical multiple conditional logistic regression analysis are presented in Table 5. These measures with their respective cutoff points each one of them would offer were at least 90% or higher sensitivity and they reached 80% or higher in the specificity as shown in Table 5.
Table 5.
ROC analysis to detect the failure of DPMS according to the index of propensity score of each step of the hierarchical multiple logistic regression model (n = 117).
| AUC | 95% CI | Cutoffs | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Step I – (model I: analgesic use (four or more than 4 days per week) and HPT) | |||||
| 0.681 | (0.58–0.78) | 0.2185269 | 0.982 | 0.983 | |
| 0.2283567 | 0.965 | 0.967 | |||
| 0.2412153 | 0.965 | 0.950 | |||
| 0.2471011 | 0.965 | 0.933 | |||
| 0.2535917 | 0.947 | 0.933 | |||
| 0.2612195 | 0.947 | 0.917 | |||
| Step II – (model II: model I along with catastrophizing pain (cutoff-Q25 = 33), BDNF (cutoff-Q75 = 58.11) and PSQI (cutoff-Q75 ⩾ 17)) | |||||
| 0.785 | (0.70–0.87) | 0.0564277 | 1.000 | 0.983 | |
| 0.0736317 | 1.000 | 0.967 | |||
| 0.0814282 | 1.000 | 0.950 | |||
| 0.0838870 | 0.982 | 0.950 | |||
| 0.0892560 | 0.982 | 0.933 | |||
| 0.0942823 | 0.982 | 0.917 | |||
| 0.0962838 | 0.982 | 0.900 | |||
| Step III – (model III: model II along with number of psychiatric diagnosis according to the MINI (major depressive episode with dysthymia, maniac-depressive disorder and generalized anxiety disorder) | |||||
| 0.818 | (0.74–0.89) | 0.0153715 | 1.000 | 0.983 | |
| 0.0305837 | 1.000 | 0.967 | |||
| 0.0388753 | 1.000 | 0.950 | |||
| 0.0442709 | 1.000 | 0.933 | |||
| 0.0537171 | 1.000 | 0.917 | |||
| 0.0593224 | 1.000 | 0.900 | |||
| .0610924 | 0.982 | 0.900 | |||
| Step IV – global index (model 4: model III along with FIQ (cutoff-Q75 = ⩾78) | |||||
| 0.832 | (0.75–0.90) | 0.0089371 | 1.000 | 0.983 | |
| 0.0139259 | 1.000 | 0.967 | |||
| 0.0193599 | 1.000 | 0.950 | |||
| 0.0263755 | 1.000 | 0.933 | |||
| 0.0318990 | 1.000 | 0.917 | |||
| 0.0349366 | 1.000 | 0.900 | |||
AUC: area under the curve; BDNF: brain-derived neurotrophic factor; CI: confidence interval; ROCs: receiver operator characteristics; FIQ: fibromyalgia impact questionnaire; PSQI: Pittsburgh sleep quality index; HTP: heat pain threshold.
The ability of each set of variables that constitute indexes to discriminate non-responders to CPM-task from responders is presented in Figure 3. Setting the AUC showed that the severity of fibromyalgia symptoms, together with the neuroplasticity biomarkers, permitted us to construct a score that offers 100% sensitivity and 98% specificity to screening patients with a failure of the DPMS upon the paradigm to non-responders to the CPM-task.
Figure 3.
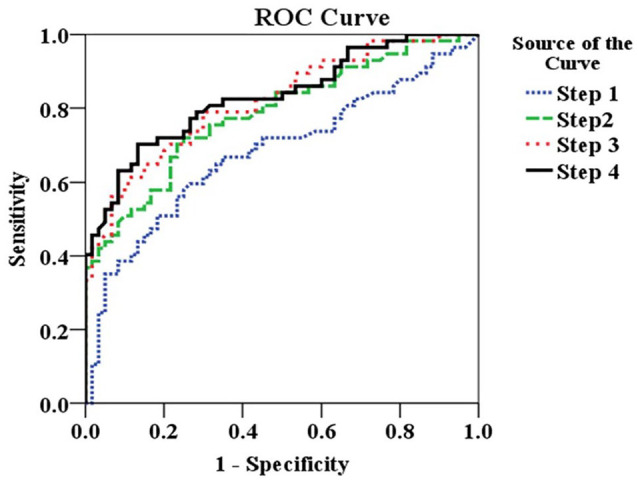
Nonparametric receiver operating characteristics (ROC) analyses. The area under the curve (AUC) with exact binomial 95% confidence intervals (CIs) according to each step of hierarchical multiple regression analysis is presented in Table 4. The ROC shows the discriminant properties of the set of variables retained in each block. Variable retained in step I: analgesic use (four or more than 4 days per week) and heat pain threshold (HPT). Step II: step I along with catastrophizing pain (cutoff-Q25 = 33), BDNF (cutoff-Q75 = 58.11) and PSQI (cutoff-Q25 > 17). Step III: step II along with the number of psychiatric diagnoses according to the MINI (major depressive episode with dysthymia, manic-depressive disorder and generalized anxiety disorder). Step IV: step III along with FIQ (cutoff-Q75 = >78) to assess the impact of symptoms on quality of life.
Discussion
We constructed a framework that integrates the severity symptoms related to fibromyalgia with the changes in the neuroplasticity state to comprehend their relationships with the paradigm of the efficiency of DPMS according to the spectrum of the responders and non-responders to the CPM-task. More precisely, under a conceptual perspective, our findings might explain the pathophysiological processes that underlie the fibromyalgia from a biopsychosocial heuristic model. Thus, they bring attention towards their value to improve the diagnosis with the perspective to the appraisal of some of its mediators in serum could accelerate the translation of results to the bedside. In the sense, CSS is a primary mechanism of the fibromyalgia pathophysiology, characterized to be a continuum phenomenon in which patients may lie at one of its extremes.41 It is worth noting that as expected that the severity of this cluster of fibromyalgia symptoms can be useful to identify patients prone to have a higher dysfunction of DPMS, which could be used as biofeedback for helping patients engage in therapeutics and rehabilitation interventions. Likewise, it aids in reducing the psychological and economic burden and could steer resources towards cost-effective interventions.
Aligned with this perspective, the reliability of serum BDNF reached 67% according to AUC, that is, its positive predictive value to identify non-responder to CPM-task. This result is supported by an earlier study that found that the inefficiency of DPMS was associated with higher serum BDNF in the myofascial pain syndrome31 and likewise, results were observed in patients with different conditions of musculoskeletal pain.42 Although the BDNF effect on pain processing may be specific of each region of the central nervous system (i.e. spine, brainstem, hippocampus, cortex, etc.),43 our findings suggest that it can be a proxy marker to indicate the imbalance of DPMS. According to preclinical study, the BDNF at the brainstem DPMS, either periaqueductal grey (PAG) or rostral ventromedial medulla (RVM) is originated from neurons in PAG, and the activation of TrkB signalling by BDNF in RVM induces facilitation in the descending pain pathway.44 Likewise, the BDNF activates specific nucleons of raphe magnus (NRM) responsible for facilitation in the DPMS. Although the serum levels of BDNF are an indirect measure of the BDNF in the brain, the literature data show that the brain contributes to 70–80% of circulating BDNF.2,3,45 Thus, changes in serum BDNF can indicate a dysfunction of the central nervous system, given the growing literature showing a lower serum BDNF in patients with major depression, whereas in fibromyalgia, its levels are high.46,47 These differences suggest that the serum BDNF indicates that a neuroplasticity process underpins the pathophysiological mechanisms of each of the two conditions, which is over-activated in chronic pain. However, we need parsimony in the interpretation of these results, and more data are required to conclude that the generation of BDNF is a compensatory mechanism related to the physiopathology of fibromyalgia.
According to the AUC of S100B protein, its reliability properties to identify non-responder to CPM-task is closer to 0.50, which is mildly better than chance. Although a higher level of 100B protein in brain regions has been associated with pain signalling, this is the first study to extent data of its relationship with the DPMS function. According to a study using mice models with S100B, overexpressing decreased significantly the tactile threshold in comparison to wild-type mice. Whereas, S100B knockout mice did not develop mechanical hypersensitivity after spinal nerve transection. Likewise, a clinical study found that higher serum S100-B was associated with lower pain pressure threshold in fibromyalgia.48 Also, in healthy females, the serum S100B was correlated with the concentration in oligodendrocytes, particularly in the frontal, parietal, corpus callosum, dorsolateral prefrontal and temporal white matter,10,49 and, to a lesser degree, in the dorsolateral prefrontal and temporal cortices.10 Increased S100B in minor depression has been suggested early glial pathology that precedes specific neuronal changes such as in major depression.50 Overall, these data suggest that its serum level can be a proxy marker of white matter structural changes, and it could indirectly indicate the role of neuroglia in the physiopathology of chronic pain. Given the close link between neuroinflammation and chronic pain and the limited reliability of serum levels of S100B to discriminate the dysfunction of DPMS, we could consider that the S100B protein may have a critical role in mediating central pain sensitization and this process can include the dysfunction of DPMS.
Our study showed that the dysfunction of DPMS is associated with the emotional burden (e.g. depressive symptoms, number of psychiatric diagnoses, pain catastrophizing and disability due to pain). This result is aligned with what we observe in a clinical setting, where the severity of psychological symptoms is a factor associated with the severity of the disease, and it has been demonstrated that it can be a predictive factor of treatment response.51–53 The multivariate model showed that these symptoms are positively correlated to dysfunction in the DPMS. Thus, this result confirms our hypothesis that the severity of fibromyalgia symptoms, related to psychological characteristics, may indicate the failure of DPMS.
Scores equal or higher than the value of the cutoff point to identify non-responders to CPM-task in the composite index showed a [AUC] = 0.83, sensitivity = 100% and specificity = 98%. Noteworthy, these results confirm our hypothesis that the dysfunction of DPMS is correlated with the fibromyalgia symptoms’ severity and that it interferes with functional disability. They permit clinicians to identify patients with a higher propensity to have a failure of DPMS based on the impact of fibromyalgia symptoms on personal and professional relationships, physical activity, work and social commitments. The relevance of these findings is even more significant because they indicate that only the intensity of diffuse pain does not provide support to identify the severity of the inefficacy of the DPMS, which may be an intermediate signal to enable more effective diagnosis and better management of symptoms.
Scores higher than 0.056 on index in the step II (PSQI indicating bad sleep quality, higher level of pain catastrophizing and high levels of BDNF), together with use of analgesic (four or more than four days per week) determined to identify dysfunction of DPMS by the spectrum of the non-responders to CPM-task showing [AUC] = 0.78, sensitivity = 100% and specificity = 98%. According to previous studies, better functioning of pain inhibition was positively associated with sleep efficiency and with the sleep duration in different pain conditions (i.e. temporomandibular joint disorders,54 rheumatoid arthritis55 and fibromyalgia).56 On neurobiological grounds, these findings may be explained by common neurotransmitters involved in sleep, depressive symptoms and DPMS function, including noradrenaline (NA), serotonin (5HT) and dopamine (DA). As far pain modulation, according to preclinical studies, the DPMS depends on endogenous opioids in the PAG and noradrenergic projections from the locus coeruleus57–59 which dampens nociceptive afferents at the spinal dorsal horn;60 noteworthy, the PAG matter – a fundamental DPMS structure27,61 – has also been shown to be correlated with sleep effectiveness.62 In this line, an experimental study using functional magnetic resonance imaging (fMRI) showed that, relative to controls, fibromyalgia patients deactivated the rostral anterior cingulate cortex (rACC).63 This functional imaging study suggests that in fibromyalgia brainstem dysfunctional structures involved in pain inhibition are also included in sleep regulation, depressive symptoms and pain catastrophizing. In humans, it seems that the perceived level of the conditioned stimulus pain can be affected by cognitive manipulation. These results suggest that the mood can influence the CPM response rather than solely on its physical intensity.26 Although the design of this study does not permit to determine the causal directionality, it is possible that impaired DPMS engenders pain catastrophizing, depressive symptoms and sleep disturbances in fibromyalgia patients.
Previous studies have demonstrated that pain catastrophizing is processed in brain areas less activated during the CPM test, such as the secondary somatosensory cortex, the dorsolateral prefrontal cortex, the anterior cingulate cortex and the cerebellum.64–67 In addition, another study showed that pain catastrophizing was positively correlated with blood pressure68 and a similar correlation was observed between blood pressure levels and the CPM responses.69,70 According to meta-analysis, a positive correlation of pain catastrophizing with electrical-based CPM could be mediated by the autonomic nervous system.26 Also, a higher pain catastrophizing could lead to enhanced pain perception, with a higher CPM efficiency by the attention bias.71 From a treatment perspective, a better therapeutic approach (i.e. cognitive therapy, antidepressant, mindfulness, etc.) might restore the DPMS efficacy and the cluster of fibromyalgia symptoms. Although further prospective studies will need to sort out the directionality of this relationship between the failures of DPMS with these main symptoms of fibromyalgia, their relevance is their potential as a marker of therapeutic efficacy.
Concerns are related to the design and interpretation of these results. The downward negative spiral of DPMS dysfunction has severe clinical consequences, as follows: first, we included only women because it is recognized that exist sex differences in the pain threshold, pain processing in the brain and in the circuitry of descending pain modulation (from midbrain to brainstem to spinal cord) has been found to be robustly sexually dimorphic.72 Besides, the majority of studies described higher CPM responses in males.73 Although a more homogeneous sample reduces the potential for confounding bias, it limits the external validity. Second, we include young and middle-aged adults since it has demonstrated an age-dependent decline in CPM responses (i.e. middle-aged adults showed decreased CPM responses compared with younger adults but not with older adults).73 Third, AUC corresponds to the probability of correctly identifying the condition of interest from ‘noise’ and is generally accepted as satisfactory when >0.7. The ROC curve is the inherent validity of a test for identifying a condition of non-responders to CPM-task requires analysis of the tradeoff between sensitivity and specificity. Finally, we constructed a propensity score-adjusted index to adjust the relationship between dependent variables according to the spectrum of the responders and non-responders to CPM-task and all patients received a standardized stimulus in order not to bias towards the paradigm to measure CPM-task.
These results showed in an integrative perspective that in fibromyalgia the severity of main symptoms might identify patients prone to the inefficiency of DPMS according to the spectrum of responders and non-responders to CPM-task. Also, they highlight that the neuroplasticity mediators, such as BDNF and S100-B protein, could corroborate to validate the CPM-task as a test that allows for inference regarding the loss of descending pain inhibition. Thus, the CPM-task is a simple, useful test to help in the individualized clinical decision-making process based on its potential predictive properties for patient’s response to therapy.
Footnotes
Contributorship: Matheus Dorigatti Soldatelli, Vinicius Souza dos Santos, Iraci Lucena da S. Torres, Felipe Fregni and Wolnei Caumo were involved in the study concept and design, acquisition of data or analysis and interpretation of data; Matheus Dorigatti Soldatelli, Timo Siepmann, Bem Min-Woo Illigens, Felipe Fregni and Wolnei Caumo contributed to drafting/revising the manuscript for important intellectual content; and all the authors contributed to the approval of the final version to be published.
Authors’ note: This work is part of a Master’s thesis of the master’s Program in Clinical Research, Center for Clinical Research and Management Education, Division of Health Care Sciences, Dresden International University, Dresden, Germany.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This research was supported by grants and material support from the following Brazilian agencies: Committee for the Development of Higher Education Personnel – CAPES – PNPD/CAPES (material support); National Council for Scientific and Technological Development – CNPq (grants to Dr Wolnei Caumo and Dr Iraci Lucena da S. Torres); Postgraduate Research Group at the Hospital de Clínicas de Porto Alegre (material support); Foundation for Support of Research at Rio Grande do Sul (FAPERGS) (material support); and Brazilian Innovation Agency (FINEP), Process No. 1245/12 (Dr Wolnei Caumo).
Guarantor: W.C. is the guarantor of this article.
ORCID iD: Wolnei Caumo  https://orcid.org/0000-0002-5083-4658
https://orcid.org/0000-0002-5083-4658
References
- 1. Asmundson GJ, Norton PJ, Norton GR. Beyond pain: the role of fear and avoidance in chronicity. Clin Psychol Rev 1999; 19(1): 97–119. [DOI] [PubMed] [Google Scholar]
- 2. Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998; 37(12): 1553–1561. [DOI] [PubMed] [Google Scholar]
- 3. Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res 1996; 36(2): 280–286. [DOI] [PubMed] [Google Scholar]
- 4. Coull JAM, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005; 438(7070): 1017–1021. [DOI] [PubMed] [Google Scholar]
- 5. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10(9): 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018; 19(3): 138–152. [DOI] [PubMed] [Google Scholar]
- 7. Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 2007; 36(6): 339–356. [DOI] [PubMed] [Google Scholar]
- 8. Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 2008; 37(6): 339–352. [DOI] [PubMed] [Google Scholar]
- 9. Caumo W, Antunes LC, Elkfury JL, et al. The central sensitization inventory validated and adapted for a Brazilian population: psychometric properties and its relationship with brain-derived neurotrophic factor. J Pain Res 2017; 10: 2109–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steiner J, Bernstein HG, Bielau H, et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci 2007; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schroeter ML, Abdul-Khaliq H, Krebs M, et al. Serum markers support disease-specific glial pathology in major depression. J Affect Disord 2008; 111(2–3): 271–280. [DOI] [PubMed] [Google Scholar]
- 12. Schroeter M, Sacher J, Steiner J, et al. Serum S100B represents a new biomarker for mood disorders. Curr Drug Targets 2013; 14(11): 1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kowianski P, Lietzau G, Czuba E, et al. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol 2018; 38(3): 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stefani LC, Torres IL, de Souza IC, et al. BDNF as an effect modifier for gender effects on pain thresholds in healthy subjects. Neurosci Lett 2012; 514(1): 62–66. [DOI] [PubMed] [Google Scholar]
- 15. Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010; 14: 339–339. [DOI] [PubMed] [Google Scholar]
- 16. Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 1979; 6: 283–304. [DOI] [PubMed] [Google Scholar]
- 17. Baba Y, Kohase H, Oono Y, et al. Effects of dexmedetomidine on conditioned pain modulation in humans. Eur J Pain 2012; 16(8): 1137–1147. [DOI] [PubMed] [Google Scholar]
- 18. Treister R, Pud D, Eisenberg E. The dopamine agonist apomorphine enhances conditioned pain modulation in healthy humans. Neurosci Lett 2013; 548: 115–119. [DOI] [PubMed] [Google Scholar]
- 19. Lindstedt F, Berrebi J, Greayer E, et al. Conditioned pain modulation is associated with common polymorphisms in the serotonin transporter gene. PLoS ONE 2011; 6(3): e18252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laurito CE, Baughman VL, Becker GL, et al. The effectiveness of oral clonidine as a sedative/anxiolytic and as a drug to blunt the hemodynamic responses to laryngoscopy. J Clin Anesth 1991; 3: 186–193. [DOI] [PubMed] [Google Scholar]
- 21. Hughes JW, Watkins L, Blumenthal JA, et al. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res 2004; 57(4): 353–358. [DOI] [PubMed] [Google Scholar]
- 22. Horjales-Araujo E, Demontis D, Lund EK, et al. Polymorphism in serotonin receptor 3B is associated with pain catastrophizing. PLoS ONE 2013; 8(11): e78889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karg K, Burmeister M, Shedden K, et al. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited. Arch Gen Psychiatry 2011; 68(5): 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caumo W, Schmidt AP, Schneider CN, et al. Risk factors for postoperative anxiety in adults. Anaesthesia 2001; 56(8): 720–728. [DOI] [PubMed] [Google Scholar]
- 25. Racine M, Tousignant-Laflamme Y, Kloda LA, et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception – Part 1: are there really differences between women and men. Pain 2012; 153(3): 602–618. [DOI] [PubMed] [Google Scholar]
- 26. Nahman-Averbuch H, Nir R-R, Sprecher E, et al. Psychological factors and conditioned pain modulation. Clin J Pain 2016; 32: 541–554. [DOI] [PubMed] [Google Scholar]
- 27. Boyer N, Dallel R, Artola A, et al. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain 2014; 155(7): 1196–1205. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol 2011; 7: 518–527. [DOI] [PubMed] [Google Scholar]
- 29. Schestatsky P, Valls-Sole J, Costa J, et al. Skin autonomic reactivity to thermoalgesic stimuli. Clin Auton Res 2007; 17(6): 349–355. [DOI] [PubMed] [Google Scholar]
- 30. Schestatsky P, Stefani LC, Sanches PR, et al. Validation of a Brazilian quantitative sensory testing (QST) device for the diagnosis of small fiber neuropathies. Arq Neuropsiquiatr 2011; 69(6): 943–948. [DOI] [PubMed] [Google Scholar]
- 31. Botelho LM, Morales-Quezada L, Rozisky JR, et al. A framework for understanding the relationship between descending pain modulation, motor corticospinal, and neuroplasticity regulation systems in chronic myofascial pain. Front Hum Neurosci 2016; 10: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marques P, Barsante Santos AM, Matsutani LA, et al. Validation of the Brazilian version of the fibromyalgia impact questionnaire (FIQ). Rev Bras Reum 2006; 46: 4570. [Google Scholar]
- 33. Sehn FF, Chachamovich EMDP, Vidor LP, et al. Cross-cultural adaptation and validation of the Brazilian Portuguese version of the pain catastrophizing scale. Pain Med 2012; 13(11): 1425–1435. [DOI] [PubMed] [Google Scholar]
- 34. Kersten P, White PJ, Tennant A. Is the pain visual analogue scale linear and responsive to change? An exploration using Rasch analysis. PLoS ONE 2014; 9(6): e99485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995; 7: 524–532. [Google Scholar]
- 36. Gomes-Oliveira MH, Gorenstein C, Lotufo Neto F, et al. Validation of the Brazilian Portuguese version of the beck depression inventory-II in a community sample. Braz J Psychiatry 2012; 34(4): 389–394. [DOI] [PubMed] [Google Scholar]
- 37. Bertolazi AN, Fagondes SC, Hoff LS, et al. Validation of the Brazilian Portuguese version of the Pittsburgh sleep quality index. Sleep Med 2011; 12(1): 70–75. [DOI] [PubMed] [Google Scholar]
- 38. Amorim P. Mini international neuropsychiatric interview (MINI): validation of a short structured diagnostic psychiatric interview. Rev Bras Psiquiatr 2000; 22: 106–115. [PubMed] [Google Scholar]
- 39. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143: 29–36. [DOI] [PubMed] [Google Scholar]
- 40. Manuscript A, Plasticity CN, Latremoliere A, et al. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolfe F, Walitt B. Culture, science and the changing nature of fibromyalgia. Nat Rev Rheumatol 2013; 9(12): 751–755. [DOI] [PubMed] [Google Scholar]
- 42. Caumo W, Deitos A, Carvalho S, et al. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Front Hum Neurosci 2016; 10: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spezia Adachi LN, Quevedo AS, de Souza A, et al. Exogenously induced brain activation regulates neuronal activity by top-down modulation: conceptualized model for electrical brain stimulation. Exp Brain Res 2015; 233(5): 1377–1389. [DOI] [PubMed] [Google Scholar]
- 44. Guo W, Robbins MT, Wei F, et al. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci 2006; 26(1): 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laske C, Eschweiler G. Brain-derived neurotrophic factor: from nerve growth factor to modulator of brain plasticity in cognitive processes and psychiatric diseases. Nervenari 77: 523–537. [DOI] [PubMed] [Google Scholar]
- 46. Molendijk ML, Bus BAA, Spinhoven P, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry 2011; 16(11): 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stefani LC, Leite FM, da Graça L, et al. BDNF and Serum S100B levels according the spectrum of structural pathology in chronic pain patients. Neurosci Lett 2019; 706: 105–109. [DOI] [PubMed] [Google Scholar]
- 48. Zanette SA, Dussan-Sarria JA, Souza A, et al. Higher serum S100B and BDNF levels are correlated with a lower pressure-pain threshold in fibromyalgia. Mol Pain 2014; 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Streitburger DP, Arelin K, Kratzsch J, et al. Validating serum S100B and neuron-specific enolase as biomarkers for the human brain – a combined serum, gene expression and MRI study. PLoS ONE 2012; 7(8): e43284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polyakova M, Sander C, Arelin K, et al. First evidence for glial pathology in late life minor depression: S100B is increased in males with minor depression. Front Cell Neurosci 2015; 9: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schreiber KL, Loggia ML, Kim J, et al. Painful after-sensations in fibromyalgia are linked to catastrophizing and differences in brain response in the medial temporal lobe. J Pain 2017; 18(7): 855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Heer EW, Vriezekolk JE, van der Feltz-Cornelis CM. Poor illness perceptions are a risk factor for depressive and anxious symptomatology in fibromyalgia syndrome: a longitudinal cohort study. Front Psychiat 2017; 8: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lawson K. Emerging pharmacological strategies for the treatment of fibromyalgia Emerging pharmacological strategies for the treatment of fibromyalgia. World J Pharmacol 2017; 6: 1–10. [Google Scholar]
- 54. Edwards RR, Grace E, Peterson S, et al. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain 2009; 13(10): 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee YC, Lu B, Edwards RR, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum 2013; 65(1): 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Keskindag B, Karaaziz M. The association between pain and sleep in fibromyalgia. Saudi Med J 2017; 38: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Millan MJ. Descending control of pain. Prog Neurobiol 2002; 66: 355–474. [DOI] [PubMed] [Google Scholar]
- 58. Isaac SO, Berridge CW. Wake-promoting actions of dopamine D1 and D2 receptor stimulation. J Pharmacol Exp Ther 2003; 307(1): 386–394. [DOI] [PubMed] [Google Scholar]
- 59. Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci 2006; 26(1): 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci 2004; 25(12): 613–617. [DOI] [PubMed] [Google Scholar]
- 61. Willer JC, Bouhassira D, Le Bars D. Bases neurophysiologiques du phénomène de contre-irritation: les contrôles inhibiteurs diffus induits par stimulations nociceptives. Neurophysiol Clin Neurophysiol 1999; 29: 379–400. [DOI] [PubMed] [Google Scholar]
- 62. Sastre JP, Buda C, Kitahama K, et al. Importance of the ventrolateral region of the periaqueductal gray and adjacent tegmentum in the control of paradoxical sleep as studied by muscimol microinjections in the cat. Neuroscience 1996; 74(2): 415–426. [DOI] [PubMed] [Google Scholar]
- 63. Jensen KB, Kosek E, Petzke F, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 2009; 144(1–2): 95–100. [DOI] [PubMed] [Google Scholar]
- 64. Sprenger C, Bingel U, Buchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain 2011; 152(2): 428–439. [DOI] [PubMed] [Google Scholar]
- 65. Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004; 127(Pt4): 835–843. [DOI] [PubMed] [Google Scholar]
- 66. Piche M, Bouin M, Arsenault M, et al. Decreased pain inhibition in irritable bowel syndrome depends on altered descending modulation and higher-order brain processes. Neuroscience 2011; 195: 166–175. [DOI] [PubMed] [Google Scholar]
- 67. Nahman-Averbuch H, Martucci KT, Granovsky Y, et al. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain 2014; 155(12): 2491–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Leonard MT, Chatkoff DK, Gallaway M. Association between pain catastrophizing, spouse responses to pain, and blood pressure in chronic pain patients: a pathway to potential comorbidity. Int J Behav Med 2013; 20(4): 590–598. [DOI] [PubMed] [Google Scholar]
- 69. Chalaye P, Devoize L, Lafrenaye S, et al. Cardiovascular influences on conditioned pain modulation. Pain 2013; 154(8): 1377–1382. [DOI] [PubMed] [Google Scholar]
- 70. Chalaye P, Lafrenaye S, Goffaux P, et al. The role of cardiovascular activity in fibromyalgia and conditioned pain modulation. Pain 2014; 155(6): 1064–1069. [DOI] [PubMed] [Google Scholar]
- 71. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009; 9(5): 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mogil JS. Sex-based divergence of mechanisms underlying pain and pain inhibition. Curr Opin Behav Sci 2018; 23: 113–117. [Google Scholar]
- 73. Grashorn W, Sprenger C, Forkmann K, et al. Age-dependent decline of endogenous pain control: exploring the effect of expectation and depression. PLoS ONE 2013; 8(9): e75629. [DOI] [PMC free article] [PubMed] [Google Scholar]