Abstract
Background
World is in grip of coronavirus disease-2019 (COVID-19) pandemic right now. Majority of studies center around its epidemiological and clinical characteristics. Information regarding secondary bacterial infections is limited. This retrospective observational study was done to determine the prevalence and characteristics of bloodstream infections in COVID-19 patients admitted in a tertiary care center in Jaipur.
Materials and methods
All blood cultures received from COVID-19 positive patients admitted in designated COVID care ICUs and wards were included in the study. A predesigned pretested questionnaire was used to collect relevant data. Blood cultures were done using BD BACTEC™ FX40, and identification and antimicrobial susceptibility testing of isolates were done by VITEK® 2 COMPACT.
Results
One thousand five hundred seventy-eight (1578) COVID-19 positive patients were admitted in center during 5-month study period from whom 158 blood cultures were received. Out of these, 15 (9.4%) were positive. Median age of patients with positive blood culture was 54 years and included 10 males and 5 females. Ten (67%) patients needed intensive care in ICU. Significant correlation of blood culture positivity was found with parameters like ICU admission, presence of an indwelling device, underlying comorbidity, raised biochemical markers, and adverse clinical outcome.
Conclusions
Incidence of bloodstream infections is low for COVID-19 patients. Antibiotic prophylaxis needs to be used with caution, and prompt discontinuation should be done based on clinical judgment.
How to cite this article
Rajni E, Garg VK, Bacchani D, Sharma R, Vohra R, Mamoria V, et al. Prevalence of Bloodstream Infections and their Etiology in COVID-19 Patients Admitted in a Tertiary Care Hospital in Jaipur. Indian J Crit Care Med 2021;25(4):369–373.
Keywords: Antibiotic stewardship, Antimicrobial resistance, Bacteremia, Blood culture, Blood epidemiology, Bloodstream, Coinfections, COVID-19, Sepsis
Introduction
The world is in grip of coronavirus disease-2019 (COVID-19) pandemic right now. It originated in the city of Wuhan, Hubei Province, Central China, and has spread globally at an alarming rate. The newly identified SARS-CoV-2 has caused a large number of deaths with tens of thousands of confirmed cases worldwide, posing a serious threat to public health. The global healthcare system appears to be completely overwhelmed by the current pandemic.1,2 With no clinically approved vaccine or specific therapeutic drug available, research on the newly emerged virus is urgently needed.
Scientists all over the world are engaged in unraveling the mysteries associated with the virus. Information regarding its etiopathogenesis, treatment, and clinical outcome is still evolving. Rapid identification of asymptomatic and mildly symptomatic contacts is the priority for outbreak control till an effective and safe vaccine is developed and becomes available to all or most of the world's population. At the same time, tracking the clinical course and complications occurring in confirmed cases is essential to prepare a robust action plan for understanding the disease and treating patients.1–3
Viral infections are often associated with secondary bacterial infections, thus complicating clinical outcome and prognosis.4–7 Majority of studies during the existing coronavirus pandemic have centered on its epidemiological and clinical characteristics.8–10 Information regarding secondary bacterial infections is limited. Thus, this retrospective observational study was done to determine the prevalence and spectrum of bloodstream infections in COVID-19 patients, admitted in a tertiary care center in Jaipur. The study also sheds light on antimicrobial sensitivity pattern of these isolates and clinical outcome of patients.
Materials and Methods
This is a retrospective laboratory-based observational study conducted in the Department of Microbiology, Mahatma Gandhi Medical College, Jaipur, an approved and high-volume COVID treatment center from March 23, 2020, to August 23, 2020. This is a multispecialty center catering to a wide catchment area in and around Jaipur. Since the onset of pandemic, the hospital has been designated as a COVID care center by the Government of Rajasthan. At the hospital, a separate block of 220 ward beds and 30 intensive care unit (ICU) beds has been completely dedicated for care of COVID-positive patients.
Due approval was taken from Institutional Ethical Committee before undertaking the present study (MGMCH/IEC/JPR/2020/181). The definition of a confirmed case was in accordance with World Health Organization interim guidance.3 The diagnosis of COVID-19 was confirmed by positive result of real-time reverse-transcription polymerase chain reaction (RT-PCR) for respiratory specimens (throat and/or nasopharyngeal swabs).
All blood cultures received from COVID-positive patients admitted in designated COVID care ICUs and wards were included in the study. Blood cultures were done using BD BACTEC™ FX40, and identification and antimicrobial susceptibility testing of isolates were done by VITEK® 2 COMPACT. The interval from time of blood culture collection to time of gram stain was used to calculate the time to blood culture positivity during the study period.
A predesigned and pretested questionnaire was used to collect the epidemiological, demographic, laboratory, clinical management, and outcome data of the patients.
The data were entered in Microsoft Office Excel Worksheet. Mean and standard deviation were calculated. Appropriate statistical tests were used to find significant association. p value <0.05 was considered statistically significant.
Results
One thousand five hundred seventy-eight (1578) COVID-19 positive patients were admitted in center during the 5-month study period (March 23, 2020 to August 23, 2020). A total of 158 blood cultures were received from these inpatients in the Department of Microbiology, Mahatma Gandhi Medical College, Jaipur. Out of these, 15 (9.4%) showed positive blood cultures. Only one isolate per patient was included for study purpose.
The median age of COVID-19 patients showing a positive blood culture was 54 years (interquartile range: 25–69 years), and these included 10 males and 5 females. The 15 patients with a positive blood culture included 10 (67%) in ICU and 5 (33%) in the ward.
Table 1 gives a summary of demographic, epidemiological, clinical features, and outcome data of COVID-19 patients showing a positive blood culture in our institute.
Table 1.
Summary of demographic features, comorbid illnesses, clinical condition, and outcomes in bacteremia COVID-19 patients
| Parameters | Number of patients (N = 15) n (%) |
|---|---|
| Median age | 54 years (IQR: 25–69 years) |
| Gender | |
| Male | 10 (67%) |
| Female | 5 (33%) |
| Location | |
| ICUs | 10 (67%) |
| Wards | 5 (33%) |
| Severity score | |
| On oxygen therapy | 13 out of 15 patients (86%) |
| On ventilatory support | 8 out of 10 ICU patients (80%) |
| Presence of indwelling device | |
| Urinary catheter | 11 (73.3%) |
| Central catheter | 9 (60%) |
| Endotracheal tubes | 8 (53.3%) |
| Dialysis line | 3 (20%) |
| Presenting complaints | |
| Breathlessness | 9 (60%) |
| Fever | 8 (53.3%) |
| Cough | 6 (40%) |
| Underlying comorbid conditions | |
| Diabetes | 8 (53.3%) |
| Hypertension | 6 (40%) |
| Lung disease | 4 (26.6%) |
| Renal disease | 3 (20%) |
| On dialysis | 3 (20%) |
| Cardiac disease | 3 (20%) |
| Liver disease | 2 (13.3%) |
| Malignancy | 2 (13.3%) |
| Raised biochemical markers | |
| Ferritin | 12 (80%) |
| D-dimer | 12 (80%) |
| Procalcitonin | 10 (66.6%) |
| C-reactive protein | 9 (60%) |
| Final clinical outcome | |
| Discharged (stable) | 7 (46%) |
| Deceased | 4 (26.6%) |
| Shifted from ICU to ward | 3 (20%) |
| Cont. mechanical ventilation | 1 (6.6%) |
| Median period from symptom onset to death | 10 days (IQR: 3–30 days) |
Table 2 provides a comparison of various parameters among COVID-19 patients with positive and negative blood cultures.
Table 2.
Comparison of various parameters among COVID-19 patients with positive and negative blood cultures
| Blood culture | Chi-square | p-value | |||
|---|---|---|---|---|---|
| S. No. | Parameters | Positive | Negative | ||
| 1 | Age: (median ± SD) | 54 ± 13.56 years | 51.9 ± 18.94 | – | 0.677 |
| 2 | Sex | 0.13 (1) | 0.971 | ||
| Male | 10 | 96 | |||
| Female | 5 | 47 | |||
| 3 | Location | ||||
| ICU | 10 | 28 | 16.5 (1) | 0.000 | |
| Ward | 5 | 115 | |||
| 4 | Presence of indwelling device | ||||
| Yes | 13 | 61 | 10.56 (1) | 0.0011 | |
| No | 2 | 82 | |||
| 5 | Presence of comorbid conditions | ||||
| Yes | 8 | 14 | 21.5 (1) | 0.000 | |
| No | 7 | 129 | |||
| 6 | Raised biochemical markers | ||||
| Yes | 12 | 8 | 92.7 (1) | 0.000 | |
| No | 3 | 135 | |||
| 7 | Final outcome | ||||
| Recovered and discharged | 7 | 140 | 27.6 (1) | 0.000 | |
| Death | 4 | 3 | |||
The predominant isolate in positive blood cultures was coagulase-negative Staphylococcus 11 (73.3%) (Fig. 1).
Fig. 1.
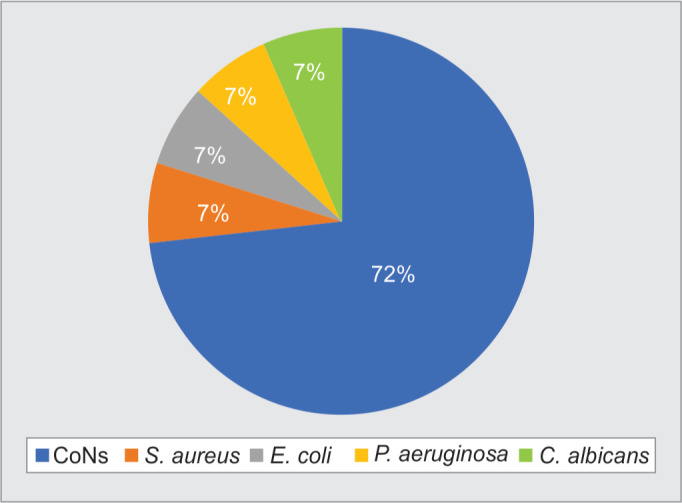
Distribution of clinical isolates from positive blood cultures (n = 15)
Ninety percent of gram-positive isolates were found to be methicillin-resistant. A high level of resistance (70–90%) was also noted for commonly used drugs like macrolides, clindamycin, and quinolones. They were, however, found susceptible to gentamicin, cotrimoxazole, vancomycin, linezolid, and daptomycin.
Among the gram-negative isolates, Pseudomonas aeruginosa was sensitive to all drugs tested except ticarcillin. Escherichia coli was multidrug-resistant, found sensitive only to tigecycline and colistin.
Candida albicans 1/15 was found sensitive to voriconazole, caspofungin, anidulafungin, flucytosine, and amphotericin but resistant to fluconazole.
Among the 15 positive blood cultures, the incubation period required for flagging positive was also recorded. The vast majority (87%) signaled positive within 1 - 2 days of incubation, with remaining 13% becoming positive on day 3.
Discussion
Bacteremia has been described as a complication of COVID-19 infection.4–7,11–14 Both these conditions have a number of overlapping clinical and biochemical features. Blood culture thus constitutes an important investigation for bringing more clarity into clinical judgment. While most patients with COVID-19 are treated with empirical antibiotics for potential bacterial coinfections, very little data exist regarding rate of bacteremia among these patients. Early identification of bloodstream pathogens and their antibiotic resistance profile is necessary to improve clinical outcome.15 This study was conducted to identify the prevalence, characteristics, etiological agents involved, antimicrobial sensitivity pattern, and clinical outcomes in COVID-19 patients with bloodstream infections admitted in our tertiary care hospital.
Majority (two-thirds) of patients who developed bacteremia in our study were critically ill and admitted in ICU. Association of blood culture positivity and need for intensive care in ICU was found to be significantly high (p = 0.000) (Table 1). Oxygen therapy was required in 13/15 patients. Eight out of ten patients in ICU were on ventilatory support. Most of the patients (11/15) also had hypotension requiring vasopressors. Thirteen out of fifteen patients (86%) had an indwelling device such as endotracheal tube, urinary catheter, central line catheter, peripherally inserted central catheter, and/or dialysis catheter. Association of the presence of an indwelling device with blood culture positivity was also found to be significant. (p = 0.0011) (Table 2). There is ample literature suggesting that the occurrence of secondary bacterial infections is affected by the severity of COVID-19. Lansbury et al. too in their meta-analysis reported a higher proportion of bacterial coinfections from ICU patients.11,12,16
Shortness of breath (60%) and fever (53.3%) were the most common presenting symptoms. The symptoms began 6 ± 4 days before admission. 53% of patients had a comorbid condition with diabetes being most common, followed by hypertension, preexisting lung, and renal disease (Table 1). Also, the presence of an underlying comorbidity was found to be significantly associated with a positive blood culture finding (Table 2). Similar findings have been reported by other studies too.8–11,16–18
All patients were tested for a battery of biochemical markers, which included ferritin, D-dimer, C-reactive protein, and procalcitonin. It was observed that ferritin and D-dimer levels were increased in 12 patients (80%) each. Procalcitonin and C-reactive protein levels were raised in 10 (67%) and 9 patients (60%), respectively (Table 1). Also, the presence of raised biochemical markers was found significantly high in patients with a positive blood culture (Table 2). Various studies have reiterated this association of raised biochemical markers with a more severe disease.16,18,19
Different researchers from all over the world have given variable data regarding nosocomial infection rates among COVID-19 patients. He et al. reported nosocomial infection rate of 7.1% among COVID-19 patients with bacteremia accounting for 24.6%.20 Li et al. reported bacteremia to be a complication in 7.7% of COVID-19 patients from Wuhan.21 According to a meta-analysis by Rawson et al., 8% of patients were reported as experiencing bacterial/fungal coinfection during hospital admission.4 In our study, we found 9.4% (15/158) prevalence of bloodstream infection among COVID-19 positive patients. Goyal et al. in their study from the USA reported a 6% prevalence of bacteremia among their admitted patients.9
Likewise, Sepulveda et al. too in their study found a low rate of bacteremia among COVID-19 patients in their retrospective cohort analysis of 88,201 blood cultures. The authors also reported a high proportion of organisms to be belonging to skin commensal flora correlating well with our findings when 11/15 isolates were coagulase-negative Staphylococcus.13 The source of organisms may be difficult to elucidate. These may be commensal microflora carried by patients before they developed COVID-19, especially for those with underlying diseases or nosocomial environment. Ninety-eight percent of blood cultures in their study flagged positive within 4 days of incubation. In our study, all blood cultures were positive within 3 days of incubation. Lansbury et al. had reported the commonest bacteria as being Mycoplasma pneumonia, Pseudomonas aeruginosa, and Haemophilus influenzae.12
In yet another retrospective study from the UK including 836 COVID-positive patients, blood cultures were collected from 643 (77%) patients. A positive blood culture was found in 7.1%, of which almost two-thirds were classified as contaminants. Candida albicans was isolated from three cases of central line-associated bloodstream infection.14 Likewise, Candida albicans was isolated from 1/15 case of bloodstream infection in our study.
In our study, coagulase-negative staphylococci were the most predominant bacterial isolate (11/15). Reasons for this finding are not clear but may be related to fear and uncertainty among healthcare providers when dealing with COVID-positive patients. Rapid upswing in number of patients has put tremendous pressure on existing healthcare system, especially critical care.1,2,22 This may have resulted in suboptimal skin preparation before blood sample collection. It is difficult to determine whether these isolates were involved in pathogenicity or were mere colonizers. Broad-spectrum antibiotics administered at admission may have also altered the microbiological and clinical milieu. High-end antibiotics should be considered only after the pathogenic role of these isolates has been confirmed. It is believed that clean ICU environment and strict adherence to infection control guidelines are crucial for a low rate of infection.
Very few studies have reported fungal coinfections in COVID patients.2,22 Candida albicans was isolated from blood culture of a single patient in our study. The fact that this isolate was fluconazole-resistant necessitates stringent surveillance on the possibility of nosocomial fungal infections among COVID patients. Candida albicans was also reported from respiratory and urinary tract in six patients from the UK.12 Chaudhary et al. have recently reported bloodstream infections caused by multidrug-resistant C. auris in a COVID-19 ICU in New Delhi, India. Overall, candidemia was detected in 15 (2.5%) of the 596 ICU patients.22 There is also evidence of Aspergillus being isolated from patients in Europe.23,24 Role of serum markers like galactomannan and β-d-glucan may become more important in workup and treatment of such patients.
Concerns have been raised by several healthcare agencies regarding how the current pandemic may be impacting already existing issue of antimicrobial resistance (AMR). There is a definite increase in exposure to healthcare settings and invasive procedures. There may be a compromise in following infection control and hand hygiene protocols because of this massive influx of patients. All these provide a favorable niche for superbugs to multiply and disseminate. The usage of prophylactic antibiotics in COVID-positive patients to keep secondary bacterial infections at bay only complicates the scenario.4,10,15
All COVID-positive symptomatic patients in our hospital were given antibiotic azithromycin empirically. However, for patients in whom secondary bacterial infection was suspected, further antibiotics were added on a case-to-case basis after blood culture sample collection. Later, therapy was tailored according to antibiotic susceptibility reports provided by the laboratory. The patients in our study also received Inj methylprednisolone 0.5 - 1 mg/kg IV in two divided doses for 5 to 10 days. The dose and duration of methylprednisolone varied depending on disease severity.
It has been noted from a review of data from Asia that more than 70% of patients received antimicrobial treatment despite less than 10% having bacterial or fungal coinfections.4,10,11 Another review of studies identified that while 72% (1450/2010) of patients received antibiotics, only 8% (62/806) demonstrated superimposed bacterial or fungal coinfections.4 All these necessitate the urgency of instituting and following antibiotic stewardship guidelines. Recent interim guidance on the clinical management of COVID-19 by WHO incorporates antibiotic stewardship principles with specific recommendations.25
Each patient in our study had at least 14 days of follow-up. At the time of data collection, four patients with positive blood culture were still admitted, three in ward while one patient required mechanical ventilation and was in ICU. Among the 15 blood culture-positive patients, 4 succumbed to infection. The cause of death was septicemia and multiorgan dysfunction syndrome in each. It was observed that at least one comorbid condition was present in each of these four patients, median period from symptom onset to death being 10 days (interquartile range: 3–30 days). Various studies correlate the presence of coinfections with an unfavorable clinical outcome.16–18 A similar correlation has been reinforced by our findings wherein mortality was significantly high in patients with a positive blood culture (Table 2). Zhou et al. reported observation of secondary bacterial infection in 15% of patients admitted to hospitals in China and 98% of patients with secondary bacterial infection succumbed.18 Among the deceased, men outnumbered women by three times. This gender association with a more severe disease and higher mortality has been noted in other contemporary studies too.26,27 A possible explanation for this could be behavioral and biological differences in immunity between the two.
Given the rapidly evolving nature of research and resultant literature on SARS-CoV-2, clear-cut policies need to be developed for supporting the optimal selection of empirical therapies and also rapid de-escalation of treatment as and when needed. It is essential to collect more data and evidence to support antimicrobial use in COVID-19 patients. Antibiotic stewardship policies specific for current ongoing pandemic need to be formulated. Given the low prevalence of bloodstream infections, default ordering of blood cultures as part of the initial workup for patients with suspected COVID-19 being admitted is probably not required, especially in resource-limited setting like ours. One potential solution to support antimicrobial prescribing in COVID-19 is the use of biomarkers like procalcitonin. It helps in differentiating between bacterial and viral infection, and its use has been reported in several other COVID-19-related studies.19
Strengths
Our study is the first in this geographical area to report on bloodstream infections in COVID-19 patients. The strength of our study lies in the fact that in addition to data on prevalence of bloodstream infection, causative culture isolates and their antimicrobial susceptibility profile are also provided. This will help in developing specific guidelines regarding clinical status of patients, biochemical parameters, and culture findings so as to guide which patients require hospital admission and vigilant care. Also, specific antibiotic stewardship guidelines may be formulated and implemented.
Limitations
The study, however, has a few limitations. Because it is retrospective in nature, poor control on various confounding factors is unavoidable. Ensuing panic and confusion regarding virus may have resulted in a lower rate of collection of samples. During the course of pandemic, many experimental therapies were proposed including use of hydroxychloroquine, macrolides, tetracyclines, and quinolone. Their potential role in low rates of bacterial infections among hospitalized patients needs to be evaluated. Also, our study is restricted to a short-term follow-up of patients. Long-term follow-up may be done to further our understanding of this novel virus.
Conclusion
Incidence of bloodstream infections appears to be low for COVID-19 patients. Antibiotic prophylaxis may be used with caution, and prompt discontinuation should be done based on clinical judgment. More such studies need to be carried out as the pandemic continues to evolve on a global level. The authors reiterate strict emphasis on cleanliness and disinfection of hospital equipment and environment for better patient outcomes.
Orcid
Ekadashi Rajni https://orcid.org/0000-0003-3742-9620
Vishnu K Garg https://orcid.org/0000-0002-6729-4088
Daisy Bacchani https://orcid.org/0000-0003-4265-9607
Richa Sharma https://orcid.org/0000-0001-9747-0700
Rajat Vohra https://orcid.org/0000-0003-4193-0995
Vedprakash Mamoria https://orcid.org/0000-0003-1622-209X
Hemant Malhotra https://orcid.org/0000-0001-5613-5375
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. DOI: [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Geneva: World Health Organization; 2020. [Google Scholar]
- 4.Rawson TM, Moore LSP, Zhu N, Nishanthy R, Keira S, Mark G, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clinical Infect Dis. 2020;2(1010):1–7. doi: 10.1093/CID/CIAA530. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore SE, Wilde AM, Song M, Bohn BC, Patross CJ, Denham B, et al. A patient with Escherichia coli bacteremia and COVID-19 co-infection: a case report for the Louisville COVID-19 epidemiology study. J Resp Infect. 2020;4(1):1–4. doi: 10.18297/jri/vol4/iss1/15. DOI: [DOI] [Google Scholar]
- 6.Elzein F, Alsherbeeni N, Almatrafi K, Shosha D, Naoufel K. COVID-19 co-infection in a patient with Brucella bacteremia. Respir Med Case Rep. 2020;31:1–2. doi: 10.1016/j.rmcr.2020.101183. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osakwe N. A case of Bacillus Cereus bacteremia in a COVID-19 patient treated with steroids. IDCases. 2020;21:1–2. doi: 10.1016/j.idcr.2020.e00855. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anant M, Tiwari P, Bhatnagar S, Ankit P, Abhishek M, Lalit D, et al. Clinico-demographic profile & hospital outcomes of COVID-19 patients admitted at a tertiary care centre in north India. Indian J Med Res. 2020;152(1):61–69. doi: 10.4103/ijmr.IJMR_1788_20. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W, Ni Z, Hu Y, Liang W, Qu C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Y, Yang Q, Xu M, Kong H, Chen H, Fu Y, et al. Secondary bacterial infections in critical ill patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(6):1–4. doi: 10.1093/ofid/ofaa220. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepulveda J, Westblade LF, Whittier S, Satlin MJ, Greendyke WG, Aaron JG, et al. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J Clin Microbiol. 2020;58(8):1–7. doi: 10.1128/JCM.00875-20. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP, et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–1399. doi: 10.1016/j.cmi.2020.06.025. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadri SS, Lai YL, Warner S, Strich JK, Babiker A, Ricotta EE, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2020;21:241–251. doi: 10.1016/s1473-3099(20)30477-1. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippi G, Plebani M. Procalcitonin in patients with severe corona-virus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Li W, Wang Z, Chen H, Tian L, Liu D. Nosocomial infection among patients with COVID-19: a retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol. 2020;41(8):1–2. doi: 10.1017/ice.2020.126. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006S0091-6749(20)30495-4. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg Infect Dis. 2020;26(11):2694–2696. doi: 10.3201/eid2611.203504. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alanio A, Dellière S, Fodil S, Bretagne S, Megarbane B. High prevalence of putative invasive pulmonary Aspergillosis in critically ill COVID-19 patients. Lancet Respir Med. 2020;8(6):e48–e49. doi: 10.2139/ssrn.3575581. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaize M, Mayaux J, Nabet C, Lampros A, Marcelin AG, Thellier M. Fatal invasive Aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26(7):1636–1637. doi: 10.3201/eid2607.201603. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Clinical management of COVID-19: interim guidance. Geneva: World Health Organization; 2020. pp. 1–62. Available from: [Google Scholar]
- 26.Bwire GM. Coronavirus: why men are more vulnerable to Covid-19 than women? SN Compr Clin Med. 2020;2:1–3. doi: 10.1007/s42399-020-00341-w. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:1–6. doi: 10.3389/fpubh.2020.00152/full. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]


