Abstract
Breast cancer is believed to be driven by epigenetic regulation of genes implicated in cell proliferation, survival, and differentiation. Recently, aberrant N6-methyladenosine (m6A) decorations turned up as crucial epigenetic regulator for malignant breast cancer, which may serve as new targets for breast cancer treatment. Here we briefly outline the functions of m6A and its regulatory proteins, including m6A “writers,” “readers,” and “erasers” on RNA life fate, recapitulate the latest breakthroughs in understanding m6A modification and its regulatory proteins, and the underlying molecular mechanisms that contribute to the carcinogenesis and the progression of breast cancer, so as to provide potential epigenetic targets for diagnosis, treatment and prognosis in breast cancer.
Keywords: m6A modification, Epigenetics, Methyltransferase, Demethylase, m6A reader, Breast cancer
Introduction
Breast cancer is the leading cancer and the main cause of cancer-related mortality for women all around the world according to the research in 2021 [1]. Although progress has been made in both understanding and treating breast cancer, nearly 30 percent of patients suffer relapse or metastasis which is the major cause of breast cancer-related mortality due to the shortage of effective treatment or preventive strategy [2]. Despite progressive genetic abnormalities, accumulating studies revealed that breast cancer can be also driven by epigenetic alterations [3]. However, the molecular mechanisms by which epigenetic alterations are regulated and drive breast cancer remain elusive.
N6-methyladenosine (m6A) modification was discovered and partially characterized in a great variety of cellular mRNAs in the 70s decade [4,5]. It is the most abundant internal methylation in mRNA [6]. Establishment of methylated RNA immunoprecipitation sequencing (MeRIP-seq/m6A-seq) enables the investigation of the m6A RNA methylomes and the mapping over 18,000 m6A sites in the transcripts of more than 7,000 human genes [7,8]. Approximately 90% of all m6A sites are conserved with G-A-C and A-A-C motifs [9]. m6A modification is a post-transcriptional methylation which formed by transferring the methyl group to the nitrogen atoms at 6th position of adenosine from S-adenosylmethionine [10]. m6A modification is regulated by the methyltransferase (“writer”), demethyltransferase (“eraser”) and m6A recognized RNA binding protein (“reader”), and determines RNA life fate, including RNA splicing, translocation, stability and translation [11,12]. Accumulating evidence indicates that dysregulation of m6A modification and its corresponding proteins contribute to the tumorigenesis and the progression of cancer [13].
In the current review, we briefly outline the roles of m6A modification and its regulatory proteins on RNA life fate, recapitulate the recent advances in understanding m6A modification and its regulatory proteins, and the underlying molecular mechanisms that assist breast cancer carcinogenesis and progression, so as to provide potential epigenetic targets for diagnosis, treatment and prognosis in breast cancer.
m6A modification determines RNA life fate
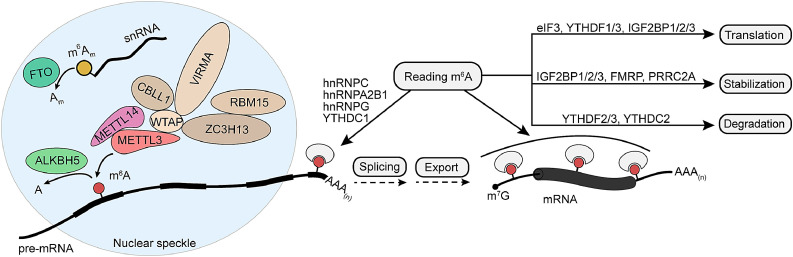
The reversible modification of m6A is regulated by the cooperation of methyltransferase (“writer”) and demethyltransferase (“eraser”) [11]. The m6A “writer” complex methyltransferase complex is composed of core methylation subunits, including methyltransferase-like 3 (METTL3) and methyltransferase-like 14 (METTL14), and methylation cofactors, including Wilms tumor 1-associated protein (WTAP), RNA binding motif protein 15/15B (RBM15/15B), Vir like m6A methyltransferase associated protein (VIRMA, synonym: KIAA1429), zinc finger CCCH-type containing 13 (ZC3H13) and Cbl proto-oncogene like 1 (CBLL1, synonym: HAKAI) [14]. Among them, METTL3 plays a methyltransferase catalytic role, METTL14 structurally serves as an upholder for METTL3 [15]. Methylation cofactors, such as WTAP, promotes m6A modification through guiding METTL3 and METTL14 to the nuclear speckles, while RBM15/RBM15B could interact with METTL3 in a WTAP-dependent manner and participate in the modulation of m6A modification of certain RNAs [12]. VIRMA associates with alternative polyadenylation and preferentially mediates m6A methylation of mRNA close to the stop codon and 3′ UTR [16]. ZC3H13 cooperates with other cofactors, like WTAP, to affect 3ꞌ UTR methylation [17]. CBLL1 interacts with WTAP/ZC3H13/VIRMA complex and affects some extents of m6A [18]. In addition, METTL16 was currently discovered as an m6A “writer” that primarily targets ncRNAs, lncRNAs, and pre‐mRNAs [19]. Two reported demethyltransferases those erase m6A modification are AlkB homolog 5 (ALKBH5) and fat mass and obesity-associated (FTO) protein, both belonging to the AlkB dioxygenase family which demethylate N-methylated nucleic acids [12]. Notably, FTO has moderate effect on m6A or m6Am levels in mRNA but preferred to demethylate N6, 2’-O-dimethyladenosine (m6Am) in snRNA [20]. m6Am is abundant in both mRNA and snRNA, and is involved in the RNA's stability and splicing [21,22]. m6A modification and m6A “readers” have an important role in the metabolism and the translation of mRNA [6]. Different sub-location of “readers” may exert different functions on RNA life fate [23]. The “readers” located in the nucleus, such as heterogeneous nuclear ribonucleoproteins (hnRNPs), including HNRNPG, HNRNPC, and HNRNPA2B1 and YTH domain containing 1 (YTHDC1) are involved in RNA stabilization, RNA splicing, RNA export, RNA structure switching, X chromosome inactivation and microRNA maturation [23,24]. The “readers” located in the cytoplasm, such as YTH domain-containing family protein 1-3 (YTHDF1-3), YTH domain containing 2 (YTHDC2), Fragile X mental retardation 1 (FMR1), proline-rich coiled-coil 2A (PRRC2A), insulin-like growth factor 2 mRNA-binding proteins 1-3 (IGF2BP1-3) and eukaryotic initiation factor 3 (eIF3), mainly contribute to the translation and degradation of mRNA [6,24,25] (Fig. 1).
Fig. 1.
m6A modification determines RNA life fate. Shown here is the working model of methyltransferase complex, demethylases, and m6A binding proteins. A, adenosine; m6A, N6-methyladenosine; m6Am, N6, 2’-O-dimethyladenosine; Am, 2’-O-methyladenosine; snRNA, small nuclear RNA; m7G, N7-methylguanosine; AAA(n), polyadenylation.
The expression of m6A modification and regulatory proteins in breast cancer
Epigenetic marks, such as DNA methylation, histone modifications, can be prognostic and predictive biomarkers in oncology. As the most abundant internal methylation in mRNA, m6A modification along with its regulatory proteins can serve as promising biomarkers for breast cancer.
The expression of m6A writers in breast cancer
Although, several studies reported that the expressions of m6A methylation, METTL3 and METTL14 were increased in breast tumor and promoted breast cancer progression [26−29], another studies showed opposite perspective that METTL3 and METTL14 may be low expressed in breast cancer [30]. Wu et al explored the mRNA expression of m6A regulators in both breast cancer samples and public datasets (TCGA and ONCOMINE), revealing that METTL3 and METTL14 were down-regulated in breast cancer [30]. Additionally, Gong et al also analyzed the expression of METTL14 and ZC3H13 in multiple bioinformatics databases, and found that these 2 genes negatively associated with the overall survival and may play a tumor-suppressing role in breast cancer [31]. While the inconsistency between serval previous studies and Wu's or Gong's study may due to the heterogeneity of cancer cell and the unequal expression of proteins and mRNAs which is frequently occurred in cancer. It is probably that METTL3 and METTL14 are indeed not prognostic markers in breast cancer, but involved in specific process and stage of pathogenesis which contribute to the regulation of breast cancer. TCGA data show that the expression of WTAP is reduced 1.9-fold in breast cancer samples as compared with normal tissue. Moreover, low mRNA level of WTAP is associated with ER (+) or PR (+) status, and high mRNA level is found to be associated with basal-like and normal breast-like breast cancer [30]. The expression of VIRMA mRNA is high and negatively related to patients’ survival time in breast cancer [32].
The expression of m6A erasers in breast cancer
The m6A “erasers”, FTO and ALKBH5, were reported to be tumor promotor in breast cancer [33,34]. The increase of FTO was observed in human HER2(+) breast cancer and highly correlated with poor prognosis of patients [33]. TCGA data also reveal that the mRNA expression of ALKBH5 is elevated 1.5-fold in breast cancer [30]. However, analysis of data from ONCOMINE revealed that FTO and ALKBH5, were decreased in breast cancer [30].
The expression of m6A readers in breast cancer
YTHDF1/2/3 and YTHDC1/2 have recently been identified as “reader” of m6A modification on mRNA, they display a preferential reorganization for m6A-methylated mRNA [35]. A recent study reported that YTHDF1 and YTHDF3 were most frequently amplified and associated with poor survival time of breast cancer patients through analyzing TCGA data [36]. IGF2BP family proteins (IGF2BP1/2/3) have been recognized as important regulators in breast cancer progression for a long time [37,38]. Although the expression level of IGF2BP1 is low in breast cancer, it is necessary for clonogenic growth of breast cancer cells [39]. IGF2BP2 and IGF2BP3 are aberrantly overexpressed in triple-negative breast cancer (TNBC) and associated with poor patients’ survival [38]. eIF3 is a huge complex comprised of 13 non-identical subunits, from eIF3a to eIF3m, in human cells. It has been reported that the expressions of eIF3d and eIF3e are increased in breast cancer cell lines and promote carcinogenesis of breast cancer [40,41]. It was reported that eIF3f was significantly down-regulated in ER (+) breast cancer cells [42], while the expressions of eIF3m and eIF3h are obviously higher in TNBC than those in non-TNBC or in normal breast tissues [43,44]. The expression levels of HNRNPA2B1 and HNRNPC are also higher in breast cancer cells [45,46]. However, Liu, et al reported that in their study, higher expression of hnRNPA2B1 correlated with longer median survival time of breast cancer patients [47].
In summary, m6A modification is probably not a prognostic marker for breast cancer, and the expression of m6A regulatory proteins is more complex in breast cancer or cell lines than m6A modification. The targets regulated by m6A modification and m6A regulatory proteins probably exert the function in breast cancer, which raise an open question and need to be understood.
m6A modification regulates breast cancer progression
Recently, accumulating evidence indicates that m6A modification plays important roles in breast cancer progression. m6A modification and m6A regulatory proteins regulate the expressions of proto-oncogenes or tumor suppressor genes through modulating the RNA life fate during breast cancer progression [27,33]. Alternatively, m6A regulatory proteins also participate in breast cancer progression via m6A independent manner [32,48]. m6A regulatory proteins are highly connected with breast cancer cell proliferation, metastasis, invasion, drug-resistance, and so on [30]. Thus, m6A regulatory proteins are promising targets for breast cancer treatment.
m6A modification modulates the growth of breast tumor
m6A “writer” complex components exhibit either promoting or inhibiting effects on the growth of breast tumor. The interfering of METTL3 expression could decrease cell proliferation and promote apoptosis in breast cancer. Using gene-specific m6A-qPCR, Wang et al identified Bcl-2 as the downstream effector of METTL3 [26]. Another study revealed that METTL3 showed high co-expression with hepatitis B X-interacting protein (HBXIP) which is reported as an oncogene for breast cancer[27]. HBXIP is able to increase the expression METTL3 through inhibiting miRNA let-7g. In turn, increased METTL3 upregulates the expression of HBXIP through enhancing m6A modification in HBXIP's mRNA which results in tumor malignant growth [27]. A recent study revealed that METTL3 regulated m6A-induced expression of LINC00958. m6A-modified LINC00958 is up-regulated in breast cancer cells and facilitates the tumor progression via miR-378a-3p/YY1 axis [49]. During METTL14-mediated m6A methylation in breast cancer, long non-coding RNA LNC942 exerts carcinogenic function as the upstream regulator for METTL14, whereas, CXCR4 and CYP1B1 are identified as direct targets of METTL14 which is also regulated by LNC942. LNC942 is able to promote the mRNA stability of CXCR4 and CYP1B1 for breast cancer proliferation and progression [29]. However, another study demonstrated opposite results that overexpression of METTL14 significantly suppressed cell viability and colony formation ability of MDA-MB-231 cells [30]. Due to the conflict results, we queried breast cancer cell lines data from DepMap portal (https://depmap.org/portal/), which is a cancer dependency database generated by cell depletion assay using either CRISPR or RNAi method, to help evaluate the role of METTL14. Using CRISPR method, METTL14 was essential in most of breast cancer cell lines. However, using RNAi method, METTL14 was non-essential in most of breast cancer cell lines (Table 1), which suggests low expression of METTL14 is enough to exert its function on the viability of breast cancer cell. A recent study showed that VIRMA was able to promote breast cancer proliferation. Through RIP-seq analysis, Qian et al found that the mRNA of CDK1 in the cell cycle was mostly associated with the oncogenic activities of VIRMA. Interestingly, VIRMA increased the stability of CDK1 mRNA in an m6A-independent manner [32]. CBLL1 negatively contributes to the carcinogenesis and malignant of breast cancer. CBLL1 functions as a co-repressor of ERα through interfering with the recruitment of SRC-1 and SRC-2 which are coactivators of ERα. Functionally, Gong et al revealed that CBLL1 overexpression could hindered the proliferation and migration of breast cancer cells [50].
Table 1.
List of reported functions of m6A regulatory proteins in breast cancer.
| Gene name | Role in RNA modification | Role in cancer | Mechanism | m6A regulation | References | DepMapa |
|---|---|---|---|---|---|---|
| METTL3 | writer | Oncogene | Promoting the expression of Bcl-2 through m6A modification | m6A dependent | [26] | Essential |
| Oncogene | Promoting the expression of HBXIP through enhancing m6A modification and be suppressed by let-7g | m6A dependent | [27] | |||
| Oncogene | Promoting the expression of LINC00958 through enhancing m6A | m6A dependent | [49] | |||
| Tumor suppressor | Inhibiting the expression of COL3A1 by m6A modification | m6A dependent | [48] | |||
| Oncogene | Promoting the expression of miR-221-3p by increasing pri-miR-221-3p m6A mRNA modification | m6A dependent | [65] | |||
| METTL14 | writer | Oncogene | Promoting the expression of hsa-miR-146a-5p | Unkown | [28] | Essentialb |
| Oncogene | Promoting the expression of CXCR4 and CYP1B1 through m6A modification which are the targets of LNC942 | m6A dependent | [29] | |||
| VIRMA | cofactor | Oncogene | Increasing the stability of CDK1 mRNA | m6A independent | [32] | Very essentialb |
| CBLL1 | cofactor | Tumor suppressor | Interfering with the recruitment of coactivators of ERα: SRC-1 and SRC-2 | m6A independent | [50] | Essential |
| FTO | eraser | Oncogene | Inducing BNIP3 mRNA degradation through modulating m6A | m6A dependent | [33] | Partial-essentialc |
| Oncogene | Promoting glycolysis and lactic acid production through PI3K/AKT signaling pathway | Unkown | [52] | |||
| Oncogene | Promoting miR-181b-3p/ARL5B signaling pathway | Unkown | [58] | |||
| ALKBH5 | eraser | Oncogene | Inducing by hypoxia and promoting the mRNA stability and the expression of NANOG | m6A dependent | [34] | Partial-essential |
| YTHDF3 | reader | Oncogene | Promoting the translation of ST6GALNAC5, GJA1 and EGFR through m6A modification | m6A dependent | [62] | No data |
| IGF2BP1 | reader | Tumor suppressor | Promoting the degradation of UCA1 through recruiting the CCR4-NOT1 deadenylase complex | Unkown | [63] | Partial-essentialc |
| Oncogene | Interacting with lncRNA KB-1980E6.3 and promoting c-Myc mRNA stability through modulating m6A | m6A dependent | [66] | |||
| IGF2BP2 | reader | Oncogene | Interacting with pseudogene-transcribed RPSAP52 | Unkown | [53] | Partial-essential |
| Oncogene | Suppressing the transcription of miR-200a by destabilizing the mRNA of the progesterone receptor (PR) | Unkown | [38] | |||
| IGF2BP3 | reader | Oncogene | Promoting the expression of CD44 via binding the mRNA of CD44 | Unkown | [54] | Partial-essentialc |
| Oncogene | Inhibiting TRIM25 mRNA degradation mediated by miR-3614 through blocking the maturation of miR-3614 | Unkown | [55] | |||
| Oncogene | Promoting the mRNA stability of CERS6 as an RNA sponge for long non-coding RNA CERS6-AS1 | Unkown | [56] | |||
| Oncogene | Suppressing the transcription of miR-200a by destabilizing the mRNA of the progesterone receptor (PR) | Unkown | [38] | |||
| Oncogene | Promoting the expression of SOX2 by binding to the mRNA of SLUG | Unkown | [64] | |||
| Oncogene | Promoting the expression of BCRP via binding to BCRP mRNA | Unkown | [70] | |||
| eIF3d | reader | Oncogene | Promoting Wnt/β-catenin signaling | Unkown | [40] | Very essentialc |
| eIF3h | reader | Oncogene | Catalyzing the deubiquitylation of YAP and resulting in the stabilization of YAP | m6A independent | [44] | Essentialc |
| HNRNPC | reader | Oncogene | Regulating Alu-enriched dsRNA and the down-stream interferon response | Unkown | [46] | Very essential |
| HNRNPA2B1 | reader | Oncogene | Promoting ERK1/2 and STAT3 pathway | Unkown | [58] | Partial-essentialc |
| Tumor suppressor | Regulating ERK-MAPK/Twist, GR-β/TCF4, STAT3 and WNT/TCF4 signaling pathways | Unkown | [47] |
Data were retrieved from breast cancer cell lines dataset from DepMap portal (https://depmap.org/portal/). Very essential, dependency score ≤ -1.0; Essential, -0.1 < dependency score ≤ -0.5; Partial essential, -0.5 < dependency score ≤ 0; Non-essential, dependency score > 0. For RNAi data, Combined RNAi (Broad, Novartis, Marcotte) dataset was queried. For CRISPR data, CRISPR (Avana) Public 21Q1 dataset was queried. Lower dependency score from either CRISPR or RNAi data was chosen for default.
Conflict with RNAi data.
Supported by both RNAi data and CRISPR data.
Like the “writer” complex, m6A “eraser” also exert essential roles during breast cancer cell proliferation [51]. FTO enhances cell proliferation and colony formation of breast cancer cells [33]. Mechanistically, FTO induces the mRNA degradation of BNIP3, an apoptosis-inducing protein, through modulating m6A in the 3′UTR [33]. FTO also play an oncogenic role in breast cancer through modulating energy metabolism, which involves the promotion of glycolysis and lactic acid production through PI3K/AKT signaling pathway [52]. Knockdown of ALKBH5 inhibits breast cancer cell proliferation and colony formation [34]. The expression of ALKBH5 can be induced by hypoxia. Induced ALKBH5 promotes the mRNA stability and the expression of NANOG, which in turn enhances breast cancer stem cells (BCSCs) [34]. According to these independent studies, m6A “erasers” play an oncogenic role in breast cancer. Thus, the m6A “erasers” may be promising targets for breast cancer treatment. m6A “readers” are major players in breast cancer due to their RNA binding property. The expression of IGF2BP1 is low in breast cancer cells, but it is necessary for the clonogenic growth of breast cancer cells [39]. Furthermore, IGF2BP2 interacts with pseudogene-transcribed RPSAP52 to stimulate the proliferative pathways in breast cancer [53]. Consistently, IGF2BP3 contributes to breast cancer cell proliferation via binding the mRNA of CD44 and enhancing the expression of CD44, which increases IGF2 levels in fibroblasts [54]. Additionally, IGF2BP3 promotes cell proliferation by blocking the maturation of miR-3614, which protects TRIM25 mRNA from degradation mediated by miR-3614 [55]. Additionally, IGF2BP3 serves as an RNA sponge for long non-coding RNA CERS6-AS1. Due to the binding of CERS6-AS1 to IGF2BP3, the mRNA stability of CERS6 is increased, which results in the promotion of cell proliferation and the suppression of cell apoptosis [56]. Although the authors didn't investigate whether these functions of IGF2BPs relied on m6A modification of the RNA, the functions of IGF2BPs was exerted through their RNA binding capacity, indicating that m6A modification might contribute to the recognition of RNAs. Knockdown of eIF3d suppresses breast cancer cell proliferation through inhibiting Wnt/β-catenin signaling [40]. eIF3h serves as a prognostic marker, of which amplification or overexpression regulates the proliferation, survival, and transformation of breast cancer cells [57]. Inhibition of HNRNPC hindered the proliferation of MCF-7 and T47D cells [46]. In this process, dsRNA sensor RIG‐I induced interferon response, resulted in the suppression of cell proliferation. Moreover, suppression of HNRNPC also induces Alu-enriched dsRNA which eventually resulted in nonsense-mediated RNA decay [46]. HNRNPA2B1 increases the carcinogenesis of breast cancer which is attributable to ERK1/2 and STAT3 pathway stimulation [58].
m6A modification modulates the metastasis of breast tumor
Breast cancer metastasis is an enormous challenge for treatment. m6A modification and regulatory proteins also participate in the process of metastasis. COL3A1 promotes the metastatic ability of TNBC cells. METTL3 can methylate the mRNA of COL3A1 resulting in decreased expression of COL3A1 and inhibited metastasis [48]. Additionally, increased expression of METTL14 and METTL14-mediated m6A modifications enhance the metastasis capacity of breast cancer cells through reshaping the miRNA/mRNA network which is most enriched in cancer [28]. hsa‑miR‑146a‑5p is identified as one of the miRNA targets of METTL14 in this remodeled miRNA/mRNA network [28]. VIRMA is able to promote breast cancer metastasis via increasing the mRNA stability of CDK1 [32]. CBLL1 inhibits breast cancer cell migration through suppressing ERα activation [50]. RBM15/15B is reported positively correlated with invasive breast carcinoma [59,60]. m6A “writer” most likely modulates a complicated group of targets, which form a network and feed multiple cancer pathways. Further researches need to consider to extend their downstream investigation to a larger scope, which might figure out the discordance presented in the current independent studies. m6A “erasers”, FTO and ALKBH5, were also supposed to contribute to breast cancer metastasis [33,34]. FTO is able to promote the migration and the invasion of HER2(+) breast cancer cells through miR-181b-3p/ARL5B axis [61]. m6A “readers” are also major players in breast cancer metastasis. Recently, an important study reported that YTHDF3 was correlated with the prognosis of breast cancer and promoted breast cancer brain metastasis [62]. Chang et al found that YTHDF3 knockdown inhibited transmigration across the blood-brain barrier in a mouse model [62]. Mechanistically, YTHDF3 increased the translation of the key brain metastasis genes such as ST6GALNAC5, GJA1 and EGFR in an m6A-dependent manner [62]. Another study showed that IGF2BP1 inhibited lncRNA UCA1-mediated breast cancer cell invasion. IGF2BP1 interacts with lncRNA UCA1 and triggers UCA1 degradation via recruiting the CCR4-NOT1 deadenylase complex [63]. Similarly, IGF2BP2 and IGF2BP3 were reported to promote the metastasis of TNBC collaboratively through recruiting CCR4-NOT1 deadenylase complex [38]. They can suppress the transcription of miR-200a by destabilizing the mRNA of the progesterone receptor (PR). In turn, miR-200a could directly target the mRNAs of IGF2BP2 and IGF2BP3 [38]. Interestingly, Zhou, et al have confirmed that elevated eIF3h in breast cancer cells exerts an oncogenic role as deubiquitylating enzyme which catalyzes the deubiquitylation of YAP, resulting in the stabilization of YAP and breast tumor invasion and metastasis [44]. It is worth noting that eIF3h is known as a subunit of translation initiation factor eIF3, which is also known as m6A reader. eIF3h functioned neither as translation initiation factor nor m6A reader in Zhou's research. eIF3d can also contribute to breast cancer invasion through Wnt/β-catenin pathway [40]. Liu, et al revealed that HNRNPA2B1 expression was not only negatively associated with breast cancer metastasis. Through multiple in vitro and in vivo experiments, Liu, et al also confirmed that HNRNPA2B1 could inhibit breast cancer metastasis, which involved complicated regulation of ERK-MAPK/Twist, GR-β/TCF4, STAT3 and WNT/TCF4 signaling pathways [47]. However, whether HNRNPA2B1 worked depending on its m6A reader activity remains unclear (Fig. 2).
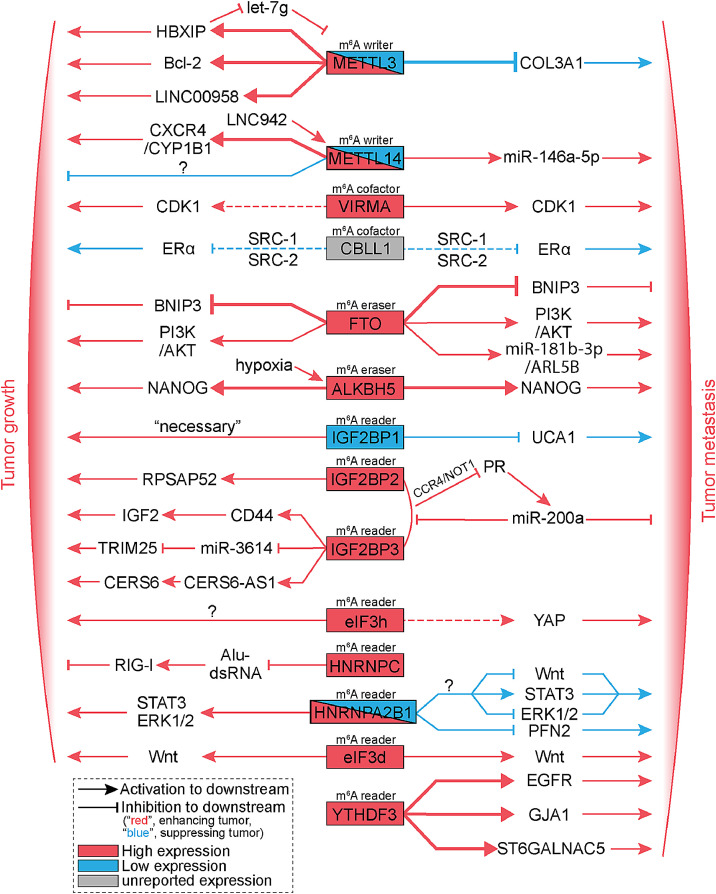
Fig. 2.
m6A modification modulates the growth and the metastasis of breast tumor. Shown here is molecules and pathways regulated by m6A regulatory proteins resulting in enhanced or suppressed breast tumor growth and metastasis. Arrows indicate activation to downstream molecules or pathways, and flat lines indicate inhibition to downstream molecules or pathways. Bold arrows indicate m6A dependent manner, dashed lines indicate m6A independent manner, and solid arrows or lines indicate unknown manner. Red arrows or flat lines indicate to enhance breast tumor growth and metastasis, blue arrows or flat lines indicate to suppress breast tumor growth and metastasis. The red box means high expression, the blue box means low expression, and the gray box means unreported expression in breast cancer. “?”, unknown mechanism. (Color version of figure is available online.)
m6A modification modulates the clinical outcomes of breast cancer
Drug tolerance and stemness of cancer cells are 2 important things that affect clinical outcomes of breast cancer [64]. METTL3/miR-221-3p/HIPK2/Che-1 axis is found to be involved in Adriamycin resistance in MCF-7 breast cancer cells. METTL3 is able to methylate pri-miR-221-3p resulting in elevated expression of mature miR-221-3p. Consequently, miR-221-3p down-regulates HIPK2 through targeting the 3’UTR of HIPK2 resulting in increased level of Che-1 which is a target of HIPK2. Thus, the METTL3/miR-221-3p/HIPK2/Che-1 axis enhances drug resistance in adriamycin-resistant MCF-7 cells [65]. A recent study revealed that a hypoxia-induced lncRNA KB-1980E6.3 could promote the stem-like properties of breast cancer stem cells (BCSCs) through the recruitment of IGF2BP1. The interaction between IGF2BP1 and lncRNA KB-1980E6.3 is important for m6A-induced mRNA stability of c-Myc [66]. IGF2BP3 is also reported to associate with TNBC [67] and facilitates the initiation of tumor and the stemness of BCSCs in TNBC through regulating SOX2 expression. In this process, SLUG, as an important functional downstream of IGF2BP3, mediates the regulation of SOX2 by IGF2BP3 [68]. Besides, IGF2BP3 was shown to be essential for the stemness of breast cancer cells, which could be regulated by miR-34a [69]. Preferential expression of IGF2BP3 in TNBC contributes to the resistance to many chemotherapeutics [70]. Suppression of IGF2BP3 in TNBC cells significantly increased the sensitivity to doxorubicin and mitoxantrone of cancer cells. The mechanism underlines that IGF2BP3 regulates breast cancer resistance protein (BCRP), which is a major effector of drug resistance in breast cancer [70].
The expression of HNRNPA2B1 is higher in tamoxifen-resistant LCC9 cells than that in MCF-7 cells which are tamoxifen-sensitive cells. In MCF-7 cells, ectopic expression of HNRNPA2B1 could alter miRNA transcriptome [45]. However, the involved pathways, the roles of altered miRNA transcriptome and downstream effectors involving in endocrine-resistance necessitates further elucidation of detailed mechanisms [45].
Since some conflict results were observed in multiple independent studies. We also checked the roles of m6A regulatory proteins in DepMap portal. Through the results we can conclude that most of m6A regulatory proteins are at least partial essential in breast cancer cell lines (Table 1).
Experimental methods to identify m6A specific regulation of individual RNA
It has been difficult for researchers to pick out individual RNAs of interest that bear m6A marks and this has required the development of a number of new techniques. Researchers also could not exclude the interferences from contaminating RNA species which was co-purified with target mRNA. A lot of studies didn't distinguish whether their purposed model worked in m6A dependent manner. m6A-seq (or MeRIP-seq) engaging immunoprecipitation and high-throughput sequencing technologies opens up the era of “Epitranscriptome” [71]. Dedicated and detailed reviews on technologies investigating epitranscriptome were previously published [23]. To facilitate the following studies focus on m6A modification or m6A regulatory proteins in breast cancer, we summarized necessary experiments those identifying individual RNAs that contained m6A, and those exclude the interferences from contaminating RNA species which was co-purified with target mRNA.
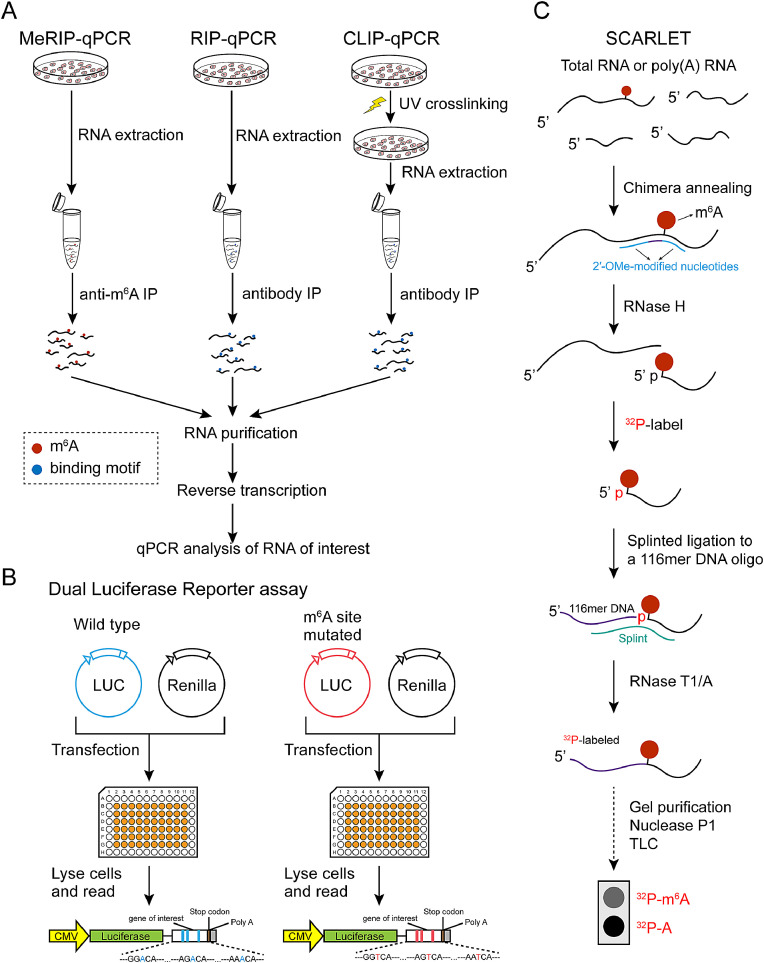
MeRIP-qPCR
Methylated RNA immunoprecipitation (MeRIP) is widely used in m6A related studies. Immunoprecipitated and purified with m6A antibody, m6A marked RNAs could be subjected to reverse transcription and qPCR to confirm the m6A enrichment of RNA of interest (ROI) or to compare m6A enrichment of ROI between different samples/treatments [7] (Fig. 3A).
Fig. 3.
Experiments identify m6A specific regulation of individual RNA. Shown here is schematic diagrams of (A) methylated RNA immunoprecipitation (MeRIP)-qPCR, RNA immunoprecipitation (RIP)-qPCR, cross-linking immunoprecipitation (CLIP)-qPCR, (B) dual luciferase reporter assay, (C) site-specific cleavage, and radioactive-labeling followed by ligation-assisted extraction and thin-layer chromatography (SCARLET) technologies. m6A, N6-methyladenosine; IP, immunoprecipitation; qPCR, quantitative polymerase chain reaction; LUC, luciferase; CMV, cytomegalovirus promoter; A, adenosine; TLC, thin-layer chromatography.
RIP-qPCR or CLIP-qPCR
RNA immunoprecipitation (RIP)-qPCR or cross-linking immunoprecipitation (CLIP)-qPCR is useful for the determination of interaction between the protein of interest and the RNA of interest. Both techniques depend on antibody to immunoprecipitate RNA-protein complex, and include reverse transcription, and qPCR analysis of RNA of interest. The differences are CLIP-qPCR uses ultraviolet (UV) light to crosslink proteins to RNAs that are in close proximity, and the following isolation is done stringently [72] (Fig. 3A).
Dual luciferase reporter assay
Dual luciferase reporter assay uses a reporter DNA conjugated with luciferase to quantify the regulation to the reporter. Weng, et al inserted wild type or m6A sites-mutated (A to T) 3’ coding region of MYB or MYC right before the stop codon of luciferase to show that METTL14 regulated the mRNA stability of MYB or MYC through m6A sites [73]. This method is an alternative easy way to confirm an m6A specific regulation of target RNA, when the m6A sites in RNA of interest are known (Fig. 3B).
Site-specific cleavage and radioactive-labeling followed by ligation-assisted extraction and thin-layer chromatography (SCARLET)
SCARLET was established to accurately decide whether an m6A is presented at a given site of mRNA/lncRNA [74]. The method uses a chimeric DNA oligonucleotide with 2’-OMe/2’-H modifications to guide RNase H to create a site-specific cleavage at the 5’ end of candidate m6A site. The cleaved site is radiolabeled with 32P and splint-ligated to a 116 nt ssDNA oligonucleotide. The resulted fused RNA-DNA oligonucleotide is purified and digested to generate mononucleotides with 5’ phosphate and determined by thin-layer chromatography [74]. The method can confirm any m6A site at mRNA or lncRNA from a total-RNA sample without additional purification step (Fig. 3C).
Potential targeted therapeutic strategies based on m6A modification in breast cancer
Several new drugs targeting DNA methylases or histone modifying enzymes have been approved for the treatment of cancer, and epigenetic chemical intervention to influence gene expression has become an active field of international research on new drug development [75,76]. Given the critical role of m6A regulatory proteins in breast cancer, they are expected to be promising drug targets for cancer treatment. Metformin, which was previously regarded as anti-diabetic medicine, is capable of inhibiting breast cancer cell proliferation by down-regulating the expression of METTL3. This study suggests that drug repurposing is a valuable approach for the screening of m6A inhibitors, due to the possibility that FDA approved medications may exert m6A regulatory functions [77]. Recently, the researchers developed small molecule inhibitors CS1 and CS2, which target m6A “eraser” FTO in cancer [78]. The inhibitor could occupy FTO catalytic "pocket" to stop the m6A modified oligonucleotides into function, thus inhibiting the demethylation function of FTO. The inhibitors showed effective anti-leukemia effect in PDX mouse model, and the clinical trials showed a satisfactory anti-cancer effect. Moreover, it is worth noting that the anti-cancer effect of CS1 and CS2 in diverse solid tumors was verified, including breast cancer [78]. Their study suggests that to develop applicable selective and effective inhibitors of m6A regulatory protein for clinical use may well provide more effective strategies to treat breast cancer, especially when combined with other therapeutic agents for cancers that are resistant to existing therapies.
Conclusion
Although m6A has been the focus of many studies in recent years, our knowledge about it is far from complete. From the current studies, METTL3, METTL14, FTO, ALKBH5, YTHDF3 and IGF2BP1 are proved to exert functions dependent on m6A modification in breast cancer. However, whether the other m6A modulators regulate breast cancer progression replying on m6A modification is unanswered (Table 1).
Given that cancer is proven to be driven by epigenetic alteration, and alteration of mRNA controls the expression of oncogenes, targeting m6A regulatory proteins can serve as a new approach for precisely modifying epitranscriptome of cancer and benefiting cancer treatment. Development of enzyme inhibitor of m6A “writer” and “eraser” can control the addition and removal of m6A marks which control RNA life fate. Development of competence of m6A reader can specify the RNA to be regulated. Combined interventions of “writer”, “eraser” and “reader” may make specific control of RNA life fate come true.
Author contributions
RF: Conceptualization, Writing - original draft. LY: Writing - review & editing. HS: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China under Grant No. 81802856 (to Hui Shi); the Projects of Shandong Province Higher Education Science and Technology under Grant No. J17KA230 (to Hui Shi). The funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
Conflicts of interest: The authors declare no competing interests.
Contributor Information
Lihong Ye, Email: yelihong@nankai.edu.cn.
Hui Shi, Email: 8858shihui@mail.jnmc.edu.cn.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Patel S. Breast cancer: Lesser-known facets and hypotheses. Biomed Pharmacother. 2018;98:499–506. doi: 10.1016/j.biopha.2017.12.087. [DOI] [PubMed] [Google Scholar]
- 3.Bao X., Anastasov N., Wang Y., Rosemann M. A novel epigenetic signature for overall survival prediction in patients with breast cancer. J Transl Med. 2019;17:380. doi: 10.1186/s12967-019-2126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer K.P. RNA synthesis and processing reactions in a subcellular system from mouse L cells. Hoppe Seylers Z Physiol Chem. 1982;363:33–43. doi: 10.1515/bchm2.1982.363.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 9.Lan Q., Liu P.Y., Haase J., Bell J.L., Huttelmaier S., Liu T. The critical role of RNA m(6)A methylation in cancer. Cancer Res. 2019;79:1285–1292. doi: 10.1158/0008-5472.CAN-18-2965. [DOI] [PubMed] [Google Scholar]
- 10.Niu Y., Zhao X., Wu Y.S., Li M.M., Wang X.J., Yang Y.G. N6-methyl-adenosine (m6A) in RNA: an old modification with a novel epigenetic function. Genomics Proteomics Bioinformatics. 2013;11:8–17. doi: 10.1016/j.gpb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z.X., Li L.M., Sun H.L., Liu S.M. Link between m6A modification and cancers. Front Bioeng Biotechnol. 2018;6:89. doi: 10.3389/fbioe.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L., Li H., Wu A., Peng Y., Shu G., Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176. doi: 10.1186/s12943-019-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang R., Chen X., Zhang S., Shi H., Ye Y., Shi H., Zou Z., Li P., Guo Q., Ma L. EGFR/SRC/ERK-stabilized YTHDF2 promotes cholesterol dysregulation and invasive growth of glioblastoma. Nat Commun. 2021;12:177. doi: 10.1038/s41467-020-20379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L., Jia G., Yu M., Lu Z., Deng X. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yue Y., Liu J., Cui X., Cao J., Luo G., Zhang Z., Cheng T., Gao M., Shu X., Ma H. VIRMA mediates preferential m(6)A mRNA methylation in 3′UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knuckles P., Lence T., Haussmann I.U., Jacob D., Kreim N., Carl S.H., Masiello I., Hares T., Villasenor R., Hess D. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen J., Lv R., Ma H., Shen H., He C., Wang J., Jiao F., Liu H., Yang P., Tan L. Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69:1028–1038. doi: 10.1016/j.molcel.2018.02.015. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warda A.S., Kretschmer J., Hackert P., Lenz C., Urlaub H., Hobartner C., Sloan K.E., Bohnsack M.T. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18:2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauer J., Sindelar M., Despic V., Guez T., Hawley B.R., Vasseur J.J., Rentmeister A., Gross S.S., Pellizzoni L., Debart F. FTO controls reversible m(6)Am RNA methylation during snRNA biogenesis. Nat Chem Biol. 2019;15:340–347. doi: 10.1038/s41589-019-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J., Liu F., Lu Z., Fei Q., Ai Y., He P.C., Shi H., Cui X., Su R., Klungland A. Differential m(6)A, m(6)Am, and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell. 2018;71:973–985. doi: 10.1016/j.molcel.2018.08.011. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulias K., Toczydlowska-Socha D., Hawley B.R., Liberman N., Takashima K., Zaccara S., Guez T., Vasseur J.J., Debart F., Aravind L. Identification of the m(6)Am methyltransferase PCIF1 reveals the location and functions of m(6)Am in the transcriptome. Mol Cell. 2019;75:631–643. doi: 10.1016/j.molcel.2019.06.006. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaccara S., Ries R.J., Jaffrey S.R. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20:608–624. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- 24.Shi H., Wei J., He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu R., Li A., Sun B., Sun J.G., Zhang J., Zhang T., Chen Y., Xiao Y., Gao Y., Zhang Q. A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019;29:23–41. doi: 10.1038/s41422-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Xu B., Shi J. N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene. 2020;722 doi: 10.1016/j.gene.2019.144076. [DOI] [PubMed] [Google Scholar]
- 27.Cai X., Wang X., Cao C., Gao Y., Zhang S., Yang Z., Liu Y., Zhang X., Zhang W., Ye L. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–19. doi: 10.1016/j.canlet.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Yi D., Wang R., Shi X., Xu L., Yilihamu Y., Sang J. METTL14 promotes the migration and invasion of breast cancer cells by modulating N6methyladenosine and hsamiR146a5p expression. Oncol Rep. 2020;43:1375–1386. doi: 10.3892/or.2020.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun T., Wu Z., Wang X., Wang Y., Hu X., Qin W., Lu S., Xu D., Wu Y., Chen Q. LNC942 promoting METTL14-mediated m(6)A methylation in breast cancer cell proliferation and progression. Oncogene. 2020;39:5358–5372. doi: 10.1038/s41388-020-1338-9. [DOI] [PubMed] [Google Scholar]
- 30.Wu L., Wu D., Ning J., Liu W., Zhang D. Changes of N6-methyladenosine modulators promote breast cancer progression. BMC Cancer. 2019;19:326. doi: 10.1186/s12885-019-5538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong P.J., Shao Y.C., Yang Y., Song W.J., He X., Zeng Y.F., Huang S.R., Wei L., Zhang J.W. Analysis of N6-methyladenosine methyltransferase reveals METTL14 and ZC3H13 as tumor suppressor genes in breast cancer. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.578963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian J.Y., Gao J., Sun X., Cao M.D., Shi L., Xia T.S., Zhou W.B., Wang S., Ding Q., Wei J.F. KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6-methyladenosine-independent manner. Oncogene. 2019;38:6123–6141. doi: 10.1038/s41388-019-0861-z. [DOI] [PubMed] [Google Scholar]
- 33.Niu Y., Lin Z., Wan A., Chen H., Liang H., Sun L., Wang Y., Li X., Xiong X.F., Wei B. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18:46. doi: 10.1186/s12943-019-1004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C., Samanta D., Lu H., Bullen J.W., Zhang H., Chen I., He X., Semenza G.L. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H., Wang X., Lu Z., Zhao B.S., Ma H., Hsu P.J., Liu C., He C. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anita R., Paramasivam A., Priyadharsini J.V., Chitra S. The m6A readers YTHDF1 and YTHDF3 aberrations associated with metastasis and predict poor prognosis in breast cancer patients. Am J Cancer Res. 2020;10:2546–2554. [PMC free article] [PubMed] [Google Scholar]
- 37.Degrauwe N., Suva M.L., Janiszewska M., Riggi N., Stamenkovic I. IMPs: an RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer. Genes Dev. 2016;30:2459–2474. doi: 10.1101/gad.287540.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H.Y., Ha Thi H.T., Hong S. IMP2 and IMP3 cooperate to promote the metastasis of triple-negative breast cancer through destabilization of progesterone receptor. Cancer Lett. 2018;415:30–39. doi: 10.1016/j.canlet.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 39.Fakhraldeen S.A., Clark R.J., Roopra A., Chin E.N., Huang W., Castorino J., Wisinski K.B., Kim T., Spiegelman V.S., Alexander C.M. Two isoforms of the RNA binding protein, coding region determinant-binding protein (CRD-BP/IGF2BP1), are expressed in breast epithelium and support clonogenic growth of breast tumor cells. J Biol Chem. 2015;290:13386–13400. doi: 10.1074/jbc.M115.655175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y., Guo Y. Knockdown of eIF3D inhibits breast cancer cell proliferation and invasion through suppressing the Wnt/beta-catenin signaling pathway. Int J Clin Exp Pathol. 2015;8:10420–10427. [PMC free article] [PubMed] [Google Scholar]
- 41.Grzmil M., Rzymski T., Milani M., Harris A.L., Capper R.G., Saunders N.J., Salhan A., Ragoussis J., Norbury C.J. An oncogenic role of eIF3e/INT6 in human breast cancer. Oncogene. 2010;29:4080–4089. doi: 10.1038/onc.2010.152. [DOI] [PubMed] [Google Scholar]
- 42.Cuesta R., Berman A.Y., Alayev A., Holz M.K. Estrogen receptor alpha promotes protein synthesis by fine-tuning the expression of the eukaryotic translation initiation factor 3 subunit f (eIF3f) J Biol Chem. 2019;294:2267–2278. doi: 10.1074/jbc.RA118.004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han W., Zhang C., Shi C.T., Gao X.J., Zhou M.H., Shao Q.X., Shen X.J., Wu C.J., Cao F., Hu Y.W. Roles of eIF3m in the tumorigenesis of triple negative breast cancer. Cancer Cell Int. 2020;20:141. doi: 10.1186/s12935-020-01220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z., Zhou H., Ponzoni L., Luo A., Zhu R., He M., Huang Y., Guan K.L., Bahar I., Liu Z. EIF3H orchestrates hippo pathway-mediated oncogenesis via catalytic control of YAP stability. Cancer Res. 2020;80:2550–2563. doi: 10.1158/0008-5472.CAN-19-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klinge C.M., Piell K.M., Tooley C.S., Rouchka E.C. HNRNPA2/B1 is upregulated in endocrine-resistant LCC9 breast cancer cells and alters the miRNA transcriptome when overexpressed in MCF-7 cells. Sci Rep. 2019;9:9430. doi: 10.1038/s41598-019-45636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y., Zhao W., Liu Y., Tan X., Li X., Zou Q., Xiao Z., Xu H., Wang Y., Yang X. Function of HNRNPC in breast cancer cells by controlling the dsRNA-induced interferon response. EMBO J. 2018;37 doi: 10.15252/embj.201899017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y., Li H., Liu F., Gao L.B., Han R., Chen C., Ding X., Li S., Lu K., Yang L. Heterogeneous nuclear ribonucleoprotein A2/B1 is a negative regulator of human breast cancer metastasis by maintaining the balance of multiple genes and pathways. EBioMedicine. 2020;51 doi: 10.1016/j.ebiom.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi Y., Zheng C., Jin Y., Bao B., Wang D., Hou K., Feng J., Tang S., Qu X., Liu Y. Reduced expression of METTL3 promotes metastasis of triple-negative breast cancer by m6A methylation-mediated COL3A1 up-regulation. Front Oncol. 2020;10:1126. doi: 10.3389/fonc.2020.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rong D., Dong Q., Qu H., Deng X., Gao F., Li Q., Sun P. m(6)A-induced LINC00958 promotes breast cancer tumorigenesis via the miR-378a-3p/YY1 axis. Cell Death Discov. 2021;7:27. doi: 10.1038/s41420-020-00382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong E.Y., Park E., Lee K. Hakai acts as a coregulator of estrogen receptor alpha in breast cancer cells. Cancer Sci. 2010;101:2019–2025. doi: 10.1111/j.1349-7006.2010.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan A., Dang Y., Chen G., Mo Z. Overexpression of the fat mass and obesity associated gene (FTO) in breast cancer and its clinical implications. Int J Clin Exp Pathol. 2015;8:13405–13410. [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Wang R., Zhang L., Li J., Lou K., Shi B. The lipid metabolism gene FTO influences breast cancer cell energy metabolism via the PI3K/AKT signaling pathway. Oncol Lett. 2017;13:4685–4690. doi: 10.3892/ol.2017.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveira-Mateos C., Sanchez-Castillo A., Soler M., Obiols-Guardia A., Pineyro D., Boque-Sastre R., Calleja-Cervantes M.E., Castro de Moura M., Martinez-Cardus A., Rubio T. The transcribed pseudogene RPSAP52 enhances the oncofetal HMGA2-IGF2BP2-RAS axis through LIN28B-dependent and independent let-7 inhibition. Nat Commun. 2019;10:3979. doi: 10.1038/s41467-019-11910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y., Yu C., Wu Y., Sun X., Su Q., You C., Xin H. CD44(+) fibroblasts increases breast cancer cell survival and drug resistance via IGF2BP3-CD44-IGF2 signalling. J Cell Mol Med. 2017;21:1979–1988. doi: 10.1111/jcmm.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z., Tong D., Han C., Zhao Z., Wang X., Jiang T., Li Q., Liu S., Chen L., Chen Y. Blockade of miR-3614 maturation by IGF2BP3 increases TRIM25 expression and promotes breast cancer cell proliferation. EBioMedicine. 2019;41:357–369. doi: 10.1016/j.ebiom.2018.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao G., Huang J., Pan W., Li X., Zhou T. Long noncoding RNA CERS6-AS1 functions as a malignancy promoter in breast cancer by binding to IGF2BP3 to enhance the stability of CERS6 mRNA. Cancer Med. 2020;9:278–289. doi: 10.1002/cam4.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmood S.F., Gruel N., Chapeaublanc E., Lescure A., Jones T., Reyal F., Vincent-Salomon A., Raynal V., Pierron G., Perez F. A siRNA screen identifies RAD21, EIF3H, CHRAC1 and TANC2 as driver genes within the 8q23, 8q24.3 and 17q23 amplicons in breast cancer with effects on cell growth, survival and transformation. Carcinogenesis. 2014;35:670–682. doi: 10.1093/carcin/bgt351. [DOI] [PubMed] [Google Scholar]
- 58.Hu Y., Sun Z., Deng J., Hu B., Yan W., Wei H., Jiang J. Splicing factor hnRNPA2B1 contributes to tumorigenic potential of breast cancer cells through STAT3 and ERK1/2 signaling pathway. Tumour Biol. 2017;39 doi: 10.1177/1010428317694318. [DOI] [PubMed] [Google Scholar]
- 59.Shahriyari L., Abdel-Rahman M., Cebulla C. BAP1 expression is prognostic in breast and uveal melanoma but not colon cancer and is highly positively correlated with RBM15B and USP19. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens T.A., Meech R. BARX2 and estrogen receptor-alpha (ESR1) coordinately regulate the production of alternatively spliced ESR1 isoforms and control breast cancer cell growth and invasion. Oncogene. 2006;25:5426–5435. doi: 10.1038/sj.onc.1209529. [DOI] [PubMed] [Google Scholar]
- 61.Xu Y., Ye S., Zhang N., Zheng S., Liu H., Zhou K., Wang L., Cao Y., Sun P., Wang T. The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun (Lond) 2020;40:484–500. doi: 10.1002/cac2.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang G., Shi L., Ye Y., Shi H., Zeng L., Tiwary S., Huse J.T., Huo L., Ma L., Ma Y. YTHDF3 induces the translation of m(6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020 doi: 10.1016/j.ccell.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Y., Meng X., Chen S., Li W., Li D., Singer R., Gu W. IMP1 regulates UCA1-mediated cell invasion through facilitating UCA1 decay and decreasing the sponge effect of UCA1 for miR-122-5p. Breast Cancer Res. 2018;20:32. doi: 10.1186/s13058-018-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saygin C., Matei D., Majeti R., Reizes O., Lathia J.D. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell. 2019;24:25–40. doi: 10.1016/j.stem.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 65.Pan X., Hong X., Li S., Meng P., Xiao F. METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. Exp Mol Med. 2021;53:91–102. doi: 10.1038/s12276-020-00510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu P., He F., Hou Y., Tu G., Li Q., Jin T., Zeng H., Qin Y., Wan X., Qiao Y. A novel hypoxic long noncoding RNA KB-1980E6.3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-Myc mRNA stability. Oncogene. 2021 doi: 10.1038/s41388-020-01638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohashi R., Sangen M., Namimatsu S., Yanagihara K., Yamashita K., Sakatani T., Takei H., Naito Z. Prognostic value of IMP3 expression as a determinant of chemosensitivity in triple-negative breast cancer. Pathol Res Pract. 2017;213:1160–1165. doi: 10.1016/j.prp.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Samanta S., Sun H., Goel H.L., Pursell B., Chang C., Khan A., Greiner D.L., Cao S., Lim E., Shultz L.D. IMP3 promotes stem-like properties in triple-negative breast cancer by regulating SLUG. Oncogene. 2016;35:1111–1121. doi: 10.1038/onc.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Q.D., Zheng S.R., Cai Y.J., Chen D.L., Shen Y.Y., Lin C.Q., Hu X.Q., Wang X.H., Shi H., Guo G.L. IMP3 promotes TNBC stem cell property through miRNA-34a regulation. Eur Rev Med Pharmacol Sci. 2018;22:2688–2696. doi: 10.26355/eurrev_201805_14965. [DOI] [PubMed] [Google Scholar]
- 70.Samanta S., Pursell B., Mercurio A.M. IMP3 protein promotes chemoresistance in breast cancer cells by regulating breast cancer resistance protein (ABCG2) expression. J Biol Chem. 2013;288:12569–12573. doi: 10.1074/jbc.C112.442319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang S., Zhao B.S., Zhou A., Lin K., Zheng S., Lu Z., Chen Y., Sulman E.P., Xie K., Bogler O. m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606. doi: 10.1016/j.ccell.2017.02.013. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon J.H., Gorospe M. Cross-linking immunoprecipitation and qPCR (CLIP-qPCR) analysis to map interactions between long noncoding RNAs and RNA-binding proteins. Methods Mol Biol. 2016;1402:11–17. doi: 10.1007/978-1-4939-3378-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weng H., Huang H., Wu H., Qin X., Zhao B.S., Dong L., Shi H., Skibbe J., Shen C., Hu C. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell. 2018;22:191–205. doi: 10.1016/j.stem.2017.11.016. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu N., Parisien M., Dai Q., Zheng G., He C., Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19:1848–1856. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han Y., Wang Z., Sun S., Zhang Z., Liu J., Jin X., Wu P., Ji T., Ding W., Wang B. Decreased DHRS2 expression is associated with HDACi resistance and poor prognosis in ovarian cancer. Epigenetics. 2020;15:122–133. doi: 10.1080/15592294.2019.1656155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Das M. Venetoclax with decitabine or azacitidine for AML. Lancet Oncol. 2018;19:e672. doi: 10.1016/S1470-2045(18)30824-6. [DOI] [PubMed] [Google Scholar]
- 77.Cheng L., Zhang X., Huang Y.Z., Zhu Y.L., Xu L.Y., Li Z., Dai X.Y., Shi L., Zhou X.J., Wei J.F. Metformin exhibits antiproliferation activity in breast cancer via miR-483-3p/METTL3/m(6)A/p21 pathway. Oncogenesis. 2021;10:7. doi: 10.1038/s41389-020-00290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su R., Dong L., Li Y., Gao M., Han L., Wunderlich M., Deng X., Li H., Huang Y., Gao L. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96. doi: 10.1016/j.ccell.2020.04.017. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]