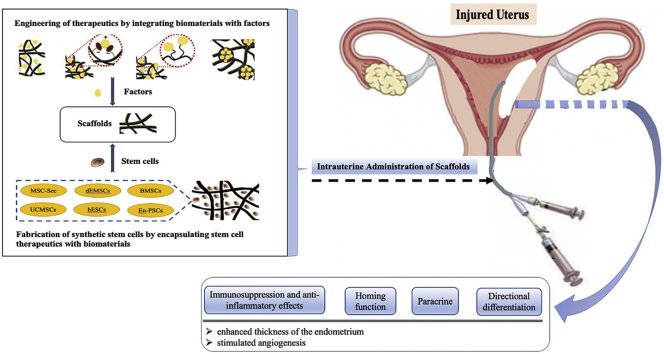
Abstract
Intrauterine adhesions (IUAs) refer to the repair disorder after endometrial injury and may lead to uterine infertility, recurrent miscarriage, abnormal menstrual bleeding, and other obstetric complications. It is a pressing public health issue among women of childbearing age. Presently, there are limited clinical treatments for IUA, and there is no sufficient evidence that these treatment modalities can effectively promote regeneration after severe endometrial injury or improve pregnancy outcome. The inhibitory pathological micro-environment is the main factor hindering the repair of endometrial damaged tissues. To address this, tissue engineering and regenerative medicine have been achieving promising developments. Particularly, biomaterials have been used to load stem cells or therapeutic factors or construct an in situ delivery system as a treatment strategy for endometrial injury repair. This article comprehensively discusses the characteristics of various bio-scaffold materials and their application as stem cell or therapeutic factor delivery systems constructed for uterine tissue regeneration.
Keywords: Scaffold-based therapeutics delivery systems, Asherman's syndrome/endometrium regeneration, Stem cell, Therapeutic factor, Biological scaffold material
Abbreviations: IUA, Intrauterine adhesions; D&C, Dilatation and curettage; MSC-Sec, Mesenchymal stem cell-secretome; dEMSCs, endometrial stromal cells; BMSCs, bone marrow mesenchymal stem cells; UCMSCs, umbilical cord derived mesenchymal stem cells; hESCs, human embryonic stem cells; En-PSC, endometrial perivascular cells; BMNCs, autologous bone marrow mononuclear cells; bFGF, basic fibroblast growth factors; ECM, extracellular matrix; VEGF, vascular endothelial growth factor; SDF-1α, stromal cell-derived factor-1α; KGF, Keratinocyte growth factor
Graphical abstract
1. Introduction
The human endometrium is a dynamically remodeling mucosa and has a remarkable regenerative capacity, undergoing 400–500 monthly cycles of morphological and functional changes during a woman's reproductive life [1]. The cycling of the endometrium suggests that endometrial stem cells are involved in the regeneration of a new functional layer mainly in the stroma of the basalis layer. Congenital anomalies and acquired severe uterine damage, such as intrauterine adhesions (IUAs) caused by curettage and infections or scar formation after undergoing previous caesarean section and myomectomy, can affect the uterine cavity and contractility and embryo implantation, ultimately leading to uterus factor infertility, recurrent miscarriage, abnormal menstrual bleeding, and other obstetrical complications [2]. Dilatation and curettage (D&C), a primary risk factor for IUA, causes damage to the basal layer of the endometrium, thereby leading to endometrial fibrosis, in which fibrous tissues replace stromal tissues and is accompanied by a decrease in or disappearance of glands. As a result, the uterine cavity and/or the cervical canal become partially or completely obliterated [3]. According to reports, 36–53 million pregnancies worldwide are terminated yearly, of which approximately 90% occur in the first trimester [4]. The incidence of IUA following early pregnancy loss is approximately 6.3% [5]. Therefore, IUA is a critical public health concern among premenopausal women.
The central premise of its treatment strategy is to promote endometrial regeneration and functional recovery. Common treatments include hysteroscopic lysis of adhesions, application of artificial hormone therapy, and placement of intrauterine devices [6]. Currently, hysteroscopy adhesiolysis is considered as the gold standard for IUA treatment. Despite a number of ancillary treatments applied after surgery, including physical barriers and hormone therapy, the effect remains poor in severe cases [7,8]. Moreover, severe IUA has a high recurrence rate of more than 62%, with unfavorable prognosis despite a good initial therapeutic effect [9]. In general, the formation of severe fibrotic tissues and scar is irreversible [2]. Presently, stem cell therapy is the most attractive treatment of tissue injury and fibrosis in response to damage. Because stem cells are undifferentiated and can self-replicate, they provide benefits in both morphological and functional aspects through the secretion of trophic support, promotion of cell regeneration, and regulation of immune and anti-inflammation factors [10,11]. Current research has found that stem cell transplantation for endometrial repair has limitations in terms of long-term safety and effectiveness [15]. Stem cells and cancer cells share similar characteristics, including high proliferative capacity, the self-renewal ability, and a high degree of plasticity. Bone marrow-derived stem cells could also contribute to endometriosis and may be relevant to the formation of endometrial carcinomas [14].
The latest progress in tissue engineering and regenerative medicine is to use biomaterials mediated by bioactive molecules for in situ tissue engineering, and implant bioactive scaffolds modified or eluted by bioactive factors into tissue defect sites, so as to achieve intrauterine repair the role of membrane damage [18]. Endometrial fibrosis is the main pathological feature of IUA, where the normal hormonal responsive endometrium is replaced by the excessive deposition of ECM, and the endometrial cavity is obstructed with avascular and non-functional fibrotic synechiae bundles [17]. Biological scaffold materials combined with stem cell transplantation treatment strategies have shown good prospects for the functional repair of the endometrium. According to reports, IUA patients have been transplanted with bone marrow mesenchymal stem cells (BM-MSCs), endometrial mesenchymal stem cells (eMSCs), and menstrual blood–derived stem cells (MenSCs) into the uterine cavity through biological scaffolds, which can stimulate the growth of the endometrium [12,13]. Many efforts have been attempted to explore specific stem cell–based therapies [16].
This article summarizes the therapeutic strategies of bio-scaffold materials loaded with stem cells, as well as the therapeutic factors involved in repairing endometrial injury through in situ delivery. We discuss the characteristics of each kind of biological scaffold material and the advancements in delivery systems constructed for endometrial regenerative medicine. We also introduce the latest developments on biomaterials, i.e., modified or combined mode and loaded with stem cells or therapeutic factor delivery system.
2. Challenges in endometrium regeneration
Human endometrium is composed of endometrial glands and endometrial stroma. It is a regenerative tissue that undergoes more than 400 cycles of proliferation, differentiation, and shedding during the female reproductive life. According to its histological features, the endometrium is classified as consisting of columnar epithelia and stroma. The upper epithelium contains luminal epithelial cells and glands, whereas the stroma is composed of supporting mesenchymal cells, stromal fibroblasts, the vasculature, and leukocytes. To understand the characteristics of the endometrium based on its structure and function, it can also be classified based on the functional layer and an inner basal layer [19]. The basalis of the endometrium is a permanent layer, which generates a new functionalis through intrinsic stem or progenitor cells every menstrual cycle to prepare for blastocyst implantation [6]. In humans, the endometrial stem cell niche is located in the endothelium of the spiral arterioles in the basal layer, providing support to both the epithelial and stromal compartments [20]. In addition, the endometrium has a remarkable capacity to regenerate the functional layer from its basalis during the reproductive period and throughout the entire life-span if hormonal supplementation is administered, due to the existence of endometrial stem cells [21].
IUA, also known as Asherman syndrome, is a consequence of trauma to the endometrium. IUA can be implicated in uterine cavity surgeries, such as artificial abortion, dilatation and curettage, post-abortion hemorrhage and curettage, manual placenta removal, polypectomy, myomectomy, and conization of the cervix [9]. Infections represent another established cause of IUA [22]. A recent study revealed that 40% of patients affected by IUAs present symptoms of chronic endometritis (CE) and a higher recurrence of adhesion. These findings indicate that chronic inflammation may play a role in the development and recurrence of IUA [23]. In addition, there are different factors that can cause the destruction of the endometrium through to its basal compartment, affecting the local cellular niche [10]. The damaged endometrium then cannot be properly repaired, leading to the formation of fibrotic synechiae across the cavity, in which the glands are replaced by inactive cubocolumnar endometrial epithelium unresponsive to hormonal stimulation. The newly formed fibrotic tissue is usually avascular, although thin-walled telangiectatic vessels can be observed, and the glands may be sparse and inactive or cystically dilated, causing reduced blood perfusion and atrophy. Deformation, stenosis, and fibrous scarring of the uterine cavity also occur [24]. Studies, these result in clinical manifestations including amenorrhea, dysmenorrhea, infertility, miscarriage, chronic pelvic pain, and placenta accreta [25].
Currently, the pathogenesis of IUA remains unclear; however, several hypotheses exist, such as the fibrosis hyperplasia theory, neural reflex theory, abnormal differentiation of stem cells, changes in the microenvironment of uterine cavity and fibrosis, abnormal signal pathway regulation, and inflammatory response induced by adherent fibroblasts. Among these, the fibrosis hyperplasia theory is the most widely studied [26]. The transforming growth factor-β (TGF-β) has been identified as highly involved in the pathogenesis of IUA by promoting endometrial fibrosis via the canonical TGF-β/Smad signaling pathway. Furthermore, the role of TGF-β in IUA has been speculated to be associated with ER-α, CCN2, and MMP-9 [9]. The Hippo signaling pathway has been previously demonstrated to participate in endometrial fibrosis in IUAs and cross-talks with other signaling pathways, such as those of TGF-β and Wnt/β-catenin through a variety of mechanisms [[27], [28], [29]]. Impaired homeostasis of the extracellular matrix (ECM) is also involved in the creation of adhesions [30]. More recently, impaired NF-κB signaling has also been implicated in IUA. Such signaling is a marker of aberrant inflammation that can create a vicious fibrotic cycle [31]. Additionally, a recent study has reported the upregulation of ΔNp63 in women suffering from IUA, thus causing endometrial stem cell quiescence and leading to adhesion formation [32]. These mechanism studies not only contribute to understanding the pathogenesis of adhesion development but also provide a basis for searching effective therapeutics for IUA treatment and prevention. Hysteroscopy adhesiolysis is considered as the gold standard for IUA treatment, other treatment methods studies include hormone therapy with estrogen, stem cells, TGF-β inhibitor, G-CSF, and some potential targets (miRNAs and KDR).
The main pathological feature of IUA belongs to endometrial fibrosis. Targeting fibrosis is significant for the treatment and prevention of IUA, which will be aided by studies on the specific mechanism of endometrial fibrosis, to inhibit endometrial fibrosis while promoting endometrial hyperplasia. Thus, improving treatment efficacy and reducing the recurrence rate of IUA post-surgical repair of transcervical resection of adhesion (TCRA) should also be the focus points for future studies. The concept of regenerative medicine is considered the future for patients with IUA. Evidences accumulated above support the notion that stem cells or its paracrine mechanism acts as one of the main candidates of new therapy, which provides a theoretical method for the development of potential reagents for the treatment of IUA.
3. Hyaluronic acid in endometrial regenerative medicine
Among biopolymers, hyaluronic acid (HA) represents one of the most used materials in the design of hydrogels for biomedical applications owing to its biocompatibility, native biofunctionality, biodegradability, non-immunogenicity, and versatility. HA is a natural linear polysaccharide comprising alternating units of d-glucuronic acid and N-acetyl-d-glucosamine that are connected by β-1,3- and β-1,4-glycosidic bonds. Furthermore, it is a non-sulfated glycosaminoglycan that is widely found in the epithelial and connective tissues of vertebrates and is the major component of the ECM. HA is synthesized by hyaluronan synthase at the plasma membrane and is then extruded to the ECM [33]. In the ECM of most tissues, HA contributes to maintaining mechanical integrity, homeostasis, viscoelasticity, and lubrication owing to its high molecular weight and its humectant property [34]. Furthermore, it also plays an important role in various intracellular functions. Particularly, due to its binding to cell surface-specific receptors (such as CD44 or RHAMM), HA can regulate cell adhesion, migration, proliferation, and differentiation and consequently, processes such as inflammation, wound healing, tissue development, morphogenesis, tumor progression, and metastasis.
For these reasons, hydrogels built from HA have been recently developed and investigated for biomedical applications, including tissue regeneration, tissue engineering, drug delivery, gene therapy, and diagnostics [33]. As an endogenous component of soft tissues, HA could not only provide essential structural and mechanical support for the nearby cells but also exhibit biological effects. HA could decrease the incidence of adhesions by downregulating the inflammatory response and promoting the vascularization process, thus emerging as one of the significant gel-based physical barriers for the IUA treatment [35]. Treatment with HA gel can reduce the incidence of IUA and increase pregnancy rates following an intrauterine operation [36,37]. Meta-analysis of clinical trials showed that HA gel could prevent IUAs, particularly those with moderate severity and a lower adhesion score. However, for those with severe IUAs, the usage of HA gel alone as a physical barrier was insufficient to prevent the reoccurrence of adhesion. Furthermore, HA gel alone showed no significant effect on the postoperative pregnancy rate [38,39]. In a randomized controlled trial, the application of HA gel during hysteroscopy in patients with moderate to severe IUA had improved the quality of the endometrium and uterine receptivity, thereby enhancing the clinical pregnancy rate after IVF/CSI and FET [40].
Meanwhile, pharmaceutical scientists have long been employing HA gel as a therapeutic delivery carrier to slowly release cargoes at the pathological site [41]. Therefore, highly biocompatible HA gel could act as a physical barrier to suppress re-adhesion, while being potentially loaded with multiple therapeutics to facilitate endometrium regeneration, which is of significant importance for IUA prevention. In addition, HA is widely used in the design of engineered hydrogels owing to its biofunctionality and presence of numerous sites for modification with reactive groups. There are numerous examples of modified HA macromers that form either covalent or physical hydrogels via crosslinking reactions, such as click chemistry or the supramolecular assemblies of guest–host pairs. HA hydrogels range from relatively static matrices to those that exhibit spatiotemporally dynamic properties through external triggers, such as light. Such hydrogels are being explored for in vitro cell culture, in vivo carriers for cells, and therapeutic delivery, including in an environmentally responsive manner [42].
In addition to bioactive proteins, some studies have focused on secreted extracellular vesicles from stem cells for uterus repair [12]. Liu et al. [43] innovatively created a stem cell secretome modified-HA hydrogel that increases the release of a number of regeneration-related growth factors, such as epidermal growth factor (EGF), FGF, IGF-1, and IGF binding protein (IGFBP). The crosslinked HA gel served as a carrier and prolonged the in vivo retention time of the stem cell secretome, thus leading to a thicker endometrium and more glands compared with gel application alone. The nanoscale functionalization of endometrium scaffolds mimics the natural environment, provides a steady release of bioactive molecules, and transmits signals from extracellular vesicles during uterus regeneration.
Furthermore, Kim et al. [44] fabricated decidualized endometrial stromal cells (dEMSCs) that are encapsulated in HA hydrogel and applied them in a murine uterine infertility model. Within a short duration of 2 weeks, hydrogel treatment decreased the number of fibrous tissues and increased the thickness of the endometrium. Importantly, the regenerated endometrium demonstrated functional recovery as evidenced by the expression and secretion of molecules essential for embryonic implantation, such as Desmin, CD44, PECAM, and IGF-1. The successful implantation of the transferred embryos was followed by normal development and live birth of offspring after the dEMSC-loaded HA hydrogel treatment. The selection of bioprocessed isotopic cells shortened the recovery time significantly when compared with bone marrow mesenchymal stem cells (BMSCs) or human embryonic stem cell (hESC)-derived endometrium-like cells. In addition, HA is suitable for the repair of endometrium where plenty of hyaluronidases could degrade HA. Furthermore, the limiting mobility of the cross-linkage with porosity allowed seeding cells to remain in the injured site and provided the ideal scaffold stiffness for endometrium regeneration. Thus, HA is a promising therapeutic material to treat IUAs (Table 1).
Table 1.
Biomaterials used as scaffold for endometrium regeneration.
| Scaffold type | Cells/factors | Model | Reference |
|---|---|---|---|
| HA hydrogel | MSC-Sec | Rat | Liu et al., 2019 [43] |
| HA hydrogel | dEMSCs | Mouse | Kim et al., 2019 [44] |
| Poloxamer hydrogel | 17β-estradiol | Rat | Zhang et al., 2017 [51] |
| Poloxamer hydrogel | 17β-estradiol | Rat | Zhang et al., 2020 [54] |
| Aloe/poloxamer hydrogel | 17β-estradiol | Rat | Yao et al., 2020 [55] |
| Poloxamer hydrogel | KGF | Rat | Xu et al., 2017 [59] |
| Poloxamer hydrogel | KGF | Rat | Xu et al., 2017 [60] |
| Poloxamer hydrogel | Vitamin C | Rat | Yang et al., 2017 [62] |
| ECM | / | Rat | Miyazaki et al., 2014 [71] |
| ECM | / | Rat | Santoso et al., 2014 [72] |
| ECM | / | Rat | Hellström et al., 2016 [73] |
| ECM | / | Pig | Campo et al., 2017 [74] |
| ECM | / | Rat | Daryabari et al., 2019 [75] |
| ECM | / | Rat | Miki et al., 2011 [76] |
| ECM | 17β-estradiol | Human amnion | Chen et al., 2020 [84] |
| UBM | / | Rat | Zhang et al., 2020 [77] |
| GP-PC-dUECM | / | Rat | Yao et al., 2020 [78] |
| Collagen scaffolds | BMSCs | Rat | Ding et al., 2014 [87] |
| Collagen scaffolds | BMNCs | Patient | Zhao et al., 2017 [32] |
| Collagen scaffolds | UCMSCs | Patient | Cao et al., 2018 [13] |
| Collagen scaffolds | UCMSCs | Rat | Xu et al., 2017 [89] |
| Collagen scaffolds | UCMSCs | Rat | Xin et al., 2019 [90] |
| Collagen scaffolds | hESCs | Rat | Song et al., 2015 [91] |
| Collagen scaffolds | En-PSCs | Rat | Li et al., 2019 [92] |
| Collagen scaffolds | bFGF | Rat | Li et al., 2011 [96] |
| Collagen scaffolds | VEGF | Rat | Lin et al., 2012 [97] |
| Collagen scaffolds | bFGF | Patient | Jiang et al., 2019 [98] |
| PGS scaffolds | BMSCs | Rat | Xiao et al., 2019 [100] |
| Chitosan-heparin hydrogels | SDF-1α | Rat | Wenbo et al., 2020 [103] |
| SF-BC membrane | SDF-1α | Rat | Cai et al., 2019 [109] |
| GelMA-Na-alginate-loaded porous scaffold | bFGF | Rat | Cai et al., 2019 [113] |
4. Poloxamers in endometrial regenerative medicine
Poloxamers or Pluronics are a class of water-soluble, non-ionic, triblock copolymers formed by polar (polyethylene oxide) and non-polar (poly propylene oxide) blocks, which confer amphiphilic and surface-active properties to polymers. Poloxamers have been approved by the FDA and are included in the United States and European Pharmacopoeia. Because they are non-toxic and non-irritant, they can be used as solubilizer, emulsifier, and stabilizer and can be administered through oral, parenteral, topical routes. As wetting agents, they are useful in ointments, suppository bases, and gels. Commonly used poloxamers include P188 (F-68 grade), P237 (F-87 grade), P338 (F-108 grade), and P407 (F-127 grade) types, which are freely soluble in water [45,46]. They can be considered smart polymers owing to their stimuli-sensitive properties. These polymers behave differently and can modify their structure as a function of pH, temperature, and salt concentration [47]. This allows the preparation of thermosensitive hydrogels with different properties, e.g. critical gelation concentration and gelation time at physiological conditions [48]. More recently, such hydrogels have become especially attractive for tissue engineering as matrices for repairing and regenerating a wide variety of tissues and organs [49]. This section introduces the recent advances in the use of Poloxamers in repairing the endometrium (Table 1).
Estrogen is used to promote the antifibrosis effect, prevent adhesion recurrence or post-operative adhesion, and induce endometrial repair after TCRA [50]. We have previously reported a novel poloxamer 127 (HP) thermosensitive hydrogel loaded with estradiol (E2-HP hydrogel) to achieve the localized delivery and sustained release of estradiol for IUA medication [51]. Estradiol-loaded HP solution could transform into a gel at 37 °C and remain in the uterus at least 48 h, whereas the estradiol solution was quickly cleared by the body within the first 2 h. Estradiol was slowly released from the HP hydrogel for 72 h. The E2 sustained-release selectively reduced fibrotic tissue areas and stimulated vascularization to provide more nutrients, oxygen, and hormones to the injured tissues [52,53]. In this study, our group demonstrated that the uterine injectable E2-HP hydrogel was effective in preventing mechanical injury-induced IUAs in a rat model. Moreover, its therapeutic effect was associated with the inhibition of ER stress-induced apoptosis and the augmentation of the vascular endothelial growth factor (VEGF)-stimulated angiogenesis. The E2-HP hydrogel on the recovery of IUA was closely associated with the upregulation of kisspeptin through activating the ERK1/2 and MAPKs p38 pathways [54].
Recently, we have designed a nanocomposite aloe/poloxamer hydrogel for β-estradiol (E2) intrauterine delivery. We demonstrated that it exerted multitherapeutic effects and promoted endometrial regeneration. Briefly, nanoparticulate-decellularized uterus (uECMNPs) was prepared to encapsulate E2 (E2@uECMNPs), which improved the solubility and prolonged the cargo release. Then, E2@uECMNPs were further embedded into the thermosensitive aloe-poloxamer hydrogel (E2@uECMNPs/AP). Multiple components from the E2@uECMNPs/AP system could collectively promote proliferation and inhibit the apoptosis of endometrial stromal cells. E2@uECMNPs/AP significantly increased morphological recovery and decreased the uterine fibrosis rate compared with IUA rats in other groups. Additionally, the levels of Ki67, cytokeratin, and estrogen receptor β were all upregulated, along with the decreased expression of TGF-β1 and TNF-α in the uterus of rats receiving E2@uECMNPs/AP therapy. Taken together, the in situ administration of E2@uECMNPs/AP hydrogel could effectively promote endometrial regeneration and prevent re-adhesion (Fig. 1) [55].
Fig. 1.
Experimental scheme of the fabrication and testing of the E2@uECMNPs/AP hydrogel system that exhibited multitherapeutic effects and promoted endometrial regeneration to prevent of intrauterine adhesion. Histological analysis and collagen deposition of the uterus from IUA rats receiving different treatments. A. Representative H&E staining of the uterus on days 3 and 7 (40× and 100×). After 7 days of the drug intervention, H&E staining of uterus appeared well organized with epithelial tissue and many glands in both E2@uECMNPs/AP and AP group. Meanwhile, there were few glands that appeared in the endometrium in the uterus from commercially available E2 gels group, E2 alone group, and IUA group. B. Representative images of Masson stained-uterus on days 3 and 7 (200×). Masson staining appeared a significant decrease in collagen deposition in the AP group on day 7. Compared with the AP group, less fibrosis was observed in the group of E2@uECMNPs/AP, which suggested a trend toward significance. (Reproduced with permission of Ref. [55]).
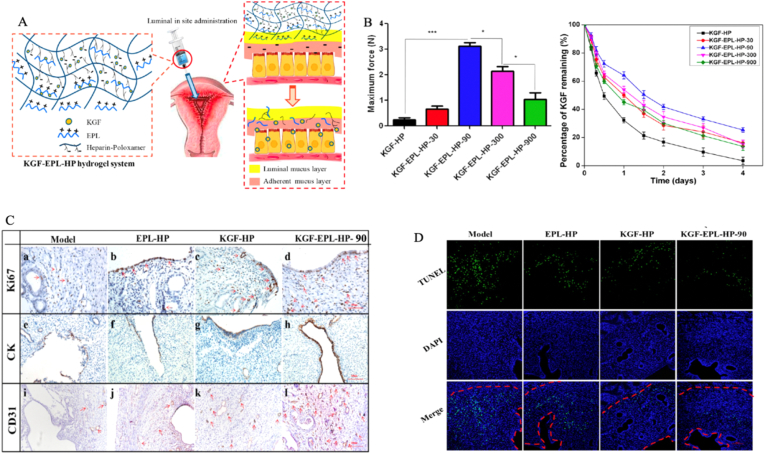
With the versatile role of the hydrogel in drug delivery, it can also encapsulate growth factors to develop regenerative medicine [56,57]. As expected, many growth factors loaded in hydrogels provide benefits for topical uterine regeneration, which is usually limited by quick inactivation and fast elimination after administration. Exogenous KGF applied to mucosal injury significantly enhanced wound healing, but its use is often hampered by a short biological half-life [58]. In our previous study [59], we have developed a thermosensitive heparin-modified poloxamer hydrogel to encapsulate the keratinocyte growth factor (KGF) for IUA treatment. Heparin modification significantly increased KGF affinity and prolonged the release duration of KGF to 7 days without losing its bioactivity. IUA rats receiving the KGF-hydrogel showed better morphological and functional recovery of the mechanically damaged uterus when compared with those in the KGF solution group. In a follow-up study, we added a naturally derived poly (amino acid) polymer, ε-polylysine (PLL), into the thermosensitive hydrogel system to load KGF [60]. This addition significantly enhanced the bioadhesivity of the hydrogel system as evidenced by the adhesion force rising to 3.18 N (10-fold higher than that of the non-PLL hydrogel). The KGF-modified hydrogel scaffold facilitated cell autophagy by inhibiting the mammalian target of rapamycin (mTOR) signaling pathway and increasing CD31 expression levels, endothelial migration, and proliferation of endometrial glandular and luminal epithelial cells (Fig. 2). Functional epithelial repair was achieved due to the restoration of appropriate micro-milieu by reducing inflammation and immune responses [61].
Fig. 2.
Scheme of thermosensitive bioadhesive KGF-EPL-HP hydrogel for injured uterus (A). Adhesive evaluation of the KGF-EPL-HP hydrogel. The adhesive force of KGF-EPL-HP hydrogels against gelatin substrate in comparison with HP hydrogels on the left. The remaining percentage of KGF on excised rabbit uterine mucosa for KGF-EPL-HP hydrogels with various EPL concentrations after continuous rinsing with PBS on the right (∗p<0.05; ∗∗∗p<0.001; n = 3) (B). Immunohistochemistry images of Ki67 (a–d), CK (e–h) and CD31 (i–l) staining on day 3 after treatment. Positively stained cells were marked by red arrows (scale bar = 100 μm) (C). The number of Ki67-positive cells in rats treated with KGF-EPL-HP hydrogel was significantly higher than that of rats treated with other groups (a–d). The obvious keratinization of epithelial cells of the endometrium was observed in groups of KGF-HP hydrogel or KGF-EPL-HP-90 hydrogel (e−h). There was a large number of neonatal microvessels were formed in group of KGF-EPL-HP- 90 hydrogel (i–l). TUNEL assay kit analysis of the injured uterus 3 days after treatment. Red line: the border of the basal layer; blue: cell nuclei, DAPI; green: apoptotic cells. Original magnification: ×200, scale = 1 μm (D). Reprinted (adapted) with permission from Ref. [60]. Copyright (YEAR) American Chemical Society. (Reproduced with permission of Ref. [60])
Therefore, the hydrogel system could be easily modified to possess functional properties, including thermosensitivity and bioadhesivity, and improve therapeutic outcomes. Hydrogel offers great promises to deliver multiple therapeutics with totally different characteristics and increased therapeutic concentration at the pathological site. Yang et al. reported that BMSCs were encapsulated by the thermo-responsive gelation of pluronic F-127 (PF-127) and vitamin C, which was added for membrane stability [62]. In addition, vitamin C as a prominent antioxidant downregulated the secretion of tumor necrosis factor α (TNF-a) and interleukin 6 (IL-6), thus maintaining redox homeostasis and facilitating a pro-regenerative tendency by increasing the IL-10 level [63,64]. Vitamin C further alleviated the cytotoxic effect of PF-127 and promoted cell survival and growth on rat BMSC encapsulation. The BMSC/PF-127/vitamin C hydrogel recovered endometrial thickness and decreased the fibrotic regions of the stromal tissues of the endometrium [65].
5. Decellularized matrix in endometrial regenerative medicine
Decellularized extracellular matrix (dECM) is an isolated ECM of tissues derive from its original inhabiting cells and has emerged as a promising natural biomaterial for tissue engineering aiming to support, replace, or regenerate damaged tissues. The dECM can be easily obtained from tissues/organs of various species using adequate decellularization methods and can mimic the structure and composition of the native ECM, thereby providing a favorable cellular environment [66]. In natural tissues, the ECM is a 3D structure that contains various extracellular macromolecules, which are mainly proteins such as polysaccharides, collagen, and proteoglycans [[67], [68], [69]]. The ECM provides physical support for cell adhesion and significantly influences cell behaviors including migration, proliferation, and differentiation. Decellularized scaffolds can also be converted into ECM hydrogels or coatings. These injectable ECM hydrogels undergo a non-toxic, collagen-based self-assembly process to form a nanofibrous hydrogel when incubated at 37 °C or introduced in vivo, thereby making them an interesting option for regenerative medicine, especially for minimally invasive procedures [70].
Miyazaki et al. [71] fabricated a decellularized uterine matrix scaffold from rat uterus via aortic perfusion with detergents. The scaffold provided not only mechanical support for the uterus but also vascular architecture for blood perfusion. In addition, it induced recellularization, uterus regeneration, and high pregnancy rate, similar to that of the uninjured uterus. Santoso et al. [72] used different methods for decellularization of the uterine matrix from rat uterus, including sodium dodecyl sulfate (SDS) and high hydrostatic pressure (HHP), and found that the latter had more preserved ECM and was more efficient in cell removal. The HHP method also avoided collagen denaturation and reduction in protein contents. Hellström et al. [73] created a uterine patch from rat uterus using the perfusion method for scaffold decellularization and found that the scaffold was biocompatible after recellularization in vivo. In addition, Campo et al. [74] used decellularization and recellularization techniques in the fabrication of a scaffold from porcine uterus and achieved an excellent vascular network in the ECM after recellularization by human side population stem cells. Similarly, Daryabari et al. [75] reported a whole-organ perfusion decellularization method for the production of a scaffold from ovine uterus and implanted its segments into rats. The scaffold successfully retained the vascular structure after decellularization and started recellularization in the endometrium and myometrium after implantation, potentially due to homing of the circulating and local stem cells. Moreover, the excellent biomechanical properties guaranteed uterine regeneration in vivo for a long term. Miki et al. [76] found that the orientation of smooth muscle cells and ECM was a vital factor for correct tissue topology and functional uterine regeneration after studying a decellularized scaffold derived from rat uterus. Zhang et al. [77] transplanted a urinary bladder matrix (UBM) into the uterine horns in Sprague–Dawley rats and found thicker endometria, increased numbers of glands, fewer fibrotic areas, and increased proliferation of cells and blood vessels. They revealed that the transplantation of UBM reduced the mRNA levels of proinflammatory cytokines (tumor necrosis factor α) and increased those of anti-inflammatory cytokines (basic fibroblast growth factor [bFGF]). More embryos were seen in the UBM group than in the injured group.
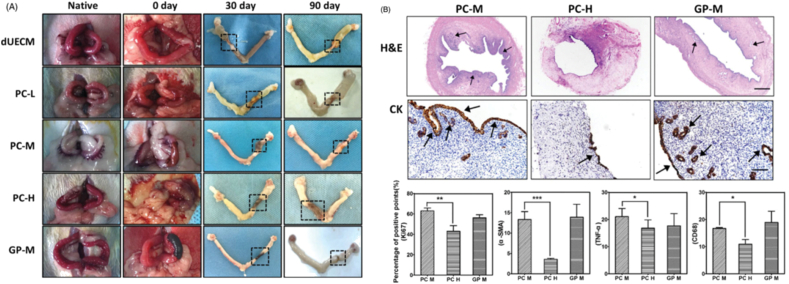
ECM has also been considered as a promising scaffold in xenotransplantation, although natural tissue ECM is often mechanically weak and rapidly degraded, which might compromise outcomes. How to restore its mechanical strength and optimize its in vivo degradation, while maintaining the microstructure and maximally suppressing immune rejection remains challenging. Naturally derived genipin (GP) and procyanidins (PCs) have been cross-linked with dUECM to produce a mechanically enhanced cross-linked-dUECM with prolonged enzymatic degradation rate. The in vivo transplantation of GP- and PC-cross-linked dUECM to a uterus circular excised rat yielded excellent recellularization ability and promoted uterus regeneration after 90 days. While the reconstruction efficacy of crosslinked dUECM is highly dependent on the crosslinking degree, crosslinking conditions must also be carefully evaluated to balance the mechanical and biological support it provides for promotion of tissue regeneration (Fig. 3) [78]. These studies have indicated that decellularized biomaterials are helpful for functional uterus regeneration due to their biocompatibility, regulation of cell survival and homing, and topological support [70]. ECM hydrogels may be used in the future as part of the treatment of Asherman's syndrome and endometrial atrophy (Table 1).
Fig. 3.
In vivo evaluation of cross-linked dECM for uterus regeneration and its fabrication using a xenogeneic rat model. Dotted lines indicate the repair sites. Thirty days after surgery, dUECM without crosslinking treatment were entirely degraded and the rat uterus only connected by the outer adhesive mucosa. The cross-linked dUECM remained at the implanted site but significantly decreased in size. After 90 days, PC-M-crosslinking group exhibited best regeneration results with limited scaffold remained and apparent uterus regeneration as evidenced by newborn uterus tissues near the implanted area (A). H&E and immunohistochemical staining of Ki67, ɑ-SMA, TNF-ɑ, and CD68 after xenotransplantation of cross-linked dUECM at 90 days. Positive staining is indicated by the red arrows. The scale bar represents 100 μm. ∗p< 0.05; ∗∗∗p< 0.001; n = 3. Data were expressed as mean ± standard deviation. At a minimum, three samples were represented for each data point. Statistical analysis of experimental data was accomplished using two-tailed analysis of variance, performed with a statistical computer program (Student's t-test), and p values < 0.05 were considered statistically significant (B). PC-L, low concentration of procyanidin (PC) (0.05%) cross-linked dUECM. PC-M, middle concentration of procyanidin (PC) (0.1%) cross-linked dUECM. PC-H, high concentration of procyanidin (PC) (0.2%) cross-linked dUECM. GP-M, middle concentration of genipin (GP) (0.625%) crosslinked dUECM. (Reproduced with permission of Ref. [78])
The amnion membrane (AM), a prospective biomaterial, can not only act as a mechanical barrier that separates the wounded surface but also secrete various growth factors to activate the regulation of endometrial cell proliferation, migration, and differentiation, thereby achieving morphological and functional recovery of the uterus [79,80]. In the clinical setting, both the fresh and lyophilized AM have been used for IUA therapy [81,82]. However, AM may induce an immune response when integral membranes are transplanted into the majority of human tissues. Decellularized AM can reduce potential immunogenicity as the extracellular components of tissues are well tolerated and can even be used as xenografts [83]. Chen et al. [84] prepared human amnion extracellular matrix (HAECM)–derived scaffolds for endometrial cell growth and drug delivery. Using cell proliferation experiments, they showed that the ‘slow and even’ release of E2 by the scaffolds is more conducive to the growth of endometrial cells than that of free E2. They demonstrated that EGF and IGF-1 are involved in endometrial proliferation, and that HAECM partly promotes the regeneration of endometrium through the EGF and IGF-1 families as intermediaries. Meanwhile, they found that the expression of cytokines and their receptors peaked in the E2-MS-HAECM (17β-estradiol by HAECM scaffold integrated with PLGA microspheres) group, suggesting a synergistic effect between the HAECM scaffold and E2 on endometrial regeneration, which was also supported by the results of cell proliferation assay (Fig. 4). Therefore, besides creating a framework for simple mechanical support, advanced scaffolds provide higher functionality for the biologic guidance of endometrium regeneration.
Fig. 4.
Schematic diagram of E2-MS-human amnion extracellular matrix (HAECM) scaffold as an intrauterine controlled release system for endometrium regeneration and scanning electron microscopy photograph of E2-MS (502H), HAECM scaffold and E2-MS- HAECM scaffold (A). Quantitative PCR analysis of EGF, IGF-1, EGFR, and IGF-1R mRNA levels in Ishikawa cells treated with different preparations after 72 h. ∗p < 0.05, ∗∗p < 0.01 vs. control group. Data are presented as the mean ± standard deviation; n = 3 (B). (Reproduced with permission of Ref. [84]).
6. Collagen scaffold in endometrial regenerative medicine
Collagen is one of the basic structural elements of the ECM, which has been widely used in wound healing and tissue repair owing to its abundant source, biocompatibility, and biodegradability. Collagen can act not only as a framework for mechanical support but also as an additive to regulate cell behavior. It has been reported that porous collagen sponges with micrometer-sized pores could be used for cell cultures, such as fibroblast, marrow cells, and keratinocytes [85]. BMSCs have been largely used for the regeneration of the endometrium and uterus due to their easy isolation, abundant sources, and reparative potential (Table 1) [86].
6.1. Collagen scaffold loaded with cells
Ding et al. [87] investigated the effect of collagen/bone marrow-derived mesenchymal stem cells (BM-MSCs) constructs in the healing of severe uterine injury in rats. BM-MSCs constructs mainly migrated and accumulated in the basal layer of the regenerative endometrium after transplantation. BMSCs could adhere to collagen through interactions with its surface receptor. In addition, the collagen scaffold showed a porous structure with pore diameters of several tens to hundreds of micrometers, thus allowing cell attachment and the diffusion of oxygen and nutrients. BMSCs could attach and proliferate on the collagen matrix both in vitro and in vivo. Four weeks of post-transplantation, the wounded area adhered to the collagen/BMSCs constructs, showing high expression levels of bFGF, IGF-1, TGF-b1, and VEGF and prominent microvasculature regeneration. Therefore, collagen scaffold holds great promise as a cell delivery vehicle by anchoring to the injured tissue and promoting uterine tissue regeneration.
Recently, this technology has progressed to clinical trials. Five patients with severe IUA and secondary infertility received transplantations of autologous bone marrow mononuclear cells carried by collagen scaffolds to the inner uterine surface. All patients achieved live births with normal placentas [32]. In this study, ΔNp63 was also demonstrated to be a biomarker of IUA that also induced endometrial quiescence. Consistently, ΔNp63-positive cells exhibited a unique IUA stemness alteration. It was speculated that BM-MSCs reversed ΔNp63 and induced quiescence and stemness alteration in the endometria of patients with IUA. Although the sample size was not sufficiently large, this clinical trial demonstrated that the collagen/BM-MSC delivery system may be an alternative treatment strategy for women with IUA.
Umbilical cord-derived mesenchymal stem cells (UC-MSCs) have displayed their merits owing to suitable sources, pain-free acquisition, and excellent proliferation capacity [88]. Cao et al. [13] designed a phase I clinical trial involving 26 patients with repeated IUA. Allogeneic UC-MSCs on the collagen scaffolds were implanted into the uterine cavity of patients undergoing hysteroscopic lysis of adhesions. Once the scaffold was in place, hormone replacement therapy was applied to induce and promote a natural menstrual cycle in all the patients. After 3 months, the average thickness of the endometrium increased, and the IUA score decreased compared with those before the UC-MSC/collagen treatment. The upregulation of ERα, Ki67, and vWF expression after treatment suggested improved endometrial differentiation and neovascularization. At the end of the 30-month follow-up period, 10 patients were pregnant. The researchers proposed that the collagen scaffold provides an attachment framework for MSCs and maintains high-density UC-MSCs at the damaged site of the uterine cavity. Umbilical cord stem cell transplantation was thus deemed as an effective treatment for IUAs.
Xu et al. [89] investigated the effect of UC-MSCs mixed with degradable collagen fibers in IUA rat models. Scaffold/UC-MSC complexes and UC-MSCs were injected into two groups of IUA rats. On day 60, the number of cells positively expressing MMP-9 was significantly higher in the scaffold/UC-MSCs group than in the other groups. The cell-scaffold composite degraded collagen in scarring areas by increasing matrix metalloprotein 9 (MMP-9), FGF-2, and VEGF levels while promoting angiogenesis and endometrial cyclic regeneration. Thus, the scaffold/UC-MSCs group showed prominent angiogenesis and insignificant scarring in the injured site. Xin et al. [90] fabricated a collagen scaffold (CS) loaded with human UCMSCs for endometrial regeneration. The CS/UC-MSCs promoted human endometrial stromal cell proliferation and inhibited apoptosis in vitro via paracrine effects. In a model of endometrial damage, transplantation with the CS/UC-MSCs maintained normal luminal structure and promoted endometrial regeneration and collagen remodeling. It also induced intrinsic endometrial cell proliferation and epithelium recovery, while enhancing the expression of estrogen receptor α and progesterone receptor. Thereby, the ability of the regenerated endometrium to receive embryos was improved.
Song et al. [91] effectively produced human embryonic stem cells (hESCs) analogous to endometrial cells, which, in combination with a collagen scaffold, were found to repair endometrial structure and function. The hESCs were co-cultured with endometrial stromal cells during induction. The hESC-derived cells were subsequently transplanted into a rat uterus with full-thickness injury after having been put onto collagen scaffolds to assess cell function in vivo. Large quantities of endometrium-like cells improved endometrial function and development by simulating an in vivo endometrium stem cell niche and secreting growth factors that modulated the effects of estrogen- and progesterone-driven basal layer repair. Cell loading on the scaffolds improved the biological activity of biomaterials and maintained their physiochemical properties to support the mechanical stability of tissue regrowth. Li et al. [92] selected cell types for uterine repair by focusing on their ability to restore angiogenesis and inhibit scar-tissue formation. Endometrial perivascular cells (CD146 + platelet-derived growth factor receptor [PDGFR] b +) (En-PSCs) worked similarly as stem cells in the endometrial layer, and the cysteine-rich angiogenic inducer 61 (CYR61) contributed to vascular formation. Zhao et al. [93] designed a CYR61-transfected En-PSC-loaded collagen scaffold and found that it significantly increased blood vessel density as the scaffold stimulated the release of angiogenic factors from the ECM and accelerated neovascularization in vivo.
Given these results, the biologically degradable collagen scaffold has shown great potential as a carrier for cell transplantation into the uterine cavity area to treat recurrent IUA patients. However, cell survival upon the implantation of collagen/BMSCs scaffold is a limiting factor. Additionally, because collagen is mainly a natural animal-derived protein biomaterial, variation between batches is inevitable during the extraction process. Its poor reproducibility could lead to several problems, such as severe allergic reactions, disease transmission, and immune rejection, thereby raising some concerns for clinical use [94].
6.2. Collagen scaffold loaded with therapeutic factor
The bFGF and VEGF are essential for endometrial proliferation and angiogenesis during the menstrual cycle [95]. However, either by local application or systemic administration, they have short half-life in vivo and low concentrations at the injury site, which lowers their effects and causes side-effects on the surrounding tissues due to the burst effect. Increasing the local concentration and prolonging the biological effect of the growth factors are thus of great significance [2]. Therefore, an optimized bFGF delivery system i.e. a collagen membrane loaded with bFGF fused to the collagen-binding domain (CBD) has been constructed. This combination significantly reduced the random diffusion of bFGF in vivo and increased target delivery at the endometrium. The recombinant proteins were transported in a location-specific manner with collagen, and the effective concentration was maintained in the injured area.
In a previous study, Li et al. [96] applied the CBD-bFGF delivery system to reconstruct a severely damaged uterine wall and found that CBD-bFGF could promote its functional regeneration. The pregnancy rate of the CBD-bFGF group (86.67%) was close to that of the sham-operated group (100%), suggesting a nearly full uterine recovery from injury. VEGF, one of the most commonly studied angiogenic growth factors, is critical to the proliferation of endothelial cells, formation of new vessels, and re-epithelialization of the endometrium. In another study, a CBD-VEGF delivery system was injected into the scarred area of a full-thickness injury model of rat uterus. Healthy, well-vascularized tissues with thick uterine walls and organized smooth muscles were generated. Remarkably, embryos were successfully implanted into the scar sites of the CBD-VEGF group, whereas in the native VEGF or PBS groups, they were only implanted in normal tissues. This indicates that CBD-VEGF has a beneficial effect on the remodeling of the uterus and can even repair endometrial scarring [97].
In another study [98], the sustained release of the collagen-binding bFGF was administrated around the scarred endometrium guided by ultrasound every 4 weeks in 18 patients (2–4 times). After treatment, menstrual blood volume, endometrial thickness, and the scarred endometrial area improved. Histological analysis results showed that blood vessel density increased noticeably. Three patients (3/18) achieved pregnancy over 20 gestational weeks. Therefore, the administration of bFGF to the surrounding scarred endometrium may provide a new therapeutic approach for patients with endometrial fibrosis.
7. Application of other scaffold materials in endometrial regenerative medicine
Poly(glycerol sebacate) (PGS) is a representative synthetic bioelastomer with the characteristics of controllable degradation, high plasticity, and excellent biocompatibility. Therefore, PGS has been widely used in a variety of biomedical applications, especially for soft tissue regeneration [99]. One of its most important features is robust elasticity, which enables it to easily sustain and restore various deformations in soft tissues and in dynamic mechanical environments, without mechanical irritations to the surroundings. PGS films have also been demonstrated to prevent postoperative adhesions. Furthermore, PGS porous scaffolds have been shown to be good carriers for numerous types of cells, such as human umbilical vein endothelial cells, mesenchymal stem cells, and bone marrow stromal cells. Xiao et al. [100] loaded BMSCs on a synthetic PGS scaffold, which potentially recovered the different deformations of soft tissues in various dynamic conditions, without external irritations. They compared the effect of PGS with those of poly(lactic-co-glycolic acid) (PLGA) and collagen scaffolds in the resumption of damaged rat uteruses. PGS scaffold significantly prolonged the retention time of BMSCs in a wounded rat uterus model. More importantly, BMSCs can directly differentiate into endometrial stromal cells after the transplantation of PGS/BMSCs constructs, but not PLGA/BMSCs and collagen/BMSCs. In addition, the levels of TGF-β1, bFGF, VEGF, and IGF in the injured endometrium adjacent to PGS/BMSCs constructs were higher than the other group. Lastly, transplantation of PGS/BMSCs resulted in better morphology and recovery of the damaged uterus. The receptive fertility of PGS/BMSCs is similar to that of collagen/BMSCs but is significantly higher than that of PLGA/BMSCs. PGS showed more improvement in proliferation and endometrial morphology.
Chitosan is natural polysaccharide usually extracted from marine sources. It is soluble in low pH aqueous solutions and is biocompatible, biodegradable, non-toxic, and anti-bacterial. Chitosan can be easily chemically modified based on its amino group to improve properties such as solubility, antioxidant activities, and cell adhesion [101,102]. Meanwhile, heparin is a sulfated natural glycosaminoglycan widely found in biological tissues. It has affinities to growth factors and could extend their half-lives while increasing their bioactivities [18]. In situ drug delivery system has become increasingly popular because of its physical barrier function and controlled release of drug carrier. Wenbo et al. [103] reported a facile approach for fabricating chitosan-heparin hydrogels in a controlled-release manner and their application for IUA. The in situ drug delivery of SDF-1α for controlled release of hydrogels to an injured rat uterus showed that endogenous c-kit positive stem cells (HSCs) were recruited to the injury site, thus promoting wound recovery. After 7 days of treatment, the uterus treated with SDF-1α-releasing hydrogels showed no difference in terms of endometrial thickness, number of glands, and fibrosis level with the control group. Chitosan-heparin hydrogel could thus be a candidate for the uterine injury healing, as well as other wound dressing drug delivery systems.
In addition to direct cell-loading in the scaffolds, many strategies are focused on the surface, or on structure modification, to improve biocompatibility and enhance absorption for cell attachment and delivery of bioactive growth factors, hormones, and extracellular vesicles [104,105]. Bacterial cellulose (BC) is a biocompatible and water absorbable bacteria scaffold and has been used in bone, blood vessel, and nerve repair. The dense structure of BC networks has pores whose sizes are not large enough to allow migration. Supplementation of other materials such as silk fibroin (SF) increased pore size and subsequently, induced a significant increase in cell adhesion and cell viability in comparison with pure BC [[106], [107], [108]]. Cai et al. [109] developed a chemotactic composite scaffold by incorporating recombinant human stromal cell–derived factor-1α (rhSDF-1α) into a silk fibroin-bacterial cellulose (SF-BC) membrane carrier. In this study, they demonstrated that the SF-BC membrane possessed good physical, chemical, and biocompatibility properties in vitro. Because the plasticity and tensile strength of the composite membrane scaffold were similar to those of the rat uterus, this could promote the regeneration of the uterus after injury and further support the growth of implanted embryos. SDF-1, released from SF-BC, may promote the migration of adjacent uterine cells to the injury site, induce the regeneration of full-thickness uterine injury, and improve the pregnancy outcome of the damaged uterus. Hence, the better pregnancy outcomes observed in this study may be due to SDF-1 promoting vascularization and greater formation of mature endometrium.
Droplet-based systems have been directly used to synthesize particles and encapsulate many biological entities for biomedical applications due to their powerful encapsulation capability and facile versatility [110]. Microfluidics are systems with integrated microchannels, where small quantities of fluids could flow in a controlled manner. Droplet-based microfluidics have been recognized as a promising approach to building cell–loaded microspheres owing to their ability to control pore size, morphology, and microstructure [111,112]. Cai et al. [113] first reported the use of droplet microfluidics for the porous scaffold construction for IUA treatment. They presented a new drug-loaded porous scaffold based on a microfluidic droplet template, which combines the characteristics of the artificial biocompatible material GelMA and the natural polysaccharide material Na-alginate. Thus, the scaffold not only possessed compressibility but also facilitated drug delivery and release. The system improved neovascularization, cellularized the damaged tissues, and repaired the endometrium. These features confirm that drug-loaded porous scaffolds can be alternatives to improve postoperative IUA.
8. Summary
Despite the considerable advances in the etiology and treatment of IUA, the cellular and molecular mechanisms underlying its development remain unclear. To date, no efficient therapy has been developed to prevent its recurrence. Comprehensive treatment with hysteroscopy is the main treatment, among other popular IUA treatment methods including hormone therapy with estrogen, stem cells, TGF-β inhibitor, G-CSF, and potential targets. Although these strategies have been explored in IUA, none have been proved to be effective or achieve the desired result in clinical treatment. Despite having a few ethical disputes, stem cells are easy to obtain and possess the function of self-immune regulation. However, stem-cell treatment is only under the preliminary research stage, and it cannot be widely applied in the clinical setting because of their low survival rate, difficulty of inducing differentiation, and stem cell tumorigenesis.
Current studies have demonstrated that bio-scaffolds combined with stem cells or therapeutic factors for targeted intrauterine administration have achieved certain effects, either in the treatment of IUA patients or in model animals, thereby reducing the harm caused by systemic administration. However, information on the long-term safety and the recurrence of IUA remain limited. Biological scaffolds can support the attachment and proliferation of endometrial cells and can deliver stem cells or bioactive molecules to promote endometrial regeneration. However, the biocompatibility and mechanical properties of biomaterials are key issues for consideration. To improve the effectiveness of stem cells or therapeutic factors, many studies on delivery strategies have focused on the surface or structural modifications of bio-scaffold materials to achieve better biocompatibility and stronger adsorption capacity, thus improving cell attachment and the delivery of growth factors, hormones, and extracellular vesicles. The reconstruction of the biomimetic repair microenvironment after endometrial injury may require the synergy among therapeutic factors and stem cells. Future studies should focus on multimodal combination using formulation technology to combine therapeutic factors with stem cells and achieve better therapeutic effect, such as the combination strategy of microparticle systems loaded with therapeutic factors and biological scaffolds loaded with stem cells. The combination of slow and controlled release technology of pharmaceutical preparations with local targeted drug delivery technology and tissue engineering is expected to solve the bottleneck of currently available clinical IUA treatment (Fig. 5).
Fig. 5.
Biomaterial-based approaches to uterus regeneration include (i) bonding strategies of therapeutic factor-loaded scaffolds (A. direct loading or adsorption, B. immobilization through the formation of ionic complexes, C. immobilization through specific heparin-mediated interaction, and D. particulate systems) and (ii) fabrication of synthetic stem cells by encapsulating stem cell therapeutics with biomaterials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was partially supported by the Natural Science Foundation of Zhejiang Province [grant number LYY19H300002]; the Hangzhou Health Science and Technology Plan [grant number A20200682]; the Clinical Research Foundation of Zhejiang Province Medical Association [grant number 2020ZYC-A411]; and the Natural Science Foundation of Hangzhou Medical College [grant number 2016XZA02].
Contributor Information
H.-F. Lv, Email: qingganyiyang@163.com.
Y.-Z. Zhao, Email: zyzpharm@163.com.
References
- 1.Gurung S., Deane J.A., Masuda H., Maruyama T., Gargett C.E. Stem cells in endometrial physiology. Semin. Reprod. Med. 2015;33:326–332. doi: 10.1055/s-0035-1558405. [DOI] [PubMed] [Google Scholar]
- 2.Zhu X., Péault B., Yan G., Sun H., Hu Y., Ding L. Stem cells and endometrial regeneration: from basic research to clinical trial. Curr. Stem Cell Res. Ther. 2019;14:293–304. doi: 10.2174/1574888X14666181205120110. [DOI] [PubMed] [Google Scholar]
- 3.Chen G., Liu L., Sun J., Zeng L., Cai H., He Y. Foxf2 and Smad6 co-regulation of Collagen 5A2 transcription is involved in the pathogenesis of intrauterine adhesion. J. Cell Mol. Med. 2020;24:2802–2818. doi: 10.1111/jcmm.14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooker A., Fraenk D., Brölmann H., Huirne J. Prevalence of intrauterine adhesions after termination of pregnancy: a systematic review. Eur. J. Contracept. Reprod. Health Care. 2016;21:329–335. doi: 10.1080/13625187.2016.1199795. [DOI] [PubMed] [Google Scholar]
- 5.Gilman A.R., Dewar K.M., Rhone S.A., Fluker M.R. Intrauterine adhesions following miscarriage: look and learn. J. Obstet. Gynaecol. Can. 2016;38:453–457. doi: 10.1016/j.jogc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Hou X., Liu Y., Streuli I., Dällenbach P., Dubuisson J., Ansaldi Y., Pluchino N. Endometrial regeneration in Asherman's syndrome: clinical and translational evidence of stem cell therapies. Curr. Stem Cell Res. Ther. 2019;14:454–459. doi: 10.2174/1574888X14666190213100528. [DOI] [PubMed] [Google Scholar]
- 7.Johary J., Xue M., Zhu X., Xu D., Velu P.P. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J. Minim. Invasive Gynecol. 2014;21:44–54. doi: 10.1016/j.jmig.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Lin X.N., Zhou F., Wei M.L., Yang Y., Li Y., Li T.C., Zhang S.Y. Randomized, controlled trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis. Fertil. Steril. 2015;104:235–240. doi: 10.1016/j.fertnstert.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Abudukeyoumu A., Li M.Q., Xie F. Transforming growth factor-β1 in intrauterine adhesion. Am. J. Reprod. Immunol. 2020;84 doi: 10.1111/aji.13262. [DOI] [PubMed] [Google Scholar]
- 10.Cervelló I., Gil-Sanchis C., Santamaría X., Cabanillas S., Díaz A., Faus A., Pellicer A., Simón C. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil. Steril. 2015;104 doi: 10.1016/j.fertnstert.2015.08.032. 1552–1560.e1. [DOI] [PubMed] [Google Scholar]
- 11.Azizi R., Aghebati-Maleki L., Nouri M., Marofi F., Negargar S., Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: stem cell-based therapy. Biomed. Pharmacother. 2018;102:333–343. doi: 10.1016/j.biopha.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 12.Simoni M., Taylor H.S. Therapeutic strategies involving uterine stem cells in reproductive medicine. Curr. Opin. Obstet. Gynecol. 2018;30:209–216. doi: 10.1097/GCO.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y., Sun H., Zhu H., Zhu X., Tang X., Yan G., Wang J., Bai D., Wang J., Wang L., Zhou Q., Wang H., Dai C., Ding L., Xu B., Zhou Y., Hao J., Dai J., Hu Y. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res. Ther. 2018;9:192. doi: 10.1186/s13287-018-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F., Hu S., Wang S., Cheng K. Cell and biomaterial-based approaches to uterus regeneration. Regen. Biomater. 2019;6:141–148. doi: 10.1093/rb/rbz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai X., Liu J., Cao S., Wang L. Mechanisms of endometrial fibrosis and the potential application of stem cell therapy. Discov. Med. 2019;27:267–279. [PubMed] [Google Scholar]
- 16.Jahanbani Y., Davaran S., Ghahremani-Nasab M., Aghebati-Maleki L., Yousefi M. Scaffold-based tissue engineering approaches in treating infertility. Life Sci. 2020;240:117066. doi: 10.1016/j.lfs.2019.117066. [DOI] [PubMed] [Google Scholar]
- 17.Evans-Hoeker E.A., Young S.L. Endometrial receptivity and intrauterine adhesive disease. Semin. Reprod. Med. 2014;32:392–401. doi: 10.1055/s-0034-1376358. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W., Jin K., Li J., Qiu X., Li S. Delivery of stromal cell-derived factor 1α for in situ tissue regeneration. J. Biol. Eng. 2017;11:22. doi: 10.1186/s13036-017-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salazar C.A., Isaacson K., Morris S. A comprehensive review of Asherman's syndrome: causes, symptoms and treatment options. Curr. Opin. Obstet. Gynecol. 2017;29:249–256. doi: 10.1097/GCO.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 20.Murakami K., Lee Y.H., Lucas E.S., Chan Y.W., Durairaj R.P., Takeda S., Moore J.D., Tan B.K., Quenby S., Chan J.K.Y., Gargett C.E., Brosens J.J. Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology. 2014;155:4542–4553. doi: 10.1210/en.2014-1370. [DOI] [PubMed] [Google Scholar]
- 21.Mutlu L., Hufnagel D., Taylor H.S. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol. Reprod. 2015;92:138. doi: 10.1095/biolreprod.114.126771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma J.B., Roy K.K., Pushparaj M., Gupta N., Jain S.K., Malhotra N., Mittal S. Genital tuberculosis: an important cause of Asherman's syndrome in India. Arch. Gynecol. Obstet. 2008;277:37–41. doi: 10.1007/s00404-007-0419-0. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Liu L., Luo Y., Chen M., Huan Y., Fang R. Prevalence and Impact of chronic endometritis in patients with intrauterine adhesions: a prospective cohort study. J. Minim. Invasive Gynecol. 2017;24:74–79. doi: 10.1016/j.jmig.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Santamaria X., Isaacson K., Simón C. Asherman's syndrome: it may not be all our fault. Hum. Reprod. 2018;33:1374–1380. doi: 10.1093/humrep/dey232. [DOI] [PubMed] [Google Scholar]
- 25.Sonan Y., Aoki S., Enomoto K., Seki K., Miyagi E. Placenta accreta following hysteroscopic lysis of adhesions caused by Asherman's syndrome: a case report and literature review. Case Rep. Obstet. Gynecol. 2018;2018:6968382. doi: 10.1155/2018/6968382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai H., Li H., He Y. Interceed and estrogen reduce uterine adhesions and fibrosis and improve endometrial receptivity in a rabbit model of intrauterine adhesions. Reprod. Sci. 2016;23:1208–1216. doi: 10.1177/1933719116632923. [DOI] [PubMed] [Google Scholar]
- 27.Seo E., Kim W.Y., Hur J., Kim H., Nam S.A., Choi A., Kim Y.M., Park S.H., Chung C., Kim J., Min S., Myung S.J., Lim D.S., Kim Y.K. The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci. Rep. 2016;6:31931. doi: 10.1038/srep31931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorgenson A.J., Choi K.M., Sicard D., Smith K.M.J., Hiemer S.E., Varelas X., Tschumperlin D.J. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am. J. Physiol. Cell Physiol. 2017;312:C277–C285. doi: 10.1152/ajpcell.00205.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H.Y., Ge T.X., Pan Y.B., Zhang S.Y. Advanced role of hippo signaling in endometrial fibrosis: implications for intrauterine adhesion. Chin. Med. J. (Engl.) 2017;130:2732–2737. doi: 10.4103/0366-6999.218013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D., Ha C., Zhang X., Zhang Z., Liu P. Molecular implication of ADAM-15 and −17 in intrauterine adhesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;170:264–269. doi: 10.1016/j.ejogrb.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., Ma N., Sun Q., Huang C., Liu Y., Luo X. Elevated NF-κB signaling in Asherman syndrome patients and animal models. Oncotarget. 2017;8:15399–15406. doi: 10.18632/oncotarget.14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao G., Cao Y., Zhu X., Tang X., Ding L., Sun H., Li J., Li X., Dai C., Ru T., Zhu H., Lu J., Lin C., Wang J., Yan G., Wang H., Wang L., Dai Y., Wang B., Li R., Dai J., Zhou Y., Hu Y. Transplantation of collagen scaffold with autologous bone marrow mononuclear cells promotes functional endometrium reconstruction via downregulating ΔNp63 expression in Asherman's syndrome. Sci. China Life Sci. 2017;60:404–416. doi: 10.1007/s11427-016-0328-y. [DOI] [PubMed] [Google Scholar]
- 33.Trombino S., Servidio C., Curcio F., Cassano R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics. 2019;11:407. doi: 10.3390/pharmaceutics11080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dicker K.T., Gurski L.A., Pradhan-Bhatt S., Witt R.L., Farach-Carson M.C., Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10:1558–1570. doi: 10.1016/j.actbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasvani S., Kulkarni P., Rawtani D. Hyaluronic acid: a review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020;151:1012–1029. doi: 10.1016/j.ijbiomac.2019.11.066. [DOI] [PubMed] [Google Scholar]
- 36.Zheng F., Xin X., He F., Liu J., Cui Y. Meta-analysis on the use of hyaluronic acid gel to prevent intrauterine adhesion after intrauterine operations. Exp. Ther. Med. 2020;19:2672–2678. doi: 10.3892/etm.2020.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fei Z., Xin X., Fei H., Yuechong C. Meta-analysis of the use of hyaluronic acid gel to prevent intrauterine adhesions after miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;244:1–4. doi: 10.1016/j.ejogrb.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Liu H., Xu Y., Yi N., Yi W. Efficacy and safety of hyaluronic acid gel for the prevention of intrauterine adhesion: a meta-analysis of randomized clinical trials. Gynecol. Obstet. Invest. 2018;83:227–233. doi: 10.1159/000486674. [DOI] [PubMed] [Google Scholar]
- 39.Fei Z., Bin Z., Xin X., Fei H., Yuechong C. Meta-analysis on the use of hyaluronic acid gel to prevent recurrence of intrauterine adhesion after hysteroscopic adhesiolysis. Taiwan. J. Obstet. Gynecol. 2019;58:731–736. doi: 10.1016/j.tjog.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Mao X., Tao Y., Cai R., Zhang J., Gao H., Chen Q., Kuang Y., Zhang S. Cross-linked hyaluronan gel to improve pregnancy rate of women patients with moderate to severe intrauterine adhesion treated with IVF: a randomized controlled trial. Arch. Gynecol. Obstet. 2020;301:199–205. doi: 10.1007/s00404-019-05368-6. [DOI] [PubMed] [Google Scholar]
- 41.Kou L., Jiang X., Xiao S., Zhao Y.Z., Yao Q., Chen R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J. Contr. Release. 2020;318:25–37. doi: 10.1016/j.jconrel.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Highley C.B., Prestwich G.D., Burdick J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016;40:35–40. doi: 10.1016/j.copbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Liu F., Hu S., Yang H., Li Z., Huang K., Su T., Wang S., Cheng K. Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of Asherman's syndrome. Adv. Healthc. Mater. 2019;8 doi: 10.1002/adhm.201900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim Y.Y., Park K.H., Kim Y.J., Kim M.S., Liu H.C., Rosenwaks Z., Ku S.Y. Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater. 2019;89:139–151. doi: 10.1016/j.actbio.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 45.Russo E., Villa C. Poloxamer hydrogels for biomedical applications. Pharmaceutics. 2019;11:671. doi: 10.3390/pharmaceutics11120671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zarrintaj P., Ramsey J.D., Samadi A., Atoufi Z., Yazdi M.K., Ganjali M.R., Amirabad L.M., Zangene E., Farokhi M., Formela K., Saeb M.R., Mozafari M., Thomas S., Poloxamer A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020;110:37–67. doi: 10.1016/j.actbio.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 47.Sponchioni M., Capasso Palmiero U.C., Moscatelli D. Thermo-responsive polymers: applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C: Mater. Biol. Appl. 2019;102:589–605. doi: 10.1016/j.msec.2019.04.069. [DOI] [PubMed] [Google Scholar]
- 48.Gioffredi E., Boffito M., Calzone S., Giannitelli S.M., Rainer A., Trombetta M., Mozetic P., Chiono V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia CIRP. 2016;49:125–132. doi: 10.1016/j.procir.2015.11.001. [DOI] [Google Scholar]
- 49.Holland I., Logan J., Shi J., McCormick C., Liu D., Shu W. 3D biofabrication for tubular tissue engineering. Biodes Manuf. 2018;1:89–100. doi: 10.1007/s42242-018-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chi Y., He P., Lei L., Lan Y., Hu J., Meng Y., Hu L. Transdermal estrogen gel and oral aspirin combination therapy improves fertility prognosis via the promotion of endometrial receptivity in moderate to severe intrauterine adhesion. Mol. Med. Rep. 2018;17:6337–6344. doi: 10.3892/mmr.2018.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang S.S., Xia W.T., Xu J., Xu H.L., Lu C.T., Zhao Y.Z., Wu X.Q. Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17beta-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int. J. Nanomed. 2017;12:5643–5657. doi: 10.2147/IJN.S137237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng S., Zhang Y., Zhang J., Wang H., Ren B. ERK in learning and memory: a review of recent research. Int. J. Mol. Sci. 2010;11:222–232. doi: 10.3390/ijms11010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsumura S., Ohta T., Yamanouchi K., Liu Z., Sudo T., Kojimahara T., Seino M., Narumi M., Tsutsumi S., Takahashi T., Takahashi K., Kurachi H., Nagase S. Activation of estrogen receptor α by estradiol and cisplatin induces platinum-resistance in ovarian cancer cells. Canc. Biol. Ther. 2017;18:730–739. doi: 10.1080/15384047.2016.1235656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S.S., Xu X.X., Xiang W.W., Zhang H.H., Lin H.L., Shen L.E., Lin Q., Lin F., Zhou Z.Y. Using 17β-estradiol heparin-poloxamer thermosensitive hydrogel to enhance the endometrial regeneration and functional recovery of intrauterine adhesions in a rat model. FASEB J. 2020;34:446–457. doi: 10.1096/fj.201901603RR. [DOI] [PubMed] [Google Scholar]
- 55.Yao Q., Zheng Y.W., Lan Q.H., Wang L.F., Huang Z.W., Chen R., Yang Y., Xu H.L., Kou L., Zhao Y.Z. Aloe/poloxamer hydrogel as an injectable β-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur. J. Pharmaceut. Sci. 2020;148:105316. doi: 10.1016/j.ejps.2020.105316. [DOI] [PubMed] [Google Scholar]
- 56.Liu X., Yang Y., Niu X., Lin Q., Zhao B., Wang Y., Zhu L. An in situ photo-crosslinkable platelet rich plasma-Complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017;62:179–187. doi: 10.1016/j.actbio.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 57.Li H., Ham T.R., Neill N., Farrag M., Mohrman A.E., Koenig A.M., Leipzig N.D. A hydrogel bridge incorporating immobilized growth factors and neural stem/progenitor cells to treat spinal cord injury. Adv. Healthc. Mater. 2016;5:802–812. doi: 10.1002/adhm.201500810. [DOI] [PubMed] [Google Scholar]
- 58.Park J.W., Hwang S.R., Yoon I.S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules. 2017;22:1259. doi: 10.3390/molecules22081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu H.L., Xu J., Zhang S.-S., Zhu Q.Y., Hui Jin B.-, ZhuGe D.L., Shen B.X., Wu X.Q., Xiao J., Zhao Y.Z. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017;24:867–881. doi: 10.1080/10717544.2017.1333173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu H.L., Xu J., Shen B.X., Zhang S.S., Jin B.H., Zhu Q.Y., ZhuGe D.L., Wu X.Q., Xiao J., Zhao Y.Z. Dual regulations of thermosensitive heparin-poloxamer hydrogel using ε-Polylysine: bioadhesivity and controlled KGF release for enhancing wound healing of endometrial injury. ACS Appl. Mater. Interfaces. 2017;9:29580–29594. doi: 10.1021/acsami.7b10211. [DOI] [PubMed] [Google Scholar]
- 61.Gargett C.E., Chan R.W.S., Schwab K.E. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol. Cell. Endocrinol. 2008;288:22–29. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 62.Yang H., Wu S., Feng R., Huang J., Liu L., Liu F., Chen Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res. Ther. 2017;8:267. doi: 10.1186/s13287-017-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El Banna N.E., Hatem E., Heneman-Masurel A., Léger T., Baïlle D., Vernis L., Garcia C., Martineau S., Dupuy C., Vagner S., Camadro J.M., Huang M.E. Redox modifications of cysteine-containing proteins, cell cycle arrest and translation inhibition: involvement in vitamin C-induced breast cancer cell death. Redox Biol. 2019;26:101290. doi: 10.1016/j.redox.2019.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qi Y., Lohman J., Bratlie K.M., Peroutka-Bigus N., Bellaire B., Wannemuehler M., Yoon K.J., Barrett T.A., Wang Q. Vitamin C and B3 as new biomaterials to alter intestinal stem cells. J. Biomed. Mater. Res. A. 2019;107:1886–1897. doi: 10.1002/jbm.a.36715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han Q., Du Y. Advances in the application of biomimetic endometrium interfaces for uterine bioengineering in female infertility. Front. Bioeng. Biotechnol. 2020;8:153. doi: 10.3389/fbioe.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao Q., Zheng Y.W., Lan Q.H., Kou L., Xu H.L., Zhao Y.Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C: Mater. Biol. Appl. 2019;104:109942. doi: 10.1016/j.msec.2019.109942. [DOI] [PubMed] [Google Scholar]
- 67.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Yao Q., Choi J.H., Dai Z., Wang J., Kim D., Tang X., Zhu L. Improving tumor specificity and anticancer activity of dasatinib by dual-targeted polymeric micelles. ACS Appl. Mater. Interfaces. 2017;9:36642–36654. doi: 10.1021/acsami.7b12233. [DOI] [PubMed] [Google Scholar]
- 70.Campo H., Cervelló I., Pellicer A. Bioengineering strategies of the uterus towards improving current investigative models and female reproductive health. Facts Views Vis. Obgyn. 2019;11:87–99. [PMC free article] [PubMed] [Google Scholar]
- 71.Miyazaki K., Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials. 2014;35:8791–8800. doi: 10.1016/j.biomaterials.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 72.Santoso E.G., Yoshida K., Hirota Y., Aizawa M., Yoshino O., Kishida A., Osuga Y., Saito S., Ushida T., Furukawa K.S. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PloS One. 2014;9 doi: 10.1371/journal.pone.0103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hellström M., Moreno-Moya J.M., Bandstein S., Bom E., Akouri R.R., Miyazaki K., Maruyama T., Brännström M. Bioengineered uterine tissue supports pregnancy ina rat model. Fertil. Steril. 2016;106 doi: 10.1016/j.fertnstert.2016.03.048. 487–496.e1. [DOI] [PubMed] [Google Scholar]
- 74.Campo H., Baptista P.M., López-Pérez N., Faus A., Cervelló I., Simón C. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol. Reprod. 2017;96:34–45. doi: 10.1095/biolre/bio143396. [DOI] [PubMed] [Google Scholar]
- 75.Daryabari S.S., Kajbafzadeh A.M., Fendereski K., Ghorbani F., Dehnavi M., Rostami M., Garajegayeh B.A., Tavangar S.M. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J. Assist. Reprod. Genet. 2019;36:1211–1223. doi: 10.1007/s10815-019-01463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miki F., Maruyama T., Miyazaki K., Takao T., Yoshimasa Y., Katakura S., Hihara H., Uchida S., Masuda H., Uchida H., Nagai T., Shibata S., Tanaka M. The orientation of a decellularized uterine scaffold determines the tissue topology and architecture of the regenerated uterus in rats. Biol. Reprod. 2019;100:1215–1227. doi: 10.1093/biolre/ioz004. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H., Zhang Q., Zhang J., Sheng F., Wu S., Yang F., Li W. Urinary bladder matrix scaffolds improve endometrial regeneration in a rat model of intrauterine adhesions. Biomater. Sci. 2020;8:988–996. doi: 10.1039/C9BM00651F. [DOI] [PubMed] [Google Scholar]
- 78.Yao Q., Zheng Y.W., Lin H.L., Lan Q.H., Huang Z.W., Wang L.F., Chen R., Xiao J., Kou L., Xu H.L., Zhao Y.Z. Exploiting crosslinked decellularized matrix to achieve uterus regeneration and construction. Artif. Cells Nanomed. Biotechnol. 2020;48:218–229. doi: 10.1080/21691401.2019.1699828. [DOI] [PubMed] [Google Scholar]
- 79.Shakouri-Motlagh A., Khanabdali R., Heath D.E., Kalionis B. The application of decellularized human term fetal membranes in tissue engineering and regenerative medicine (TERM) Placenta. 2017;59:124–130. doi: 10.1016/j.placenta.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Gan L., Duan H., Xu Q., Tang Y.-Q., Li J.-J., Sun F.-Q., Wang S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy. 2017;19:603–616. doi: 10.1016/j.jcyt.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Cai H., Qiao L., Song K., He Y. Oxidized, regenerated cellulose adhesion barrier plus intrauterine device prevents recurrence after adhesiolysis for moderate to severe intrauterine adhesions. J. Minim. Invasive Gynecol. 2017;24:80–88. doi: 10.1016/j.jmig.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 82.Li C., Cai A., Sun C., Wu B., Chen X., Mao Y., Zhang Y., Gou Y., Yu J., Wang Y., Yu H., Wang J. The study on the safety and efficacy of amnion graft for preventing the recurrence of moderate to severe intrauterine adhesions. Genes Dis. 2020;7:266–271. doi: 10.1016/j.gendis.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aamodt J.M., Grainger D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016;86:68–82. doi: 10.1016/j.biomaterials.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Y., Fei W., Zhao Y., Wang F., Zheng X., Luan X., Zheng C. Sustained delivery of 17β-estradiol by human amniotic extracellular matrix (HAECM) scaffold integrated with PLGA microspheres for endometrium regeneration. Drug Deliv. 2020;27:1165–1175. doi: 10.1080/10717544.2020.1801891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mafi P., Hindocha S., Mafi R., Khan W.S. Evaluation of biological protein-based collagen scaffolds in cartilage and musculoskeletal tissue engineering--a systematic review of the literature. Curr. Stem Cell Res. Ther. 2012;7:302–309. doi: 10.2174/157488812800793045. [DOI] [PubMed] [Google Scholar]
- 86.Xia L., Meng Q., Xi J., Han Q., Cheng J., Shen J., Xia Y., Shi L. The synergistic effect of electroacupuncture and bone mesenchymal stem cell transplantation on repairing thin endometrial injury in rats. Stem Cell Res. Ther. 2019;10:244. doi: 10.1186/s13287-019-1326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ding L., Li X., Sun H., Su J., Lin N., Péault B., Song T., Yang J., Dai J., Hu Y. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials. 2014;35:4888–4900. doi: 10.1016/j.biomaterials.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 88.Lee H.J., Choi B., Kim Y., Lee S.E., Jin H.J., Lee H.S., Chang E.J., Kim S.W. The upregulation of toll-like receptor 3 via autocrine IFN-β signaling drives the senescence of human umbilical cord blood-derived mesenchymal stem cells through JAK1. Front. Immunol. 2019;10:1659. doi: 10.3389/fimmu.2019.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu L., Ding L., Wang L., Cao Y., Zhu H., Lu J., Li X., Song T., Hu Y., Dai J. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res. Ther. 2017;8:84. doi: 10.1186/s13287-017-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xin L., Lin X., Pan Y., Zheng X., Shi L., Zhang Y., Ma L., Gao C., Zhang S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019;92:160–171. doi: 10.1016/j.actbio.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 91.Song T., Zhao X., Sun H., Li X., Lin N., Ding L., Dai J., Hu Y. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng. A. 2015;21:353–361. doi: 10.1089/ten.tea.2014.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Z., Yan G., Diao Q., Yu F., Li X., Sheng X., Liu Y., Dai Y., Zhou H., Zhen X., Hu Y., Péault B., Ding L., Sun H., Li H. Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat. Stem Cell Res. Ther. 2019;10:179. doi: 10.1186/s13287-019-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao G., Huang B.L., Rigueur D., Wang W., Bhoot C., Charles K.R., Baek J., Mohan S., Jiang J., Lyons K.M. CYR61/CCN1 regulates sclerostin levels and bone maintenance. J. Bone Miner. Res. 2018;33:1076–1089. doi: 10.1002/jbmr.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong C., Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers. 2016;8:42. doi: 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei P., Chen X.L., Song X.X., Han C.S., Liu Y.X. VEGF, bFGF, and their receptors in the endometrium of rhesus monkey during menstrual cycle and early pregnancy. Mol. Reprod. Dev. 2004;68:456–462. doi: 10.1002/mrd.20104. [DOI] [PubMed] [Google Scholar]
- 96.Li X., Sun H., Lin N., Hou X., Wang J., Zhou B., Xu P., Xiao Z., Chen B., Dai J., Hu Y. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials. 2011;32:8172–8181. doi: 10.1016/j.biomaterials.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 97.Lin N., Li X., Song T., Wang J., Meng K., Yang J., Hou X., Dai J., Hu Y. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials. 2012;33:1801–1807. doi: 10.1016/j.biomaterials.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 98.Jiang P., Tang X., Wang H., Dai C., Su J., Zhu H., Song M., Liu J., Nan Z., Ru T., Li Y., Wang J., Yang J., Chen B., Dai J., Hu Y. Collagen-binding basic fibroblast growth factor improves functional remodeling of scarred endometrium in uterine infertile women: a pilot study. Sci. China Life Sci. 2019;62:1617–1629. doi: 10.1007/s11427-018-9520-2. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y., Tian K., Hao J., Yang T., Geng X., Zhang W. Biomimetic poly(glycerol sebacate)/polycaprolactone blend scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2019;30:53. doi: 10.1007/s10856-019-6257-3. [DOI] [PubMed] [Google Scholar]
- 100.Xiao B., Yang W., Lei D., Huang J., Yin Y., Zhu Y., You Z., Wang F., Sun S. PGS scaffolds promote the in vivo survival and directional differentiation of bone marrow mesenchymal stem cells restoring the morphology and function of wounded rat uterus. Adv. Healthc. Mater. 2019;8 doi: 10.1002/adhm.201801455. [DOI] [PubMed] [Google Scholar]
- 101.Rasool A., Ata S., Islam A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019;203:423–429. doi: 10.1016/j.carbpol.2018.09.083. [DOI] [PubMed] [Google Scholar]
- 102.Zhang L., Ma Y., Pan X., Chen S., Zhuang H., Wang S. A composite hydrogel of chitosan/heparin/poly (gamma-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018;180:168–174. doi: 10.1016/j.carbpol.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 103.Wenbo Q., Lijian X., Shuangdan Z., Jiahua Z., Yanpeng T., Xuejun Q., Xianghua H., Jingkun Z. Controlled releasing of SDF-1α in chitosan-heparin hydrogel for endometrium injury healing in rat model. Int. J. Biol. Macromol. 2020;143:163–172. doi: 10.1016/j.ijbiomac.2019.11.184. [DOI] [PubMed] [Google Scholar]
- 104.Li C., Ouyang L., Pence I.J., Moore A.C., Lin Y., Winter C.W., Armstrong J.P.K., Stevens M.M. Buoyancy-driven gradients for biomaterial fabrication and tissue engineering. Adv. Mater. 2019;31 doi: 10.1002/adma.201900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shadish J.A., Benuska G.M., DeForest C.A. Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater. 2019;18:1005–1014. doi: 10.1038/s41563-019-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang Y., Wang J., Yang F., Shao Y., Zhang X., Dai K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C: Mater. Biol. Appl. 2017;75:1034–1041. doi: 10.1016/j.msec.2017.02.174. [DOI] [PubMed] [Google Scholar]
- 107.Wang X., Chen Z., Zhou B., Duan X., Weng W., Cheng K., Wang H., Lin J. Cell-sheet-derived ECM coatings and their effects on BMSCs responses. ACS Appl. Mater. Interfaces. 2018;10:11508–11518. doi: 10.1021/acsami.7b19718. [DOI] [PubMed] [Google Scholar]
- 108.Rebelo A.R., Liu C., Schäfer K.H., Saumer M., Yang G., Liu Y. Poly(4-vinylaniline)/Polyaniline Bilayer-functionalized bacterial cellulose for flexible electrochemical biosensors. Langmuir. 2019;35:10354–10366. doi: 10.1021/acs.langmuir.9b01425. [DOI] [PubMed] [Google Scholar]
- 109.Cai H., Wu B., Li Y., Liu Y., Shi L., Gong L., Xia Y., Heng B.C., Wu H., Ouyang H., Zhu Z., Zou X. Local delivery of silk-cellulose incorporated with stromal cell-derived factor-1α functionally improves the uterus repair. Tissue Eng. A. 2019;25:1514–1526. doi: 10.1089/ten.tea.2018.0283. [DOI] [PubMed] [Google Scholar]
- 110.Feng H., Zheng T., Li M., Wu J., Ji H., Zhang J., Zhao W., Guo J. Droplet-based microfluidics systems in biomedical applications. Electrophoresis. 2019;40:1580–1590. doi: 10.1002/elps.201900047. [DOI] [PubMed] [Google Scholar]
- 111.Song Y., Shimanovich U., Michaels T.C.T., Ma Q., Li J., Knowles T.P.J., Shum H.C. Fabrication of fibrillosomes from droplets stabilized by protein nanofibrils at all-aqueous interfaces. Nat. Commun. 2016;7:12934. doi: 10.1038/ncomms12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lopa S., Mondadori C., Mainardi V.L., Talò G., Costantini M., Candrian C., Święszkowski W., Moretti M. Translational application of microfluidics and bioprinting for stem cell-based cartilage repair. Stem Cell. Int. 2018;2018:1–14. doi: 10.1155/2018/6594841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cai Y., Wu F., Yu Y., Liu Y., Shao C., Gu H., Li M., Zhao Y. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019;84:222–230. doi: 10.1016/j.actbio.2018.11.016. [DOI] [PubMed] [Google Scholar]