Summary
Non-IBD colitides (NIBDC) are intestinal diseases clinically and endoscopically overlapping with Inflammatory Bowel Diseases (IBD), sometimes with a similar histological picture. NIBDC include entities such as infectious colitis, ischemic colitis, pseudomembranous colitis, eosinophilic colitis, autoimmune enterocolitis, segmental colitis associated with diverticulosis, drug-induced colitis, radiation-induced colitis, diversion colitis, and microscopic colitis, this last including two entities: collagenous and lymphocytic colitis. The knowledge of the most useful histological features and the main clinical data for each entity is mandatory in daily clinical practice, for correct pathological diagnosis and clinical management.
Key words: colitis, infectious colitis, ischemic colitis, drug-induced colitis, microscopic colitis
Introduction
Non-Inflammatory Bowel Disease (IBD) colitides (NIBDC) are pathological conditions having a similar clinical presentation and endoscopic appearance with Inflammatory Bowel Disease (IBD), but characterized by peculiar histological features, different etiology, clinical course and therapy. NIBDC include infectious colitis (IFC), ischemic colitis (ISC), pseudomembranous colitis (PMC), cosinophilic colitis (ESC), autoimmune enterocolitis (AIE), segmental colitis associated with diverticular disease (SCAD), drug-induced colitis (DIC), radiation-induced colitis (RIC), diversion colitis (DVC) and microscopic colitis (MC), this latest one consisting of two entities: lymphocytic colitis (LC) and collagenous colitis (CC) 1,2.
Below the histopathological features of the most frequent NIBDCs are summarized. The main differential diagnosis is with IBD, especially at the onset stage of IBD, for the characterization of which we refer to ‘IBD-colitides’ in this special issue of Pathologica 3.
Infectious colitis (IFC)
Being associated with global human movement due to tourist and business purposes, infectious colitis (IFC), which is caused by enteric pathogens, is a worldwide condition 4. Moreover, IFCs are the most frequent cause of childhood death in the developing countries and the second leading cause of death, after cardiovascular disease, in the entire world 4. Many aspects of modern living also contribute to the transmission of enteric pathogens, such as water sports, camping, and consumption of raw or poorly cooked meat. Finally, the most recent immunosuppressive therapies in oncologic field increase the number of IFC cases 5. Although many IFCs have a self-limited clinical course, post-infectious bowel syndrome with prolonged diarrhea or abdominal pain can occur in some patients, which requires a more in-depth clinical investigation. Endoscopic findings range from a quite normal to diffuse erythematous aspect of the intestinal mucosa; in some cases, an IBD-like appearance with aphthous ulcers can be discovered, raising the suspicion of Crohn’s or ulcerative colitis 5. Histologically, many of these cases show a nonspecific inflammatory pattern associated with edema and congestion of the stroma but with no crypt distortion nor basal plasmacytosis 6. In these cases, clinical integration with serological data and coproculture are needed for a correct diagnosis and identification of the pathogens 5. On the other hand, infections due to spirochetes or protozoa can be easily detected on the mucosal surface, if present. Spirochetes appear as luminal fringes adherent to the epithelium (Fig. 1A), highlighted with Giemsa, Warthin-Starry histochemical stain or with immunohistochemistry based on specific antibodies7. Protozoans such as Entamoeba histolytica (Fig. 1B) are typically found clustered within necrotic debris, and appear morphologically similar to histiocytes containing phagocytosed erythrocytes. PAS and Grocott’s stains can be used for the identification of histoplasma spores 8 (Fig. 1C). Strongyloides stercoralis infection is characterized by adult worms and larvae typically located in the crypt lumina or the lamina propria, and it is surrounded by an inflammatory infiltrate rich in eosinophils. Cytomegalovirus (CMV)-infected cells, typically detected in ulcer debris or in endothelial, stromal or epithelial cells, can be recognized morphologically or highlighted immunohistochemically with the specific anti-CMV antibody (Fig. 1E) 9. It is important to remember that CMV colitis can complicate IBD and make its recognition difficult, and that quantification is crucial for establishing the correct therapy 3. More specific, or even diagnostic, morphological patterns are characterized by granulomatous inflammation: PAS-positive schistosomal eggs, at times calcified, are contained in microgranulomas associated to fibrotic submucosal thickening and lymphoid hyperplasia 10. Mycobacterium can be identified by Ziehl-Neelsen stain or with bio-molecular PCR-based methods in caseating necrotic granuloma surrounded by multinucleated giant cells 11. Yersinia enterocolitica infection causes intramural non-caseating granulomas often accompanied by stellate abscesses, usually located in the terminal ileum, mimicking the Crohn’s disease (IBD-like pattern) 5. Finally, other pathogens, such as Enterohemorragic Escherichia coli and Clostridium perfringens, can lead to an ischemic-like pattern with haemorrhage associated to acute inflammation, marked oedema, crypt withering, and lamina propria hyalinization with mucosal necrosis 5.
Figure 1.
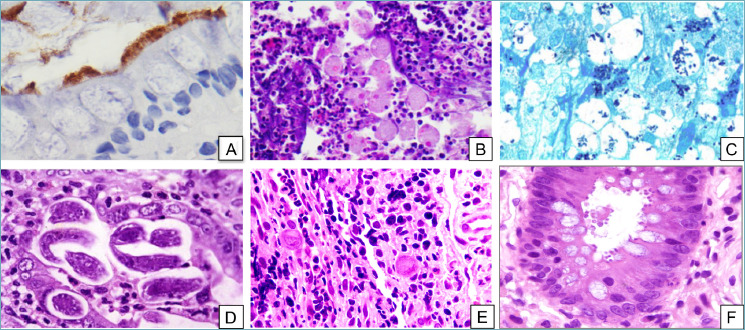
Infectious colitis. (A) Intestinal spirochetosis. The fuzzy luminal border is highlighted by immunohistochemistry for Treponema pallidum (peroxidase-diaminobenzidine, 40x). (B) Entamoeba histolytica trophozoites containing engulfed erythrocytes can be seen among neutrophils and inflammatory debris (H&E, 20x). (C) Grocott’s stain makes Histoplasma capsulatum stand out as intensely-stained small oval structures within the cytoplasm of macrophages (Grocott’s methenamine silver stain, 20x). (D) Strongyloides stercoralis can be found lurking in the crypts (H&E, 20x). (E) CMV-infected cells stand out as markedly enlarged cells with typical “owl’s eye” intranuclear inclusions (H&E, 20x). (F) Cryptosporidium parvum forms small round bodies on the luminal border of enterocytes (H&E, 20x). !
KEY POINTS:
Inflammation in IFC is usually a non-specific pattern; IBD-like and ischemic-like patterns may also be recognized;
features of chronicity, such as crypt distortion and basal plasmacytosis, are usually absent;
an accurate evaluation can sometimes help to identify the responsible pathogen, either directly (e.g. Entamoeba, Strongyloides) or by its effects (e.g. cytomegalovirus);
clinical and serological integration with coproculture is often imperative to discriminate between onset-stage IBD and IFC.
Pseudomembranous colitis (PMC)
Pseudomembranous colitis (PMC) is a distinctive infective colitis associated with Clostridium difficile infection. Clostridium difficile is a Gram-positive anaerobic bacillus causing various mucosal alterations, histologically ranging from a mild inflammation to a deeper pattern of involvement, morphologically resembling ischemic injury but with characteristic findings 12 (Fig. 2). Cryptitis and crypt abscesses are detectable in biopsy or surgical samples. Typical “mushroom-shaped” pseudomembranes can be found on the luminal surface and contain fibrin, epithelial debris, and inflammatory cells intermingled with mucus. Although the histological detection of these morphological alterations of the colon can be suggestive of Clostridium difficile infection, definitive diagnosis is based on microbiological isolation of the organism and detection of the bacterial toxin 12. PMC can also present without the characteristic pseudomembranes on the superficial epithelium, in particular in patients using immunosuppressive agents 13.
Figure 2.
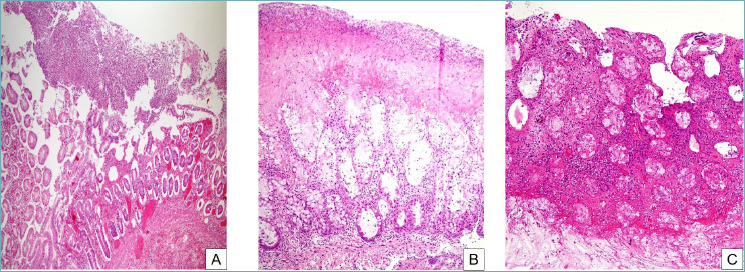
Pseudomembranous colitis. (A) At low magnification, a mushroom-shaped necroinflammatory exudate overlies an acutely injured mucosa (H&E, 10x). (B) The pseudomembrane is composed of inflammatory cells, necrotic debris, mucus and fibrin. Cryptitis and crypt abscesses are evident in the mucosa (H&E, 20x). (C) The severity of the inflammation can vary widely, from a mild picture to severe and deep acute inflammation which can obscure other morphological features (H&E, 20x).
KEY POINTS:
Characteristic mushroom-shaped pseudomembranes composed of mucus, fibrin, neutrophils and necrotic cells overlie the mucosa in PMC;
mucosal damage can range from mild to severe, with cryptitis, crypt abscesses and gland dilation without basal plasmocytosis.
Drug-induced colitis (DIC)
Drug-induced injury of the gastrointestinal tract is a relatively frequent but often underestimated event due to several factors, including the lack of knowledge of the side effects of drugs (amplified by the frequent abuse of some drugs, as is the case with non-steroidal anti-inflammatory drugs -NSAIDs-) 14. Knowledge of the temporal relationships between the medication start date and the onset of symptoms is critical15. Different patterns of DIC have been described: pseudomembranous colitis pattern, microscopic colitis pattern, IBD-type pattern, ischemic-type colitis pattern and, eosinophilic-type colitis pattern 16. The diagnosis is essentially based on clinical suspicion and the awareness that a patient is taking drugs, as in most cases of DIC colonoscopy is unremarkable. Often, endoscopy shows flat or slightly elevated circular hyper- or hypochromic lesions compared to the surrounding mucosa, sometimes with a erythematous border, distributed along a small vessel “cherry tree” appearance) 17. The microscopic spectrum ranges in hematoxylin and eosin stained sections (H&E), from mild oedema, to fulminant colitis with severe lesions including extensive necrosis (Fig. 3) 15,16. The most common but not specific finding is an intense eosinophilic infiltrate 17,18. Other abnormalities are hemorrhages, hematomas, and epithelial apoptosis. Cyclosporin can promote villous transformation and epithelial regeneration in ulcerative colitis, at times associated with histological changes that can mimic dysplasia 15,16. The acute colitis pattern has been described following treatment with laxatives, such as bisphosphonate enemas and bisacodyl, with carbamazepine, isotretinoin, and with NSAIDs such as mefenamic acid, diclofenac, naproxen and pirprofen 15,16. The ulcerative colitis-like and the microscopic colitis patterns were reported with gold salts treatment for rheumatoid arthritis, diclofenac and aminoglutethimide (an antineoplastic agent), but the morphological description is not always accurate 15,16. A Crohn’s disease-like pattern with granulomas has been reported with diclofenac, naproxen and clofazimine (with this latter, crystals can be demonstrated in the granulomas). A graft-versus-host-disease-(GVHD) like pattern has also been described with Mycophenolate mofetil 15,16. The apoptotic pattern was recently described with brentuximab, a monoclonal anti-CD30 antibody used to treat relapses of Hodgkin lymphoma 19. Pseudomelanosis, melanosis and pseudolipofuscinosis coli are the result of long-term use and abuse of anthraquinone-containing laxatives. In this condition, the lipofuscin-type pigment is detectable in the macrophages of the lamina propria of the large bowel (including the appendix). Kayexalate-sorbitol (sodium polystyrene sulfonate), used for the treatment of hyperkalemia, has been reported to induce intestinal necrosis in uremic patients; the presence of the typical crystals is the hallmark of this condition 15,16. Necrosis is observed in endoscopic and surgical specimens of the stomach, small intestine and colon 15. The use of cocaine and related products has been associated with mesenteric thrombosis, perforation and visceral ischemia in the lower GI tract. Case reports have described the occurrence of colon ischemia in association with several amphetamines, including dextroamphetamine, methylphenidate and methamphetamine, commonly known as “speed”, “crank”, “ice” or ecstasy”. Their mechanism of action is that of a sympathomimetic drug, causing vasoconstriction 15,16. Intestinal ischemia has been reported in patients treated with interleukin-2 (IL-2) in combination with interferon, which has been linked to the ischemia also as a single agent 15,16. Ischemia was noted predominantly in the ascending colon. Histopathology revealed thrombi in capillaries and venules. Ergot compounds are generally safe, but, in some instances, colitis with bowel wall necrosis and perforation or strictures have been recorded. Diuretics have been implicated in the development of both non-occlusive mesenteric ischemia and colonic ischemia. Oral contraceptives have been reported to cause focal and segmental colitis with aphthoid ulcers on a background of normal mucosa and rectal sparing 15,16. Biopsies showed focal, nonspecific ulceration on a background of a normal mucosa. Cessation of oral contraceptive use resulted in prompt relief of symptoms and healing 15,16. Finally, the most recent biological agents acting via inhibition of key regulatory molecules are capable of adverse gastrointestinal effects, most notably in the colon, as ipilimumab (a monoclonal antibody used in advanced melanoma) causing GVHD pattern colitis, brentuximab (as described above) 19, rituximab (an anti-CD20 monoclonal antibody used in the treatment of hematologic malignancies and rheumatologic disorders), and etanercept that can cause the onset of IBD, (both Crohn’s disease and ulcerative colitis) and microscopic colitis 20,21.
Figure 3.
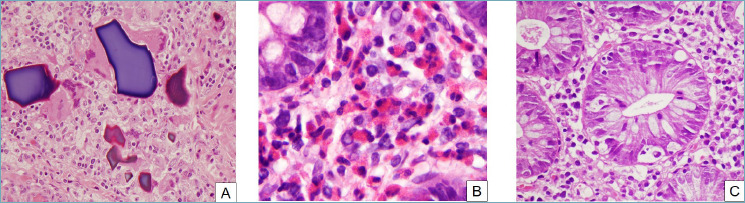
Drug-induced colitis. Various histological patterns. (A) Drug crystals and remnants can sometimes be seen in a colonic biopsy. In some cases, such as this one, they are surrounded by a foreign-body reaction (H&E, 20x). (B) Eosinophilic colitis can be caused by drugs. In this picture, numerous eosinophils infiltrate the lamina propria and crypt epithelium, forming microabscesses and sometimes degranulating (H&E, 40x). (C) Prominent apoptotic bodies can be seen in some cases of drug-induced colitis, especially with some laxatives and antineoplastic agents (H&E, 40x).
Before closing this paragraph, we deem it important to recall Professor Chandrasoma: “Many of these conditions, when encountered in biopsy or surgical resection specimens, usually receive nonspecific pathologic diagnoses. Careful clinical correlation and the knowledge of dosages and temporal relationship of drug usage is required by the pathologist to even attempt to reach the conclusion that the pathology is caused by drug toxicity: unfortunately, this information is rarely available to the pathologist” 22.
KEY POINTS:
The possible patterns of injury in DIC are numerous and variegated;
most patterns of DIC are not specific, but they rather mimic another disease;
when the histological features are not convincing and/or they do not match the clinical picture, knowledge of the drugs taken by the patient is fundamental to suspect DIC.
Eosinophilic colitis (ESC)
Eosinophils are normal constituents in the lamina propria of the colon. Intestinal tissue hypereosinophilia may represent a primary eosinophilic disorder or a secondary response to other diseases; therefore, the diagnosis of primitive eosinophilic colitis (ESC) is usually made after the exclusion of other pathological conditions and taking into consideration the site of the endoscopic sampling 23. In fact, eosinophils are more abundant in the right colon, while they are rarely seen in the left colon and in the rectum, with some individual variability due to age, geographical region, climate, exposure to allergenic foods, and infective agents 24. ESC is an uncommon chronic disease which can affect individuals of any age; a combination of genetic predisposition, dysbiosis, and the environment (ingested or inhaled allergens) is likely to be its etiology; patients may report a history of allergy (e.g. asthma, rhinitis, drug allergy and/or eczema). By definition, peripheral blood eosinophilia in ESC is absent; if present, an intestinal localization of systemic hypereosinophilic syndrome is the correct diagnosis 23. There is a growing evidence suggesting that primitive ESC is an allergic disorder mediated by Th2-type cytokines 24. Patients with ESC have non-specific symptoms and signs similar to IBDs, such as nausea, vomiting, diarrhea and/or clinical manifestations secondary to malabsorption. Most ESCs in adults are related to drug injury, which can be ascribed mainly to NSAIDs (Fig. 3B) 23,24. Other secondary causes of tissue eosinophilia in the intestinal mucosa are infections, parasites, IBD, vasculitis, connective tissue disorders, fungal infection, neoplasia, systemic disorders of eosinophils and mast cells, and radiation effect. For these reasons, the distinction between normal and pathologically increased eosinophil count is difficult, which has complicated attempts to define standardized histological criteria for diagnosis of ESC23,24. At the moment, there are still no widely accepted, evidence-based criteria for defining a pathological increase in the number of eosinophils and how many high power field (HPF) have to be considered in the count. The site-specific ranges of eosinophil counts and distribution pattern of eosinophils are more useful as a guide for when to consider an eosinophilic disorder and prompt a search for secondary causes, than as absolute thresholds 23,24.
Histological findings very suggestive of ESC are: detection of sheets or aggregates of eosinophils in the lamina propria and muscolaris mucosae, as well as accumulation of eosinophils in the crypt epithelium 25. The cut-off value in general is over 60 x 10 HPF based on the experience of Odze in children 26 but field there is no complete agreement, for example, for eosinophilic esophagitis 24. In advanced or severe disease, ascites sometimes associated with symptoms of intestinal obstruction has been described, and the surgical specimen shows an intense, transmural eosinophilic infiltration with serosal and perintestinal fat involvement 27.
KEY POINTS:
ESC is a diagnosis of exclusion;
the minimum number of eosinophils required for a diagnosis of ESC is > 60 x 10 HPF in the left colon, sigma and rectum;
other useful morphological features include eosinophilic microabscesses, degranulation and exocytosis in the gland epithelium.
Autoimmune enterocolitis (AIE)
Autoimmune enterocolitis (AIE) is an uncommon disease, clinically characterized by protracted diarrhea that can occur in both pediatric and adult patients, frequently those affected by primary immunodeficiencies. Different clinical forms of the disease are recognized 28: 1) primary (pediatric); 2) syndromic (pediatric); 3) primary (sporadic) of adults; 4) secondary (iatrogenic) of adults; and 5) paraneoplastic. The endoscopic aspects are non-specific and vary from mucosal hyperemia to scalloping of the mucosa, ulcerations, and a mosaic-like appearance 29. Histological patterns encompass: a) active duodenitis, b) celiac disease-like pattern (characterized by villous atrophy, glandular crypt hyperplasia, and marked increase in the number of T lymphocytes in the surface epithelium (> 25 lymphocytes/100 epithelial cells), c) acute graft versus host disease (GVHD)-like, with hyperplasia of the crypts and the presence of apoptotic bodies, and d) mixed pattern mainly prevalent in adults (Fig. 4) 30.
Figure 4.
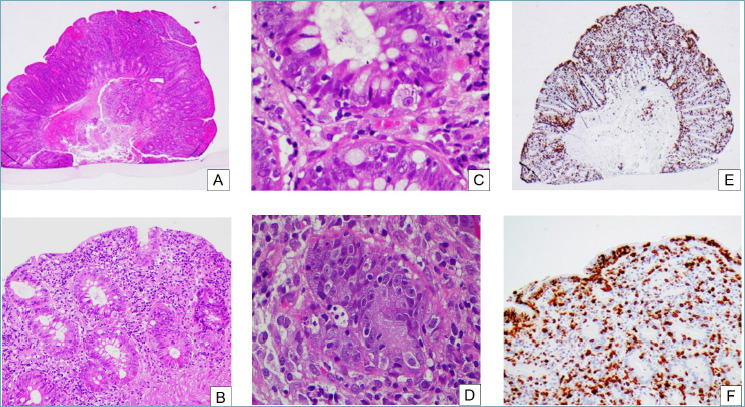
Autoimmune enteropathy. (A) At low power the mucosa appears reactive, with regenerative crypts and an hypercellular lamina propria (H&E, 2x). (B) Crypts are regenerative, with mucin depletion and surface epithelial damage (H&E, 10x). (C) On closer inspection, there are prominent apoptotic bodies in the surface and crypt epithelium (H&E, 40x). (D) Sometimes, satellite lymphocytes can be seen around the apoptotic bodies (H&E, 40x). (E) CD3 immunohistochemistry reveals a greatly increased T lymphocyte population (H&E, 2x). (F) The T lymphocytes can be seen in the lamina propria as well as in crypt and surface epithelium (H&E, 20x).
KEY POINTS:
AIE is a group of diseases with different clinical, endoscopic and histological features. Careful clinical-pathological correlation is required for diagnosis;
in addition to active inflammation, increased crypt apoptosis can be striking to the extent that it can resemble GVHD;
lymphocyte-predominant cases can resemble lymphocytic colitis, and goblet cells can be absent in cases with anti-goblet cell antibodies.
Microscopic colitis (MC)
Microscopic colitis (MC) is an umbrella term for two distinct diseases characterized by clinical history of chronic watery (non-bloody) diarrhea, normal endoscopic appearance of the ileo-colic mucosa and one of two peculiar histopathological patterns: collagenous colitis (CC) and lymphocytic colitis (LC) 31. The pathogenesis of MC is still unknown, but it is likely to be multifactorial; moreover, abnormal immune response, impaired intestinal barrier function, and myofibroblast dysfunction (the latest in CC) are believed to play a major role32. Smoking and several drugs, in particular NSAIDs, have also been associated with the development of this disease. Finally, MC is, for some authors, an autoimmune disease: in fact, MC is associated with other autoimmune conditions, such as rheumatoid arthritis, collagen vascular diseases, thyroid disorders, and celiac disease 32. Because the histological damage may be patchy and discontinuous, there may be a significant variation between specimens from different portions of the bowel or even within a single biopsy specimen. Usually, CC is more common in the right colon and less frequent in the sigmoid and rectum, whereas LC has a more ‘patchy’ distribution in the colonic mucosa 32,33. For these reasons, multiple biopsy sample should be obtained throughout the whole colon and submitted in separate vials 32. The diagnosis of CC on routine H&E stained sections is based on the presence of a thick amorphous hyaline eosinophilic band immediately beneath the superficial epithelium of the mucosa, of thickness greater than 10 μm, associated with inflammatory features in the lamina propria 33,34 (Fig. 5A). The mucosal architecture is well preserved. Masson trichrome stain is useful for the detection of the thickened collagen band (Fig. 5B). The histological diagnosis of LC is based upon a diffuse increase of intraepithelial T lymphocytes (IELs) (> 20 IELs per 100 epithelial cells) in the surface epithelium accompanied by an increase of lamina propria inflammatory cells 31 (Fig. 5C,D). In general, the finding of more than 20 intraepithelial lymphocytes is considered diagnostic for LC. Inflammation may be less prominent in the left colon, and retains the normal CD3/CD8- phenotype 33. A particular variant of MC is named microscopic colitis, incomplete (MCi) 32. This entity is characterized by the presence of clinical features of MC without the morphological criteria necessary for a diagnosis of LC or CC. Histology shows an abnormal collagenous band < 10 μm in thickness, or an increased number of IELs < 20 per 100 epithelial cells 32,35. Unusual localization of increase IELs, such as within the crypt epithelium, or association of MC with giant cells and pseudomembranes are also been described 32,35.
Figure 5.
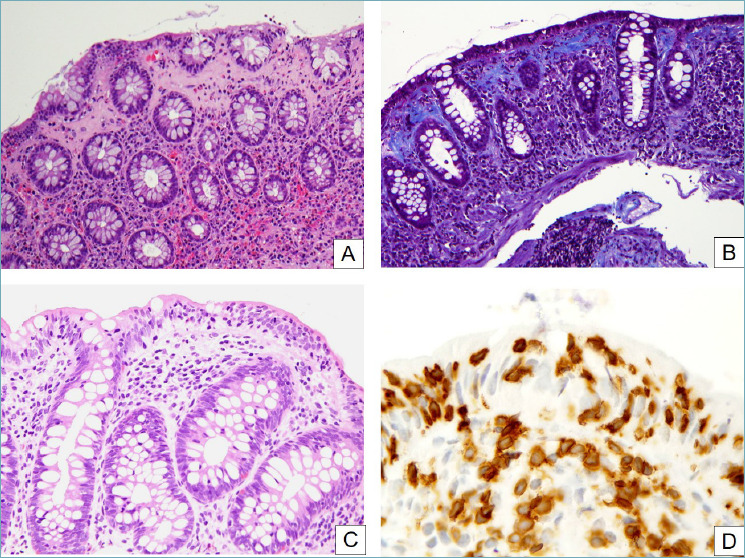
Microscopic colitis. (A) In collagenous colitis, a thick and irregular collagen band underlies the surface epithelium and often entraps inflammatory cells. Surface epithelial detachment, shown here, is also typical. The underlying lamina propria is inflamed and shows an increased amount of eosinophils (H&E, 20x). (B) A Masson trichrome clearly highlights the irregularly thickened subepithelial collagen band (Masson trichrome, 20x). (C) In lymphocytic colitis, numerous T lymphocytes infiltrate the gland epithelium (H&E, 20x). (D) CD3 stain clearly highlights the pathologically increased intraepithelial lymphocytes (peroxidase-diaminobenzidine, 40x).
KEY POINTS:
MC is an umbrella term for two diseases which share the clinical picture and endoscopic aspects: collagenous colitis and lymphocytic colitis;
CC is histologically characterized by an irregularly thickened (> 10 μm) subepithelial collagen band, associated with inflammatory features in the lamina propria;
LC shows an increase in intrapithelial T lymphocytes (> 20/100 epithelial cells) associated with lamina propria inflammation.
Ischemic colitis (ISC)
Ischemic colitis (ISC) is an entity resulting from reversible vascular occlusion, occurring in both older and young patients. In the elderly, ISC is more frequently due to vascular diseases such as hypertension, coronary artery disease, atrial fibrillation, chronic renal disease, multi-drugs therapy and diabetes mellitus; while in young patients, it is more frequently associated with cocaine or vascular autoimmune systemic disease, including Behçet disease or systemic lupus erythematosus 36. Manifestations of ISC injury into the gastrointestinal tract are variable: only 10% of patients present with gangrenous colitis, whereas most other patients have transient colitis, reversible colopathy, chronic segmental colitis or strictures, or, in a minority of cases (2.5%), fulminant pancolitis; this latter leading to surgical resection 37.
The endoscopic appearance of ISC is fairly characteristic in fulminant or severe disease, as well as in patients with chronic injury. Mild mucosal changes are often patchy and include areas of pale and edematous mucosa with scattered petechial hemorrhages. Longitudinal superficial ulcers may be evident as injury progresses. Severe injury appears as gray-green or black discoloration of the mucosa, often with pseudomembranes. Chronic ischemia can produce strictures, fistulae, and mucosal atrophy or granularity that can suggests an IBD diagnosis 37.
Histological findings on H&E from bioptic samples include atrophic crypts lined by small, irregular cells with eosinophilic cytoplasm, dark nuclei and evident nucleoli dislocated in congested or hemorrhagic hyalinized stroma in acute cases; coagulative necrosis and capillary microthrombi may be found, mainly in the acute early stage 38. Damage to capillaries and/or medium-size vessels can be documented in autoimmune disorders. Sometimes, different etiologies can be added together showing ISC on bioptic samples. On surgical resection, areas of necrosis are usually so extensive that the underlying etiology is not apparent on histological sections. Mesenteric vessels can show abnormal size and thrombi. The wide range of etiologies of ISC with their clinical manifestations are very peculiar and specific and, for more details, we invite the reader to refer to a selected bibliography 37.
KEY POINTS:
Ischemic colitis shows a predilection for the elderly, but young patients with predisposing factors can also be affected;
the histological picture is dominated by coagulative necrosis in acute cases, crypt and epithelial cell atrophy in a hyalinized and sometimes hemorrhagic stroma in chronic cases;
a wide spectrum of causes are described, with peculiar clinical findings, leading to a similar histological picture.
Radiation-induced colitis (RIC)
Local radiation therapy, often combined with chemotherapy, is one of the treatments of different malignancies including rectal, prostatic and gynecological carcinomas, leading to intestinal mucosal damage, often limited to the rectum, known as radiation-induced colitis (RIC) 39. Histology of acute phase of RIC shows dilated crypts covered by reactive mucine-depleted epithelial cells, exhibiting bizarre nuclei and increased apoptosis (Fig. 6). A transmucosal inflammation with diffuse fibrosis and dilated vessels is observed in the chronic stage 40. In long-standing cases, glandular atrophy usually predominates; inflammation is mild to absent; and fibrosis appears 39.
Figure 6.
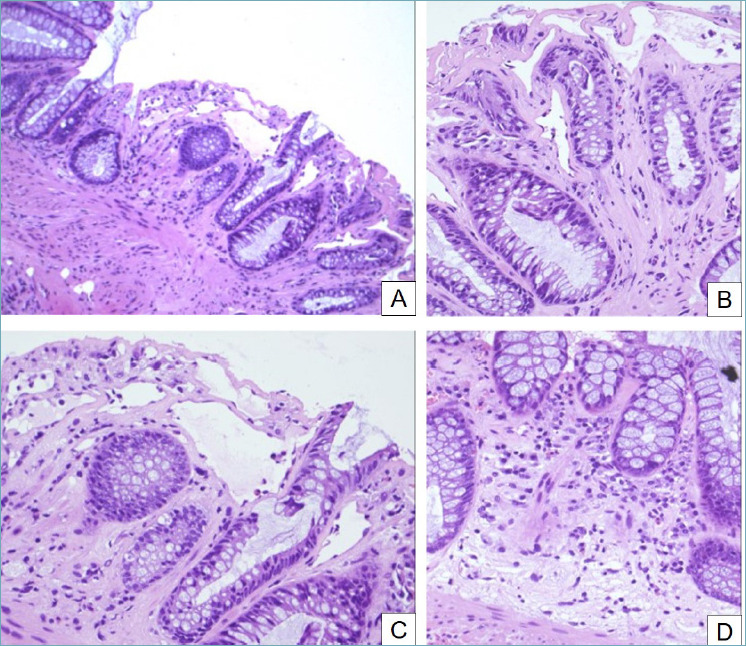
Radiation-induced colitis. (A) Crypt distortion, dilation and atrophy are evident (H&E, 10x). (B) Fibrosis of lamina propria, hyperplasia of crypts and dilated vascular channels (H&E, 20x). (C) Crypt atrophy, apoptotic bodies, goblet cell depletion and ectasia of small vessels are typical of radiation-induced colitis (H&E, 20x). (D) The lamina propria shows an inflammatory infiltrate that tends to decrease in long-standing cases (H&E, 20x).
KEY POINTS:
RIC is characterized by peculiar histological features at all stages;
the most evident feature is represented by markedly dilated capillaries, with prominent endothelial cell nuclei;
in acute phase, the glands show dilation, bizarre epithelial cell nuclei and some architectural distortion;
in chronic phase, the lamina propria is hyalinized and fibrotic; and sometimes stromal cells can have prominent nuclei, mimicking dysplasia.
Diversion colitis (DVC)
Diversion colitis (DVC) occurs in segments of the colon that are diverted from the fecal stream following surgery for congenital malformation, IBD or malignancies 41. Histological pictures include diversion colitis, proctitis and pouchitis, characterized by atrophic mucosa with mucin depletion and regenerative epithelial hyperplasia, associated with lymphoid follicular hyperplasia, and at times granuloma formation 41.
KEY POINTS:
Diagnosis of DVC cannot be made without surgical history;
characteristic histological features include a dense superficial lymphoplasmacytic infiltrate associated with prominent follicular hyperplasia;
the glands are regenerative and mucin-depleted; architectural distortion is not a feature but can be caused by the prominent inflammatory infiltrate.
Segmental colitis associated with diverticular disease (SCAD)
A small subset of patients with diverticulosis may develop segmental colitis associated with diverticular disease (SCAD). By definition, SCAD is a pathological entity characterized by a chronic inflammatory response involving the interdiverticular mucosa of the colonic segment involved. The rectum, by definition, is free of inflammation 42. The endoscopic pattern is characterized by a combination of congestion, hemorrhage, granularity and sometimes superficial ulceration involving the sigmoid colon and sparing both rectal and proximal colonic mucosa. In the involved tract, lesions are usually confined on the crests of colonic fold and sparing the orifices of diverticula. These findings represent a peculiarity of the disorders and need to be carefully detected, also for differential diagnosis with IBD. SCAD is histologically characterized by transmucosal chronic inflammation associated with crypt distortion and basal plasmocytosis 43. It is possible to identify two different histological settings: ulcerative-like pattern with abscesses and more evident crypt distortion, and Crohn-like pattern characterized by granulomas and florid lymphoid follicles in the deep lamina propria 44 (Fig. 7). Limitation of the mucosal lesion to the diverticular segment is the most important diagnostic criterion for SCAD. Therefore, to make a correct diagnosis, it is fundamental to obtain biopsy samples from the rectum and ascending and descending colon to distinguish SCAD from IBD 42.
Figure 7.
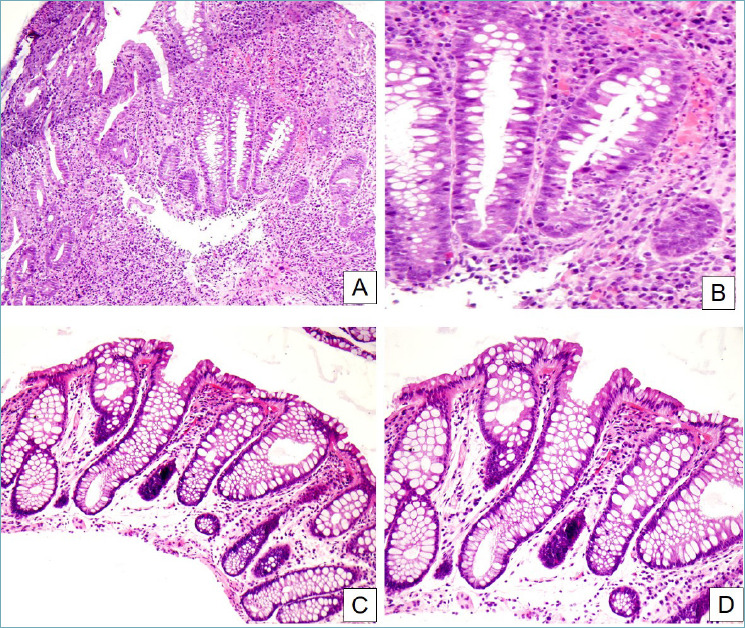
Segmental colitis associated with diverticular disease. (A) In this case, crypt distortion, cryptitis and basal plasmacytosis dominate the picture (H&E, 10x). (B) Detail of the previous image. Active inflammation and cryptitis can be appreciated, as well as the increased number of plasma cells and eosinophils in the inflammatory infiltrate (H&E, 40x). (C, D) In this other case, inflammation is much less florid, while crypt distortion and stromal edema are predominant (H&E, 20x and 40x respectively).
KEY POINTS:
SCAD can closely mimic histologically and clinically both Crohn’s disease and ulcerative colitis;
most cases mimic ulcerative colitis, with architectural distortion, cryptitis and crypt abscesses, and a dense lymphoplasmacytic infiltrate;
the key to the differential diagnosis with IBD is the distribution of the disease: in SCAD, only the interdiverticular mucosa is affected, and the rectum and proximal colon are usually spared.
Conclusions
Adequate sampling, careful histopathological examination of biopsies, close collaboration between gastroenterologists, endoscopists and pathologists, and the clinical history with laboratory data are needed in order to make an accurate diagnosis and to providethe most effective therapy in NIBDC.
Figures and tables
Acknowledgements
We are grateful to Ms. Chiara Di Giorgio (Scientific Direction of Foundation IRCCS Ospedale Casa Sollievo della Sofferenza, San Giovanni Rotondo) for editing and proofreading the manuscript. Special thanks to AITIC (Italian Association of Laboratory Technicians) for scientific support regarding methodology and handling of biopsy samples.
References
- 1.Schofield JB, Haboubi N. Histopathological mimics of inflammatory bowel disease. Inflamm Bowel Dis 2020. June 18;26(7):994-1009. https://doi.org/10.1093/ibd/izz232 10.1093/ibd/izz232 [DOI] [PubMed] [Google Scholar]
- 2.Villanacci V, Reggiani-Bonetti L, Caprioli F, et al. Histopathology of inflammatory bowel disease - Position statement of the Pathologists of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) and Italian Group of Gastrointestinal Pathologists (GIPAD-SIAPEC). Dig Liv Dis 2020;52:262-7. https://doi.org/10.1016/j.dld.2019.11.005 10.1016/j.dld.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Villanacci V, Reggiani-Bonetti L, Salviato T, et al. Histopathology of IBD Colitis. A practical approach from the pathologists of the Italian Group for the study of the gastrointestinal tract (GIPAD). Pathologica 2021;113:39-53. https://doi.org/10.32074/1591-951X-235 10.32074/1591-951X-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leder K, Torresi J, Brownstein JS, et al. Travel-associated illness trends and clusters, 2000-2010. Emerg Infect Dis 2015;19:1049-73. https://doi.org/10.3201/eid1907.121573 10.3201/eid1907.121573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamps LW. Update on infectious enterocolitis and the diseases that they mimic. Histopathology 2015;66:3-14. https://doi.org/10.1111/his.12582 10.1111/his.12582 [DOI] [PubMed] [Google Scholar]
- 6. MB, Shepherd NA. Diagnostic dilemmas in chronic inflammatory bowel disease Virchows Arch 2018;472:81-97. https://doi.org/10.1007/s00428-017-2235-7 10.1007/s00428-017-2235-7 [DOI] [PubMed] [Google Scholar]
- 7.Lemmens R T, Hauser B, et al. Intestinal spirochetosis: a case series and review of the literature. Pediatr Gastroenterol Hepatol Nutr 2019;22:193-200. https://doi.org/10.5223/pghn.2019.22.2.193 10.5223/pghn.2019.22.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahi CJ, Wheat LJ, Allen SD, et al. Gastrointestinal histoplasmosis. Am J Gastroenterol 2005;100:220-31. https://doi.org/10.1111/j.1572-0241.2005.40823.x 10.1111/j.1572-0241.2005.40823.x [DOI] [PubMed] [Google Scholar]
- 9.Reggiani-Bonetti L, Losi L, Di Gregorio C, et al. Cytomegalovirus infection of the upper gastrointestinal tract: a clinical and pathological study of 30 cases. Scand J Gastroenterol 2011;1228-35. https://doi.org/10.3109/00365521.2011.594083 10.3109/00365521.2011.594083 [DOI] [PubMed] [Google Scholar]
- 10.Slavik T, Lauwers GY. Navigating the jungles of tropical infectious gastrointestinal pathology: a pattern-based approach to the endoscopic biopsy. Virchows Arch 2018;472:135-47. https://doi.org/10.1007/s00428-017-2166-3 10.1007/s00428-017-2166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.I MP. Granulomas in the gastrointestinal tract: deciphering the Pandora’s box. Virchows Arch 2018;472:3-14. https://doi.org/10.1007/s00428-017-2210-3 10.1007/s00428-017-2210-3 [DOI] [PubMed] [Google Scholar]
- 12.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013;108:478-98; quiz 499. https://doi.org/10.1038/ajg.2013.4 10.1038/ajg.2013.4 [DOI] [PubMed] [Google Scholar]
- 13.Nomura K, Fujimoto Y, Yamashita M, et al. Absence of pseudomembranes in Clostridium difficile-associated diarrhea in patients using immunosuppression agents. Scand J Gastroenterol 2009;44:74-8. https://doi.org/10.1080/00365520802321238 10.1080/00365520802321238 [DOI] [PubMed] [Google Scholar]
- 14.Villanacci V, Casella G, Bassotti G. The spectrum of drug-related colitides: important entities, though frequently overlooked. Dig Liv Disease 2011;43:523-8. https://doi.org/10.1016/j.dld.2010.12.016 10.1016/j.dld.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 15.De Petris G, Caldero SG, Chen L, et al. Histopathological changes in the gastrointestinal tract due to drugs: an update for the surgical pathologist (part I of II). Int J Surg Pathol 2014;22:120-8. https://doi.org/10.1177/1066896913502229 10.1177/1066896913502229 [DOI] [PubMed] [Google Scholar]
- 16.De Petris G, Caldero SG, Chen L, et al. Histopathological changes in the gastrointestinal tract due to medications: an update for the surgical pathologist (part II of II) Int J Surg Pathol 2014;22:202-11.https://doi.org/10.1177/1066896913502230 10.1177/1066896913502230 [DOI] [PubMed] [Google Scholar]
- 17.Dore MP, Villanacci V, Manca A, et al. Cherry-tree colon: colonoscopic appearance suggesting drug-induced mucosal injury. Intern Emerg Med 2014;9:405-9. https://doi.org/10.1007/s11739-013-0930-1 10.1007/s11739-013-0930-1 [DOI] [PubMed] [Google Scholar]
- 18.Casella G, Villanacci V, Fisogni S, et al. Colonic left-side increase of eosinophils: a clue to drug-related colitis in adults. Aliment Pharmacol Ther 2009;29:535-41. https://doi.org/10.1111/j.1365-2036.2008.03913.x 10.1111/j.1365-2036.2008.03913.x [DOI] [PubMed] [Google Scholar]
- 19.Parente P, Graziano P, Scalzulli P, et al. Brentuximab-related apoptotic colopathy, Pathology 2020;52:483-4. https://doi.org/10.1016/j.pathol.2020.02.011 10.1016/j.pathol.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 20.Eckmann JD, Chedid V, Quinn KP, et al. De Novo colitis associated with rituximab in 21 patients at a tertiary center. Clin Gastroenterol Hepatol 2020;18:252-3. https://doi.org/10.1016/j.cgh.2019.03.027 10.1016/j.cgh.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 21.Korzenik J, Due Larsen M, Nielsen J, et al. Increased risk of developing Crohn’s disease or ulcerative colitis in 17 018 patients while under treatment with anti-TNFα agents, particularly etanercept, for autoimmune diseases other than inflammatory bowel disease. Aliment Pharmacol Ther 2019;50:289-94. https://doi.org/10.1111/apt.15370 10.1111/apt.15370 [DOI] [PubMed] [Google Scholar]
- 22.Chandrasoma P. Gastrointestinal pathology. McGraw-Hill Professional; 1998. [Google Scholar]
- 23.Walker MM, Potter M, Talley NJ. Eosinophilic gastroenteritis and other eosinophilic gut diseases distal to the oesophagus. Lancet Gastroenterol Hepatol 2018;3:271-80.https://doi.org/10.1016/S2468-1253(18)30005-0 10.1016/S2468-1253(18)30005-0 [DOI] [PubMed] [Google Scholar]
- 24.Conner JR, Kirsch R: The pathology and causes of tissue eosinophilia in the gastrointestinaltract. Histopathol 2017;71:177-99. https://doi.org/10.1111/his.13228 10.1111/his.13228 [DOI] [PubMed] [Google Scholar]
- 25.Geboes K. Eosinophilic colitis. Geboes K, Nemolato S, Leo M, Faa G, eds. Colitis: a practical approach to colon biopsy interpretation. Springer International Publishing Switzerland; 2014, pp. 151-153. https://doi.org/10.1007/978-3-319-08028-4 10.1007/978-3-319-08028-4 [DOI] [Google Scholar]
- 26.Odze RD, Wershil BK, Leichtner AM, et al. Allergic colitis in infants. J Pediatr 1995;126:163-70 [DOI] [PubMed] [Google Scholar]
- 27.McCarthy AJ, Sheahan K. Classification of eosinophilic disorders of the small and large intestine. Virchows Arch 2018;472:15-28. https://doi.org/10.1007/s00428-017-2249-1 10.1007/s00428-017-2249-1 [DOI] [PubMed] [Google Scholar]
- 28.Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep 2012;14:380-5. https://doi.org/10.1007/s11894-012-0276-2 10.1007/s11894-012-0276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montalto M, D’Onofrio F, Santoro L, et al. Autoimmune enteropathy in children and adults. Scand J Gastroenterol 2009;44:1029-36. https://doi.org/10.1080/00365520902783691 10.1080/00365520902783691 [DOI] [PubMed] [Google Scholar]
- 30.Villanacci V, Lougaris V, Ravelli A, et al. Clinical manifestations and gastrointestinal pathology in 40 patients with autoimmune enteropathy. Clin Immunol 2019;207:10-7. https://doi.org/10.1016/j.clim.2019.07.001 10.1016/j.clim.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 31.Bjørnbak C, Engel PJ, Nielsen PL, et al. Microscopic colitis: clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther 2011;34:1225-34. https://doi.org/10.1111/j.1365-2036.2011.04865.x 10.1111/j.1365-2036.2011.04865.x [DOI] [PubMed] [Google Scholar]
- 32.Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015;66:613-26. https://doi.org/10.1111/his.12592 10.1111/his.12592 [DOI] [PubMed] [Google Scholar]
- 33.Warren BF, Edwards CM, Travis SP. Microscopic colitis: classification and terminology. Histopathology 2002;40:374-6. https://doi.org/10.1046/j.1365-2559.2002.01341.x 10.1046/j.1365-2559.2002.01341.x [DOI] [PubMed] [Google Scholar]
- 34.Lazenby AJ. Collagenous and lymphocytic colitis. Semin Diagn Pathol 2005;22:295-300. https://doi.org/10.1053/j.semdp.2006.04.006 10.1053/j.semdp.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 35.Goudkade D, Fiehn A-MK, Landolfi S, et al. An investigation of European pathologists’ approach to diagnose microscopic colitis. Ann Diagn Pathol 2020;46:151520. https://doi.org/10.1016/j.anndiagpath.2020.151520 10.1016/j.anndiagpath.2020.151520 [DOI] [PubMed] [Google Scholar]
- 36.Brandt LJ, Feuerstadt P, Longstreth GF, et al. American College of Gastroenterology. ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol 2015;110:18-45. https://doi.org/10.1038/ajg.2014.395 10.1038/ajg.2014.395 [DOI] [PubMed] [Google Scholar]
- 37.Uberti G, Goldblum JR, Allende DS. Ischemic enterocolites and its differential diagnosis, Sem Diagn Pathol 2014;31:152-64. https://doi.org/10.1053/j.semdp.2014.02.001 10.1053/j.semdp.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 38.Fenster M, Feuerstadt P, Brandt LJ, et al. Real-world multicentre experience of the pathological features of colonic ischaemia and their relationship to symptom duration, disease distribution and clinical outcome. Colorectal Dis 2018;20:1132-41. https://doi.org/10.1111/codi.14323 10.1111/codi.14323 [DOI] [PubMed] [Google Scholar]
- 39.Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiothr Oncol 2014;112:140-4. https://doi.org/10.1016/j.radonc.2014.03.024 10.1016/j.radonc.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 40.Reggiani-Bonetti L, Domati F, Farinetti A, et al. Radiotherapy-induced mesorectum alterations: histological evaluation of 90 consecutive cases. Scand J Gastroenterol 2015;50:197-203. https://doi.org/10.3109/00365521.2014.983153 10.3109/00365521.2014.983153 [DOI] [PubMed] [Google Scholar]
- 41.Tominaga K, Kamimura K, Takahashi K, et al. Diversion colitis and pouchitis: A mini-review. World J Gastroenterol 2018;24:1734. https://doi.org/10.3748/wjg.v24.i16.1734 10.3748/wjg.v24.i16.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuomo R, Barbara G, Pace F, et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United European Gastroenterol J 2014;2:413-42. https://doi.org/10.1177/2050640614547068 10.1177/2050640614547068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schembri J, Bonello J, Christodoulou DK, et al. Segmental colitis associated with diverticulosis: is it the coexistence of colonic diverticulosis and inflammatory bowel disease? Ann Gastroenterol 2017;30:257-61. https://doi.org/10.20524/aog.2017.0126 10.20524/aog.2017.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tursi A, Inchingolo CD, Picchio M, et al. Histopathology of segmental colitis associated with diverticulosis resembles inflammatory bowel diseases. J Clin Gastroenterol 2015;49:350-1. https://doi.org/10.1097/MCG.0000000000000268 10.1097/MCG.0000000000000268 [DOI] [PubMed] [Google Scholar]


